Alterations in Resident Immune Cells in Prenatal Trisomy 21 Lungs
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Tissue/Ethics
2.2. Single-Cell Sequencing
2.3. Immune Cell Isolation
2.4. Quantitative Real-Time PCR Analyses
2.5. Fluorescent In Situ Hybridization (FISH) and Immunofluorescence (IF) Staining
2.6. Image Analysis
2.7. Statistical Analyses
3. Results
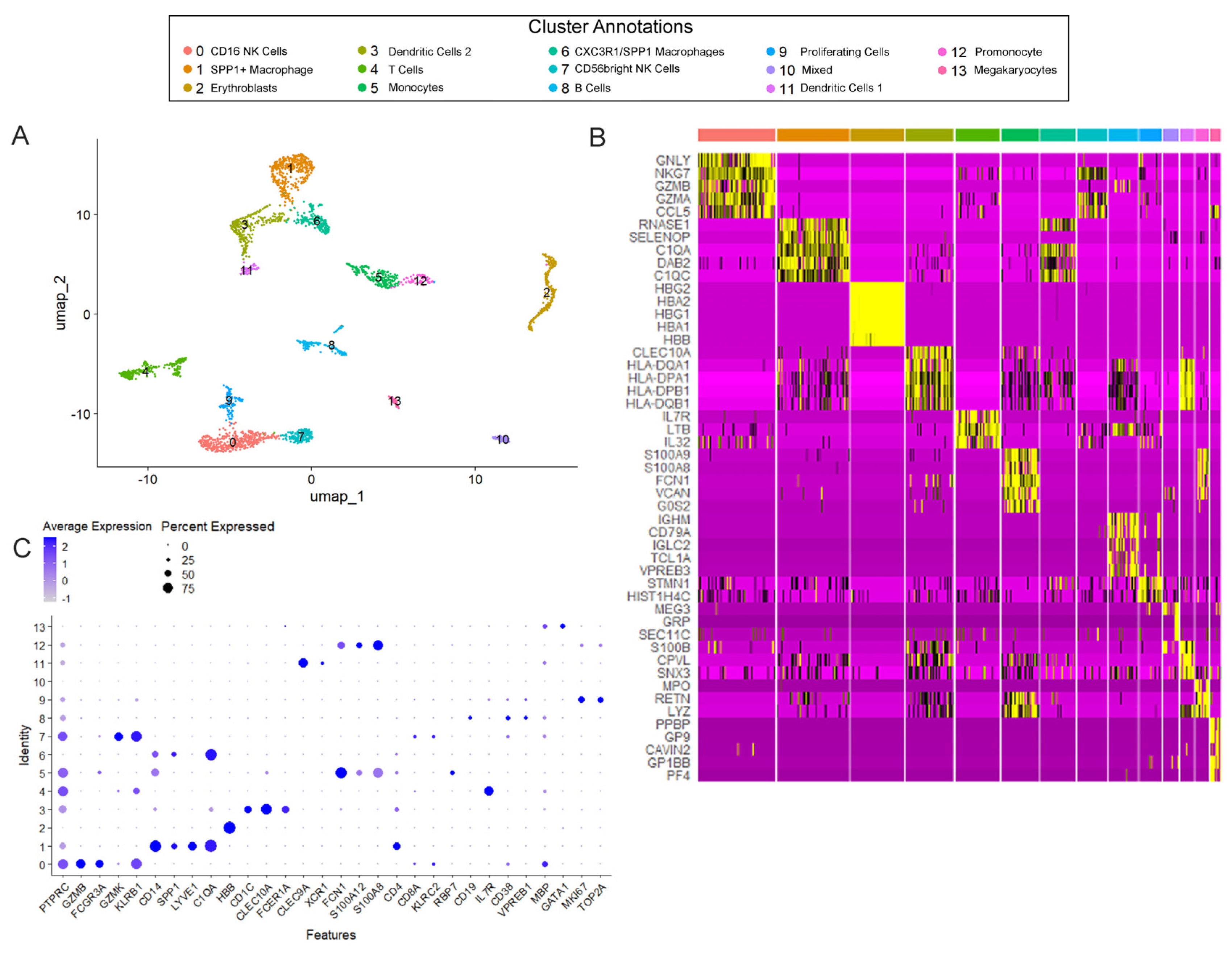
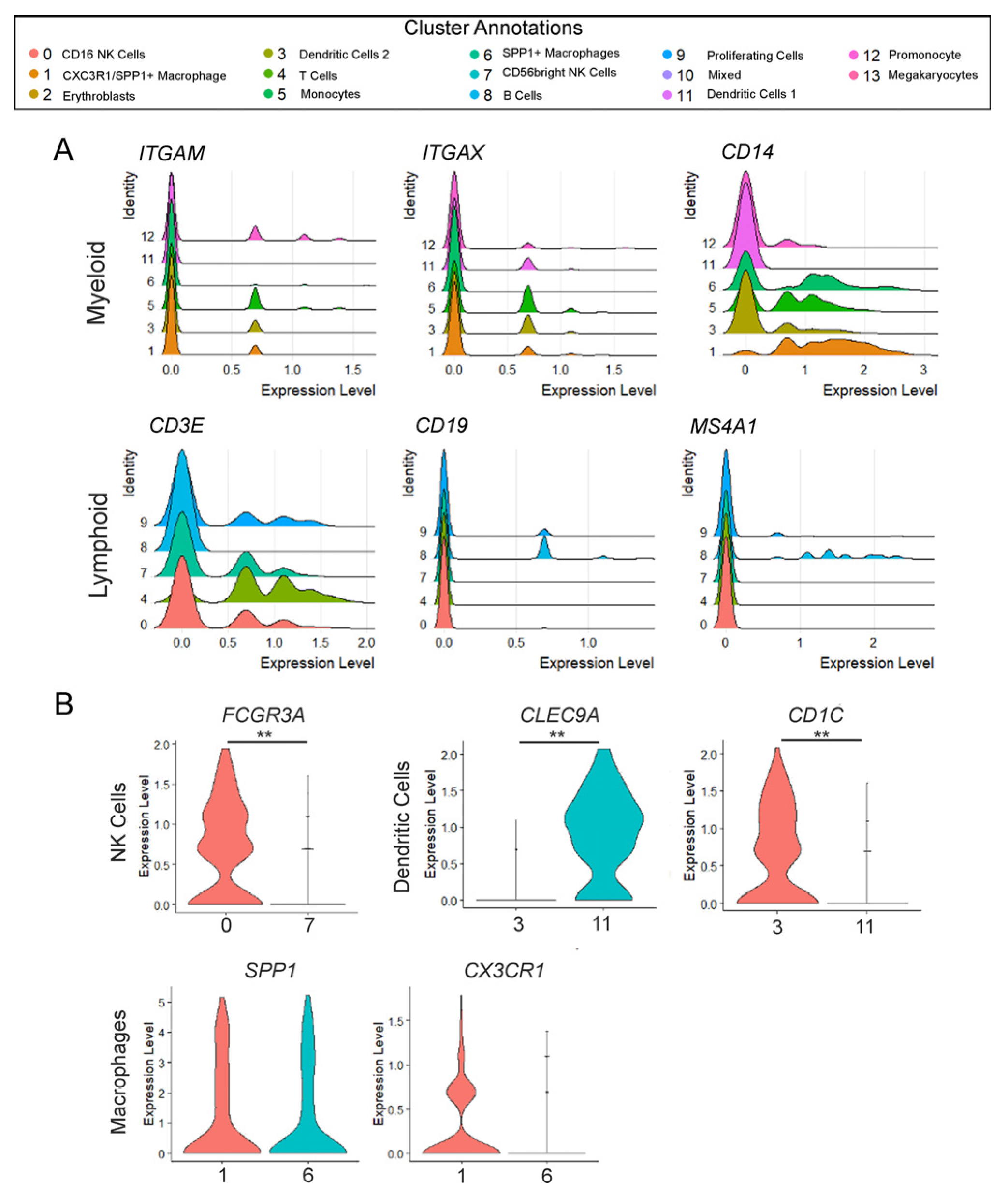
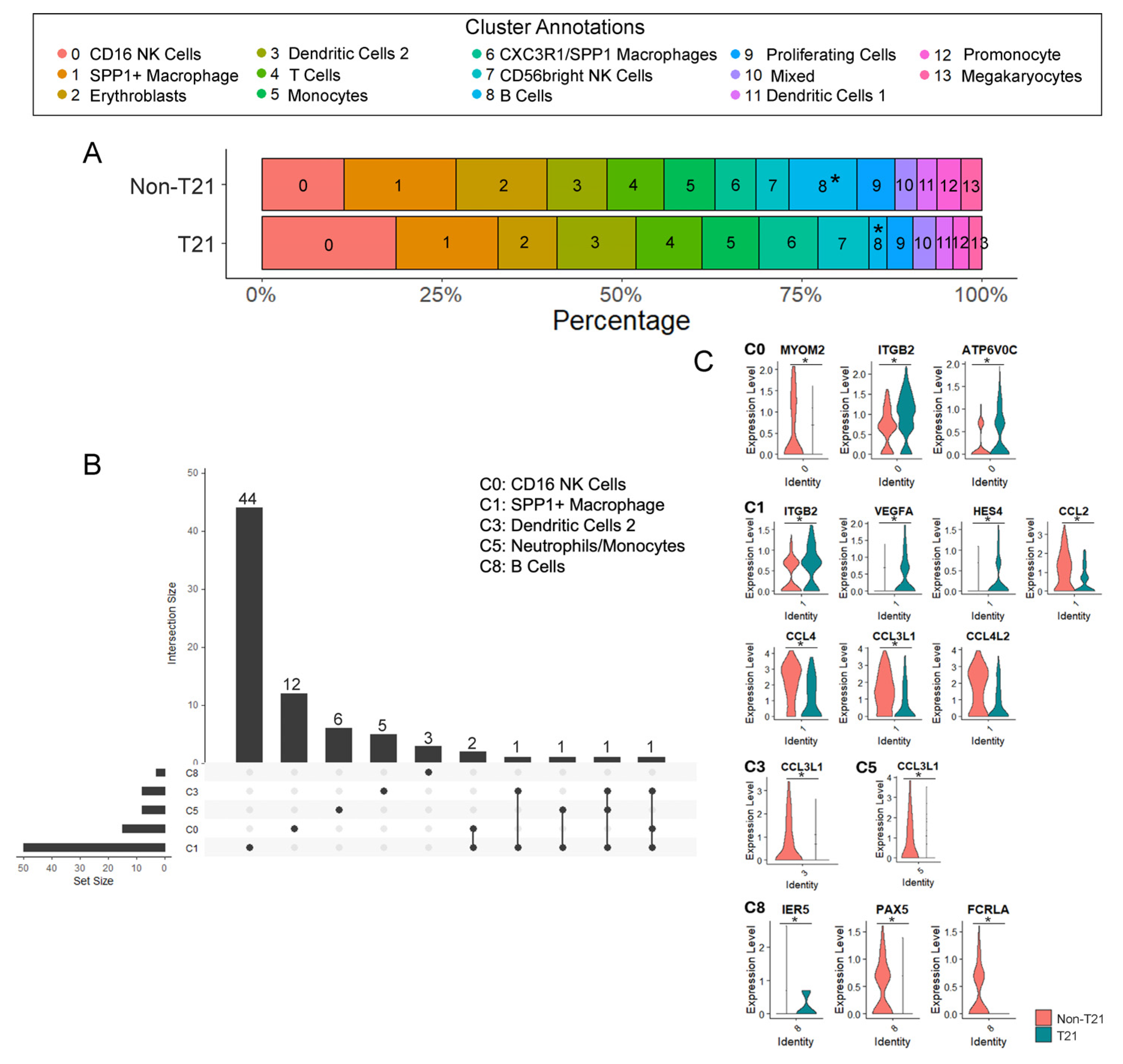
3.1. Immune Cell Populations in T21 Prenatal Lungs
3.2. The Developing Human Lung Has Different Immune Cell States
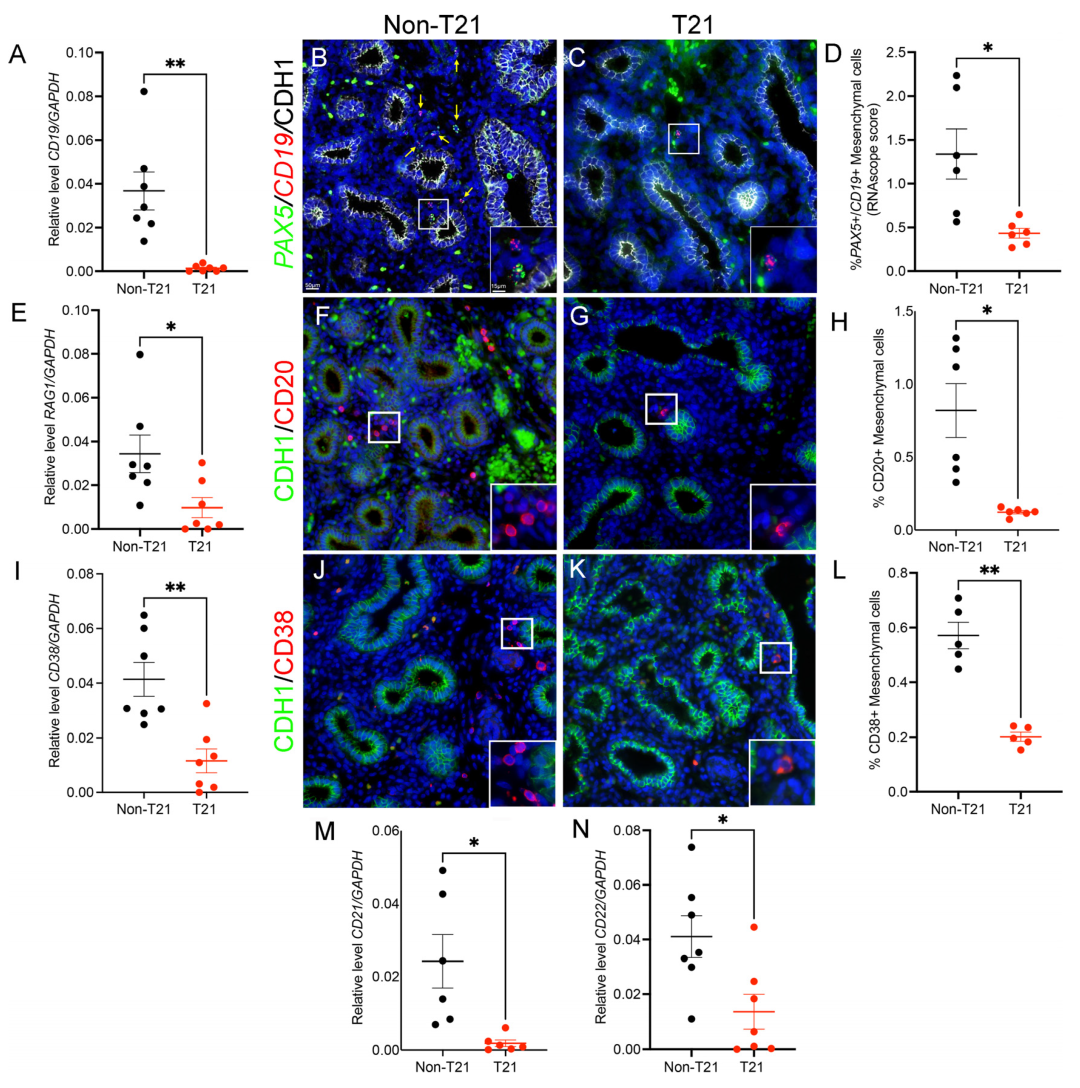
3.3. Significantly Fewer B Cells in Prenatal T21 Lungs
3.4. B Cell Development and Maturation Is Hindered in Prenatal T21 Lungs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donovan, M.G.; Eduthan, N.P.; Smith, K.P.; Britton, E.C.; Lyford, H.R.; Araya, P.; Granrath, R.E.; Waugh, K.A.; Enriquez Estrada, B.; Rachubinski, A.L.; et al. Variegated overexpression of chromosome 21 genes reveals molecular and immune subtypes of Down syndrome. Nat. Commun. 2024, 15, 5473. [Google Scholar] [CrossRef]
- Waugh, K.A.; Minter, R.; Baxter, J.; Chi, C.; Galbraith, M.D.; Tuttle, K.D.; Eduthan, N.P.; Kinning, K.T.; Andrysik, Z.; Araya, P.; et al. Triplication of the interferon receptor locus contributes to hallmarks of Down syndrome in a mouse model. Nat. Genet. 2023, 55, 1034–1047. [Google Scholar] [CrossRef]
- Davidson, M.A. Primary care for children and adolescents with Down syndrome. Pediatr. Clin. N. Am. 2008, 55, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.M.; Fitzgerald, D.A.; Cooper, D.M. Respiratory morbidity of hospitalized children with Trisomy 21. J. Paediatr. Child Health 1999, 35, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.L.; Yeager, M.E. What people with Down Syndrome can teach us about cardiopulmonary disease. Eur. Respir. Rev. 2017, 26, 160098. [Google Scholar] [CrossRef] [PubMed]
- Uppal, H.; Chandran, S.; Potluri, R. Risk factors for mortality in Down syndrome. J. Intellect. Disabil. Res. 2015, 59, 873–881. [Google Scholar] [CrossRef]
- Chan, M.; Park, J.J.; Shi, T.; Martinon-Torres, F.; Bont, L.; Nair, H.; Respiratory Syncytial Virus, N. The burden of respiratory syncytial virus (RSV) associated acute lower respiratory infections in children with Down syndrome: A systematic review and meta-analysis. J. Glob. Health 2017, 7, 020413. [Google Scholar] [CrossRef]
- Beckhaus, A.A.; Castro-Rodriguez, J.A. Down Syndrome and the Risk of Severe RSV Infection: A Meta-analysis. Pediatrics 2018, 142, e20180225. [Google Scholar] [CrossRef]
- Barnes, J.L.; Yoshida, M.; He, P.; Worlock, K.B.; Lindeboom, R.G.H.; Suo, C.; Pett, J.P.; Wilbrey-Clark, A.; Dann, E.; Mamanova, L.; et al. Early human lung immune cell development and its role in epithelial cell fate. Sci. Immunol. 2023, 8, eadf9988. [Google Scholar] [CrossRef]
- Suo, C.; Dann, E.; Goh, I.; Jardine, L.; Kleshchevnikov, V.; Park, J.E.; Botting, R.A.; Stephenson, E.; Engelbert, J.; Tuong, Z.K.; et al. Mapping the developing human immune system across organs. Science 2022, 376, eabo0510. [Google Scholar] [CrossRef]
- Bloemers, B.L.; van Bleek, G.M.; Kimpen, J.L.; Bont, L. Distinct abnormalities in the innate immune system of children with Down syndrome. J. Pediatr. 2010, 156, 804–809.e5. [Google Scholar] [CrossRef] [PubMed]
- de Hingh, Y.C.; van der Vossen, P.W.; Gemen, E.F.; Mulder, A.B.; Hop, W.C.; Brus, F.; de Vries, E. Intrinsic abnormalities of lymphocyte counts in children with down syndrome. J. Pediatr. 2005, 147, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.D.; Evans, D.; Pandey, A.; Hraha, T.H.; Smith, K.P.; Markham, N.; Rachubinski, A.L.; Wolter-Warmerdam, K.; Hickey, F.; Espinosa, J.M.; et al. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci. Rep. 2017, 7, 14818. [Google Scholar] [CrossRef]
- Danopoulos, S.; Bhattacharya, S.; Deutsch, G.; Nih, L.R.; Slaunwhite, C.; Mariani, T.J.; Al Alam, D. Prenatal histological, cellular, and molecular anomalies in trisomy 21 lung. J. Pathol. 2021, 255, 41–51. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Frauenpreis, A.; Cherry, C.; Deutsch, G.; Glass, I.A.; Mariani, T.J.; Al Alam, D.; Danopoulos, S.; Birth Defects Research, L. The Transcriptional Landscape of Developing Human Trisomy 21 Lungs. Am. J. Respir. Cell Mol. Biol. 2025. [Google Scholar] [CrossRef]
- Danopoulos, S.; Bhattacharya, S.; Mariani, T.J.; Al Alam, D. Transcriptional characterisation of human lung cells identifies novel mesenchymal lineage markers. Eur. Respir. J. 2020, 55, 1900746. [Google Scholar] [CrossRef]
- Guo, M.; Wikenheiser-Brokamp, K.A.; Kitzmiller, J.A.; Jiang, C.; Wang, G.; Wang, A.; Preissl, S.; Hou, X.; Buchanan, J.; Karolak, J.A.; et al. Single Cell Multiomics Identifies Cells and Genetic Networks Underlying Alveolar Capillary Dysplasia. Am. J. Respir. Crit. Care Med. 2023, 208, 709–725. [Google Scholar] [CrossRef]
- Viswanathan, G.; Kirshner, H.F.; Nazo, N.; Ali, S.; Ganapathi, A.; Cumming, I.; Zhuang, Y.; Choi, I.; Warman, A.; Jassal, C.; et al. Single-Cell Analysis Reveals Distinct Immune and Smooth Muscle Cell Populations that Contribute to Chronic Thromboembolic Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2023, 207, 1358–1375. [Google Scholar] [CrossRef]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef]
- Yang, S.; Corbett, S.E.; Koga, Y.; Wang, Z.; Johnson, W.E.; Yajima, M.; Campbell, J.D. Decontamination of ambient RNA in single-cell RNA-seq with DecontX. Genome Biol. 2020, 21, 57. [Google Scholar] [CrossRef]
- Ilicic, T.; Kim, J.K.; Kolodziejczyk, A.A.; Bagger, F.O.; McCarthy, D.J.; Marioni, J.C.; Teichmann, S.A. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 2016, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M., 3rd; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Lim, K.; Sun, D.; Pett, J.P.; Jeng, Q.; Polanski, K.; Dong, Z.; Bolt, L.; Richardson, L.; Mamanova, L.; et al. A human fetal lung cell atlas uncovers proximal-distal gradients of differentiation and key regulators of epithelial fates. Cell 2022, 185, 4841–4860.e25. [Google Scholar] [CrossRef]
- Nizzoli, G.; Krietsch, J.; Weick, A.; Steinfelder, S.; Facciotti, F.; Gruarin, P.; Bianco, A.; Steckel, B.; Moro, M.; Crosti, M.; et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013, 122, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, S.; Yang, Y.; Iwanowycz, S.; Gutierrez, J.; Li, Y.; Williams, C.; Hill, M.; Chung, D.; Allen, C.; Liu, B. Migrating Type 2 Dendritic Cells Prime Mucosal Th17 Cells Specific to Small Intestinal Commensal Bacteria. J. Immunol. 2022, 209, 1200–1211. [Google Scholar] [CrossRef]
- MacLean, G.A.; McEldoon, J.; Huang, J.; Allred, J.; Canver, M.C.; Orkin, S.H. Downregulation of Endothelin Receptor B Contributes to Defective B Cell Lymphopoiesis in Trisomy 21 Pluripotent Stem Cells. Sci. Rep. 2018, 8, 8001. [Google Scholar] [CrossRef]
- Araya, P.; Waugh, K.A.; Sullivan, K.D.; Nunez, N.G.; Roselli, E.; Smith, K.P.; Granrath, R.E.; Rachubinski, A.L.; Enriquez Estrada, B.; Butcher, E.T.; et al. Trisomy 21 dysregulates T cell lineages toward an autoimmunity-prone state associated with interferon hyperactivity. Proc. Natl. Acad. Sci. USA 2019, 116, 24231–24241. [Google Scholar] [CrossRef]
- Guazzarotti, L.; Trabattoni, D.; Castelletti, E.; Boldrighini, B.; Piacentini, L.; Duca, P.; Beretta, S.; Pacei, M.; Caprio, C.; Vigan Ago, A.; et al. T lymphocyte maturation is impaired in healthy young individuals carrying trisomy 21 (Down syndrome). Am. J. Intellect. Dev. Disabil. 2009, 114, 100–109. [Google Scholar] [CrossRef]
- Cossarizza, A.; Ortolani, C.; Forti, E.; Montagnani, G.; Paganelli, R.; Zannotti, M.; Marini, M.; Monti, D.; Franceschi, C. Age-related expansion of functionally inefficient cells with markers of natural killer activity in Down’s syndrome. Blood 1991, 77, 1263–1270. [Google Scholar] [CrossRef]
- Kanzaki, M.; Shibata, H.; Mogami, H.; Kojima, I. Expression of calcium-permeable cation channel CD20 accelerates progression through the G1 phase in Balb/c 3T3 cells. J. Biol. Chem. 1995, 270, 13099–13104. [Google Scholar] [CrossRef]
- Guelpa-Fonlupt, V.; Tonnelle, C.; Blaise, D.; Fougereau, M.; Fumoux, F. Discrete early pro-B and pre-B stages in normal human bone marrow as defined by surface pseudo-light chain expression. Eur. J. Immunol. 1994, 24, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Huggard, D.; Doherty, D.G.; Molloy, E.J. Immune Dysregulation in Children With Down Syndrome. Front. Pediatr. 2020, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Szczawinska-Poplonyk, A.; Poplonyk, N.; Awdi, K. Down Syndrome in Children: A Primary Immunodeficiency with Immune Dysregulation. Children 2024, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Giladi, A.; Gorki, A.D.; Solodkin, D.G.; Zada, M.; Hladik, A.; Miklosi, A.; Salame, T.M.; Halpern, K.B.; David, E.; et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 2018, 175, 1031–1044 e1018. [Google Scholar] [CrossRef]
- Gemen, E.F.; Verstegen, R.H.; Leuvenink, J.; de Vries, E. Increased circulating apoptotic lymphocytes in children with Down syndrome. Pediatr. Blood Cancer 2012, 59, 1310–1312. [Google Scholar] [CrossRef]
- Elsayed, S.M.; Elsayed, G.M. Phenotype of apoptotic lymphocytes in children with Down syndrome. Immun. Ageing 2009, 6, 2. [Google Scholar] [CrossRef]
- Carsetti, R.; Valentini, D.; Marcellini, V.; Scarsella, M.; Marasco, E.; Giustini, F.; Bartuli, A.; Villani, A.; Ugazio, A.G. Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. Eur. J. Immunol. 2015, 45, 903–914. [Google Scholar] [CrossRef]
- Verstegen, R.H.J.; Driessen, G.J.; Bartol, S.J.W.; van Noesel, C.J.M.; Boon, L.; van der Burg, M.; van Dongen, J.J.M.; de Vries, E.; van Zelm, M.C. Defective B-cell memory in patients with Down syndrome. J. Allergy Clin. Immunol. 2014, 134, 1346–1353.e9. [Google Scholar] [CrossRef]
- Santoro, S.L.; Baloh, C.H.; Hart, S.J.; Horick, N.; Kishnani, P.S.; Krell, K.; Oreskovic, N.M.; Shaffer, M.; Talib, N.; Torres, A.; et al. Pneumonia vaccine response in individuals with Down syndrome at three specialty clinics. Am. J. Med. Genet. C Semin. Med. Genet. 2023, 193, e32070. [Google Scholar] [CrossRef]
- Kusters, M.A.; Bok, V.L.; Bolz, W.E.; Huijskens, E.G.; Peeters, M.F.; de Vries, E. Influenza A/H1N1 vaccination response is inadequate in down syndrome children when the latest cut-off values are used. Pediatr. Infect. Dis. J. 2012, 31, 1284–1285. [Google Scholar] [CrossRef]
- Mortimer, G.L.; Gillespie, K.M. Early Onset of Autoimmune Diabetes in Children with Down Syndrome-Two Separate Aetiologies or an Immune System Pre-Programmed for Autoimmunity? Curr. Diab Rep. 2020, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Moo, K.G.; Arnett, A.; Goel, G.; Hu, A.; Flynn, K.J.; Speake, C.; Wiedeman, A.E.; Gersuk, V.H.; Linsley, P.S.; et al. Deep immune phenotyping reveals similarities between aging, Down syndrome, and autoimmunity. Sci. Transl. Med. 2022, 14, eabi4888. [Google Scholar] [CrossRef] [PubMed]
- Ramba, M.; Bogunovic, D. The immune system in Down Syndrome: Autoimmunity and severe infections. Immunol. Rev. 2024, 322, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Gansa, W.; Da Rosa, J.M.C.; Menon, K.; Sazeides, C.; Stewart, O.; Bogunovic, D. Dysregulation of the Immune System in a Natural History Study of 1299 Individuals with Down Syndrome. J. Clin. Immunol. 2024, 44, 130. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Lewis, H.C.; Hill, A.A.; Pandey, A.; Jackson, L.P.; Cabral, J.M.; Smith, K.P.; Liggett, L.A.; Gomez, E.B.; Galbraith, M.D.; et al. Trisomy 21 consistently activates the interferon response. eLife 2016, 5, e16220. [Google Scholar] [CrossRef]
- Kong, X.F.; Worley, L.; Rinchai, D.; Bondet, V.; Jithesh, P.V.; Goulet, M.; Nonnotte, E.; Rebillat, A.S.; Conte, M.; Mircher, C.; et al. Three Copies of Four Interferon Receptor Genes Underlie a Mild Type I Interferonopathy in Down Syndrome. J. Clin. Immunol. 2020, 40, 807–819. [Google Scholar] [CrossRef]
- Malle, L.; Bogunovic, D. Down syndrome and type I interferon: Not so simple. Curr. Opin. Immunol. 2021, 72, 196–205. [Google Scholar] [CrossRef]
- Galbraith, M.D.; Rachubinski, A.L.; Smith, K.P.; Araya, P.; Waugh, K.A.; Enriquez-Estrada, B.; Worek, K.; Granrath, R.E.; Kinning, K.T.; Paul Eduthan, N.; et al. Multidimensional definition of the interferonopathy of Down syndrome and its response to JAK inhibition. Sci. Adv. 2023, 9, eadg6218. [Google Scholar] [CrossRef]
- Izzo, A.; Mollo, N.; Nitti, M.; Paladino, S.; Cali, G.; Genesio, R.; Bonfiglio, F.; Cicatiello, R.; Barbato, M.; Sarnataro, V.; et al. Mitochondrial dysfunction in down syndrome: Molecular mechanisms and therapeutic targets. Mol. Med. 2018, 24, 2. [Google Scholar] [CrossRef]
- Tan, K.L.; Lee, H.C.; Cheah, P.S.; Ling, K.H. Mitochondrial Dysfunction in Down Syndrome: From Pathology to Therapy. Neuroscience 2023, 511, 1–12. [Google Scholar] [CrossRef]
- Dierssen, M.; Fructuoso, M.; Martinez de Lagran, M.; Perluigi, M.; Barone, E. Down Syndrome Is a Metabolic Disease: Altered Insulin Signaling Mediates Peripheral and Brain Dysfunctions. Front. Neurosci. 2020, 14, 670. [Google Scholar] [CrossRef]
- Belgacemi, R.; Cherry, C.; Thompson, M.; Koloko Ngassie, M.; Rehan, A.; El Alam, I.; Jourdan Le Saux, C.; Glass, I.; Britt, R.D., Jr.; Prakash, Y.S.; et al. Elevated Senescence Markers in Developing Trisomy 21 Human Lungs. Am. J. Respir. Cell Mol. Biol. 2025, 73, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Mano, H.; Aoki, K.; Hayashi, T.; Muto, A.; Nambu, Y.; Takahashi, K.; Itoh, K.; Taketani, S.; Nutt, S.L.; et al. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nat. Commun. 2015, 6, 6750. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.R.; Ahsan, F.M.; Wolf, D.M.; Shirihai, O.; Teitell, M.A. Initial B Cell Activation Induces Metabolic Reprogramming and Mitochondrial Remodeling. iScience 2018, 5, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, H.; Kodali, S.; Wang, J. Regulation of B cell fate, survival, and function by mitochondria and autophagy. Mitochondrion 2018, 41, 58–65. [Google Scholar] [CrossRef]
- Cossarizza, A.; Monti, D.; Montagnani, G.; Ortolani, C.; Masi, M.; Zannotti, M.; Franceschi, C. Precocious aging of the immune system in Down syndrome: Alteration of B lymphocytes, T-lymphocyte subsets, and cells with natural killer markers. Am. J. Med. Genet. Suppl. 1990, 7, 213–218. [Google Scholar] [CrossRef]
- Mac Donald, A.; Guipouy, D.; Lemieux, W.; Harvey, M.; Bordeleau, L.J.; Guay, D.; Romero, H.; Li, Y.; Dion, R.; Beland, K.; et al. KLRC1 knockout overcomes HLA-E-mediated inhibition and improves NK cell antitumor activity against solid tumors. Front. Immunol. 2023, 14, 1231916. [Google Scholar] [CrossRef]
- Kiani, Z.; Dupuy, F.P.; Bruneau, J.; Lebouche, B.; Retiere, C.; Geraghty, D.E.; Bernard, N.F. The Education of NK Cells Determines Their Responsiveness to Autologous HIV-Infected CD4 T Cells. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Waugh, K.A.; Araya, P.; Pandey, A.; Jordan, K.R.; Smith, K.P.; Granrath, R.E.; Khanal, S.; Butcher, E.T.; Estrada, B.E.; Rachubinski, A.L.; et al. Mass Cytometry Reveals Global Immune Remodeling with Multi-lineage Hypersensitivity to Type I Interferon in Down Syndrome. Cell Rep. 2019, 29, 1893–1908.4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frauenpreis, A.; Bhattacharya, S.; Belgacemi, R.; Sokolskiy, P.; Deutsch, G.; Jendzjowsky, N.; Glass, I.A.; Mariani, T.J.; Al Alam, D.; Danopoulos, S. Alterations in Resident Immune Cells in Prenatal Trisomy 21 Lungs. Cells 2025, 14, 1866. https://doi.org/10.3390/cells14231866
Frauenpreis A, Bhattacharya S, Belgacemi R, Sokolskiy P, Deutsch G, Jendzjowsky N, Glass IA, Mariani TJ, Al Alam D, Danopoulos S. Alterations in Resident Immune Cells in Prenatal Trisomy 21 Lungs. Cells. 2025; 14(23):1866. https://doi.org/10.3390/cells14231866
Chicago/Turabian StyleFrauenpreis, Andrew, Soumyaroop Bhattacharya, Randa Belgacemi, Pauline Sokolskiy, Gail Deutsch, Nicholas Jendzjowsky, Ian A. Glass, Thomas J. Mariani, Denise Al Alam, and Soula Danopoulos. 2025. "Alterations in Resident Immune Cells in Prenatal Trisomy 21 Lungs" Cells 14, no. 23: 1866. https://doi.org/10.3390/cells14231866
APA StyleFrauenpreis, A., Bhattacharya, S., Belgacemi, R., Sokolskiy, P., Deutsch, G., Jendzjowsky, N., Glass, I. A., Mariani, T. J., Al Alam, D., & Danopoulos, S. (2025). Alterations in Resident Immune Cells in Prenatal Trisomy 21 Lungs. Cells, 14(23), 1866. https://doi.org/10.3390/cells14231866





