Targeting Regulated Cell Death Pathways in COPD: Mechanisms and Therapeutic Strategies
Abstract
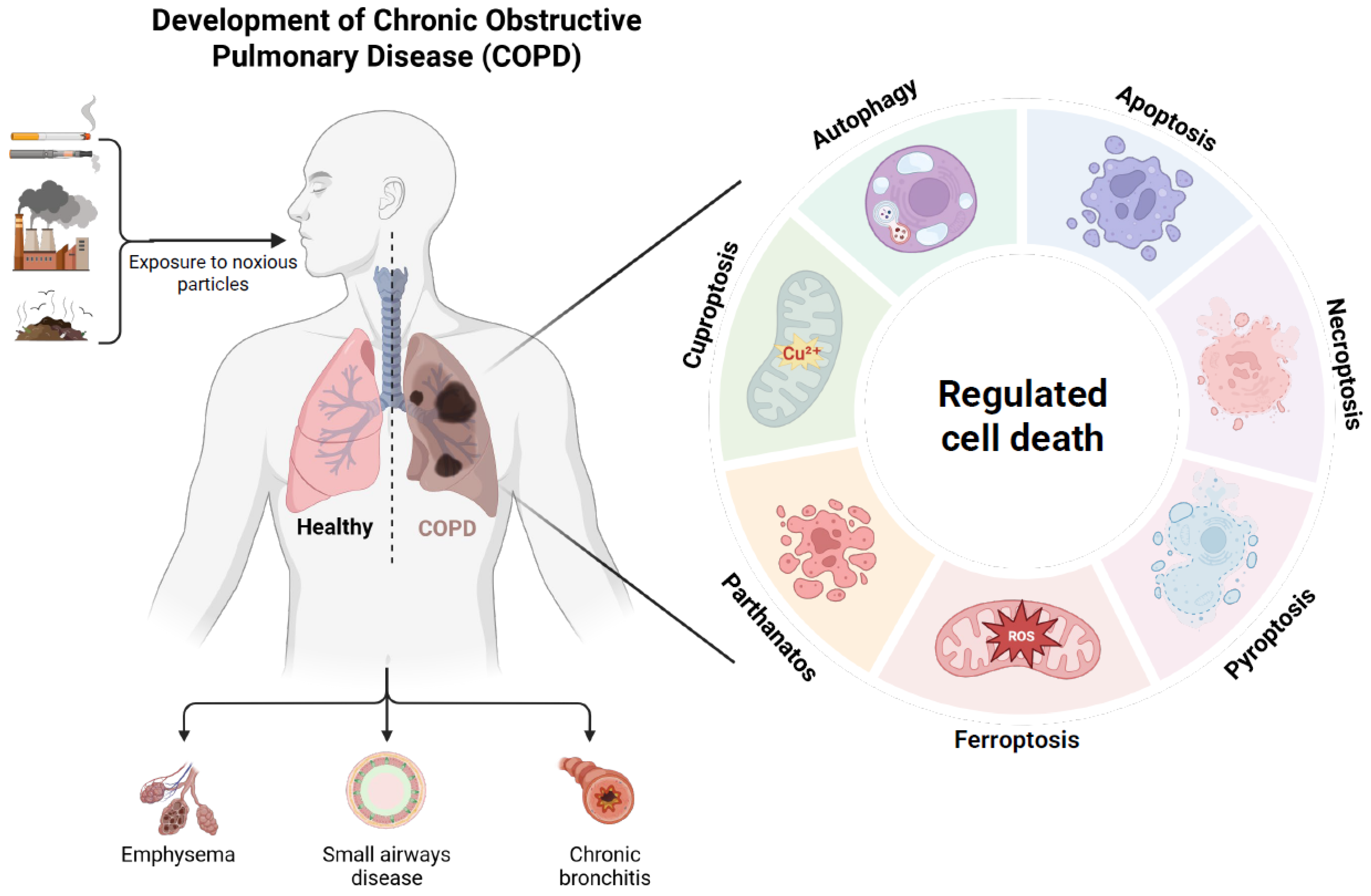
1. Introduction
2. Apoptosis
2.1. Overview of Apoptosis
2.2. Apoptosis in COPD
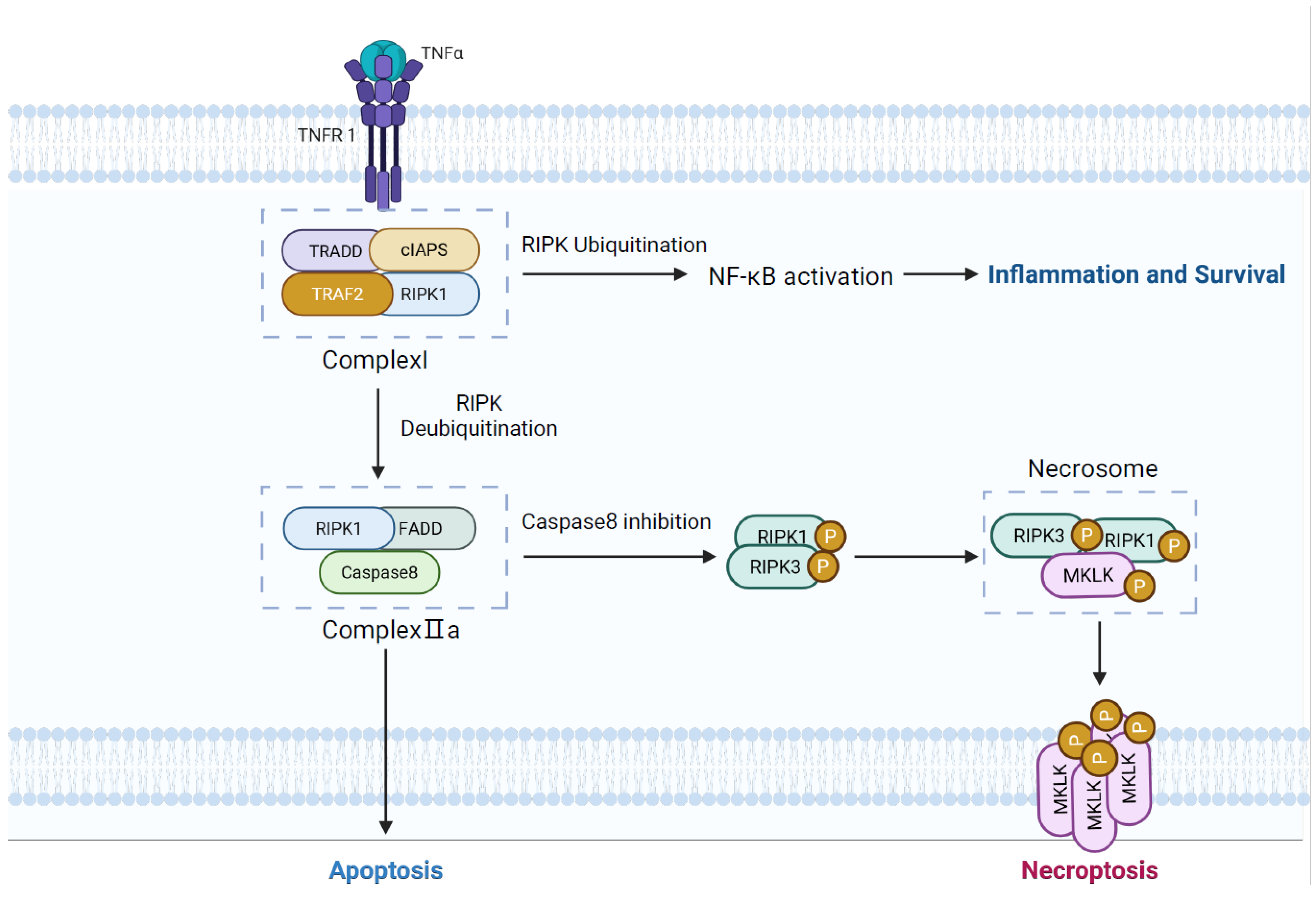
3. Necroptosis
3.1. Overview of Necroptosis
3.2. Necroptosis in COPD
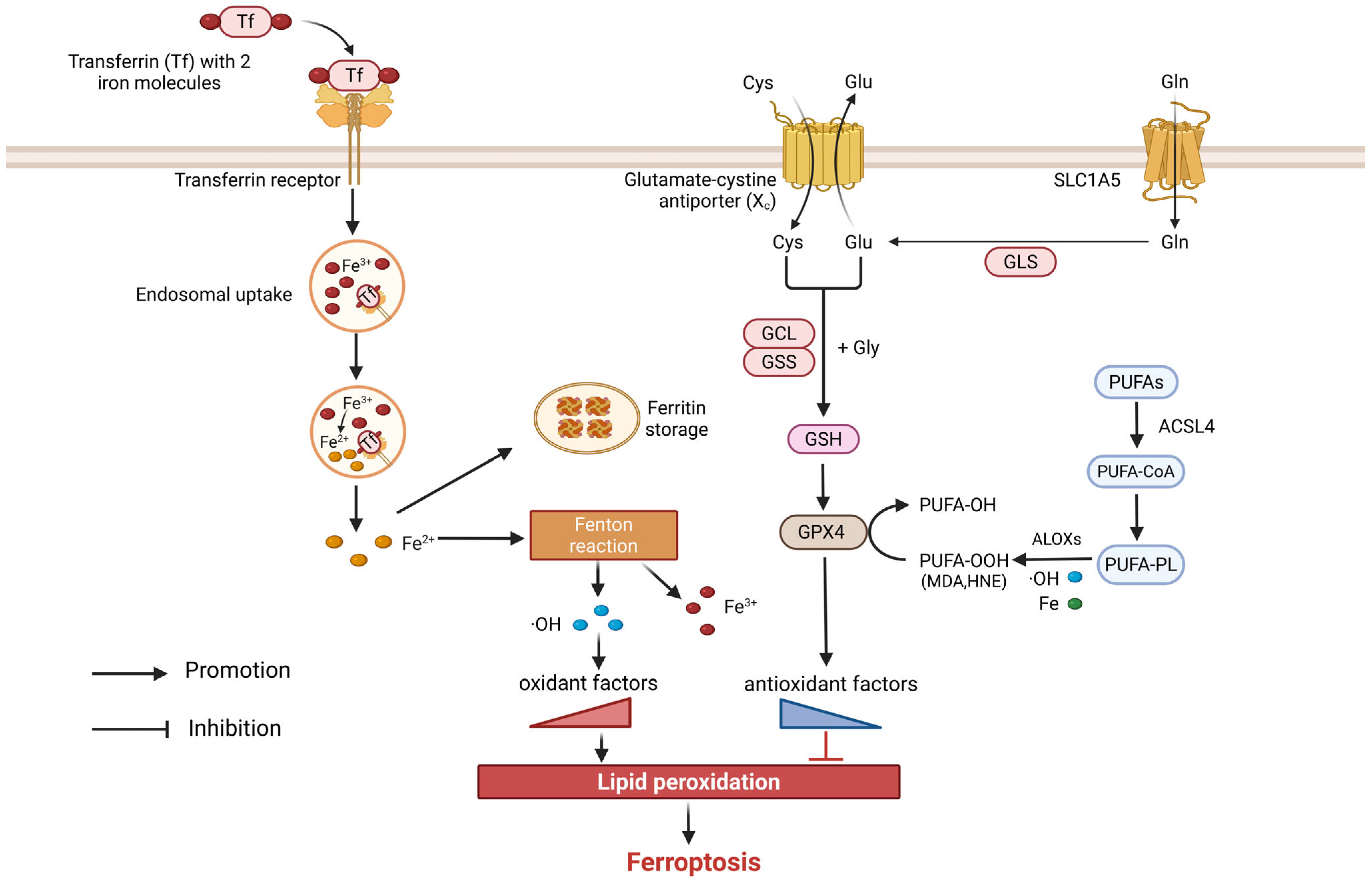
4. Ferroptosis
4.1. Overview of Ferroptosis
4.2. Ferroptosis in COPD
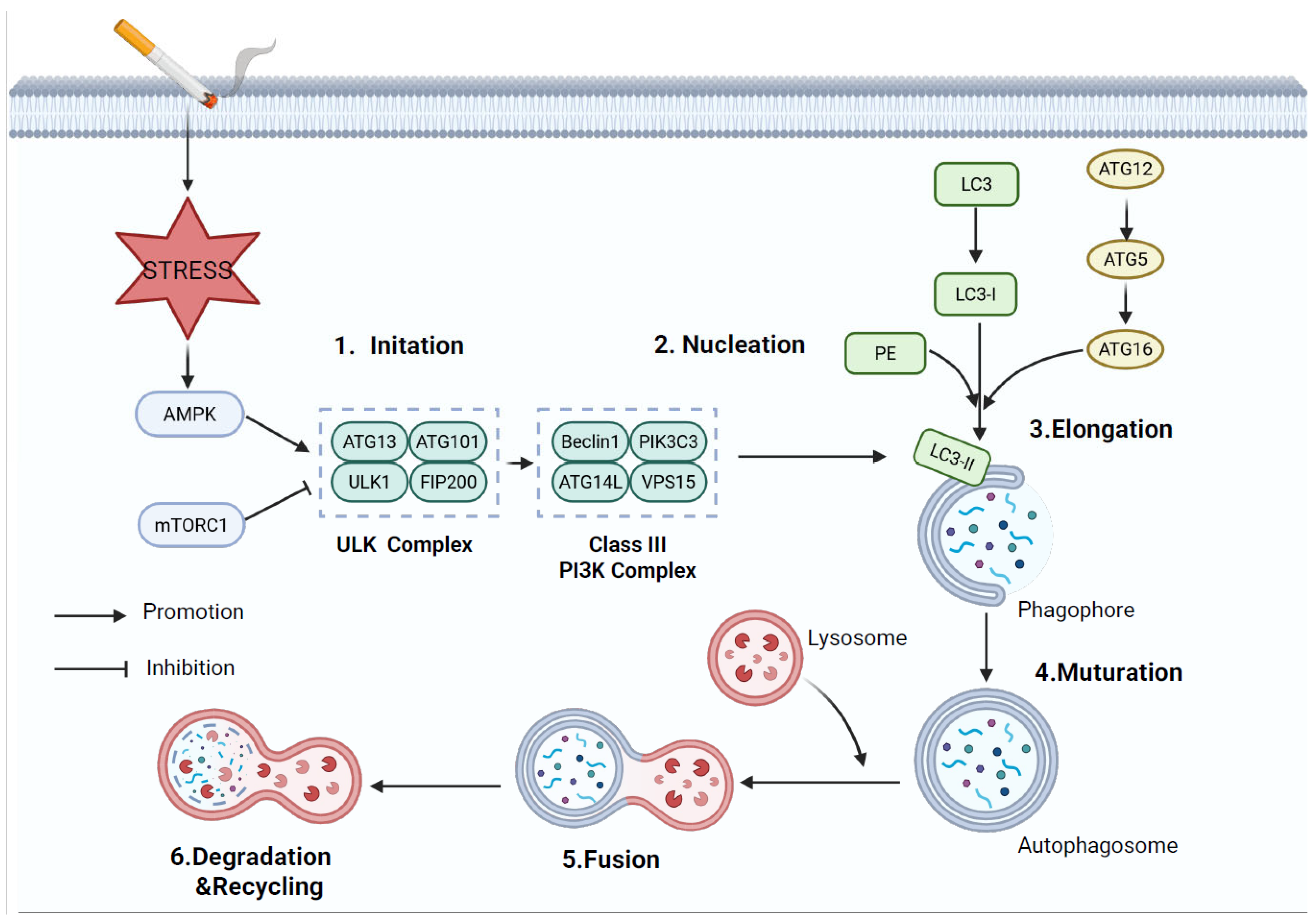
5. Autophagy
5.1. Overview of Autophagy
5.2. Autophagy in COPD
6. Pyroptosis
6.1. Overview of Pyroptosis
6.2. Pyroptosis in COPD
7. Other Forms of RCD
7.1. Cuproptosis
7.2. Parthanatos
8. Therapeutic Potential
8.1. Targeting Apoptosis
8.2. Targeting Necroptosis
8.3. Targeting Ferroptosis
8.4. Targeting Autophagy
8.5. Targeting Pyroptosis
9. The Future of Translational Research in RCD for COPD
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- de Oca, M.M.; Perez-Padilla, R.; Celli, B.; Aaron, S.D.; Wehrmeister, F.C.; Amaral, A.F.S.; Mannino, D.; Zheng, J.; Salvi, S.; Obaseki, D.; et al. The global burden of COPD: Epidemiology and effect of prevention strategies. Lancet Respir. Med. 2025, 13, 709–724. [Google Scholar] [CrossRef]
- Agustí, A.; Melén, E.; DeMeo, D.L.; Breyer-Kohansal, R.; Faner, R. Pathogenesis of chronic obstructive pulmonary disease: Understanding the contributions of gene-environment interactions across the lifespan. Lancet Respir. Med. 2022, 10, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Stolz, D.; Mkorombindo, T.; Schumann, D.M.; Agusti, A.; Ash, S.Y.; Bafadhel, M.; Bai, C.; Chalmers, J.D.; Criner, G.J.; Dharmage, S.C.; et al. Towards the elimination of chronic obstructive pulmonary disease: A Lancet Commission. Lancet 2022, 400, 921–972. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef]
- Rao, W.; Wang, S.; Duleba, M.; Niroula, S.; Goller, K.; Xie, J.; Mahalingam, R.; Neupane, R.; Liew, A.-A.; Vincent, M.; et al. Regenerative Metaplastic Clones in COPD Lung Drive Inflammation and Fibrosis. Cell 2020, 181, 848–864.E18. [Google Scholar] [CrossRef]
- Sauler, M.; Bazan, I.S.; Lee, P.J. Cell Death in the Lung: The Apoptosis-Necroptosis Axis. Annu. Rev. Physiol. 2019, 81, 375–402. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, Y.; Liu, S.; Li, C.; Dong, J.; Kong, X.; Ji, X.; Cheng, X.; Zhang, L. RIPK3 signaling and its role in regulated cell death and diseases. Cell Death Discov. 2024, 10, 200. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. The evolution of regulated cell death pathways in animals and their evasion by pathogens. Physiol. Rev. 2022, 102, 411–454. [Google Scholar] [CrossRef]
- Hadian, K.; Stockwell, B.R. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat. Rev. Drug Discov. 2023, 22, 723–742. [Google Scholar] [CrossRef]
- Minagawa, S.; Yoshida, M.; Araya, J.; Hara, H.; Imai, H.; Kuwano, K. Regulated Necrosis in Pulmonary Disease. A Focus on Necroptosis and Ferroptosis. Am. J. Respir. Cell Mol. Biol. 2020, 62, 554–562. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.; Yangzhong, X.; Zhang, X.; Zu, A.; Hou, Y.; Li, L.; Sun, S. Pyroptosis in inflammation-related respiratory disease. J. Physiol. Biochem. 2022, 78, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, J.; Zheng, J.; Zhou, Y.; Jia, D.; Wang, J. Recent Progress of Ferroptosis in Lung Diseases. Front. Cell Dev. Biol. 2021, 9, 789517. [Google Scholar] [CrossRef] [PubMed]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liu, Z.; Cai, S.; Fu, H.; Gan, Y.; Li, X.; Wang, X.; Liu, C.; Ma, W.; Chen, J.; et al. Copper homeostasis and cuproptosis in myocardial infarction: Molecular mechanisms, treatment strategies and potential therapeutic targets. Front. Pharmacol. 2025, 16, 1525585. [Google Scholar] [CrossRef]
- Liu, C.; Lai, F.; Zhang, T.; Mao, K.; Wan, H.; He, Y. Roles and therapeutic potential of PARP-1 in neurodegenerative diseases. Biochem. Pharmacol. 2025, 242, 117373. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, S.; Baima, Q.; Zhen, Y.; Hang, X.; Meng, X. Dual role mechanisms of regulated cell death in apical periodontitis: From pathogenic destruction to therapeutic potential. Cell Death Discov. 2025, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.-A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Lien, C.-I.; Tseng, Y.-C.; Tu, Y.-F.; Kulczyk, A.W.; Lu, Y.-C.; Wang, Y.-T.; Su, T.-W.; Hsu, L.-C.; Lo, Y.-C.; et al. Deciphering DED assembly mechanisms in FADD-procaspase-8-cFLIP complexes regulating apoptosis. Nat. Commun. 2024, 15, 3791. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.-D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Holmes, M.; Reynolds, P.N. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur. Respir. J. 2005, 25, 447–454. [Google Scholar] [CrossRef]
- Chen, L.; Luo, L.; Kang, N.; He, X.; Li, T.; Chen, Y. The Protective Effect of HBO1 on Cigarette Smoke Extract-Induced Apoptosis in Airway Epithelial Cells. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 15–24. [Google Scholar] [CrossRef]
- Song, Q.; Chen, P.; Liu, X.-M. The role of cigarette smoke-induced pulmonary vascular endothelial cell apoptosis in COPD. Respir. Res. 2021, 22, 39. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Jeong, Y.; Kim, S.; Yeom, J.E.; Lee, J.; Lee, W.; Bae, G.; Kang, J. PRMT1 Ablation in Endothelial Cells Causes Endothelial Dysfunction and Aggravates COPD Attributable to Dysregulated NF-κB Signaling. Adv. Sci. 2025, 12, 2411514. [Google Scholar] [CrossRef]
- Kasahara, Y.; Tuder, R.M.; Cool, C.D.; Lynch, D.A.; Flores, S.C.; Voelkel, N.F. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir. Crit. Care Med. 2001, 163, 737–744. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.Y.; Chen, R.; Zhou, M.; Guo, Y. Hedgehog signaling modulates cigarette-induced COPD development. Exp. Ther. Med. 2021, 22, 729. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zong, D.; Chen, Y.; Chen, P. Anti-apoptotic effect of the Shh signaling pathway in cigarette smoke extract induced MLE 12 apoptosis. Tob. Induc. Dis. 2019, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, K.; Zheng, Y.; Zhao, J.; Xie, T.; Ding, Y. Upregulation of ARHGAP18 by miR-613 Inhibits Cigarette Smoke Extract-Induced Apoptosis and Epithelial-Mesenchymal Transition in Bronchial Epithelial Cells. Int. J. Chron. Obstruct. Pulmon. Dis. 2025, 20, 2525–2537. [Google Scholar] [CrossRef]
- Li, T.; He, X.; Luo, L.; Zeng, H.; Ren, S.; Chen, Y. F-Box Protein FBXW17-Mediated Proteasomal Degradation of Protein Methyltransferase PRMT6 Exaggerates CSE-Induced Lung Epithelial Inflammation and Apoptosis. Front. Cell Dev. Biol. 2021, 9, 599020. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, L.; Liu, Y.; Chen, H.; Hu, Q.; Xie, C.; Meng, X.; Shen, X. Scutellarein alleviates chronic obstructive pulmonary disease through inhibition of ferroptosis by chelating iron and interacting with arachidonate 15-lipoxygenase. Phytother. Res. 2023, 37, 4587–4606. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, Q.; Li, S.; Su, Q.; Fan, H. Inflammation mechanism and research progress of COPD. Front. Immunol. 2024, 15, 1404615. [Google Scholar] [CrossRef]
- Byrne, A.J.; Mathie, S.A.; Gregory, L.G.; Lloyd, C.M. Pulmonary macrophages: Key players in the innate defence of the airways. Thorax 2015, 70, 1189–1196. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, T.; Zhu, Z.; Homer, R.J.; Riese, R.J.; Chapman, H.A.; Shapiro, S.D.; Elias, J.A. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J. Exp. Med. 2000, 192, 1587–1600. [Google Scholar] [CrossRef]
- Kheradmand, F.; Zhang, Y.; Corry, D.B. Contribution of adaptive immunity to human COPD and experimental models of emphysema. Physiol. Rev. 2023, 103, 1059–1093. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, A.; Yu, G.; Wang, H. Endoplasmic Reticulum Stress in Chronic Obstructive Pulmonary Disease: Mechanisms and Future Perspectives. Biomolecules 2022, 12, 1637. [Google Scholar] [CrossRef]
- Feng, H.; Li, M.; Altawil, A.; Yin, Y.; Zheng, R.; Kang, J. Cigarette smoke extracts induce apoptosis in Raw264.7 cells via endoplasmic reticulum stress and the intracellular Ca2+/P38/STAT1 pathway. Toxicol. Vitr. 2021, 77, 105249. [Google Scholar] [CrossRef]
- Chen, R.; Michaeloudes, C.; Liang, Y.; Bhavsar, P.K.; Chung, K.F.; Ip, M.S.M.; Mak, J.C.W. ORMDL3 regulates cigarette smoke–induced endoplasmic reticulum stress in airway smooth muscle cells. J. Allergy Clin. Immunol. 2022, 149, 1445–1457.e5. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, F.; Xie, W.; Niu, Y.; Wang, H.; Li, G.; Zhao, L.; Wang, X.; Xie, W. Induced Necroptosis and Its Role in Cancer Immunotherapy. Int. J. Mol. Sci. 2024, 25, 10760. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, W.; He, L.; Liu, D.; Zhao, L.; Wang, X. A Glimpse of necroptosis and diseases. Biomed. Pharmacother. 2022, 156, 113925. [Google Scholar] [CrossRef]
- Seo, J.; Nam, Y.W.; Kim, S.; Oh, D.-B.; Song, J. Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators. Exp. Mol. Med. 2021, 53, 1007–1017. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, B.-W. Recognition of necroptosis: From molecular mechanisms to detection methods. Biomed. Pharmacother. 2024, 178, 117196. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, Y.; Xiong, X.; Zheng, Y.; Liu, X.; Wang, W.; Meng, G. Roles of the adaptor protein tumor necrosis factor receptor type 1-associated death domain protein (TRADD) in human diseases. Biomed. Pharmacother. 2022, 153, 113467. [Google Scholar] [CrossRef]
- Lu, Z.; Van Eeckhoutte, H.P.; Liu, G.; Nair, P.M.; Jones, B.; Gillis, C.M.; Nalkurthi, B.C.; Verhamme, F.; Buyle-Huybrecht, T.; Vandenabeele, P.; et al. Necroptosis Signaling Promotes Inflammation, Airway Remodeling, and Emphysema in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 204, 667–681. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.-K.; Wu, P.-P.; Wang, Y.; Ren, L.-Y.; Xu, A.-H. Necroptosis Mediates Cigarette Smoke-Induced Inflammatory Responses in Macrophages. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 1093–1101. [Google Scholar] [CrossRef]
- Van Eeckhoutte, H.P.; Donovan, C.; Kim, R.Y.; Conlon, T.M.; Ansari, M.; Khan, H.; Jayaraman, R.; Hansbro, N.G.; Dondelinger, Y.; Delanghe, T.; et al. RIPK1 kinase-dependent inflammation and cell death contribute to the pathogenesis of COPD. Eur. Respir. J. 2023, 61, 2201506. [Google Scholar] [CrossRef]
- Mizumura, K.; Cloonan, S.M.; Nakahira, K.; Bhashyam, A.R.; Cervo, M.; Kitada, T.; Glass, K.; Owen, C.A.; Mahmood, A.; Washko, G.R.; et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Investig. 2014, 124, 3987–4003. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X.; Xiang, F.; He, X.; Li, J.; Liu, H.; Xie, L. MicroRNA-21 plays a role in exacerbating chronic obstructive pulmonary disease by regulating necroptosis and apoptosis in bronchial epithelial cells. Tob. Induc. Dis. 2025, 23, 10.18332. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.-S.; Xu, X.-C.; Li, Z.-Y.; Chen, H.-P.; Ying, S.-M.; Li, W.; Shen, H.-H.; Chen, Z.-H. Endoplasmic reticulum chaperone GRP78 mediates cigarette smoke-induced necroptosis and injury in bronchial epithelium. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gregory, A.D.; Li, X.; Wei, J.; Burton, C.L.; Gibson, G.; Scott, S.J.; St Croix, C.M.; Zhang, Y.; Shapiro, S.D. RIP3-dependent necroptosis contributes to the pathogenesis of chronic obstructive pulmonary disease. JCI Insight 2021, 6, e144689. [Google Scholar] [CrossRef]
- Mizumura, K.; Ozoe, R.; Nemoto, Y.; Furusho, N.; Kurosawa, Y.; Kozu, Y.; Oki, T.; Maruoka, S.; Gon, Y. Cigarette Smoke Extract-Induced Necroptosis Causes Mitochondrial DNA Release and Inflammation of Bronchial Epithelial Cells. Int. J. Chron. Obstruct. Pulmon. Dis. 2025, 20, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef]
- Jin, X.; Tang, J.; Qiu, X.; Nie, X.; Ou, S.; Wu, G.; Zhang, R.; Zhu, J. Ferroptosis: Emerging mechanisms, biological function, and therapeutic potential in cancer and inflammation. Cell Death Discov. 2024, 10, 45. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yoshida, M.; Minagawa, S.; Araya, J.; Sakamoto, T.; Hara, H.; Tsubouchi, K.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T.; et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 2019, 10, 3145. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wu, Y.; Zhao, J.; Cheng, C.; Lin, J.; Yang, Y.; Lu, L.; Xiang, Q.; Bian, T.; Liu, Q. N6-Methyladenosine-modified circSAV1 triggers ferroptosis in COPD through recruiting YTHDF1 to facilitate the translation of IREB2. Cell Death Differ. 2023, 30, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, S. Relationship Between ACSL4-Mediated Ferroptosis and Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 99–111. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Xu, D.; Zhu, D.; Zhou, Y.; Chen, Z.; Huang, X. Overexpression of USP8 inhibits inflammation and ferroptosis in chronic obstructive pulmonary disease by regulating the OTUB1/SLC7A11 signaling pathway. Allergol. Immunopathol. 2024, 52, 60–67. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, T.; Liu, X.; Ma, Y.; Luo, L.; Wang, Z.; Zhao, Z.; Li, H.; He, X.; Zeng, H.; et al. DNA dioxygenases TET2 deficiency promotes cigarette smoke induced chronic obstructive pulmonary disease by inducing ferroptosis of lung epithelial cell. Redox Biol. 2023, 67, 102916. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, S.; Shi, G.-J.; Meng, G.-L.; Yang, S.-J. Distinct Types of Regulated Cell Death in Melanoma. Cells 2025, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gu, X.; Wang, G.; Zhong, Y.; Ma, F.; Liu, Q.; Xie, J. Regulated Cell Death of Alveolar Macrophages in Acute Lung Inflammation: Current Knowledge and Perspectives. J. Inflamm. Res. 2024, 17, 11419–11436. [Google Scholar] [CrossRef]
- Huang, X.; Yan, H.; Xu, Z.; Yang, B.; Luo, P.; He, Q. The inducible role of autophagy in cell death: Emerging evidence and future perspectives. Cell Commun. Signal. 2025, 23, 151. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Autophagy in asthma and chronic obstructive pulmonary disease. Clin. Sci. 2022, 136, 733–746. [Google Scholar] [CrossRef]
- Mercado, N.; Colley, T.; Baker, J.R.; Vuppussetty, C.; Kono, Y.; Clarke, C.; Tooze, S.; Johansen, T.; Barnes, P.J. Bicaudal D1 impairs autophagosome maturation in chronic obstructive pulmonary disease. FASEB Bioadv. 2019, 1, 688–705. [Google Scholar] [CrossRef]
- Fujii, S.; Hara, H.; Araya, J.; Takasaka, N.; Kojima, J.; Ito, S.; Minagawa, S.; Yumino, Y.; Ishikawa, T.; Numata, T.; et al. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology 2012, 1, 630–641. [Google Scholar] [CrossRef]
- Kono, Y.; Colley, T.; To, M.; Papaioannou, A.I.; Mercado, N.; Baker, J.R.; To, Y.; Abe, S.; Haruki, K.; Ito, K.; et al. Cigarette smoke-induced impairment of autophagy in macrophages increases galectin-8 and inflammation. Sci. Rep. 2021, 11, 335. [Google Scholar] [CrossRef]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef]
- Liao, S.-X.; Zhang, L.-Y.; Shi, L.-M.; Hu, H.-Y.; Gu, Y.-H.; Wang, T.-H.; Ouyang, Y.; Sun, P.-P. Integrating bulk and single-cell RNA sequencing data: Unveiling RNA methylation and autophagy-related signatures in chronic obstructive pulmonary disease patients. Sci. Rep. 2025, 15, 4005. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-F.; Li, Z.-Y.; Dong, L.-L.; Li, W.-J.; Wu, Y.-P.; Wang, J.; Chen, H.-P.; Liu, H.-W.; Li, M.; Jin, C.-L.; et al. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation. Autophagy 2020, 16, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef]
- Yang, R.; Zhan, Y.; Deng, Z.; Zhang, J.; Chen, S.; Zhang, Y.; Fu, H.; Meng, X.; Wu, J.; Gu, Y.; et al. Arylsulfatase K attenuates airway epithelial cell senescence in COPD by regulating parkin-mediated mitophagy. Redox Biol. 2025, 86, 103793. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Q.; He, K.; Liao, G.; Cheng, L.; Li, M.; He, Z. Mechanism of cigarette smoke in promoting small airway remodeling in mice via STAT 3/PINK 1-Parkin/EMT. Free Radic. Biol. Med. 2024, 224, 447–456. [Google Scholar] [CrossRef]
- Wang, Y.; Han, G.; Yang, J.; Xue, L.; Chen, Y. Hydrogen sulfide attenuates PM2.5-induced COPD by inhibiting cellular senescence via the Klotho/Parkin-mediated mitophagy signaling pathway. Ecotoxicol. Environ. Saf. 2025, 293, 117987. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Chen, T.T.; Zhang, D.W.; Zhang, Y.; Li, F.; Ding, Y.C.; Wang, M.Y.; Zhang, L.; Chen, K.G.; Fei, G.H. Microplastics exacerbate ferroptosis via mitochondrial reactive oxygen species-mediated autophagy in chronic obstructive pulmonary disease. Autophagy 2025, 21, 1717–1743. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, J.; Chen, Y.; Ding, Y. Nicotine promotes chronic obstructive pulmonary disease via inducing pyroptosis activation in bronchial epithelial cells. Mol. Med. Rep. 2022, 25, 92. [Google Scholar] [CrossRef]
- Fu, X.; Hong, W.; Li, S.; Chen, Z.; Zhou, W.; Dai, J.; Deng, X.; Zhou, H.; Li, B.; Ran, P. Wood smoke particulate matter (WSPM2.5) induces pyroptosis through both Caspase-1/IL-1β/IL-18 and ATP/P2Y-dependent mechanisms in human bronchial epithelial cells. Chemosphere 2022, 307, 135726. [Google Scholar] [CrossRef]
- Buscetta, M.; Cristaldi, M.; Cimino, M.; La Mensa, A.; Dino, P.; Bucchieri, F.; Rappa, F.; Amato, S.; Aronica, T.S.; Pace, E.; et al. Cigarette smoke promotes inflammasome-independent activation of caspase-1 and -4 leading to gasdermin D cleavage in human macrophages. FASEB J. 2022, 36, e22525. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, F.; Lu, H.; Chen, J.; Huang, X.; Chen, Y.; Lin, L. S1PR2 is Important for Cigarette Smoke-induced Pyroptosis in Human Bronchial Epithelial Cells. Arch. Med. Res. 2023, 54, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, M.; Liu, Y.; Zhang, Z.; Bai, Z. Cigarette Smoke Extract and Lipopolysaccharide Induce Pyroptosis in Pulmonary Microvascular Endothelial Cells of Rats. Bull. Exp. Biol. Med. 2023, 174, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.B.; Hamon, R.; Jersmann, H.; Ween, M.P.; Asare, P.; Haberberger, R.; Pant, H.; Hodge, S.J. AIM2 nuclear exit and inflammasome activation in chronic obstructive pulmonary disease and response to cigarette smoke. J. Inflamm. 2021, 18, 19. [Google Scholar] [CrossRef]
- Ruwanpura, S.M.; McLeod, L.; Dousha, L.F.; Seow, H.J.; West, A.C.; West, A.J.; Weng, T.; Alanazi, M.; MacDonald, M.; King, P.T.; et al. Cross-talk between IL-6 trans-signaling and AIM2 inflammasome/IL-1β axes bridge innate immunity and epithelial apoptosis to promote emphysema. Proc. Natl. Acad. Sci. USA 2022, 119, e2201494119. [Google Scholar] [CrossRef]
- Ju, J.; Liu, Y.; Liang, H.; Yang, B. The role of pyroptosis in endothelial dysfunction induced by diseases. Front. Immunol. 2022, 13, 1093985. [Google Scholar] [CrossRef]
- Wu, Y.; Di, X.; Zhao, M.; Li, H.; Bai, L.; Wang, K. The role of the NLRP3 inflammasome in chronic inflammation in asthma and chronic obstructive pulmonary disease. Immun. Inflamm. Dis. 2022, 10, e750. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Kang, N.; Yu, Y.; Mi, Y.; Guo, J.; Wu, J.; Weng, C.-F. Polyphenols, flavonoids and inflammasomes: The role of cigarette smoke in COPD. Eur. Respir. Rev. 2022, 31, 220028. [Google Scholar] [CrossRef]
- Nachmias, N.; Langier, S.; Brzezinski, R.Y.; Siterman, M.; Stark, M.; Etkin, S.; Avriel, A.; Schwarz, Y.; Shenhar-Tsarfaty, S.; Bar-Shai, A. NLRP3 inflammasome activity is upregulated in an in-vitro model of COPD exacerbation. PLoS ONE 2019, 14, e0214622. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y. Pyroptosis and respiratory diseases: A review of current knowledge. Front. Immunol. 2022, 13, 920464. [Google Scholar] [CrossRef]
- Sawada, M.; Kawayama, T.; Imaoka, H.; Sakazaki, Y.; Oda, H.; Takenaka, S.; Kaku, Y.; Azuma, K.; Tajiri, M.; Edakuni, N.; et al. IL-18 Induces Airway Hyperresponsiveness and Pulmonary Inflammation via CD4+ T Cell and IL-13. PLoS ONE 2013, 8, e54623. [Google Scholar] [CrossRef] [PubMed]
- Faner, R.; Sobradillo, P.; Noguera, A.; Gomez, C.; Cruz, T.; López-Giraldo, A.; Ballester, E.; Soler, N.; Arostegui, J.I.; Pelegrín, P.; et al. The inflammasome pathway in stable COPD and acute exacerbations. ERJ Open Res. 2016, 2, 00002-2016. [Google Scholar] [CrossRef] [PubMed]
- Gellner, C.A.; Belluzzi, J.D.; Leslie, F.M. Self-administration of nicotine and cigarette smoke extract in adolescent and adult rats. Neuropharmacology 2016, 109, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Y.; Jiang, Y.-X.; Yang, Y.-C.; Liu, J.-Y.; Huo, C.; Ji, X.-L.; Qu, Y.-Q. Cigarette smoke extract induces pyroptosis in human bronchial epithelial cells through the ROS/NLRP3/caspase-1 pathway. Life Sci. 2021, 269, 119090. [Google Scholar] [CrossRef]
- Rao, Y.; Gai, X.; Xiong, J.; Le, Y.; Sun, Y. Transient Receptor Potential Cation Channel Subfamily V Member 4 Mediates Pyroptosis in Chronic Obstructive Pulmonary Disease. Front. Physiol. 2021, 12, 783891. [Google Scholar] [CrossRef]
- Hou, T.; Zhu, L.; Zhang, Y.; Tang, Y.; Gao, Y.; Hua, S.; Ci, X.; Peng, L. Lipid peroxidation triggered by the degradation of xCT contributes to gasdermin D-mediated pyroptosis in COPD. Redox Biol. 2024, 77, 103388. [Google Scholar] [CrossRef]
- Mo, R.; Li, J.; Chen, Y.; Ding, Y. lncRNA GAS5 promotes pyroptosis in COPD by functioning as a ceRNA to regulate the miR-223-3p/NLRP3 axis. Mol. Med. Rep. 2022, 26, 219. [Google Scholar] [CrossRef]
- Buscetta, M.; Di Vincenzo, S.; Miele, M.; Badami, E.; Pace, E.; Cipollina, C. Cigarette smoke inhibits the NLRP3 inflammasome and leads to caspase-1 activation via the TLR4-TRIF-caspase-8 axis in human macrophages. FASEB J. 2020, 34, 1819–1832. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Tian, Z.; Jiang, S.; Zhou, J.; Zhang, W. Copper homeostasis and cuproptosis in mitochondria. Life Sci. 2023, 334, 122223. [Google Scholar] [CrossRef]
- Boaru, D.L.; Leon-Oliva, D.D.; Castro-Martinez, P.D.; Garcia-Montero, C.; Fraile-Martinez, O.; García-González, B.; Pérez-González, I.; Michael Alhaddadin, M.N.; Barrena-Blázquez, S.; Lopez-Gonzalez, L.; et al. Cuproptosis: Current insights into its multifaceted role in disease, cancer, and translational/therapeutic opportunities. Biomed. Pharmacother. 2025, 190, 118422. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhu, W.; Qi, H.; He, L.; Wang, Q.; Shen, J.; Song, Y.; Shen, Y.; Zhu, Q.; Zhou, J. The cuproptosis-related gene glutaminase promotes alveolar macrophage copper ion accumulation in chronic obstructive pulmonary disease. Int. Immunopharmacol. 2024, 129, 111585. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Huang, J.-B.; Zhu, M.; Ji, D.-J.; Huang, S.-J.; Li, J. Identification of Cuproptosis-Related Genes and Their Potential Role in COPD Pathogenesis: A Bioinformatics Analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2025, 20, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yue, Y.; Zhang, Q.; Wang, X. Copper homeostasis dysregulation in respiratory diseases: A review of current knowledge. Front. Physiol. 2024, 15, 1243629. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and its associated nucleases in DNA damage response. DNA Repair 2019, 81, 102651. [Google Scholar] [CrossRef]
- Mashimo, M.; Bu, X.; Aoyama, K.; Kato, J.; Ishiwata-Endo, H.; Stevens, L.A.; Kasamatsu, A.; Wolfe, L.A.; Toro, C.; Adams, D.; et al. PARP1 inhibition alleviates injury in ARH3-deficient mice and human cells. JCI Insight 2019, 4, e124519. [Google Scholar] [CrossRef]
- Pandey, N.; Black, B.E. Rapid Detection and Signaling of DNA Damage by PARP-1. Trends Biochem. Sci. 2021, 46, 744–757. [Google Scholar] [CrossRef]
- Künzi, L.; Holt, G.E. Cigarette smoke activates the parthanatos pathway of cell death in human bronchial epithelial cells. Cell Death Discov. 2019, 5, 127. [Google Scholar] [CrossRef]
- Huang, P.; Chen, G.; Jin, W.; Mao, K.; Wan, H.; He, Y. Molecular Mechanisms of Parthanatos and Its Role in Diverse Diseases. Int. J. Mol. Sci. 2022, 23, 7292. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Zijlstra, G.J.; van der Toorn, M.; Hesse, L.; Gras, R.; Ten Hacken, N.H.T.; Krysko, D.V.; Vandenabeele, P.; de Vries, M.; van Oosterhout, A.J.M.; et al. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L377–L386. [Google Scholar] [CrossRef]
- Zhu, F.; Ji, Y.; You, Q.; Dong, Q.; Tang, Y.; Zhang, Y. Asiaticoside Mitigates Chronic Obstructive Pulmonary Disease by Modulating TRIM27 Stability and Activating PGC-1α/Nrf2 Signaling. Appl. Biochem. Biotechnol. 2025, 197, 6234–6254. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, J.-H.; Kim, C.-Y.; Jeong, J.-S.; Ko, J.-W.; Kim, T.-W. Korean Red Ginseng suppresses emphysematous lesions induced by cigarette smoke condensate through inhibition of macrophage-driven apoptosis pathways. J. Ginseng Res. 2024, 48, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, X.; Zeng, R.; Lai, X.; Wang, J.; Liu, H.; Wu, H.; He, J.; Liu, L.; Zhu, Z.; et al. Formononetin attenuates cigarette smoke-induced COPD in mice by suppressing inflammation, endoplasmic reticulum stress, and apoptosis in bronchial epithelial cells via AhR/CYP1A1 and AKT/mTOR signaling pathways. Phytother. Res. 2024, 38, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, Y.; Cui, H.; Li, S.; Duan, L.; Jiao, Z. N-acetyl-L-cysteine protects rat lungs and RLE-6TN cells from cigarette smoke-induced oxidative stress. Mol. Med. Rep. 2025, 31, 97. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, J.; Zhang, J.; Li, N.; Yi, Y.; Lu, H.; Li, M.; Liu, R.; Chen, Y.; Liu, X. Anthrahydroquinone-2,6-Disulfonate Restores Lung Function in COPD Through Keap1/Nrf2 Pathway Activation. J. Inflamm. Res. 2025, 18, 9559–9579. [Google Scholar] [CrossRef]
- Min, H.-Y.; Sim, J.Y.; Ahn, J.H.; Kang, N.-W.; Boo, H.-J.; Kim, J.; Yu, N.-Y.; Bottacin, M.; Huh, J.; Park, C.-S.; et al. Gaylussacin, a stilbene glycoside, inhibits chronic obstructive pulmonary disease in mice. Redox Biol. 2025, 85, 103744. [Google Scholar] [CrossRef]
- Xie, B.; Chen, Q.; Dai, Z.; Jiang, C.; Chen, X. Progesterone (P4) ameliorates cigarette smoke-induced chronic obstructive pulmonary disease (COPD). Mol. Med. 2024, 30, 123. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Park, S.-M.; Yang, S.-R. An Update on the Role of Nrf2 in Respiratory Disease: Molecular Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 8406. [Google Scholar] [CrossRef]
- Feng, Q.; Crochet, J.R.; Dai, Q.; Leppert, P.C.; Price, T.M. Expression of a Mitochondrial Progesterone Receptor (PR-M) in Leiomyomata and Association with Increased Mitochondrial Membrane Potential. J. Clin. Endocrinol. Metab. 2014, 99, E390–E399. [Google Scholar] [CrossRef]
- Luan, G.; Zhu, Z.; Wu, K.; Yin, S. Theaflavin-3,3’-digallate attenuates cigarette smoke extract-induced pulmonary emphysema in mice by suppressing necroptosis. Exp. Ther. Med. 2022, 23, 11. [Google Scholar] [CrossRef]
- Dera, A.A.; Al Fayi, M.; Otifi, H.; Alshyarba, M.; Alfhili, M.; Rajagopalan, P. Thymoquinone (Tq) protects necroptosis induced by autophagy/mitophagy-dependent oxidative stress in human bronchial epithelial cells exposed to cigarette smoke extract (CSE). J. Food Biochem. 2020, 44, e13366. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Xu, Z.-P.; Guan, Y.-Q.; Wang, Y.-Y.; Wen, X.-S.; Li, G.-H.; Wang, X.-N.; Shen, T. Ethyl acetate fraction of Thesium chinense Turcz. alleviates chronic obstructive pulmonary disease through inhibition of ferroptosis mediated by activating Nrf2/SLC7A11/GPX4 axis. J. Ethnopharmacol. 2025, 337, 118776. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, B.; Xuan, Y.; Jiang, Y.; Chen, J.; Liao, H.; Feng, J.; Zhang, J. Fluorofenidone alleviates cigarette smoke exposure-induced chronic lung injury by targeting ferroptosis. Sci. Rep. 2024, 14, 32149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liao, S.; Pan, Z.; Jiang, S.; Fan, J.; Yu, S.; Xue, L.; Yang, J.; Ma, S.; Liu, T.; et al. Hydrogen sulfide alleviates particulate matter-induced emphysema and airway inflammation by suppressing ferroptosis. Free Radic. Biol. Med. 2022, 186, 1–16. [Google Scholar] [CrossRef]
- Tang, X.; Li, Z.; Yu, Z.; Li, J.; Zhang, J.; Wan, N.; Zhang, J.; Cao, J. Effect of curcumin on lung epithelial injury and ferroptosis induced by cigarette smoke. Hum. Exp. Toxicol. 2021, 40, S753–S762. [Google Scholar] [CrossRef]
- Lian, N.; Zhang, Q.; Chen, J.; Chen, M.; Huang, J.; Lin, Q. The Role of Ferroptosis in Bronchoalveolar Epithelial Cell Injury Induced by Cigarette Smoke Extract. Front. Physiol. 2021, 12, 751206. [Google Scholar] [CrossRef]
- Sul, O.J.; Park, S.H.; Choi, H.W.; Kim, D.J.; Ra, S.W. NADPH oxidase-dependent heme oxygenase-1 expression mediates cigarette smoke-induced ferroptosis via intracellular Fe(II) accumulation. Free Radic. Biol. Med. 2025, 237, 131–146. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Yang, Y.; Di, T.; Wu, Y.; Bian, T. NCOA4-Mediated Ferroptosis in Bronchial Epithelial Cells Promotes Macrophage M2 Polarization in COPD Emphysema. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 667–681. [Google Scholar] [CrossRef]
- Tan, W.; Liang, Z.; Tan, X.; Tan, G. Ginsenoside Rg1 improves cigarette smoke-induced ferroptosis in COPD by regulating PERK/ATF4 axis to inhibit endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2024, 739, 150946. [Google Scholar] [CrossRef]
- Xu, A.; Xu, Y.; Chen, H.; Xiang, L.; Zhao, X. Ginkgo biloba extract alleviates ferroptosis in lung epithelial cells induced by cigarette smoke extract through miR-3,619-5p/GPX4 axis. Toxicol. Res. 2025, 14, tfae225. [Google Scholar] [CrossRef]
- Liu, L.; Wen, T.; Xiao, Y.; Chen, H.; Yang, S.; Shen, X. Sea buckthorn extract mitigates chronic obstructive pulmonary disease by suppression of ferroptosis via scavenging ROS and blocking p53/MAPK pathways. J. Ethnopharmacol. 2025, 336, 118726. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Dong, J.; Zhang, C.; Tang, J. Acacetin Attenuates Cigarette Smoke Extract-Induced Human Bronchial Epithelial Cell Injury by Activating NRF2/SLC7A11/GPX4 Signaling to Inhibit Ferroptosis. Cell Biochem. Biophys. 2025, 83, 2499–2510. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Luo, L.; Zong, D.; Li, H.; Zeng, Z.; Cui, Y.; Meng, W.; Chen, Y. Dihydroquercetin suppresses cigarette smoke induced ferroptosis in the pathogenesis of chronic obstructive pulmonary disease by activating Nrf2-mediated pathway. Phytomedicine 2022, 96, 153894. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, Z.; Chang, J.; He, L.; Zhang, Z.; Song, X.; Hou, X.; Fan, F.; Jiang, Z. Sodium pyruvate exerts protective effects against cigarette smoke extract-induced ferroptosis in alveolar and bronchial epithelial cells through the GPX4/Nrf2 axis. J. Inflamm. 2023, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; You, C.-G. Lipoxin alleviates oxidative stress: A state-of-the-art review. Inflamm. Res. 2022, 71, 1169–1179. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Liu, K.; Shi, M.; Zeng, X.; Liu, X. LXA4 alleviates inflammation and ferroptosis in cigarette smoke induced chronic obstructive pulmonary disease via the ALX/FPR2 receptor. Int. Immunopharmacol. 2025, 151, 114322. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yang, M.; Tian, Y.; Liu, X.; Li, J.; Zhao, P. Bufei Yishen formula alleviates airway epithelial cell senescence in COPD by activating AMPK-Sirt1-FoxO3a pathway and promoting autophagy. Sci. Rep. 2025, 15, 16584. [Google Scholar] [CrossRef]
- Jia, L.; Liu, X.; Liu, X.; Guan, Q.; Tian, Y.; Li, J.; Zhao, P. Bufei Yishen formula protects the airway epithelial barrier and ameliorates COPD by enhancing autophagy through the Sirt1/AMPK/Foxo3 signaling pathway. Chin. Med. 2024, 19, 32. [Google Scholar] [CrossRef]
- Liu, Y.; Di, N.; Li, C.; Cui, Y.; He, J.; Wei, L. Dihydromyricetin restores mucus hypersecretion in Air–Liquid interface cultures in COPD by targeting the SRC-MAPK signaling pathway. Eur. J. Pharmacol. 2025, 1000, 177703. [Google Scholar] [CrossRef]
- Zhou, H.; Lai, Y.; Zhu, Y.; Shao, F.; Ma, G.; Yang, N.; Ma, X.; Sun, Y.; Shi, Q. Quercetin improves airway remodeling in COPD rats by suppressing phenotypic switch of ASMCs via inhibiting the Wnt5a/β-catenin pathway. Phytomedicine 2025, 139, 156491. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, J.; Xu, J.; Zhu, L.; Zhai, C.; Wang, Y.; Wang, Y.; Feng, Y.; Cao, H. Vardenafil alleviates cigarette smoke-induced chronic obstructive pulmonary disease by activating autophagy via the AMPK/mTOR signalling pathway: An in vitro and in vivo study. Cell. Dev. Biol. Anim. 2023, 59, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-S.; Lee, H.J.; Hyun, M.; Kim, H.-J.; Kim, J.-H.; Hwang, K.-H.; Kim, W.-S.; Choi, J.; Heo, J.D. Diindolylmethane Inhibits Cadmium-Induced Autophagic Cell Death via Regulation of Oxidative Stress in HEL299 Human Lung Fibroblasts. Molecules 2022, 27, 5215. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, W.; Wang, J.; Xie, Y.; Wang, W. Puerarin inhibits FUNDC1-mediated mitochondrial autophagy and CSE-induced apoptosis of human bronchial epithelial cells by activating the PI3K/AKT/mTOR signaling pathway. Aging 2022, 14, 1253–1264. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, C.; Yang, G.; Hu, Y.; Qu, Y. Cytoprotective role of resveratrol in cigarette smoke-induced pyroptosis through Nrf2 pathway activation. Cell Stress Chaperones 2025, 30, 100107. [Google Scholar] [CrossRef]
- Hou, T.; Tang, Y.; Wang, L.; Peng, L.; Ci, X. Dihydromyricetin alleviates lipid peroxidation-induced Pyroptosis by inhibiting xCT ubiquitination and degradation in experimental COPD model. Phytomedicine 2025, 144, 156929. [Google Scholar] [CrossRef]
- Zeng, J.; Liao, S.; Liang, Z.; Li, C.; Luo, Y.; Wang, K.; Zhang, D.; Lan, L.; Hu, S.; Li, W.; et al. Schisandrin A regulates the Nrf2 signaling pathway and inhibits NLRP3 inflammasome activation to interfere with pyroptosis in a mouse model of COPD. Eur. J. Med. Res. 2023, 28, 217. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, L.; Tian, D.; Wang, P.; Men, K.; Ge, Y.; Zhang, C. Tanshinone increases Hemopexin expression in lung cells and macrophages to protect against cigarette smoke-induced COPD and enhance antiviral responses. Cell Cycle 2023, 22, 645–665. [Google Scholar] [CrossRef]
- Sul, O.J.; Choi, H.W.; Oh, J.; Ra, S.W. GSPE attenuates CSE-induced lung inflammation and emphysema by regulating autophagy via the reactive oxygen species/TFEB signaling pathway. Food Chem. Toxicol. 2023, 177, 113795. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; Hua, C.; Peng, L.; Ci, X. Daphnetin ameliorates PM2.5-induced airway inflammation by inhibiting NLRP3 inflammasome-mediated pyroptosis in CS-exposed mice. Biomed. Pharmacother. 2023, 165, 115047. [Google Scholar] [CrossRef]
- Chen, P.; Li, Q.; Su, X.; Zhang, Z.-Q.; Li, G.-P. Osthole, an ingredient from Cnidium monnieri, reduces the pyroptosis and apoptosis in bronchial epithelial cells. J. Asian Nat. Prod. Res. 2023, 25, 999–1011. [Google Scholar] [CrossRef]
- Xu, L.-T.; Wang, T.; Fang, K.-L.; Zhao, Y.; Wang, X.-N.; Ren, D.-M.; Shen, T. The ethanol extract of flower buds of Tussilago farfara L. attenuates cigarette smoke-induced lung inflammation through regulating NLRP3 inflammasome, Nrf2, and NF-κB. J. Ethnopharmacol. 2022, 283, 114694. [Google Scholar] [CrossRef]
- Tian, X.; Xue, Y.; Xie, G.; Zhou, Y.; Xiao, H.; Ding, F.; Zhang, M. (−)-Epicatechin ameliorates cigarette smoke-induced lung inflammation via inhibiting ROS/NLRP3 inflammasome pathway in rats with COPD. Toxicol. Appl. Pharmacol. 2021, 429, 115674. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Cheng, S.; Li, R.; Wang, Y.; Zeng, D.; Jiang, H.; Liang, Y.; Huang, R.; Pan, H.; Wu, X.; et al. Isoforskolin Alleviates AECOPD by Improving Pulmonary Function and Attenuating Inflammation Which Involves Downregulation of Th17/IL-17A and NF-κB/NLRP3. Front. Pharmacol. 2021, 12, 721273. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Luan, G.; Hu, J.; Zhu, Z.; Kong, Z.; Yin, S. Astragaloside IV Reduces Lung Epithelial Cell Pyroptosis via TXNIP-NLRP3-GSDMD pathway. Cell Biochem. Biophys. 2024, 82, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Gong, J.; Yan, Y.; Cui, X.; Yu, Q.; Shi, Y.; Tian, X.; Jiang, Q.; Tan, Z.; et al. Tianlong kechuanling ameliorated COPD-PH through DNMT3A-GSDMD axis-dependent regulation of endothelial cell pyroptosis. J. Ethnopharmacol. 2025, 353, 120332. [Google Scholar] [CrossRef]
- Gao, J.; Han, L.; Zhang, Y.; Zhang, X.; Fei, X.; Zhang, M. Disulfiram alleviates epithelial barrier disruption in ozone-induced chronic obstructive pulmonary disease mouse models via inhibiting Gasdermin D-mediated pyroptosis. Int. Immunopharmacol. 2025, 159, 114887. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Chen, Q.; Zhu, X.; Hong, J. Propofol modulates Nrf2/NLRP3 signaling to ameliorate cigarette smoke-induced damage in human bronchial epithelial cells. Tissue Cell 2024, 88, 102341. [Google Scholar] [CrossRef]
- Wang, L.; Meng, J.; Wang, C.; Wang, Y.; Yang, C.; Li, Y. Hydrogen sulfide attenuates cigarette smoke-induced pyroptosis through the TLR4/NF-κB signaling pathway. Int. J. Mol. Med. 2022, 49, 56. [Google Scholar] [CrossRef]
- Donovan, C.; Kim, R.Y.; Galvao, I.; Jarnicki, A.G.; Brown, A.C.; Jones-Freeman, B.; Gomez, H.M.; Wadhwa, R.; Hortle, E.; Jayaraman, R.; et al. Aim2 suppresses cigarette smoke-induced neutrophil recruitment, neutrophil caspase-1 activation and anti-Ly6G-mediated neutrophil depletion. Immunol. Cell Biol. 2022, 100, 235–249. [Google Scholar] [CrossRef]
- Jia, G.; Yu, S.; Sun, W.; Yang, J.; Wang, Y.; Qi, Y.; Chen, Y. Hydrogen Sulfide Attenuates Particulate Matter-Induced Emphysema and Airway Inflammation Through Nrf2-Dependent Manner. Front. Pharmacol. 2020, 11, 29. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, L.; Tian, C.; Qian, B. Magnesium isoglycyrrhizinate inhibits airway inflammation in rats with chronic obstructive pulmonary disease. BMC Pulm. Med. 2021, 21, 371. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Molino, A.; Imitazione, P.; Somma, P.; Rega, R.; Saccomanno, A.; Aquino, R.P.; Pinto, A.; Sorrentino, R. AIM2 Inflammasome Activation Leads to IL-1α and TGF-β Release from Exacerbated Chronic Obstructive Pulmonary Disease-Derived Peripheral Blood Mononuclear Cells. Front. Pharmacol. 2019, 10, 257. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Lu, J.; Liu, Z.; Zhao, S.; Zhang, M.; Lu, M.; Xu, W.; Sun, F.; Wu, Q.; et al. Characteristics of Prognostic Programmed Cell Death–Related Long Noncoding RNAs Associated with Immune Infiltration and Therapeutic Responses to Colon Cancer. Front. Immunol. 2022, 13, 828243. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Q.; Cao, S.; Fang, H.; Li, J.; Shi, X.; Pang, C.; Lu, D.; Zhao, X.; Li, J.; et al. Programmed cell death-related genes define distinct molecular subtypes and risk profiles in hepatocellular carcinoma. Sci. Rep. 2025, 15, 37117. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, R.; Li, M.; Zhai, Q.; Dong, N.; Dong, H.; Zhang, G.; Zhang, Y. Development of a prognostic prediction signature for idiopathic pulmonary fibrosis by integrating multiple programmed cell death-related genes and machine learning algorithms. J. Thorac. Dis. 2025, 17, 7056–7073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, C.-L.; Xu, L.; Wu, J.J.; Wang, L. Pyroptosis-Related Genes as Prognostic Biomarkers and Immune Infiltration Features in Sepsis-Induced ARDS: A Single-Cell and Bulk RNA-Sequencing Analysis. J. Inflamm. Res. 2025, 18, 14261–14282. [Google Scholar] [CrossRef]
- Ghadban, C.; García-Unzueta, M.; Agüero, J.; Martín-Audera, P.; Lavín, B.A.; Guerra, A.R.; Berja, A.; Aranda, N.; Guzun, A.; Insua, A.I.; et al. Associations between serum levels of ferroptosis-related molecules and outcomes in stable COPD: An exploratory prospective observational study. Intern. Emerg. Med. 2025, 20, 1761–1773. [Google Scholar] [CrossRef]
- Clarke, N.; Thornton, P.; Reader, V.; Lindsay, N.; Digby, Z.; Mullen, B.; Gorman, M.; Jacobson, E.; Langdon, G.; Johnstone, H.; et al. Anti-Neuroinflammatory and Anti-Inflammatory Effects of the NLRP3 Inhibitor NT-0796 in Subjects with Parkinson’s Disease. Mov. Disord. 2025, 40, 2199–2208. [Google Scholar] [CrossRef]
- Gatlik, E.; Mehes, B.; Voltz, E.; Sommer, U.; Tritto, E.; Lestini, G.; Liu, X.; Pal, P.; Velinova, M.; Denney, W.S.; et al. First-in-human safety, tolerability, and pharmacokinetic results of DFV890, an oral low-molecular-weight NLRP3 inhibitor. Clin. Transl. Sci. 2024, 17, e13789. [Google Scholar] [CrossRef]
- Oliveira, A.P.d.; Figueiredo-Junior, A.T.; Mineiro, P.C.d.O.; Mota, E.C.; Amorim, C.S.d.; Valenca, H.d.M.; Gomes, A.C.C.d.A.; Serra, S.S.d.S.; Silva, P.L.; Takiya, C.M.; et al. Modulation of Pulmonary Inflammation and the Redox Pathway In Vitro and In Vivo by Fumaric Ester. Antioxidants 2025, 14, 1141. [Google Scholar] [CrossRef] [PubMed]
- Bomprezzi, R. Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: An overview. Ther. Adv. Neurol. Disord. 2015, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Lauletta, G.; Tommasino, N.; Salsano, A.; Battista, T.; Ruggiero, A.; Martora, F.; Potestio, L. Management of Psoriasis Patients with Serious Infectious Diseases. Adv. Ther. 2024, 41, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Farenc, C.; Clot, P.-F.; Badalamenti, S.; Kruger, A.J.; Pomponio, R.J.; Krahnke, T.; Staudinger, H.; Lin, Y. A Randomized Phase I Trial Evaluating Safety and Pharmacokinetics of Single and Multiple Ascending Doses of Eclitasertib, a RIPK1 Inhibitor, in Healthy Participants. Adv. Ther. 2025, 42, 3993–4012. [Google Scholar] [CrossRef]
- Sun, A.L.A.; Gillies, J.D.; Shen, Y.; Deng, H.; Xue, F.; Ma, Y.; Song, L. A phase I randomized study to evaluate safety, pharmacokinetics, and pharmacodynamics of SIR2446M, a selective RIPK1 inhibitor, in healthy participants. Clin. Transl. Sci. 2024, 17, e13857. [Google Scholar] [CrossRef]
- Lickliter, J.; Wang, S.; Zhang, W.; Zhu, H.; Wang, J.; Zhao, C.; Shen, H.; Wang, Y. A phase I randomized, double-blinded, placebo-controlled study assessing the safety and pharmacokinetics of RIPK1 inhibitor GFH312 in healthy subjects. Clin. Transl. Sci. 2023, 16, 1691–1703. [Google Scholar] [CrossRef]
- Chen, R.; Hu, X.; Huang, Y.; Jiang, Y.; Chen, G.; Shan, Q.; Xu, X.; Zheng, S. Regulated Cell Death in Lenvatinib Resistance of Hepatocellular Carcinoma: From Molecular Mechanisms to Therapeutic Strategies. Int. J. Biol. Sci. 2025, 21, 2012–2026. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Chen, M.; Zeng, M. Hexavalent chromium-induced apoptosis in Hep3B cells is accompanied by calcium overload, mitochondrial damage, and AIF translocation. Ecotoxicol. Environ. Saf. 2021, 208, 111391. [Google Scholar] [CrossRef]
- Khawas, S.; Sharma, N. Cell death crosstalk in respiratory diseases: Unveiling the relationship between pyroptosis and ferroptosis in asthma and COPD. Mol. Cell. Biochem. 2025, 480, 1305–1326. [Google Scholar] [CrossRef]


| Compound | RCD Types | Target | COPD Model | Functions | Ref. |
|---|---|---|---|---|---|
| Anthrahydroquinone-2,6-disulfonate (AH2QDS) | Apoptosis | Nrf2, HO-1, NQO1↑ | SD rats | Attenuates CS-induced lung inflammation | [126] |
| Asiaticoside (AS) | Apoptosis | PGC-1α, Nrf2↑ | BEAS-2B cells | Reduces CSE-induced TNF-α, IL-6 and EMT | [122] |
| Formononetin (FMN) | Apoptosis | AhR, CYP1A1, AKT, mTOR↓ | BEAS-2B cells | Reduces CSE-induced TNF-α, IL-1β | [124] |
| Gaylussacin | Apoptosis | SIRT1↑, MMP-12↓ | Mice | Attenuates Pb/Cd-induced emphysema and lung inflammation | [127] |
| Apoptosis | SIRT1↑, MMP-12↓ | MH-S cells | Reduces Pb/Cd-induced TNF-α, IL-1β | [127] | |
| Korean Red Ginseng (KRG) | Apoptosis | Bax, Caspase 3↓; Bcl-2↑ | NCI-H292 cells | Reduces CSC-induced TNF-α, IL-1β | [123] |
| Apoptosis | Bax, Caspase 3↓; Bcl-2↑ | Mice | Attenuates CS-induced emphysema and lung inflammation | [123] | |
| N-acetyl-L-cysteine (NAC) | Apoptosis | p53, Caspase 3↓; Bcl-2, HO-1, GSH↑ | RLE-6TN cells | - | [125] |
| Apoptosis | p53, Caspase 3↓; Bcl-2, HO-1, GSH↑ | Wistar rats | Attenuates CS-induced emphysema and lung inflammation | [125] | |
| Progesterone (P4) | Apoptosis | SIRT1, PGC-1α, Nrf1↑ | Mice | Attenuates CS-induced emphysema and lung inflammation | [128] |
| Compound | RCD Types | Target | COPD Model | Functions | Ref. |
|---|---|---|---|---|---|
| GSK’547 | Necroptosis | RIPK1 kinase inhibitor | Mice | Attenuates CS-induced parenchymal inflammation, airway remodeling and emphysema | [52] |
| Theaflavin-3,3′-digallate (TF-3) | Necroptosis | p-RIPK3, p-MLKL↓ | Mice | Attenuates CS-induced emphysema and lung inflammation | [131] |
| GSK’872 | Necroptosis | RIPK3 kinase inhibitor | Mice | Attenuates CS-induced emphysema and lung inflammation | [56] |
| Necroptosis | RIPK3 kinase inhibitor | MLE-12 cells | Reduces CSE-induced TNF-α, IL-6 and cell death | [56] | |
| Necroptosis | RIPK3 kinase inhibitor | BMDMs | Reduces CSE-induced CXCL1, CXCL2, IL-6 and cell death | [51] | |
| Necrostatin-1 (NEC-1) | Necroptosis | RIPK1 kinase inhibitor | BMDMs | Reduces CSE-induced CXCL1, CXCL2, IL-6 and cell death | [51] |
| Necroptosis | RIPK1 kinase inhibitor | Mice | Suppresses the CS-induced neutrophilic airway inflammation | [121] | |
| Thymoquinone (Tq) | Necroptosis | p-MLKL, RIP-1, RIP-3↓ | BEAS-2B cells | Reduces cell death | [132] |
| Compound | RCD Types | Target | COPD Model | Functions | Ref. |
|---|---|---|---|---|---|
| Ginkgo biloba extract (GBE) | Ferroptosis | GSH, GPX4, FTH1↑; ACSL4↓ | Mice | Alleviates PM2.5-induced emphysema and airway inflammation | [141] |
| Acacetin | Ferroptosis | GSH, GPX4, SLC7A11↑; Fe2+↓ | 16HBE cells | Decreases cell death | [143] |
| Ginsenoside Rg1 | Ferroptosis | GSH, GPX4↑; Fe2+↓ | BEAS-2B cells | Reduces CSE-induced IL-6, TNF-α and IL-1β | [140] |
| Ethyl acetate fraction of Thesium chinense Turcz (TCEA) | Ferroptosis | GPX4, SLC7A11↑; ACSL4, ALOX15↓ | Mice | Attenuates CS-induced emphysema and lung inflammation | [133] |
| Fluorofenidone (AKF) | Ferroptosis | GSH, GPX4, SLC7A11↑; MDA, Fe2+↓ | BEAS-2B cells | Reduces CSE-induced IL-6, TNF-α and IL-1β | [134] |
| Ferroptosis | GSH, GPX4, SLC7A11↑; MDA, Fe2+↓ | Mice | Attenuates CS-induced emphysema, lung fibrosis and lung inflammation | [134] | |
| Sea buckthorn extract (SBE) | Ferroptosis | SLC7A11, GSH, GPX4↑; MDA, ACSL4↓ | Mice | Alleviates CS-induced emphysema and airway inflammation | [142] |
| Sodium pyruvate (NaPyr) | Ferroptosis | GSH, GPX4, NRF2↑; COX2↓ | A549 and BEAS-2B cells | Reduces CSE-induced TNF-α and IL-8 | [145] |
| Ferrostatin-1 (Fer-1) | Ferroptosis | NRF2, GPX4↑; COX2↓ | A549 and BEAS-2B cells | Reduces CSE-induced TNF-α and IL-8 | [145] |
| Ferroptosis | Fe2+, ALOX15↓ | Mice | Attenuates LPS/CS-induced airway inflammation and MUC5AC | [36] | |
| Ferroptosis | GPX4↑; COX2↓ | BEAS-2B cells | alleviates PM2.5-induced IL-6, IL-8, TNF-α | [135] | |
| Ferroptosis | GPX4, SLC7A11↑ | BEAS-2B cells | Reduces CSE-induced TNF-α, IL-6 and cell death | [136] | |
| Ferroptosis | GPX4, SLC7A11↑ | Bronchoalveolar epithelial cells (BAECs) | Reduces CSE-induced TNF-α, IL-6 and cell death | [137] | |
| Ferroptosis | GPX4↑ | 16HBE cells | Decreases cell death | [137] | |
| Ferroptosis | GPX4↑ | BEAS-2B cells | Decreases cell death | [138] | |
| Scutellarein (STR) | Ferroptosis | GPX4, NRF2↑; ALOX15↓ | Mice | Attenuates LPS/CS-induced airway inflammation and MUC5AC | [36] |
| Sodium hydrosulfide | Ferroptosis | NRF2, GPX4, NCOA4, PPAR-γ↑; COX2↓ | Mice | Alleviates PM2.5-induced emphysema and airway inflammation | [135] |
| Ferroptosis | NRF2, GPX4, NCOA4, PPAR-γ↑; COX2↓ | BEAS-2B cells | Alleviates PM2.5-induced IL-6, IL-8, TNF-α | [135] | |
| Deferoxamine (DFO) | Ferroptosis | GPX4↑, COX2↓ | BEAS-2B cells | Alleviates PM2.5-induced IL-6, IL-8, TNF-α | [135] |
| Ferroptosis | GPX4, SLC7A11↑ | BEAS-2B cells | Reduces CSE-induced TNF-α, IL-6 and cell death | [136] | |
| Ferroptosis | GPX4↑ | 16HBE cells | Decreases cell death | [62] | |
| Dihydroquercetin (DHQ) | Ferroptosis | GPX4, SLC7A11↑ | Mice | Alleviates CS-induced emphysema | [148,149] |
| Curcumin (CUR) | Ferroptosis | GPX4, SLC7A11↑ | BEAS-2B cells | Reduces CSE-induced TNF-α, IL-6 and cell death | [136] |
| Compound | RCD Types | Target | COPD Model | Functions | Ref. |
|---|---|---|---|---|---|
| Bufei Yishen Formula (BYF) | Autophagy | LC3BII↑, p62↓ | 16HBE cells | Reduces CSE-induced IL-1β, IL-6, TNF-α, MMP-2, MMP-9 | [148] |
| Autophagy | LC3BII↑ | BEAS-2B cells | Reduces CSE-induced IL-1β, IL-6, TNF-α; maintains epithelial barrier integrity | [149] | |
| Dihydromyricetin (DHM) | Autophagy | Beclin1, LC3BII↑, p62↓ | Airway organoids | Reduces mucus hypersecretion and repairs ciliary function | [150] |
| Diindolylmethane (DIM) | Autophagy | LC3BII↓, p62↑ | HEL299 cells | Reduces CdCl2-induced oxidative stress | [153] |
| Quercetin | Autophagy | p62↑; ROS, LC3BII, MDA↓ | SD rats | Alleviates CS-induced collagen deposition and airway inflammation; improves lung function | [151] |
| Vardenafil | Autophagy | Beclin1, LC3BII↑, p62↓ | Mice | Alleviates CS-induced emphysema and airway inflammation | [152] |
| Hydrogen sulfide (H2S) | Mitophagy | PINK1, Parkin↑, ROS, MDA↓ | Mice | Alleviates CS-induced emphysema and airway inflammation; improves lung function | [88] |
| Puerarin | Mitophagy | PINK1, Parkin, DRP1, FUNDC1↓ | 16HBE cells | Reduces CSE-induced cell death and restores mitochondrial function | [154] |
| Compound | RCD Types | Target | COPD Model | Functions | Ref. |
|---|---|---|---|---|---|
| Tianlong kechuanling (TL) | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | Mice | Alleviates LPS + CS + Hx-induced lung function decline, pulmonary hypertension and airway inflammation | [166] |
| Resveratrol | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓; NRF2/HO-1↑ | BEAS-2B, 16HBE and A549 cells | Reduces CSE-induced IL-18, IL-1β and cell death | [155] |
| Dihydromyricetin | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓; SLC7A11, GPX4↑ | Mice | Alleviates LPS + CS-induced lung function decline and airway inflammation | [156] |
| Disulfiram (DSF) | Pyroptosis | GSDMD-N↓ | Mice | Alleviates O3-induced emphysema and airway inflammation; maintains epithelial barrier integrity | [167] |
| Astragaloside IV | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | BEAS-2B cells | Reduces cell death | [165] |
| Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | Mice | Alleviates LPS + CS-induced emphysema and airway inflammation | [165] | |
| Propofol | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓; NRF2↑ | 16HBE cells | Ameliorates CSE-induced IL-6, TNF-α, IL-1β and reduces cell death | [168] |
| Schisandrin A (SchA) | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓; NRF2/HO-1↑ | Mice | Alleviates CS-induced emphysema and airway inflammation; improves lung function | [157] |
| Tanshinone (TS) | Pyroptosis | - | Mice | Alleviates LPS + CS + H1N1-induced lung function decline and airway inflammation | [158] |
| Pyroptosis | NLRP3 inflammasome activation↓; NF-κB signaling activation↓ | BEAS-2B and Raw264.7 cells | Alleviates LPS + CSE-induced IL-6, IL-8, TNF-α and IL-1β | [158] | |
| grape seed proanthocyanidin extract (GSPE) | Pyroptosis | - | Mice | Ameliorates lung inflammation and emphysema induced by intraperitoneal injection of CSE. | [159] |
| Pyroptosis | NLRP3 inflammasome activation↓ | RAW 264.7 cells | - | [159] | |
| Daphnetin (Daph) | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | Mice | Alleviates PM2.5/PM2.5 + CS-induced airway inflammation and hypersecretion | [160] |
| Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | BEAS-2B | Alleviates PM2.5/PM2.5 + CSE-induced IL-1β and cell death | [160] | |
| MCC950 (Also known as cRId3) | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | Mice | Alleviates PM2.5/PM2.5 + CS-induced airway inflammation | [160] |
| Pyroptosis | The NLRP3 inhibitor | 16HBE cells | Reduces CSE-induced IL-1β and TLR4 | [169] | |
| Pyroptosis | The NLRP3 inhibitor | Mice | Alleviates CS-induced airway inflammation | [170] | |
| Osthole | Pyroptosis | NLRP3 inflammasome activation↓ | 16HBE cells | Reduces CSE-induced IL-6, TNF-α and IL-1β | [161] |
| The flower buds of Tussilago farfara L. (FTF) | Pyroptosis | NLRP3 inflammasome activation↓ | Mice | Alleviates CS-induced airway inflammation | [162] |
| Hydrogen sulfide | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | SD rats | Attenuates CS-induced lung inflammation | [169] |
| Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | 16HBE cells | Reduces CSE-induced IL-1β | [169] | |
| Pyroptosis | NLRP3 inflammasome activation↓ | Mice | Attenuates CS-induced airway inflammation and emphysema | [171] | |
| Pyroptosis | NLRP3 inflammasome activation↓ | A549 cells | Alleviates PM2.5-induced IL-1β and cell death | [171] | |
| VX-765 | Pyroptosis | Specific Caspase-1 inhibitor | 16HBE cells | Alleviates PM2.5-induced IL-1β, IL-6, IL-8, CXCL-1 and CXCL-2 | [91] |
| Pyroptosis | Specific Caspase-1 inhibitor | 16HBE cells | Alleviates CSE-induced IL-1β, IL-18 and cell death | [105] | |
| Magnesium isoglycyrrhizinate (MgIG) | Pyroptosis | NLRP3 inflammasome activation↓ | Wistar rats | Alleviates LPS + CS-induced lung function decline, airway inflammation, airway remodeling and emphysema | [172] |
| Isoforskolin (ISOF) | Pyroptosis | NLRP3 inflammasome activation↓ | Mice | Alleviates CS + H1N1-induced lung function decline and airway inflammation | [164] |
| (−)-Epicatechin (EC) | Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | Rats | Alleviates CS-induced lung inflammation and emphysema | [163] |
| Pyroptosis | NLRP3 inflammasome activation↓; GSDMD-N↓ | BEAS-2B cells | Reduces CSE-induced IL-18, IL-1β and cell death | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Huang, Q.; Xie, J. Targeting Regulated Cell Death Pathways in COPD: Mechanisms and Therapeutic Strategies. Cells 2025, 14, 1874. https://doi.org/10.3390/cells14231874
Fu H, Huang Q, Xie J. Targeting Regulated Cell Death Pathways in COPD: Mechanisms and Therapeutic Strategies. Cells. 2025; 14(23):1874. https://doi.org/10.3390/cells14231874
Chicago/Turabian StyleFu, Hao, Qian Huang, and Jungang Xie. 2025. "Targeting Regulated Cell Death Pathways in COPD: Mechanisms and Therapeutic Strategies" Cells 14, no. 23: 1874. https://doi.org/10.3390/cells14231874
APA StyleFu, H., Huang, Q., & Xie, J. (2025). Targeting Regulated Cell Death Pathways in COPD: Mechanisms and Therapeutic Strategies. Cells, 14(23), 1874. https://doi.org/10.3390/cells14231874






