A Macrophage-Derived Factor on Human iPSC-Derived Cardiomyocyte Function: The Role of Osteopontin
Highlights
- Our findings reveal that distinct macrophage subtypes differentially influence the function of hiPSC-CM.
- M2 macrophages secrete significantly high levels of OPN, which can modulate hiPSC-CM function.
- Macrophage-derived factors contribute to the communication between macrophages and cardiomyocytes, resulting in functional changes.
- OPN presents a candidate target for therapeutic intervention in cardiac disease.
Abstract
1. Introduction
2. Materials and Methods
2.1. Human-Induced Pluripotent Stem Cell (hiPSC) Culture
2.2. Differentiation of HiPSC into Cardiomyocytes
2.3. HiPSC-CM Dissociation and Enrichment
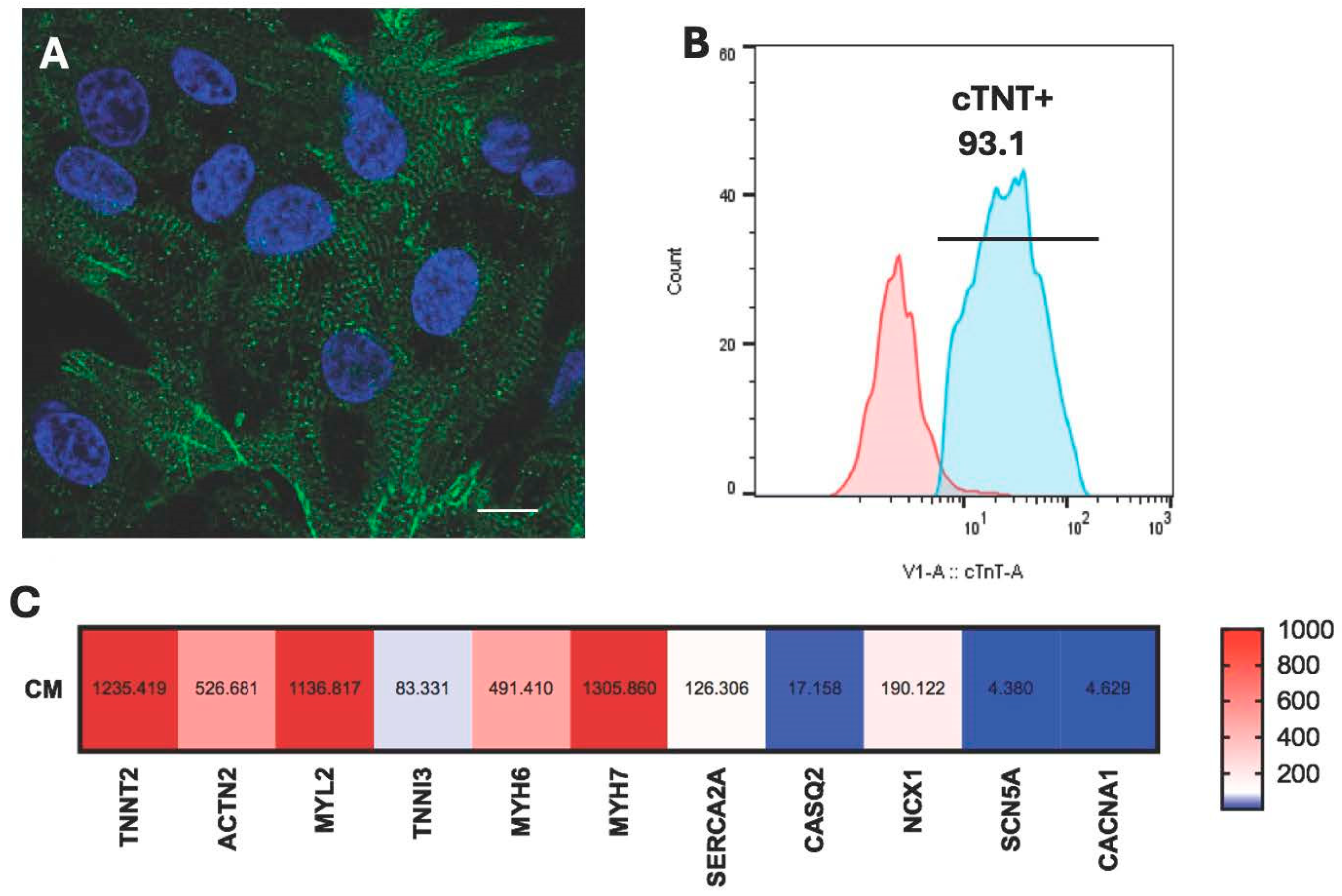
2.4. Characterization of HiPSC-CM
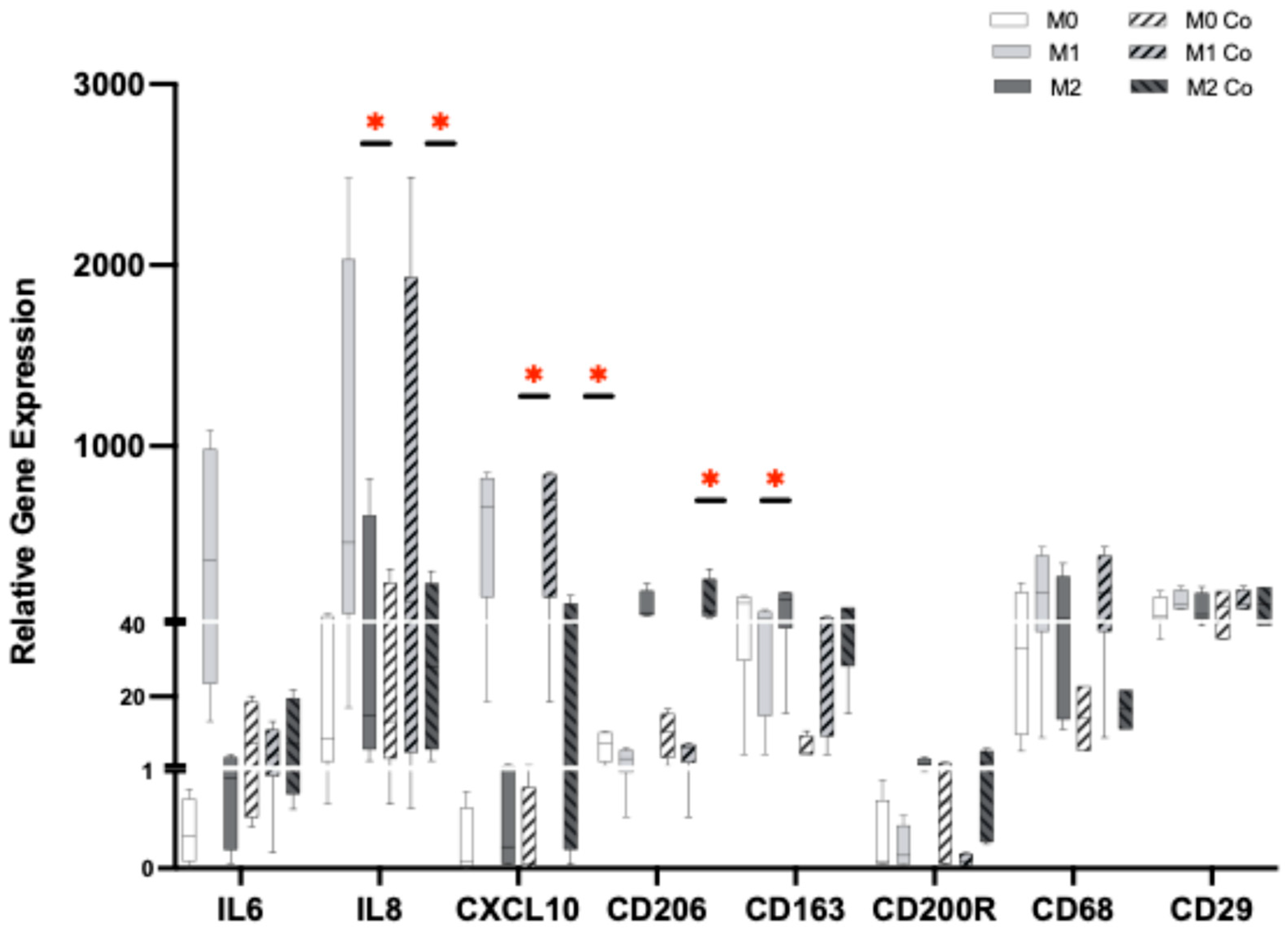
2.5. HiPSC-Macrophage Culture and Differentiation
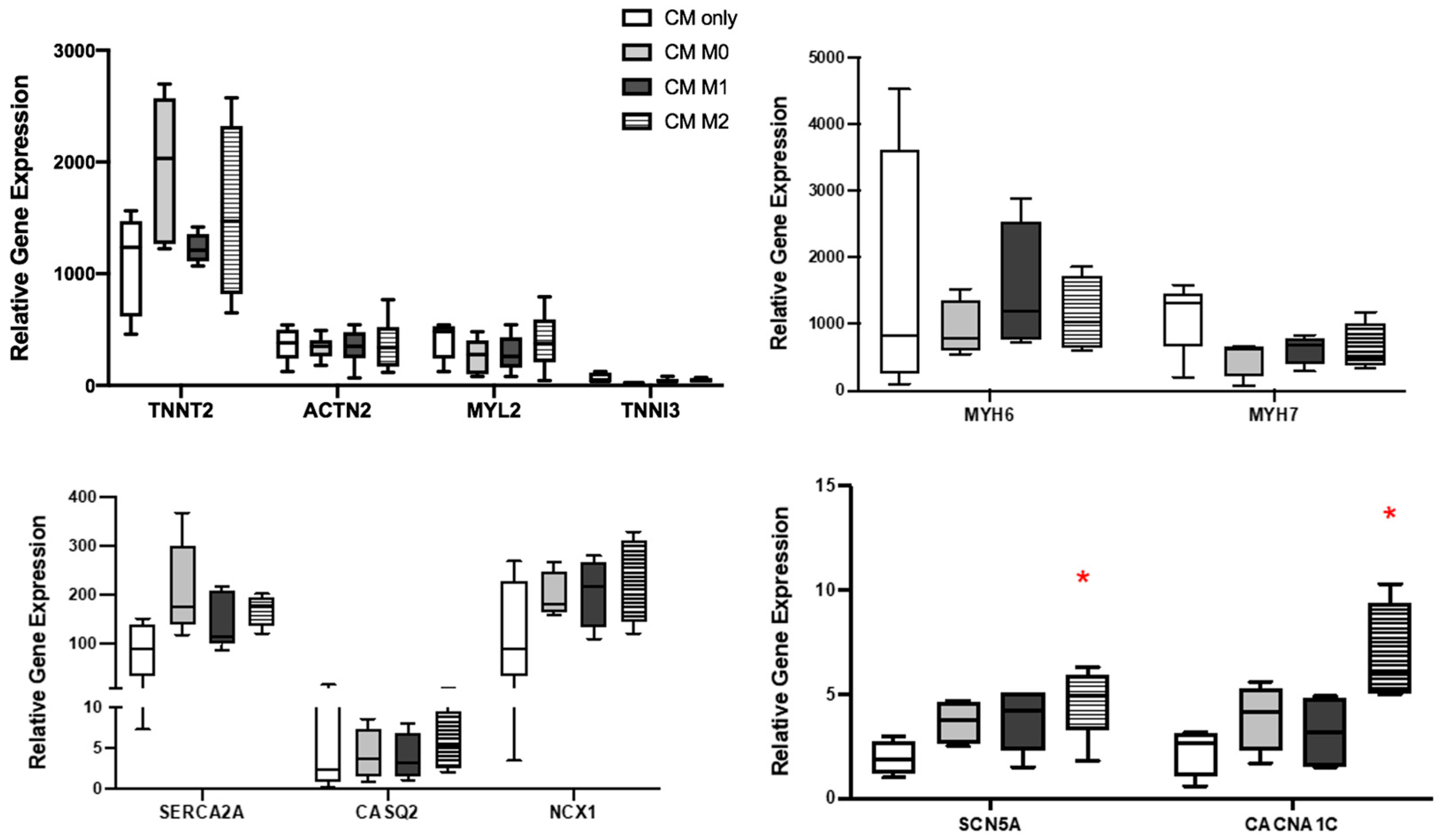
2.6. HiPSC-CM and HiPSC-Macrophages Co-Culture
2.7. RT-PCR
2.8. Triple Transient Measurement (TTM)
2.9. OPN Experiments
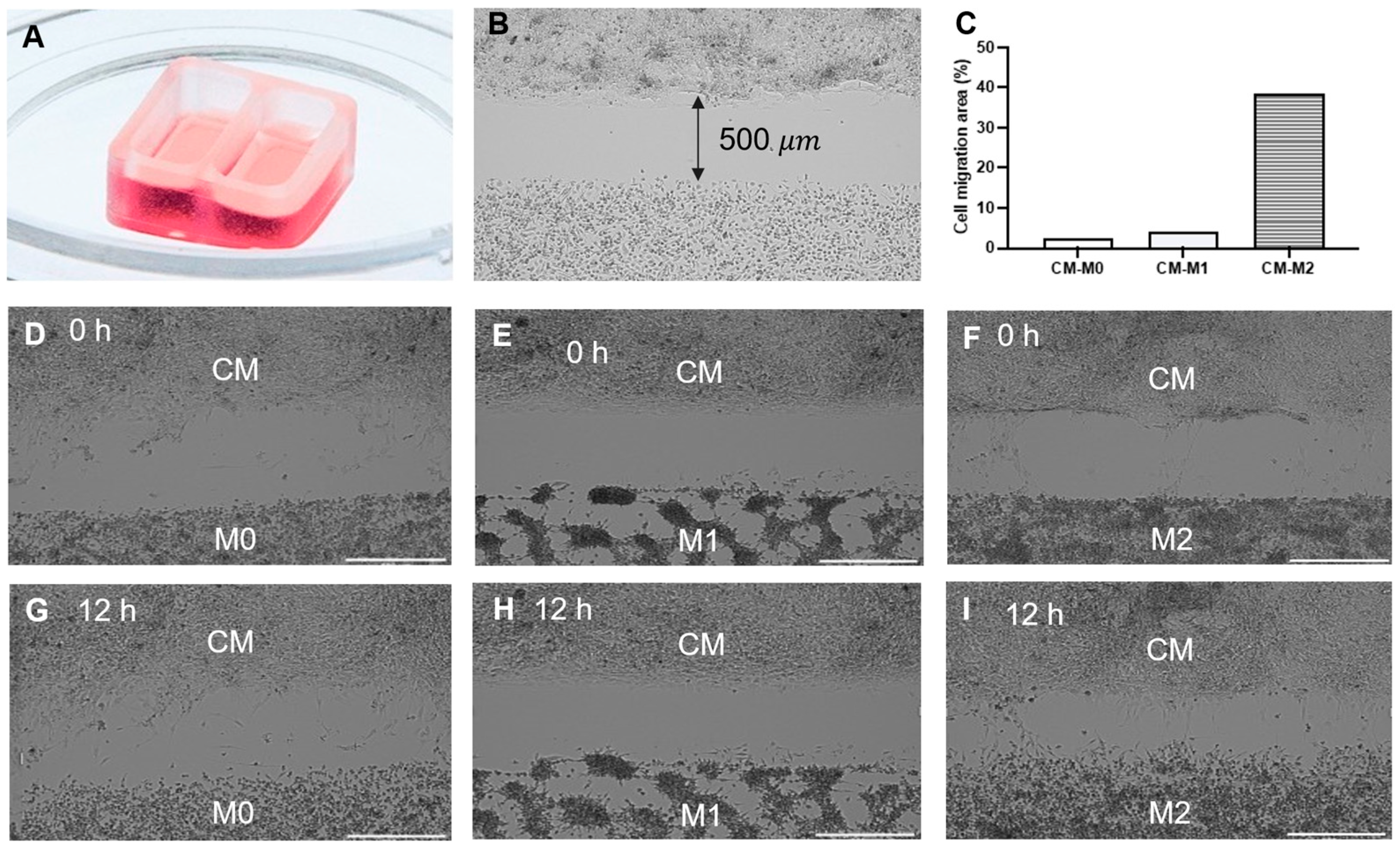
2.10. Cell Migration
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, S.L.; Xu, J.; Kochanek, K.D.; Arias, E. Mortality in the United States. NCHS Data Brief 2017, 2018, 1–8. [Google Scholar]
- Li, T.; Yan, Z.; Fan, Y.; Fan, X.; Li, A.; Qi, Z.; Zhang, J. Cardiac repair after myocardial infarction: A two-sided role of inflammation-mediated. Front. Cardiovasc. Med. 2023, 9, 1077290. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Dobaczewski, M.; Gonzalez-Quesada, C.; Frangogiannis, N.G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J. Mol. Cell. Cardiol. 2010, 48, 504–511. [Google Scholar] [CrossRef]
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-myocardial infarction heart failure. JACC Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef]
- Kim, Y.; Nurakhayev, S.; Nurkesh, A.; Zharkinbekov, Z.; Saparov, A. Macrophage polarization in cardiac tissue repair following myocardial infarction. Int. J. Mol. Sci. 2021, 22, 2715. [Google Scholar] [CrossRef]
- Sager, H.B.; Kessler, T.; Schunkert, H. Monocytes and macrophages in cardiac injury and repair. J. Thorac. Dis. 2016, 9, S30–S35. [Google Scholar] [CrossRef]
- Alvarez-Argote, S.; O’Meara, C.C. The evolving roles of cardiac macrophages in homeostasis, regeneration, and repair. Int. J. Mol. Sci. 2021, 22, 7923. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.-H.; Xu, J.-D.; Sun, S.-N.; Li, Y.; Zhou, Z.; Li, H.; Liu, X.; Deng, J.-P.; Huang, Y.-S.; Chen, Z.-X. Single-cell transcriptomic analyses of cardiac immune cells reveal that rel-driven cd72-positive macrophages induce cardiomyocyte injury. Cardiovasc. Res. 2022, 118, 1303–1320. [Google Scholar] [CrossRef]
- Kubota, A.; Frangogiannis, N.G. Macrophages in myocardial infarction. Am. J. Physiol. Cell Physiol. 2022, 323, C1304–C1324. [Google Scholar] [CrossRef]
- Denes, A.; Hansen, C.E.; Oezorhan, U.; Figuerola, S.; de Vries, H.E.; Sorokin, L.; Planas, A.M.; Engelhardt, B.; Schwaninger, M. Endothelial cells and macrophages as allies in the healthy and diseased brain. Acta Neuropathol. 2024, 147, 38. [Google Scholar] [CrossRef]
- Shimodaira, T.; Matsuda, K.; Uchibori, T.; Sugano, M.; Uehara, T.; Honda, T. Upregulation of osteopontin expression via the interaction of macrophages and fibroblasts under il-1b stimulation. Cytokine 2018, 110, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Hitscherich, P.G.; Xie, L.-H.; Del Re, D.; Lee, E.J. The effects of macrophages on cardiomyocyte calcium-handling function using in vitro culture models. Physiol. Rep. 2019, 7, e14137. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Berse, B.; Van De Water, L.; Papadopoulos-Sergiou, A.; Perruzzi, C.; Manseau, E.; Dvorak, H.; Senger, D. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell 1992, 3, 1169–1180. [Google Scholar] [CrossRef]
- Kato, A.; Okura, T.; Hamada, C.; Miyoshi, S.; Katayama, H.; Higaki, J.; Ito, R. Cell stress induces upregulation of osteopontin via the erk pathway in type ii alveolar epithelial cells. PLoS ONE 2014, 9, e100106. [Google Scholar] [CrossRef] [PubMed]
- Mazzali, M.; Kipari, T.; Ophascharoensuk, V.; Wesson, J.; Johnson, R.; Hughes, J. Osteopontin—A molecule for all seasons. Qjm 2002, 95, 3–13. [Google Scholar] [CrossRef]
- O’Brien, E.R.; Garvin, M.R.; Stewart, D.K.; Hinohara, T.; Simpson, J.B.; Schwartz, S.M.; Giachelli, C.M. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 1648–1656. [Google Scholar] [CrossRef]
- Nystrom, T.; Duner, P.; Hultgardh-Nilsson, A. A constitutive endogenous osteopontin production is important for macrophage function and differentiation. Exp. Cell Res. 2007, 313, 1149–1160. [Google Scholar] [CrossRef]
- Murry, C.E.; Giachelli, C.M.; Schwartz, S.M.; Vracko, R. Macrophages express osteopontin during repair of myocardial necrosis. Am. J. Pathol. 1994, 145, 1450–1462. [Google Scholar]
- Yousefi, K.; Irion, C.I.; Takeuchi, L.M.; Ding, W.; Lambert, G.; Eisenberg, T.; Sukkar, S.; Granzier, H.L.; Methawasin, M.; Lee, D.I. Osteopontin promotes left ventricular diastolic dysfunction through a mitochondrial pathway. J. Am. Coll. Cardiol. 2019, 73, 2705–2718. [Google Scholar] [CrossRef]
- Gordin, D.; Forsblom, C.; Panduru, N.M.; Thomas, M.C.; Bjerre, M.; Soro-Paavonen, A.; Tolonen, N.; Sandholm, N.; Flyvbjerg, A.; Harjutsalo, V. Osteopontin is a strong predictor of incipient diabetic nephropathy, cardiovascular disease, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2014, 37, 2593–2600. [Google Scholar] [CrossRef]
- Abdelaziz Mohamed, I.; Gadeau, A.-P.; Hasan, A.; Abdulrahman, N.; Mraiche, F. Osteopontin: A promising therapeutic target in cardiac fibrosis. Cells 2019, 8, 1558. [Google Scholar] [CrossRef]
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; van Helden, R.W.J.; Krotenberg Garcia, A.; Mircea, M.; Kostidis, S.; Davis, R.P.; et al. Human-ipsc-derived cardiac stromal cells enhance maturation in 3d cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 2020, 26, 862–879.e811. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, E.; Bellin, M.; Sala, L.; van Meer, B.J.; Tertoolen, L.G.; Orlova, V.V.; Mummery, C.L. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 2017, 144, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; van den Hil, F.E.; Mummery, C.L.; Orlova, V.V. Generation and functional characterization of monocytes and macrophages derived from human induced pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 2020, 52, e108. [Google Scholar] [CrossRef] [PubMed]
- van Meer, B.J.; Krotenberg, A.; Sala, L.; Davis, R.P.; Eschenhagen, T.; Denning, C.; Tertoolen, L.G.J.; Mummery, C.L. Simultaneous measurement of excitation-contraction coupling parameters identifies mechanisms underlying contractile responses of hipsc-derived cardiomyocytes. Nat. Commun. 2019, 10, 4325. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Z.; Huang, W.; Zhang, J.; Zou, L.; Wang, H. Communications between macrophages and cardiomyocytes. Cell Commun. Signal. 2023, 21, 206. [Google Scholar] [CrossRef]
- Jian, Y.; Zhou, X.; Shan, W.; Chen, C.; Ge, W.; Cui, J.; Yi, W.; Sun, Y. Crosstalk between macrophages and cardiac cells after myocardial infarction. Cell Commun. Signal. 2023, 21, 109. [Google Scholar] [CrossRef]
- Grandi, E.; Navedo, M.F.; Saucerman, J.J.; Bers, D.M.; Chiamvimonvat, N.; Dixon, R.E.; Dobrev, D.; Gomez, A.M.; Harraz, O.F.; Hegyi, B. Diversity of cells and signals in the cardiovascular system. J. Physiol. 2023, 601, 2547–2592. [Google Scholar] [CrossRef]
- Almeida Paiva, R.; Martins-Marques, T.; Jesus, K.; Ribeiro-Rodrigues, T.; Zuzarte, M.; Silva, A.; Reis, L.; da Silva, M.; Pereira, P.; Vader, P. Ischaemia alters the effects of cardiomyocyte-derived extracellular vesicles on macrophage activation. J. Cell. Mol. Med. 2019, 23, 1137–1151. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, D.; Xie, X.; Wang, S.; Wang, Z.; Zhao, W.; Xu, H.; Zheng, L. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res. Cardiol. 2016, 111, 63. [Google Scholar] [CrossRef]
- Sugita, J.; Fujiu, K.; Nakayama, Y.; Matsubara, T.; Matsuda, J.; Oshima, T.; Liu, Y.; Maru, Y.; Hasumi, E.; Kojima, T. Cardiac macrophages prevent sudden death during heart stress. Nat. Commun. 2021, 12, 1910. [Google Scholar] [CrossRef]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G. Macrophages facilitate electrical conduction in the heart. Cell 2017, 169, 510–522.e520. [Google Scholar] [CrossRef]
- Whitehead, A.J.; Engler, A.J. Regenerative cross talk between cardiac cells and macrophages. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H2211–H2221. [Google Scholar] [CrossRef]
- Lambert, J.M.; Lopez, E.F.; Lindsey, M.L. Macrophage roles following myocardial infarction. Int. J. Cardiol. 2008, 130, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Wang, S.; Yu, L.; Tang, J.; Zhou, S. The role of cardiac macrophage and cytokines on ventricular arrhythmias. Front. Physiol. 2020, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Stuart, S.D.F.; De Jesus, N.M.; Lindsey, M.L.; Ripplinger, C.M. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J. Mol. Cell. Cardiol. 2016, 91, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, G.; Alarcón, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepúlveda, M. Macrophage-dependent il-1β production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.-D.; Wang, Q.; Hou, J.-W.; Li, W.; Cai, X.-X.; Yang, Y.-L.; Zhang, L.-H.; Wei, Z.-X.; Chen, T.-Z.; Wang, Y.-P. Macrophages facilitate post myocardial infarction arrhythmias: Roles of gap junction and kca3. 1. Theranostics 2019, 9, 6396. [Google Scholar] [CrossRef] [PubMed]
- Zaklyazminskaya, E.; Dzemeshkevich, S. The role of mutations in the scn5a gene in cardiomyopathies. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Segura, E.; Marsolais, M.; Parent, L. A cacna1c variant associated with cardiac arrhythmias provides mechanistic insights in the calmodulation of L-type Ca2+ channels. J. Biol. Chem. 2022, 298, 102632. [Google Scholar] [CrossRef]
- Bhat, S.; Dao, D.T.; Terrillion, C.E.; Arad, M.; Smith, R.J.; Soldatov, N.M.; Gould, T.D. Cacna1c (cav1. 2) in the pathophysiology of psychiatric disease. Prog. Neurobiol. 2012, 99, 1–14. [Google Scholar] [CrossRef]
- Tanabe, N.; Wheal, B.D.; Kwon, J.; Chen, H.H.; Shugg, R.P.; Sims, S.M.; Goldberg, H.A.; Dixon, S.J. Osteopontin signals through calcium and nuclear factor of activated t cells (nfat) in osteoclasts: A novel rgd-dependent pathway promoting cell survival. J. Biol. Chem. 2011, 286, 39871–39881. [Google Scholar] [CrossRef]
- Li, L.; Shi, W.; Liu, M.; Bai, X.; Sun, Y.; Zhu, X.; Su, H.; Ji, Y.; Zhu, F.; Liu, X. Single-cell secretion analysis in the engineered tumor microenvironment reveals differential modulation of macrophage immune responses. Anal. Chem. 2021, 93, 4198–4207. [Google Scholar] [CrossRef]
- Lenga, Y.; Koh, A.; Perera, A.S.; McCulloch, C.A.; Sodek, J.; Zohar, R. Osteopontin expression is required for myofibroblast differentiation. Circ. Res. 2008, 102, 319–327. [Google Scholar] [CrossRef]
- Waller, A.H.; Sanchez-Ross, M.; Kaluski, E.; Klapholz, M. Osteopontin in cardiovascular disease: A potential therapeutic target. Cardiol. Rev. 2010, 18, 125–131. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Osteopontin in cardiovascular diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef]
- Moorman, H.R.; Poschel, D.; Klement, J.D.; Lu, C.; Redd, P.S.; Liu, K. Osteopontin: A key regulator of tumor progression and immunomodulation. Cancers 2020, 12, 3379. [Google Scholar] [CrossRef]
- Tan, Y.; Zhao, L.; Yang, Y.-G.; Liu, W. The role of osteopontin in tumor progression through tumor-associated macrophages. Front. Oncol. 2022, 12, 953283. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in bone metabolism and bone diseases. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919159-51. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Fibroblast—Extracellular matrix interactions in tissue fibrosis. Curr. Pathobiol. Rep. 2016, 4, 11–18. [Google Scholar] [CrossRef]
- Trueblood, N.A.; Xie, Z.; Communal, C.; Sam, F.; Ngoy, S.; Liaw, L.; Jenkins, A.W.; Wang, J.; Sawyer, D.B.; Bing, O.H.; et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ. Res. 2001, 88, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Balligand, J.L.; Fischer, T.A.; Smith, T.W.; Kelly, R.A. Glucocorticoids increase osteopontin expression in cardiac myocytes and microvascular endothelial cells. Role in regulation of inducible nitric oxide synthase. J. Biol. Chem. 1995, 270, 28471–28478. [Google Scholar] [CrossRef]
- Cho, H.-J.; Cho, H.-J.; Kim, H.-S. Osteopontin: A multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr. Atheroscler. Rep. 2009, 11, 206–213. [Google Scholar] [CrossRef]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Sarkar-Dutta, M.; McRae, S.; Ramachandran, A.; Kumar, B.; Waris, G. Osteopontin regulates hepatitis c virus (hcv) replication and assembly by interacting with hcv proteins and lipid droplets and by binding to receptors αvβ3 and cd44. J. Virol. 2018, 92, e02116–e02117. [Google Scholar] [CrossRef]
- Driver, J.; Weber, C.E.; Callaci, J.J.; Kothari, A.N.; Zapf, M.A.; Roper, P.M.; Borys, D.; Franzen, C.A.; Gupta, G.N.; Wai, P.Y.; et al. Alcohol inhibits osteopontin-dependent transforming growth factor-β1 expression in human mesenchymal stem cells. J. Biol. Chem. 2015, 290, 9959–9973. [Google Scholar] [CrossRef]
- Liu, G.-X.; Sun, J.-T.; Yang, M.-X.; Qi, X.-M.; Shao, Q.-Q.; Xie, Q.; Qu, X.; Wei, F.-C.; Sun, S.-Z. Opn promotes survival of activated t cells by up-regulating cd44 in patients with oral lichen planus. Clin. Immunol. 2011, 138, 291–298. [Google Scholar] [CrossRef]
- Takahashi, K.; Takahashi, F.; Hirama, M.; Tanabe, K.K.; Fukuchi, Y. Restoration of cd44s in non-small cell lung cancer cells enhanced their susceptibility to the macrophage cytotoxicity. Lung Cancer 2003, 41, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Sottnik, J.L.; Theodorescu, D. Cd44: A metastasis driver and therapeutic target. Oncoscience 2016, 3, 320. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Rigamonti, F.; Burger, F.; Roth, A.; Bertolotto, M.; Spinella, G.; Pane, B.; Palombo, D.; Pende, A.; Bonaventura, A. Serum levels of osteopontin predict major adverse cardiovascular events in patients with severe carotid artery stenosis. Int. J. Cardiol. 2018, 255, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in vascular disease: Friend or foe? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yoshida, N.; Yamamoto, T.; Isobe, S.; Moriyama, H.; Goto, S.; Kitakata, H. Il (interleukin)-10–stat3–galectin-3 axis is essential for osteopontin-producing reparative macrophage polarization after myocardial infarction. Circulation 2018, 138, 2021–2035. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, L.; Lee, E.J. A Macrophage-Derived Factor on Human iPSC-Derived Cardiomyocyte Function: The Role of Osteopontin. Cells 2025, 14, 1881. https://doi.org/10.3390/cells14231881
Hao L, Lee EJ. A Macrophage-Derived Factor on Human iPSC-Derived Cardiomyocyte Function: The Role of Osteopontin. Cells. 2025; 14(23):1881. https://doi.org/10.3390/cells14231881
Chicago/Turabian StyleHao, Lei, and Eun Jung Lee. 2025. "A Macrophage-Derived Factor on Human iPSC-Derived Cardiomyocyte Function: The Role of Osteopontin" Cells 14, no. 23: 1881. https://doi.org/10.3390/cells14231881
APA StyleHao, L., & Lee, E. J. (2025). A Macrophage-Derived Factor on Human iPSC-Derived Cardiomyocyte Function: The Role of Osteopontin. Cells, 14(23), 1881. https://doi.org/10.3390/cells14231881





