Histone Deacetylase Inhibition Enhances AQP3 Levels in Human Corneal Epithelial Cells and Corneal Wound Healing in Normoglycemic and Diabetic Male Mice
Highlights
- SAHA improved corneal wound healing in diabetic and normoglycemic mice.
- AQP3 expression was increased at the wound’s edge in the cornea during healing.
- SAHA could potentially be used clinically to promote corneal wound healing.
- AQP3 upregulation at the edge of corneal wounds is likely important for healing.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Cell Treatment Plan for SAHA and RGFP966
2.3. Cell Plating and Treatment Timeline for Determining the Effect of Medium Glucose Level
2.4. Cell Migration and Proliferation Assay
2.5. Immunofluorescence Microscopy on Wounded Corneas
2.6. Corneal Wound Healing In Vivo
2.7. Statistical Analysis
3. Results
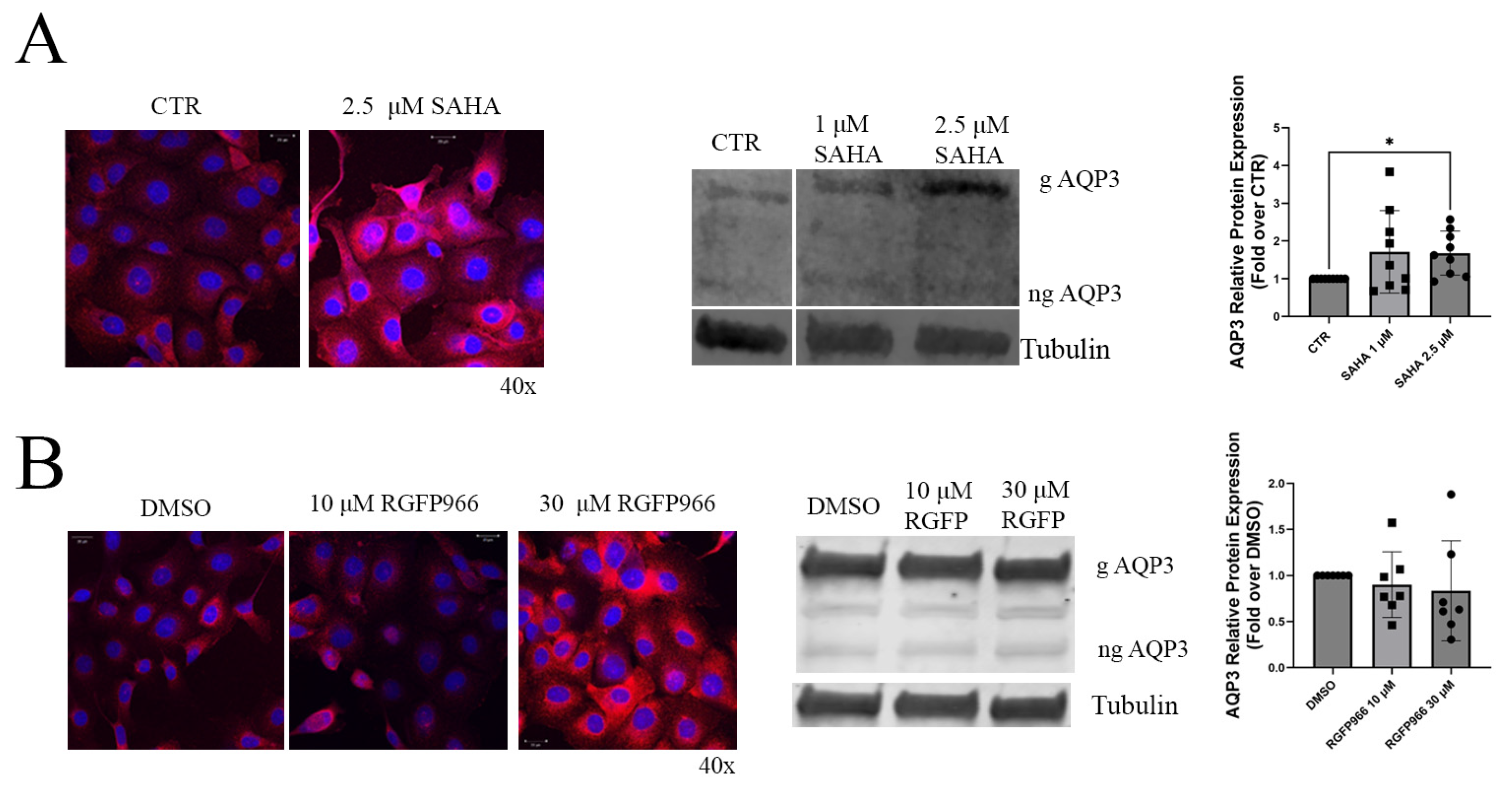
3.1. HDAC Inhibition Increased AQP3 Levels in HCECs Grown in Standard (Hyperglycemic) Conditions
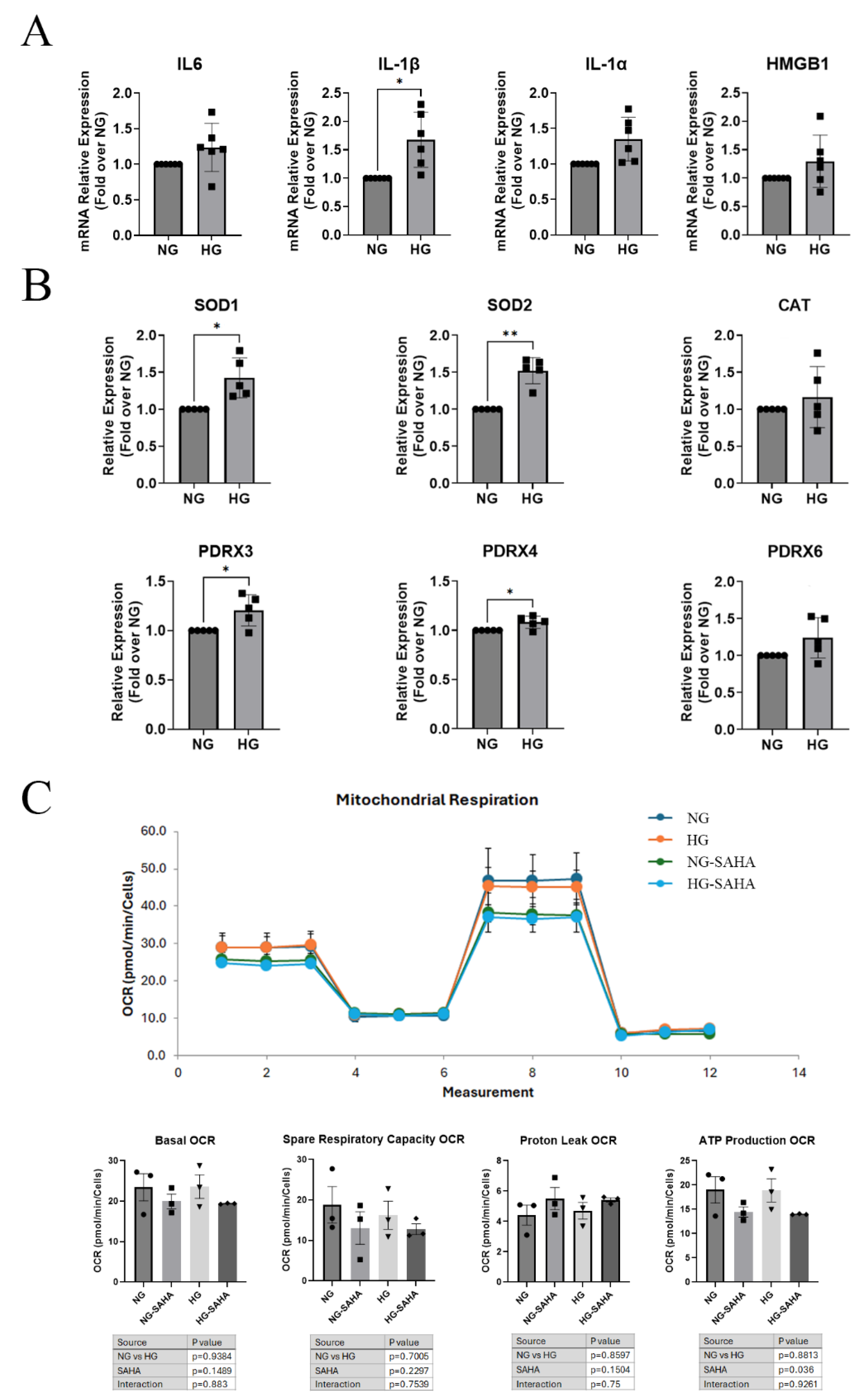
3.2. Hyperglycemic Conditions Altered Inflammatory and Reactive Oxygen Species (ROS) Scavenger Gene Expression but Had Little or No Effect on Mitochondrial Function or ROS Production
3.3. Acute Hyperglycemic Conditions Did Not Affect HDAC mRNA Levels or Activity, AQP3 Expression, or Migration, but HDAC Inhibition Increased Glycerol Uptake
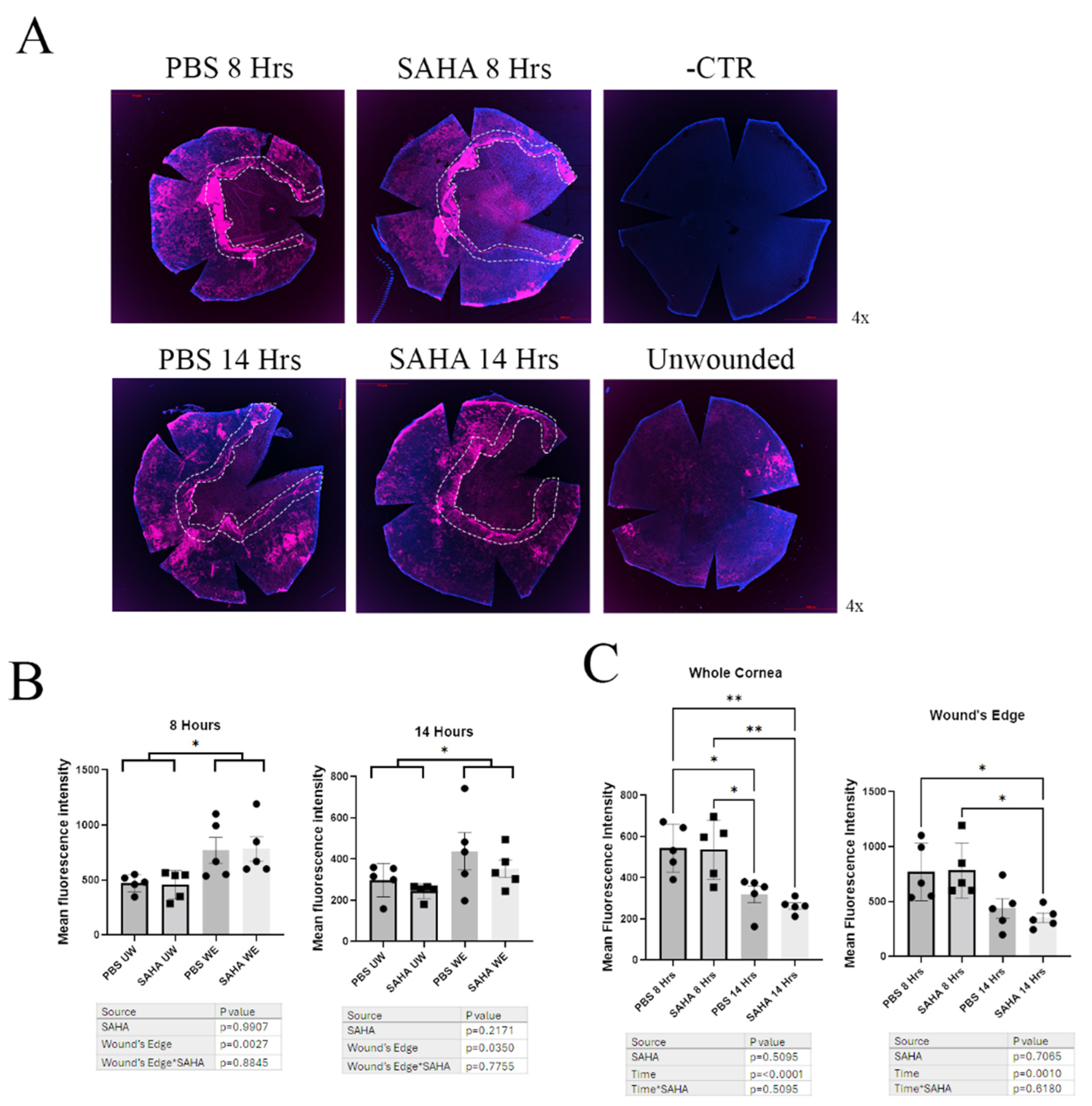
3.4. Wounded Corneas Showed No Difference in Aqp3 Expression Levels by Immunofluorescence of Whole-Mount Corneas, but Aqp3 Levels at the Wound’s Edge Were Increased
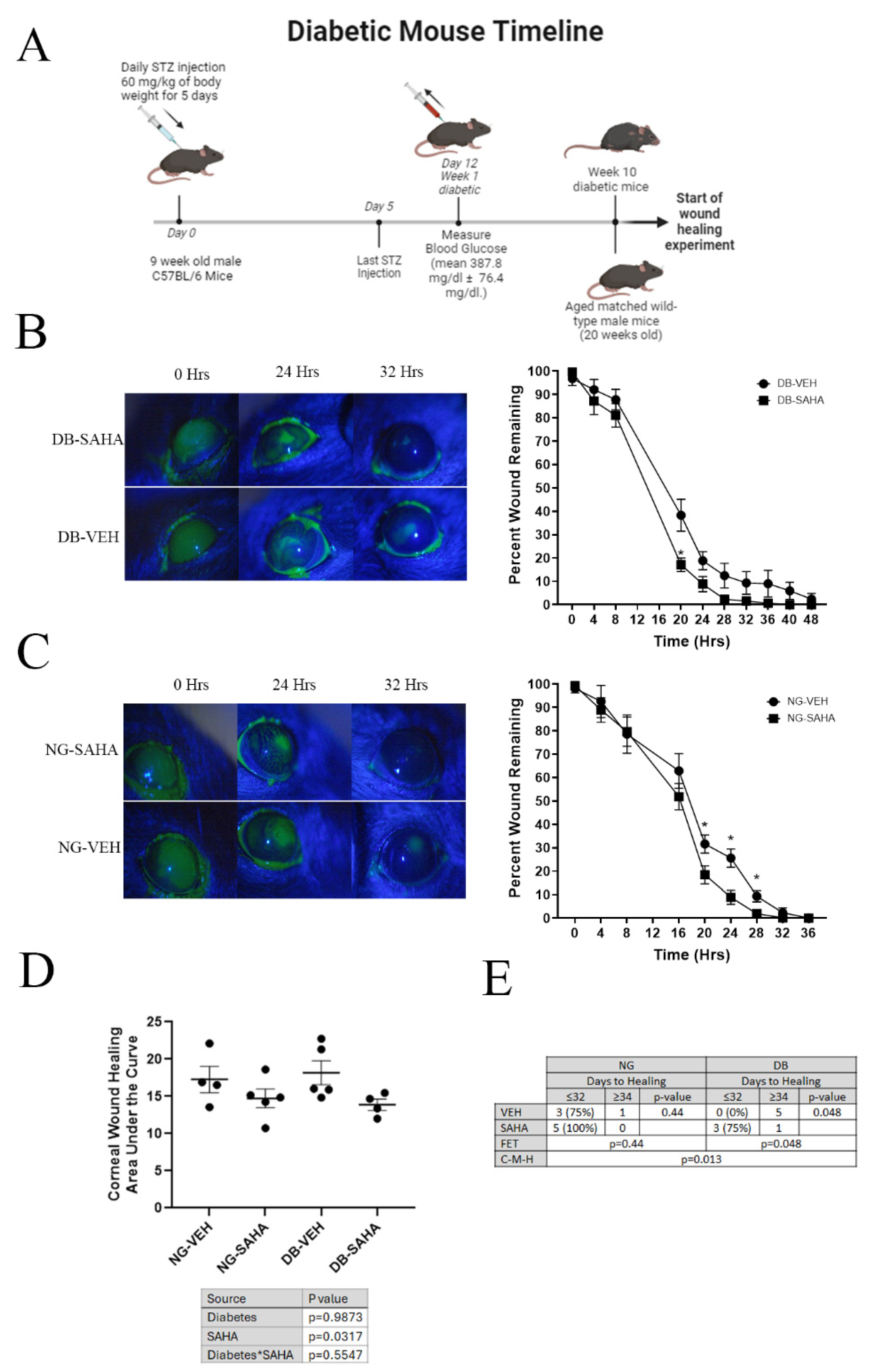
3.5. Inhibition of HDACs by SAHA Improved Wound Healing in Diabetic and Normoglycemic Male Mice but Not Female Mice
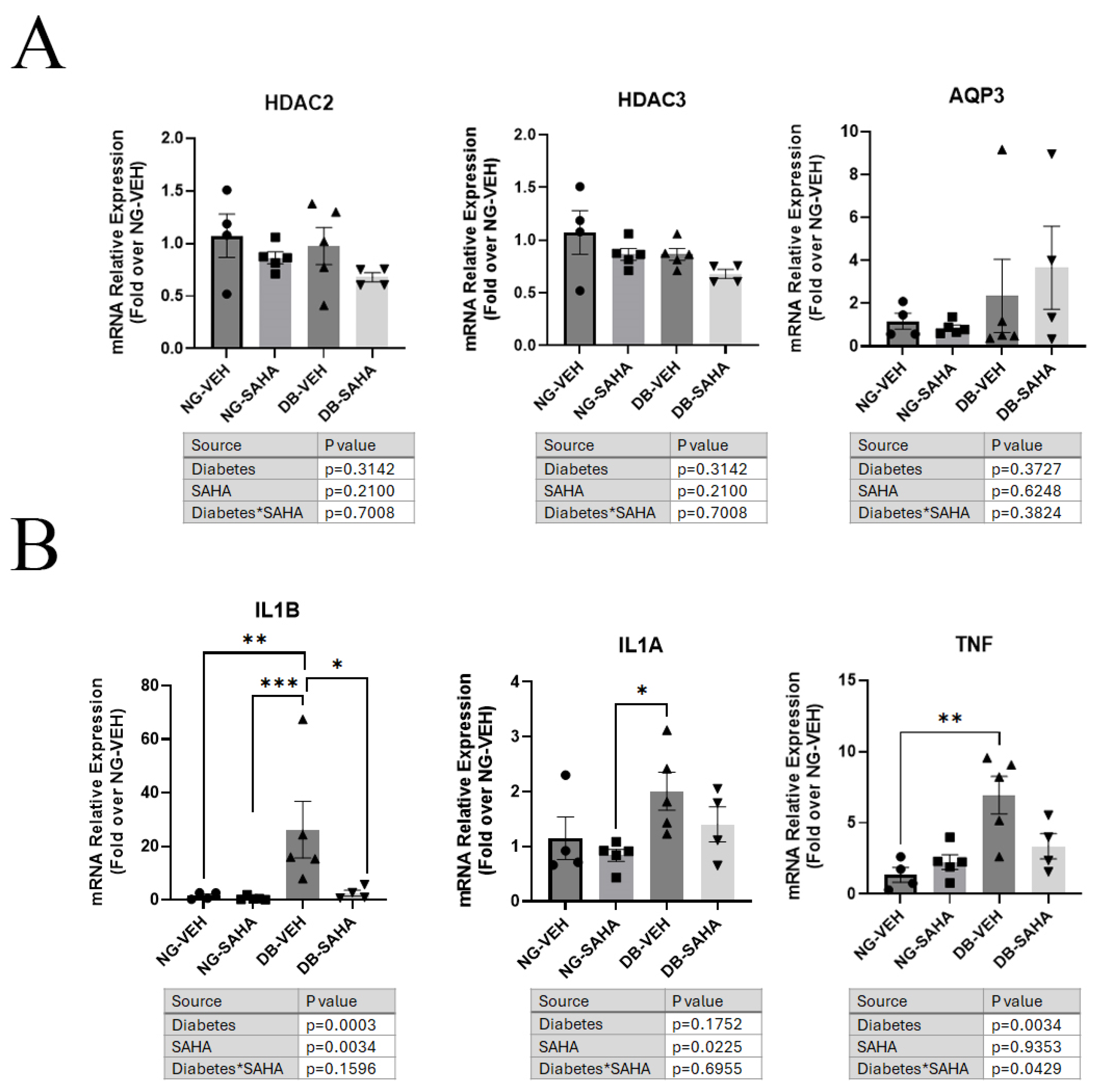
3.6. Healed Corneas Showed Significant Differences in Inflammatory Marker Expression in Vehicle-Treated Compared to SAHA-Treated Diabetic Male Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AQP3 | Aquaporin-3 |
| DMSO | Dimethylsulfoxide |
| FDA | Food and Drug Administration |
| HCEC | Human corneal epithelial cells |
| HDAC | Histone deacylatase |
| IL-1β | Interleukin-1beta |
| Il-1α | Interleukin-1alpha |
| PBS | Phosphate-buffered saline |
| PRDX | Peroxiredoxin |
| ROS | Reactive oxygen species |
| SAHA | Suberoylanilide hydroxamic acid |
| SEM | Standard error of the mean |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| TNF | Tumor necrosis factor |
References
- Chen, W.L.; Lin, C.T.; Ko, P.S.; Yeh, P.T.; Kuan, Y.H.; Hu, F.R.; Yang, C.M. In vivo confocal microscopic findings of corneal wound healing after corneal epithelial debridement in diabetic vitrectomy. Ophthalmology 2009, 116, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V. Diabetic complications in the cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.P.; Li, Y.; Ljubimov, A.V.; Yu, F.S. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 2009, 58, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Morita, I.; Takase, H.; Ohno-Matsui, K.; Mochizuki, M. Prolonged exposure to high glucose impaired cellular behavior of normal human corneal epithelial cells. Curr. Eye Res. 2003, 27, 197–203. [Google Scholar] [CrossRef]
- Hara, M.; Verkman, A.S. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 7360–7365. [Google Scholar] [CrossRef]
- Ma, T.; Hara, M.; Sougrat, R.; Verbavatz, J.M.; Verkman, A.S. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 2002, 277, 17147–17153. [Google Scholar] [CrossRef]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Kaneko, M.; Mizukami, N.; Kusunoki, Y.; Sugiyama, K. Relationship between Aging-Related Skin Dryness and Aquaporins. Int. J. Mol. Sci. 2017, 18, 1559. [Google Scholar] [CrossRef]
- Levin, M.H.; Verkman, A.S. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4365–4372. [Google Scholar] [CrossRef]
- Ikarashi, N.; Mizukami, N.; Pei, C.; Uchino, R.; Fujisawa, I.; Fukuda, N.; Kon, R.; Sakai, H.; Kamei, J. Role of Cutaneous Aquaporins in the Development of Xeroderma in Type 2 Diabetes. Biomedicines 2021, 9, 104. [Google Scholar] [CrossRef]
- Ikarashi, N.; Mizukami, N.; Kon, R.; Kaneko, M.; Uchino, R.; Fujisawa, I.; Fukuda, N.; Sakai, H.; Kamei, J. Study of the Mechanism Underlying the Onset of Diabetic Xeroderma Focusing on an Aquaporin-3 in a Streptozotocin-Induced Diabetic Mouse Model. Int. J. Mol. Sci. 2019, 20, 3782. [Google Scholar] [CrossRef]
- Sugimoto, T.; Huang, L.; Minematsu, T.; Yamamoto, Y.; Asada, M.; Nakagami, G.; Akase, T.; Nagase, T.; Oe, M.; Mori, T.; et al. Impaired aquaporin 3 expression in reepithelialization of cutaneous wound healing in the diabetic rat. Biol. Res. Nurs. 2013, 15, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Ullmann, Y.; Egozi, D.; Keren, A.; Daod, E.; Anis, O.; Kabha, H.; Belokopytov, M.; Ashkar, M.; Shofti, R.; et al. Topical Erythropoietin Treatment Accelerates the Healing of Cutaneous Burn Wounds in Diabetic Pigs Through an Aquaporin-3-Dependent Mechanism. Diabetes 2017, 66, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, D.; Rouzaire, M.; Marceau, G.; Prat, C.; Pereira, B.; Lemarie, R.; Deruelle, P.; Fajardy, I.; Gallot, D.; Blanchon, L.; et al. Aquaporins and Fetal Membranes From Diabetic Parturient Women: Expression Abnormalities and Regulation by Insulin. J. Clin. Endocrinol. Metab. 2015, 100, E1270–E1279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, J.; Liu, C.; Chen, Y. Association between levels of aquaporin 3 in the placenta and adiponectin in the umbilical cord blood with gestational diabetes mellitus and pregnancy outcome. Mol. Med. Rep. 2020, 22, 1498–1506. [Google Scholar] [CrossRef]
- Lawlor, L.; Yang, X.B. Harnessing the HDAC-histone deacetylase enzymes, inhibitors and how these can be utilised in tissue engineering. Int. J. Oral Sci. 2019, 11, 20. [Google Scholar] [CrossRef]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef]
- Lundby, A.; Lage, K.; Weinert, B.T.; Bekker-Jensen, D.B.; Secher, A.; Skovgaard, T.; Kelstrup, C.D.; Dmytriyev, A.; Choudhary, C.; Lundby, C.; et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012, 2, 419–431. [Google Scholar] [CrossRef]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef]
- Choudhary, V.; Olala, L.O.; Kagha, K.; Pan, Z.Q.; Chen, X.; Yang, R.; Cline, A.; Helwa, I.; Marshall, L.; Kaddour-Djebbar, I.; et al. Regulation of the Glycerol Transporter, Aquaporin-3, by Histone Deacetylase-3 and p53 in Keratinocytes. J. Investig. Dermatol. 2017, 137, 1935–1944. [Google Scholar] [CrossRef]
- Szigety, K.M.; Liu, F.; Yuan, C.Y.; Moran, D.J.; Horrell, J.; Gochnauer, H.R.; Cohen, R.N.; Katz, J.P.; Kaestner, K.H.; Seykora, J.T.; et al. HDAC3 ensures stepwise epidermal stratification via NCoR/SMRT-reliant mechanisms independent of its histone deacetylase activity. Genes Dev. 2020, 34, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, C.; Prabu, P.; Balakumar, M.; Lenin, R.; Prabhu, D.; Anjana, R.M.; Mohan, V.; Balasubramanyam, M. Augmentation of histone deacetylase 3 (HDAC3) epigenetic signature at the interface of proinflammation and insulin resistance in patients with type 2 diabetes. Clin. Epigenetics 2016, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tong, Q.; Zhang, Z.; Wang, S.; Zheng, Y.; Liu, Q.; Qian, L.B.; Chen, S.Y.; Sun, J.; Cai, L. Inhibition of HDAC3 prevents diabetic cardiomyopathy in OVE26 mice via epigenetic regulation of DUSP5-ERK1/2 pathway. Clin. Sci. 2017, 131, 1841–1857. [Google Scholar] [CrossRef] [PubMed]
- Teena, R.; Dhamodharan, U.; Ali, D.; Rajesh, K.; Ramkumar, K.M. Gene Expression Profiling of Multiple Histone Deacetylases (HDAC) and Its Correlation with NRF2-Mediated Redox Regulation in the Pathogenesis of Diabetic Foot Ulcers. Biomolecules 2020, 10, 1466. [Google Scholar] [CrossRef]
- Karnam, K.; Sedmaki, K.; Sharma, P.; Routholla, G.; Goli, S.; Ghosh, B.; Venuganti, V.V.K.; Kulkarni, O.P. HDAC6 inhibitor accelerates wound healing by inhibiting tubulin mediated IL-1β secretion in diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165903. [Google Scholar] [CrossRef]
- Karnam, K.; Sedmaki, K.; Sharma, P.; Mahale, A.; Ghosh, B.; Kulkarni, O.P. Pharmacological blockade of HDAC3 accelerates diabetic wound healing by regulating macrophage activation. Life Sci. 2023, 321, 121574. [Google Scholar] [CrossRef]
- Anumanthan, G.; Sharma, A.; Waggoner, M.; Hamm, C.W.; Gupta, S.; Hesemann, N.P.; Mohan, R.R. Efficacy and Safety Comparison Between Suberoylanilide Hydroxamic Acid and Mitomycin C in Reducing the Risk of Corneal Haze After PRK Treatment In Vivo. J. Refract. Surg. 2017, 33, 834–839. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Mylarapu, N.; Watsky, M.A. Effects of 1,25 and 24,25 Vitamin D on Corneal Epithelial Proliferation, Migration and Vitamin D Metabolizing and Catabolizing Enzymes. Sci. Rep. 2017, 7, 16951. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, X.; Mylarapu, N.; Kuthyar, S.; Sakhalkar, O.; Watsky, M.A. A Method for Eliminating Fibroblast Contamination in Mouse and Human Primary Corneal Epithelial Cell Cultures. Curr. Eye Res. 2023, 48, 981–991. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Lu, J.; Watsky, M.A. Effects of 1,25-Vitamin D3 and 24,25-Vitamin D3 on Corneal Nerve Regeneration in Diabetic Mice. Biomolecules 2023, 13, 1754. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Szalai, E.; Deak, E.; Modis, L., Jr.; Nemeth, G.; Berta, A.; Nagy, A.; Felszeghy, E.; Kaposzta, R.; Malik, R.A.; Csutak, A. Early Corneal Cellular and Nerve Fiber Pathology in Young Patients With Type 1 Diabetes Mellitus Identified Using Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Shibuya, M.; Nakashima, H.; Hisamura, R.; Masuda, N.; Imagawa, T.; Uehara, M.; Tsubota, K. Involvement of oxidative stress on corneal epithelial alterations in a blink-suppressed dry eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, B.T.; Schlotzer-Schrehardt, U.; Skeie, J.M.; Burckart, K.A.; Schmidt, G.A.; Reed, C.R.; Zimmerman, M.B.; Kruse, F.E.; Greiner, M.A. Mitochondrial and Morphologic Alterations in Native Human Corneal Endothelial Cells Associated With Diabetes Mellitus. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2130–2138. [Google Scholar] [CrossRef]
- Skeie, J.M.; Aldrich, B.T.; Goldstein, A.S.; Schmidt, G.A.; Reed, C.R.; Greiner, M.A. Proteomic analysis of corneal endothelial cell-descemet membrane tissues reveals influence of insulin dependence and disease severity in type 2 diabetes mellitus. PLoS ONE 2018, 13, e0192287. [Google Scholar] [CrossRef]
- Adcock, I.M. HDAC inhibitors as anti-inflammatory agents. Br. J. Pharmacol. 2007, 150, 829–831. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, L.-Y.; Wen, R.; Yang, N.; Zhang, T.-N. Histone deacetylases and their inhibitors in inflammatory diseases. Biomed. Pharmacother. 2024, 179, 117295. [Google Scholar] [CrossRef]
- Fortingo, N.; Melnyk, S.; Sutton, S.H.; Watsky, M.A.; Bollag, W.B. Innate Immune System Activation, Inflammation and Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 14933. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Jiang, Q.-w.; Kaili, D.; Freeman, J.; Lei, C.-y.; Geng, B.-c.; Tan, T.; He, J.-f.; Shi, Z.; Ma, J.-j.; Luo, Y.-h.; et al. Diabetes inhibits corneal epithelial cell migration and tight junction formation in mice and human via increasing ROS and impairing Akt signaling. Acta Pharmacol. Sin. 2019, 40, 1205–1211. [Google Scholar] [CrossRef]
- Trengove, N.J.; Bielefeldt-Ohmann, H.; Stacey, M.C. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000, 8, 13–25. [Google Scholar] [CrossRef]
- Mirza, R.; Koh, T.J. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011, 56, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, C.; Kämpfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and Sustained Induction of Chemokines during Impaired Wound Healing in the Genetically Diabetic Mouse: Prolonged Persistence of Neutrophils and Macrophages during the Late Phase of Repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Dong, M.; Li, Y.; Zhou, Q.; Yang, L. The proinflammatory cytokines IL-1β and TNF-α modulate corneal epithelial wound healing through p16Ink4a suppressing STAT3 activity. J. Cell. Physiol. 2020, 235, 10081–10093. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.E.; Fang, M.M.; Weinheimer-Haus, E.M.; Ennis, W.J.; Koh, T.J. Sustained Inflammasome Activity in Macrophages Impairs Wound Healing in Type 2 Diabetic Humans and Mice. Diabetes 2014, 63, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.; DiPietro, L.A.; Koh, T.J. Selective and Specific Macrophage Ablation Is Detrimental to Wound Healing in Mice. Am. J. Pathol. 2009, 175, 2454–2462. [Google Scholar] [CrossRef]
- Zhu, H.; Shan, L.; Schiller, P.W.; Mai, A.; Peng, T. Histone deacetylase-3 activation promotes tumor necrosis factor-alpha (TNF-alpha) expression in cardiomyocytes during lipopolysaccharide stimulation. J. Biol. Chem. 2010, 285, 9429–9436. [Google Scholar] [CrossRef]
- Kim, B.; Kim, Y.Y.; Nguyen, P.T.-T.; Nam, H.; Suh, J.G. Sex differences in glucose metabolism of streptozotocin-induced diabetes inbred mice (C57BL/6J). Appl. Biol. Chem. 2020, 63, 59. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Zhang, Z.; Dan, J.; Zhou, Q.; Wang, X.; Li, W.; Zhou, L.; Yang, L.; Xie, L. Insulin Promotes Corneal Nerve Repair and Wound Healing in Type 1 Diabetic Mice by Enhancing Wnt/β-Catenin Signaling. Am. J. Pathol. 2020, 190, 2237–2250. [Google Scholar] [CrossRef]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P Promotes Diabetic Corneal Epithelial Wound Healing Through Molecular Mechanisms Mediated via the Neurokinin-1 Receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; Kakazu, A.; He, J.; Bazan, H.E.P. Mouse strains and sexual divergence in corneal innervation and nerve regeneration. FASEB J. 2019, 33, 4598–4609. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Hu, K.M.; Seamon, K.J.; Mani, V.; Chen, Y.; Gronert, K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 2012, 26, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Giuliano, E.A.; Gafen, H.B.; Gupta, S.; Martin, L.M.; Sinha, P.R.; Rodier, J.T.; Fink, M.K.; Hesemann, N.P.; Chaurasia, S.S.; et al. Is sex a biological variable in corneal wound healing? Exp. Eye Res. 2019, 187, 107705. [Google Scholar] [CrossRef]
- Abu-Romman, A.; Scholand, K.K.; Govindarajan, G.; Yu, Z.; Pal-Ghosh, S.; Stepp, M.A.; de Paiva, C.S. Age-Related Differences in the Mouse Corneal Epithelial Transcriptome and Their Impact on Corneal Wound Healing. Investig. Ophthalmol. Vis. Sci. 2024, 65, 21. [Google Scholar] [CrossRef]
- Aoki, M.; Shimozuru, M.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Sex differences in behavioral and corticosterone responses to mild stressors in ICR mice are altered by ovariectomy in peripubertal period. Zool. Sci. 2010, 27, 783–789. [Google Scholar] [CrossRef]
- Petroutsos, G.; Guimaraes, R.; Giraud, J.P.; Pouliquen, Y. Corticosteroids and corneal epithelial wound healing. Br. J. Ophthalmol. 1982, 66, 705–708. [Google Scholar] [CrossRef]
- Xue, Y.; Xu, P.; Hu, Y.; Liu, S.; Yan, R.; Liu, S.; Li, Y.; Liu, J.; Fu, T.; Li, Z. Stress systems exacerbate the inflammatory response after corneal abrasion in sleep-deprived mice via the IL-17 signaling pathway. Mucosal Immunol. 2024, 17, 323–345. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The Role of Oxidative Stress in Diabetic Neuropathy: Generation of Free Radical Species in the Glycation Reaction and Gene Polymorphisms Encoding Antioxidant Enzymes to Genetic Susceptibility to Diabetic Neuropathy in Population of Type I Diabetic Patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [CrossRef]
- Mussi, N.; Stuard, W.L.; Sanches, J.M.; Robertson, D.M. Chronic Hyperglycemia Compromises Mitochondrial Function in Corneal Epithelial Cells: Implications for the Diabetic Cornea. Cells 2022, 11, 2567. [Google Scholar] [CrossRef]
- Shetty, R.; Kumar, N.R.; Subramani, M.; Krishna, L.; Murugeswari, P.; Matalia, H.; Khamar, P.; Dadachanji, Z.V.; Mohan, R.R.; Ghosh, A.; et al. Safety and efficacy of combination of suberoylamilide hydroxyamic acid and mitomycin C in reducing pro-fibrotic changes in human corneal epithelial cells. Sci. Rep. 2021, 11, 4392. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Hanus, J.; Anderson, C.; Zhang, H.; Dellinger, M.; Brekken, R.; Wang, S. Inhibition of multiple pathogenic pathways by histone deacetylase inhibitor SAHA in a corneal alkali-burn injury model. Mol. Pharm. 2013, 10, 307–318. [Google Scholar] [CrossRef]
- Bollag, W.B.; Olala, L.O.; Xie, D.; Lu, X.; Qin, H.; Choudhary, V.; Patel, R.; Bogorad, D.; Estes, A.; Watsky, M. Dioleoylphosphatidylglycerol Accelerates Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ray, S.; Bollag, W.B. Modulation of phospholipase D-mediated phosphatidylglycerol formation by differentiating agents in primary mouse epidermal keratinocytes. Biochim. Biophys. Acta 2003, 1643, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Zhong, X.; Dodd, M.E.; Hardy, D.M.; Zheng, X.; Allred, W.T. Phospholipase d signaling and extracellular signal-regulated kinase-1 and -2 phosphorylation (activation) are required for maximal phorbol ester-induced transglutaminase activity, a marker of keratinocyte differentiation. J. Pharmacol. Exp. Ther. 2005, 312, 1223–1231. [Google Scholar] [CrossRef]
- Bollag, W.B.; Xie, D.; Zheng, X.; Zhong, X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: Production of a phosphatidylglycerol signaling lipid. J. Investig. Dermatol. 2007, 127, 2823–2831. [Google Scholar] [CrossRef]
- Melnyk, S.; Bollag, W.B. Aquaporins in the Cornea. Int. J. Mol. Sci. 2024, 25, 3748. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Rahimi, A. The relation between hydration and mechanical behavior of bovine cornea in tension. J. Mech. Behav. Biomed. Mater. 2014, 36, 90–97. [Google Scholar] [CrossRef]
- Rodríguez-López, R.; Webb, J.N.; Erdi, M.; Kofinas, P.; Franco, W.; Zhang, H.; Randleman, J.B.; Scarcelli, G. Determining the Relationship Between Corneal Stiffening and Tissue Dehydration After Corneal Cross-Linking. Investig. Ophthalmol. Vis. Sci. 2024, 65, 14. [Google Scholar] [CrossRef]
- Díez-Ajenjo, M.A.; Luque-Cobija, M.J.; Peris-Martínez, C.; Ortí-Navarro, S.; García-Domene, M.C. Refractive changes and visual quality in patients with corneal edema after cataract surgery. BMC Ophthalmol. 2022, 22, 242. [Google Scholar] [CrossRef]
- Echevarria, M.; Windhager, E.E.; Frindt, G. Selectivity of the renal collecting duct water channel aquaporin-3. J. Biol. Chem. 1996, 271, 25079–25082. [Google Scholar] [CrossRef]
- Yang, B.; Verkman, A.S. Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 1997, 272, 16140–16146. [Google Scholar] [CrossRef]
- Sebastian, R.; Chau, E.; Fillmore, P.; Matthews, J.; Price, L.A.; Sidhaye, V.; Milner, S.M. Epidermal aquaporin-3 is increased in the cutaneous burn wound. Burns 2015, 41, 843–847. [Google Scholar] [CrossRef]
- Prangenberg, J.; Doberentz, E.; Witte, A.L.; Madea, B. Aquaporin 1 and 3 as local vitality markers in mechanical and thermal skin injuries. Int. J. Leg. Med. 2021, 135, 1837–1842. [Google Scholar] [CrossRef]
- Toki, M.I.; Cecchi, F.; Hembrough, T.; Syrigos, K.N.; Rimm, D.L. Proof of the quantitative potential of immunofluorescence by mass spectrometry. Lab. Investig. 2017, 97, 329–334. [Google Scholar] [CrossRef]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnyk, S.; Lu, X.; Ronderos, V.; Choudhary, V.; Johnson, M.H.; Watsky, M.A.; Bollag, W.B. Histone Deacetylase Inhibition Enhances AQP3 Levels in Human Corneal Epithelial Cells and Corneal Wound Healing in Normoglycemic and Diabetic Male Mice. Cells 2025, 14, 1880. https://doi.org/10.3390/cells14231880
Melnyk S, Lu X, Ronderos V, Choudhary V, Johnson MH, Watsky MA, Bollag WB. Histone Deacetylase Inhibition Enhances AQP3 Levels in Human Corneal Epithelial Cells and Corneal Wound Healing in Normoglycemic and Diabetic Male Mice. Cells. 2025; 14(23):1880. https://doi.org/10.3390/cells14231880
Chicago/Turabian StyleMelnyk, Samuel, Xiaowen Lu, Victoria Ronderos, Vivek Choudhary, Maribeth H. Johnson, Mitchell A. Watsky, and Wendy B. Bollag. 2025. "Histone Deacetylase Inhibition Enhances AQP3 Levels in Human Corneal Epithelial Cells and Corneal Wound Healing in Normoglycemic and Diabetic Male Mice" Cells 14, no. 23: 1880. https://doi.org/10.3390/cells14231880
APA StyleMelnyk, S., Lu, X., Ronderos, V., Choudhary, V., Johnson, M. H., Watsky, M. A., & Bollag, W. B. (2025). Histone Deacetylase Inhibition Enhances AQP3 Levels in Human Corneal Epithelial Cells and Corneal Wound Healing in Normoglycemic and Diabetic Male Mice. Cells, 14(23), 1880. https://doi.org/10.3390/cells14231880





