Abstract
Epigenetics encompasses heritable but reversible modifications of gene expression that occur without changes in the DNA sequence and involve mechanisms such as DNA and RNA methylation and histone modifications. These mechanisms modulate chromatin architecture, genome stability, and cellular responses to environmental stressors, and their dysregulation contributes to oncogenesis and cancer progression. In parallel, radiotherapy remains a cornerstone of cancer treatment; furthermore, ionizing radiation induces epigenetic modifications alongside direct DNA double-strand breaks and oxidative damage. Radiation-induced epigenetic changes, including global or locus-specific DNA methylation shifts (e.g., genes promoter CpG islets), histone acetylation and methylation imbalances, are increasingly recognized as key contributors to molecular radioresistance. These adaptive responses may enhance tumor cell survival, affect therapeutic efficacy, and promote metastasis. Understanding the interplay between radiation exposure and epigenetic remodeling opens new perspectives for precision oncology and diagnostics. Epigenetic biomarkers hold potential for predicting treatment response and prognosis, while epigenetic modifiers may sensitize tumors to radiation. This review summarizes current evidence on radiation-induced epigenetic mechanisms and evaluates their diagnostic, prognostic, and therapeutic implications in cancer management.
Keywords:
epigenetics; radiation; cancer; RNA and DNA methylation; histone modifications; anticancer 1. Introduction
Epigenetics is a mechanism of cellular regulation that controls gene expression without altering the DNA sequence [1,2,3,4,5]. Through mechanisms such as DNA and RNA methylation, histone modifications, or non-coding RNA activity, epigenetic regulation controls chromatin structure, genome stability, gene expression, and cell response to various signals [1,6,7,8]. Importantly, epigenetic changes have been shown to play a role in the development and progression of many diseases, e.g., cancers [9]. The dynamic and reversible nature of epigenetic modifications has made them attractive targets for both diagnostic and therapeutic approaches.
At the same time, radiopharmacy focuses on the development and use of new radioactive drugs—radiopharmaceuticals, for both diagnosis and treatment [10]. Molecular imaging based on radiopharmaceuticals can help visualize biological processes at the cellular and molecular levels, thus enabling the early detection of diseases. It can also be used to target specific cells or tissues, potentially delivering radiation to destroy cancer cells or modulate gene expression [11]. On the other hand, cancer therapy can also involve the use of external sources of radiation, such as in external beam radiation therapy using high-energy photons, electrons, or protons [12]. Exposure to radiation—whether from radiopharmaceuticals or from external sources used in radiotherapy—can induce epigenetic changes within cells. In cells after radiation exposure, one of the most common events is the occurrence of epigenetic modifications, which are involved in the regulation and function of key genes and proteins in various radiation response pathways, and may also promote mechanisms of radiation resistance [12]. These radiation-induced effects often manifest as alterations in DNA methylation, histone modifications, and changes in non-coding RNA expression, all of which play critical roles in the regulation of gene expression and cellular response to radiation. Radiation directly damages DNA through the induction of double-strand breaks (DSBs) and indirectly through the formation of reactive oxygen species (ROS) [12,13]. Molecular radioresistance comprises genetic and epigenetic characteristics that are either inherent to cancer cells or acquired after exposure to radiation. This approach aims to deliver focal radiation to the tumor and surrounding at-risk tissue compartments to inhibit tumor growth, proliferation, and metastasis [12].
Despite growing evidence that radiation induces profound epigenetic alterations affecting both therapeutic response and normal tissue toxicity, the current literature largely treats radiobiology, epigenetics, and radiopharmacy as separate domains, leaving a fragmented understanding of how these mechanisms intersect in clinical practice. Existing reviews tend to focus either on molecular epigenetic changes or on radiopharmaceutical advancements, but rarely integrate these perspectives to explain how radiation-related epigenetic remodeling can be harnessed for both improved diagnosis and targeted anticancer therapy. This review synthesizes both fields to outline how radiation-triggered DNA/RNA methylation changes, histone remodeling, and enzyme dysregulation can serve as actionable biomarkers or therapeutic targets within nuclear medicine. It uniquely emphasizes the emerging potential of radiotheranostics targeting epigenetic enzymes and demonstrates how understanding radiation-epigenome interactions can guide the development of new diagnostic tracers and epigenetically informed radiosensitizing strategies. This interdisciplinary framing—bridging epigenetic biology with radiopharmaceutical innovation—constitutes the main conceptual advance of the review. This work addresses that gap by providing a unified framework that links radiation-induced epigenetic mechanisms with their implications for radiotheranostics, radiosensitization strategies, and radiation-associated toxicities. By combining a radiopharmacy perspective with emerging epigenetic insights, we highlight new opportunities for precision imaging, personalized treatment planning, and development of epigenetically informed radiotherapeutic approaches.
By understanding how radiotherapy and radiopharmaceuticals affect the epigenome, researchers aim to develop new therapeutic strategies for diseases. This could involve targeting specific epigenetic pathways to reverse detrimental changes or promote beneficial ones. In this review, we will try to summarize radiation-related epigenetic changes, such as DNA methylation and histone modifications (namely, acetylation and methylation), and how these changes can be used as diagnostic or prognostic tools in patients with cancers.
2. DNA/RNA Modifications
Epigenetic modifications of RNA and DNA have been shown to play an important role in tumorigenesis and diagnosis [14]. The main DNA-related epigenetic modifications include DNA methylation and histone modifications such as methylation, acetylation, phosphorylation, and ubiquitination [14,15]. RNA modifications include N6-methyladenosine (m6A), pseudouridine (Ψ), 5-methylcytosine (m5C), 2′-O-methylation or N7-methylguanosine (m7G) [16,17,18]. RNA and DNA modifications affect genome stability, gene expression, protein synthesis, cellular processes, immune function and regulation of repair processes [19,20]. Importantly, epigenetic modifications of RNA and DNA can be modified by radiation [21,22].
Indirect actions of radiation involve the formation of ROS, which can affect gene expression. Ionizing radiation (IR) induces DSBs both through direct high-energy damage to DNA and through free radicals generated within cells. ROS can also affect DNA by, e.g., oxidizing nucleoside bases, leading to the formation of 8-oxo guanine [23]. They can alter nucleotide sequences, dysregulate base excision repair (BER) control and repair action [24], and cause mitochondrial DNA lesions, strand breaks, and degradation of mitochondrial DNA [13,23,25]. ROS production has been implicated in radiotherapy due to its effect on downstream cell survival [26], death signaling [23,27], primary prevention [28,29], and enhancement of therapeutic responses [30,31]. Importantly, ROS can directly induce DNA and histone modifications, affect the activity of epigenetic enzymes, and influence the expression of non-coding RNAs, leading to global changes in DNA methylation [7].
In the following sections of this review, we will focus on RNA and DNA methylation in the context of radiation exposure and their roles in cancer diagnosis and therapies.
2.1. RNA Methylation
RNA methylation is an epigenetic modification that can regulate gene expression without altering the DNA sequence. Methyl groups can be added or removed by specific enzymes: RNA methyltransferases (RNMTs), which use S-adenosylmethionine (SAM) as a methyl-group donor to transfer the methyl group to RNA sequences [1,2], and RNA demethylases, which reverse these modifications and restore RNA to its original state [2,32]. Methylation modifications in RNA sequences can also be recognized by specific proteins, which affect various cellular mechanisms such as gene expression and cell signaling [2,33]. RNA methylation affects several biological processes, including RNA splicing, stability, translocation, cell growth, immunity, and aging [2,33,34,35]. These modifications are primarily found in messenger RNA (mRNA) and long non-coding RNA, where they play essential roles in various cellular functions and disease mechanisms, but they are also widespread across other RNA types, including transfer RNA (tRNA), ribosomal RNA (rRNA), and small nuclear RNA (snRNA) [18,36].
2.1.1. RNMTs (METTL3 and METTL14)
The most abundant and well-studied type of RNA modification is m6A, which plays a significant role in the regulation of gene expression at the post-transcriptional level [37,38]. m6A is involved in various processes of RNA metabolism, such as stability, splicing, transcription, translation, and degradation [38]. It is a reversible epigenetic modification, a type of methylation that occurs in non-coding RNAs in tumors [38,39] and is implicated in cancer progression [40]. The best known RNMTs include methyltransferase-like 3 (METTL3) and METTL14, which are core components of the methyltransferase complex responsible for adding a methyl group to RNA sequence to form m6A [41,42]. Catalytically active subunit responsible for methylation is METTL3, while METTL14 is a structural component that stabilizes METTL3 and helps in recognizing and binding the RNA substrate [42]. METTL3 is involved in tumor progression, invasion, growth, and drug resistance in various cancers [43]. METTL3 was shown to be involved in UV-induced DNA damage response in U2OS (osteosarcoma) and HeLa (cervical cancer) cells, where elevated METTL3 recruitment was observed [44]. Similarly, carbon-ion radiotherapy elevates METTL3 levels in non-small-cell lung cancer (NSCLC), and METTL3 knockdown impairs the proliferation, migration, and invasion of NSCLC cells, both in vivo and in vitro, by inhibiting H2A histone family member X (H2AX) mRNA degradation, which leads to enhanced DNA damage repair and increased cell survival [45]. Similarly, METTL14 was shown to enhance the expression of miR-99a-5p, which inhibits the persistence of cancer stem-like cells (CSCs) and reduces the radioresistance of esophageal squamous cell carcinoma (ESCC). Downregulation of miR-99a-5p was associated with an unfavorable prognosis in ESCC patients [46]. Furthermore, METTL14 level was shown to be involved in further facilitating the persistence of CSCs in ESCC by suppressing tribbles homolog 2 (TRIB2) expression [46]. TRIB2 is a scaffold protein involved in cell proliferation, survival, differentiation, and apoptosis through the regulation of MAPK and AKT signaling pathways [47]. METTL14 depletion was also shown not only to enhance cell resistance to highly active agents used in the treatment of advanced breast cancer (i.e., paclitaxel and doxorubicin) but also to confer radioprotective effects on ALDH+ breast cancer stem cells [48]. These findings highlight the role of METTLs in radioresistance and suggest potential therapeutic targets for cancer treatment.
2.1.2. RNA Demethylases (ALKBH5 and FTO)
On the other hand, RNA demethylases include, for example, alkB homolog 5 (ALKBH5) [49] and fat mass and obesity-associated protein (FTO) [50], which are responsible for removing m6A modifications from mRNA and non-coding RNAs. In patient-derived glioblastoma stem cells (GBMSCs), where ALKBH5 expression was downregulated, decreased GBMSC survival after irradiation was observed due to defects in DNA damage repair [51]. Simultaneously, a decrease in the expression of genes involved in homologous recombination, such as checkpoint kinase 1 (CHK1)—a crucial serine/threonine kinase in the DNA damage response [52]—and DNA repair protein RAD51 homolog 1 (RAD51), essential for repairing double-strand breaks in DNA [53], was also observed [51]. In cervical squamous cell carcinoma (CSCC), the m6A demethylase FTO promotes resistance to chemoradiotherapy by upregulating β-catenin through mRNA demethylation, which in turn enhances excision repair cross-complementation group 1 (ERCC1) activity [54]. Upregulation of FTO in radioresistant nasopharyngeal carcinoma (NPC) tissues and cells, relative to their parental radiosensitive parts, was also observed [55]. FTO by m6A modification of the OTU deubiquitinase, ubiquitin aldehyde binding 1 (OTUB1) transcripts and promotes the expression of OTUB1—a protein involved in regulating protein stability, cell proliferation, immune responses, energy metabolism, and DNA damage [56]. This mechanism inhibits radiation-induced ferroptosis in NPC cells [55]. In both in vitro and patient-derived xenograft models, treatment with the FTO inhibitor FB23-2 and the ferroptosis activator erastin enhanced the sensitivity of NPC cells to radiotherapy [55]. In HPV head and neck squamous cell carcinoma (HNSCC) models in humans and mice, genetic and pharmacological inhibition of FTO enhanced the efficiency of radiotherapy [57]. This effect was associated with increased DNA damage, reduced efficiency of homologous directed repair, and decreased formation of RAD51 foci. Importantly, FTO inhibitors do not exacerbate radiation-induced oral mucositis, a common toxic effect of HNSCC treatment [57]. Therefore, RNA demethylases such as ALKBH5 and FTO clearly play important roles in cancer therapy and radiosensitivity, and they represent promising targets for the development of more effective therapeutic strategies to improve treatment outcomes for patients. In Table 1, we summarize all RNA modifications mentioned in the main text.

Table 1.
The summary of epigenetic mechanisms involved in RNA modifications changed by cancer-related therapies and radiation.
2.2. DNA Methylation
DNA methylation is a dynamic and reversible modification of DNA. A methyl group is added to the fifth carbon position of the cytosine ring in DNA (5-methylcytosine) in a process regulated by DNA methyltransferases (DNMTs) [3,4,5]. This process can silence genes, prevent their activation, and play an important role in cell differentiation, development, and the maintenance of genome stability [2]. Importantly, DNA methylation can modify gene expression without changing the DNA sequence and can be passed from one cell to another, mainly during the cell cycle, from the parental to the daughter cell [58,59]. DNA methylation, as a dynamic process, can be influenced by diet, environmental exposures, age, or other signals from outside or inside the cell [60,61].
2.2.1. ROS and DNA Methylation
Radiation, as one of the main forms of cancer treatment, triggers DNA damage through different mechanisms, like ionization or ROS production. These changes lead to the destruction of DNA structure, DSBs, transcription complex, and polymerase dysfunction, all of which consequently result in tumor cell death [62,63,64]. ROS have been shown to downregulate AT-rich interacting domain 1 A (ARID1A) expression, a key protein kinase that plays a crucial role in maintaining genomic stability by responding to DNA replication stress and DNA damage. ARID1A is an AT-rich interacting domain 1A of brahma-related gene 1 (BRG-1)-associated factor complex (BAF), chromatin remodeler commonly mutated in cancer. What is important, ROS were found to decrease ARID1A expression through promoter methylation in ovarian cancers [65,66]. This provides clear evidence of the connection between ROS and DNA methylation.
2.2.2. DNMT1
Another example of radiation affecting DNA methylation was observed in the murine thymus, where low-dose X-ray irradiation increased global DNA hypomethylation, increased accumulation of DNA damage, and reduced expression of DNMTs [67]. Changes in DNA methylation in response to radiation have also been reported in malignant cancers, and these were associated with cellular sensitivity to radiotherapy and DNMT levels [68]. In the more malignant MDA-MB-231 human breast cancer cells (compared with MCF-7), loss of DNA methylation and altered expression of DNMT1, as well as methyl-binding proteins methyl-CpG binding protein 2 (MeCP2) and methyl-CpG binding domain protein 2 (MBD2), were observed relative to the non-tumorigenic MCF-10-2A epithelial breast cells [69]. These findings indicate that epigenetic differences among breast cancer tumor cell lines may contribute to and serve as an indicator of the development of a more aggressive tumor phenotype during tumor progression.
2.2.3. DNMT3A/B
In rhabdomyosarcoma (RMS), the most common soft tissue sarcoma in childhood, primary tumor biopsies have shown overexpression of DNMT3A and DNMT3B [70]. In embryonal RMS cells, knockdown of DNMT3A and DNMT3B followed by IR exposure led to increased radiosensitivity. Silencing of DNMT3A activates the senescence program by upregulating p16 and p21 [71], cyclin-dependent kinase inhibitors involved in the induction and maintenance of cellular senescence [72], whereas DNMT3B silencing induces significant DNA damage and impairs the DNA repair mechanisms [71]. These findings suggest that DNMT3A and DNMT3B expression can influence the efficacy of radiotherapy and that their inhibition may represent a promising radiosensitizing strategy, particularly for RMS patients. In NPC, elevated DNMT3B expression was noticed after exposure to irradiation, which was associated with the development of radioresistance. On the other hand, DNMT3B silencing in response to radiation, mediated by DNA demethylation, enhanced the activation of cell cycle regulators p53 and p21, led to G1 phase of cell cycle arrest, and promoted apoptosis [73]. These findings open new possibilities in developing novel therapies using epigenetic modulations and radiation in NPC. In prostate cancer cells, PC3 cells, DNMT3B expression was shown to be induced by X-ray radiation. Downregulation of DNMT3B by miR-145 was achieved through direct targeting, and DNMT3B knockdown increased expression of miR-145 by CpG island promoter hypomethylation, consequently sensitizing prostate cancer cells to radiation [74]. These results further support the possibility of a combination of epigenetic regulation and radiation as a combined therapeutic strategy in prostate cancer. Therefore, we can conclude that DNMT3A primarily regulates the senescence program in RMS, with its silencing increasing p16 and p21 expression and promoting cellular senescence, while DNMT3B is more directly linked to DNA damage responses and repair, and its silencing leads to impaired DNA repair and enhanced radiosensitivity across multiple cancer types.
2.2.4. DNA and CpG Island Methylation
Interestingly, epigenetic mechanisms have also been shown to play a crucial role in the arginosuccinate synthetase 1 (ASS1)-negative glioblastoma (GBM) tumor model. Treatment of (ASS1)-negative GBM with ADI-PEG20, an inhibitor of arginase 1 (Arg1), was shown to be effective, whereas ASS1-positive GBM cells were unaffected by ADI-PEG20. This differential response was associated with the absence of CpG island methylation, and only the combination of radiotherapy and ADI-PEG20 achieved complete tumor elimination by promoting macrophages/microglia recruitment and M1 repolarization [75]. In another study, biopsies from patients with inoperable breast cancer before and after radiotherapy (patients receiving 10–24 Gray) were analyzed. Eighty-two differentially methylated genes were observed in irradiated samples (compared to non-irradiated), predominantly in genes involved in immune system pathways. Among the genes significantly associated with clinical response and altered methylation were: H2A histone family, member Y (H2AFY), involved in the epigenetic response [76]; cathepsin A (CTSA), responsible for degradation intra- and extracellular substrates [77]; leukotriene C4 synthase (LTC4S), participating in the synthesis of proinflammatory lipid mediator [78]; interleukin 5 receptor subunit alpha (IL5RA), involved in immune response regulation [79]; and RB transcriptional corepressor 1 (RB1), a key cell cycle/tumor suppressor [80]. All of these genes are associated with tumor progression. Interestingly, the degree of methylation correlated with the radiation dose and was enriched for genes involved in immune response, proliferation and apoptosis pathways [81]. The data mentioned above show that changes in DNA methylation in tumors following radiotherapy correlate with methylation levels, may be dose-dependent, and can be associated with treatment response in breast cancer.
2.2.5. DNMTis
Another strategy used in radiation-related therapies involves DNMT inhibitors (DNMTis). Treatment with 3 DNMTis (psammaplin A, 5-aza-2′-deoxycytidine, and zebularine) in combination with radiation exposure was shown to induce radiosensitivity in both A549 (lung cancer) and U373MG (glioblastoma) cell lines, was observed [6]. Furthermore, psammaplin A increased the sub-G1 fraction of A549 cells, and prolonged H2AX expression, a marker of radiation-induced DSBs [82]. This was observed in the cells treated with DNMTis prior to radiation compared with those exposed to radiation alone, suggesting an association with the inhibition of DNA repair [6]. Gamma-phosphorylated H2AX (γH2AX) recruits multiple components of the DNA damage response to sites of DNA DSBs to initiate repair. Therefore, elevated ROS, which was previously observed to affect γH2AX phosphorylation, can blunt treatment response to radiation as well as worsen outcomes for some cancers [13]. In another study, a newly synthesized phthalimido-alkanamide derivative, MA17, which is a DNMTi, was used in glioblastoma cell lines (U87MG, U373MG, U138MG, and T98G) and normal human astrocytes (NHA). MA17 was shown to radiosensitize all glioblastoma cell lines without affecting normal astrocytes. Furthermore, DNMT activity and expression of the FA complementation group A (FANCA; involved in DNA repair and maintenance) genes were downregulated. Increased apoptosis, autophagy, and DSBs formation were also observed in MA17-treated cells compared with those subjected to radiotherapy alone [83]. These findings demonstrate that DNMTis exerts promising radiosensitizing effects and may represent effective adjuvant agents to enhance the efficacy of radiotherapy in cancer treatment.
2.2.6. Radiation-Driven Epigenetic Changes
Radiation used in cancer therapy has been shown to be connected with the possibility of developing radiation-induced cardiovascular disease (RICVD) [84]. As a mechanism involved in this process, next to the endothelial inflammatory activation, premature endothelial senescence, increased ROS generation, mitochondrial dysfunction, and epigenetic mechanisms, particularly DNA methylation, are pointed out [84,85]. RICVD represents a significant problem in thoracic radiotherapy [84]. In heart-irradiated rats exposed to 27.6 Gy of radiation, DNA methylation alterations persisted up to 7 months after exposure, with differential expression of cardiac-relevant differentially methylated regions [84]. Higher expression of the sarcolemma-associated protein (SLMAP) gene, involved in cell cycle control, migration and signaling [86], was correlated with hypomethylation [84]. In contrast, expression of the E2F transcription factor 6 (E2F6) gene, which regulates the cell cycle and is involved in chromatin modification and DNA methylation [87], was inversely correlated with a decreased global longitudinal strain [84] in these rats. Similarly, in radiotherapy-treated breast cancer patients, E2F6 and SLMAP exhibited differential expression directly and six months after radiotherapy, respectively [84]. These findings suggest a direct association between radiation-induced DNA methylation changes and the pathophysiology of RICVD, which could serve as a basis for future diagnostic or therapeutic approaches. The wild-type isocitrate dehydrogenase (IDH) enzyme supports DNA demethylation by producing α-ketoglutarate (α-KG) [88]. However, mutations in isocitrate dehydrogenase 1 and 2 (IDH1/2) are observed in approximately 80% of gliomas. These mutations lead to the production of oncometabolite d-2-hydroxyglutarate (d-2-HG), which results in aberrant DNA methylation. Simultaneously, IDH mutant gliomas are more sensitive to radiotherapy due to reduced levels of NAD and NADPH, which are required to counteract oxidative stress [89]. This was demonstrated in a phase III clinical study in patients with IDH mutations/non-codel tumors treated with radiotherapy and Temozolomide (TMZ). Patients with high-risk low-grade glioma showed longer progression-free survival (median PFS 55 months versus 36 months; p = 0.0043) after radiotherapy than those treated with TMZ alone [90]. These findings suggest that DNA demethylation can also play a crucial role in cancer-related treatment and radiation response.
DNA methylation is a dynamic but heritable epigenetic modification that can be reshaped by radiation and ROS, influencing gene expression, genome stability, and cellular radiosensitivity. Radiation alters the activity of DNMTs, modulates CpG island methylation, and drives gene-specific methylation changes that can either promote tumor progression or enhance radiosensitivity, depending on the cellular context. These radiation-induced methylation patterns not only affect cancer treatment outcomes through DNMT1, DNMT3A/B, and DNMTis-related mechanisms but also contribute to long-term normal-tissue effects such as radiation-induced cardiovascular disease. As a summary, we gathered together all the mentioned DNA modifications, related to anticancer treatment, mentioned in this paragraph in Table 2. We specified the mechanism of action, used cell line or model, and how epigenetic modification was modified in relation to radiation.

Table 2.
The summary of epigenetic mechanisms involved in DNA modifications changed by cancer-related therapies and radiation.
3. Histone Modifications
Chromatin is a complex of DNA and proteins (histones) that forms chromosomes within the nucleus of eukaryotic cells. Histones are small proteins that help pack long DNA strands into compact structures. Five types of histones—H1, H2A, H2B, H3, and H4—are distinguished; they are rich in basic amino acids (arginine and lysine), which promote binding to the negatively charged DNA [92]. Histone modifications are crucial not only for chromatin structure and remodeling but are also involved in DNA repair, tagging of DSBs, activation of repair regulators, cell cycle regulation, and apoptosis [7,93,94]. In preoperative chemoradiotherapy, epigenetic therapies—including DNA methylation and histone deacetylase inhibitors (HDACis)—sensitize tumors to radiopharmaceuticals, enhancing treatment synergy. Early clinical data show that the combined use of epigenetic markers and radiopharmaceuticals improves therapeutic efficacy, increases survival rates, reduces recurrence, minimizes off-target effects, enhances tumor selectivity, and facilitates tumor downstaging [95]. Elevated ROS levels have also been associated with histone protein degradation and decreased phosphorylation of H2AX, a key step in the DNA repair process. Moreover, radiation also induces histone modification, i.e., phosphorylation of histone H2AX, namely, phosphorylation at serine 139 (γ-H2AX), that is used as a measure of DSBs in early caused cellular response to IR and is crucial for repair of DSBs [7]. Moreover, ROS were shown to be able to activate enzymes involved in histone control and have a dual effect on DNMTs, which play a role in the expression of oncogenes and tumor suppressor genes [25,91]. Therefore, in the following section, we will focus on two main histone modifications, such as histone methylation and acetylation, in cancer, with particular attention to their roles in radiation-related treatments. All histone modifications discussed in the following paragraphs in the context of anticancer treatment and diagnostics are summarized in Table 3.

Table 3.
The summary of epigenetic mechanisms involved in histone methylation and acetylation modulated by cancer-related therapies and radiation.
3.1. Histone Methylation
Histone methylation is an epigenetic process involving the addition of a methyl group to lysine or arginine amino acids, on the N-terminal “tails” of histone proteins [8,116]. This process is regulated by two classes of enzymes: histone methyltransferases (HMTs), which catalyze the addition of methyl groups, and histone demethylases (HDMs), which remove them [8,117]. More than one methyl group can be added on lysine residues, mono (me1), di-(me2) or tri-(me3) and me1 or me2 on arginine [118]. Histone methylation modulates DNA–histone attraction, chromatin structure, DNA access, and transcription [8,116]. Methyl groups on histones also act as docking sites for other proteins involved in cell cycle, function, and signaling [119]. Histone methylation can have both effects on gene expression, depending on the specific histone residue [120]; e.g., H3K9me3 is associated with the formation of “closed” chromatin and blocking expression [121], while H3K4me3 leads to gene expression and “open” chromatin [108]. This dynamic process is involved in multiple biological functions, including DNA repair [122], cell cycle control [123], aging [116,123], and stress response [116]. Importantly, histone methylation is involved in the regulation of gene expression in response to radiation. Radiation caused by DNA damage also affects chromatin structure and histone methylation and can impact response to DSBs. Changes in histone methylation can affect long-term repair processes and affect cells’ radiosensitivity [124,125]. Therefore, histone methylation represents a potential target for cancer therapy, improves radiotherapies, or contributes to tumor progression and adaptation.
3.1.1. X-Ray Irradiation and Histone Methylation
Researchers have shown that even low-dose X-ray irradiation in an in vitro murine model decreased me3 of histone H4 in the thymus and reduced chromatin compactness [67]. In the MDA-MB-231 breast cancer cell line cells, observations included decreased 3me of lysine 20 of histone H4 and hyperacetylation of histone H4, compared to MCF-7 cells, as well as lower expression of suppressor of variegation 4–20 homolog 2 (Suv4-20h2) and histone methyltransferase. These changes were associated with increased malignant properties of breast cancer cells [69]. The presented data demonstrate that significant epigenetic alterations linked to histone methylation occur in human breast cancer cells, while X-ray irradiation induces histone methylation modifications in murine models. These observations suggest that histone methylation can be used as a novel target for many different treatments.
3.1.2. HDMs
The H3K4-specific histone lysine demethylase 5 (KDM5) has been shown to be overexpressed in different cancers, including breast, prostate, lung and bladder carcinomas [33,96,97]. KDM is involved in transcriptional regulation and DNA repair and, in cancer, exerts positive effects on cancer proliferation and chemoresistance [33,126,127]. In MCF-7 breast cancer cells overexpressing KDM5B, also known as JARID1B, inhibition of KDME activity increased the sensitivity of breast cancer cells to IR and enhanced radiation-induced damage by blocking the catalytic function of KDM5 enzymes [96]. Similarly, in ESCC patients presenting overexpression of KDM5B, its inhibition enhanced the H3K4me3 methylation of phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3) promoter and induced the expression of PIK3C3. In in vitro models (KYSE-150 and TE-10 cells), knockdown of KDM5B promoted apoptosis, cell cycle arrest, autophagy, and increased sensitivity to radiotherapy [97]. MiR-320a regulates a number of genes involved in various physiological processes, including tumor suppression [128]. In non-small cell lung cancer (NSCLC) clinical samples, suppressed expression of KDM5B and enhanced radioresistance of NSCLC through the downregulation of phosphatase and TENsin homolog (PTEN) expression, a crucial tumor suppressor that inhibits cell growth, proliferation, and survival [129], was reported to be caused by KDM5B [98]. Furthermore, inhibition of miR-320a in radioresistance of NSCLC was also reproduced by in vivo assay [98]. Therefore, the role of KDM5, an epigenetic enzyme, seems to be crucial for radioresistance and cancer treatment, and future directions targeting this histone methylation-related enzyme can be a promising new branch in pharmacy.
3.2. Histone Acetylation
In histone acetylation, an acetyl group is added to lysine residues on histone proteins, which neutralizes their positive charge, loosens the DNA–histone interaction, and consequently causes the chromatin to loosen, relaxed structure, calling “open” for interaction with transcription factors, which leads to increased gene expression [130]. Histone acetylation is regulated by two main classes of enzymes: histone deacetylases (HDACs) and histone acetyltransferases (HATs) [130,131]. HATs add acetyl groups to the lysine residues of histone tails, leading to decondensation of the chromatin and increased gene expression, while HDACs remove acetyl groups, leading to suppression of gene transcription [132]. Therefore, chromatin structure modifications caused by acetylation/deacetylation alter the expression of target genes, affect transcriptional activation, and regulate chromatin condensation and its availability [133,134,135]. Both HDACs and HATs play important roles in radiotherapy through mechanisms involving radiosensitization, DNA repair, and gene expression modifications [99]. HDACis have been shown to enhance the radiosensitivity of cancer cells [6,136]. Since HDACs and HATs are involved in the DNA repair process, blocking their activity may represent an effective mechanism to stop the repair of damage to cancer cells caused by radiotherapy, thereby improving treatment outcomes [107,135,137,138]. In the following section, we will summarize the roles of HATs and HDACs in cancer therapy, highlight ongoing clinical trials, and discuss future directions in the context of radiopharmacy.
3.2.1. HDACis
A major aspect of the role of HDACs in cancer treatment is associated with HDACis. HDACis represent a promising class of anticancer drugs that modulate chromatin structure and gene expression by blocking histone deacetylation. They have been shown to exert radiosensitizing effects [6], for example, in sarcomas, where HDACis augment the cellular response to radiation [139]. Furthermore, scientific reports have demonstrated that HDAC activity can also be used as a biomarker in cancer therapies [100,101]. More than a twofold increase in HDACs activity was observed in patients with invasive grade III breast carcinomas, and radioresistant patients presented high HDACs and low HATs activity after irradiation. Therefore, patients with breast cancer tumors can be designated for HDAC inhibitor-based radio-sensitization treatment, which may result in much more effective treatment [102].
Vorinostat, an inhibitor of class I and II of HDACs, is a promising anticancer agent that inhibits the proliferation of various cancer cell types, including breast carcinoma [140], sarcomas [139,141,142], or lymphomas [143,144]. The addition of vorinostat to radiotherapy in osteosarcoma cell lines induces radiation-induced apoptosis, causes cell cycle arrest, and inhibits cell proliferation and clonogenic survival, with similar effects in RMS cell lines [142]. Moreover, vorinostat enhances radiation in osteosarcoma and RMS cells by inhibiting the expression of radiation-induced DNA repair proteins (Rad51 and Ku80) [141]. Both mechanisms lead to significant radiosensitization caused by using vorinostat. The combination of vorinostat with heavy ion radiotherapy in osteosarcoma models resulted in a marked delay of tumor growth due to increased rate of apoptosis, elevated expression of p53 and p21 (proteins associated with cellular responses to stress like DNA damage), and inhibition of proliferation and angiogenesis, compared with heavy ion radiotherapy alone [145]. That shows the radiosensitization effects of vorinostat are also in heavy ion radiotherapy. One approach involves the use of nanoparticles carrying chemotherapeutic drugs. Dual-target nanoparticles using both a cisplatin prodrug—that is, a modified, inactive form, typically a platinum (IV) complex, designed to be converted into the active platinum (II) cisplatin within cancer cells, aiming to improve efficacy, reduce toxicity, or overcome drug resistance [146]—and a HDACi (vorinostat) increased DNA damage, impaired cancer cell repair ability, and promoted the effects of radiotherapy [147,148]. The radiosensitivity of cancer cells can be increased by increasing DNA damage. Interestingly, in three pancreatic cancer cell lines (Su.86.86, MIA Paca-2, T3M-4) treated with vorinostat or CUDC-101, a small molecule that simultaneously inhibits epidermal growth factor receptor (EGFR), human growth factor receptor 2 (HER2), and subsequently irradiated after 24 h, increased the radiation sensitivity of pancreatic tumor cell lines in a dose-dependent manner, reduced proliferation and clonogenic survival, and increased radiation-induced apoptosis (diminished full length Poly (ADP-ribose) polymerase [PARP-1]) [136]. These findings indicate that both compounds act as effective radiosensitizers in pancreatic cancer. HDACs inhibition was also observed in peripheral blood mononuclear cells (PBMC), tumor biopsies, and paired skin biopsies from patients with intermediate or high-risk of HNSCC, treated with CUDC-101 and HDACis in combination with external beam radiation (70 Gy to gross disease over 7 weeks) [149]. Radiotherapy can upregulate both activating and inhibitory receptors, e.g., ribonucleic acid export 1 (Rae-1) and UL16-binding protein (ULBP) [148]. Rae-1 is a cell surface glycoprotein, which is expressed on cells and functions as a ligand for the activating receptor natural killer group 2 member D (NKG2D) [150]. ULBP is a family of cell surface glycoproteins that act as ligands for the activating receptor NKG2D, which is expressed on immune cells like natural killer (NK) cells and T cells [151]. Both Rae-1 and ULBP were found to be upregulated by radiotherapy and HDACi therapy, leading to increased NK cell cytotoxicity against cancer cells [152,153].
Vorinostat shows strong preclinical potential as a radiosensitizer, consistently enhancing radiation-induced apoptosis, cell-cycle arrest, and suppression of key DNA repair proteins across multiple tumor models. Its ability to boost heavy-ion radiotherapy efficacy and increase immune-activating NKG2D ligands further broadens its therapeutic relevance. However, its clinical applicability remains limited by the predominance of cell-line and animal data, uncertain translation to measurable patient benefit, potential systemic toxicity from broad HDACs inhibition, and challenges in drug delivery that may require nanoparticle formulations to achieve optimal tumor penetration. These factors highlight the need for more rigorous clinical trials, better-defined dosing windows, and biomarker-driven patient selection before vorinostat can be reliably integrated into radiotherapy-based cancer treatment.
3.2.2. HATs
HATs, such as CREB-binding protein (CBP) and E1A binding protein p300 (p300), induce histone H3 lysine 27 acetylation (H3K27ac) at target gene promoters and modulate its transcription [154]. CBP/p300 has been shown to be overexpressed in many cancer and drug-resistant cancer cells, e.g., lung, breast, and small-cell carcinoma [100,101,103,104,105,106]. CBP/p300 activates oncogene transcription and induces cancer cell proliferation, survival, tumorigenesis, metastasis, immune evasion, and drug-resistance, and was shown to be a poor prognostic linked with increased tumor recurrence [104,106,107]. Inhibition of CBP/p300 has been shown to reduce H3K27ac, downregulate oncogene transcription, induce cancer cell growth inhibition and cell death, activate immune response, overcome drug resistance, suppress tumor progression in vivo, and enhance the anticancer efficacy of radiotherapy [107]. Therefore, new inhibitors for CBP/p300 epigenetic enzymes are promising novel anticancer agents for clinical translation because they can enhance the effectiveness of other cancer treatments, such as radiotherapy.
KATs
One of the emerging approaches integrating epigenetic mechanisms into radiotherapy is radiotherapy-triggered proteolysis-targeting chimeras (PROTAC) prodrugs. In this strategy, PROTACs and PROTAC-oriented targeted protein degradation (TPD) strategies offer more precise and effective treatment modalities for a variety of diseases, but mainly for cancers. One of the main degraders used lysine acetyltransferase (KAT) as a target, where degraders create ternary complexes comprising ligands for targeted proteins and E3 ubiquitin ligase, along with a connecting linker, allowing targeted degradation via a ubiquitination-dependent method [155]. In multiple breast cancer cell lines, upregulation of lysine acetyltransferase 7 (KAT7) has been reported, and its expression was negatively correlated with the survival of breast cancer patients [110]. KAT7 is involved in the control of cell survival, DNA replication, and transcription, and KAT7 inactivation relates to global loss of H3K14ac and decreased expression of a broad range of genes [109,156]. KAT7 overexpression enhances the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway, which is frequently dysregulated in breast cancer, promoting tumor growth, survival, and metabolism [157], and also contributes to radioresistance [110]. Moreover, silencing of KAT7 has been shown to suppress breast cancer radioresistance in vitro, highlighting its importance in radiation response [110]. Therefore, acetyltransferases such as KAT7 and CBP/p300 hold great potential for clinical application.
NAT10
Another example of using HATs in cancer radiopharmacy is the development of radiotracers for tumor imaging and theranostic applications. N-acetyltransferase 10 (NAT10), a HAT that plays a key role in regulating gene expression by adding acetyl groups to histone proteins [158], can also modify RNA acetylation, particularly in rRNA and messenger RNA [111]. NAT10 is overexpressed in many cancers, such as breast, liver, colorectal, lung, bladder, cervical, and oral cancers, as well as multiple myeloma. Its overexpression is associated with tumor progression, resistance to apoptosis, and increased cell migration and invasion [33,112,113,114,115,127]. Therefore, inhibitors of NAT10, such as [11C] remodelin, have been used to develop positron emission tomography (PET) radiotracers for tumor imaging and theranostic applications in both mouse models [159] and breast cancer patients [160]. These agents enable a more targeted approach to visualizing and treating specific cancers by exploiting the elevated NAT10 expression, making this enzyme a promising target for the development of PET radiotracers. Another HAT-associated radiosensitizer is garcinol, a HAT inhibitor that blocks the chromatin remodeling process involved in the non-homologous and joining DNA repair pathway, which cancer cells use to repair radiation-induced DNA damage [105,161]. Treatment with garcinol prevents cancer cells (A549 lung and HeLa cervical carcinoma cells) from repairing radiation-induced DSBs, leading to cell death or senescence and inhibition of proliferation [144]. That makes garcinol another promising agent used in cancer treatment that improves the radiation effect.
Histone modifications play a central role in shaping chromatin structure, regulating DNA repair, and determining cellular radiosensitivity, and radiation disrupts these marks through ROS generation, altered enzyme activity, and direct chromatin damage. While both histone methylation and acetylation pathways offer promising therapeutic entry points, through HDACis, HAT inhibitors, KDM5 blockers, or radiotherapy-activated PROTACs, the evidence remains largely preclinical, with variable responses across tumor types and concerns about off-target effects and normal-tissue toxicity. Critically, although targeting histone-modifying enzymes shows strong potential to enhance radiotherapy and enable novel radiotheranostic strategies, translation to the clinic will require clearer biomarker-guided patient selection, better understanding of long-term epigenomic impacts, and rigorous validation in human studies. Table 4 presents a compressed and comprehensive summary of all the mentioned inhibitions of epigenetic mechanisms, and their role in anticancer treatments and diagnostics related to radiation.

Table 4.
A summary of the effects of inhibition of epigenetic mechanisms in anticancer treatments and diagnostic related to radiation.
4. Summary
As demonstrated, epigenetic modifications are not only among the mechanisms used in anticancer therapies but can also radiosensitize cancer cells. Epigenetic mechanisms, including changes in the activity or expression of epigenetic enzymes, can also serve as diagnostic tools. Moreover, epigenetic heterogeneity is widespread among different individual patients, tumor lesions, and disease stages, leading to the various radiation treatment outcomes. These molecular characteristics provide potential targets for emerging epigenetic drugs (epi-drugs), which can be combined with radiotherapy to achieve better treatment results. Given the complexity and heterogeneity of tumors, epigenetic mechanisms that can distinguish different cell fractions or even different cell cycles may represent promising new targets for diagnostics and therapy, which can be widely used in the field of radiopharmacy. Another possible link between anticancer therapies, diagnostics, and epigenetic mechanisms lies in the dynamic nature of epigenetic changes, which may serve as early markers of cellular changes, without permanent changes in DNA code and gene expression. On the other hand, the complexity, multiplicity, and balance of epigenetic modifications can be another method of diagnostic approaches that integrate multiple molecular markers to precisely assess tumor progression and patient condition. There is no doubt that the combination of epigenetic modulation with radiation provides more effective therapeutic outcomes than radiation alone. Figure 1 presents a summary of the link between the initial effects of radiation, the resulting epigenetic alterations, and their impact on clinical outcomes.
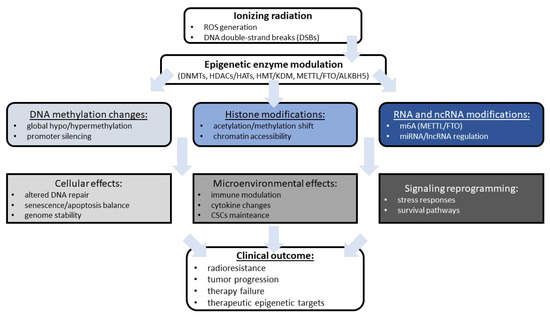
Figure 1.
The summary of radiation-induced epigenetic remodeling and tumor response discussed in the review. ALKBH5, alkB homolog 5; CSCs, cancer stem-like cells; DNMTs, DNA methyltransferases; DSBs, double-strand breaks; FTO, fat mass and obesity-associated protein; HATs, histone acetyltransferases; HDACs, histone deacetylases; HMT, histone methyltransferases; KDM, lysine demethylase; m6A, N6-methyladenosine; METTL, methyltransferase-like; lncRNA, long non-coding RNA; miRNA, microRNA; ROS, reactive oxygen species.
Future research on epigenetic radiosensitizers such as vorinostat must address several translational hurdles, including limited clinical evidence, heterogeneous tumor epigenomes, and challenges in achieving effective yet safe modulation of chromatin regulators in patients. While preclinical studies highlight strong radiosensitizing potential through impaired DNA repair, apoptosis induction, and immune activation, translating these findings into durable clinical benefit requires optimized drug delivery, biomarker-guided patient selection, and strategies that minimize systemic toxicity. Emerging radiotheranostic approaches—such as PET-based tracers targeting epigenetic enzymes like NAT10 or radiation-activated PROTACs that degrade HATs or HDAC-associated proteins—offer a promising pathway to overcome these barriers by enabling precise imaging, selective targeting, and combination regimens that exploit radiation-induced vulnerabilities. Together, these advancing technologies may bridge the gap between mechanistic insight and clinical efficacy, ultimately enabling personalized radiotherapy that integrates real-time epigenetic modulation.
Future challenges and directions include the need for more comprehensive studies to fully elucidate the complex interplay between radiation and epigenetic regulation, to identify specific and effective epigenetic-based therapies, and to translate these findings into clinical practice for improved patient outcomes. They may not only improve diagnostic approaches but also represent promising targets for the development of novel anticancer therapies in the future. However, the focus on developing new, mechanism-based epigenetic reagents should remain a key priority for researchers.
Author Contributions
Conceptualization, A.J.O. and A.G.-P.; writing—original draft preparation, A.J.O. and A.G.-P.; writing—review and editing, E.C. and N.B.; tables, A.J.O., I.S.K. and M.B.-G.; funding acquisition, A.G.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Bialystok grant number B.SUB.24.361.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
This review underwent rigorous language enhancement using ChatGPT (OpenAI’s GPT-5.1) for grammatical precision and readability optimization. The authors confirm full accountability for the intellectual content and ethical compliance of the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALKBH5 | alkB homolog 5 |
| Arg1 | arginase 1 |
| ARID1A | AT-rich interacting domain 1 A |
| ASS1 | arginosuccinate synthetase 1 |
| BAF | brahma-related gene 1-associated factor complex |
| BER | base excision repair |
| BRG-1 | brahma-related gene 1 |
| CBP | CREB-binding protein |
| CHK1 | checkpoint kinase 1 |
| CSCs | cancer stem-like cells |
| CTSA | cathepsin A |
| d-2-HG | d-2-hydroxyglutarate |
| DNMTi | DNMT inhibitors |
| DNMTs | DNA methyltransferases |
| DSBs | double-strand breaks |
| E2F6 | E2F transcription factor 6 |
| EGFR | epidermal growth factor receptor |
| ERCC1 | enhances excision repair cross-complementation group 1 |
| ESCC | esophageal squamous cell carcinoma |
| FANCA | FA complementation group A |
| FTO | fat mass and obesity-associated protein |
| GBM | glioblastoma |
| GBMSCs | glioblastoma stem cells |
| H2AFY | H2A histone family, member Y |
| H2AX | H2A histone family member X |
| HATs | histone acetyltransferases |
| HDACis | histone deacetylase inhibitors |
| HDACs | histone deacetylases |
| HDMs | histone demethylases |
| HER2 | human growth factor receptor 2 |
| HMTs | histone methyltransferases |
| HNSCC | HPV head and neck squamous cell carcinoma |
| IDH | isocitrate dehydrogenase |
| IL5RA | interleukin 5 receptor subunit alpha |
| IR | ionizing radiation |
| KAT | lysine acetyltransferase |
| KDM5 | lysine demethylase 5 |
| LTC4S | leukotriene C4 synthase |
| m5C | 5-methylcytosine |
| m6A | N6-methyladenosine |
| m7G | 2’-O-methylation or N7-methylguanosine |
| MBD2 | methyl-CpG binding domain protein 2 |
| me1 | mono-methylation |
| me2 | di-methylation |
| me3 | tri-methylation |
| MeCP2 | methyl-CpG binding protein 2 |
| METTL | methyltransferase-like |
| mRNA | messenger RNA |
| NAT10 | N-acetyltransferase 10 |
| NHA | normal human astrocytes |
| NK | natural killer |
| NKG2D | natural killer group 2 member D |
| NPC | nasopharyngeal carcinoma |
| NSCLC | non-small cell lung cancer |
| OTUB1 | ubiquitin aldehyde binding 1 |
| p300 | E1A binding protein p300 |
| PARP-1 | Poly (ADP-ribose) polymerase |
| PBMC | peripheral blood mononuclear cells |
| PIK3C3 | phosphatidylinositol 3-kinase catalytic subunit type 3 |
| PROTAC | proteolysis-targeting chimeras |
| PET | positron emission tomography |
| PTEN | phosphatase and TENsin homolog |
| RAD51 | RAD51 homolog 1 |
| Rae-1 | ribonucleic acid export 1 |
| RB1 | RB transcriptional corepressor 1 |
| RICVD | radiation-induced cardiovascular disease |
| RMS | rhabdomyosarcoma |
| RNMTs | RNA methyltransferases |
| ROS | reactive oxygen species |
| rRNA | ribosomal RNA |
| SAM | S-adenosylmethionine |
| SLMAP | sarcolemma associated protein |
| snRNA | small nuclear RNA |
| Suv4-20h2 | suppressor of variegation 4-20 homolog 2 |
| TMZ | Temozolomide |
| TPD | targeted protein degradation |
| tRNA | transfer RNA |
| TRIB2 | tribbles pseudokinase 2 |
| TSA | trichostatin A |
| ULBP | UL16-binding protein |
| α-KG | α-ketoglutarate |
| γH2AX | gamma-phosphorylated H2AX |
References
- Ochiai, Y.; Clifton, B.E.; Le Coz, M.; Terenzio, M.; Laurino, P. SUPREM: An engineered non-site-specific m6A RNA methyltransferase with highly improved efficiency. Nucleic Acids Res. 2024, 52, 12158–12172. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Z.; Yang, Y.; Zhang, C.; Liu, H.; Wan, J. The functions and mechanisms of post-translational modification in protein regulators of RNA methylation: Current status and future perspectives. Int. J. Biol. Macromol. 2023, 253, 126773. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Li, Y.; Robertson, K.D. DNA Methylation: Superior or Subordinate in the Epigenetic Hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Costello, J. DNA methylation: An epigenetic mark of cellular memory. Exp. Mol. Med. 2017, 49, e322. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Chie, E.; DaYoung, P.; Kim, I.; Kim, I. DNMT (DNA methyltransferase) inhibitors radiosensitize human cancer cells by suppressing DNA repair activity. Radiat. Oncol. 2012, 7, 39. [Google Scholar] [CrossRef]
- Belli, M.; Tabocchini, M.A. Ionizing Radiation-Induced Epigenetic Modifications and Their Relevance to Radiation Protection. Int. J. Mol. Sci. 2020, 21, 5993. [Google Scholar] [CrossRef]
- Demetriadou, C.; Koufaris, C.; Kirmizis, A. Histone N-alpha terminal modifications: Genome regulation at the tip of the tail. Epigenet. Chromatin 2020, 13, 29. [Google Scholar] [CrossRef]
- Kanwal, R.; Gupta, S. Epigenetic modifications in cancer. Clin. Genet. 2012, 81, 303–311. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Gao, X.; Chen, X.; Li, L.; Li, G.; Liu, C.; Miao, Y.; Wang, R.; Hu, K. Radiopharmaceuticals and their applications in medicine. Signal Transduct. Target. Ther. 2025, 10, 1. [Google Scholar] [CrossRef]
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef]
- Arechaga-Ocampo, E. Epigenetics as a determinant of radiation response in cancer. Int. Rev. Cell Mol. Biol. 2024, 383, 145–190. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xie, B.; Wang, Q.; Yang, R.; Sun, J.; Hu, C.; Liu, S.; Tao, Y.; Xiao, D. New insights into immune cells in cancer immunotherapy: From epigenetic modification, metabolic modulation to cell communication. MedComm 2024, 5, e551. [Google Scholar] [CrossRef] [PubMed]
- Al Aboud, N.M.; Tupper, C.; Jialal, I. Genetics, Epigenetic Mechanism; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cui, L.; Ma, R.; Cai, J.; Guo, C.; Chen, Z.; Yao, L.; Wang, Y.; Fan, R.; Wang, X.; Shi, Y. RNA modifications: Importance in immune cell biology and related diseases. Signal Transduct. Target. Ther. 2022, 7, 334. [Google Scholar] [CrossRef]
- Adamopoulos, P.G.; Athanasopoulou, K.; Daneva, G.N.; Scorilas, A. The Repertoire of RNA Modifications Orchestrates a Plethora of Cellular Responses. Int. J. Mol. Sci. 2023, 24, 2387. [Google Scholar] [CrossRef]
- Li, S.; Luo, P.; Fan, J.; Li, Y.; Tu, J.; Long, X. RNA Modifications in Health and Disease. MedComm 2025, 6, e70341. [Google Scholar] [CrossRef]
- Jimeno, S.; Balestra, F.R.; Huertas, P. The Emerging Role of RNA Modifications in DNA Double-Strand Break Repair. Front. Mol. Biosci. 2021, 8, 664872. [Google Scholar] [CrossRef]
- Stixová, L.; Tichý, V.; Bártová, E. RNA-related DNA damage and repair: The role of N7-methylguanosine in the cell nucleus exposed to UV light. Heliyon 2024, 10, e25599. [Google Scholar] [CrossRef]
- Barnes, B.M.; Shyne, A.; Gunn, D.A.; Griffiths, C.E.M.; Watson, R.E.B. Epigenetics and ultraviolet radiation: Implications for skin ageing and carcinogenesis. Skin Health Dis. 2024, 4, e410. [Google Scholar] [CrossRef]
- He, X.; Cai, L.; Tang, H.; Chen, W.; Hu, W. Epigenetic modifications in radiation-induced non-targeted effects and their clinical significance. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2023, 1867, 130386. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, X.; Xiong, Z.; Ihsan, A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Anadón, A.; Wang, X.; et al. Cancer Metabolism: The Role of ROS in DNA Damage and Induction of Apoptosis in Cancer Cells. Metabolites 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.-C.; Zhang, Y.; Wang, Y.; Wei, W.; Zhou, Y.-X.; Xie, Y.; Wang, X.; Qi, Y.-Z.; Chang, L.; Jia, Z.-P.; et al. Quantitative proteomics reveals mitochondrial respiratory chain as a dominant target for carbon ion radiation: Delayed reactive oxygen species generation caused DNA damage. Free Radic. Biol. Med. 2019, 130, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Zulato, E.; Ciccarese, F.; Agnusdei, V.; Pinazza, M.; Nardo, G.; Iorio, E.; Curtarello, M.; Silic-Benussi, M.; Rossi, E.; Venturoli, C.; et al. LKB1 loss is associated with glutathione deficiency under oxidative stress and sensitivity of cancer cells to cytotoxic drugs and γ-irradiation. Biochem. Pharmacol. 2018, 156, 479–490. [Google Scholar] [CrossRef]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- González, M.J.; Miranda-Massari, J.R.; Mora, E.M.; Guzmán, A.; Riordan, N.H.; Riordan, H.D.; Casciari, J.J.; Jackson, J.A.; Román-Franco, A. Orthomolecular Oncology Review: Ascorbic Acid and Cancer 25 Years Later. Integr. Cancer Ther. 2005, 4, 32–44. [Google Scholar] [CrossRef]
- Attique, I.; Haider, Z.; Khan, M.; Hassan, S.; Soliman, M.M.; Ibrahim, W.N.; Anjum, S. Reactive Oxygen Species: From Tumorigenesis to Therapeutic Strategies in Cancer. Cancer Med. 2025, 14, e70947. [Google Scholar] [CrossRef]
- Chang, C.; Ma, G.; Cheung, E.; Hutchins, A.P. A programmable system to methylate and demethylate N6-methyladenosine (m6A) on specific RNA transcripts in mammalian cells. J. Biol. Chem. 2022, 298, 102525. [Google Scholar] [CrossRef]
- Chen, X.; Chen, M.; Gu, X.; Zhou, Q.; Zhao, Y.; Yang, Y.; Zhang, H.; Yang, X. Roles of KDM5 demethylases in therapeutic resistance of cancers. Epigenetics Chromatin 2025, 18, 61. [Google Scholar] [CrossRef]
- Luan, J.; Kopp, J.B.; Zhou, H. N6-methyladenine RNA Methylation Epigenetic Modification and Kidney Diseases. Kidney Int. Rep. 2023, 8, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yin, H.; Hong, M.; Wang, Y.; Yu, T.; Teng, Y.; Li, T.; Wu, Q. RNA methylation in hematological malignancies and its interactions with other epigenetic modifications. Leukemia 2021, 35, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Wang, H. RNA modifications in long non-coding RNAs and their implications in cancer biology. Bioorg. Med. Chem. 2024, 113, 117922. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021, 6, 74. [Google Scholar] [CrossRef]
- Zhuang, H.; Yu, B.; Tao, D.; Xu, X.; Xu, Y.; Wang, J.; Jiao, Y.; Wang, L. The role of m6A methylation in therapy resistance in cancer. Mol. Cancer. 2023, 22, 91. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, L.; Cheng, L.; Lv, G.; Sun, B.; Wang, G.; Tang, Q. The roles of N6-methyladenosine and its target regulatory noncoding RNAs in tumors: Classification, mechanisms, and potential therapeutic implications. Exp. Mol. Med. 2023, 55, 487–501. [Google Scholar] [CrossRef]
- Wang, L.; Dou, X.; Chen, S.; Yu, X.; Huang, X.; Zhang, L.; Chen, Y.; Wang, J.; Yang, K.; Bugno, J.; et al. YTHDF2 inhibition potentiates radiotherapy antitumor efficacy. Cancer Cell 2023, 41, 1294–1308.e8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N. The role of RNA methyltransferase METTL3 in gynecologic cancers: Results and mechanisms. Front. Pharmacol. 2023, 14, 1156629. [Google Scholar] [CrossRef]
- Wang, P.; Doxtader, K.A.; Nam, Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell 2016, 63, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Feng, R.; Lu, T.; Chen, X.; Zhou, X.; Wang, X. Novel insights into the m6A-RNA methyltransferase METTL3 in cancer. Biomark. Res. 2021, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Laurent, B.; Hsu, C.-H.; Nachtergaele, S.; Lu, Z.; Sheng, W.; Xu, C.; Chen, H.; Ouyang, J.; Wang, S.; et al. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature 2017, 543, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, P.; Huang, Y.; Shi, W.; Mao, J.; Ma, N.; Kong, L.; Guo, L.; Liu, J.; Chen, J.; et al. METTL3-mediated m6A mRNA contributes to the resistance of carbon-ion radiotherapy in non-small-cell lung cancer. Cancer Sci. 2023, 114, 105–114. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, K.; Gu, S.; Wang, W.; Xie, S.; Lu, T.; Li, L.; Dong, C.; Wang, X.; Zhou, Y. A methyltransferase-like 14/miR-99a-5p/tribble 2 positive feedback circuit promotes cancer stem cell persistence and radioresistance via histone deacetylase 2-mediated epigenetic modulation in esophageal squamous cell carcinoma. Clin. Transl. Med. 2021, 11, e545. [Google Scholar] [CrossRef]
- Fang, Y.; Zekiy, A.O.; Ghaedrahmati, F.; Timoshin, A.; Farzaneh, M.; Anbiyaiee, A.; Khoshnam, S.E. Tribbles homolog 2 (Trib2), a pseudo serine/threonine kinase in tumorigenesis and stem cell fate decisions. Cell Commun. Signal. 2021, 19, 41. [Google Scholar] [CrossRef]
- Bai, X.; Liu, J.; Zhou, S.; Wu, L.; Feng, X.; Zhang, P. METTL14 suppresses the expression of YAP1 and the stemness of triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2024, 43, 307. [Google Scholar] [CrossRef]
- Qu, J.; Yan, H.; Hou, Y.; Cao, W.; Liu, Y.; Zhang, E.; He, J.; Cai, Z. RNA demethylase ALKBH5 in cancer: From mechanisms to therapeutic potential. J. Hematol. Oncol. 2022, 15, 8. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Shi, Y.; Liu, J.; Lin, L.; Chen, X. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. Am. J. Transl. Res. 2019, 11, 6084–6092. [Google Scholar]
- Kowalski-Chauvel, A.; Lacore, M.G.; Arnauduc, F.; Delmas, C.; Toulas, C.; Cohen-Jonathan-Moyal, E.; Seva, C. The m6A RNA Demethylase ALKBH5 Promotes Radioresistance and Invasion Capability of Glioma Stem Cells. Cancers 2020, 13, 40. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Z.; Aili, D.; Wang, L.; Liu, T. Exploration of the carcinogenetic and immune role of CHK1 in human cancer. J. Cancer 2024, 15, 5927–5941. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jia, R.; Wang, L.; Yang, Q.; Hu, X.; Fu, Q.; Zhang, X.; Li, W.; Ren, Y. The Emerging Roles of Rad51 in Cancer and Its Potential as a Therapeutic Target. Front. Oncol. 2022, 12, 935593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Bai, Z.; Xia, D.; Zhao, Z.; Zhao, R.; Wang, Y.; Zhe, H. FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting β-catenin through mRNA demethylation. Mol. Carcinog. 2018, 57, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-M.; Li, Z.-X.; Wu, Y.-H.; Shi, Z.-L.; Mi, J.-L.; Hu, K.; Wang, R.-S. m6A demethylase FTO renders radioresistance of nasopharyngeal carcinoma via promoting OTUB1-mediated anti-ferroptosis. Transl. Oncol. 2023, 27, 101576. [Google Scholar] [CrossRef]
- Saldana, M.; VanderVorst, K.; Berg, A.L.; Lee, H.; Carraway, K.L. Otubain 1: A non-canonical deubiquitinase with an emerging role in cancer. Endocr. Relat. Cancer. 2019, 26, R1–R14. [Google Scholar] [CrossRef]
- Ji, L.; Pu, L.; Wang, J.; Cao, H.; Melemenidis, S.; Sinha, S.; Guan, L.; Laseinde, E.E.; von Eyben, R.; Richter, S.A.; et al. FTO inhibition enhances the therapeutic index of radiation therapy in head and neck cancer. JCI Insight 2025, 10, e184968. [Google Scholar] [CrossRef]
- Lanata, C.M.; Chung, S.A.; Criswell, L.A. DNA methylation 101: What is important to know about DNA methylation and its role in SLE risk and disease heterogeneity. Lupus Sci. Med. 2018, 5, e000285. [Google Scholar] [CrossRef]
- Sallustio, F.; Gesualdo, L.; Gallone, A. New findings showing how DNA methylation influences diseases. World J. Biol. Chem. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Dhingra, R.; Nwanaji-Enwerem, J.C.; Samet, M.; Ward-Caviness, C.K. DNA Methylation Age—Environmental Influences, Health Impacts, and Its Role in Environmental Epidemiology. Curr. Environ. Health Rep. 2018, 5, 317–327. [Google Scholar] [CrossRef]
- Maleknia, M.; Ahmadirad, N.; Golab, F.; Katebi, Y.; Haj Mohamad Ebrahim Ketabforoush, A. DNA Methylation in Cancer: Epigenetic View of Dietary and Lifestyle Factors. Epigenet. Insights 2023, 16, 25168657231199892. [Google Scholar] [CrossRef]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.-C.; Tsai, C.-Y.; Tsai, M.-M.; Yeh, C.-T.; Lin, K.-H. Roles of Long Noncoding RNAs in Recurrence and Metastasis of Radiotherapy-Resistant Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1903. [Google Scholar] [CrossRef]
- Chi, H.-C.; Tsai, C.-Y.; Tsai, M.-M.; Lin, K.-H. Impact of DNA and RNA Methylation on Radiobiology and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 555. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, P.; Huang, H.-W.; Liu, L.-P.; Zhao, F. Reactive oxygen species downregulate ARID1A expression via its promoter methylation during the pathogenesis of endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4509–4515. [Google Scholar] [PubMed]
- Winarto, H.; Tan, M.; Sadikin, M.; Wanandi, S. Expression is Down-Regulated by Oxidative Stress in Endometriosis and Endometriosis-Associated Ovarian Cancer. Transl. Oncogenom. 2017, 9, 1177272716689818. [Google Scholar] [CrossRef]
- Pogribny, I.; Koturbash, I.; Tryndyak, V.; Hudson, D.; Stevenson, S.M.L.; Sedelnikova, O.; Bonner, W.; Kovalchuk, O. Fractionated low-dose radiation exposure leads to accumulation of DNA damage and profound alterations in DNA and histone methylation in the murine thymus. Mol. Cancer Res. 2005, 3, 553–561. [Google Scholar] [CrossRef]
- Peng, Q.; Weng, K.; Li, S.; Xu, R.; Wang, Y.; Wu, Y. A Perspective of Epigenetic Regulation in Radiotherapy. Front. Cell Dev. Biol. 2021, 9, 624312. [Google Scholar] [CrossRef]
- Tryndyak, V.P.; Kovalchuk, O.; Pogribny, I.P. Loss of DNA methylation and histone H4 lysine 20 trimethylation in human breast cancer cells is associated with aberrant expression of DNA methyltransferase 1, Suv4-20h2 histone methyltransferase and methyl-binding proteins. Cancer Biol. Ther. 2006, 5, 65–70. [Google Scholar] [CrossRef]
- Megiorni, F.; Camero, S.; Ceccarelli, S.; McDowell, H.P.; Mannarino, O.; Marampon, F.; Pizer, B.; Shukla, R.; Pizzuti, A.; Marchese, C.; et al. DNMT3B in vitro knocking-down is able to reverse embryonal rhabdomyosarcoma cell phenotype through inhibition of proliferation and induction of myogenic differentiation. Oncotarget 2016, 7, 79342–79356. [Google Scholar] [CrossRef]
- Camero, S.; Vitali, G.; Pontecorvi, P.; Ceccarelli, S.; Anastasiadou, E.; Cicchetti, F.; Flex, E.; Pomella, S.; Cassandri, M.; Rota, R.; et al. DNMT3A and DNMT3B Targeting as an Effective Radiosensitizing Strategy in Embryonal Rhabdomyosarcoma. Cells 2021, 10, 2956. [Google Scholar] [CrossRef]
- Yan, J.; Chen, S.; Yi, Z.; Zhao, R.; Zhu, J.; Ding, S.; Wu, J. The role of p21 in cellular senescence and aging-related diseases. Mol. Cells 2024, 47, 100113. [Google Scholar] [CrossRef]
- Wu, C.; Guo, E.; Ming, J.; Sun, W.; Nie, X.; Sun, L.; Peng, S.; Luo, M.; Liu, D.; Zhang, L.; et al. Radiation-Induced DNMT3B Promotes Radioresistance in Nasopharyngeal Carcinoma through Methylation of p53 and p21. Mol. Ther. Oncolytics. 2020, 17, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Ren, Z.; Chen, Y.; Zhu, J.; Du, Y.; Pan, D.; Li, X.; Hu, B. A feedback regulation between miR-145 and DNA methyltransferase 3b in prostate cancer cell and their responses to irradiation. Cancer Lett. 2015, 361, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hajji, N.; Garcia-Revilla, J.; Soto, M.S.; Perryman, R.; Symington, J.; Quarles, C.C.; Healey, D.R.; Guo, Y.; Orta-Vázquez, M.L.; Mateos-Cordero, S.; et al. Arginine deprivation alters microglial polarity and synergizes with radiation to eradicate non-arginine-auxotrophic glioblastoma tumors. J. Clin. Investig. 2022, 132, e142137. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Parracho, R.T.; Corujo, D.; Xie, M.; Kutkaite, G.; Olsen, T.K.; Rubies Bedos, M.; Salehi, M.; Baryawno, N.; Menden, M.P.; et al. Epigenetic regulation of cell state by H2AFY governs immunogenicity in high-risk neuroblastoma. J. Clin. Investig. 2024, 134, e175310. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Wang, Y.; Nie, Q.; Zhang, X.; Yang, Y.; Mao, G. Cathepsin a upregulation in glioma: A potential therapeutic target associated with immune infiltration. J. Med. Biochem. 2022, 41, 459–465. [Google Scholar] [CrossRef]
- Fujimori, K.; Uno, S.; Kuroda, K.; Matsumoto, C.; Maehara, T. Leukotriene C4 synthase is a novel PPARγ target gene, and leukotriene C4 and D4 activate adipogenesis through cysteinyl LT1 receptors in adipocytes. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2022, 1869, 119203. [Google Scholar] [CrossRef]
- Laffey, K.G.; Du, J.; Schrum, A.G.; Ackerman, S.J. Transcriptional Regulation of the Human IL5RA Gene through Alternative Promoter Usage during Eosinophil Development. Int. J. Mol. Sci. 2021, 22, 10245. [Google Scholar] [CrossRef]
- Berry, J.L.; Polski, A.; Cavenee, W.K.; Dryja, T.P.; Murphree, A.L.; Gallie, B.L. The RB1 Story: Characterization and Cloning of the First Tumor Suppressor Gene. Genes 2019, 10, 879. [Google Scholar] [CrossRef]
- Halvorsen, A.R.; Helland, Å.; Fleischer, T.; Haug, K.M.; Grenaker Alnæs, G.I.; Nebdal, D.; Syljuåsen, R.G.; Touleimat, N.; Busato, F.; Tost, J.; et al. Differential DNA methylation analysis of breast cancer reveals the impact of immune signaling in radiation therapy. Int. J. Cancer. 2014, 135, 2085–2095. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Hassan, A.M.E.; Ali, A.A.; Hassan, N.M.; Yousif, A.A.; Elbashir, F.E.; Omer, A.; Abdalla, O.M. Detection of Radiation-Induced DNA Damage in Breast Cancer Patients by Using Gamma H2AX Biomarker: A Possible Correlation with Their Body Mass Index. Genome Integr. 2022, 13, 1. [Google Scholar]
- Wee, C.W.; Kim, J.H.; Kim, H.J.; Kang, H.-C.; Suh, S.Y.; Shin, B.S.; Ma, E.; Kim, I.H. Radiosensitization of Glioblastoma Cells by a Novel DNA Methyltransferase-inhibiting Phthalimido-Alkanamide Derivative. Anticancer Res. 2019, 39, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Benotmane, M.A.; Baatout, S.; Guns, P.-J.; Aerts, A. Radiation-induced cardiovascular disease: An overlooked role for DNA methylation? Epigenetics 2022, 17, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Mysara, M.; Benotmane, M.A.; Tamarat, R.; Santos, S.C.R.; Crijns, A.P.G.; Spoor, D.; Van Nieuwerburgh, F.; Deforce, D.; Baatout, S.; et al. DNA Methylation Alterations in Fractionally Irradiated Rats and Breast Cancer Patients Receiving Radiotherapy. Int. J. Mol. Sci. 2022, 23, 16214, Correction in Int. J. Mol. Sci. 2023, 24, 17590. https://doi.org/10.3390/ijms242417590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tojjari, A.; Giles, F.J.; Vilbert, M.; Saeed, A.; Cavalcante, L. SLAM Modification as an Immune-Modulatory Therapeutic Approach in Cancer. Cancers 2023, 15, 4808. [Google Scholar] [CrossRef]
- Dahlet, T.; Truss, M.; Frede, U.; Al Adhami, H.; Bardet, A.F.; Dumas, M.; Vallet, J.; Chicher, J.; Hammann, P.; Kottnik, S.; et al. E2F6 initiates stable epigenetic silencing of germline genes during embryonic development. Nat. Commun. 2021, 12, 3582. [Google Scholar] [CrossRef]
- Molenaar, R.J.; Radivoyevitch, T.; Maciejewski, J.P.; van Noorden, C.J.F.; Bleeker, F.E. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim. Biophys. Acta 2014, 1846, 326–341. [Google Scholar] [CrossRef]
- Molenaar, R.J.; Botman, D.; Smits, M.A.; Hira, V.V.; van Lith, S.A.; Stap, J.; Henneman, P.; Khurshed, M.; Lenting, K.; Mul, A.N.; et al. Radioprotection of IDH1-Mutated Cancer Cells by the IDH1-Mutant Inhibitor AGI-5198. Cancer Res. 2015, 75, 4790–4802. [Google Scholar] [CrossRef]
- Baumert, B.G.; Hegi, M.E.; van den Bent, M.J.; von Deimling, A.; Gorlia, T.; Hoang-Xuan, K.; Brandes, A.A.; Kantor, G.; Taphoorn, M.J.B.; Hassel, M.B.; et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016, 17, 1521–1532. [Google Scholar] [CrossRef]
- Ma, X.; Fan, W.; Zhang, T.; Luo, L.; Wang, K. The interplay between oxidative stress and epigenetic reprogramming in cancer. Int. J. Cancer 2025, 157, 2004–2018. [Google Scholar] [CrossRef]
- Williamson, E.A.; Wray, J.W.; Bansal, P.; Hromas, R. Overview for the histone codes for DNA repair. Prog. Mol. Biol. Transl. Sci. 2012, 110, 207–227. [Google Scholar] [CrossRef]
- Méndez-Acuña, L.; Di Tomaso, M.V.; Palitti, F.; Martínez-López, W. Histone post-translational modifications in DNA damage response. Cytogenet. Genome Res. 2010, 128, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, N.B.; Durante, M. Protein acetylation within the cellular response to radiation. J. Cell Physiol. 2011, 226, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, S. Epigenomics and Radiopharmaceuticals in Preoperative Chemoradiotherapy: Advancing Personalized Cancer Treatment Protocols. Cancer Biother. Radiopharm. 2025. [Google Scholar] [CrossRef]
- Pippa, S.; Mannironi, C.; Licursi, V.; Bombardi, L.; Colotti, G.; Cundari, E.; Mollica, A.; Coluccia, A.; Naccarato, V.; La Regina, G.; et al. Small Molecule Inhibitors of KDM5 Histone Demethylases Increase the Radiosensitivity of Breast Cancer Cells Over-expressing JARID1B. Molecules 2019, 24, 1739. [Google Scholar] [CrossRef]
- Wang, X.; Gu, M.; Ju, Y.; Zhou, J. Overcoming radio-resistance in esophageal squamous cell carcinoma via hypermethylation of PIK3C3 promoter region mediated by KDM5B loss. J. Radiat. Res. 2022, 63, 331–341. [Google Scholar] [CrossRef]
- Xu, L.-M.; Yu, H.; Yuan, Y.-J.; Zhang, J.; Ma, Y.; Cao, X.-C.; Wang, J.; Zhao, L.-J.; Wang, P. Overcoming of Radioresistance in Non-small Cell Lung Cancer by microRNA-320a Through HIF1α-Suppression Mediated Methylation of PTEN. Front. Cell Dev. Biol. 2020, 8, 553733. [Google Scholar] [CrossRef]
- Everix, L.; Seane, E.N.; Ebenhan, T.; Goethals, I.; Bolcaen, J. Introducing HDAC-Targeting Radiopharmaceuticals for Glio-blastoma Imaging and Therapy. Pharmaceuticals 2023, 16, 227. [Google Scholar] [CrossRef]
- Zhou, M.; Yuan, M.; Zhang, M.; Lei, C.; Aras, O.; Zhang, X.; An, F. Combining histone deacetylase inhibitors (HDACis) with other therapies for cancer therapy. Eur. J. Med. Chem. 2021, 226, 113825. [Google Scholar] [CrossRef]
- Antrobus, J.; Mackinnon, B.; Melia, E.; Hughes, J.R.; Parsons, J.L. HDAC Inhibitors Can Enhance Radiosensitivity of Head and Neck Cancer Cells Through Suppressing DNA Repair. Cancers 2024, 16, 4108. [Google Scholar] [CrossRef]
- Sharda, A.; Rashid, M.; Shah, S.G.; Sharma, A.K.; Singh, S.R.; Gera, P.; Chilkapati, M.K.; Gupta, S. Elevated HDAC activity and altered histone phospho-acetylation confer acquired radio-resistant phenotype to breast cancer cells. Clin. Epigenet. 2020, 12, 4. [Google Scholar] [CrossRef]
- Xiao, X.-S.; Cai, M.-Y.; Chen, J.-W.; Guan, X.-Y.; Kung, H.-F.; Zeng, Y.-X.; Xie, D. High Expression of p300 in Human Breast Cancer Correlates with Tumor Recurrence and Predicts Adverse Prognosis. Chin. J. Cancer Res. 2011, 23, 201–207. [Google Scholar] [CrossRef]
- Gao, Y.; Geng, J.; Hong, X.; Qi, J.; Teng, Y.; Yang, Y.; Qu, D.; Chen, G. Expression of p300 and CBP is associated with poor prognosis in small cell lung cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 760–767. [Google Scholar] [PubMed]
- Oike, T.; Ogiwara, H.; Torikai, K.; Nakano, T.; Yokota, J.; Kohno, T. Garcinol, a histone acetyltransferase inhibitor, radiosensi-tizes cancer cells by inhibiting non-homologous end joining. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.S.Z.; Stagni, V.; Iuzzolino, A.; Rotili, D.; Mai, A.; Del Bufalo, D.; Lavia, P.; Degrassi, F.; Trisciuoglio, D. Pharmaco-logical targeting of CBP/p300 drives a redox/autophagy axis leading to senescence-induced growth arrest in non-small cell lung cancer cells. Cancer Gene Ther. 2023, 30, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, B.; Liu, X.; Zhang, X.D.; Zhang, L.; Liu, T. Histone acetyltransferases CBP/p300 in tumorigenesis and CBP/p300 inhibitors as promising novel anticancer agents. Theranostics 2022, 12, 4935–4948. [Google Scholar] [CrossRef]
- Wang, H.; Guan, T.; Hu, R.; Huang, Z.; Liang, Z.; Lin, X.; Qiu, Y.; Liao, P.; Guo, X.; Ke, Y.; et al. Targeting KAT7 inhibits the progression of colorectal cancer. Theranostics 2025, 15, 1478–1495. [Google Scholar] [CrossRef]
- Wang, M.; Mu, G.; Qiu, B.; Wang, S.; Tao, C.; Mao, Y.; Zhao, X.; Liu, J.; Chen, K.; Li, Z.; et al. Competitive antagonism of KAT7 crotonylation against acetylation affects procentriole formation and colorectal tumorigenesis. Nat. Commun. 2025, 16, 2379. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Ding, T.; Zhang, H.; Zhang, Q.; Dai, H.; Zhang, H.; Tang, J.; Wang, X. KAT7 promotes radioresistance through upregulating PI3K/AKT signaling in breast cancer. J. Radiat. Res. 2023, 64, 448–456. [Google Scholar] [CrossRef]
- Ito, S.; Horikawa, S.; Suzuki, T.; Kawauchi, H.; Tanaka, Y.; Suzuki, T.; Suzuki, T. Human NAT10 Is an ATP-dependent RNA Acetyltransferase Responsible for N4-Acetylcytidine Formation in 18 S Ribosomal RNA (rRNA). J. Biol. Chem. 2014, 289, 35724–35730. [Google Scholar] [CrossRef]
- Rodrigues, P.; Bangali, H.; Ali, E.; Nauryzbaevish, A.S.; Hjazi, A.; Fenjan, M.N.; Alawadi, A.; Alsaalamy, A.; Alasheqi, M.Q.; Mustafa, Y.F. The mechanistic role of NAT10 in cancer: Unraveling the enigmatic web of oncogenic signaling. Pathol. Res. Pract. 2024, 253, 154990. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Du, Y.; Yan, Z.; Gao, J.; Zhang, H.; Wu, Q.; Nian, B.; Huang, X.; Da, M. Acetyltransferase NAT10 promotes gastric cancer progression by regulating the Wnt/β-catenin signaling pathway and enhances chemotherapy resistance. Discov. Oncol. 2025, 16, 173. [Google Scholar] [CrossRef]
- Li, L.; Li, F.; Zhou, C.; Ni, K.; Hu, H.; Wang, M.; Wang, H.; Gao, L. N-acetyltransferase 10 as a novel prognostic biomarker in papillary renal cell carcinoma: A machine learning and experimental validation study. Transl. Cancer Res. 2024, 13, 6364–6380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Z.; Sun, S.; Xie, S.; Jiang, M.; Chen, B.; Gu, C.; Yang, Y. NAT10 acetylates BCL-XL mRNA to promote the proliferation of multiple myeloma cells through PI3K-AKT pathway. Front. Oncol. 2022, 12, 967811. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, E.; Turberfield, A.H.; Klose, R.J. Histone demethylases in chromatin biology and beyond. EMBO Rep. 2015, 16, 1620–1639. [Google Scholar] [CrossRef]
- Yang, Y.; Luan, Y.; Feng, Q.; Chen, X.; Qin, B.; Ren, K.-D.; Luan, Y. Epigenetics and Beyond: Targeting Histone Methylation to Treat Type 2 Diabetes Mellitus. Front. Pharmacol. 2021, 12, 807413. [Google Scholar] [CrossRef]
- Alam, H.; Gu, B.; Lee, M.G. Histone methylation modifiers in cellular signaling pathways. Cell. Mol. Life Sci. 2015, 72, 4577–4592. [Google Scholar] [CrossRef]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef]
- Monaghan, L.; Massett, M.E.; Bunschoten, R.P.; Hoose, A.; Pirvan, P.-A.; Liskamp, R.M.J.; Jørgensen, H.G.; Huang, X. The Emerging Role of H3K9me3 as a Potential Therapeutic Target in Acute Myeloid Leukemia. Front. Oncol. 2019, 9, 705. [Google Scholar] [CrossRef]
- Gong, F.; Miller, K.M. Histone methylation and the DNA damage response. Mutat. Res. Rev. Mutat. Res. 2019, 780, 37–47. [Google Scholar] [CrossRef]
- McCauley, B.S.; Dang, W. Histone methylation and aging: Lessons learned from model systems. Biochim. Biophys. Acta 2014, 1839, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.A.; Mazurek, B.; Seiler, D.M. Radiation-induced alterations in histone modification patterns and their potential impact on short-term radiation effects. Front. Oncol. 2012, 2, 117. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, P.; Chen, C.; Ding, W.; Tillement, O.; Bai, H.; Zhang, S. Targeting epigenetic regulators as a promising avenue to overcome cancer therapy resistance. Signal Transduct. Target. Ther. 2025, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Ohguchi, Y.; Ohguchi, H. Diverse Functions of KDM5 in Cancer: Transcriptional Repressor or Activator? Cancers 2022, 14, 3270. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Shu, X.; Song, C.-X. Mapping epigenetic modifications by sequencing technologies. Cell Death Differ. 2025, 32, 56–65. [Google Scholar] [CrossRef]
- De-Ugarte, L.; Balcells, S.; Guerri-Fernandez, R.; Grinberg, D.; Diez-Perez, A.; Nogues, X.; Garcia-Giralt, N. Effect of the Tumor Suppressor miR-320a on Viability and Functionality of Human Osteosarcoma Cell Lines Compared to Primary Osteoblasts. Appl. Sci. 2020, 10, 2852. [Google Scholar] [CrossRef]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Guerini Rocco, E.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Yi, S.-J.; Kim, K. Gene regulation by histone-modifying enzymes under hypoxic conditions: A focus on histone methylation and acetylation. Exp. Mol. Med. 2022, 54, 878–889. [Google Scholar] [CrossRef]
- Hutt, D.M.; Roth, D.M.; Marchal, C.; Bouchecareilh, M. Using Histone Deacetylase Inhibitors to Analyze the Relevance of HDACs for Translation. Methods Mol. Biol. 2017, 1510, 77–91. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Olichwier, A.; Sowka, A.; Balatskyi, V.V.; Gan, A.-M.; Dziewulska, A.; Dobrzyn, P. SCD1-related epigenetic modifications affect hormone-sensitive lipase (Lipe) gene expression in cardiomyocytes. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119608. [Google Scholar] [CrossRef] [PubMed]
- Verza, F.A.; Das, U.; Fachin, A.L.; Dimmock, J.R.; Marins, M. Roles of Histone Deacetylases and Inhibitors in Anticancer Therapy. Cancers 2020, 12, 1664. [Google Scholar] [CrossRef] [PubMed]
- Moertl, S.; Payer, S.; Kell, R.; Winkler, K.; Anastasov, N.; Atkinson, M.J. Comparison of Radiosensitization by HDAC Inhibitors CUDC-101 and SAHA in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3259. [Google Scholar] [CrossRef]
- Gil, J.; Ramírez-Torres, A.; Encarnación-Guevara, S. Lysine acetylation and cancer: A proteomics perspective. J. Proteomics. 2017, 150, 297–309. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ding, Y.; Wang, L.; Wang, S.; Wang, H.; Qin, Y. DNA Methylation: From Cancer Biology to Clinical Perspectives. Front. Biosci. (Landmark Ed.) 2022, 27, 326. [Google Scholar] [CrossRef]
- Tang, F.; Choy, E.; Tu, C.; Hornicek, F.; Duan, Z. Therapeutic applications of histone deacetylase inhibitors in sarcoma. Cancer Treat. Rev. 2017, 59, 33–45. [Google Scholar] [CrossRef]
- Wawruszak, A.; Borkiewicz, L.; Okon, E.; Kukula-Koch, W.; Afshan, S.; Halasa, M. Vorinostat (SAHA) and Breast Cancer: An Overview. Cancers 2021, 13, 4700. [Google Scholar] [CrossRef]
- Blattmann, C.; Oertel, S.; Ehemann, V.; Thiemann, M.; Huber, P.E.; Bischof, M.; Witt, O.; Deubzer, H.E.; Kulozik, A.E.; Debus, J.; et al. Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 237–245. [Google Scholar] [CrossRef]
- Blattmann, C.; Thiemann, M.; Stenzinger, A.; Christmann, A.; Roth, E.; Ehemann, V.; Debus, J.; Kulozik, A.E.; Weichert, W.; Huber, P.E.; et al. Radiosensitization by histone deacetylase inhibition in an osteosarcoma mouse model. Strahlenther. Onkol. 2013, 189, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, S.H.; Hernandez-Ilizaliturri, F.J.; Clements, J.L.; Czuczman, M.S. Acquired resistance to rituximab is associated with chemotherapy resistance resulting from decreased Bax and Bak expression. Clin. Cancer Res. 2008, 14, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Gu, J.J.; Zhang, Q.; Mavis, C.; Hernandez-Ilizaliturri, F.J.; Czuczman, M.S.; Guo, Y. Vorinostat, a histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest and re-sensitizes rituximab- and chemo-resistant lymphoma cells to chemotherapy agents. J. Cancer Res. Clin. Oncol. 2016, 142, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Blattmann, C.; Oertel, S.; Thiemann, M.; Dittmar, A.; Roth, E.; Kulozik, A.E.; Ehemann, V.; Weichert, W.; Huber, P.E.; Stenzinger, A.; et al. Histone deacetylase inhibition sensitizes osteosarcoma to heavy ion radiotherapy. Radiat. Oncol. 2015, 10, 146. [Google Scholar] [CrossRef]
- Lv, R.; Shi, P.; Lu, Z.; Wei, T.; Yang, J.; Liao, X.; Yang, B.; Gao, C. The novel platinum(IV) prodrug of cisplatin axially conjugated with cannabidiol induces mitochondrial dysfunction and synergistically enhances anti-tumor effects. J. Inorg. Biochem. 2025, 272, 113003. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Q.; Zhu, Z.; Wang, Q.; Dou, J.; Zhao, Y.; Lv, W.; Zhong, F.; Yao, Y.; Zhang, G.; et al. Cancer Chemoradiotherapy Duo: Nano-Enabled Targeting of DNA Lesion Formation and DNA Damage Response. ACS Appl. Mater. Interfaces 2018, 10, 35734–35744. [Google Scholar] [CrossRef]
- Guo, S.; Yao, Y.; Tang, Y.; Xin, Z.; Wu, D.; Ni, C.; Huang, J.; Wei, Q.; Zhang, T. Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct. Target. Ther. 2023, 8, 205. [Google Scholar] [CrossRef]
- Galloway, T.J.; Wirth, L.J.; Colevas, A.D.; Gilbert, J.; Bauman, J.E.; Saba, N.F.; Raben, D.; Mehra, R.; Ma, A.W.; Atoyan, R.; et al. A Phase I Study of CUDC-101, a Multitarget Inhibitor of HDACs, EGFR, and HER2, in Combination with Chemoradiation in Patients with Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015, 21, 1566–1573. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, L.; Li, X.; Lu, W.; Lu, F.; Wang, S.; Wang, Y.; Hua, L.; Cui, C.; Dong, B.; et al. Rae1 drives NKG2D binding-dependent tumor development in mice by activating mTOR and STAT3 pathways in tumor cells. Cancer Sci. 2020, 111, 2234–2247. [Google Scholar] [CrossRef]
- Lee, G.H.; An, H.J.; Kim, T.H.; Kim, G.; Park, K.-S.; Park, H.; Lee, T.H.; Kwon, A.-Y. Clinical Impact of Natural Killer Group 2D Receptor Expression and That of Its Ligand in Ovarian Carcinomas: A Retrospective Study. Yonsei Med. J. 2021, 62, 288. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Chiang, Y.; Hsu, F.-M.; Tsai, C.-L.; Cheng, J.C.-H. Radiosensitization effect by HDAC inhibition improves NKG2D-dependent natural killer cytotoxicity in hepatocellular carcinoma. Front. Oncol. 2022, 12, 1009089. [Google Scholar] [CrossRef]
- Jung, E.K.; Chu, T.-H.; Vo, M.-C.; Nguyen, H.P.Q.; Lee, D.H.; Lee, J.K.; Lim, S.C.; Jung, S.-H.; Yoon, T.-M.; Yoon, M.S.; et al. Natural killer cells have a synergistic anti-tumor effect in combination with chemoradiotherapy against head and neck cancer. Cytotherapy 2022, 24, 905–915. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Zhang, Y. CBP/p300 and HDAC activities regulate H3K27 acetylation dynamics and zygotic genome activation in mouse preimplantation embryos. EMBO J. 2022, 41, e112012. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Qiao, X.; Fang, Y.; Guo, R.; Bai, P.; Liu, S.; Li, T.; Jiang, Y.; Wei, S.; Na, Z.; et al. Epigenetics-targeted drugs: Current paradigms and future challenges. Signal Transduct. Target. Ther. 2024, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Helin, K. Roles of H3K4 methylation in biology and disease. Trends Cell Biol. 2025, 35, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, Y.; Cao, J.; Lin, L.; Chen, X.; Zhou, Z.; Pu, J.; Chen, G.; Ma, X.; Deng, Q.; et al. N-acetyltransferase NAT10 controls cell fates via connecting mRNA cytidine acetylation to chromatin signaling. Sci. Adv. 2024, 10, eadh9871. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, Y.; Kumata, K.; Xie, L.; Kurihara, Y.; Ogawa, M.; Kokufuta, T.; Nengaki, N.; Wang, F.; Zhang, M.-R.R. The N-acetyltransferase 10 inhibitor [11C]remodelin: Synthesis and preliminary positron emission tomography study in mice. EJNMMI Radiopharm. Chem. 2025, 10, 6. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, H.; Wu, J.; Chen, W.; Guan, X. Inhibition of N-acetyltransferase 10 using remodelin attenuates doxorubicin resistance by reversing the epithelial-mesenchymal transition in breast cancer. Am. J. Transl. Res. 2018, 10, 256–264. [Google Scholar]
- Kopytko, P.; Piotrowska, K.; Janisiak, J.; Tarnowski, M. Garcinol-A Natural Histone Acetyltransferase Inhibitor and New Anti-Cancer Epigenetic Drug. Int. J. Mol. Sci. 2021, 22, 2828. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).