Effects of Imposed Defocus on Inhibitor of DNA-Binding Gene Expression in Chick Posterior Ocular Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Lens Treatments
2.2. Tissue Sample Collection
2.3. RNA Purification
2.4. Real-Time PCR
2.5. Statistical Analysis
3. Results
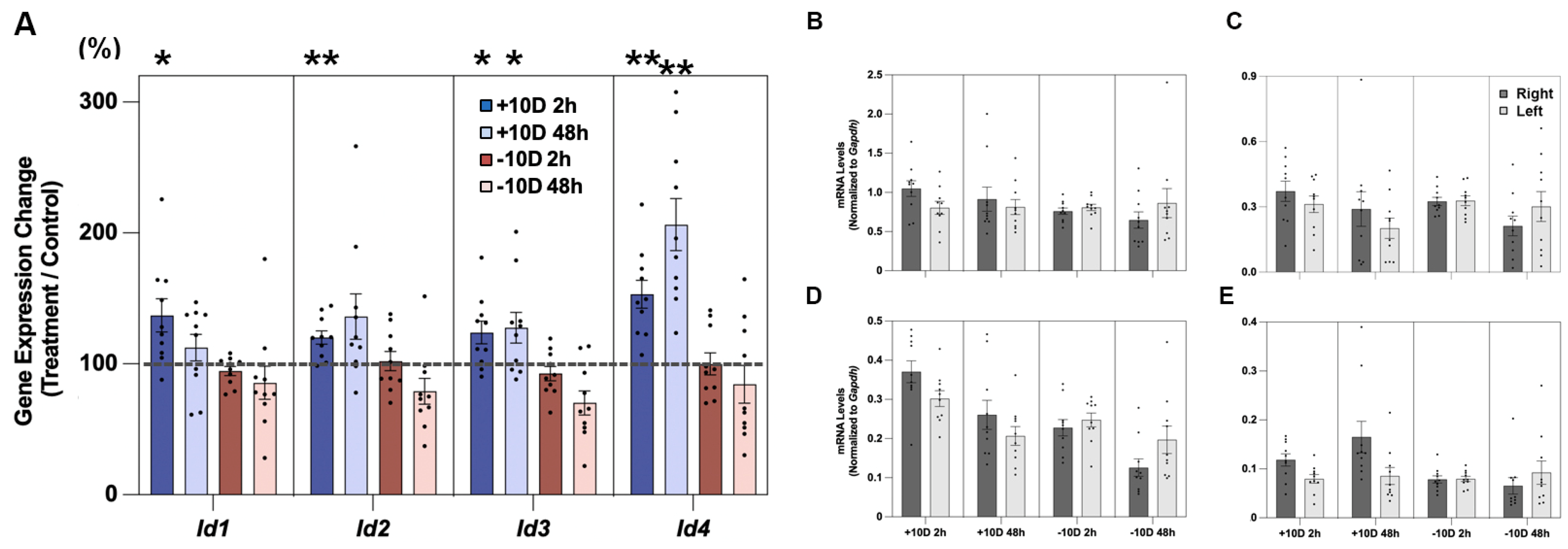
3.1. Id Gene Expression in the Retina and Effects of Lens Treatment
3.2. Id Gene Expression in the RPE and Effects of Lens Treatment
3.3. Id Gene Expression in the Choroid and Effects of Lens Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMP | Bone Morphogenetic Protein |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| Id | Inhibitors of DNA-binding gene |
| RPE | Retinal Pigment Epithelium |
| TGF-β | Transforming Growth Factor Beta |
References
- Vitale, S.; Sperduto, R.D.; Ferris, F.L. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch. Ophthalmol. 2009, 127, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Ang, M.; Wong, C.W.; Hoang, Q.V.; Cheung, G.C.M.C.; Lee, S.Y.; Chia, A.; Saw, S.M.; Matsui, K.O.; Schmetterer, L. Imaging in myopia: Potential biomarkers, current challenges and future developments. Br. J. Ophthalmol. 2019, 103, 855–862. [Google Scholar] [CrossRef]
- Schiefer, U.; Kraus, C.; Baumbach, P.; Ungewiß, J.; Michels, R. Refractive errors. Dtsch. Arzteblatt. Int. 2016, 113, 693–702. [Google Scholar] [CrossRef]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef]
- McBrien, N.A.; Norton, T.T. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri). Vis. Res. 1992, 32, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Wallman, J.; Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Hung, L.F. The role of optical defocus in regulating refractive development in infant monkeys. Vis. Res. 1999, 39, 1415–1435. [Google Scholar] [CrossRef]
- Troilo, D.; Smith, E.L.; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.; et al. IMI—Report on experimental models of emmetropization and myopia. Invest. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef]
- Morgan, I.G.; Rose, K.A.; Ashby, R.S. Animal models of experimental myopia: Limitations and synergies with studies on human myopia. In Pathologic Myopia, 2nd ed.; Spaide, R.F., Ohno-Matsui, K., Yannuzzi, L.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 67–85. [Google Scholar]
- Morgan, I.G.; Ashby, R.S.; Nickla, D.L. Form deprivation and lens-induced myopia: Are they different? Ophthalmic. Physiol. Opt. 2013, 33, 355–361. [Google Scholar] [CrossRef]
- Wildsoet, C.; Wallman, J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis. Res. 1995, 35, 1175–1194. [Google Scholar] [CrossRef]
- Brown, D.M.; Mazade, R.; Clarkson-Townsend, D.; Hogan, K.; Roy, R.M.D.; Pardue, M.T. Candidate pathways for retina to scleral signaling in refractive eye growth. Exp. Eye Res. 2022, 219, 109071. [Google Scholar] [CrossRef]
- Stone, R.A.; Tobias, J.W.; Wei, W.; Carlstedt, X.; Zhang, L.; Iuvone, P.M.; Nickla, D.L. Diurnal gene expression patterns in retina and choroid distinguish myopia progression from myopia onset. PLoS ONE 2024, 19, e0307091. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wildsoet, C.F. RPE and choroid mechanisms underlying ocular growth and myopia. Prog. Mol. Biol. Transl. Sci. 2015, 134, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Q.; Song, W.; Chuang, G.M.; Sun, D.; Cheung, K.; Chou, A.; He, A.; Shoghi, E.; Wildsoet, C.F. Dynamic BMP gene expression regulation in chick RPE during recovery from short term optical defocus and form-deprivation. PLoS ONE 2024, 19, e0311505. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Muroy, S.E.; Zhang, Y.; Saijo, K.; Kolora, S.R.R.; Zhu, Q.; Wildsoet, C.F. Gene expression signatures of contact lens-induced myopia in guinea pig retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2022, 63, 25. [Google Scholar] [CrossRef]
- Zhang, Y.; Azmoun, S.; Hang, A.; Zeng, J.; Eng, E.; Wildsoet, C.F. Retinal defocus and form-deprivation induced regional differential gene expression of BMPs in chick RPE. J. Comp. Neurol. 2020, 528, 2864–2873. [Google Scholar] [CrossRef]
- Zhang, Y.; Phan, E.; Wildsoet, C.F. Retinal defocus and form-deprivation exposure duration affects RPE BMP gene expression. Sci. Rep. 2019, 9, 7332. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Hang, A.; Phan, E.; Wildsoet, C.F. Differential gene expression of BMP2 and BMP receptors in chick retina & choroid induced by imposed optical defocus. Vis. Neurosci. 2016, 33, E015. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ho, C.; Wildsoet, C.F. Effects of imposed defocus of opposite sign on temporal gene expression patterns of BMP4 and BMP7 in chick RPE. Exp. Eye Res. 2013, 109, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Wildsoet, C.F. Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6072–6080. [Google Scholar] [CrossRef]
- Ke, J.; Wu, R.; Chen, Y.; Abba, M.L. Inhibitor of DNA binding proteins: Implications in human cancer progression and metastasis. Am. J. Transl. Res. 2018, 10, 3887–3910. [Google Scholar]
- Sikder, H.A.; Devlin, M.K.; Dunlap, S.; Ryu, B.; Alani, R.M. Id proteins in cell growth and tumorigenesis. Cancer Cell 2003, 3, 525–530. [Google Scholar] [CrossRef]
- Zebedee, Z.; Hara, E. Id proteins in cell cycle control and cellular senescence. Oncogene 2001, 20, 8317–8325. [Google Scholar] [CrossRef]
- Chu, Y.H.; Lin, J.; Nath, S.; Schachtrup, C. Id proteins: Emerging roles in CNS disease and targets for modifying neural stem cell behavior. Cell Tissue Res. 2022, 387, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Roschger, C.; Cabrele, C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun. Signal. 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Miyazawa, K. Id: A target of BMP signaling. Sci. STKE 2002, 2002, pe40. [Google Scholar] [CrossRef]
- Ogata, T.; Wozney, J.M.; Benezra, R.; Noda, M. Bone morphogenetic protein 2 transiently enhances expression of a gene, Id (inhibitor of differentiation), encoding a helix-loop-helix molecule in osteoblast-like cells. Proc. Natl. Acad. Sci. USA 1993, 90, 9219–9222. [Google Scholar] [CrossRef]
- Hollnagel, A.; Oehlmann, V.; Heymer, J.; Rüther, U.; Nordheim, A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem. 1999, 274, 19838–19845. [Google Scholar] [CrossRef]
- Ho, C.C.; Zhou, X.; Mishina, Y.; Bernard, D.J. Mechanisms of bone morphogenetic protein 2 (BMP2) stimulated inhibitor of DNA binding 3 (Id3) transcription. Mol. Cell. Endocrinol. 2011, 332, 242–252. [Google Scholar] [CrossRef]
- Kamaid, A.; Neves, J.; Giráldez, F. Id Gene regulation and function in the prosensory domains of the chicken inner ear: A link between Bmp signaling and Atoh1. J. Neurosci. 2010, 30, 11426–11434. [Google Scholar] [CrossRef]
- Kee, Y.; Bronner-Fraser, M. Id4 expression and its relationship to other Id genes during avian embryonic development. Mech. Dev. 2001, 109, 341–345. [Google Scholar] [CrossRef]
- Liu, Y.; Wildsoet, C. The effective add inherent in 2-zone negative lenses inhibits eye growth in myopic young chicks. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5085–5093. [Google Scholar] [CrossRef]
- Loh, K.M.; Ang, L.T.; Zhang, J.; Kumar, V.; Ang, J.; Auyeong, J.Q.; Lee, K.L.; Choo, S.H.; Lim, C.Y.Y.; Nichane, M.; et al. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell 2014, 14, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Ehata, S.; Miyazono, K. Bone morphogenetic protein signaling in cancer; some topics in the recent 10 Years. Front. Cell Dev. Biol. 2022, 10, 883523. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Li, Y.; Southwood, M.; Ye, L.; Long, L.; Al-Lamki, R.S.; Morrell, N.W. Id proteins are critical downstream effectors of BMP signaling in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L312–L321. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Nichols, J.; Chambers, I.; Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003, 115, 281–292. [Google Scholar] [CrossRef]
- Shu, D.Y.; Wojciechowski, M.C.; Lovicu, F.J. Bone morphogenetic protein-7 suppresses TGFβ2-induced epithelial-mesenchymal transition in the lens: Implications for cataract prevention. Investig. Ophthalmol. Vis. Sci. 2017, 58, 781–796. [Google Scholar] [CrossRef]
- Saika, S.; Ikeda, K.; Yamanaka, O.; Flanders, K.C.; Ohnishi, Y.; Nakajima, Y.; Muragaki, Y.; Ooshima, A. Adenoviral gene transfer of BMP-7, Id2, or Id3 suppresses injury-induced epithelial-to-mesenchymal transition of lens epithelium in mice. Am. J. Physiol. Cell Physiol. 2006, 290, C282–C289. [Google Scholar] [CrossRef]
- Ueki, Y.; Wilken, M.S.; Cox, K.E.; Chipman, L.B.; Bermingham-McDonogh, O.; Reh, T.A. A transient wave of BMP signaling in the retina is necessary for Müller glial differentiation. Development 2015, 142, 533. [Google Scholar] [CrossRef]
- Du, Y.; Xiao, Q.; Yip, H.K. Regulation of retinal progenitor cell differentiation by bone morphogenetic protein 4 is mediated by the Smad/Id cascade. Investig. Opthalmol. Vis. Sci. 2010, 51, 3764. [Google Scholar] [CrossRef]
- Ostrin, L.A.; Harb, E.; Nickla, D.L.; Read, S.A.; Alonso-Caneiro, D.; Schroedl, F.; Kaser-Eichberger, A.; Zhou, X.; Wildsoet, C.F. IMI—The dynamic choroid: New insights, challenges, and potential significance for human myopia. Investig. Ophthalmol. Vis. Sci. 2023, 64, 4. [Google Scholar] [CrossRef]
- Ito, K.; Hoerig, C.; Dan, Y.S.; McFadden, S.A.; Mamou, J.; Hoang, Q.V. Biomechanical changes occur in myopic choroidal stroma and mirror those in the adjacent sclera. Commun. Eng. 2024, 3, 139. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef] [PubMed]
- Sironen, R.K.; Karjalainen, H.M.; Elo, M.A.; Kaarniranta, K.; Törrönen, K.; Takigawa, M.; Helminen, H.J.; Lammi, M.J. cDNA array reveals mechanosensitive genes in chondrocytic cells under hydrostatic pressure. Biochim. Biophys. Acta 2002, 1591, 45–54. [Google Scholar] [CrossRef]
- Fong, S.; Itahana, Y.; Sumida, T.; Singh, J.; Coppe, J.P.; Liu, Y.; Richards, P.C.; Bennington, J.L.; Lee, N.M.; Debs, R.J.; et al. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc. Natl. Acad. Sci. USA 2003, 100, 13543–13548. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, J.T., Jr.; Norton, T.T. Selective regulation of MMP and TIMP mRNA levels in during minus lens compensation and recovery. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3484–3492. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Lv, H.; Jiang, X.; Zhang, M.; Li, X. α-adrenergic agonist brimonidine control of experimentally induced myopia in guinea pigs: A pilot study. Mol. Vis. 2017, 23, 785–798. [Google Scholar]
- Xiang, A.; Peng, Z.; He, H.; Meng, X.; Luo, Y.; Yang, J.; Zeng, F.; Chen, X.; Zhong, X. The potential of brimonidine for myopia treatment: Targeting MMP-2 to regulate choroidal thickness and control eye growth. Heliyon 2024, 10, e37416. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Zhang, Y.; Vyas, S.A.; Zhu, Q.; Wildsoet, Q.Z. Changes in expression in BMP2 and two closely related genes in guinea pig retinal pigment epithelium during Induction and recovery from myopia. Biomolecules 2023, 13, 1373. [Google Scholar] [CrossRef] [PubMed]





| Gene | NCBI Access Number | Sequences (5′–3′) | Efficiency | Amplicon |
|---|---|---|---|---|
| Id1 | NM_204590.2 | Forward: 5′-CGCGGCTAGTAACCTTCTCAGA-3′ Reverse: 5′-TTCTCCGGCATCATTGTAATATACA-3′ | 91.8% | 69 bp |
| Id2 | NM_205002.1 | Forward: 5′-CCCTACAGGCAGCCGAGTT-3′ Reverse: 5′-TCAGCCACAGAGCGCTTTG-3 | 97.7% | 65 bp |
| Id3 | NM_204589.1 | Forward:5′-TGCTCCAAAGACGAGAGAAGTTT-3′ Reverse: 5′-TGTTTGCTAATCGGCACTGATG-3′ | 94.4% | 64 bp |
| Id4 | NM_204282.1 | Forward: 5′-TCCCTGCAGGAATGTTGCA-3′ Reverse: 5′ ATCTCTCTATGTACACGGTATGAAATGTC-3′ | 97.2% | 68 bp |
| Gapdh | NM_204305.1 | Forward: 5′-AGATGCAGGTGCTGAGTATGTTG-3′ Reverse: 5′-GATGAGCCCCAGCCTTCTC-3′ | 95.6% | 71 bp |
| Right (n = 5) | Left (n = 5) | % Change (Right/Left) | |
|---|---|---|---|
| Id1 | 1.2 × 10−4 [6.0 × 10−5] | 1.2 × 10−4 [5.5 × 10−5] | 109.2 [10.3] |
| Id2 | 1.0 × 10−2 [9.6 × 10−4] | 1.0 × 10−2 [1.2 × 10−3] | 110.7 [9.5] |
| Id3 | 8.8 × 10−4 [5.4 × 10−5] | 8.2 × 10−4 [8.2 × 10−5] | 110.4 [7.5] |
| Id4 | 6.7 × 10−2 [7.4 × 10−3] | 6.3 × 10−2 [4.3 × 10−3] | 105.5 [5.1] |
| Id1 | Id2 | Id3 | Id4 | |
|---|---|---|---|---|
| +10 D 2 h (n = 11) | 331.3 [216.8] | 123.3 [7.8] * | 129.1 [17.1] | 112.1 [5.7] |
| +10 D 48 h (n = 6) | 68.4 [13.0] | 131.2 [9.2] * | 117.4 [9.9] | 107.2 [5.3] |
| −10 D 2 h (n = 11) | 80.9 [10.4] | 107.4 [5.9] | 116.2 [6.7] * | 108.5 [6.0] |
| −10 D 48 h (n = 8) | 75.6 [4.9] ** | 117.7 [19.5] | 104.0 [13.2] | 116.3 [6.7] * |
| Id1 | Id2 | Id3 | Id4 | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | |
| +10 D 2 h (n = 11) | 6.6 × 10−4 [4.5 × 10−4] | 1.7 × 10−4 [3.4 × 10−5] | 1.1 × 10−2 [1.0 × 10−3] | 9.2 × 10−3 [8.2 × 10−4] | 1.2 × 10−3 [1.6 × 10−4] | 1.0 × 10−3 [1.2 × 10−4] | 8.1 × 10−2 [7.3 × 10−3] | 7.2 × 10−2 [5.8 × 10−3] |
| +10 D 48 h (n = 6) | 2.2 × 10−4 [1.1 × 10−5] | 4.4 × 10−4 [1.3 × 10−4] | 1.1 × 10−2 [1.8 × 10−3] | 7.8 × 10−3 [1.1 × 10−3] | 9.5 × 10−4 [8.4 × 10−5] | 8.3 × 10−4 [9.4 × 10−5] | 8.6 × 10−2 [2.6 × 10−3] | 8.1 × 10−2 [3.5 × 10−3] |
| −10 D 2 h (n = 11) | 1.5 × 10−4 [4.0 × 10−5] | 2.0 × 10−4 [4.6 × 10−5] | 9.3 × 10−3 [8.0 × 10−4] | 8.8 × 10−3 [7.2 × 10−4] | 1.0 × 10−3 [7.5 × 10−5] | 8.7 × 10−4 [1.0 × 10−4] | 8.2 × 10−2 [9.0 × 10−3] | 7.5 × 10−2 [6.6 × 10−3] |
| −10 D 48 h (n = 8) | 2.4 × 10−4 [2.2 × 10−5] | 3.1 × 10−4 [2.8 × 10−5] | 1.1 × 10−2 [1.6 × 10−3] | 1.3 × 10−2 [4.0 × 10−3] | 7.2 × 10−4 [1.0 × 10−4] | 7.3 × 10−4 [1.0 × 10−4] | 1.0 × 10−1 [3.1 × 10−3] | 8.7 × 10−2 [3.1 × 10−3] |
| Right (n = 3) | Left (n = 3) | % Change (Right/Left) | |
|---|---|---|---|
| Id1 | 5.3 × 10−2 [3.2 × 10−2] | 1.4 × 10−2 [3.8 × 10−3] | 399.0 [181.8] |
| Id2 | 1.6 × 10−1 [2.1 × 10−2] | 1.5 × 10−1 [3.7 × 10−2] | 128.3 [52.8] |
| Id3 | 2.3 × 10−2 [7.0 × 10−3] | 1.7 × 10−2 [3.4 × 10−3] | 150.4 [49.2] |
| Id4 | 6.7 × 10−2 [2.6 × 10−2] | 6.3 × 10−2 [1.7 × 10−2] | 118.8 [41.2] |
| Id1 | Id2 | Id3 | Id4 | |
|---|---|---|---|---|
| +10 D 2 h (n = 7) | 274.4 [106.0] | 247.7 [84.2] | 327.4 [124.1] | 190.5 [76.3] |
| +10 D 48 h (n = 7) | 387.7 [211.1] | 156.8 [19.8] * | 661.8 [162.8] * | 426.7 [200.8] |
| −10 D 2 h (n = 10) | 268.7 [94.2] | 105.6 [27.1] | 161.2 [69.0] | 211.2 [56.8] |
| −10 D 48 h (n = 6) | 63.5 [5.0] * | 104.9 [26.5] | 147.5 [68.8] | 114.4 [27.7] |
| Id1 | Id2 | Id3 | Id4 | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | |
| +10 D 2 h (n = 7) | 2.4 × 10−2 [6.4 × 10−3] | 1.8 × 10−2 [6.3 × 10−3] | 2.5 × 10−1 [4.9 × 10−2] | 1.5 × 10−1 [2.7 × 10−2] | 5.0 × 10−2 [1.3 × 10−2] | 2.2 × 10−2 [4.2 × 10−3] | 2.6 × 10−2 [7.0 × 10−3] | 3.7 × 10−2 [1.3 × 10−2] |
| +10 D 48 h (n = 7) | 2.7 × 10−2 [1.2 × 10−2] | 8.7 × 10−3 [1.0 × 10−3] | 5.4 × 10−1 [1.0 × 10−1] | 3.9 × 10−1 [9.8 × 10−2] | 1.6 × 10−1 [4.9 × 10−2] | 2.8 × 10−2 [6.6 × 10−3] | 1.6 × 10−2 [7.1 × 10−3] | 3.7 × 10−3 [3.1 × 10−4] |
| −10 D 2 h (n = 10) | 3.3 × 10−2 [1.5 × 10−2] | 3.1 × 10−2 [1.8 × 10−2] | 1.4 × 10−1 [3.6 × 10−2] | 1.5 × 10−1 [3.0 × 10−2] | 3.4 × 10−2 [1.0 × 10−2] | 3.7 × 10−2 [7.6 × 10−3] | 4.6 × 10−2 [1.2 × 10−2] | 3.2 × 10−2 [8.4 × 10−3] |
| −10 D 48 h (n = 6) | 5.9 × 10−3 [4.7 × 10−4] | 9.5 × 10−3 [9.9 × 10−4] | 1.8 × 10−1 [4.0 × 10−2] | 1.9 × 10−1 [4.4 × 10−2] | 2.4 × 10−2 [7.0 × 10−3] | 2.0 × 10−2 [2.3 × 10−3] | 1.3 × 10−2 [6.4 × 10−3] | 2.0 × 10−2 [1.3 × 10−2] |
| Right (n = 6) | Left (n = 6) | % Change (Right/Left) | |
|---|---|---|---|
| Id1 | 7.6 × 10−1 [7.4 × 10−2] | 7.6 × 10−1 [8.6 × 10−2] | 106.4 [14.8] |
| Id2 | 2.4 × 10−1 [2.2 × 10−2] | 2.2 × 10−1 [2.6 × 10−2] | 110.7 [13.8] |
| Id3 | 2.1 × 10−1 [3.0 × 10−2] | 1.7 × 10−1 [1.9 × 10−2] | 127.3 [14.5] |
| Id4 | 7.6 × 10−2 [7.7 × 10−3] | 7.0 × 10−2 [7.1 × 10−3] | 110.8 [8.6] |
| Id1 | Id2 | Id3 | Id4 | |
|---|---|---|---|---|
| +10 D 2 h (n = 10) | 137.0 [12.7] * | 120.0 [5.1] ** | 124.0 [8.7] * | 153.2 [10.7] ** |
| +10 D 48 h (n = 10) | 112.4 [10.2] | 136.1 [17.4] | 127.5 [11.8] * | 206.3 [19.9] ** |
| −10 D 2 h (n = 10) | 94.6 [3.4] | 102.0 [7.3] | 92.6 [5.4] | 100.0 [8.4] |
| −10 D 48 h (n = 10) | 85.5 [12.6] | 78.9 [9.9] | 70.2 [9.2] | 84.4 [14.3] |
| Id1 | Id2 | Id3 | Id4 | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | |
| +10 D 2 h (n = 10) | 1.0 [1.0 × 10−1] | 8.0 × 10−1 [8.3 × 10−2] | 3.7 × 10−1 [4.6 × 10−2] | 3.1 × 10−1 [3.8 × 10−2] | 3.7 × 10−1 [2.8 × 10−2] | 3.0 × 10−1 [2.0 × 10−2] | 1.2 × 10−1 [1.3 × 10−2] | 8.0 × 10−2 [8.9 × 10−3] |
| +10 D 48 h (n = 10) | 9.1 × 10−1 [1.5 × 10−1] | 8.1 × 10−1 [9.5 × 10−2] | 2.9 × 10−1 [8.0 × 10−2] | 2.0 × 10−1 [4.6 × 10−2] | 2.6 × 10−1 [3.7 × 10−2] | 2.1 × 10−1 [2.4 × 10−2] | 1.7 × 10−1 [3.2 × 10−2] | 8.5 × 10−2 [1.7 × 10−2] |
| −10 D 2 h (n = 10) | 7.6 × 10−1 [4.1 × 10−2] | 8.1 × 10−1 [4.1 × 10−2] | 3.2 × 10−1 [1.9 × 10−2] | 3.3 × 10−1 [2.2 × 10−2] | 2.3 × 10−1 [2.1 × 10−2] | 2.5 × 10−1 [1.8 × 10−2] | 7.9 × 10−2 [7.4 × 10−3] | 8.0 × 10−2 [5.3 × 10−3] |
| −10 D 48 h (n = 10) | 6.5 × 10−1 [1.0 × 10−1] | 8.6 × 10−1 [1.9 × 10−1] | 2.1 × 10−1 [4.6 × 10−2] | 3.0 × 10−1 [6.8 × 10−2] | 1.3 × 10−1 [2.2 × 10−2] | 2.0 × 10−1 [3.5 × 10−2] | 6.5 × 10−2 [1.7 × 10−2] | 9.2 × 10−2 [2.4 × 10−2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tseng, C.; Hang, A.; Sun, D.; Song, W.; Wildsoet, C.F. Effects of Imposed Defocus on Inhibitor of DNA-Binding Gene Expression in Chick Posterior Ocular Tissues. Cells 2025, 14, 1883. https://doi.org/10.3390/cells14231883
Zhang Y, Tseng C, Hang A, Sun D, Song W, Wildsoet CF. Effects of Imposed Defocus on Inhibitor of DNA-Binding Gene Expression in Chick Posterior Ocular Tissues. Cells. 2025; 14(23):1883. https://doi.org/10.3390/cells14231883
Chicago/Turabian StyleZhang, Yan, Connor Tseng, Abraham Hang, Daniel Sun, Wulian Song, and Christine F. Wildsoet. 2025. "Effects of Imposed Defocus on Inhibitor of DNA-Binding Gene Expression in Chick Posterior Ocular Tissues" Cells 14, no. 23: 1883. https://doi.org/10.3390/cells14231883
APA StyleZhang, Y., Tseng, C., Hang, A., Sun, D., Song, W., & Wildsoet, C. F. (2025). Effects of Imposed Defocus on Inhibitor of DNA-Binding Gene Expression in Chick Posterior Ocular Tissues. Cells, 14(23), 1883. https://doi.org/10.3390/cells14231883






