Reciprocal Paracrine Signaling and Dynamic Coordination of Transitional States in the Alveolar Epithelial Type 2 Cells and Associated Alveolar Lipofibroblasts During Homeostasis, Injury and Repair
Highlights
- Reciprocal epithelial–mesenchymal signaling between AT2 cells and fibroblasts governs whether the lung regenerates or progresses toward fibrosis.
- AT2/AT1 transitional states and fibroblast plasticity (LIF-iLIF-aMYF) are dynamically regulated by key pathways including AREG–EGFR, FGF10–FGFR2b, and TGF-β.
- Targetable pathways such as AREG–EGFR, PPARγ activation, LOX/PCSK9, and microRNA offer opportunities to both halt fibrosis and promote alveolar regeneration.
- WI-38 fibroblast systems and alveolosphere co-cultures provide an important human-relevant platform for screening antifibrotic and pro-regenerative drugs, supporting translational pipeline development for IPF and COPD therapies.
Abstract
1. Introduction
1.1. Progressive AT2 to AT1 Differentiation Involves the Formation of Intermediate AT2/AT1 States
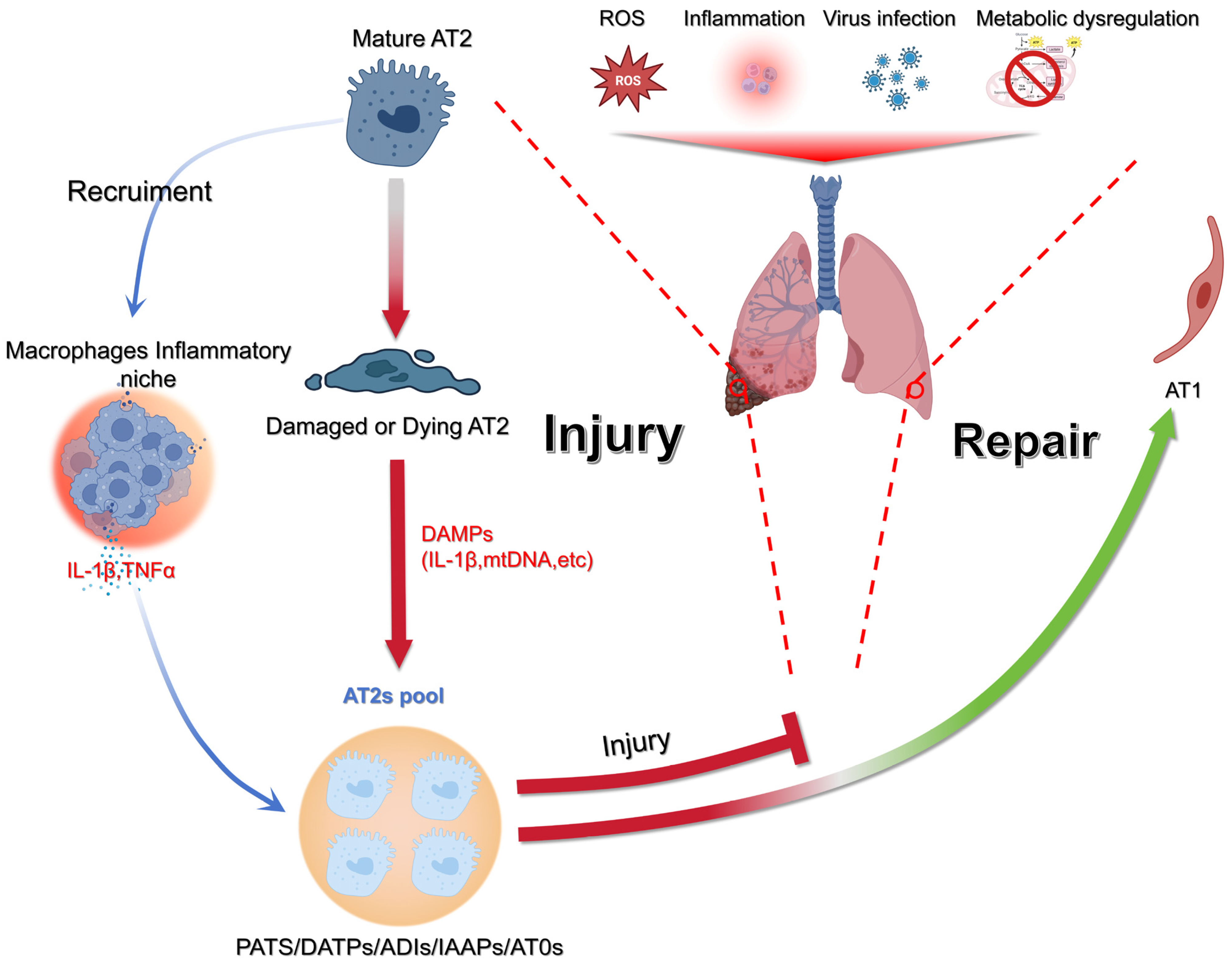
1.2. From Injury to Identity: Many Names, One Character
| Name | Markers | Reference |
|---|---|---|
| Pre-alveolar type-1 transitional cells (PATS) | Krt8, Krt18, Krt19, Cldn4, Ctgf, Sfn | [3] |
| Damage-associated transient progenitors (DATPs) | Cldn4, Krt8, Ndrg1, Sprr1a | [15] |
| Alveolar differentiation intermediates (ADIs) | Krt8+, p53, Myc, Tnfα | [5] |
| AT0 | SFTPC, AGER, SCGB3A2, SOX2 | [21] |
| RAS | SCGB3A2, SCGB1A1 | [23] |
1.3. The Chicken or the Egg Paradigm: In the End, It Is the Epithelium
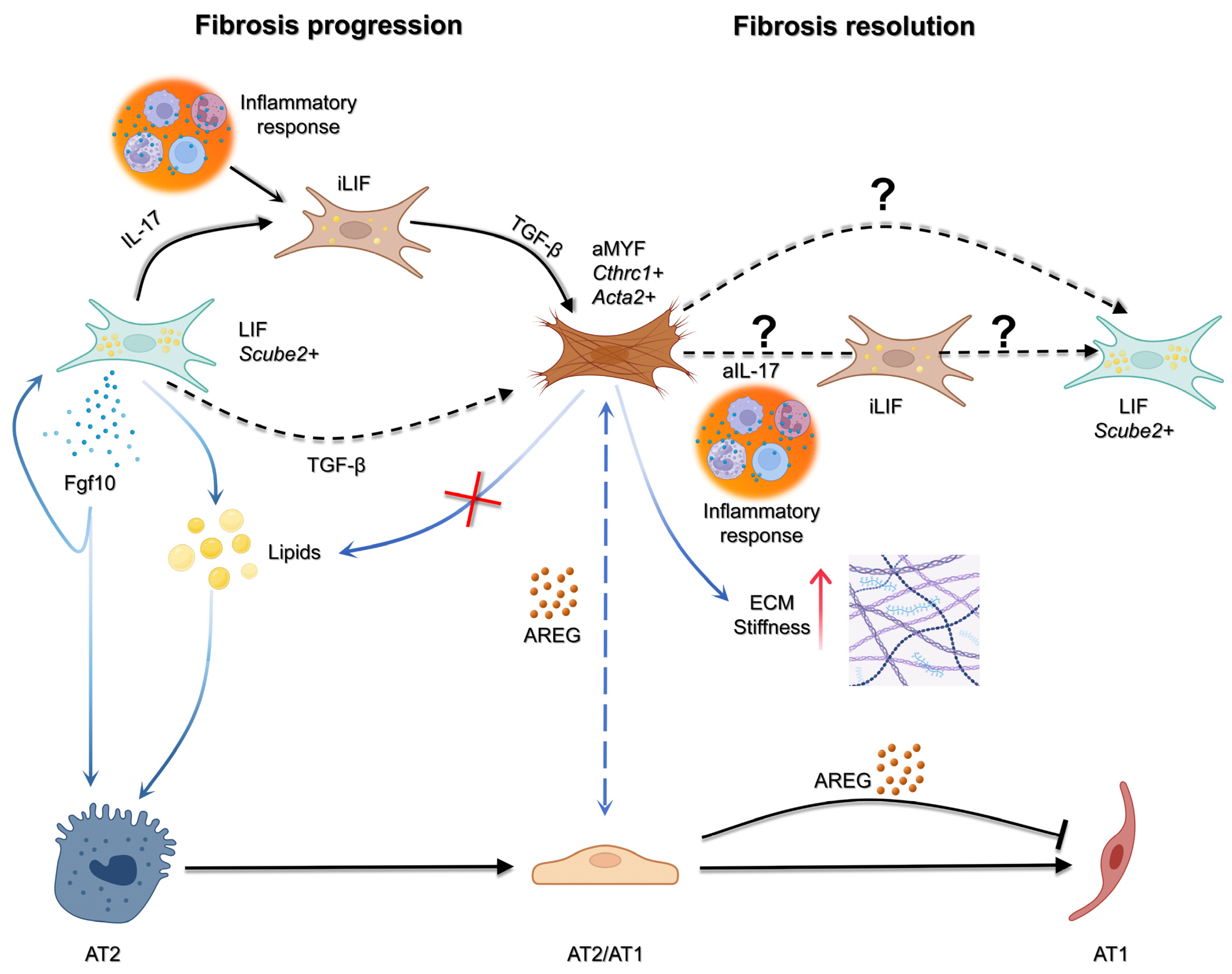
1.4. Reversible and Progressive LIF to MYF Differentiation Switch During Fibrosis Formation and Resolution
1.5. Use of WI-38 Cells to Investigate LIF to MYF Reversible Switch
1.6. WI-38 as a Screen for FDA-Approved Drugs
1.7. Investigating the Impact of Drugs to Induce LIFs
1.8. Investigating the Impact of Drugs on AT2s
1.9. Investigating AT2/LIF Dynamic Interaction Using Alveolospheres
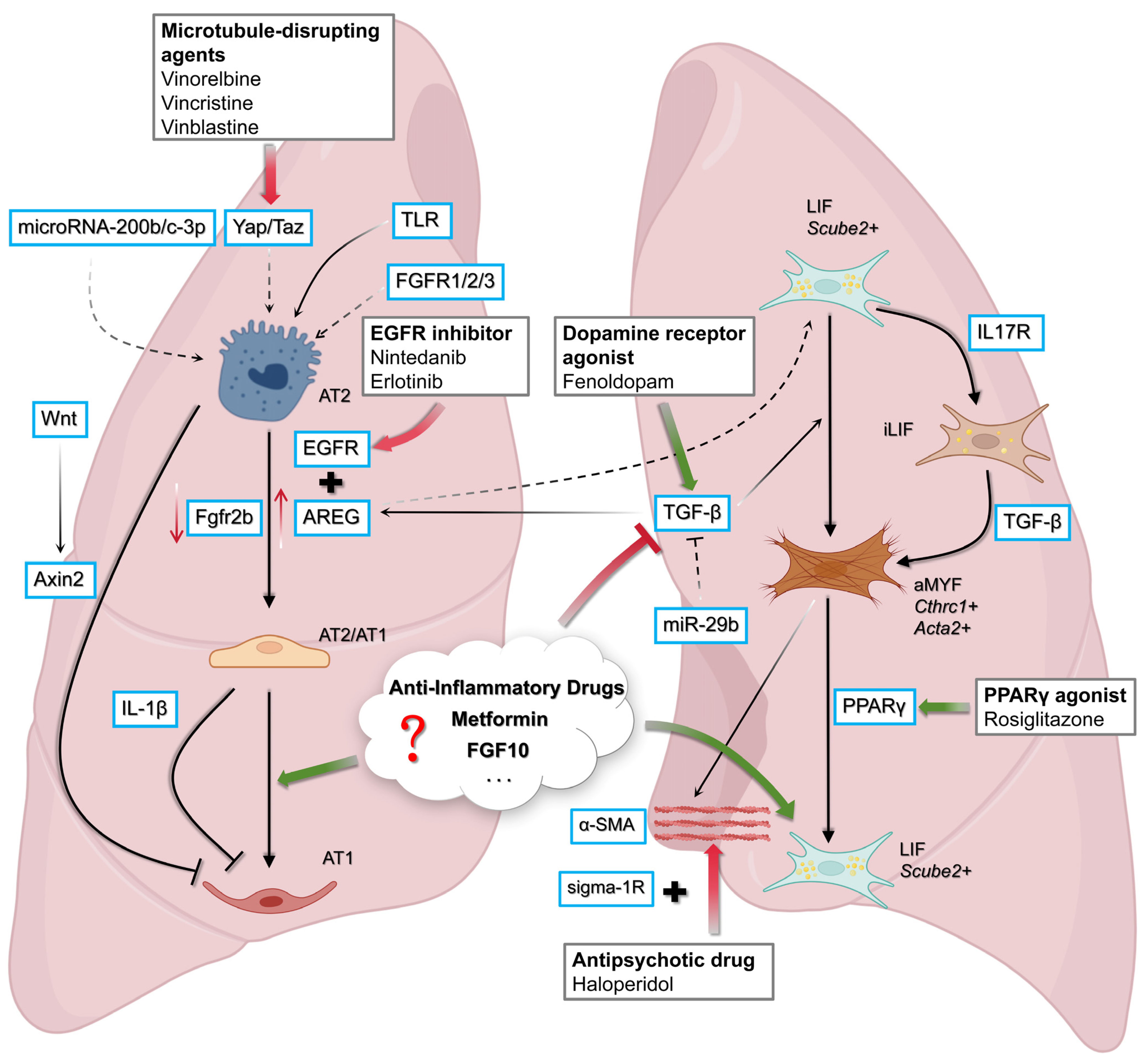
1.10. Devising Therapeutic Intervention to Resume AT2/AT1 Differentiation Towards AT1 or Reverse AT2/AT1 Differentiation Towards AT2
1.11. Nintedanib Target: AREG/EGFR Signaling Takes Central Stage
1.12. LOX and PCSK9 as Upstream Modulators of the Fibroblast–Epithelium Axis
1.13. Targeting MicroRNAs to Restore Alveolar Differentiation Dynamics
1.14. Rethinking AT1 Cell Plasticity: A Conceptual Framework
1.15. Guardians of the Alveolus: FGFR2 and the Fate of AT2 Cells
1.16. Functional Diversity of Alveolar Type II Cells: Coordinators of Regeneration and Immune Homeostasis
1.17. Killing 2 Birds with One Stone: Finding Antifibrotic and Pro-Regenerative Drugs Using the WI-38 Cell-Based Model
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTA2 | Alpha Smooth Muscle Actin |
| AEC(s) | Alveolar Epithelial Cell(s) |
| ADIs | Alveolar Differentiation Intermediates |
| AEP(s) | Alveolar Epithelial Progenitor(s) (Axin2+ AT2 subset) |
| AGER | Advanced Glycosylation End-Product Specific Receptor (AT1 marker) |
| AKT | Protein Kinase B |
| ALK5 | Activin Receptor-Like Kinase 5 (TGF-β type I receptor) |
| AREG | Amphiregulin |
| AT0 | Alveolar Type 0 transitional cell (human) |
| AT1 | Alveolar Type I epithelial cell |
| AT2 | Alveolar Type II epithelial cell |
| AT2/AT1 | Transitional AT2-to-AT1 intermediate state |
| ATAC-seq | Assay for Transposase-Accessible Chromatin using sequencing |
| AXIN2 | Axis Inhibition Protein 2 (Wnt pathway component) |
| BLM | Bleomycin (injury model) |
| BMP | Bone Morphogenetic Protein |
| CCL5 (RANTES) | Chemokine (C-C motif) Ligand 5 |
| CDC42 | Cell Division Control Protein 42 |
| CLDN4 | Claudin-4 |
| COPD | Chronic Obstructive Pulmonary Disease |
| CTGF | Connective Tissue Growth Factor |
| CTHRC1 | Collagen Triple-Helix Repeat Containing-1 (myofibroblast marker) |
| CXCL8 (IL-8) | C-X-C Motif Chemokine Ligand 8 |
| CXCL10/11 | C-X-C Motif Chemokine Ligand 10/11 |
| DAMP(s) | Damage-Associated Molecular Pattern(s) |
| DATP(s) | Damage-Associated Transient Progenitor(s) |
| DREAM | DP, RB-like, E2F and MuvB complex |
| ECM | Extracellular Matrix |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| ELN | Elastase (injury model) |
| EMT/MET | Epithelial-to-Mesenchymal Transition/Mesenchymal-to-Epithelial Transition |
| ER stress | Endoplasmic Reticulum stress |
| ERK/MAPK | Extracellular Signal-Regulated Kinase/Mitogen-Activated Protein Kinase |
| FGF10 | Fibroblast Growth Factor 10 |
| FGFR2b | Fibroblast Growth Factor Receptor 2b |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GFP | Green Fluorescent Protein |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HIF-1α | Hypoxia-Inducible Factor-1 alpha |
| HO-1 | Heme Oxygenase-1 |
| HOPX | Homeodomain Only Protein Homeobox |
| IAAP(s) | Injury-Activated Alveolar Progenitor(s) (Pd-l1+/TomLow subset) |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IFN-β | Interferon beta |
| IGFBP2 | Insulin-like Growth Factor-Binding Protein 2 |
| IL-1β | Interleukin-1 beta |
| IL-17 | Interleukin-17 |
| IPF | Idiopathic Pulmonary Fibrosis |
| ISG(s) | Interferon-Stimulated Gene(s) |
| KRT8/18/19 | Keratin-8/18/19 |
| LR-MSC(s) | Lung-Resident Mesenchymal Stem Cell(s) |
| LIF | Lipofibroblast |
| LOX | Lysyl Oxidase |
| iLIF | Inflammatory Lipofibroblast (intermediate) |
| aMYF | Activated Myofibroblast |
| MAPK14 | p38α Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein-1 (CCL2) |
| mTOR | Mammalian Target of Rapamycin |
| MRI | Magnetic Resonance Imaging |
| MDA5 | Melanoma Differentiation-Associated protein 5 |
| M-CSF | Macrophage Colony-Stimulating Factor |
| miRNA | MicroRNA |
| MRP1 | Multidrug Resistance-Associated Protein 1 |
| NDRG1 | N-Myc Downstream Regulated Gene 1 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| Notch1 | Neurogenic locus notch homolog protein 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ox-LDL | Oxidized Low-Density Lipoprotein Cholesterol |
| PAPC(s) | Primed Alveolar Progenitor Cell(s) |
| PATS | Pre-Alveolar Type-1 Transitional cell(s) |
| PCLAF (PAF/KIAA0101) | PCNA Clamp Associated Factor |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin Type 9 |
| PD-L1 | Programmed Death-Ligand 1 (see CD274) |
| PCLS | Precision-Cut Lung Slice(s) |
| PI3K | Phosphoinositide 3-Kinase |
| PLC | Phospholipase C |
| PNX | Pneumonectomy |
| PPARγ | Peroxisome Proliferator-Activated Receptor gamma |
| RAS (cell) | Respiratory Airway Secretory cell |
| RB | Retinoblastoma (family protein) |
| RLE-6TN | Rat Lung Epithelial cell line (type II) |
| RSMC(s) | Resident-Smooth Muscle Cell(s) |
| RT-qPCR | Reverse Transcription quantitative PCR |
| scRNA-seq | Single-cell RNA-sequencing |
| SCGB1A1/SCGB3A2 | Secretoglobin family 1A1/3A2 |
| SFTPB/SFTPC | Surfactant Protein B/C |
| SFK | Src-Family Kinase(s) |
| Sfn | Stratifin (14-3-3 sigma) |
| Sox2/SOX2 | SRY-Box Transcription Factor 2 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| TGF-β | Transforming Growth Factor beta |
| TLR | Toll-Like Receptor |
| TNFα | Tumor Necrosis Factor alpha |
| WI-38 | Human embryonic lung fibroblast cell line |
| Wnt/β-catenin | Wingless/β-catenin signaling pathway |
| YAP/TAZ | Yes-Associated Protein/Transcriptional co-Activator with PDZ-binding motif |
References
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, G.-D.; Vasquez-Pacheco, E.; Chu, X.; Seeger, W.; El Agha, E.; Bellusci, S.; Lingampally, A. Revisiting pulmonary fibrosis: Inflammatory dynamics of the lipofibroblast-to-inflammatory lipofibroblast-to-activated myofibroblast reversible switch. Front. Immunol. 2025, 16, 1609509. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tata, A.; Konkimalla, A.; Katsura, H.; Lee, R.F.; Ou, J.; Banovich, N.E.; Kropski, J.A.; Tata, P.R. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat. Cell Biol. 2020, 22, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.C.; Mercer, P.F. Mechanisms of Alveolar Epithelial Injury, Repair, and Fibrosis. Ann. Am. Thorac. Soc. 2015, 12, S16–S20. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Z.; Wang, G.; Geng, J.; Wu, H.; Liu, X.; Bin, E.; Sui, J.; Dai, H.; Tang, N. Sustained amphiregulin expression in intermediate alveolar stem cells drives progressive fibrosis. Cell Stem Cell 2024, 31, 1344–1358.e6. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, V.S.; Zhang, W.; Rehman, J.; Malik, A.B. Activation of Type II Cells into Regenerative Stem Cell Antigen-1+ Cells during Alveolar Repair. Am. J. Respir. Cell Mol. Biol. 2015, 53, 113–124. [Google Scholar] [CrossRef]
- Liu, Y.; Sadikot, R.T.; Adami, G.R.; Kalinichenko, V.V.; Pendyala, S.; Natarajan, V.; Zhao, Y.-Y.; Malik, A.B. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J. Exp. Med. 2011, 208, 1473–1484. [Google Scholar] [CrossRef]
- Porter, D.W.; Hubbs, A.F.; Mercer, R.R.; Robinson, V.A.; Ramsey, D.; McLaurin, J.; Khan, A.; Battelli, L.; Brumbaugh, K.; Teass, A.; et al. Pulmonary inflammation and fibrosis following subacute inhalational exposure to silica: Determinants of progression. Pathology 1993, 25, 91–99. [Google Scholar] [CrossRef]
- Fubini, B.; Fenoglio, I.; Ceschino, R.; Ghiazza, M.; Martra, G.; Tomatis, M.; Turci, F. Silica-induced pulmonary fibrosis: Pathogenesis and therapeutic opportunities. Curr. Med. Chem. 2013, 20, 2978–2994. [Google Scholar] [CrossRef]
- Liu, T.; Santos, F.G.D.L.; Ding, L.; Wu, Z.; Phan, S.H. Amphiregulin Promotes Fibroblast Activation in Pulmonary Fibrosis. FASEB J. 2016, 30, 50.6. [Google Scholar] [CrossRef]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Wan, R.; Pan, X.; Li, B.; Zhao, H.; Yang, J.; Zhao, W.; Wang, S.; Wang, Q.; et al. Single-Cell RNA Sequencing Provides New Insights into Therapeutic Roles of Thyroid Hormone in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2023, 69, 456–469. [Google Scholar] [CrossRef]
- Verheyden, J.M.; Sun, X. A transitional stem cell state in the lung. Nat. Cell Biol. 2020, 22, 1025–1026. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.-E.; Tsagkogeorga, G.; Yanagita, M.; Koo, B.-K.; Han, N.; Lee, J.-H. Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors that Mediate Alveolar Regeneration. Cell Stem Cell 2020, 27, 366–382.e7. [Google Scholar] [CrossRef]
- Riemondy, K.A.; Jansing, N.L.; Jiang, P.; Redente, E.F.; Gillen, A.E.; Fu, R.; Miller, A.J.; Spence, J.R.; Gerber, A.N.; Hesselberth, J.R.; et al. Single-cell RNA sequencing identifies TGF-β as a key regenerative cue following LPS-induced lung injury. J. Clin. Investig. 2019, 4. [Google Scholar] [CrossRef]
- Kim, B.; Huang, Y.; Ko, K.-P.; Zhang, S.; Zou, G.; Zhang, J.; Kim, M.J.; Little, D.; Ellis, L.V.; Paschini, M.; et al. PCLAF-DREAM drives alveolar cell plasticity for lung regeneration. Nat. Commun. 2024, 15, 9169. [Google Scholar] [CrossRef]
- Chong, L.; Ahmadvand, N.; Noori, A.; Lv, Y.; Chen, C.; Bellusci, S.; Zhang, J.-S. Injury activated alveolar progenitors (IAAPs): The underdog of lung repair. Cell. Mol. Life Sci. 2023, 80, 145. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, W.J.; Frank, D.B.; Zepp, J.A.; Morley, M.P.; Alkhaleel, F.A.; Kong, J.; Zhou, S.; Cantu, E.; Morrisey, E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 2018, 555, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-Q.; Dhlamini, Q.; Chen, C.; Li, X.; Bellusci, S.; Zhang, J.-S. FGF10 and Lipofibroblasts in Lung Homeostasis and Disease: Insights Gained From the Adipocytes. Front. Cell Dev. Biol. 2021, 9, 645400. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.K.L.; Sontake, V.; Tata, A.; Kobayashi, Y.; Macadlo, L.; Okuda, K.; Conchola, A.S.; Nakano, S.; Gregory, S.; Miller, L.A.; et al. Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature 2022, 604, 111–119. [Google Scholar] [CrossRef]
- Han, S.; Budinger, G.S.; Gottardi, C.J. Alveolar epithelial regeneration in the aging lung. J. Clin. Investig. 2023, 133, e170504. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Cardenas-Diaz, F.L.; Kathiriya, J.J.; Morley, M.P.; Carl, J.; Brumwell, A.N.; Katzen, J.; Slovik, K.J.; Babu, A.; Zhou, S.; et al. Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature 2022, 604, 120–126. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Huang, H.; Li, J.; Wang, Z.; Yu, Y.; Zhang, C.; Li, J.; Dai, H.; Wang, F.; et al. Pulmonary alveolar type I cell population consists of two distinct subtypes that differ in cell fate. Proc. Natl. Acad. Sci. USA 2018, 115, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Ciminieri, C.; Woest, M.E.; Reynaert, N.L.; Heijink, I.H.; Wardenaar, R.; Spierings, D.C.J.; Brandsma, C.-A.; Königshoff, M.; Gosens, R. IL-1β Induces a Proinflammatory Fibroblast Microenvironment that Impairs Lung Progenitors’ Function. Am. J. Respir. Cell Mol. Biol. 2023, 68, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Rudd, R.; Haslam, P.; Turnerwarwick, M. Cryptogenic fibrosing alveolitis—Relationships of pulmonary physiology and bronchoalveolar lavage to response to treatment and prognosis. Am. Rev. Respir. Dis. 1981, 124, 1–8. [Google Scholar]
- Turner-Warwick, M.; Burrows, B.; Johnson, A. Cryptogenic fibrosing alveolitis: Clinical features and their influence on survival. Thorax 1980, 35, 171–180. [Google Scholar] [CrossRef]
- Jones, M.R.; Lingampally, A.; Ahmadvand, N.; Chong, L.; Wu, J.; Wilhem, J.; Vazquez-Armendariz, A.I.; Ansari, M.; Herold, S.; Ornitz, D.M.; et al. FGFR2b signalling restricts lineage-flexible alveolar progenitors during mouse lung development and converges in mature alveolar type 2 cells. Cell. Mol. Life Sci. 2022, 79, 609. [Google Scholar] [CrossRef]
- Ahmadvand, N.; Lingampally, A.; Khosravi, F.; Vazquez-Armendariz, A.I.; Rivetti, S.; Jones, M.R.; Wilhelm, J.; Herold, S.; Barreto, G.; Koepke, J.; et al. Fgfr2b signaling is essential for the maintenance of the alveolar epithelial type 2 lineage during lung homeostasis in mice. Cell. Mol. Life Sci. 2022, 79, 302. [Google Scholar] [CrossRef]
- Lv, Y.-Q.; Cai, G.-F.; Zeng, P.-P.; Dhlamini, Q.; Chen, L.-F.; Chen, J.-J.; Lyu, H.-D.; Mossahebi-Mohammadi, M.; Ahmadvand, N.; Bellusci, S.; et al. FGF10 Therapeutic Administration Promotes Mobilization of Injury-Activated Alveolar Progenitors in a Mouse Fibrosis Model. Cells 2022, 11, 2396. [Google Scholar] [CrossRef]
- Yuan, T.; Volckaert, T.; Redente, E.F.; Hopkins, S.; Klinkhammer, K.; Wasnick, R.; Chao, C.-M.; Yuan, J.; Zhang, J.-S.; Yao, C.; et al. FGF10-FGFR2B Signaling Generates Basal Cells and Drives Alveolar Epithelial Regeneration by Bronchial Epithelial Stem Cells after Lung Injury. Stem Cell Rep. 2019, 12, 1041–1055. [Google Scholar] [CrossRef]
- Dorry, S.J.; Ansbro, B.O.; Ornitz, D.M.; Mutlu, G.M.; Guzy, R.D. FGFR2 Is Required for AEC2 Homeostasis and Survival after Bleomycin-induced Lung Injury. Am. J. Respir. Cell Mol. Biol. 2020, 62, 608–621. [Google Scholar] [CrossRef]
- Günther, A.; Lübke, N.; Ermert, M.; Schermuly, R.T.; Weissmann, N.; Breithecker, A.; Markart, P.; Ruppert, C.; Quanz, K.; Ermert, L.; et al. Prevention of Bleomycin-induced Lung Fibrosis by Aerosolization of Heparin or Urokinase in Rabbits. Am. J. Respir. Crit. Care Med. 2003, 168, 1358–1365. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Huang, H.; Hu, Y.; Fu, S.; Wang, Z.; Shi, M.; Zhao, X.; Yuan, J.; Li, J.; et al. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell 2020, 180, 107–121.e17. [Google Scholar] [CrossRef]
- Umbayev, B.; Safarova, Y.; Yermekova, A.; Nessipbekova, A.; Syzdykova, A.; Askarova, S. Role of a small GTPase Cdc42 in aging and age-related diseases. Biogerontology 2023, 24, 27–46. [Google Scholar] [CrossRef] [PubMed]
- El Agha, E.; Moiseenko, A.; Kheirollahi, V.; De Langhe, S.; Crnkovic, S.; Kwapiszewska, G.; Szibor, M.; Kosanovic, D.; Schwind, F.; Schermuly, R.T.; et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 2017, 20, 261–273.e3. [Google Scholar] [CrossRef]
- Lingampally, A.; Truchi, M.; Mauduit, O.; Delcroix, V.; Vasquez-Pacheco, E.; Gautier-Isola, M.; Chu, X.; Khadim, A.; Chao, C.-M.; Zabihi, M.; et al. Evidence for a lipofibroblast-to-Cthrc1+myofibroblast reversible switch during the development and resolution of lung fibrosis in young mice. Eur. Respir. J. 2024, 65, 2300482. [Google Scholar] [CrossRef] [PubMed]
- Lingampally, A.; Truchi, M.; Shi, X.; Zhou, Y.; Vasquez-Pacheco, E.; Panagiotidis, G.; Hadzic, S.; Koepke, J.; Vazquez-Armendariz, A.I.; Herold, S.; et al. Unraveling Alveolar Fibroblast and Activated Myofibroblast Heterogeneity and Differentiation Trajectories During Lung Fibrosis Development and Resolution in Young and Old Mice. Aging Cell 2025, 24, e14503. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Wolters, P.J.; Sheppard, D. Alveolar fibroblast lineage orchestrates lung inflammation and fibrosis. Nature 2024, 631, 627–634. [Google Scholar] [CrossRef]
- Vásquez-Pacheco, E.; Marega, M.; Lingampally, A.; Fassy, J.; Truchi, M.; Goth, K.; Trygub, L.; Taghizadeh, S.; Bartkuhn, M.; Alexopoulos, I.; et al. Highlighting fibroblast plasticity in lung fibrosis: The WI-38 cell line as a model for investigating the myofibroblast and lipofibroblast switch. Theranostics 2024, 14, 3603–3622. [Google Scholar] [CrossRef]
- Kheirollahi, V.; Wasnick, R.M.; Biasin, V.; Vazquez-Armendariz, A.I.; Chu, X.; Moiseenko, A.; Weiss, A.; Wilhelm, J.; Zhang, J.-S.; Kwapiszewska, G.; et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat. Commun. 2019, 10, 2987. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Fuentes, H.A.; Barreto, G.; Al-Suhaimi, E.A.; Liehn, E.A. Fibroblast plasticity in pulmonary and cardiovascular fibrosis: Mechanistic insights and emerging therapeutic targets. Arch. Med. Res. 2025, 56, 103173. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Vodret, S.; Braga, L.; Guarnaccia, C.; Celsi, F.; Rossetti, G.; Martinelli, V.; Battini, T.; Long, C.; Vukusic, K.; et al. High-throughput screening discovers antifibrotic properties of haloperidol by hindering myofibroblast activation. J. Clin. Investig. 2019, 4, e123987. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Liu, J.; Pan, T.; Wang, Q.; Miao, K.; Xu, Y.; Xiong, W.; Yu, J. Dopamine receptor agonists ameliorate bleomycin-induced pulmonary fibrosis by repressing fibroblast differentiation and proliferation. Biomed. Pharmacother. 2021, 139, 111500. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.A.; Daugherty, L.E.; Thatcher, T.H.; Lakatos, H.F.; Ray, D.M.; Redonnet, M.; Phipps, R.P.; Sime, P.J. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: Implications for therapy of lung fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2005, 288, L1146–L1153. [Google Scholar] [CrossRef]
- Rehan, V.K.; Wang, Y.; Patel, S.; Santos, J.; Torday, J.S. Rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr. Pulmonol. 2006, 41, 558–569. [Google Scholar] [CrossRef]
- Gan, C.; Zhang, Q.; Liu, H.; Wang, G.; Wang, L.; Li, Y.; Tan, Z.; Yin, W.; Yao, Y.; Xie, Y.; et al. Nifuroxazide ameliorates pulmonary fibrosis by blocking myofibroblast genesis: A drug repurposing study. Respir. Res. 2022, 23, 32. [Google Scholar] [CrossRef]
- Gupta, P.; Ashar, Y.V.; Ashby, C.R.; Lin, L.; Chen, Z.-S. The Oncogenic Protein, Breakpoint Cluster (BCR)-Abelson Kinase (ABL) and Chronic Myelocytic Leukemia (CML): Insight Into the Drug Resistance Mechanisms and Approaches for Targeting BCR-ABL in CML. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 312–328. [Google Scholar] [CrossRef]
- Portugal, C.C.; Almeida, T.O.; Socodato, R.; Relvas, J.B. Src family kinases (SFKs): Critical regulators of microglial homeostatic functions and neurodegeneration in Parkinson’s and Alzheimer’s diseases. FEBS J. 2021, 289, 7760–7775. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Ahangari, F.; Becker, C.; Foster, D.G.; Chioccioli, M.; Nelson, M.; Beke, K.; Wang, X.; Justet, A.; Adams, T.; Readhead, B.; et al. Saracatinib, a Selective Src Kinase Inhibitor, Blocks Fibrotic Responses in Preclinical Models of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2022, 206, 1463–1479. [Google Scholar] [CrossRef]
- Feng, L.; Li, W.; Chao, Y.; Huan, Q.; Lu, F.; Yi, W.; Jun, W.; Binbin, C.; Na, L.; Shougang, Z. Synergistic Inhibition of Renal Fibrosis by Nintedanib and Gefitinib in a Murine Model of Obstructive Nephropathy. Kidney Dis. 2020, 7, 34–49. [Google Scholar] [CrossRef]
- Willis, B.C.; Liebler, J.M.; Luby-Phelps, K.; Nicholson, A.G.; Crandall, E.D.; du Bois, R.M.; Borok, Z. Induction of Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells by Transforming Growth Factor-β1. Am. J. Pathol. 2005, 166, 1321–1332. [Google Scholar] [CrossRef]
- Higashiyama, H.; Yoshimoto, D.; Kaise, T.; Matsubara, S.; Fujiwara, M.; Kikkawa, H.; Asano, S.; Kinoshita, M. Inhibition of activin receptor-like kinase 5 attenuates Bleomycin-induced pulmonary fibrosis. Exp. Mol. Pathol. 2007, 83, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018, 359, 1118–1123. [Google Scholar] [CrossRef]
- McGowan, S.E. Retinoids and pulmonary alveolar regeneration: Rationale and therapeutic challenges. Drug Discov. Today Dis. Mech. 2006, 3, 77–84. [Google Scholar] [CrossRef]
- Nabeyrat, E.; Besnard, V.; Corroyer, S.; Cazals, V.; Clement, A. Retinoic acid-induced proliferation of lung alveolar epithelial cells: Relation with the IGF system. Am. J. Physiol. Cell. Mol. Physiol. 1998, 275, L71–L79. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Choi, E.-H.; Kim, Y.; Kim, M.; Choi, D.; Kim, W.; Cha, B. Identification of YAP regulators through high-throughput screening and NanoBiT-based validation-drug repositioning for cancer therapy. Anim. Cells Syst. 2025, 29, 325–338. [Google Scholar] [CrossRef]
- LaCanna, R.; Liccardo, D.; Zhang, P.; Tragesser, L.; Wang, Y.; Cao, T.; Chapman, H.A.; Morrisey, E.E.; Shen, H.; Koch, W.J.; et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Investig. 2019, 129, 2107–2122. [Google Scholar] [CrossRef]
- Shoyab, M.; McDonald, V.L.; Bradley, J.G.; Todaro, G.J. Amphiregulin: A bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc. Natl. Acad. Sci. USA 1988, 85, 6528–6532. [Google Scholar] [CrossRef] [PubMed]
- Avraham, R.; Yarden, Y. Feedback regulation of EGFR signalling: Decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 2011, 12, 104–117. [Google Scholar] [CrossRef]
- Berasain, C.; Castillo, J.; Perugorría, M.; Prieto, J.; Avila, M. Amphiregulin: A new growth factor in hepatocarcinogenesis. Cancer Lett. 2007, 254, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Busser, B.; Sancey, L.; Brambilla, E.; Coll, J.-L.; Hurbin, A. The multiple roles of amphiregulin in human cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2011, 1816, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Avila, M.A. Amphiregulin. Semin. Cell Dev. Biol. 2014, 28, 31–41. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, J.-Y.; Lee, C.-M.; Cho, W.-K.; Kang, M.-J.; Koff, J.L.; Yoon, P.-O.; Chae, J.; Park, H.-O.; Elias, J.A.; et al. Amphiregulin, an Epidermal Growth Factor Receptor Ligand, Plays an Essential Role in the Pathogenesis of Transforming Growth Factor-β-induced Pulmonary Fibrosis. J. Biol. Chem. 2012, 287, 41991–42000. [Google Scholar] [CrossRef]
- Kindermann, M.; Knipfer, L.; Atreya, I.; Wirtz, S. ILC2s in infectious diseases and organ-specific fibrosis. Semin. Immunopathol. 2018, 40, 379–392. [Google Scholar] [CrossRef]
- Nakagome, K.; Nagata, M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx 2011, 38, 555–563. [Google Scholar] [CrossRef]
- Richeldi, L.; Kreuter, M.; Selman, M.; Crestani, B.; Kirsten, A.-M.; AWuyts, W.; Xu, Z.; Bernois, K.; Stowasser, S.; Quaresma, M.; et al. Long-term treatment of patients with idiopathic pulmonary fibrosis with nintedanib: Results from the TOMORROW trial and its open-label extension. Thorax 2017, 73, 581–583. [Google Scholar] [CrossRef]
- da Silva, R.F.; Dhar, D.; Raina, K.; Kumar, D.; Kant, R.; Cagnon, V.H.A.; Agarwal, C.; Agarwal, R. Nintedanib inhibits growth of human prostate carcinoma cells by modulating both cell cycle and angiogenesis regulators. Sci. Rep. 2018, 8, 9540. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Yao, F.; Huang, C. Lysyl oxidase family proteins: Prospective therapeutic targets in cancer. Int. J. Mol. Sci. 2022, 23, 12270. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, P.A.; Jones, C.M.; Rotile, N.J.; Koerner, S.K.; Ferreira, D.S.; Chen, H.H.; Probst, C.K.; Tager, A.M.; Caravan, P. Molecular magnetic resonance imaging of lung fibrogenesis with an oxyamine-based probe. Angew. Chem. Int. Ed. Engl. 2017, 56, 9825–9828. [Google Scholar] [CrossRef]
- Haak, A.J.; Tan, Q.; Tschumperlin, D.J. Matrix biomechanics and dynamics in pulmonary fibrosis. Matrix Biol. 2018, 73, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.E.; Alsafadi, H.N.; Mitash, N.; Justet, A.; Hu, Q.; Pineda, R.; Staab-Weijnitz, C.; Korfei, M.; Gvazava, N.; Wannemo, K.; et al. Inhibition of epithelial cell YAP–TEAD/LOX signaling attenuates pulmonary fibrosis in preclinical models. Nat. Commun. 2025, 16, 7099. [Google Scholar] [CrossRef]
- Lin, J.; Pan, Z.; Sun, J.; Wang, X.; Yin, D.; Huo, C.; Guo, Q. PCSK9 inhibitor alleviates experimental pulmonary fibrosis–induced pulmonary hypertension via attenuating epithelial–mesenchymal transition by suppressing Wnt/β-catenin signaling in vivo and in vitro. Front. Med. 2024, 11, 1509168. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Theus, S.; Deng, X.; Fan, Y.; Zhou, S.; Mehta, J.L. PCSK9 regulates expression of scavenger receptors and ox-LDL uptake in macrophages. Cardiovasc. Res. 2018, 114, 1145–1153. [Google Scholar] [CrossRef]
- Cui, C.; Yan, A.; Huang, S.; Chen, Y.; Zhao, J.; Li, C.; Wang, X.; Yang, J. PCSK9 manipulates lipid metabolism and the immune microenvironment in cancer. Onco Targets Ther. 2025, 18, 411–427. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Prattichizzo, F.; Marfella, R.; Sardu, C.; Martino, E.; Scisciola, L.; Marfella, L.; Grotta, R.; Frigé, C.; Paolisso, G.; et al. SIRT3 mediates the effects of PCSK9 inhibitors on inflammation, autophagy, and oxidative stress in endothelial cells. Theranostics 2023, 13, 531–542. [Google Scholar] [CrossRef]
- Mehta, J.L.; Sanada, N.; Hu, C.P.; Chen, J.; Dandapat, A.; Sugawara, F.; Satoh, H.; Inoue, K.; Kawase, Y.; Jishage, K.; et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed a high-cholesterol diet. Circ. Res. 2007, 100, 1634–1642. [Google Scholar] [CrossRef]
- Pandit, K.V.; Milosevic, J. MicroRNA regulatory networks in idiopathic pulmonary fibrosis. Biochem. Cell Biol. 2015, 93, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.V.; Schwartz, D.A. Epigenetic Control of Gene Expression in the Lung. Am. J. Respir. Crit. Care Med. 2011, 183, 1295–1301. [Google Scholar] [CrossRef]
- Carraro, G.; El-Hashash, A.; Guidolin, D.; Tiozzo, C.; Turcatel, G.; Young, B.M.; De Langhe, S.P.; Bellusci, S.; Shi, W.; Parnigotto, P.P.; et al. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev. Biol. 2009, 333, 238–250. [Google Scholar] [CrossRef]
- Subramanyam, D.; Lamouille, S.; Judson, R.L.; Liu, J.Y.; Bucay, N.; Derynck, R.; Blelloch, R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 443–448. [Google Scholar] [CrossRef]
- Ali, M.; Zhang, X.; LaCanna, R.; Tomar, D.; Elrod, J.W.; Tian, Y. MICU1-dependent mitochondrial calcium uptake regulates lung alveolar type 2 cell plasticity and lung regeneration. J. Clin. Investig. 2022, 7, e154447. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Lian, X.; Zhu, Z.; Chen, X.; Pei, W.; Li, S.; Abbas, A.; Wang, Y.; Tian, L. MicroRNA-29b Mediates Lung Mesenchymal-Epithelial Transition and Prevents Lung Fibrosis in the Silicosis Model. Mol. Ther.-Nucleic Acids 2019, 14, 20–31. [Google Scholar] [CrossRef]
- Castaldi, A.; Horie, M.; Rieger, M.E.; Dubourd, M.; Sunohara, M.; Pandit, K.; Zhou, B.; Offringa, I.A.; Marconett, C.N.; Borok, Z. Genome-wide integration of microRNA and transcriptomic profiles of differentiating human alveolar epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L173–L184. [Google Scholar] [CrossRef]
- Dehmel, S.; Weiss, K.J.; El-Merhie, N.; Callegari, J.; Konrad, B.; Mutze, K.; Eickelberg, O.; Königshoff, M.; Krauss-Etschmann, S. microRNA Expression Profile of Purified Alveolar Epithelial Type II Cells. Genes 2022, 13, 1420. [Google Scholar] [CrossRef]
- Oglesby, I.K.; Vencken, S.F.; Agrawal, R.; Gaughan, K.; Molloy, K.; Higgins, G.; McNally, P.; McElvaney, N.G.; Mall, M.A.; Greene, C.M. miR-17 overexpression in cystic fibrosis airway epithelial cells decreases interleukin-8 production. Eur. Respir. J. 2015, 46, 1350–1360. [Google Scholar] [CrossRef]
- Guo, W.; Benlhabib, H.; Mendelson, C.R. The MicroRNA 29 Family Promotes Type II Cell Differentiation in Developing Lung. Mol. Cell. Biol. 2016, 36, 2141. [Google Scholar] [CrossRef]
- Moimas, S.; Salton, F.; Kosmider, B.; Ring, N.; Volpe, M.C.; Bahmed, K.; Braga, L.; Rehman, M.; Vodret, S.; Graziani, M.L.; et al. miR-200 family members reduce senescence and restore idiopathic pulmonary fibrosis type II alveolar epithelial cell transdifferentiation. ERJ Open Res. 2019, 5, 00138–02019. [Google Scholar] [CrossRef]
- Callaway, D.A.; Penkala, I.J.; Zhou, S.; Knowlton, J.J.; Cardenas-Diaz, F.; Babu, A.; Morley, M.P.; Lopes, M.; Garcia, B.A.; Morrisey, E.E. TGF-β controls alveolar type 1 epithelial cell plasticity and alveolar matrisome gene transcription in mice. J. Clin. Investig. 2024, 134, e172095. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Kolliputi, N.; Wang, Y.; Gou, D.; Chintagari, N.R.; Liu, L. Trans-differentiation of Alveolar Epithelial Type II Cells to Type I Cells Involves Autocrine Signaling by Transforming Growth Factor β1 through the Smad Pathway. J. Biol. Chem. 2007, 282, 3968–3976. [Google Scholar] [CrossRef]
- Zhao, L.; Yee, M.; O’Reilly, M.A. Transdifferentiation of alveolar epithelial type II to type I cells is controlled by opposing TGF-β and BMP signaling. Am. J. Physiol. Cell. Mol. Physiol. 2013, 305, L409–L418. [Google Scholar] [CrossRef]
- Xiao, J.; Meng, X.-M.; Huang, X.R.; Chung, A.C.; Feng, Y.-L.; Hui, D.S.; Yu, C.-M.; Sung, J.J.; Lan, H.Y. miR-29 Inhibits Bleomycin-induced Pulmonary Fibrosis in Mice. Mol. Ther. 2012, 20, 1251–1260. [Google Scholar] [CrossRef]
- Juul, N.H.; Yoon, J.-K.; Martinez, M.C.; Rishi, N.; Kazadaeva, Y.I.; Morri, M.; Neff, N.F.; Trope, W.L.; Shrager, J.B.; Sinha, R.; et al. KRAS(G12D) drives lepidic adenocarcinoma through stem-cell reprogramming. Nature 2023, 619, 860–867. [Google Scholar] [CrossRef]
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B.L.M. Fibroblast Growth Factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124, 4867–4878. [Google Scholar] [CrossRef]
- Lebeche, D.; Malpel, S.; Cardoso, W.V. Fibroblast growth factor interactions in the developing lung. Mech. Dev. 1999, 86, 125–136. [Google Scholar] [CrossRef]
- Chang, D.R.; Alanis, D.M.; Miller, R.K.; Ji, H.; Akiyama, H.; McCrea, P.D.; Chen, J. Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 18042–18051. [Google Scholar] [CrossRef]
- Abler, L.L.; Mansour, S.L.; Sun, X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev. Dyn. 2009, 238, 1999–2013. [Google Scholar] [CrossRef]
- MacKenzie, B.; Henneke, I.; Hezel, S.; Al Alam, D.; El Agha, E.; Chao, C.-M.; Quantius, J.; Wilhelm, J.; Jones, M.; Goth, K.; et al. Attenuating endogenous Fgfr2b ligands during bleomycin-induced lung fibrosis does not compromise murine lung repair. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L1014–L1024. [Google Scholar] [CrossRef]
- Brownfield, D.G.; de Arce, A.D.; Ghelfi, E.; Gillich, A.; Desai, T.J.; Krasnow, M.A. Alveolar cell fate selection and lifelong maintenance of AT2 cells by FGF signaling. Nat. Commun. 2022, 13, 7137. [Google Scholar] [CrossRef]
- AMiura, T.; Holmes, K.V. Host-pathogen interactions during coronavirus infection of primary alveolar epithelial cells. J. Leukoc. Biol. 2009, 86, 1145–1151. [Google Scholar] [CrossRef]
- Chuquimia, O.D.; Petursdottir, D.H.; Rahman, M.J.; Hartl, K.; Singh, M.; Fernández, C. The Role of Alveolar Epithelial Cells in Initiating and Shaping Pulmonary Immune Responses: Communication between Innate and Adaptive Immune Systems. PLoS ONE 2012, 7, e32125. [Google Scholar] [CrossRef]
- Striz, I.; Brabcova, E.; Kolesar, L.; Liu, X.; Brabcova, I.; Sekerkova, A.; Poole, J.; Jaresova, M.; Slavcev, A.; Rennard, S. Epithelial cells modulate genes associated with NF kappa B activation in co-cultured human macrophages. Immunobiology 2011, 216, 1110–1116. [Google Scholar] [CrossRef][Green Version]
- Droemann, D.; Goldmann, T.; Branscheid, D.; Clark, R.; Dalhoff, K.; Zabel, P.; Vollmer, E. Toll-like receptor 2 is expressed by alveolar epithelial cells type II and macrophages in the human lung. Histochem. Cell Biol. 2003, 119, 103–108. [Google Scholar] [CrossRef]
- Beppu, A.K.; Zhao, J.; Yao, C.; Carraro, G.; Israely, E.; Coelho, A.L.; Drake, K.; Hogaboam, C.M.; Parks, W.C.; Kolls, J.K.; et al. Epithelial plasticity and innate immune activation promote lung tissue remodeling following respiratory viral infection. Nat. Commun. 2023, 14, 5814. [Google Scholar] [CrossRef]
- Froom, Z.S.; Callaghan, N.I.; Huyer, L.D. Cellular crosstalk in fibrosis: Insights into macrophage and fibroblast dynamics. J. Biol. Chem. 2025, 301, 110203. [Google Scholar] [CrossRef]
- Keenum, M.C.; Chatterjee, P.; Atalis, A.; Pandey, B.; Jimenez, A.; Roy, K. Single-cell epitope-transcriptomics reveal lung stromal and immune cell response kinetics to nanoparticle-delivered RIG-I and TLR4 agonists. Biomaterials 2023, 297, 122097. [Google Scholar] [CrossRef]
- Yang, N.; Luna, J.M.; Dai, P.; Wang, Y.; Rice, C.M.; Deng, L. Lung type II alveolar epithelial cells collaborate with CCR2+ inflammatory monocytes in host defense against poxvirus infection. Nat. Commun. 2022, 13, 1671. [Google Scholar] [CrossRef]
- Chuquimia, O.D.; Petursdottir, D.H.; Periolo, N.; Fernández, C. Alveolar Epithelial Cells Are Critical in Protection of the Respiratory Tract by Secretion of Factors Able To Modulate the Activity of Pulmonary Macrophages and Directly Control Bacterial Growth. Infect. Immun. 2013, 81, 381–389. [Google Scholar] [CrossRef]
- Qian, Z.; Travanty, E.A.; Oko, L.; Edeen, K.; Berglund, A.; Wang, J.; Ito, Y.; Holmes, K.V.; Mason, R.J. Innate Immune Response of Human Alveolar Type II Cells Infected with Severe Acute Respiratory Syndrome–Coronavirus. Am. J. Respir. Cell Mol. Biol. 2013, 48, 742–748. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Hussain, S.; Zheng, Y.; Sanjabi, S.; Ouaaz, F.; ABeg, A. Distinct Roles of Different NF-κB Subunits in Regulating Inflammatory and T Cell Stimulatory Gene Expression in Dendritic Cells. J. Immunol. 2007, 178, 6777–6788. [Google Scholar] [CrossRef]
- Ahmadvand, N.; Khosravi, F.; Lingampally, A.; Wasnick, R.; Vazquez-Armendariz, A.I.; Carraro, G.; Heiner, M.; Rivetti, S.; Lv, Y.; Wilhelm, J.; et al. Identification of a novel subset of alveolar type 2 cells enriched in PD-L1 and expanded following pneumonectomy. Eur. Respir. J. 2021, 58, 2004168. [Google Scholar] [CrossRef]
- Xu, X.; Rock, J.R.; Lu, Y.; Futtner, C.; Schwab, B.; Guinney, J.; Hogan, B.L.M.; Onaitis, M.W. Evidence for type II cells as cells of origin of K-Ras–induced distal lung adenocarcinoma. Proc. Natl. Acad. Sci. USA 2012, 109, 4910–4915. [Google Scholar] [CrossRef]
- Sutherland, K.D.; Berns, A. Cell of origin of lung cancer. Mol. Oncol. 2010, 4, 397–403. [Google Scholar] [CrossRef]
- Lin, C.; Song, H.; Huang, C.; Yao, E.; Gacayan, R.; Xu, S.-M.; Chuang, P.-T. Alveolar Type II Cells Possess the Capability of Initiating Lung Tumor Development. PLoS ONE 2012, 7, e53817. [Google Scholar] [CrossRef]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef]
- Tong, L.; Zhou, J.; Rong, L.; Seeley, E.J.; Pan, J.; Zhu, X.; Liu, J.; Wang, Q.; Tang, X.; Qu, J.; et al. Fibroblast Growth Factor-10 (FGF-10) Mobilizes Lung-resident Mesenchymal Stem Cells and Protects Against Acute Lung Injury. Sci. Rep. 2016, 6, 21642. [Google Scholar] [CrossRef]
- Chu, X.; Lingampally, A.; Moiseenko, A.; Kheirollahi, V.; Vazquez-Armendariz, A.I.; Koepke, J.; Khadim, A.; Kiliaris, G.; Felordi, M.S.; Zabihi, M.; et al. GLI1+ cells are a source of repair-supportive mesenchymal cells (RSMCs) during airway epithelial regeneration. Cell. Mol. Life Sci. 2022, 79, 581. [Google Scholar] [CrossRef]
- Teague, T.T.; Payne, S.R.; Kelly, B.T.; Dempsey, T.M.; McCoy, R.G.; Sangaralingham, L.R.; Limper, A.H. Evaluation for clinical benefit of metformin in patients with idiopathic pulmonary fibrosis and type 2 diabetes mellitus: A national claims-based cohort analysis. Respir. Res. 2022, 23, 91. [Google Scholar] [CrossRef]
- Jovanovic, D.; Šterclová, M.; Mogulkoc, N.; Lewandowsk, K.; Müller, V.; Hájková, M.; Kramer, M.; Tekavec-Trkanjec, J.; Studnicka, M.; Stoeva, N.; et al. The effect of metformin on clinically relevant outcomes in 3,144 IPF patients from the EMPIRE registry. Eur. Respir. J. 2020, 56 (Suppl. S64), 1791. [Google Scholar] [CrossRef]
- Kahnert, K.; Andreas, S.; Kellerer, C.; Lutter, J.I.; Lucke, T.; Yildirim, Ö.; Lehmann, M.; Seissler, J.; Behr, J.; Frankenberger, M.; et al. Reduced decline of lung diffusing capacity in COPD patients with diabetes and metformin treatment. Sci. Rep. 2022, 12, 1435. [Google Scholar] [CrossRef]
- Zhu, A.; Teng, Y.; Ge, D.; Zhang, X.; Hu, M.; Yao, X. Role of metformin in treatment of patients with chronic obstructive pulmonary disease: A systematic review. J. Thorac. Dis. 2019, 11, 4371–4378. [Google Scholar] [CrossRef]
- Tao, F.; Zhou, Y.; Wang, M.; Wang, C.; Zhu, W.; Han, Z.; Sun, N.; Wang, D. Metformin alleviates chronic obstructive pulmonary disease and cigarette smoke extract-induced glucocorticoid resistance by activating the nuclear factor E2-related factor 2/heme oxygenase-1 signaling pathway. Korean J. Physiol. Pharmacol. 2022, 26, 95–111. [Google Scholar] [CrossRef]
- Antunes, M.A.; Rocco, P.R. Elastase-induced pulmonary emphysema: Insights from experimental models. An. Acad. Bras. Cienc. 2011, 83, 1385–1396. [Google Scholar] [CrossRef]
- Snider, G.L.; Lucey, E.C.; Stone, P.J. Animal Models of Emphysema1–3. Am. Rev. Respir. Dis. 1986, 133, 149–169. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Königshoff, M.; Kneidinger, N.; Eickelberg, O. TGF-ß signaling in COPD: Deciphering genetic and cellular susceptibilities for future therapeutic regimen. Swiss Med Wkly. 2009, 139, 554–563. [Google Scholar] [CrossRef]
- Kang, H.-R.; Lee, J.-Y.; Lee, C.G. TGF-β1 as a therapeutic target for pulmonary fibrosis and COPD. Expert Rev. Clin. Pharmacol. 2008, 1, 547–558. [Google Scholar] [CrossRef]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Morita, A.; Ishii, M.; Asakura, T.; Yotsukura, M.; Hegab, A.E.; Kusumoto, T.; Namkoong, H.; Ogawa, T.; Nakatake, Y.; Oda, M.; et al. Direct reprogramming of mouse fibroblasts into self-renewable alveolar epithelial-like cells. npj Regen. Med. 2025, 10, 30. [Google Scholar] [CrossRef]
- Lingampally, A.; Jones, M.R.; Bagari, S.; Chen, C.; Rivetti, S.; Bellusci, S. Use of the Reversible Myogenic to Lipogenic Transdifferentiation Switch for the Design of Pre-clinical Drug Screening in Idiopathic Pulmonary Fibrosis. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Lingampally, A.; Truchi, M.; Mauduit, O.; Delcroix, V.; Hadzic, S.; Koepke, J.; Vazquez-Armendariz, A.I.; Herold, S.; Samakovlis, C.; Makarenkova, H.P. Lineage tracing of Acta2+ cells in aged mice during lung fibrosis formation and resolution supports the lipofibroblast-to-myofibroblast reversible switch. bioRxiv 2024. [Google Scholar] [CrossRef]
- Khadim, A.; Kiliaris, G.; Vazquez-Armendariz, A.I.; Procida-Kowalski, T.; Glaser, D.; Bartkuhn, M.; Malik, T.; Chu, X.; Moiseenko, A.; Kuznetsova, I.; et al. Myofibroblasts emerge during alveolar regeneration following influenza-virus-induced lung injury. Cell Rep. 2025, 44, 115248. [Google Scholar] [CrossRef]
- Lin, C.-R.; Bahmed, K.; Kosmider, B. Impaired Alveolar Re-Epithelialization in Pulmonary Emphysema. Cells 2022, 11, 2055. [Google Scholar] [CrossRef]
- Xu, Y.; Li, M.; Bai, L. Pulmonary Epithelium Cell Fate Determination: Chronic Obstructive Pulmonary Disease, Lung Cancer, or Both. Am. J. Respir. Cell Mol. Biol. 2024, 71, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, C.-A.; de Vries, M.; Costa, R.; Woldhuis, R.R.; Königshoff, M.; Timens, W. Lung ageing and COPD: Is there a role for ageing in abnormal tissue repair? Eur. Respir. Rev. 2017, 26, 170073. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Ora, J.; Matera, M.G. Canakinumab for the treatment of chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2015, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Cottage, C.T.; Peterson, N.; Kearley, J.; Berlin, A.; Xiong, X.; Huntley, A.; Zhao, W.; Brown, C.; Migneault, A.; Zerrouki, K.; et al. Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun. Biol. 2019, 2, 307. [Google Scholar] [CrossRef]
| Disease Stage | Key Pathobiology | Therapeutic Logic | Example Interventions |
|---|---|---|---|
| Early Injury | AT2 injury, DAMP release (IL-1β, TNFα) AREG–EGFR signaling stalls AT2/AT1 intermediates | Block excessive inflammation and EGFR-driven stalling while preserving AT2 pool | Targeted anti-inflammatory modulation (IL-1β, TNFα inhibitors) EGFR/AREG blockade (nintedanib, erlotinib) |
| Intermediate | Transitional AT2/AT1 states persistence Fibroblasts: LIF → iLIF → aMYF | Promote resolution & regenerative programs in both epithelium and mesenchyme | PPARγ agonists (rosiglitazone, metformin) for fibroblast reprogramming FGF10, YAP/TAZ activation, microtubule disruptors (vinorelbine, mebendazole) for epithelial repair miRNAs (miR-29b, miR-200 family) to restore AT2 → AT1 differentiation |
| Late Fibrosis | Persistent aMYFs, ECM deposition Loss of alveolar structure, impaired regeneration | Actively reverse fibrosis and mobilize regenerative reserves | Anti-fibrotics (TGF-β/ALK5 inhibitors, saracatinib) Neuromodulatory antifibrotics (haloperidol, dopamine receptor agonists) Fibroblast lipogenic reprogramming (PPARγ agonists, metformin) rFGF10 to reverse alveolar epithelial intermediates |
| Proposed Hypothetical Pipeline for Therapeutic Discovery | |
|---|---|
| 1. Fibroblast State Manipulation | Use WI-38 fibroblasts as a manipulable system. Drive fibroblast states: LIF → iLIF → aMYF (via metformin, IL-17, TGF-β). Model reversibility: promote reprogramming back toward lipogenic LIF. |
| 2. Alveolosphere Co-culture Readouts | Combine WI-38 fibroblast states with AT2 cells. Measure epithelial outcomes: AT2→AT1 differentiation, intermediate state persistence. Readouts: organoid size, alveolar-forming efficiency, immunofluorescence, RT-qPCR. |
| 3. Multi-omics Profiling | Perform bulk RNA-seq, scRNA-seq, proteomics. Species-separated readouts (mouse epithelium vs. human fibroblast). Identify epithelial–mesenchymal signaling pathways (AREG/EGFR, TGF-β, FGF10, YAP/TAZ, miRNAs). |
| 4. Therapeutic Prioritization | Rank drug candidates (e.g., metformin, PPARγ agonists, haloperidol, miRNAs, saracatinib, rFGF10). Prioritize interventions that promote AT2→AT1 maturation, reverse fibroblast activation, and enhance alveolosphere regeneration. Feedback into successful testing in alveolosphere + WI-38 models. Advance to in vivo model, and clinical trial. |
| Target Pathway | Cellular Target | Mechanistic Effect | Representative Agents | Therapeutic Outcome | References |
|---|---|---|---|---|---|
| AREG–EGFR | AT2 cells/fibroblasts | Inhibition of profibrotic EGFR signaling; release of stalled AT2/AT1 intermediates | Nintedanib, Erlotinib | Restores AT2→AT1 differentiation and reduces fibrosis | [6,52,69] |
| TGF-β/SMAD2/3 | Fibroblasts/AT2 cells | Blockade of myofibroblast activation and epithelial–mesenchymal transition (EMT) | ALK5 inhibitors, Saracatinib, miR-29b | Attenuates ECM deposition and promotes epithelial regeneration | [47,51,84,93] |
| FGF10–FGFR2b | AT2 cells/mesenchyme | Activation of epithelial survival and progenitor renewal pathways | Recombinant FGF10 (rFGF10) | Enhances alveolar repair | [18,29,30,112] |
| PPARγ Activation | Fibroblasts | Induction of lipogenic reprogramming; reversal of activated myofibroblast (aMYF) phenotype | Rosiglitazone, Metformin | Promotes fibrosis resolution and restoration of LIF phenotype | [36,40,41,45] |
| YAP/TAZ (Hippo Signaling) | AT2 cells | Activation of regenerative and proliferative responses post-injury | Microtubule disruptors (vinorelbine, mebendazole, colchicine) | Stimulates AT2 proliferation and regeneration | [58,59] |
| Wnt/β-Catenin | AT2 cells | Regulation of differentiation plasticity through Axin2-dependent signaling | Tankyrase inhibitors, Wnt modulators | Promotes AT2→AT1 differentiation | [55] |
| microRNAs | AT2 cells | Restoration of differentiation balance; inhibition of fibrotic signaling | miR-29 family, miR-200 family | Reverses EMT and promotes AT1 maturation | [84,89] |
| Neuromodulatory Signaling | Fibroblasts/AT2 cells | Modulation of sigma-1R and dopamine receptor pathways to limit myofibroblast activation | Haloperidol, Fenoldopam | Reduces fibroblast activation and enhances tissue repair | [43,44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagiotidis, G.-D.; Chen, M.; Yang, X.; Marega, M.; Rivetti, S.; Chu, X.; Bellusci, S. Reciprocal Paracrine Signaling and Dynamic Coordination of Transitional States in the Alveolar Epithelial Type 2 Cells and Associated Alveolar Lipofibroblasts During Homeostasis, Injury and Repair. Cells 2025, 14, 1869. https://doi.org/10.3390/cells14231869
Panagiotidis G-D, Chen M, Yang X, Marega M, Rivetti S, Chu X, Bellusci S. Reciprocal Paracrine Signaling and Dynamic Coordination of Transitional States in the Alveolar Epithelial Type 2 Cells and Associated Alveolar Lipofibroblasts During Homeostasis, Injury and Repair. Cells. 2025; 14(23):1869. https://doi.org/10.3390/cells14231869
Chicago/Turabian StylePanagiotidis, Georgios-Dimitrios, Mengqing Chen, Xiuyue Yang, Manuela Marega, Stefano Rivetti, Xuran Chu, and Saverio Bellusci. 2025. "Reciprocal Paracrine Signaling and Dynamic Coordination of Transitional States in the Alveolar Epithelial Type 2 Cells and Associated Alveolar Lipofibroblasts During Homeostasis, Injury and Repair" Cells 14, no. 23: 1869. https://doi.org/10.3390/cells14231869
APA StylePanagiotidis, G.-D., Chen, M., Yang, X., Marega, M., Rivetti, S., Chu, X., & Bellusci, S. (2025). Reciprocal Paracrine Signaling and Dynamic Coordination of Transitional States in the Alveolar Epithelial Type 2 Cells and Associated Alveolar Lipofibroblasts During Homeostasis, Injury and Repair. Cells, 14(23), 1869. https://doi.org/10.3390/cells14231869






