The Binding of Concanavalin A to the Surface of Intact and Denuded Sea Urchin Eggs Affects the Fertilization Process by Altering the Structural Dynamics of Actin Filaments
Abstract
1. Introduction
2. Materials and Methods
2.1. Gamete Preparation and Fertilization
2.2. Time-Lapse Movies of the Fertilization Process and Visualization of the Sperm in Intact and Denuded Eggs
2.3. Scanning Electron Microscopy (SEM)
2.4. Transmission Electron Microscopy (TEM)
2.5. Chemicals, Reagents, and Recombinant Proteins
2.6. Microinjection, Ca2+ Imaging, Fluorescence, and Confocal Microscopy
2.7. Visualization of Actin Filaments and Lectin Binding
2.8. Statistical Analysis
3. Results
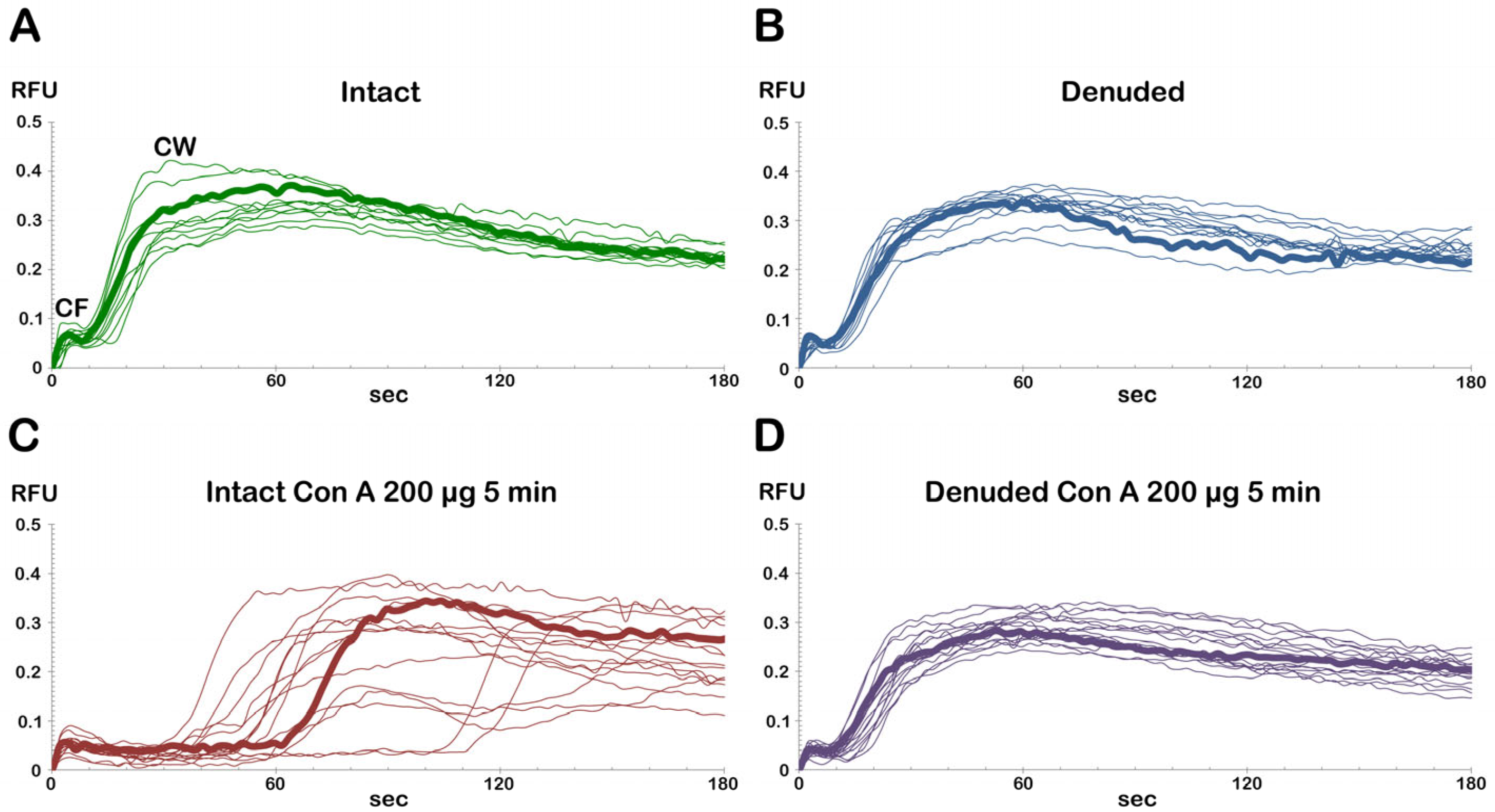
3.1. Effect of Concanavalin A Exposure on the Fertilization Envelope Elevation and Sperm Entry
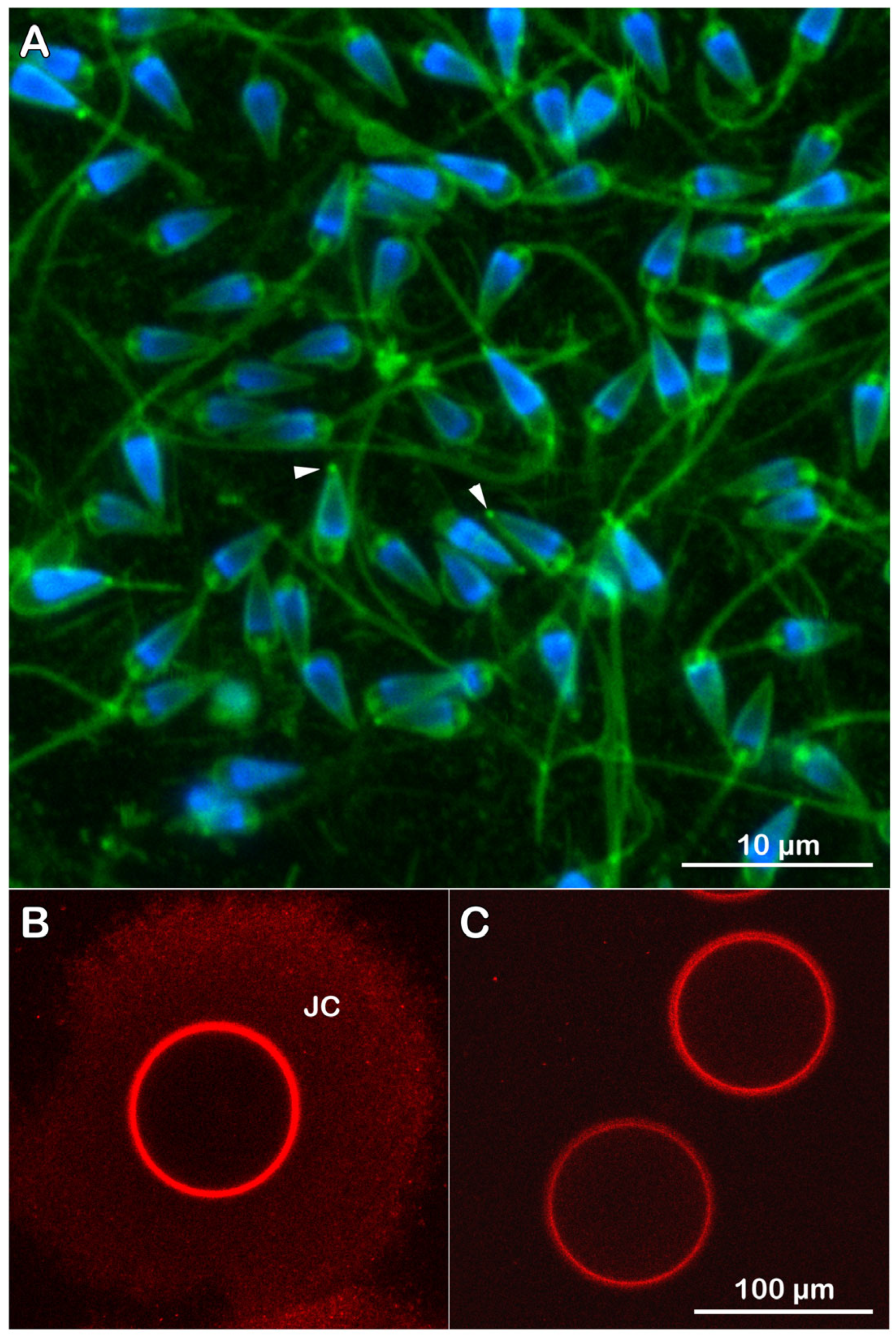
3.2. Visualization of Con A Binding on the Egg Surface
3.3. Effect of Concanavalin A Exposure on the Surface of Intact Sea Urchin Eggs Before and After Fertilization, Visualized with Electron Microscopy (Scanning and Transmission)
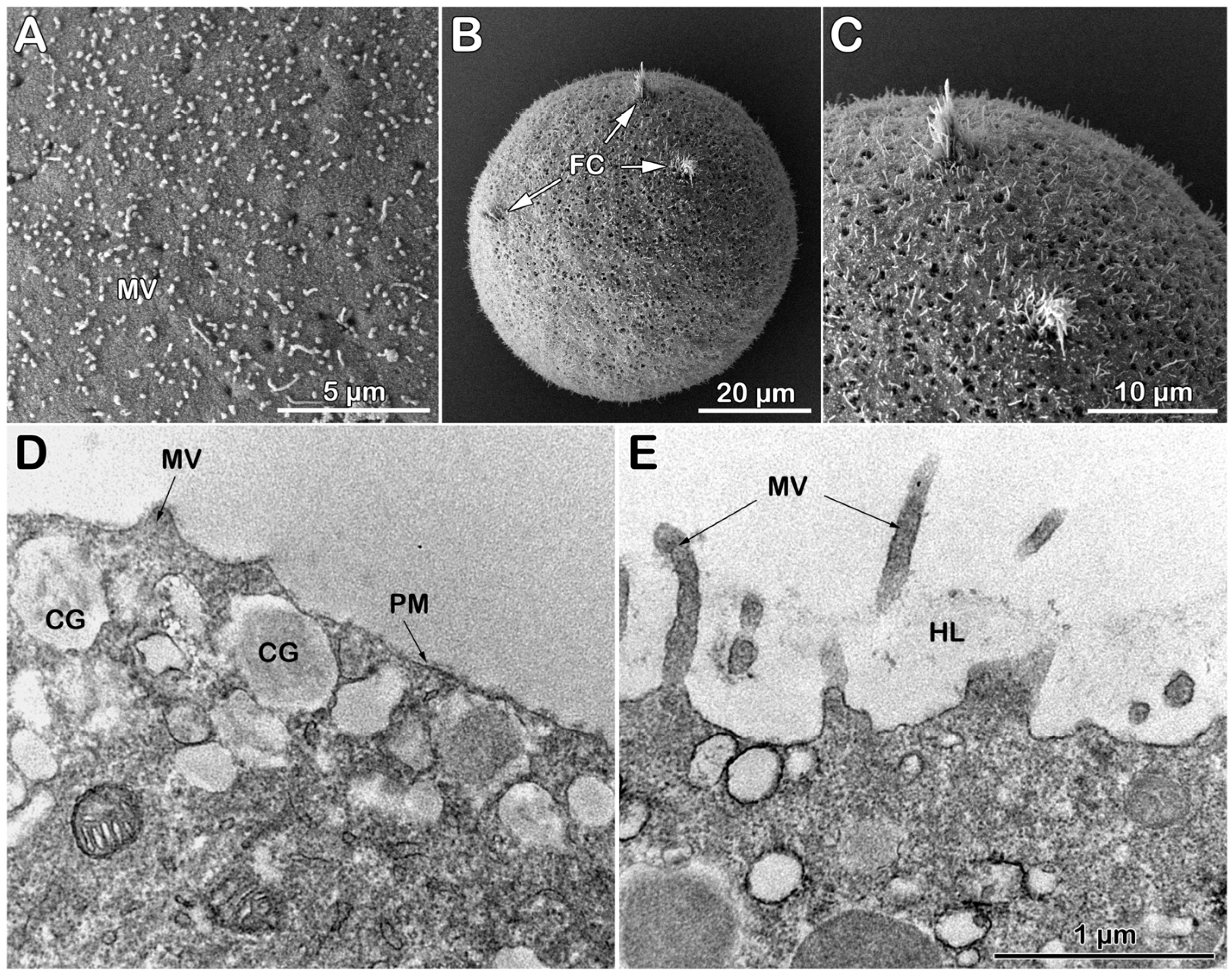
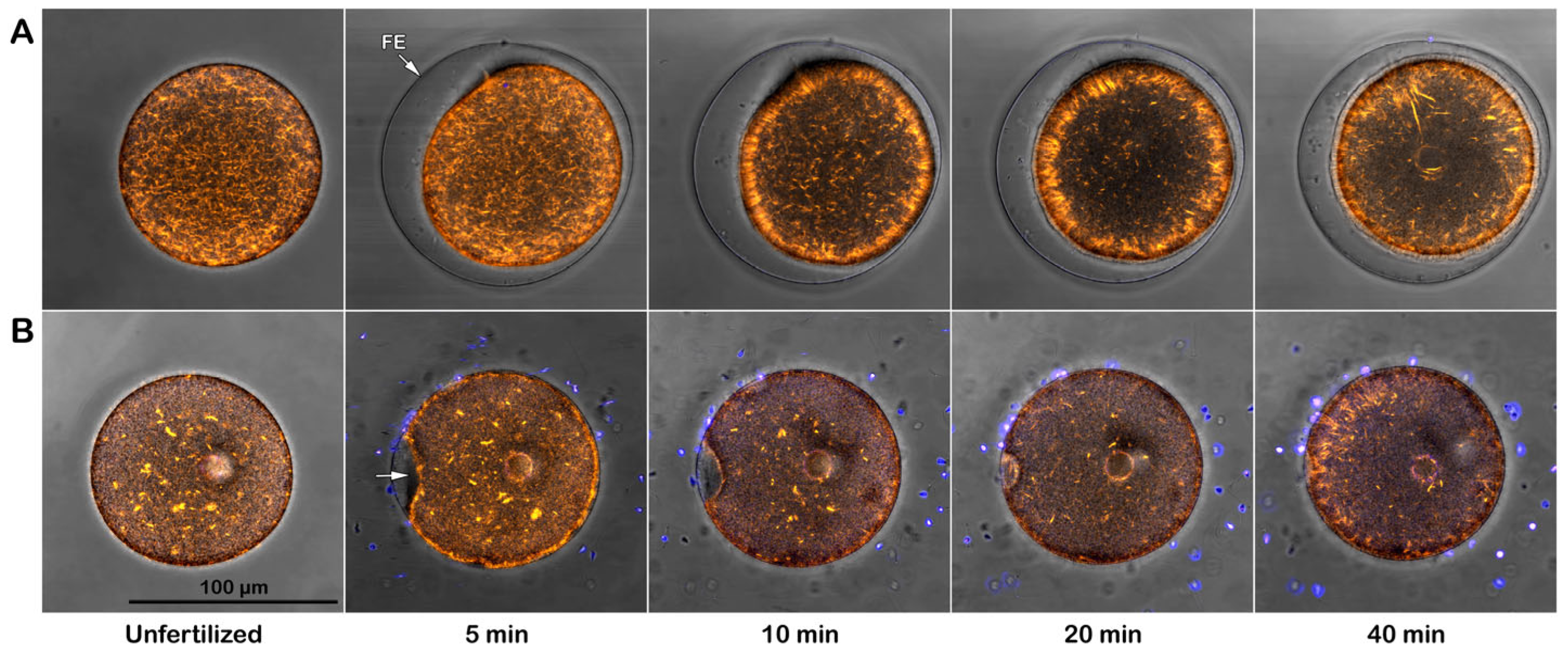
3.3.1. Topography and Ultrastructure of Intact P. lividus Eggs Before and After Fertilization
3.3.2. Effect of Con A Preincubation on the Topography and Ultrastructure of Intact Eggs
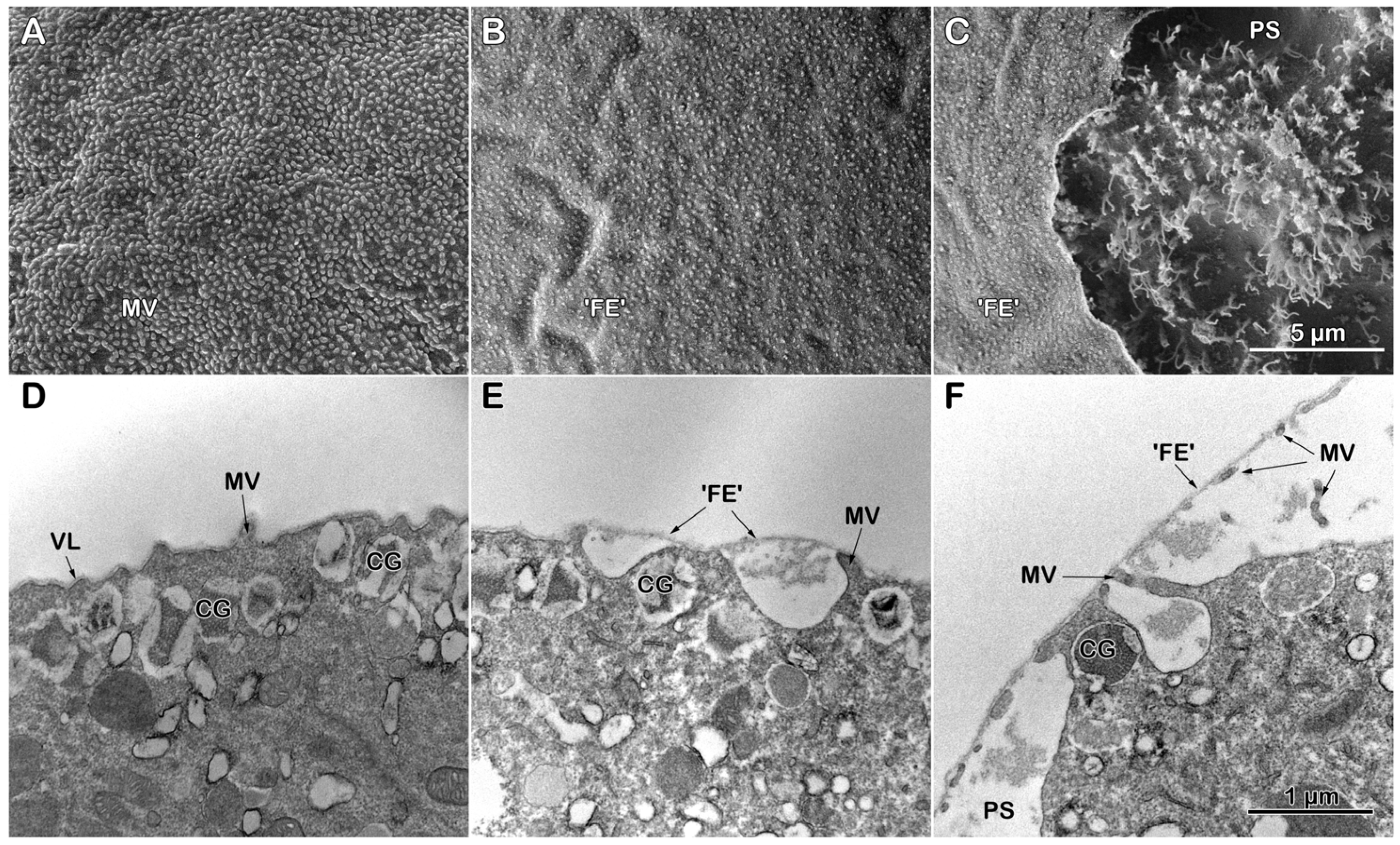
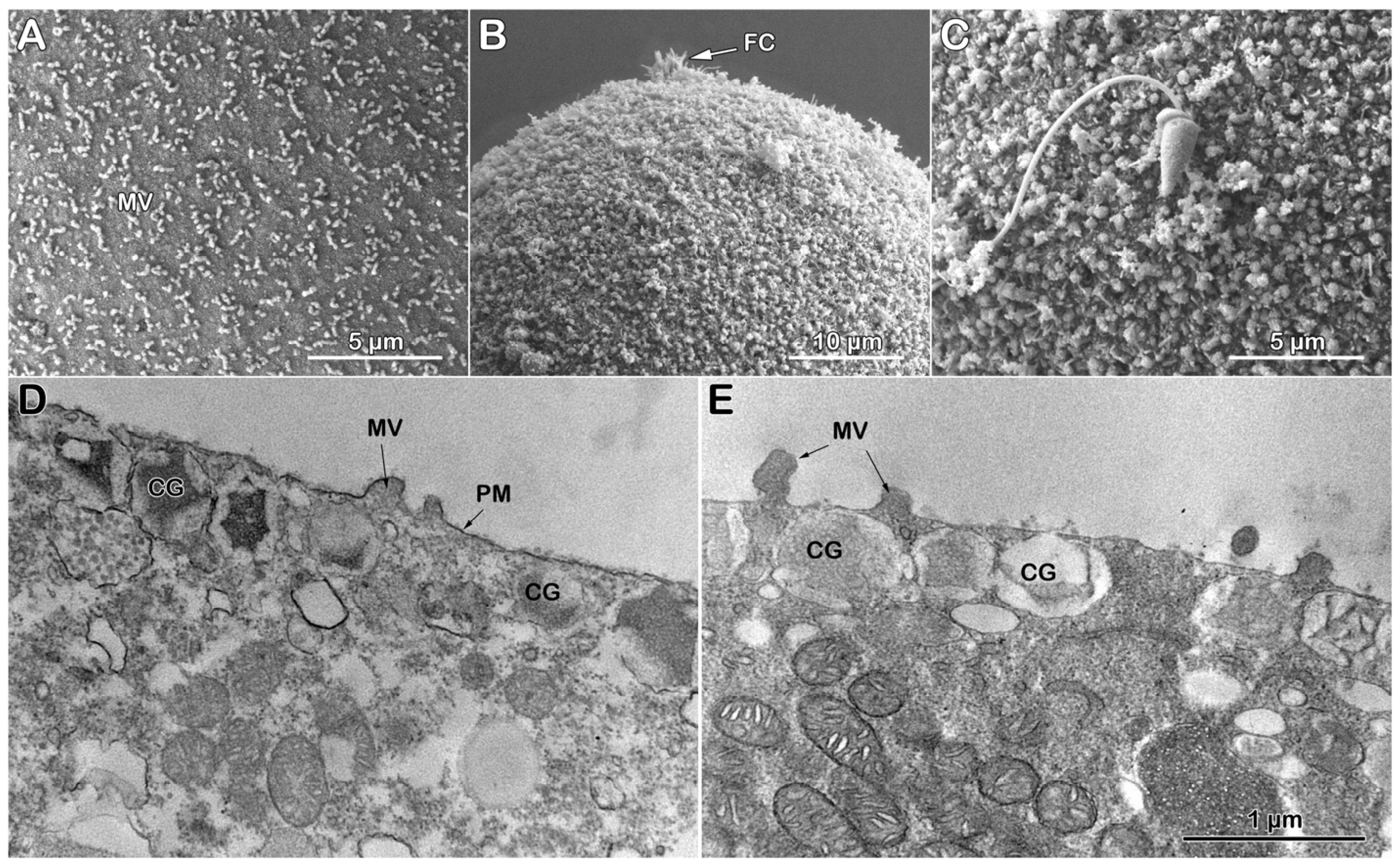
3.3.3. Topography and Ultrastructure of Denuded Eggs Before and After Fertilization
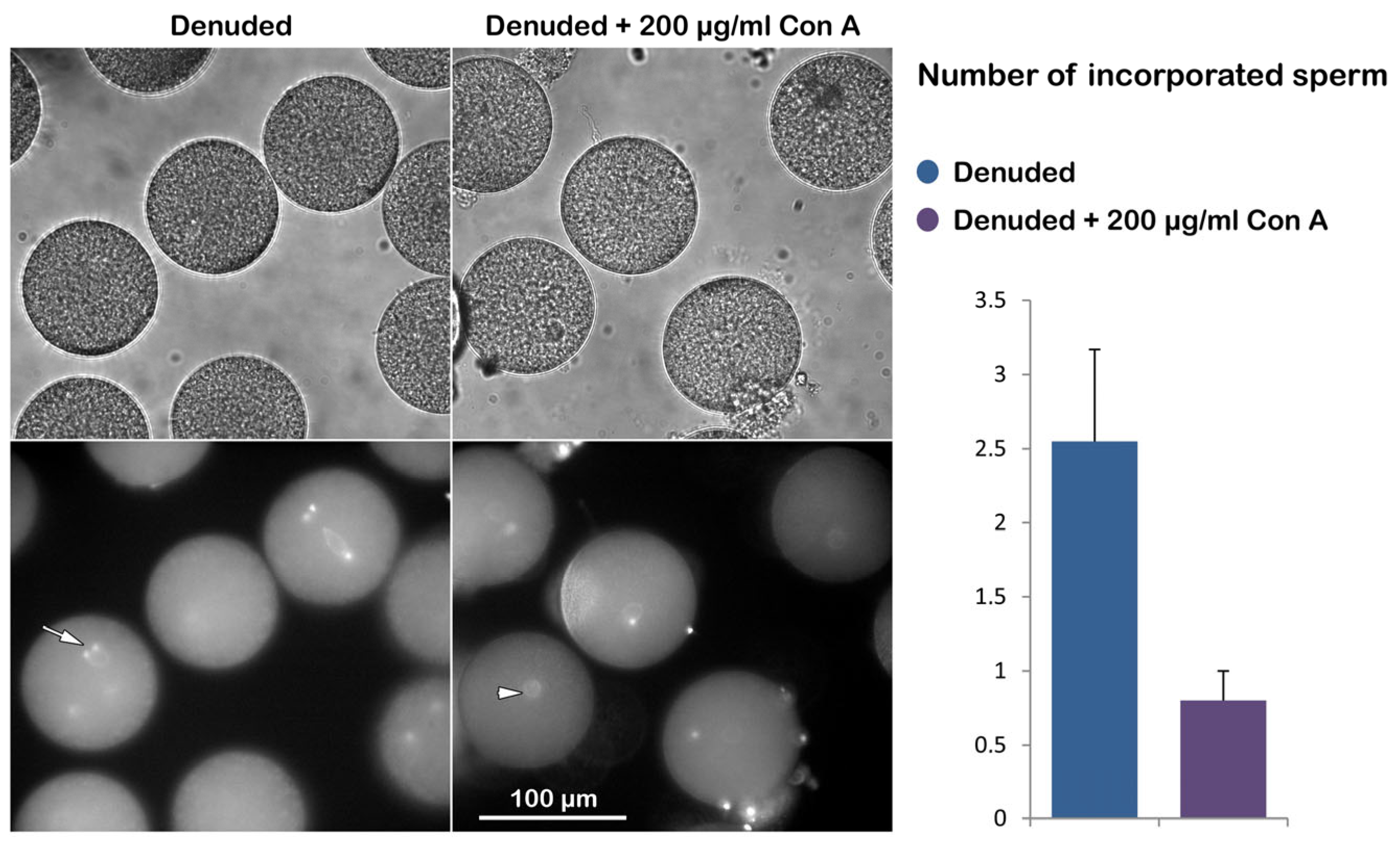
3.3.4. Morphological Effect of Con A on the Denuded Eggs Before and After Fertilization
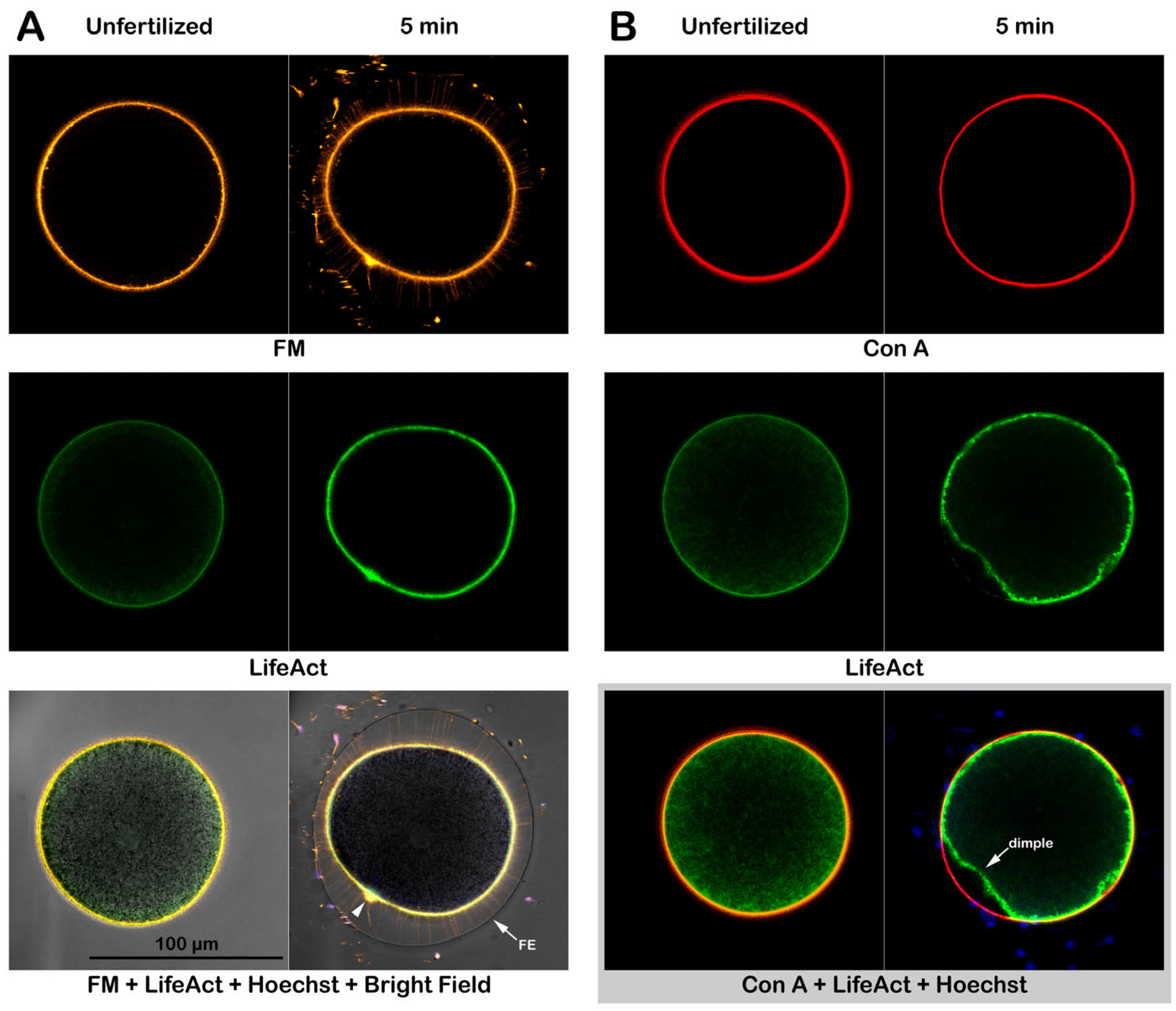
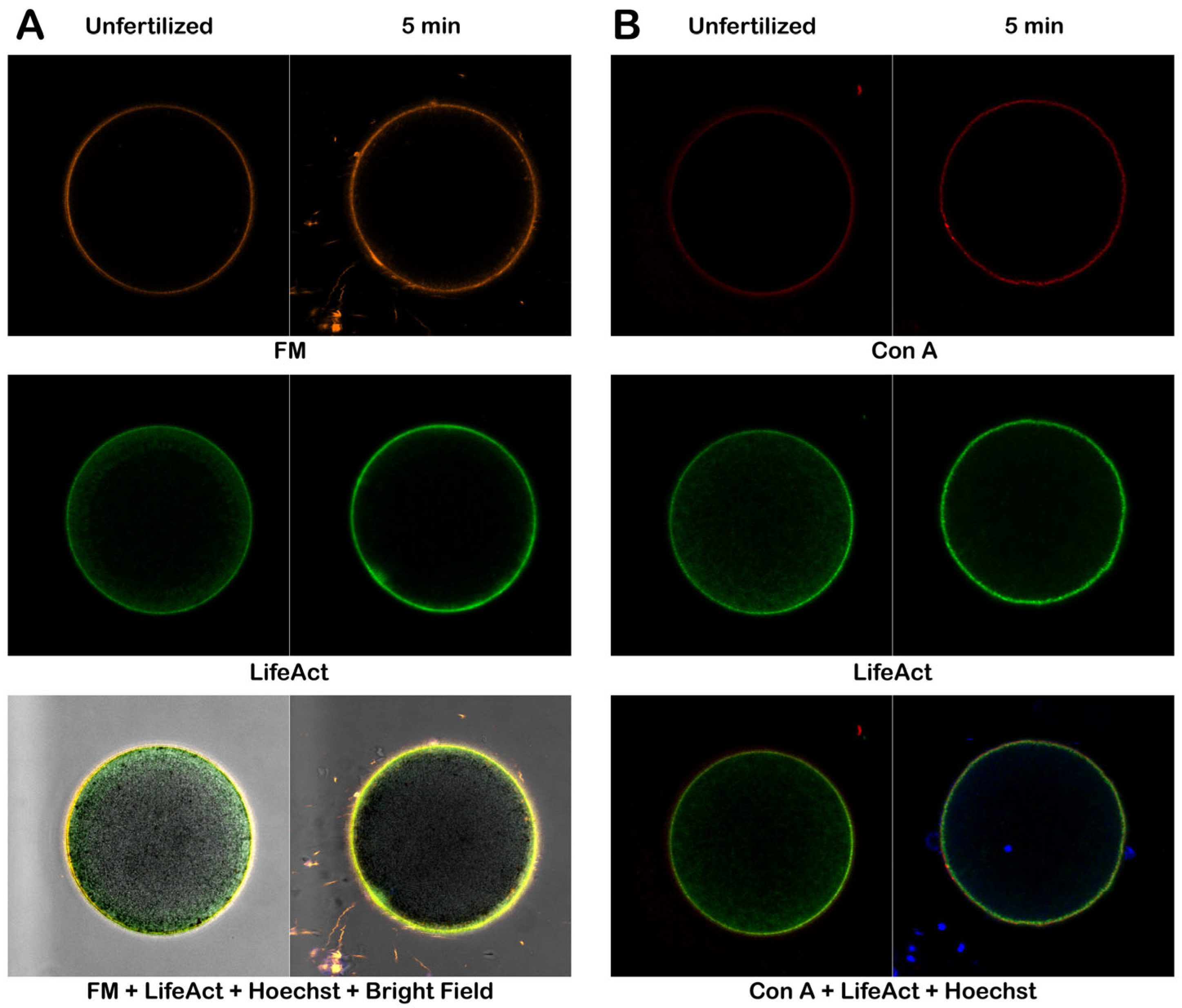
3.4. Alteration of the Cortical F-actin in Intact and Denuded Eggs in Response to Con A
3.4.1. Effect of Con A on Intact Eggs at Fertilization
3.4.2. Effect of Con A on Denuded Eggs at Fertilization
3.4.3. Effect of Con A on the Actin Filaments of Intact Eggs Probed by Alexa Phalloidin
3.4.4. Fluorescent Labeling of Live P. lividus Gametes with Probes for Extracellular Matrix
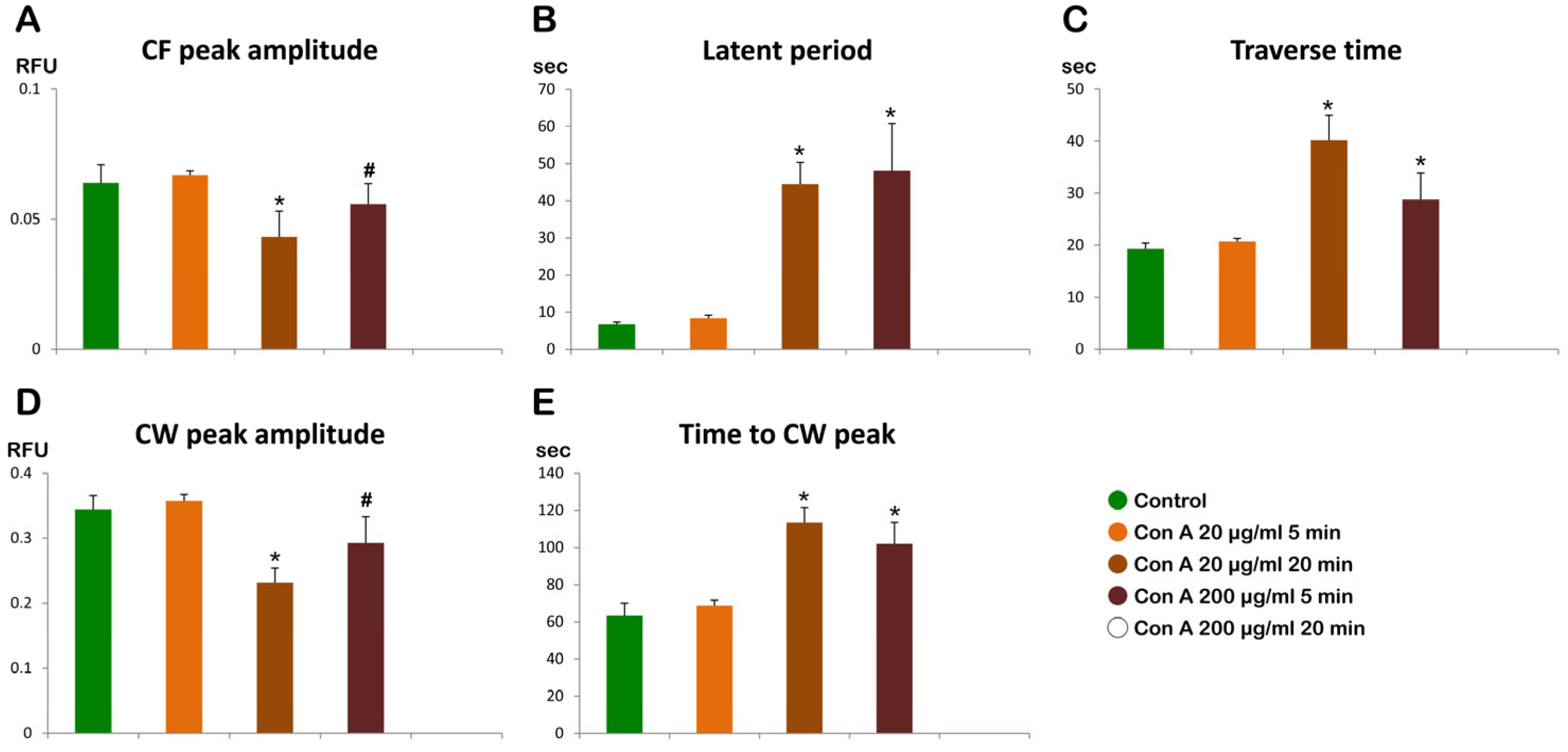
3.5. Con A Binding to the Egg Surface Affects Sperm-Induced Ca2+ Signals in Eggs
3.5.1. Ca2+ Responses in Intact Eggs Pretreated with Con A Before Insemination
3.5.2. Ca2+ Responses in Denuded Eggs Pretreated with Con A Before Insemination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirohashi, N.; Kamei, N.; Kubo, H.; Sawada, H.; Matsumoto, M.; Hoshi, M. Egg and sperm recognition systems during fertilization. Dev. Growth Differ. 2008, 50, S221–S238. [Google Scholar] [CrossRef]
- Dan, J.C. Studies on the Acrosome. I. Reaction to Egg-Water and Other Stimuli. Biol. Bull. 1952, 103, 54–66. [Google Scholar] [CrossRef]
- SeGall, G.; Lennarz, W.J. Chemical characterization of the components of the jelly coat from sea urchin eggs responsible for induction of the acrosome reaction. Dev. Biol. 1979, 71, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.P.; Mulloy, B.; Diniz, J.A.; Mourão, P.A. Sulfated polysaccharides from the egg jelly layer are species-specific inducers of acrosomal reaction in sperms of sea urchins. J. Biol. Chem. 1997, 272, 6965–6971. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D.; Moy, G.W. Isolation of bindin: The protein responsible for adhesion of sperm to sea urchin eggs. Proc. Natl. Acad. Sci. USA 1977, 74, 2456–2460. [Google Scholar] [CrossRef]
- Hirohashi, N.; Vacquier, V.D. High molecular mass egg fucose sulfate polymer is required for opening both Ca2+ channels involved in triggering the sea urchin sperm acrosome reaction. J. Biol. Chem. 2002, 277, 1182–1189. [Google Scholar] [CrossRef]
- Neill, A.T.; Vacquier, V.D. Ligands and receptors mediating signal transduction in sea urchin spermatozoa. Reproduction 2004, 127, 141–149. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; Wood, C.; Treviño, C.L.; Felix, R.; Beltran, C. Calcium channels and Ca2+ fluctuations in sperm physiology. Int. Rev. Cytol. 2005, 243, 79–172. [Google Scholar] [CrossRef]
- SeGall, G.K.; Lennarz, W.J. Jelly coat and induction of the acrosome reaction in echinoid sperm. Dev. Biol. 1981, 86, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Glabe, C.G.; Vacquier, V.D. Egg surface glycoprotein receptor for sea urchin sperm bindin. Proc. Natl. Acad. Sci. USA 1978, 75, 881–885. [Google Scholar] [CrossRef]
- Kidd, P. The jelly and vitelline coats of the sea urchin egg: New ultrastructure features. J. Ultrastruct. Res. 1978, 64, 204–215. [Google Scholar] [CrossRef]
- Chandler, D.E.; Heuser, J. The vitelline layer of the sea urchin egg and its modification during fertilization. A freeze-fracture study using quick-freezing and deep-etching. J. Cell Biol. 1980, 84, 618–632. [Google Scholar] [CrossRef]
- Glabe, C.G.; Grabel, L.B.; Vacquier, V.D.; Rosen, S.D. Carbohydrate specificity of sea urchin sperm bindin: A cell surface lectin mediating sperm-egg adhesion. J. Cell Biol. 1982, 94, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Aketa, K.; Onitake, K.; Tsuzuki, H. Tryptic disruption of sperm-binding site of sea urchin egg surface. Exp. Cell Res. 1972, 71, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D.; Epel, D.; Douglas, L.A. Sea urchin eggs release protease activity at fertilization. Nature 1972, 237, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D.; Tegner, M.J.; Epel, E. Protease activity establishes the block against polyspermy in sea urchin eggs. Nature 1972, 240, 352–353. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Payne, J.E. Methods for quantitating sea urchin sperm-egg binding. Exp. Cell Res. 1973, 82, 227–235. [Google Scholar] [CrossRef]
- Carroll, E.J., Jr.; Epel, D. Isolation and biological activity of the proteases released by sea urchin eggs following fertilization. Dev. Biol. 1975, 44, 22–32. [Google Scholar] [CrossRef]
- Epel, D. The Program of Fertilization. Sci. Am. 1975, 237, 128–138. [Google Scholar] [CrossRef]
- Bryan, J. The isolation of a major structural element of the sea urchin fertilization membrane. J. Cell Biol. 1970, 44, 635–644. [Google Scholar] [CrossRef]
- Kay, E.S.; Shapiro, B.M. Ovoperoxidase assembly into the sea urchin fertilization envelope and dityrosine crosslinking. Dev. Biol. 1987, 121, 325–333. [Google Scholar] [CrossRef]
- Larabell, C.; Chandler, D.E. Fertilization-Induced changes in the vitelline envelope of echinoderm and amphibian eggs: Self-assembly of an extracellular matrix. J. Electron. Microsc. Tech. 1981, 17, 294–318. [Google Scholar] [CrossRef]
- Epel, D.; Weaver, A.M.; Mazia, D. Methods for removal of the vitelline membrane of sea urchin eggs: I. Use of dithiothreitol (Cleland Reagent). Exp. Cell Res. 1970, 61, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Glabe, C.; Buchalter, M.; Lennarz, W.J. Studies on the interactions of sperm with the surface of the sea urchin eggs. Dev. Biol. 1981, 84, 397–406. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Chun, J.T.; Santella, L. Sodium-mediated fast electrical depolarization does not prevent polyspermic fertilization in Paracentrotus lividus eggs. Zygote 2019, 27, 241–249. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Schmitt, J.-L.; Lehn, J.-M.; Santella, L. The Effect of Synthetic Polyamine BPA-C8 on the Fertilization Process of Intact and Denuded Sea Urchin Eggs. Cells 2024, 13, 1477. [Google Scholar] [CrossRef] [PubMed]
- Wessel, G.M.; Wada, Y.; Yajima, M.; Kiyomoto, M. Bindin is essential for fertilization in the sea urchin. Proc. Natl. Acad. Sci. USA 2021, 118, e2109636118. [Google Scholar] [CrossRef]
- Hagström, B.E. Studies on the fertilization of jelly-free sea urchin eggs. Exp. Cell Res. 1956, 10, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Limatola, N.; Chun, J.T.; Cherraben, S.; Schmitt, J.L.; Lehn, J.M.; Santella, L. Effects of Dithiothreitol on Fertilization and Early Development in Sea Urchin. Cells 2021, 10, 3573. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Species-Specific Gamete Interaction during Sea Urchin Fertilization: Roles of the Egg Jelly and Vitelline Layer. Cells 2022, 11, 2984. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Regulation of the Actin Cytoskeleton-Linked Ca2+ Signaling by Intracellular pH in Fertilized Eggs of Sea Urchin. Cells 2022, 11, 1496. [Google Scholar] [CrossRef]
- Dale, B.; De Felice, L.J.; Taglietti, V. Membrane noise and conductance increase during single spermatozoon-egg interaction. Nature 1978, 275, 217–219. [Google Scholar] [CrossRef]
- Dale, B.; Dan-Sohkawa, M.; De Santis, A.; Hoshi, M. Fertilization of the starfish Astropecten aurantiacus. Exp. Cell Res. 1981, 132, 505–510. [Google Scholar] [CrossRef]
- Chun, J.T.; Limatola, N.; Vasilev, F.; Santella, L. Early events of fertilization in sea urchin eggs are sensitive to actin-binding organic molecules. Biochem. Biophys. Res. Commun. 2014, 450, 1166–1174. [Google Scholar] [CrossRef]
- Spudich, A.; Wrenn, J.T.; Wessells, N.K. Unfertilized sea urchin eggs contain a discrete cortical shell of actin that is subdivided into two organizational states. Cell Motil. Cytoskel. 1988, 9, 85–96. [Google Scholar] [CrossRef]
- Gillot, I.; Ciapa, B.; Payan, P.; Sardet, C. The calcium content of cortical granules and the loss of calcium from sea urchin eggs at fertilization. Dev. Biol. 1991, 146, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Santella, L.; Limatola, N.; Chun, J.T. Calcium and actin in the saga of awakening oocytes. Biochem. Biophys. Res. Commun. 2015, 460, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, F.; Limatola, N.; Chun, J.T.; Santella, L. Contributions of suboolemmal acidic vesicles and microvilli to the intracellular Ca2+ increase in the sea urchin eggs at fertilization. Int. J. Biol. Sci. 2019, 15, 757. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.D.; Griffin, J.L. The time sequence of early events in the fertilization of sea urchin eggs. Exp. Cell Res. 1958, 15, 163–173. [Google Scholar] [CrossRef]
- Green, J.D.; Summers, R.G. Formation of the cortical concavity at fertilization in the sea urchin egg. Dev. Growth Differ. 1980, 22, 821–829. [Google Scholar] [CrossRef]
- Stack, C.; Lucero, A.J.; Shuster, C.B. Calcium-responsive contractility during fertilization in sea urchin eggs. Dev. Dyn. 2006, 235, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Mangini, M.; Limatola, N.; Ferrara, M.A.; Coppola, G.; Chun, J.T.; De Luca, A.C.; Santella, L. Application of Raman spectroscopy to the evaluation of F-actin changes in sea urchin eggs at fertilization. Zygote 2024, 32, 38–48. [Google Scholar] [CrossRef]
- Steinhardt, R.A.; Mazia, D. Development of K+-conductance and membrane potentials in unfertilized sea urchin eggs after exposure to NH4OH. Nature 1973, 241, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.M.; Grainger, J.L. Mechanism of action of NH4Cl and other weak bases in the activation of sea urchin eggs. Nature 1978, 273, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.S.; Steinhardt, R.A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature 1978, 272, 253–254. [Google Scholar] [CrossRef]
- Carron, C.P.; Longo, F.J. Relation of cytoplasmic alkalinization to microvillar elongation and microfilament formation in the sea urchin egg. Dev. Biol. 1982, 89, 128–137. [Google Scholar] [CrossRef]
- Begg, D.A.; Rebhun, L.I. pH regulates the polymerization of actin in the sea urchin egg cortex. J. Cell Biol. 1979, 83, 241–248. [Google Scholar] [CrossRef]
- Yonemura, S.; Mabuchi, I. Wave of Cortical Actin Polymerization in the Sea Urchin Egg. Cell Motil. Cytoskel. 1987, 7, 46–53. [Google Scholar] [CrossRef]
- Longo, F.J.; Anderson, E. The effects of nicotine on fertilization in the sea urchin, Arbacia punctulata. J. Cell Biol. 1970, 46, 308–325. [Google Scholar] [CrossRef]
- Tilney, L.G.; Jaffe, L.A. Actin, microvilli, and the fertilization cone of sea urchin eggs. J. Cell Biol. 1980, 87, 771–782. [Google Scholar] [CrossRef]
- Limatola, N.; Vasilev, F.; Santella, L.; Chun, J.T. Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics. Cells 2020, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. Lectins: Cell-agglutinating and sugar-specific proteins. Science 1972, 177, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Lallier, R. Effects of concanavalin on the development of sea urchin egg. Exp. Cell Res. 1972, 72, 157–163. [Google Scholar] [CrossRef]
- Veron, M.; Shapiro, B.M. Binding of concanavalin A to the surface of sea urchin eggs and its alteration upon fertilization. J. Biol. Chem. 1977, 252, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Veron, M.; Foerder, C.; Eddy, E.M.; Shapiro, B.M. Sequential biochemical and morphological events during assembly of the fertilization membrane of the sea urchin. Cell 1977, 10, 321–328. [Google Scholar] [CrossRef]
- Shapiro, B.M.; Eddy, E.M. When Sperm Meets Egg: Biochemical Mechanisms of Gamete Interaction. Int. Rev. Cytol. 1980, 66, 257–302. [Google Scholar] [CrossRef]
- Longo, F.J. Effects of concanavalin A on the fertilization of sea urchin eggs. Dev. Biol. 1981, 82, 197–202. [Google Scholar] [CrossRef]
- Riedl, G.; Crevenna, A.H.; Kessenbrock, K.; Yu, J.H.; Neukirchen, D.; Bista, M.; Bradke, F.; Jenne, D.; Holak, T.A.; Werb, Z.; et al. Lifeact: A versatile marker to visualize F-actin. Nat. Methods. 2008, 5, 605–607. [Google Scholar] [CrossRef]
- Santella, L.; Chun, J.T. Structural actin dynamics during oocyte maturation and fertilization. Biochem. Biophys. Res. Commun. 2022, 633, 13–16. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Schneider, S.C.; Schmitt, J.-L.; Lehn, J.-M.; Santella, L. The Effect of Acidic and Alkaline Seawater on the F-Actin-Dependent Ca2+ Signals Following Insemination of Immature Starfish Oocytes and Mature Eggs. Cells 2023, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D. The quest for the sea urchin egg receptor for sperm. Biochem. Biophys. Res. Commun. 2012, 425, 583–587. [Google Scholar] [CrossRef]
- Lim, D.; Lange, K.; Santella, L. Activation of oocytes by latrunculin A. FASEB J. 2002, 16, 1050–1056. [Google Scholar] [CrossRef]
- Terasaki, M. Actin Filament Translocations in SeaUrchin Eggs. Cell Motil. Cytoskelet. 1996, 34, 48–56. [Google Scholar] [CrossRef]
- Limatola, N.; Chun, J.T.; Santella, L. Effects of Salinity and pH of Seawater on the Reproduction of the Sea Urchin Paracentrotus lividus. Biol. Bull. 2020, 239, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Chen, S.H.; Zhou, H.; Vacquier, V.D. Tandem mass spectrometry identifies proteins phosphorylated by cyclic AMP-dependent protein kinase when sea urchin sperm undergo the acrosome reaction. Dev. Biol. 2005, 285, 116–125. [Google Scholar] [CrossRef]
- Schatten, G.; Mazia, D. The penetration of the spermatozoon through the sea urchin egg surface at fertilization. Observations from the outside on whole eggs and from the inside on isolated surfaces. Exp. Cell Res. 1976, 98, 325–337. [Google Scholar] [CrossRef]
- Epel, D. Mechanisms of Activation of Sperm and Egg During Fertilization of Sea Urchin Gametes. Curr. Top. Dev. Biol. 1978, 12, 185–246. [Google Scholar] [CrossRef]
- Vacquier, V.D. The fertilizing capacity of sea urchin sperm rapidly decreases after induction of the acrosome reaction. Dev. Growth Differ. 1979, 21, 61–69. [Google Scholar] [CrossRef]
- Chun, J.T.; Vasilev, F.; Limatola, N.; Santella, L. Fertilization in Starfish and Sea Urchin: Roles of Actin. Results Probl. Cell Differ. 2018, 65, 33–47. [Google Scholar] [CrossRef] [PubMed]
- García-Soto, J.; Darszon, A. High pH-induced acrosome reaction and Ca2+ uptake in sea urchin sperm suspended in Na+-free seawater. Dev. Biol. 1985, 110, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Lopo, A.C.; Glabe, C.G.; Lennarz, W.J.; Vacquier, V.D. Sperm-egg binding events during sea urchin fertilization. Ann. N. Y. Acad. Sci. 1982, 383, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D.; Swanson, W.J.; Hellberg, M.E. What have we learned about sea urchin bindin? Develop. Growth Differ. 1995, 37, 1–10. [Google Scholar] [CrossRef]
- Jaffe, L.A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature 1976, 261, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Just, E.E. The fertilization reaction in Echinarachnius parma. Biol. Bull. 1919, 36, 1–10. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Carlson, A.E. Ion channels and signaling pathways used in the fast polyspermy block. Mol. Reprod. Dev. 2019, 87, 350–357. [Google Scholar] [CrossRef]
- Barresi, M.J.; Gilbert, S.F. Chapter 7. Fertilization. Beginning a new organism. In Developmental Biology, 13th ed.; Oxford University Press: New York, NY, USA, 2024; pp. 211–246. [Google Scholar]
- Schuel, H. The prevention of polyspermic fertilization in sea urchins. Biol. Bull. 1984, 167, 271–309. [Google Scholar] [CrossRef]
- Just, E.E. The Biology of the Cell Surface; P. Blakiston’s Son & Co.: Philadelphia, PA, USA, 1939. [Google Scholar]
- Dale, B.; De Santis, A. The effect of cytochalasin B and D on the fertilization of sea urchins. Dev. Biol. 1981, 83, 232–237. [Google Scholar] [CrossRef]
- Créton, R.; Jaffe, L.F. Role of calcium influx during the latent period in sea urchin fertilization. Dev. Growth Differ. 1995, 37, 703–709. [Google Scholar] [CrossRef]
- Lange, K. Microvillar Ca++ signaling: A new view of an old problem. J. Cell. Physiol. 1999, 180, 19–34. [Google Scholar] [CrossRef]
- Yonemura, S.; Tsukita, S.; Mabuchi, I. Structural Analysis of the Sea Urchin Egg Cortex Isolated on a Substratum. In Cell Dynamics; Protoplasma; Tazawa, M., Ed.; Springer: Vienna, Austria, 1988; Volume 2. [Google Scholar] [CrossRef]
- Steinhardt, R.; Epel, D.; Carroll, E.J., Jr.; Yanagimachi, R. Is calcium ionophore a universal activator for unfertilised eggs? Nature 1974, 252, 41–43. [Google Scholar] [CrossRef]
- Schmidt, T.; Patton, C.; Epel, D. Is there a role for the Ca2+ influx during fertilization of the sea urchin egg? Dev. Biol. 1982, 90, 284–290. [Google Scholar] [CrossRef]
- Stricker, S.A.; Centonze, V.E.; Paddock, S.W.; Schatten, G. Confocal microscopy of fertilization-induced calcium dynamics in sea urchin eggs. Dev. Biol. 1992, 149, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Galione, A.; McDougall, A.; Busa, W.B.; Willmott, N.; Gillot, I.; Whitaker, M. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science 1993, 261, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.J.; Swann, K. Lighting the fuse at fertilization. Development 1993, 117, 1–12. [Google Scholar] [CrossRef]
- Galione, A. Cyclic ADP-ribose, the ADP-ribosyl cyclase pathway and calcium signaling. Mol. Cell. Endocrinol. 1994, 98, 125–131. [Google Scholar] [CrossRef]
- Carroll, D.J.; Albay, D.T.; Terasaki, M.; Jaffe, L.A.; Foltz, K.R. Identification of PLCgamma-dependent and -independent events during fertilization of sea urchin eggs. Dev. Biol. 1999, 206, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Parrington, J.; Davis, L.C.; Galione, A.; Wessel, G. Flipping the switch: How a sperm activates the egg at fertilization. Dev. Dyn. 2007, 236, 2027–2038. [Google Scholar] [CrossRef]
- Jiang, H.; Wen, X.; Zhang, X.; Zhang, B. Concanavalin A inhibits human liver cancer cell migration by regulating F-actin redistribution and assembly via MAPK signaling pathway. Oncol. Lett. 2022, 24, 405. [Google Scholar] [CrossRef]
- Huldani, H.; Rashid, A.I.; Turaev, K.N.; Opulencia, M.G.C.; Abdelbasset, W.K.; Bokov, D.O.; Mustafa, Y.F.; Al-Gazally, M.E.; Hammid, A.T.; Kadhim, M.M.; et al. Concanavalin A as a promising lectin-based anti-cancer agent: The molecular mechanisms and therapeutic potential. Cell Commun. Signal. 2022, 20, 167. [Google Scholar] [CrossRef]


| Status of the Egg Extracellular Matrix | Con A Dose in the Media (μg/mL), 5 Min Preincubation | Washing Out Con A Before Insemination | Sperm Counts in Egg: Mean ± S.D. (Number of Eggs Examined) |
| Intact | 0 | no | 1 ± 0 (n = 60) |
| 1 | no | 1 ± 0 (n = 60) | |
| 20 | no | 1 ± 0 (n = 60) | |
| 200 | no | 0.33 ± 0.23 * (n = 60) | |
| 1000 | no | 0 ± 0 * (n = 60) | |
| Intact | 0 | yes | 1 ± 0 (n = 40) |
| 1 | yes | 1.02 ± 0.08 (n = 40) | |
| 20 | yes | 1.02 ± 0.08 (n = 40) | |
| 200 | yes | 0.32 ± 0.23 * (n = 40) | |
| 1000 | yes | 0 ± 0 * (n = 40) | |
| Denuded | 0 | no | 2.55 ± 0.62 (n = 60) |
| 200 | no | 0.8 ± 0.2 * (n = 60) |
| Intact Eggs | Intact Eggs + Con A (200 μg/mL) | |
| Before fertilization | 0.86 ± 0.34 (n = 6) | 0.88 ± 0.17 (n = 6) |
| 5 min after fertilization | 1.97 ± 0.21 (n = 6) | 1.20 ± 0.48 (n = 6) |
| * Paired t-Test | p < 0.001 | N.S. |
| Denuded Eggs | Denuded Eggs + Con A (200 μg/mL) | |
| Before fertilization | 0.76 ± 0.22 (n = 9) | 1.24 ± 0.20 (n = 8) |
| 5 min after fertilization | 1.16 ± 0.44 | 1.55 ± 0.19 (n = 8) |
| Paired t-Test | p < 0.05 | p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limatola, N.; Pirozzi, M.; Caramiello, D.; Chun, J.T.; Santella, L. The Binding of Concanavalin A to the Surface of Intact and Denuded Sea Urchin Eggs Affects the Fertilization Process by Altering the Structural Dynamics of Actin Filaments. Cells 2025, 14, 1867. https://doi.org/10.3390/cells14231867
Limatola N, Pirozzi M, Caramiello D, Chun JT, Santella L. The Binding of Concanavalin A to the Surface of Intact and Denuded Sea Urchin Eggs Affects the Fertilization Process by Altering the Structural Dynamics of Actin Filaments. Cells. 2025; 14(23):1867. https://doi.org/10.3390/cells14231867
Chicago/Turabian StyleLimatola, Nunzia, Marinella Pirozzi, Davide Caramiello, Jong Tai Chun, and Luigia Santella. 2025. "The Binding of Concanavalin A to the Surface of Intact and Denuded Sea Urchin Eggs Affects the Fertilization Process by Altering the Structural Dynamics of Actin Filaments" Cells 14, no. 23: 1867. https://doi.org/10.3390/cells14231867
APA StyleLimatola, N., Pirozzi, M., Caramiello, D., Chun, J. T., & Santella, L. (2025). The Binding of Concanavalin A to the Surface of Intact and Denuded Sea Urchin Eggs Affects the Fertilization Process by Altering the Structural Dynamics of Actin Filaments. Cells, 14(23), 1867. https://doi.org/10.3390/cells14231867








