Highlights
What are the main findings?
- Reciprocal epithelial–mesenchymal signaling between AT2 cells and fibroblasts governs whether the lung regenerates or progresses toward fibrosis.
- AT2/AT1 transitional states and fibroblast plasticity (LIF-iLIF-aMYF) are dynamically regulated by key pathways including AREG–EGFR, FGF10–FGFR2b, and TGF-β.
What are the implications of the main findings?
- Targetable pathways such as AREG–EGFR, PPARγ activation, LOX/PCSK9, and microRNA offer opportunities to both halt fibrosis and promote alveolar regeneration.
- WI-38 fibroblast systems and alveolosphere co-cultures provide an important human-relevant platform for screening antifibrotic and pro-regenerative drugs, supporting translational pipeline development for IPF and COPD therapies.
Abstract
Single-cell RNA-sequencing has transformed our understanding of alveolar epithelial type 2 (AT2) cells and alveolar lipofibroblasts (LIFs) during lung injury and repair. Both cell types undergo dynamic transitions through intermediate states that determine whether the lung proceeds toward regeneration or fibrosis. Emerging evidence highlights reciprocal paracrine signaling between AT2/AT1 transitional cells and LIF-derived myofibroblasts (aMYFs) as a key regulatory axis. Among these, amphiregulin (AREG)–EGFR signaling functions as a central profibrotic pathway whose inhibition can restore alveolar differentiation and repair. The human WI-38 fibroblast model provides a practical platform to study the reversible LIF–MYF switch and screen antifibrotic and pro-regenerative compounds. Candidate therapeutics including metformin, haloperidol and FGF10 show promise in reprogramming fibroblast and epithelial states through metabolic and signaling modulation. Integrating WI-38-based assays, alveolosphere co-cultures, and multi-omics profiling offers a translational framework for identifying interventions that halt fibrosis and actively induce lung regeneration. This review highlights a unifying framework in which epithelial and mesenchymal plasticity converge to define repair outcomes and identifies actionable targets for promoting alveolar regeneration in chronic lung disease.
1. Introduction
The diversity of topics covered in this review, including AT2 heterogeneity, fibroblast activation states, and key signaling pathways, reflects the complexity of alveolar regeneration rather than a shift in focus. Each of these components contributes to a unified framework in which reciprocal epithelial–mesenchymal communication determines whether lung injury resolves through regeneration or sustains toward fibrosis. By integrating molecular, cellular, and pharmacological perspectives, we aim to delineate how convergent cellular and signaling axes coordinate epithelial and mesenchymal fate that define the outcome of lung damage versus repair.
1.1. Progressive AT2 to AT1 Differentiation Involves the Formation of Intermediate AT2/AT1 States
Alveoli are the alpha and omega unit for gas exchange [1]. Alveolar epithelial type 2 (AT2)-to-alveolar epithelial type 1 (AT1) differentiation during homeostasis is highly important for gas exchange and for the progression of pulmonary diseases, particularly fibrosis, together with the LIF–iLIF–aMYF (lipofibroblast–inflammatory intermediate lipofibroblast–activated myofibroblast) axis [2,3]. AT2s are the prime source of facultative stem cells during lung regeneration/repair as progenitors for AT1s [1]. Many factors can lead to alveolar epithelial injury [4]. Following injury, AT2s proliferate and transiently dedifferentiate through an intermediate AT2-to-AT1 transitional state, as confirmed by lineage tracing in bleomycin-injured mice and single-cell RNA-sequencing of human IPF lungs [5,6]. These cells eventually differentiate into mature AT1 cells (Figure 1) [7,8].
The studies summarized in this review are based on several well-established experimental models of lung injury that illustrate the dynamics of epithelial and mesenchymal cell interactions during homeostasis, injury, and repair. Among these, the bleomycin (BLM)-induced lung injury model is the most extensively characterized and serves as a reference for delineating the sequential cellular events that drive alveolar damage and resolution. Following intratracheal or intranasal administration of BLM, the acute inflammatory phase occurs within days 3–7, characterized by epithelial injury, immune cell infiltration, and activation of fibroblasts. The fibrotic phase typically peaks between days 14–21, marked by myofibroblast accumulation, extracellular matrix deposition, and the emergence of transitional epithelial states such as damage-associated transient progenitors (DATPs) and pre-alveolar type I transitional cells (PATS). The resolution phase begins around day 21 and extends to approximately days 28–35, during which inflammatory activity declines, fibroblasts revert toward a lipogenic phenotype, and alveolar type II (AT2) cells progressively differentiate into mature alveolar type I (AT1) cells to restore epithelial integrity. While the bleomycin model remains the most widely used and best characterized system for investigating lung fibrosis, additional experimental approaches provide important complementary insights into progressive and chronic disease mechanisms. Silica and radiation exposure models induce persistent and spatially restricted fibrotic lesions that more closely resemble the irreversible scarring observed in idiopathic pulmonary fibrosis (IPF) [9,10]. Viral infection models, including influenza and SARS-CoV-2, have further revealed epithelial vulnerability, immune dysregulation, and prolonged AT2/AT1 transitional states that underlie post-viral fibrosis [5,6,11]. Moreover, genetic and toxin-induced models, such as surfactant protein C deficiency, telomerase mutations, and elastase- or pollutant-induced injury, demonstrate how epithelial senescence and oxidative stress predispose the lung to chronic fibrotic remodeling [5,6,11,12]. In addition to the BLM model, complementary systems such as elastase-induced emphysematous injury, pneumonectomy (PNX)-driven compensatory regeneration, and viral infection models (including influenza and SARS-CoV-2) have been instrumental in revealing conserved mechanisms of AT2 plasticity, epithelial–mesenchymal crosstalk, and fibroblast reprogramming. Collectively, these models provide a temporal and mechanistic framework for interpreting the cellular and molecular processes underlying alveolar regeneration and fibrosis resolution discussed throughout this review.
1.2. From Injury to Identity: Many Names, One Character
In recent years, several studies have described a transitional population of alveolar epithelial cells that emerge during lung injury and repair, though the terminology used varies across research groups. These cells, derived from alveolar type 2 (AT2) cells, exhibit characteristics of both AT2 and AT1 lineages but do not fully resemble either, occupying an intermediate state. Some researchers have termed them “pre-alveolar type-1 transitional cells” (PATS), while others refer to them as “damage-associated transient progenitors” (DATPs), “alveolar differentiation intermediates” (ADIs), or more recently, AT0s. Notably, these transitional cells are not merely driven by injury. They could represent a latent regenerative reservoir accessible therapeutically with the appropriate pharmacological interventions (Table 1).
Using lineage tracing and single-cell RNA-sequencing (scRNA-seq) data, researchers confirmed the presence of transitional cells originating from AT2s and identified distinct molecular signatures associated with this intermediate state. While the majority of these cells express keratin 8 (Krt8), subsets also exhibit the expression of claudin 4 (Cldn4), keratin 19 (Krt19), connective tissue growth factor (Ctgf), and stratifin (Sfn) [3,5,13,14,15,16]. In the study by [17], the authors uncovered a novel function of the PCLAF (PCNA Clamp Associated Factor; also known as KIAA0101 or PAF)–DREAM (the dimerization partner, retinoblastoma (RB)-like, E2F, and multi-vulval class B) signaling axis in modulating the plasticity of alveolar epithelial cells during the shift from AT2 to AT1 phenotypes. The DREAM complex plays a central role in controlling both the cell cycle dynamics and maintenance of cellular quiescence by fine-tuning the transcriptional activity of specific gene networks. In the study by [17], primed alveolar progenitor cells (PAPCs) were characterized transcriptionally as proliferative intermediate cells, and lineage tracing via SftpcCreERT2 and Rosa26Sun1-GFP demonstrated that AT2-derived progenitors label into PAPCs and eventually into AT1 cells. Using this system, Pclaf deletion in the AT2-lineage markedly reduced the emergence of Gfp+ Hopx+ AT1 cells, confirming that Pclaf KO in PAPCs impairs their maturation into AT1 cells. However, the authors demonstrated that activation of Tgf-β signaling is sufficient to restore AT1 cell differentiation even in the absence of Pclaf [17]. Pclaf KO was also found to be important on immune cell and fibroblast homeostasis, important factors in the progression of fibrosis [2].
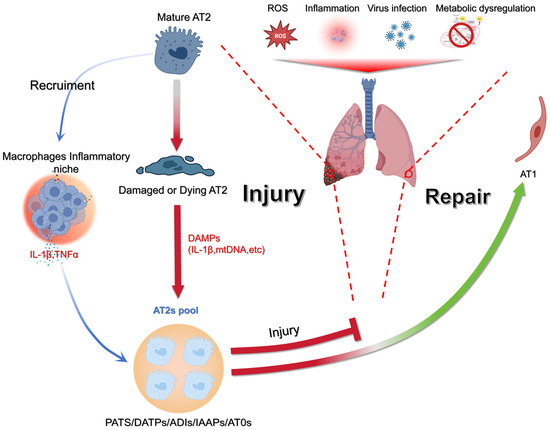
Figure 1.
Model of epithelial injury and regeneration in the lung. Environmental and cellular stressors such as ROS, infection, inflammation, or metabolic imbalance can injure AT2s. Once damaged, AT2s release DAMPs (e.g., IL-1β, mtDNA), which stimulate macrophages and create an inflammatory niche enriched in cytokines, including IL-1β and TNFα. This inflammatory response both amplifies tissue injury and signals to the remaining AT2 pool. Surviving AT2s expand and generate transitional progenitor states (PATS/DATPs/ADIs/AT0s), which ultimately affect repair by either replenishing the alveolar epithelium or failing to do so. Successful resolution leads to restoration of alveolar type 1 (AT1) cells, while failure to repair results in sustained injury. Figure created with BioRender.com.
AT2s have been described to be heterogeneous. Among them, captured using the SftpcCreERT2/+: tdTomflox/+ mice, at least four different subpopulations have been described during homeostasis: the Axin2+ AT2s (aka AEPs or alveolar epithelial progenitors), the Sca1+AT2s, the immature Pd-l1+ AT2s (aka IAAPs or injury-activated alveolar progenitors) and Pd-l1− AT2s [18].
It is difficult at this stage to define which AT2 subpopulation is preferentially engaged in differentiating into the AT1 lineage. The original proposition was that all AT2s can display such capability [1]. However, a major limitation is that the lineage tracing studies using the SftpcCreERT2 driver line did not discriminate between these four subpopulations. Further experiments using intersectional Dre/Cre-based genetics will be needed to target each of these subpopulations. In addition, in vitro evidence suggests that AEPs display a higher capability to form alveolospheres in vitro compared with the mature AT2 populations [19]. Interestingly, after bleomycin injury, it was described that the mature AT2s underwent massive cell death while the more immature IAAPs were amplified [20]. This suggests that the IAAPs may also represent an important progenitor population to replenish the missing AT2s. Whether the AT2-derived IAAPs can differentiate into AT1 cells is still unknown.
As the AT2 cells differentiate, they begin to acquire AT1 characteristics. An AT2/AT1 transitional state called pre-alveolar type-1 transitional cell state (PATS) [3] and damage-associated transient progenitors (DATPs) are characterized by the expression of Cldn4, Krt8, N-Myc Downstream Regulated 1 (Ndrg1), and Small Proline Rich Protein 1A (Sprr1a) [15]. Another group described these intermediate cells as Krt8+ ADI, exhibiting elevated expression of genes associated with epithelial–mesenchymal transition (EMT), cellular senescence, and key signaling pathways including p53, Myc, Tnfα via Nf-κb, and oxidative phosphorylation [5]. More recently, a distinct transitional population in the human lung termed alveolar type 0 (AT0) cells has been described. These cells show a hybrid molecular profile, co-expressing AT2 markers such as SFTPC, AT1 markers like AGER, and secretory cell genes including SCGB3A2 and SOX2, placing them at an intermediate position between AT2, AT1, and bronchiolar secretory cells [21]. Functionally, AT0 cells are thought to originate from AT2 cells and demonstrate bi-potency, with the capacity to give rise either to gas-exchanging AT1 cells or to secretory epithelial cells of the distal airways, thus contributing to progenitor pools at the alveolar–airway interface [22]. Respiratory bronchioles in the human lung bridge the terminal bronchioles with the alveolar ducts. Respiratory bronchioles contain respiratory airway secretory (RAS) cells, which have been proposed to be essential for airway repair and regeneration. RAS cells act as progenitors for AT2s [23]. These cells then progressively differentiate into early and late AT1 cells, initially regulated by IL-1β signaling and mainly mediated by HIF1a [15].
The early AT1s express the homeodomain only protein homeobox (Hopx) but are negative for insulin-like growth factor-binding protein 2 (Igfbp2), while the late AT1 cells are positive for both Hopx and Igfbp2 [24]. Lineage tracing of the early and late AT1s using the corresponding driver lines indicate that in the context of bleomycin injury, only the early but not the late AT1s were capable of differentiating into AT2 cells, refining the original concept that mature AT1s could differentiate into AT2s following injury [15].
From a mechanistic point of view, Interleukin 1 β (IL-1β) has been shown to prevent the differentiation of the AT2/AT1 cells toward the AT1 phenotype, leading to the accumulation of these intermediate cells [25]. This observation suggests that inflammatory processes are likely crucial for maintaining this intermediate state. This observation is counter-intuitive since anti-inflammatory steroid-based therapies have been shown to be ineffective in treating lung fibrosis in humans [26,27]. Interestingly, AT2 to AT2/AT1 differentiation is associated with the downregulation of signaling mediated by Fibroblast growth factor receptor 2 b [28], which was previously described to be critical for the survival of the AT2 cells [29]. In addition, the FGFR2b ligand FGF10 displays therapeutic activity in the context of bleomycin-induced fibrosis [29,30,31,32]. Therefore, treatments aiming at preserving or amplifying the AT2 population for patients at risk of developing fibrosis may represent a viable option.

Table 1.
Representative table of names, markers and references of published alveolar epithelial type 2/1 cells.
Table 1.
Representative table of names, markers and references of published alveolar epithelial type 2/1 cells.
| Name | Markers | Reference |
|---|---|---|
| Pre-alveolar type-1 transitional cells (PATS) | Krt8, Krt18, Krt19, Cldn4, Ctgf, Sfn | [3] |
| Damage-associated transient progenitors (DATPs) | Cldn4, Krt8, Ndrg1, Sprr1a | [15] |
| Alveolar differentiation intermediates (ADIs) | Krt8+, p53, Myc, Tnfα | [5] |
| AT0 | SFTPC, AGER, SCGB3A2, SOX2 | [21] |
| RAS | SCGB3A2, SCGB1A1 | [23] |
1.3. The Chicken or the Egg Paradigm: In the End, It Is the Epithelium
A first line of thought proposed that dysregulated proliferation and differentiation of the mesenchymal cells, leading to the deposition of high levels of extracellular matrix proteins, including collagens, was the primary cause of fibrosis. Such dysregulation led to epithelial defects, including the loss of AT2s and bronchiolization. However, based on the findings of Gunther and colleagues in [33], chronic injury to the epithelium was proposed as the causative factor for fibrosis formation [33]. This chicken or the egg paradigm regarding the origin of the fibrosis phenotype has been debated for many years. However, in 2020, a final conclusion was suggested through the inactivation of Cdc42 in AT2 cells using the SftpcCreERT2 driver line [34]. Cdc42 is a gene encoding for a Rho kinase involved in numerous cellular processes, including the regulation of actin cytoskeleton dynamics, establishment of cell polarity, shaping of cell morphology, and control of migration. It also contributes to endocytosis, exocytosis, cell cycle progression, and proliferation in a wide range of cell types. Importantly, Cdc42 has been implicated in the development of various age-related diseases, such as neurodegenerative and cardiovascular conditions, type 2 diabetes, and disorders affecting joints and bones [35]. Loss of Cdc42 leads to failed AT2-to-AT1 differentiation, accumulation of transitional AT2/AT1 intermediates, and impaired alveolar regeneration. In this model, sustained mechanical tension within the epithelial compartment activates a TGF-β–dependent stress program in AT2 cells, driving progressive fibrotic remodeling [34]. Although this study showed increased p-SMAD2 signaling in adjacent PDGFRβ+ stromal cells, the fibroblast subsets involved were not investigated. In contrast, fibroblast lineage-tracing studies demonstrate that TGF-β signaling can induce a reversible transition between LIFs and aMYFs, identifying fibroblast plasticity as a central component of fibrotic progression and resolution [36]. Thus, these findings suggest that epithelial mechanical dysfunction and fibroblast phenotypic transitions act to drive fibrosis, but their precise interdependence remains to be defined.
1.4. Reversible and Progressive LIF to MYF Differentiation Switch During Fibrosis Formation and Resolution
Lineage tracing of the activated myofibroblast in young and old Tg(Acta2-CreERT2); tdTomflox mice was recently carried out in the context of fibrosis formation and resolution [37,38]. These in vivo lineage-tracing data establish that fibroblast plasticity and partial reversion occur within the injured lung, complementing transcriptomic evidence from human fibrotic tissue. The published results demonstrated that during fibrosis formation, there is a transition from alveolar LIF to aMYF. These aMYFs exhibit a fibrotic signature and progressively differentiate from Cthrc1low LIFhigh to Cthrc1high cells. During fibrosis resolution, the reverse transition was observed, wherein Cthrc1high cells reverted to Cthrc1low LIFhigh and eventually to Acta2-negative LIFhigh fibroblasts to Cthrc1Low [37]. More recently, using the Scube2CreERT2 driver line to lineage trace the alveolar fibroblast, a novel intermediate population preceding the formation of the activated myofibroblasts was described. This population, called inflammatory alveolar fibroblasts, is negative for Acta2 expression and expresses chemokines such as (Cxcl12), serum amyloid A3 (Saa3), and lipocalin 2 (Lcn2). Their induction is driven by pro-inflammatory cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor (TNF). Inflammatory alveolar fibroblasts were proposed to form from alveolar fibroblasts upon IL17R activation and constitute a transient/intermediate population, which following exposure to TGF-β, subsequently differentiate into CTHRC1+ myofibroblasts (Figure 2) [39].
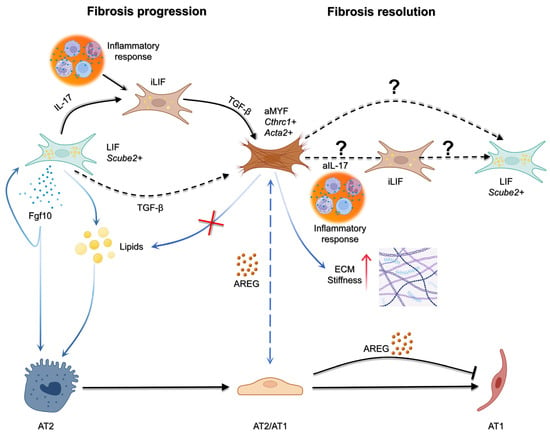
Figure 2.
Cellular interactions driving fibrosis progression and resolution. During injury, inflammatory cues such as IL-17 promote the emergence of inflammatory LIF+ fibroblasts (iLIF), which in turn release TGF-β to drive activation of myofibroblasts (aMYF; Cthrc1+ Acta2+). Activated fibroblasts reinforce fibrosis in the epithelial side through AREG stalling the epithelium in an intermediate state. In contrast, LIF+ fibroblasts (Scube2+) can provide pro-regenerative signals, including Fgf10 and lipids, that support alveolar type 2 (AT2) cell survival and differentiation into alveolar type 1 (AT1) cells. The balance between these pathways determines outcome: persistence of aMYF activity promotes fibrosis progression, while the potential reversion or remodeling of fibroblast states (aIL-17, iLIF, or LIF+ populations) may underlie fibrosis resolution. To date, no evidence indicate the reversion of aMYF to LIF through iLIF and it would be of interest to be studied. Figure created with BioRender.com.
1.5. Use of WI-38 Cells to Investigate LIF to MYF Reversible Switch
The LIF to MYF reversible switch is well described by our group [36,40,41]. This reversibility has been demonstrated in vitro using human WI-38 fibroblasts, providing a mechanistic framework that aligns with in vivo fibroblast lineage-tracing studies showing comparable transitions in murine models [37,38]. During fibrogenesis, lipofibroblasts can serve as progenitors for activated myofibroblasts. Conversely, in the resolution phase, myofibroblasts have the potential to revert to a lipofibroblast-like state. Pharmacological induction of a lipogenic program has been shown to antagonize TGF-β-driven myogenic differentiation, underscoring a potential therapeutic strategy for idiopathic pulmonary fibrosis (IPF) [36,37,38,39,40,41]. Importantly, the study by [40] emphasized the value of developing cell-based models to investigate the effects of candidate pharmacological interventions on the reversible transition between LIFs and aMYFs, with the ultimate goal of promoting fibrosis resolution.
To summarize, findings from the WI-38 model highlight its value not only as a human-derived mesenchymal platform but also as a conceptual bridge connecting cellular metabolism, fibroblast plasticity, and epithelial–mesenchymal interplay. Unlike isolated fibroblast or epithelial cultures, the WI-38 system enables the controlled dissection of paracrine signaling loops that influence both lipogenic and myogenic fibroblast phenotypes. Its responsiveness to pharmacologic agents pinpoints how metabolic reprogramming and lipid homeostasis can be leveraged to reverse fibrotic activation. This model also opens up new perspectives for studying fibroblast dynamics across different organs [42] and could, in the future, be expanded to include additional cell types, such as immune cells, to more accurately recapitulate the complex multicellular interactions that occur under pathophysiological conditions. Therefore, beyond reproducing established findings, the WI-38 paradigm provides a complex, interesting, experimental framework to mechanistically link fibroblast phenotype transitions with epithelial regenerative capacity, advancing a testable model for drug discovery and translational validation.
1.6. WI-38 as a Screen for FDA-Approved Drugs
WI-38 cells, a well-characterized human embryonic lung fibroblast line, offer a practical and accessible system for earlystage drug screening in the context of pulmonary fibrosis. These cells are responsive to lipogenic stimuli such as metformin, inflammatory stimuli, such as IL-17, and fibrotic stimuli, including TGF-β, making them suitable for modeling the transition between lipofibroblasts, intermediate inflammatory fibroblasts, and activated myofibroblasts. This feature is particularly useful for testing whether FDA-approved compounds can reverse or prevent myofibroblast activation. By combining in vitro assays with transcriptomic and proteomic analysis, WI-38 cells can serve as a first-pass platform to identify promising pharmacological candidates before moving into more complex models, such as lung organoids or in vivo systems. Their ease of culture and reproducibility make them a valuable tool in the search for drugs that could promote fibrosis resolution through lipogenic reprogramming. One interesting FDA-approved drug that can be repurposed for the treatment of fibrosis is haloperidol. Haloperidol, a well-established antipsychotic medication approved by the FDA in the 1960s, is primarily used to treat conditions such as schizophrenia, acute psychosis, and severe agitation. Emerging research suggests that this drug may also have applications beyond the field of psychiatry. A recent high-throughput screening study identified haloperidol as a strong candidate for targeting fibrotic pathways. In fibroblast cultures, it was shown to reduce the expression of α-SMA, a key marker of myofibroblast activation. This effect appears to be mediated through its interaction with the sigma-1 receptor, which influences intracellular calcium levels and triggers a mild form of endoplasmic reticulum stress. These intracellular changes ultimately disrupt Notch1 signaling, leading to reduced myogenic differentiation. What makes these findings particularly compelling is that haloperidol also showed antifibrotic effects in animal models of lung, heart, and tumor-associated fibrosis [43]. Given its clinical use and well-documented safety profile, haloperidol may offer a valuable starting point for repurposing efforts aimed at resolving fibrosis. More recently, dopamine receptor agonists, including fenoldopam (D1R-selective), have been found to mitigate myofibroblast differentiation and TGF-β/Smad2 signaling in bleomycin-induced pulmonary fibrosis models, supporting a novel neuromodulatory mechanism of fibrosis control [44]. These findings point to an unexpected but exciting possibility that antipsychotic medications, long used in mental health, might also help tackle fibrosis. Drugs like haloperidol, which have well-known safety profiles, appear to interfere with the same cellular pathways that drive fibrotic remodeling. While much remains to be tested, especially in the clinical setting, this kind of repurposing, together with the use of WI-38-based platforms, could offer a faster, more cost-effective route to developing new antifibrotic therapies.
The WI-38 fibroblast model provides a reproducible and tractable system to study fibroblast plasticity and the LIF–MYF transition, as well as to evaluate pharmacological agents that modulate mesenchymal activation. However, this model alone does not capture the epithelial injury component that initiates and sustains fibrotic remodeling. To address this limitation, the WI-38 system can be integrated with epithelial injury modules using alveolosphere co-cultures or conditioned media derived from injured epithelial cells. These injury paradigms may include exposure to inflammatory cytokines such as IL-1β or TNFα, profibrotic mediators like TGF-β1, proteolytic stress induced by elastase, oxidative injury, or cigarette smoke extract, and mechanical stretch to mimic ventilator-associated stress. Such an integrated approach allows for the simultaneous assessment of epithelial damage, differentiation blockade, and fibroblast activation within a controlled experimental context. The combined WI-38–alveolosphere platform thereby provides a physiologically relevant framework to study reciprocal epithelial–mesenchymal interactions during injury and repair and evaluate therapeutic agents that restore homeostatic signaling and promote alveolar regeneration.
1.7. Investigating the Impact of Drugs to Induce LIFs
To develop WI-38 cells into a robust screening platform for FDA-approved drugs aimed at inducing a lipofibroblast phenotype, several molecular targets show promise. One clear example is metformin, an FDA-approved anti-diabetic drug, which has demonstrated clear lipogenic effects in WI-38 cells [40,41]. One key regulator is peroxisome proliferator activated receptor γ (PPARγ), a nuclear receptor that governs lipid metabolism and adipogenic differentiation. Rosiglitazone, a PPARγ agonist, has consistently inhibited TGF-β-induced myofibroblast differentiation in human lung fibroblasts, as demonstrated by marked reductions in α-SMA and collagen production [36,45]. This is also supported by in vivo studies that confirmed rosiglitazone’s ability to prevent hyperoxia-induced LIF-to-aMYF transdifferentiation in neonatal rat lungs [46]. Nifuroxazide, an anti-diarrheal drug, suppresses the expression of inflammatory factors such as IL-6 and IL-4 along with α-SMA and collagen I expression in both TGF-β-stimulated human and bleomycin-injured mouse fibroblast models, ameliorating pulmonary fibrosis [47]. Saracatinib (a Src-family kinase inhibitor, initially developed as an anti-metastatic drug for cancer treatment [48] and later repurposed for Alzheimer’s disease [49,50]) is also an interesting target for fibrosis resolution. Transcriptomic analysis of murine lung extracts showed that saracatinib reverses several key fibrotic pathways, including those involved in epithelial-to-mesenchymal transition, immune signaling, and extracellular matrix remodeling. Notably, its ability to reduce fibrosis and dampen inflammatory responses was further validated in human precision-cut lung slices, underscoring its potential as a therapeutic option for lung fibrosis (Figure 3) [51].
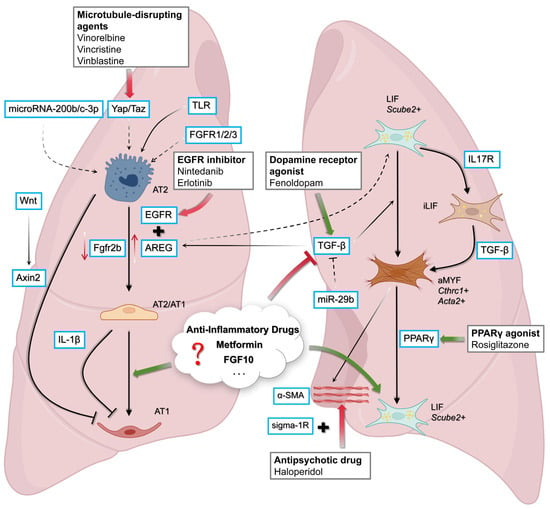
Figure 3.
Therapeutic targets and signaling pathways in alveolar repair and fibrosis. Multiple pathways regulate the balance between alveolar epithelial regeneration and fibrotic remodeling. In AT2 cells, Wnt/β-catenin signaling and EGFR/AREG activity promote differentiation towards complete AT1 or AT2/AT1, respectively. EGFR inhibitors (e.g., nintedanib, erlotinib) and dopamine receptor agonists (e.g., fenoldopam) can attenuate profibrotic signaling, whereas microtubule-disrupting agents and miR-29b act to suppress TGF-β activity. On the mesenchymal side, LIF fibroblasts (Scube2+) provide regenerative support, counterbalanced by inflammatory fibroblast states (iLIF, aMYF) that drive TGF-β-dependent matrix deposition and stiffness. Pharmacological interventions, including PPARγ agonists (rosiglitazone), antipsychotics (haloperidol, via sigma-1R), and potentially other agents such as anti-inflammatory compounds, Metformin, and FGF10, are poised to tip the balance toward epithelial repair and fibrosis resolution. Figure created with BioRender.com.
1.8. Investigating the Impact of Drugs on AT2s
The differentiation pathways of alveolar epithelial cells remain incompletely understood and are still the subject of active discussion. In the human lung, trajectory analyses indicate that AT2 cells can transition through an intermediate AT0 state (SFTPC+, AGER+, SCGB3A2+, and SOX2+) toward mature AT1 cells. This transitional concept aligns with earlier descriptions of mouse AT2/AT1 intermediates such as PATS, ADIs, IAPs, and Krt8+ transitional cells, which share overlapping features but differ in experimental context and nomenclature.
Human AT0 cells, like these other mice intermediates, exhibit high transcriptional entropy, consistent with their transient and unstable identity. Pseudotime analyses support a model in which AT2-derived AT0s can give rise to AT1s [21]. In human AT2-derived alveolosphere cultures, EGF withdrawal, but not FGF10 depletion, promoted AT0-associated markers (SCGB3A2, SFTPC, SFTPB) and induced multiluminal cystic remodeling, indicating an EGF-dependent AT2-to-AT0 differentiation. In vivo, AT0s arise in distal alveolar regions in response to lung injury and are most abundant in mildly fibrotic areas, positioned alongside normal AT2 cells, consistent with their role as an intermediate state [21]. Their presence has also been identified in human IPF tissue and reproduced in alveolosphere and organoid systems, confirming cross-species conservation of this transitional state [21]. Pharmacological studies confirmed EGFR involvement: erlotinib and inhibitors of downstream RAF and MEK pathways induced AT0 features, underscoring EGFR’s regulatory role in AT2-to-AT0 transitions. These insights provide a framework for investigating how pharmacological interventions influence AT2 plasticity [21]. Additionally, nintedanib, being an EGFR inhibitor [52], may also have an AT0-promoting effect on AT2s. TGF-β signaling, a key driver of fibrotic remodeling, can be targeted by ALK5 inhibitors, which block TGF-β-induced epithelial-to-mesenchymal transition (EMT) [53,54]. These inhibitors may also alter AT0 and AT1 phenotypes during lung injury repair.
1.9. Investigating AT2/LIF Dynamic Interaction Using Alveolospheres
We have previously reported that we can generate alveolospheres using human WI-38 cells recombined with mouse AT2 cells. It is worth mentioning that this model may reveal limitations in cross-species paracrine compatibility; however, on the other hand, it provides the advantage of tracing the transcriptomic activity of two different species population-wise. We used AT2s from SftpcGFP mice for better visualization of the differentiation status of alveolar epithelial cells. The differentiation of WI-38 cells towards the MYF or LIF can also be manipulated before assembling them with AT2s. We demonstrated that WI-38 cells differentiated to aMYF display a weaker niche activity for AT2s compared to LIF, with a reduction in both the size and colony-forming efficiency [40].
In addition, the epithelial–mesenchymal interactions occurring in the alveolospheres to drugs can also be assessed once they are formed. Using immunofluorescence (IF) and flow cytometry, it is possible to examine the impact of pro-fibrotic treatment (TGF-β) and anti-fibrotic treatment (TGF-β1 + antifibrotic treatment) on the epithelium and mesenchyme. In addition, using bulk RNA-seq on the whole organoid, bioinformatic analysis based on the fact that epithelial and mesenchymal cells arise from different species, allows us to pinpoint separately transcriptomic changes occurring in the epithelium (mouse genome) and mesenchyme (human genome) over time. These combined approaches will help to better define the mechanism of action of given drugs.
1.10. Devising Therapeutic Intervention to Resume AT2/AT1 Differentiation Towards AT1 or Reverse AT2/AT1 Differentiation Towards AT2
Persistence of ‘’transitional’’, ‘’intermediate’’, and ‘’stalled’’ AT2/AT1 states has been implicated in impaired alveolar regeneration and progressive fibrotic remodeling [34], making the promotion of efficient AT2-to-AT1 maturation an attractive therapeutic strategy. Wnt signaling has also been shown to play a major role in the AT2 to AT1 differentiation axis, with Axin2 functioning as a molecular switch. Axin2 expression is downregulated as AT2s transdifferentiate into AT1 cells. Sustained Wnt signaling prevents this transdifferentiation, whereas the inhibition of Wnt signaling facilitates it [55]. Thus, Axin2 inhibitors would tilt the balance towards AT1 differentiation, and tankyrase inhibitors, which inhibit the degradation of Axin2, might potentially lead to the dedifferentiation of AT1s back to AT2s. Retinoic acid is another interesting factor. Retinoids have been found to play a crucial role in alveologenesis and restoration following injury [56], with a direct positive effect on the number of AT2 cells [57].
The Yap/Taz axis, a member of the Hippo signaling pathway, is also an interesting target, as it has been found to positively affect alveolar regeneration after Streptococcus pneumoniae-mediated [58] inflammation [59]. In this study, mice with Yap/Taz-deficient AT2s exhibited a delay in alveolar epithelial repair, whereas in wild-type animals, increased AT2 activity was associated with the nuclear expression of Yap/Taz [59]. Given their ability to promote Yap activation [58], microtubule-disrupting agents such as vinorelbine, vincristine, vinblastine, mebendazole, colchicine, and podofilox may be of interest for supposedly promoting AT2 cell regeneration, potentially by inducing the dedifferentiation of intermediate alveolar epithelial cells back into the AT2 lineage.
1.11. Nintedanib Target: AREG/EGFR Signaling Takes Central Stage
Amphiregulin (AREG) is a member of the epidermal growth factor (EGF) family and was first identified over 25 years ago. As described by its name (amphi meaning “on both sides”), it exhibits an anti-proliferative function in certain carcinoma cell lines and a pro-proliferative function in fibroblasts [60]. Current insights suggest that AREG promotes proliferation by binding to the EGF receptor (EGFR) [61], thereby initiating tyrosine kinase signaling cascades, including MAPK, PI3K/AKT, mTOR, [61] and PLC [62,63]. These signaling pathways regulate gene expression and trigger various cellular responses, including proliferation, survival, motility, and angiogenesis [64]. Through the signaling pathways mentioned above, AREG is linked to the promotion of fibrosis through TGF-β [65]. Furthermore, AREG also plays a role in airway inflammation and asthma [66,67].
Interestingly, Ref. [6] demonstrated a link between AREG expression and intermediate alveolar type 2 (AT2) cells during fibrotic progression. This association has been validated in vivo in bleomycin-injured mouse lungs and corroborated by human single-cell datasets, indicating its physiological relevance [6,11]. Their findings revealed that in fibrotic regions of both murine models and lungs from patients with idiopathic pulmonary fibrosis (IPF), AT2 cells exhibited increased expression of intermediate AT2/AT1 markers alongside elevated Areg levels [6]. Similar observations were previously reported in a bleomycin-induced lung injury model [5]. In addition, AREG has been found to be essential for the progression of fibrosis, also in the context of fibroblast activation [11], even though it is not required for the differentiation of AT2 cells into the intermediate state. These profibrotic effects are mediated via EGFR signaling, as pharmacological inhibition of EGFR abrogated the actions of AREG [6].
Nintedanib is a tyrosine kinase receptor inhibitor [12] and an anti-fibrotic medication that limits the progression of the disease [68]. In addition to other growth factor receptors, nintedanib also exhibits affinity for EGFR in certain contexts, thereby inhibiting its function [52]. In vitro evidence from cancer research indicates that nintedanib reduces AREG levels by inhibiting EGFR signaling [69]. Thus, the link between them is indirect but mechanistically aligned as nintedanib can attenuate stalled intermediate AT2/AT1 driven fibrosis mediated by AREG, ultimately leading to alveolar regeneration.
In addition to its established profibrotic role, AREG also exerts context-dependent effects that influence both epithelial repair and mesenchymal activation. In chronic or unresolved injury, sustained AREG–EGFR signaling drives fibroblast proliferation, myofibroblast differentiation, and extracellular matrix deposition, thereby perpetuating fibrosis [6,11]. Single-cell transcriptomic analyses have revealed that AREG expression is enriched in transitional AT2/AT1 cells and activated myofibroblasts, suggesting that these cell populations may form a self-reinforcing paracrine loop that sustains pathological remodeling [5,6]. Thus, the amplitude and duration of AREG–EGFR signaling determine whether the outcome favors regeneration or fibrosis. Understanding this temporal regulation will be essential for designing targeted interventions that attenuate profibrotic AREG activity while preserving its beneficial reparative functions [5].
1.12. LOX and PCSK9 as Upstream Modulators of the Fibroblast–Epithelium Axis
Although no studies have directly examined LOX or PCSK9 in the context of LIF biology or AT2–LIF paracrine signaling, both pathways influence upstream processes that shape the fibrotic niche. Lysyl oxidase (LOX) increases extracellular matrix crosslinking and stiffness, which is known to enhance fibroblast activation and promote profibrotic mechanical feedback [70,71,72,73]. Proprotein convertase subtilisin/kexin type 9 (PCSK9), through its regulation of lipid homeostasis, oxidized LDL uptake, inflammation, and epithelial stress responses, has been shown to modulate EMT, oxidative injury, and Wnt/β-catenin signaling in alveolar epithelial cells [42,74,75,76,77,78]. While their direct involvement in LIF-to-MYF transitions remains untested, these pathways represent broader matrix–lipid regulatory axes that may indirectly influence epithelial–mesenchymal reciprocity in fibrotic lung remodeling. Their integration into future mechanistic studies could clarify whether they contribute to LIF or transitional AT2 cell dysfunction.
1.13. Targeting MicroRNAs to Restore Alveolar Differentiation Dynamics
Small non-coding RNAs, known as microRNAs (miRNAs), have emerged as pivotal regulators of lung epithelial cell fate, primarily by modulating mRNA stability and translation [79,80]. In the context of lung injury and fibrosis, specific miRNAs have been found to influence whether transitional alveolar epithelial cells continue differentiation toward a mature AT1 phenotype or revert to a progenitor-like AT2 state. Depending on the signaling context, these miRNAs can either facilitate tissue regeneration or exacerbate pathological remodeling [81,82,83,84,85,86,87,88,89]. Deciphering how individual miRNAs shape alveolar epithelial plasticity may provide new avenues for therapeutic intervention aimed at promoting regeneration while limiting fibrotic progression.
According to the study by [89], AT2 cells isolated from patients with IPF exhibited impaired differentiation into AT1 cells. However, transfection of these cells with microRNA-200b-3p and microRNA-200c-3p restored their ability to undergo differentiation into AT1 cells without affecting their proliferation [89]. In the context of alveolar morphogenesis, two studies have shown that miR-17-92 and miR-106b-25 [81] and miR-29 [88] are important for the differentiation in developing lungs. The findings of [81] revealed that miR-17-92 and miR-106b-25 in lung epithelial explants disrupted FGF10-induced budding morphogenesis, a phenotype that was rescued by supplementation with miR-17. Reduced miRNA levels decreased E-Cadherin expression and altered its localization while increasing β-catenin activity and upregulating downstream targets (Bmp4, Fgfr2b), and identified Stat3 and Mapk14 as direct targets of these miRNAs [81]. These findings suggest that these miRNAs play a critical role in embryonic lung development and should be studied in the context of disease and alveolar differentiation. In addition, the study by [88] revealed that miR-29 family expression increases in mouse fetal lung epithelial cells during alveolar morphogenesis and in human fetal lung (HFL) explants during type II cell differentiation. In cultured HFL epithelial cells, miR-29 was upregulated, while hypoxia suppressed it, with an inverse correlation with TGF-β2 [88]. Adding to that, the study of [84] revealed that silica exposure drives AT2 cells toward an epithelial–mesenchymal transition (EMT)-like state while simultaneously suppressing miR-29b. miR-29b directly dampens TGF-β1 signaling. In vitro, restoring miR-29b levels in RLE-6TN cells not only reversed EMT but also promoted mesenchymal–epithelial transition (MET), a switch accompanied by reduced TGF-β1 activity. These in vitro findings translated to silicotic mice, where miR-29b supplementation not only halted fibrosis progression but also improved lung function [84]. Beyond its alveolar morphogenic and anti-fibrotic effects, miR-29b’s modulation of TGF-β1 raises intriguing questions about its potential role in AT2-to-AT1 differentiation, a process where balanced TGF-β signaling is known to be critical [90,91,92]. This dual functionality positions miR-29b as a compelling candidate for targeting both pathological fibrosis and impaired alveolar regeneration. Evidence from [93] demonstrates that members of the miR-29 family are downregulated in AT2s following bleomycin-induced lung injury. This reduction inversely correlates with the elevated expression of extracellular matrix (ECM) components, indicating the presence of fibrosis. The molecular driver of miR-29 suppression was identified as TGF-β1. Conversely, miR-29 overexpression attenuated fibrotic gene expression in AT2s [93]. Given that TGF-β1 signaling is a known inhibitor of AT2-to-AT1 transdifferentiation [90,91,92], the capacity of miR-29 to antagonize this pathway suggests a potential role in promoting alveolar epithelial maturation. However, direct evidence for miR-29′s involvement in AT1 differentiation remains an open question requiring targeted investigation.
1.14. Rethinking AT1 Cell Plasticity: A Conceptual Framework
While current literature lacks definitive evidence that mature alveolar type 1 (AT1) cells dedifferentiate into AT2 cells under physiological conditions, the existence of intermediate AT2/AT1 transitional states (e.g., PATS, ADIs, AT0) [3,5,15,18,21] raises an intriguing possibility. These intermediates, which co-express markers of both lineages, could be pharmacologically directed toward either AT2 or AT1 fates. If such intermediates can be pushed back to an AT2 phenotype, could similar reprogramming be achieved starting from fully differentiated AT1s? Philosophically, this question challenges the traditional view of AT1 terminal differentiation and asks whether targeted interventions (e.g., WNT/FGF modulation, YAP/TAZ activation) might unlock latent plasticity in AT1 cells.
In this scenario, it could be worth philosophically reconsidering the axis of the alveolar epithelial cell fate as recent findings give important yet intriguing information. The study of [30] adds complexity to our understanding of alveolar epithelial cell fate. In a bleomycin-induced lung fibrosis model, the authors identified injury-activated alveolar progenitors (IAAPs) as a distinct cell population. At baseline (day 0), IAAPs were scarce, but their numbers rose sharply during disease progression, coinciding with a decline in AT2 cells, likely due to injury-induced cell loss. By day 16, IAAPs reached their peak abundance, while AT2 cells were at their lowest levels. During the subsequent repair phase, this relationship reversed, with IAAP numbers declining as AT2 cells recovered, a trend that continued until day 60 [30]. These findings raise a key question: do AT2 cells primarily regenerate through self-replication following injury, or do IAAPs function as a progenitor reservoir that replenishes the AT2 population?
Supporting this possibility, recent work by [94] examined the molecular status of KrasG12D, a constitutively active mutant form of the Kras oncogene, in AT1 cells using single-cell RNA-sequencing (scRNA-seq). This analysis revealed a molecular trajectory consistent with direct reversion from an AT1 to an AT2-like phenotype, suggesting that KrasG12D activation can unlock plasticity in mature AT1 cells [94].
To follow up on these ideas, in vitro studies that isolate primary AT1 cells and expose them to pro-regenerative signals such as WNT signaling manipulation, FGF10 supplementation, or YAP/TAZ activation might reveal latent plasticity. Thus, while AT1-to-AT2 dedifferentiation remains unconfirmed, the dynamic nature of alveolar epithelial transitions suggests that the boundaries between terminal and plastic states may be more permeable than previously assumed.
1.15. Guardians of the Alveolus: FGFR2 and the Fate of AT2 Cells
FGF signaling promotes proliferation and is essential for lung branching morphogenesis and repair following injury, with FGFR2 playing a critical role in epithelial patterning during development [95,96,97,98]. In adult lungs, FGF signaling is required for AT2 cell maintenance, as demonstrated by studies showing that FGFR1, FGFR2, and FGFR3 support AT2 cell survival and proliferation during homeostasis and post-injury repair [19,31,32,99]. Accordingly, FGFR signaling plays a major role in both epithelial morphogenesis and regeneration. Recent evidence using conditional FGFR2 deletion in AT2 cells (via Sftpc-Cre) reveals that this pathway is essential for their self-renewal and ability to restore alveoli after injury. When FGFR signaling is disrupted, AT2 proliferation is inhibited, thereby compromising alveolar regeneration, which demonstrates a cell-intrinsic mechanism. Organoid experiments confirm that FGFR activity governs AT2-to-AT1 differentiation, further supporting its role in driving alveolar regeneration. Complementary in vivo conditional-Fgfr2b knockout studies have confirmed the essential role of FGFR2 in epithelial survival and alveolar regeneration [29]. While representative images of these organoids are not presented here due to the review format, their morphology have been described [100]. Moreover, in vivo studies using bleomycin injury models have shown that the deletion of Fgfr2 alone in AT2 cells leads to increased mortality and lung injury, indicating that FGFR2 is the dominant receptor mediating AT2 survival during injury [29]. Taken together, the data underscore the importance of FGFR2 in preserving AT2 cell function and facilitating epithelial recovery in the injured lung.
1.16. Functional Diversity of Alveolar Type II Cells: Coordinators of Regeneration and Immune Homeostasis
The interplay between mesenchymal, epithelial, and immune cells in the lung has emerged as a key determinant of tissue homeostasis and pathophysiological responses. Among these, fibroblasts and alveolar type 2 (AT2) epithelial cells occupy a central position, not only due to their physiological roles but also because of their capacity to influence the behavior of immune cells. Fibroblasts, in particular, are capable of modulating local immune activity through the release of cytokines, chemokines, and matrix-bound signals, thereby regulating both immune and AT2 cell function [2]. AT2 cells, in turn, contribute to this network through immunomodulatory signaling and by maintaining epithelial–stromal balance under both physiological and pathological conditions [25,101,102,103,104,105,106,107,108,109,110].
Detailed mechanisms of fibroblast–immune cell interactions have been extensively reviewed in our earlier work [2]. Here, we focused on immune-related functions of AT2 cells. In a co-culture model of THP-1 macrophages and A549 epithelial-like carcinoma cells, unstimulated epithelial cells were sufficient to induce NF-κB, a major regulator of the immune response, in macrophages [111]. Specifically, 29 out of 84 NF-κB-associated genes were upregulated ≥4-fold, including cytokines, chemokines, and immune receptors accompanied by increased IL-6, IL-8, and ICAM-1 expression [103].
AT2s express and respond to Toll-like receptor (TLR) signaling. TLR2, in particular, plays a key role in initiating signaling cascades that shape downstream inflammatory responses [104]. In mice, profiling of the alveolar epithelial cells’ secretome revealed 12 cytokines, chemokines, and growth factors including Gm-csf, Mcp-1, Il-6, and Ip-10, present under basal conditions, with others, such as G-csf, M-csf, and Rantes induced only upon LPS stimulation. Alveolar epithelial cells demonstrate the ability to respond to both microbial and endogenous signals, producing a range of immunomodulatory factors that can influence monocytes, macrophages, dendritic cells, and T cells. Notably, AECs secreted T cell-attracting chemokines such as Ip-10 and Rantes following activation [102].
AT2s play a central role in lung repair, but their function is highly sensitive to the inflammatory state of the surrounding microenvironment. IL-1β, elevated during COPD exacerbations, exerts timing-dependent effects on AT2s’ behavior. While acute IL-1β exposure transiently enhances organoid growth via NF-κB signaling, prolonged exposure disrupts AT2 differentiation and repair capacity. This shift is marked by the accumulation of transitional progenitor states and the altered expression of key markers, including upregulation of Tm4sf1 and suppression of Lmo7, indicating injury-induced AT2/AT1s [25]. Beyond their role in maintaining alveolar structure and participating in tissue repair, AT2 cells also serve as pivotal regulators of pulmonary immune responses during infection. In response to vaccinia virus infection, AT2s upregulate IFN-β and downstream interferon-stimulated genes through MDA5 and STING-dependent nucleic acid sensing. This response promotes the recruitment of Ccr2+ inflammatory monocytes, which differentiate into Lyve1− interstitial macrophages capable of clearing viral particles and limiting viral replication [108]. During SARS-CoV infection, AT2 cells upregulate type I and III interferons (notably IFN-β and IL-29) and secrete a range of chemokines, including CXCL8 (IL-8), CCL5 (RANTES), CXCL10, and CXCL11, which recruit and activate various immune cell populations, such as neutrophils, monocytes, NK cells, and T lymphocytes, thus orchestrating the early innate immune response [110]. In conclusion, these findings highlight the dual identity of AT2s as both regenerative and immunologically active components of the lung microenvironment. Positioned at the intersection of epithelial, mesenchymal, and immune signaling, AT2 cells not only respond to injury and infection but also actively orchestrate immune cell recruitment and function.
IAAPs have also been identified to have immunoregulatory functions [18,29,112]. ATAC-seq analysis upon pneumonectomy (PNX) study [112] revealed distinct chromatin accessibility profiles between TomHigh (AT2) and TomLow (AT2/AT1) cells. TomLow cells exhibited strong activation of FGFR2b signaling, as evidenced by upregulation of Fgfr2b, Etv5, Sftpc, Ccnd1, and Ccnd2, together with a trend towards higher Ki67 expression, indicating the IAAPs’ phenotype [18], while TomHigh remained unresponsive under the same conditions. Transcriptomic profiling further identified immune-related surface molecules enriched in TomLow, including Cd33, Cd300lf, and particularly Cd274 (PD-L1). qPCR confirmed elevated expression of Pd-l1 in TomLow relative to TomHigh, and both immunofluorescence and flow cytometry demonstrated a high proportion of PD-L1-positive TomLow cells. Given the established role of PD-L1 as an immune checkpoint ligand and its prominent expression in lung adenocarcinoma, its enrichment in TomLow IAAPs [113,114,115,116] reinforces the concept that this population is uniquely equipped for immune regulation within the alveolar niche. Although such properties may be advantageous during injury and regeneration, they may also have implications in tumor biology. Future work should therefore investigate the dual role of these PD-L1+ IAAPs in repair, alveologenesis, and disease progression.
1.17. Killing 2 Birds with One Stone: Finding Antifibrotic and Pro-Regenerative Drugs Using the WI-38 Cell-Based Model
As outlined above, the WI-38-based system provides a robust platform for screening candidate anti-fibrotic and pro-regenerative compounds. When combined with the alveolosphere model, these approaches enable the evaluation of drug effects on both mesenchymal and epithelial counterparts, either independently or in conjunction, with the ultimate goal of driving disease regression and promoting de novo alveolar regeneration. FGF10 is indispensable for branching and alveolar morphogenesis [95]. In the therapeutic context, Ref. [30] demonstrated that exogenous rFGF10 exerts both protective and therapeutic effects against BLM-induced pulmonary fibrosis, providing fundamental preclinical support for its potential use in IPF. Consistent with the established role of FGF10/FGFR2b signaling in epithelial repair [18,29,112], rFGF10 not only limited fibrotic progression but also promoted alveolar regeneration, likely reflecting its combined impact on epithelial and mesenchymal compartments. AT2 injury through the deletion of Fgfr2b caused a marked loss of mature AT2 cells through apoptosis, whereas injury-activated alveolar progenitors (IAAPs) survived, expanded, and displayed enhanced regenerative capacity [29]. Importantly, in the study of [30], IAAPs were amplified in both murine models of BLM injury and in IPF patients, with their relative abundance peaking at the height of fibrosis when mature AT2s were most depleted. rFgf10 administration further prolonged and amplified the IAAP response, supporting the view that these progenitor-like cells, rather than canonical AT2s, may serve as the primary drivers of alveolar repair [30]. While the precise mechanisms remain to be fully resolved, these findings suggest that IAAPs may represent a key regenerative reserve in the injured lung. Targeting this population through rFgf10 holds promise for halting the progression of fibrosis and restoring alveolar architecture. In the context of the mesenchymal side, evidence shows that the intratracheal administration of Fgf10 mobilizes lung-resident-mesenchymal stem cells (LR-MSCs), which can be readily isolated by bronchoalveolar lavage and display classical MSC features and multipotency. These LR-MSCs, expanded by Fgf10, protect against diverse forms of lung injury and likely represent an organ-specific progenitor pool supporting epithelial repair [117]. Extending this concept, indications from our group demonstrate that Gli1+ cells generate repair-supportive smooth muscle-like cells (RSMCs) after injury, where they acquire Acta2 expression and accumulate in the peribronchial region. Crucially, these RSMCs serve as a privileged mesenchymal niche by producing FGF10, which sustains club cell progenitors and ensures effective epithelial regeneration, while loss of Fgf10 in Gli1+ cells markedly impair repair [118]. Studying the WI-38 model in conjunction with FGF10 could provide important insights into how FGF10 contributes to both anti-fibrotic strategies and the promotion of alveolar regeneration.
Metformin, first highlighted by our group [41], has emerged as a key candidate in anti-fibrotic research; while clinical trials report conflicting effects on patient survival, they consistently suggest that metformin may confer benefits in IPF [119,120]. Importantly, the cohort study of COPD patients (GOLD 1–4; n = 1541; mean age 64.4 y; 601 females) showed that metformin therapy was associated with a significantly attenuated annual decline in pulmonary diffusing capacity (KCO: 0.2 vs. 2.3% predicted; TLCO: 0.8 vs. 2.8% predicted; p < 0.05) relative to non-diabetic control patients [121]. These findings support a potential role for metformin in mitigating emphysema-related alveolar dysfunction. Increasingly, this indicates that it may improve health status, symptoms, hospitalization rates, and mortality in those with Type 2 Diabetes Mellitus (T2DM), while no significant benefits were observed in patients without T2DM and not receiving metformin [122]. Recent studies using rat cigarette smoke-induced COPD and in vitro models demonstrated that metformin is protective by improving alveolar structure and lung function, mitigating inflammation and oxidative stress via activation of the Nrf2/HO-1/MRP1 pathway [123]. Elastase is commonly employed in animal models to mimic emphysematous changes in COPD by degrading elastin within the extracellular matrix, leading to alveolar destruction [124,125]. In a cellular context, the WI-38–alveolosphere system combined with AT2 cells can utilize elastase to model ECM remodeling and alveolar injury. This allowed for the investigation of AT2 responses to ECM stress and the supportive role of mesenchymal fibroblasts in tissue repair. Additionally, while elastase effectively models structural alveolar damage, it does not fully reproduce the chronic inflammatory components of COPD, which may require complementary stimuli such as cytokines [126] or cigarette smoke extract [124,125]. Nevertheless, this approach provides a valuable platform for studying alveolar injury and regeneration, particularly the interactions between AT2 cells and WI-38 fibroblasts, in a controlled, human-relevant system.
The emerging paradigm of metformin is particularly compelling, and when combined with the potential of testing novel targets in the WI-38 model, it may open new avenues for IPF-COPD research. Such approaches hold promise not only for halting disease progression but also for promoting tissue regeneration. TGF-β is a master regulator promoting both conditions [127,128]. Thus, it would be of high interest to test the potential effect of TGF-β inhibitors in the WI-38 model, together with alveolosphere, in the context of TGF-β-induced IPF or elastase-induced COPD, which could give some interesting findings.
Another characteristic shared by both diseases is their reliance on inflammatory responses [2,126]. Although several anti-inflammatory targets have not yielded promising outcomes in clinical trials [129], others remain under active investigation, as comprehensively reviewed by [2]. Consequently, the field remains open to testing the effects of diverse inflammatory inhibitors in cellular models, which may provide valuable insights.
Using a cellular approach, a recent study demonstrated that mouse fibroblasts can be directly converted into alveolar epithelial-like cells by introducing specific transcription factors, including Nkx2-1, Foxa1, Foxa2, and Gata6, in an in vitro system. These reprogrammed cells showed hallmarks of alveolar type 2 cells, including lamellar body-like structures and characteristic molecular markers. While their ability to differentiate into alveolar type 1 cells in vitro was limited, when delivered into the lungs of mice with bleomycin-induced fibrosis, the cells integrated into the alveolar tissue and gave rise to both type 1 and type 2-like alveolar cells [129]. This work presents a promising approach for generating functional alveolar cells from fibroblasts, paving the way for regenerative lung therapies. This also gives rise to the probability of using human cellular model reprogramming for a potential human approach.
These findings suggest that future therapeutic strategies should focus not only on slowing disease progression but also on actively promoting alveolar regeneration. Considering the complexity of IPF and COPD, a combined approach targeting multiple cellular and molecular pathways may be most effective (Table 2). For example, stimulating progenitor cells with FGF10 to support both epithelial and mesenchymal compartments, in conjunction with metformin to reduce inflammation and oxidative stress, could enhance repair. At the same time, modulating TGF-β signaling to limit fibrosis and testing anti-inflammatory interventions to refine the microenvironment may further support regeneration. Using the WI-38–alveolosphere co-culture system under TGF-β or elastase pressure to test such combinatorial strategies could provide new insights into cellular interactions and reveal therapeutic opportunities that have not yet been explored (Table 3).

Table 2.
Conceptual framework summarizing the sequential cellular and molecular interactions between alveolar epithelial and mesenchymal compartments during injury, fibrosis, and repair. This table integrates findings discussed in the main text and should be interpreted as a schematic synthesis rather than direct experimental evidence.

Table 3.
Proposed pipeline for therapeutic discovery in lung injury and fibrosis. The workflow begins with fibroblast state manipulation (LIF/iLIF/aMYF balance), proceeds through alveolosphere co-culture readouts, integrates multi-omics profiling to dissect epithelial–mesenchymal interaction, and results in therapeutic prioritization. This pipeline is designed as a conceptual framework; interventions highlighted are hypothetical and exploratory.
2. Discussion
The dynamic interplay between alveolar epithelial type 2 (AT2) cells, lipofibroblasts (LIFs), and immune components during lung injury and repair represents a complex biological jigsaw. The emergence of AT2/AT1 intermediate states (variously termed PATS, ADIs, or AT0 cells) during alveolar repair presents a fascinating biological enigma [3,5,13,15,16,21]. These cells, characterized by the co-expression of KRT8, CLDN4, and CTGF, display remarkable phenotypic plasticity. While their persistence correlates with disease progression, their transient state during pathophysiology suggests an evolutionarily conserved role in alveolar regeneration. This notion raises important questions about the molecular switches that determine whether these cells progress to terminal AT1 differentiation or become arrested in an intermediate, disease-promoting state. The fibroblast–AT2 interaction in both homeostatic and disease states is well described [2,29,37,40,130,131,132]. However, the reciprocal signaling from activated myofibroblasts that affects the AT2/AT1 intermediate states remains incompletely characterized. Here, the WI-38–alveolosphere co-culture system provides a compelling human-relevant platform to interrogate bidirectional communication between alveolar epithelial and mesenchymal compartments. Such a platform is essential not only for understanding alveolar injury and repair but also for defining interventions that can promote true alveologenesis—a goal that represents the ultimate therapeutic win against IPF and COPD (Table 4).

Table 4.
Comparative overview of therapeutic strategies targeting epithelial–mesenchymal signaling pathways in lung injury and fibrosis.
The identification of haloperidol as an inhibitor of myofibroblast activation expands the potential pharmacological arsenal against fibrosis and chronic pulmonary conditions [43], suggesting an effective role for neuromodulation, which has received limited attention until now. In this context, the WI-38–alveolosphere assay incorporating neuromodulators or even neuronal cells would be of high interest. Similarly, the miR-29 family’s ability to suppress TGF-β signaling and promote AT2 differentiation highlights the potential of microRNA-based therapies for alveolar regeneration.
The clinical solution likely lies in precise, context-dependent interventions: simultaneously putting the brakes on destructive AREG signaling and stalled AT2/AT1 differentiation, while accelerating the pro-regenerative pathways, such as FGF10 and YAP/TAZ signaling. Notably, emerging evidence suggests these transitional cells may not be permanently lost to disease. Even in advanced fibrosis or expanding to COPD [133,134,135,136], lineage tracing reveals AT2/AT1 intermediates that, through interventions, maintain their capacity to contribute to alveolar repair [6]. This could be a clinical game-changer. A treatment paradigm must evolve beyond simply stopping chronic injury to actively jumpstart the lung’s regenerative programs and promote alveolar regeneration.
FGF10 emerges as a pivotal factor in this context. Essential for branching morphogenesis and alveolar development [95], Fgf10 promotes epithelial repair and alveolar regeneration in preclinical models of fibrosis [18,29,30,112]. Notably, injury-activated alveolar progenitors (IAAPs) survive AT2 injury, expand, and are amplified further upon rFgf10 administration, supporting the view that these progenitor-like cells drive alveolar repair. On the mesenchymal side, Fgf10 mobilizes lung-resident mesenchymal stem cells (LR-MSCs) and repair-supportive Gli1+ cells, which serve as a niche that produces Fgf10 and sustains epithelial regeneration [117,118]. Together, these findings highlight the therapeutic potential of targeting both epithelial and mesenchymal compartments through FGF10-based interventions.
Metformin similarly holds promise, with emerging evidence suggesting that it attenuates emphysema-related alveolar dysfunction and improves outcomes in COPD patients with Type 2 diabetes mellitus [121,122,123]. Using WI-38–alveolosphere systems in combination with elastase or TGF-β injury models allows for the study of ECM remodeling, alveolar damage, and the supportive role of mesenchymal fibroblasts under controlled human-relevant conditions. Such combinatorial platforms can dissect mechanisms of injury and regeneration, providing actionable insights for therapeutic development.
Beyond these canonical pathways, the pharmacological landscape expands further. A mentioned above haloperidol inhibits myofibroblast activation [43], and miR-29 family members suppress TGF-β signaling while promoting AT2 differentiation, highlighting the potential of neuromodulation and microRNA-based therapies. The convergence of these approaches underscores that precise, context-dependent interventions simultaneously limiting destructive signals, such as AREG or TGF-β, and promoting pro-regenerative pathways, like FGF10, YAP/TAZ, or Wnt/β-catenin signaling, may be required to restore alveolar architecture.
Importantly, even in advanced fibrosis or COPD, lineage tracing reveals that AT2/AT1 intermediates retain regenerative potential [6,133,134,135,136]. This suggests that stage-specific, temporally controlled interventions such as early disruption of AREG/EGFR signaling and late-stage promotion of AT1 maturation via YAP/TAZ or Wnt modulation could harness the lung’s intrinsic regenerative programs. Combinatorial strategies addressing both epithelial dysfunction (via FGFR2b agonism or IL-1β inhibition) [137,138] and mesenchymal activation (via TGF-β blockade or PPARγ-mediated LIF reprogramming) are particularly promising.
Lung injury resolution is a gradual, multifactorial process that depends on synchronized epithelial and mesenchymal remodeling rather than a simple reversal of fibrosis. During this phase, the restoration of alveolar architecture depends on the capacity of surviving AT2 cells to re-enter the cell cycle, self-renew, and differentiate into mature AT1 cells through transitional intermediates such as DATPs and PATS. In parallel, activated myofibroblasts progressively lose their contractile phenotype and reacquire lipogenic characteristics typical of quiescent lipofibroblasts. This phenotypic reversion is supported by PPARγ activation and by the re-establishment of paracrine signaling networks involving FGF10, Wnt, and temporally regulated AREG–EGFR activity. The balance between pro-fibrotic and pro-regenerative cues determines whether the lung proceeds toward structural restitution or persistent remodeling. Consequently, impaired resolution is often associated with sustained inflammatory signaling, accumulation of transitional epithelial states, and failure of fibroblast deactivation. Understanding the molecular checkpoints that govern this transition offers new opportunities to promote endogenous repair and prevent chronic fibrosis.
Ultimately, the development of human-relevant platforms such as the WI-38–alveolosphere system is crucial for testing these multiphasic, context-specific therapies. Such systems allow for the interrogation of progenitor cell behavior, epithelial–mesenchymal crosstalk, and the dynamic cellular transitions that underpin alveologenesis. Future studies should prioritize validating interventions in vivo to bridge current in vitro findings with clinically relevant outcomes. By enabling the precise modeling of both IPF and COPD pathophysiology, these platforms provide an invaluable tool for advancing therapies that not only slow disease progression but also actively restore lung architecture, offering the potential for true alveolar regeneration.
3. Conclusions
Despite rapid advances in single-cell mapping and lineage-tracing technologies, several critical knowledge gaps remain. The precise molecular cues that govern the transition between fibroblast states, the spectrum of AT2 transitional intermediates in human lungs, and the determinants of epithelial–mesenchymal reciprocity still remain to be understood. Moreover, most mechanistic insights derive from murine injury models, highlighting the need for validation in human tissue and ex vivo platforms. From a translational standpoint, integrating in vitro models such as WI-38 fibroblast–alveolosphere co-cultures and precision-cut lung slices could accelerate the identification of context-specific antifibrotic therapies. Clarifying these regulatory checkpoints will be essential to bridge fundamental discovery with clinical intervention in chronic lung diseases such as IPF and COPD.
This review underscores the dynamic reciprocity between alveolar epithelial type 2 (AT2) cells and lipofibroblasts (LIFs) as the central determinant of lung injury resolution or progression toward fibrosis. Transitional AT2/AT1 states, coupled with mesenchymal activation and AREG–EGFR signaling, define a reversible but tightly regulated network that dictates alveolar fate. Integrating human-relevant systems such as WI-38 fibroblast and alveolosphere co-culture models provides a practical framework for dissecting these epithelial–mesenchymal interactions and testing pharmacological interventions. Compounds such as metformin, haloperidol, rosiglitazone, saracatinib, and FGF10 exemplify strategies capable of reprogramming fibrotic pathways while enhancing epithelial regeneration. Together, these advances highlight the feasibility of a dual therapeutic paradigm—simultaneously halting fibrosis and restoring alveolar architecture—paving the way for truly regenerative treatments in idiopathic pulmonary fibrosis and COPD.
Author Contributions
Conceptualization, S.B.; Writing—original draft, Writing—review and editing. G.-D.P.; Writing—review and editing, M.C.; Writing—review and editing, X.Y., M.M., S.R. and X.C.; Writing—review and editing, S.B.; supervision, S.B.; project administration, S.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding from the DFG (BE4443/18-1, BE4443/1-1, BE4443/4-1, BE4443/6-1, KFO309 284237345 P7, and SFB CRC1213 268555672 projects A02 and A04), UKGM, the Universities of Giessen and Marburg Lung Center (UGMLC), and DZL.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors declare that no generative AI was used in the creation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript.
| ACTA2 | Alpha Smooth Muscle Actin |
| AEC(s) | Alveolar Epithelial Cell(s) |
| ADIs | Alveolar Differentiation Intermediates |
| AEP(s) | Alveolar Epithelial Progenitor(s) (Axin2+ AT2 subset) |
| AGER | Advanced Glycosylation End-Product Specific Receptor (AT1 marker) |
| AKT | Protein Kinase B |
| ALK5 | Activin Receptor-Like Kinase 5 (TGF-β type I receptor) |
| AREG | Amphiregulin |
| AT0 | Alveolar Type 0 transitional cell (human) |
| AT1 | Alveolar Type I epithelial cell |
| AT2 | Alveolar Type II epithelial cell |
| AT2/AT1 | Transitional AT2-to-AT1 intermediate state |
| ATAC-seq | Assay for Transposase-Accessible Chromatin using sequencing |
| AXIN2 | Axis Inhibition Protein 2 (Wnt pathway component) |
| BLM | Bleomycin (injury model) |
| BMP | Bone Morphogenetic Protein |
| CCL5 (RANTES) | Chemokine (C-C motif) Ligand 5 |
| CDC42 | Cell Division Control Protein 42 |
| CLDN4 | Claudin-4 |
| COPD | Chronic Obstructive Pulmonary Disease |
| CTGF | Connective Tissue Growth Factor |
| CTHRC1 | Collagen Triple-Helix Repeat Containing-1 (myofibroblast marker) |
| CXCL8 (IL-8) | C-X-C Motif Chemokine Ligand 8 |
| CXCL10/11 | C-X-C Motif Chemokine Ligand 10/11 |
| DAMP(s) | Damage-Associated Molecular Pattern(s) |
| DATP(s) | Damage-Associated Transient Progenitor(s) |
| DREAM | DP, RB-like, E2F and MuvB complex |
| ECM | Extracellular Matrix |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| ELN | Elastase (injury model) |
| EMT/MET | Epithelial-to-Mesenchymal Transition/Mesenchymal-to-Epithelial Transition |
| ER stress | Endoplasmic Reticulum stress |
| ERK/MAPK | Extracellular Signal-Regulated Kinase/Mitogen-Activated Protein Kinase |
| FGF10 | Fibroblast Growth Factor 10 |
| FGFR2b | Fibroblast Growth Factor Receptor 2b |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GFP | Green Fluorescent Protein |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HIF-1α | Hypoxia-Inducible Factor-1 alpha |
| HO-1 | Heme Oxygenase-1 |
| HOPX | Homeodomain Only Protein Homeobox |
| IAAP(s) | Injury-Activated Alveolar Progenitor(s) (Pd-l1+/TomLow subset) |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IFN-β | Interferon beta |
| IGFBP2 | Insulin-like Growth Factor-Binding Protein 2 |
| IL-1β | Interleukin-1 beta |
| IL-17 | Interleukin-17 |
| IPF | Idiopathic Pulmonary Fibrosis |
| ISG(s) | Interferon-Stimulated Gene(s) |
| KRT8/18/19 | Keratin-8/18/19 |
| LR-MSC(s) | Lung-Resident Mesenchymal Stem Cell(s) |
| LIF | Lipofibroblast |
| LOX | Lysyl Oxidase |
| iLIF | Inflammatory Lipofibroblast (intermediate) |
| aMYF | Activated Myofibroblast |
| MAPK14 | p38α Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein-1 (CCL2) |
| mTOR | Mammalian Target of Rapamycin |
| MRI | Magnetic Resonance Imaging |
| MDA5 | Melanoma Differentiation-Associated protein 5 |
| M-CSF | Macrophage Colony-Stimulating Factor |
| miRNA | MicroRNA |
| MRP1 | Multidrug Resistance-Associated Protein 1 |
| NDRG1 | N-Myc Downstream Regulated Gene 1 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| Notch1 | Neurogenic locus notch homolog protein 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ox-LDL | Oxidized Low-Density Lipoprotein Cholesterol |
| PAPC(s) | Primed Alveolar Progenitor Cell(s) |
| PATS | Pre-Alveolar Type-1 Transitional cell(s) |
| PCLAF (PAF/KIAA0101) | PCNA Clamp Associated Factor |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin Type 9 |
| PD-L1 | Programmed Death-Ligand 1 (see CD274) |
| PCLS | Precision-Cut Lung Slice(s) |
| PI3K | Phosphoinositide 3-Kinase |
| PLC | Phospholipase C |
| PNX | Pneumonectomy |
| PPARγ | Peroxisome Proliferator-Activated Receptor gamma |
| RAS (cell) | Respiratory Airway Secretory cell |
| RB | Retinoblastoma (family protein) |
| RLE-6TN | Rat Lung Epithelial cell line (type II) |
| RSMC(s) | Resident-Smooth Muscle Cell(s) |
| RT-qPCR | Reverse Transcription quantitative PCR |
| scRNA-seq | Single-cell RNA-sequencing |
| SCGB1A1/SCGB3A2 | Secretoglobin family 1A1/3A2 |
| SFTPB/SFTPC | Surfactant Protein B/C |
| SFK | Src-Family Kinase(s) |
| Sfn | Stratifin (14-3-3 sigma) |
| Sox2/SOX2 | SRY-Box Transcription Factor 2 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| TGF-β | Transforming Growth Factor beta |
| TLR | Toll-Like Receptor |
| TNFα | Tumor Necrosis Factor alpha |
| WI-38 | Human embryonic lung fibroblast cell line |
| Wnt/β-catenin | Wingless/β-catenin signaling pathway |
| YAP/TAZ | Yes-Associated Protein/Transcriptional co-Activator with PDZ-binding motif |
References
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, G.-D.; Vasquez-Pacheco, E.; Chu, X.; Seeger, W.; El Agha, E.; Bellusci, S.; Lingampally, A. Revisiting pulmonary fibrosis: Inflammatory dynamics of the lipofibroblast-to-inflammatory lipofibroblast-to-activated myofibroblast reversible switch. Front. Immunol. 2025, 16, 1609509. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tata, A.; Konkimalla, A.; Katsura, H.; Lee, R.F.; Ou, J.; Banovich, N.E.; Kropski, J.A.; Tata, P.R. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat. Cell Biol. 2020, 22, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.C.; Mercer, P.F. Mechanisms of Alveolar Epithelial Injury, Repair, and Fibrosis. Ann. Am. Thorac. Soc. 2015, 12, S16–S20. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Z.; Wang, G.; Geng, J.; Wu, H.; Liu, X.; Bin, E.; Sui, J.; Dai, H.; Tang, N. Sustained amphiregulin expression in intermediate alveolar stem cells drives progressive fibrosis. Cell Stem Cell 2024, 31, 1344–1358.e6. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, V.S.; Zhang, W.; Rehman, J.; Malik, A.B. Activation of Type II Cells into Regenerative Stem Cell Antigen-1+ Cells during Alveolar Repair. Am. J. Respir. Cell Mol. Biol. 2015, 53, 113–124. [Google Scholar] [CrossRef]
- Liu, Y.; Sadikot, R.T.; Adami, G.R.; Kalinichenko, V.V.; Pendyala, S.; Natarajan, V.; Zhao, Y.-Y.; Malik, A.B. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J. Exp. Med. 2011, 208, 1473–1484. [Google Scholar] [CrossRef]
- Porter, D.W.; Hubbs, A.F.; Mercer, R.R.; Robinson, V.A.; Ramsey, D.; McLaurin, J.; Khan, A.; Battelli, L.; Brumbaugh, K.; Teass, A.; et al. Pulmonary inflammation and fibrosis following subacute inhalational exposure to silica: Determinants of progression. Pathology 1993, 25, 91–99. [Google Scholar] [CrossRef]
- Fubini, B.; Fenoglio, I.; Ceschino, R.; Ghiazza, M.; Martra, G.; Tomatis, M.; Turci, F. Silica-induced pulmonary fibrosis: Pathogenesis and therapeutic opportunities. Curr. Med. Chem. 2013, 20, 2978–2994. [Google Scholar] [CrossRef]
- Liu, T.; Santos, F.G.D.L.; Ding, L.; Wu, Z.; Phan, S.H. Amphiregulin Promotes Fibroblast Activation in Pulmonary Fibrosis. FASEB J. 2016, 30, 50.6. [Google Scholar] [CrossRef]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Wan, R.; Pan, X.; Li, B.; Zhao, H.; Yang, J.; Zhao, W.; Wang, S.; Wang, Q.; et al. Single-Cell RNA Sequencing Provides New Insights into Therapeutic Roles of Thyroid Hormone in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2023, 69, 456–469. [Google Scholar] [CrossRef]
- Verheyden, J.M.; Sun, X. A transitional stem cell state in the lung. Nat. Cell Biol. 2020, 22, 1025–1026. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.-E.; Tsagkogeorga, G.; Yanagita, M.; Koo, B.-K.; Han, N.; Lee, J.-H. Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors that Mediate Alveolar Regeneration. Cell Stem Cell 2020, 27, 366–382.e7. [Google Scholar] [CrossRef]
- Riemondy, K.A.; Jansing, N.L.; Jiang, P.; Redente, E.F.; Gillen, A.E.; Fu, R.; Miller, A.J.; Spence, J.R.; Gerber, A.N.; Hesselberth, J.R.; et al. Single-cell RNA sequencing identifies TGF-β as a key regenerative cue following LPS-induced lung injury. J. Clin. Investig. 2019, 4. [Google Scholar] [CrossRef]
- Kim, B.; Huang, Y.; Ko, K.-P.; Zhang, S.; Zou, G.; Zhang, J.; Kim, M.J.; Little, D.; Ellis, L.V.; Paschini, M.; et al. PCLAF-DREAM drives alveolar cell plasticity for lung regeneration. Nat. Commun. 2024, 15, 9169. [Google Scholar] [CrossRef]
- Chong, L.; Ahmadvand, N.; Noori, A.; Lv, Y.; Chen, C.; Bellusci, S.; Zhang, J.-S. Injury activated alveolar progenitors (IAAPs): The underdog of lung repair. Cell. Mol. Life Sci. 2023, 80, 145. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, W.J.; Frank, D.B.; Zepp, J.A.; Morley, M.P.; Alkhaleel, F.A.; Kong, J.; Zhou, S.; Cantu, E.; Morrisey, E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 2018, 555, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-Q.; Dhlamini, Q.; Chen, C.; Li, X.; Bellusci, S.; Zhang, J.-S. FGF10 and Lipofibroblasts in Lung Homeostasis and Disease: Insights Gained From the Adipocytes. Front. Cell Dev. Biol. 2021, 9, 645400. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.K.L.; Sontake, V.; Tata, A.; Kobayashi, Y.; Macadlo, L.; Okuda, K.; Conchola, A.S.; Nakano, S.; Gregory, S.; Miller, L.A.; et al. Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature 2022, 604, 111–119. [Google Scholar] [CrossRef]
- Han, S.; Budinger, G.S.; Gottardi, C.J. Alveolar epithelial regeneration in the aging lung. J. Clin. Investig. 2023, 133, e170504. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Cardenas-Diaz, F.L.; Kathiriya, J.J.; Morley, M.P.; Carl, J.; Brumwell, A.N.; Katzen, J.; Slovik, K.J.; Babu, A.; Zhou, S.; et al. Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature 2022, 604, 120–126. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Huang, H.; Li, J.; Wang, Z.; Yu, Y.; Zhang, C.; Li, J.; Dai, H.; Wang, F.; et al. Pulmonary alveolar type I cell population consists of two distinct subtypes that differ in cell fate. Proc. Natl. Acad. Sci. USA 2018, 115, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Ciminieri, C.; Woest, M.E.; Reynaert, N.L.; Heijink, I.H.; Wardenaar, R.; Spierings, D.C.J.; Brandsma, C.-A.; Königshoff, M.; Gosens, R. IL-1β Induces a Proinflammatory Fibroblast Microenvironment that Impairs Lung Progenitors’ Function. Am. J. Respir. Cell Mol. Biol. 2023, 68, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Rudd, R.; Haslam, P.; Turnerwarwick, M. Cryptogenic fibrosing alveolitis—Relationships of pulmonary physiology and bronchoalveolar lavage to response to treatment and prognosis. Am. Rev. Respir. Dis. 1981, 124, 1–8. [Google Scholar]
- Turner-Warwick, M.; Burrows, B.; Johnson, A. Cryptogenic fibrosing alveolitis: Clinical features and their influence on survival. Thorax 1980, 35, 171–180. [Google Scholar] [CrossRef]
- Jones, M.R.; Lingampally, A.; Ahmadvand, N.; Chong, L.; Wu, J.; Wilhem, J.; Vazquez-Armendariz, A.I.; Ansari, M.; Herold, S.; Ornitz, D.M.; et al. FGFR2b signalling restricts lineage-flexible alveolar progenitors during mouse lung development and converges in mature alveolar type 2 cells. Cell. Mol. Life Sci. 2022, 79, 609. [Google Scholar] [CrossRef]
- Ahmadvand, N.; Lingampally, A.; Khosravi, F.; Vazquez-Armendariz, A.I.; Rivetti, S.; Jones, M.R.; Wilhelm, J.; Herold, S.; Barreto, G.; Koepke, J.; et al. Fgfr2b signaling is essential for the maintenance of the alveolar epithelial type 2 lineage during lung homeostasis in mice. Cell. Mol. Life Sci. 2022, 79, 302. [Google Scholar] [CrossRef]
- Lv, Y.-Q.; Cai, G.-F.; Zeng, P.-P.; Dhlamini, Q.; Chen, L.-F.; Chen, J.-J.; Lyu, H.-D.; Mossahebi-Mohammadi, M.; Ahmadvand, N.; Bellusci, S.; et al. FGF10 Therapeutic Administration Promotes Mobilization of Injury-Activated Alveolar Progenitors in a Mouse Fibrosis Model. Cells 2022, 11, 2396. [Google Scholar] [CrossRef]
- Yuan, T.; Volckaert, T.; Redente, E.F.; Hopkins, S.; Klinkhammer, K.; Wasnick, R.; Chao, C.-M.; Yuan, J.; Zhang, J.-S.; Yao, C.; et al. FGF10-FGFR2B Signaling Generates Basal Cells and Drives Alveolar Epithelial Regeneration by Bronchial Epithelial Stem Cells after Lung Injury. Stem Cell Rep. 2019, 12, 1041–1055. [Google Scholar] [CrossRef]
- Dorry, S.J.; Ansbro, B.O.; Ornitz, D.M.; Mutlu, G.M.; Guzy, R.D. FGFR2 Is Required for AEC2 Homeostasis and Survival after Bleomycin-induced Lung Injury. Am. J. Respir. Cell Mol. Biol. 2020, 62, 608–621. [Google Scholar] [CrossRef]
- Günther, A.; Lübke, N.; Ermert, M.; Schermuly, R.T.; Weissmann, N.; Breithecker, A.; Markart, P.; Ruppert, C.; Quanz, K.; Ermert, L.; et al. Prevention of Bleomycin-induced Lung Fibrosis by Aerosolization of Heparin or Urokinase in Rabbits. Am. J. Respir. Crit. Care Med. 2003, 168, 1358–1365. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Huang, H.; Hu, Y.; Fu, S.; Wang, Z.; Shi, M.; Zhao, X.; Yuan, J.; Li, J.; et al. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell 2020, 180, 107–121.e17. [Google Scholar] [CrossRef]
- Umbayev, B.; Safarova, Y.; Yermekova, A.; Nessipbekova, A.; Syzdykova, A.; Askarova, S. Role of a small GTPase Cdc42 in aging and age-related diseases. Biogerontology 2023, 24, 27–46. [Google Scholar] [CrossRef] [PubMed]
- El Agha, E.; Moiseenko, A.; Kheirollahi, V.; De Langhe, S.; Crnkovic, S.; Kwapiszewska, G.; Szibor, M.; Kosanovic, D.; Schwind, F.; Schermuly, R.T.; et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 2017, 20, 261–273.e3. [Google Scholar] [CrossRef]
- Lingampally, A.; Truchi, M.; Mauduit, O.; Delcroix, V.; Vasquez-Pacheco, E.; Gautier-Isola, M.; Chu, X.; Khadim, A.; Chao, C.-M.; Zabihi, M.; et al. Evidence for a lipofibroblast-to-Cthrc1+myofibroblast reversible switch during the development and resolution of lung fibrosis in young mice. Eur. Respir. J. 2024, 65, 2300482. [Google Scholar] [CrossRef] [PubMed]
- Lingampally, A.; Truchi, M.; Shi, X.; Zhou, Y.; Vasquez-Pacheco, E.; Panagiotidis, G.; Hadzic, S.; Koepke, J.; Vazquez-Armendariz, A.I.; Herold, S.; et al. Unraveling Alveolar Fibroblast and Activated Myofibroblast Heterogeneity and Differentiation Trajectories During Lung Fibrosis Development and Resolution in Young and Old Mice. Aging Cell 2025, 24, e14503. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Wolters, P.J.; Sheppard, D. Alveolar fibroblast lineage orchestrates lung inflammation and fibrosis. Nature 2024, 631, 627–634. [Google Scholar] [CrossRef]
- Vásquez-Pacheco, E.; Marega, M.; Lingampally, A.; Fassy, J.; Truchi, M.; Goth, K.; Trygub, L.; Taghizadeh, S.; Bartkuhn, M.; Alexopoulos, I.; et al. Highlighting fibroblast plasticity in lung fibrosis: The WI-38 cell line as a model for investigating the myofibroblast and lipofibroblast switch. Theranostics 2024, 14, 3603–3622. [Google Scholar] [CrossRef]
- Kheirollahi, V.; Wasnick, R.M.; Biasin, V.; Vazquez-Armendariz, A.I.; Chu, X.; Moiseenko, A.; Weiss, A.; Wilhelm, J.; Zhang, J.-S.; Kwapiszewska, G.; et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat. Commun. 2019, 10, 2987. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Fuentes, H.A.; Barreto, G.; Al-Suhaimi, E.A.; Liehn, E.A. Fibroblast plasticity in pulmonary and cardiovascular fibrosis: Mechanistic insights and emerging therapeutic targets. Arch. Med. Res. 2025, 56, 103173. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Vodret, S.; Braga, L.; Guarnaccia, C.; Celsi, F.; Rossetti, G.; Martinelli, V.; Battini, T.; Long, C.; Vukusic, K.; et al. High-throughput screening discovers antifibrotic properties of haloperidol by hindering myofibroblast activation. J. Clin. Investig. 2019, 4, e123987. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Liu, J.; Pan, T.; Wang, Q.; Miao, K.; Xu, Y.; Xiong, W.; Yu, J. Dopamine receptor agonists ameliorate bleomycin-induced pulmonary fibrosis by repressing fibroblast differentiation and proliferation. Biomed. Pharmacother. 2021, 139, 111500. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.A.; Daugherty, L.E.; Thatcher, T.H.; Lakatos, H.F.; Ray, D.M.; Redonnet, M.; Phipps, R.P.; Sime, P.J. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: Implications for therapy of lung fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2005, 288, L1146–L1153. [Google Scholar] [CrossRef]
- Rehan, V.K.; Wang, Y.; Patel, S.; Santos, J.; Torday, J.S. Rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr. Pulmonol. 2006, 41, 558–569. [Google Scholar] [CrossRef]
- Gan, C.; Zhang, Q.; Liu, H.; Wang, G.; Wang, L.; Li, Y.; Tan, Z.; Yin, W.; Yao, Y.; Xie, Y.; et al. Nifuroxazide ameliorates pulmonary fibrosis by blocking myofibroblast genesis: A drug repurposing study. Respir. Res. 2022, 23, 32. [Google Scholar] [CrossRef]
- Gupta, P.; Ashar, Y.V.; Ashby, C.R.; Lin, L.; Chen, Z.-S. The Oncogenic Protein, Breakpoint Cluster (BCR)-Abelson Kinase (ABL) and Chronic Myelocytic Leukemia (CML): Insight Into the Drug Resistance Mechanisms and Approaches for Targeting BCR-ABL in CML. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 312–328. [Google Scholar] [CrossRef]
- Portugal, C.C.; Almeida, T.O.; Socodato, R.; Relvas, J.B. Src family kinases (SFKs): Critical regulators of microglial homeostatic functions and neurodegeneration in Parkinson’s and Alzheimer’s diseases. FEBS J. 2021, 289, 7760–7775. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Ahangari, F.; Becker, C.; Foster, D.G.; Chioccioli, M.; Nelson, M.; Beke, K.; Wang, X.; Justet, A.; Adams, T.; Readhead, B.; et al. Saracatinib, a Selective Src Kinase Inhibitor, Blocks Fibrotic Responses in Preclinical Models of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2022, 206, 1463–1479. [Google Scholar] [CrossRef]
- Feng, L.; Li, W.; Chao, Y.; Huan, Q.; Lu, F.; Yi, W.; Jun, W.; Binbin, C.; Na, L.; Shougang, Z. Synergistic Inhibition of Renal Fibrosis by Nintedanib and Gefitinib in a Murine Model of Obstructive Nephropathy. Kidney Dis. 2020, 7, 34–49. [Google Scholar] [CrossRef]
- Willis, B.C.; Liebler, J.M.; Luby-Phelps, K.; Nicholson, A.G.; Crandall, E.D.; du Bois, R.M.; Borok, Z. Induction of Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells by Transforming Growth Factor-β1. Am. J. Pathol. 2005, 166, 1321–1332. [Google Scholar] [CrossRef]
- Higashiyama, H.; Yoshimoto, D.; Kaise, T.; Matsubara, S.; Fujiwara, M.; Kikkawa, H.; Asano, S.; Kinoshita, M. Inhibition of activin receptor-like kinase 5 attenuates Bleomycin-induced pulmonary fibrosis. Exp. Mol. Pathol. 2007, 83, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018, 359, 1118–1123. [Google Scholar] [CrossRef]
- McGowan, S.E. Retinoids and pulmonary alveolar regeneration: Rationale and therapeutic challenges. Drug Discov. Today Dis. Mech. 2006, 3, 77–84. [Google Scholar] [CrossRef]
- Nabeyrat, E.; Besnard, V.; Corroyer, S.; Cazals, V.; Clement, A. Retinoic acid-induced proliferation of lung alveolar epithelial cells: Relation with the IGF system. Am. J. Physiol. Cell. Mol. Physiol. 1998, 275, L71–L79. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Choi, E.-H.; Kim, Y.; Kim, M.; Choi, D.; Kim, W.; Cha, B. Identification of YAP regulators through high-throughput screening and NanoBiT-based validation-drug repositioning for cancer therapy. Anim. Cells Syst. 2025, 29, 325–338. [Google Scholar] [CrossRef]
- LaCanna, R.; Liccardo, D.; Zhang, P.; Tragesser, L.; Wang, Y.; Cao, T.; Chapman, H.A.; Morrisey, E.E.; Shen, H.; Koch, W.J.; et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Investig. 2019, 129, 2107–2122. [Google Scholar] [CrossRef]
- Shoyab, M.; McDonald, V.L.; Bradley, J.G.; Todaro, G.J. Amphiregulin: A bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc. Natl. Acad. Sci. USA 1988, 85, 6528–6532. [Google Scholar] [CrossRef] [PubMed]
- Avraham, R.; Yarden, Y. Feedback regulation of EGFR signalling: Decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 2011, 12, 104–117. [Google Scholar] [CrossRef]
- Berasain, C.; Castillo, J.; Perugorría, M.; Prieto, J.; Avila, M. Amphiregulin: A new growth factor in hepatocarcinogenesis. Cancer Lett. 2007, 254, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Busser, B.; Sancey, L.; Brambilla, E.; Coll, J.-L.; Hurbin, A. The multiple roles of amphiregulin in human cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2011, 1816, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Avila, M.A. Amphiregulin. Semin. Cell Dev. Biol. 2014, 28, 31–41. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, J.-Y.; Lee, C.-M.; Cho, W.-K.; Kang, M.-J.; Koff, J.L.; Yoon, P.-O.; Chae, J.; Park, H.-O.; Elias, J.A.; et al. Amphiregulin, an Epidermal Growth Factor Receptor Ligand, Plays an Essential Role in the Pathogenesis of Transforming Growth Factor-β-induced Pulmonary Fibrosis. J. Biol. Chem. 2012, 287, 41991–42000. [Google Scholar] [CrossRef]
- Kindermann, M.; Knipfer, L.; Atreya, I.; Wirtz, S. ILC2s in infectious diseases and organ-specific fibrosis. Semin. Immunopathol. 2018, 40, 379–392. [Google Scholar] [CrossRef]
- Nakagome, K.; Nagata, M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx 2011, 38, 555–563. [Google Scholar] [CrossRef]
- Richeldi, L.; Kreuter, M.; Selman, M.; Crestani, B.; Kirsten, A.-M.; AWuyts, W.; Xu, Z.; Bernois, K.; Stowasser, S.; Quaresma, M.; et al. Long-term treatment of patients with idiopathic pulmonary fibrosis with nintedanib: Results from the TOMORROW trial and its open-label extension. Thorax 2017, 73, 581–583. [Google Scholar] [CrossRef]
- da Silva, R.F.; Dhar, D.; Raina, K.; Kumar, D.; Kant, R.; Cagnon, V.H.A.; Agarwal, C.; Agarwal, R. Nintedanib inhibits growth of human prostate carcinoma cells by modulating both cell cycle and angiogenesis regulators. Sci. Rep. 2018, 8, 9540. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Yao, F.; Huang, C. Lysyl oxidase family proteins: Prospective therapeutic targets in cancer. Int. J. Mol. Sci. 2022, 23, 12270. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, P.A.; Jones, C.M.; Rotile, N.J.; Koerner, S.K.; Ferreira, D.S.; Chen, H.H.; Probst, C.K.; Tager, A.M.; Caravan, P. Molecular magnetic resonance imaging of lung fibrogenesis with an oxyamine-based probe. Angew. Chem. Int. Ed. Engl. 2017, 56, 9825–9828. [Google Scholar] [CrossRef]
- Haak, A.J.; Tan, Q.; Tschumperlin, D.J. Matrix biomechanics and dynamics in pulmonary fibrosis. Matrix Biol. 2018, 73, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.E.; Alsafadi, H.N.; Mitash, N.; Justet, A.; Hu, Q.; Pineda, R.; Staab-Weijnitz, C.; Korfei, M.; Gvazava, N.; Wannemo, K.; et al. Inhibition of epithelial cell YAP–TEAD/LOX signaling attenuates pulmonary fibrosis in preclinical models. Nat. Commun. 2025, 16, 7099. [Google Scholar] [CrossRef]
- Lin, J.; Pan, Z.; Sun, J.; Wang, X.; Yin, D.; Huo, C.; Guo, Q. PCSK9 inhibitor alleviates experimental pulmonary fibrosis–induced pulmonary hypertension via attenuating epithelial–mesenchymal transition by suppressing Wnt/β-catenin signaling in vivo and in vitro. Front. Med. 2024, 11, 1509168. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Theus, S.; Deng, X.; Fan, Y.; Zhou, S.; Mehta, J.L. PCSK9 regulates expression of scavenger receptors and ox-LDL uptake in macrophages. Cardiovasc. Res. 2018, 114, 1145–1153. [Google Scholar] [CrossRef]
- Cui, C.; Yan, A.; Huang, S.; Chen, Y.; Zhao, J.; Li, C.; Wang, X.; Yang, J. PCSK9 manipulates lipid metabolism and the immune microenvironment in cancer. Onco Targets Ther. 2025, 18, 411–427. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Prattichizzo, F.; Marfella, R.; Sardu, C.; Martino, E.; Scisciola, L.; Marfella, L.; Grotta, R.; Frigé, C.; Paolisso, G.; et al. SIRT3 mediates the effects of PCSK9 inhibitors on inflammation, autophagy, and oxidative stress in endothelial cells. Theranostics 2023, 13, 531–542. [Google Scholar] [CrossRef]
- Mehta, J.L.; Sanada, N.; Hu, C.P.; Chen, J.; Dandapat, A.; Sugawara, F.; Satoh, H.; Inoue, K.; Kawase, Y.; Jishage, K.; et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed a high-cholesterol diet. Circ. Res. 2007, 100, 1634–1642. [Google Scholar] [CrossRef]
- Pandit, K.V.; Milosevic, J. MicroRNA regulatory networks in idiopathic pulmonary fibrosis. Biochem. Cell Biol. 2015, 93, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.V.; Schwartz, D.A. Epigenetic Control of Gene Expression in the Lung. Am. J. Respir. Crit. Care Med. 2011, 183, 1295–1301. [Google Scholar] [CrossRef]
- Carraro, G.; El-Hashash, A.; Guidolin, D.; Tiozzo, C.; Turcatel, G.; Young, B.M.; De Langhe, S.P.; Bellusci, S.; Shi, W.; Parnigotto, P.P.; et al. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev. Biol. 2009, 333, 238–250. [Google Scholar] [CrossRef]
- Subramanyam, D.; Lamouille, S.; Judson, R.L.; Liu, J.Y.; Bucay, N.; Derynck, R.; Blelloch, R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 443–448. [Google Scholar] [CrossRef]
- Ali, M.; Zhang, X.; LaCanna, R.; Tomar, D.; Elrod, J.W.; Tian, Y. MICU1-dependent mitochondrial calcium uptake regulates lung alveolar type 2 cell plasticity and lung regeneration. J. Clin. Investig. 2022, 7, e154447. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Lian, X.; Zhu, Z.; Chen, X.; Pei, W.; Li, S.; Abbas, A.; Wang, Y.; Tian, L. MicroRNA-29b Mediates Lung Mesenchymal-Epithelial Transition and Prevents Lung Fibrosis in the Silicosis Model. Mol. Ther.-Nucleic Acids 2019, 14, 20–31. [Google Scholar] [CrossRef]
- Castaldi, A.; Horie, M.; Rieger, M.E.; Dubourd, M.; Sunohara, M.; Pandit, K.; Zhou, B.; Offringa, I.A.; Marconett, C.N.; Borok, Z. Genome-wide integration of microRNA and transcriptomic profiles of differentiating human alveolar epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L173–L184. [Google Scholar] [CrossRef]
- Dehmel, S.; Weiss, K.J.; El-Merhie, N.; Callegari, J.; Konrad, B.; Mutze, K.; Eickelberg, O.; Königshoff, M.; Krauss-Etschmann, S. microRNA Expression Profile of Purified Alveolar Epithelial Type II Cells. Genes 2022, 13, 1420. [Google Scholar] [CrossRef]
- Oglesby, I.K.; Vencken, S.F.; Agrawal, R.; Gaughan, K.; Molloy, K.; Higgins, G.; McNally, P.; McElvaney, N.G.; Mall, M.A.; Greene, C.M. miR-17 overexpression in cystic fibrosis airway epithelial cells decreases interleukin-8 production. Eur. Respir. J. 2015, 46, 1350–1360. [Google Scholar] [CrossRef]
- Guo, W.; Benlhabib, H.; Mendelson, C.R. The MicroRNA 29 Family Promotes Type II Cell Differentiation in Developing Lung. Mol. Cell. Biol. 2016, 36, 2141. [Google Scholar] [CrossRef]
- Moimas, S.; Salton, F.; Kosmider, B.; Ring, N.; Volpe, M.C.; Bahmed, K.; Braga, L.; Rehman, M.; Vodret, S.; Graziani, M.L.; et al. miR-200 family members reduce senescence and restore idiopathic pulmonary fibrosis type II alveolar epithelial cell transdifferentiation. ERJ Open Res. 2019, 5, 00138–02019. [Google Scholar] [CrossRef]
- Callaway, D.A.; Penkala, I.J.; Zhou, S.; Knowlton, J.J.; Cardenas-Diaz, F.; Babu, A.; Morley, M.P.; Lopes, M.; Garcia, B.A.; Morrisey, E.E. TGF-β controls alveolar type 1 epithelial cell plasticity and alveolar matrisome gene transcription in mice. J. Clin. Investig. 2024, 134, e172095. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Kolliputi, N.; Wang, Y.; Gou, D.; Chintagari, N.R.; Liu, L. Trans-differentiation of Alveolar Epithelial Type II Cells to Type I Cells Involves Autocrine Signaling by Transforming Growth Factor β1 through the Smad Pathway. J. Biol. Chem. 2007, 282, 3968–3976. [Google Scholar] [CrossRef]
- Zhao, L.; Yee, M.; O’Reilly, M.A. Transdifferentiation of alveolar epithelial type II to type I cells is controlled by opposing TGF-β and BMP signaling. Am. J. Physiol. Cell. Mol. Physiol. 2013, 305, L409–L418. [Google Scholar] [CrossRef]
- Xiao, J.; Meng, X.-M.; Huang, X.R.; Chung, A.C.; Feng, Y.-L.; Hui, D.S.; Yu, C.-M.; Sung, J.J.; Lan, H.Y. miR-29 Inhibits Bleomycin-induced Pulmonary Fibrosis in Mice. Mol. Ther. 2012, 20, 1251–1260. [Google Scholar] [CrossRef]
- Juul, N.H.; Yoon, J.-K.; Martinez, M.C.; Rishi, N.; Kazadaeva, Y.I.; Morri, M.; Neff, N.F.; Trope, W.L.; Shrager, J.B.; Sinha, R.; et al. KRAS(G12D) drives lepidic adenocarcinoma through stem-cell reprogramming. Nature 2023, 619, 860–867. [Google Scholar] [CrossRef]
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B.L.M. Fibroblast Growth Factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124, 4867–4878. [Google Scholar] [CrossRef]
- Lebeche, D.; Malpel, S.; Cardoso, W.V. Fibroblast growth factor interactions in the developing lung. Mech. Dev. 1999, 86, 125–136. [Google Scholar] [CrossRef]
- Chang, D.R.; Alanis, D.M.; Miller, R.K.; Ji, H.; Akiyama, H.; McCrea, P.D.; Chen, J. Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 18042–18051. [Google Scholar] [CrossRef]
- Abler, L.L.; Mansour, S.L.; Sun, X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev. Dyn. 2009, 238, 1999–2013. [Google Scholar] [CrossRef]
- MacKenzie, B.; Henneke, I.; Hezel, S.; Al Alam, D.; El Agha, E.; Chao, C.-M.; Quantius, J.; Wilhelm, J.; Jones, M.; Goth, K.; et al. Attenuating endogenous Fgfr2b ligands during bleomycin-induced lung fibrosis does not compromise murine lung repair. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L1014–L1024. [Google Scholar] [CrossRef]
- Brownfield, D.G.; de Arce, A.D.; Ghelfi, E.; Gillich, A.; Desai, T.J.; Krasnow, M.A. Alveolar cell fate selection and lifelong maintenance of AT2 cells by FGF signaling. Nat. Commun. 2022, 13, 7137. [Google Scholar] [CrossRef]
- AMiura, T.; Holmes, K.V. Host-pathogen interactions during coronavirus infection of primary alveolar epithelial cells. J. Leukoc. Biol. 2009, 86, 1145–1151. [Google Scholar] [CrossRef]
- Chuquimia, O.D.; Petursdottir, D.H.; Rahman, M.J.; Hartl, K.; Singh, M.; Fernández, C. The Role of Alveolar Epithelial Cells in Initiating and Shaping Pulmonary Immune Responses: Communication between Innate and Adaptive Immune Systems. PLoS ONE 2012, 7, e32125. [Google Scholar] [CrossRef]
- Striz, I.; Brabcova, E.; Kolesar, L.; Liu, X.; Brabcova, I.; Sekerkova, A.; Poole, J.; Jaresova, M.; Slavcev, A.; Rennard, S. Epithelial cells modulate genes associated with NF kappa B activation in co-cultured human macrophages. Immunobiology 2011, 216, 1110–1116. [Google Scholar] [CrossRef][Green Version]
- Droemann, D.; Goldmann, T.; Branscheid, D.; Clark, R.; Dalhoff, K.; Zabel, P.; Vollmer, E. Toll-like receptor 2 is expressed by alveolar epithelial cells type II and macrophages in the human lung. Histochem. Cell Biol. 2003, 119, 103–108. [Google Scholar] [CrossRef]
- Beppu, A.K.; Zhao, J.; Yao, C.; Carraro, G.; Israely, E.; Coelho, A.L.; Drake, K.; Hogaboam, C.M.; Parks, W.C.; Kolls, J.K.; et al. Epithelial plasticity and innate immune activation promote lung tissue remodeling following respiratory viral infection. Nat. Commun. 2023, 14, 5814. [Google Scholar] [CrossRef]
- Froom, Z.S.; Callaghan, N.I.; Huyer, L.D. Cellular crosstalk in fibrosis: Insights into macrophage and fibroblast dynamics. J. Biol. Chem. 2025, 301, 110203. [Google Scholar] [CrossRef]
- Keenum, M.C.; Chatterjee, P.; Atalis, A.; Pandey, B.; Jimenez, A.; Roy, K. Single-cell epitope-transcriptomics reveal lung stromal and immune cell response kinetics to nanoparticle-delivered RIG-I and TLR4 agonists. Biomaterials 2023, 297, 122097. [Google Scholar] [CrossRef]
- Yang, N.; Luna, J.M.; Dai, P.; Wang, Y.; Rice, C.M.; Deng, L. Lung type II alveolar epithelial cells collaborate with CCR2+ inflammatory monocytes in host defense against poxvirus infection. Nat. Commun. 2022, 13, 1671. [Google Scholar] [CrossRef]
- Chuquimia, O.D.; Petursdottir, D.H.; Periolo, N.; Fernández, C. Alveolar Epithelial Cells Are Critical in Protection of the Respiratory Tract by Secretion of Factors Able To Modulate the Activity of Pulmonary Macrophages and Directly Control Bacterial Growth. Infect. Immun. 2013, 81, 381–389. [Google Scholar] [CrossRef]
- Qian, Z.; Travanty, E.A.; Oko, L.; Edeen, K.; Berglund, A.; Wang, J.; Ito, Y.; Holmes, K.V.; Mason, R.J. Innate Immune Response of Human Alveolar Type II Cells Infected with Severe Acute Respiratory Syndrome–Coronavirus. Am. J. Respir. Cell Mol. Biol. 2013, 48, 742–748. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Hussain, S.; Zheng, Y.; Sanjabi, S.; Ouaaz, F.; ABeg, A. Distinct Roles of Different NF-κB Subunits in Regulating Inflammatory and T Cell Stimulatory Gene Expression in Dendritic Cells. J. Immunol. 2007, 178, 6777–6788. [Google Scholar] [CrossRef]
- Ahmadvand, N.; Khosravi, F.; Lingampally, A.; Wasnick, R.; Vazquez-Armendariz, A.I.; Carraro, G.; Heiner, M.; Rivetti, S.; Lv, Y.; Wilhelm, J.; et al. Identification of a novel subset of alveolar type 2 cells enriched in PD-L1 and expanded following pneumonectomy. Eur. Respir. J. 2021, 58, 2004168. [Google Scholar] [CrossRef]
- Xu, X.; Rock, J.R.; Lu, Y.; Futtner, C.; Schwab, B.; Guinney, J.; Hogan, B.L.M.; Onaitis, M.W. Evidence for type II cells as cells of origin of K-Ras–induced distal lung adenocarcinoma. Proc. Natl. Acad. Sci. USA 2012, 109, 4910–4915. [Google Scholar] [CrossRef]
- Sutherland, K.D.; Berns, A. Cell of origin of lung cancer. Mol. Oncol. 2010, 4, 397–403. [Google Scholar] [CrossRef]
- Lin, C.; Song, H.; Huang, C.; Yao, E.; Gacayan, R.; Xu, S.-M.; Chuang, P.-T. Alveolar Type II Cells Possess the Capability of Initiating Lung Tumor Development. PLoS ONE 2012, 7, e53817. [Google Scholar] [CrossRef]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef]
- Tong, L.; Zhou, J.; Rong, L.; Seeley, E.J.; Pan, J.; Zhu, X.; Liu, J.; Wang, Q.; Tang, X.; Qu, J.; et al. Fibroblast Growth Factor-10 (FGF-10) Mobilizes Lung-resident Mesenchymal Stem Cells and Protects Against Acute Lung Injury. Sci. Rep. 2016, 6, 21642. [Google Scholar] [CrossRef]
- Chu, X.; Lingampally, A.; Moiseenko, A.; Kheirollahi, V.; Vazquez-Armendariz, A.I.; Koepke, J.; Khadim, A.; Kiliaris, G.; Felordi, M.S.; Zabihi, M.; et al. GLI1+ cells are a source of repair-supportive mesenchymal cells (RSMCs) during airway epithelial regeneration. Cell. Mol. Life Sci. 2022, 79, 581. [Google Scholar] [CrossRef]
- Teague, T.T.; Payne, S.R.; Kelly, B.T.; Dempsey, T.M.; McCoy, R.G.; Sangaralingham, L.R.; Limper, A.H. Evaluation for clinical benefit of metformin in patients with idiopathic pulmonary fibrosis and type 2 diabetes mellitus: A national claims-based cohort analysis. Respir. Res. 2022, 23, 91. [Google Scholar] [CrossRef]
- Jovanovic, D.; Šterclová, M.; Mogulkoc, N.; Lewandowsk, K.; Müller, V.; Hájková, M.; Kramer, M.; Tekavec-Trkanjec, J.; Studnicka, M.; Stoeva, N.; et al. The effect of metformin on clinically relevant outcomes in 3,144 IPF patients from the EMPIRE registry. Eur. Respir. J. 2020, 56 (Suppl. S64), 1791. [Google Scholar] [CrossRef]
- Kahnert, K.; Andreas, S.; Kellerer, C.; Lutter, J.I.; Lucke, T.; Yildirim, Ö.; Lehmann, M.; Seissler, J.; Behr, J.; Frankenberger, M.; et al. Reduced decline of lung diffusing capacity in COPD patients with diabetes and metformin treatment. Sci. Rep. 2022, 12, 1435. [Google Scholar] [CrossRef]
- Zhu, A.; Teng, Y.; Ge, D.; Zhang, X.; Hu, M.; Yao, X. Role of metformin in treatment of patients with chronic obstructive pulmonary disease: A systematic review. J. Thorac. Dis. 2019, 11, 4371–4378. [Google Scholar] [CrossRef]
- Tao, F.; Zhou, Y.; Wang, M.; Wang, C.; Zhu, W.; Han, Z.; Sun, N.; Wang, D. Metformin alleviates chronic obstructive pulmonary disease and cigarette smoke extract-induced glucocorticoid resistance by activating the nuclear factor E2-related factor 2/heme oxygenase-1 signaling pathway. Korean J. Physiol. Pharmacol. 2022, 26, 95–111. [Google Scholar] [CrossRef]
- Antunes, M.A.; Rocco, P.R. Elastase-induced pulmonary emphysema: Insights from experimental models. An. Acad. Bras. Cienc. 2011, 83, 1385–1396. [Google Scholar] [CrossRef]
- Snider, G.L.; Lucey, E.C.; Stone, P.J. Animal Models of Emphysema1–3. Am. Rev. Respir. Dis. 1986, 133, 149–169. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Königshoff, M.; Kneidinger, N.; Eickelberg, O. TGF-ß signaling in COPD: Deciphering genetic and cellular susceptibilities for future therapeutic regimen. Swiss Med Wkly. 2009, 139, 554–563. [Google Scholar] [CrossRef]
- Kang, H.-R.; Lee, J.-Y.; Lee, C.G. TGF-β1 as a therapeutic target for pulmonary fibrosis and COPD. Expert Rev. Clin. Pharmacol. 2008, 1, 547–558. [Google Scholar] [CrossRef]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Morita, A.; Ishii, M.; Asakura, T.; Yotsukura, M.; Hegab, A.E.; Kusumoto, T.; Namkoong, H.; Ogawa, T.; Nakatake, Y.; Oda, M.; et al. Direct reprogramming of mouse fibroblasts into self-renewable alveolar epithelial-like cells. npj Regen. Med. 2025, 10, 30. [Google Scholar] [CrossRef]
- Lingampally, A.; Jones, M.R.; Bagari, S.; Chen, C.; Rivetti, S.; Bellusci, S. Use of the Reversible Myogenic to Lipogenic Transdifferentiation Switch for the Design of Pre-clinical Drug Screening in Idiopathic Pulmonary Fibrosis. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Lingampally, A.; Truchi, M.; Mauduit, O.; Delcroix, V.; Hadzic, S.; Koepke, J.; Vazquez-Armendariz, A.I.; Herold, S.; Samakovlis, C.; Makarenkova, H.P. Lineage tracing of Acta2+ cells in aged mice during lung fibrosis formation and resolution supports the lipofibroblast-to-myofibroblast reversible switch. bioRxiv 2024. [Google Scholar] [CrossRef]
- Khadim, A.; Kiliaris, G.; Vazquez-Armendariz, A.I.; Procida-Kowalski, T.; Glaser, D.; Bartkuhn, M.; Malik, T.; Chu, X.; Moiseenko, A.; Kuznetsova, I.; et al. Myofibroblasts emerge during alveolar regeneration following influenza-virus-induced lung injury. Cell Rep. 2025, 44, 115248. [Google Scholar] [CrossRef]
- Lin, C.-R.; Bahmed, K.; Kosmider, B. Impaired Alveolar Re-Epithelialization in Pulmonary Emphysema. Cells 2022, 11, 2055. [Google Scholar] [CrossRef]
- Xu, Y.; Li, M.; Bai, L. Pulmonary Epithelium Cell Fate Determination: Chronic Obstructive Pulmonary Disease, Lung Cancer, or Both. Am. J. Respir. Cell Mol. Biol. 2024, 71, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, C.-A.; de Vries, M.; Costa, R.; Woldhuis, R.R.; Königshoff, M.; Timens, W. Lung ageing and COPD: Is there a role for ageing in abnormal tissue repair? Eur. Respir. Rev. 2017, 26, 170073. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Ora, J.; Matera, M.G. Canakinumab for the treatment of chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2015, 31, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Cottage, C.T.; Peterson, N.; Kearley, J.; Berlin, A.; Xiong, X.; Huntley, A.; Zhao, W.; Brown, C.; Migneault, A.; Zerrouki, K.; et al. Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun. Biol. 2019, 2, 307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).