Unlocking the Tumor Microenvironment: Innovations in Multiplex Immunohistochemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Materials
2.3. Immunohistochemistry
2.4. Microscopic Evaluation
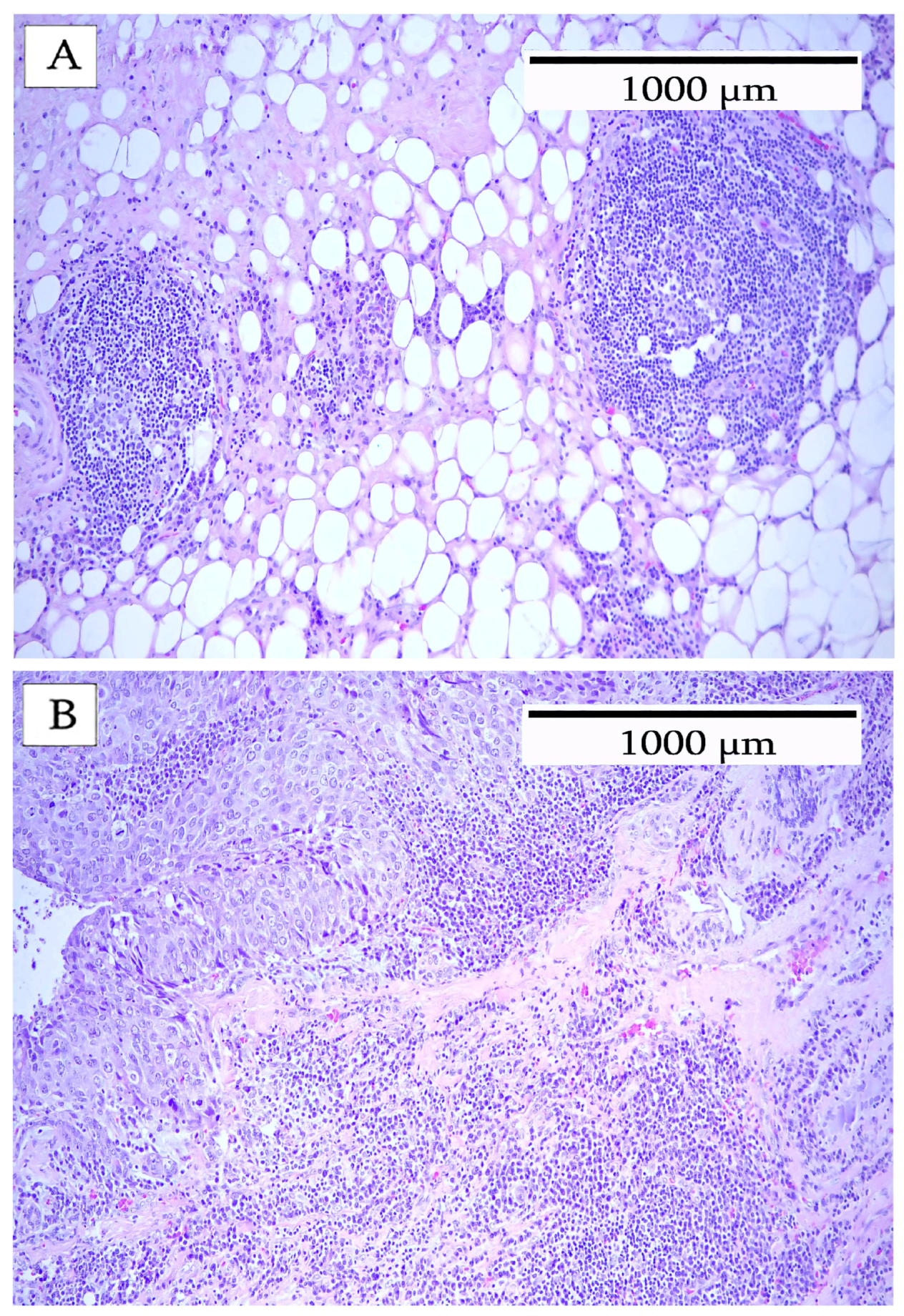
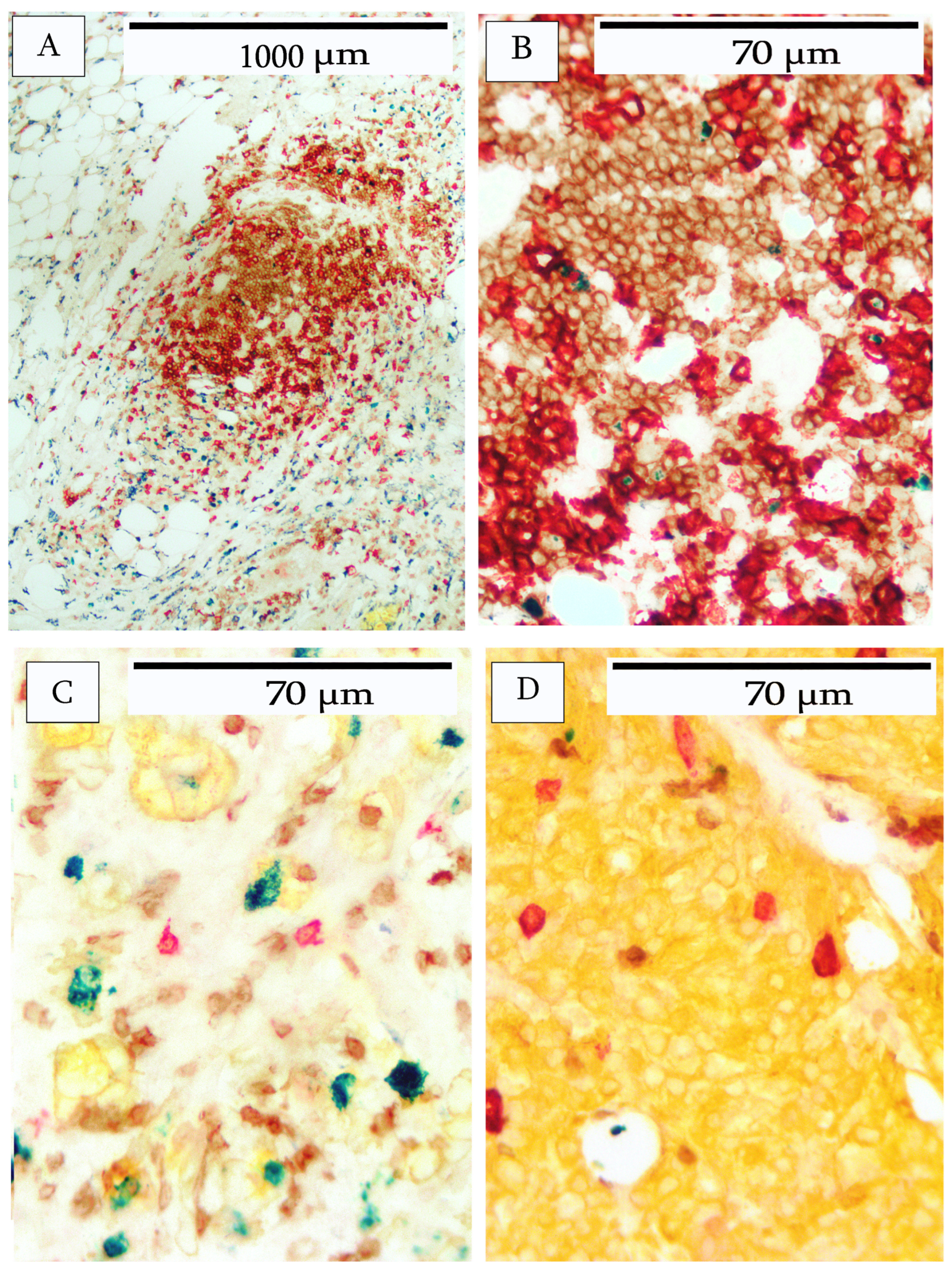
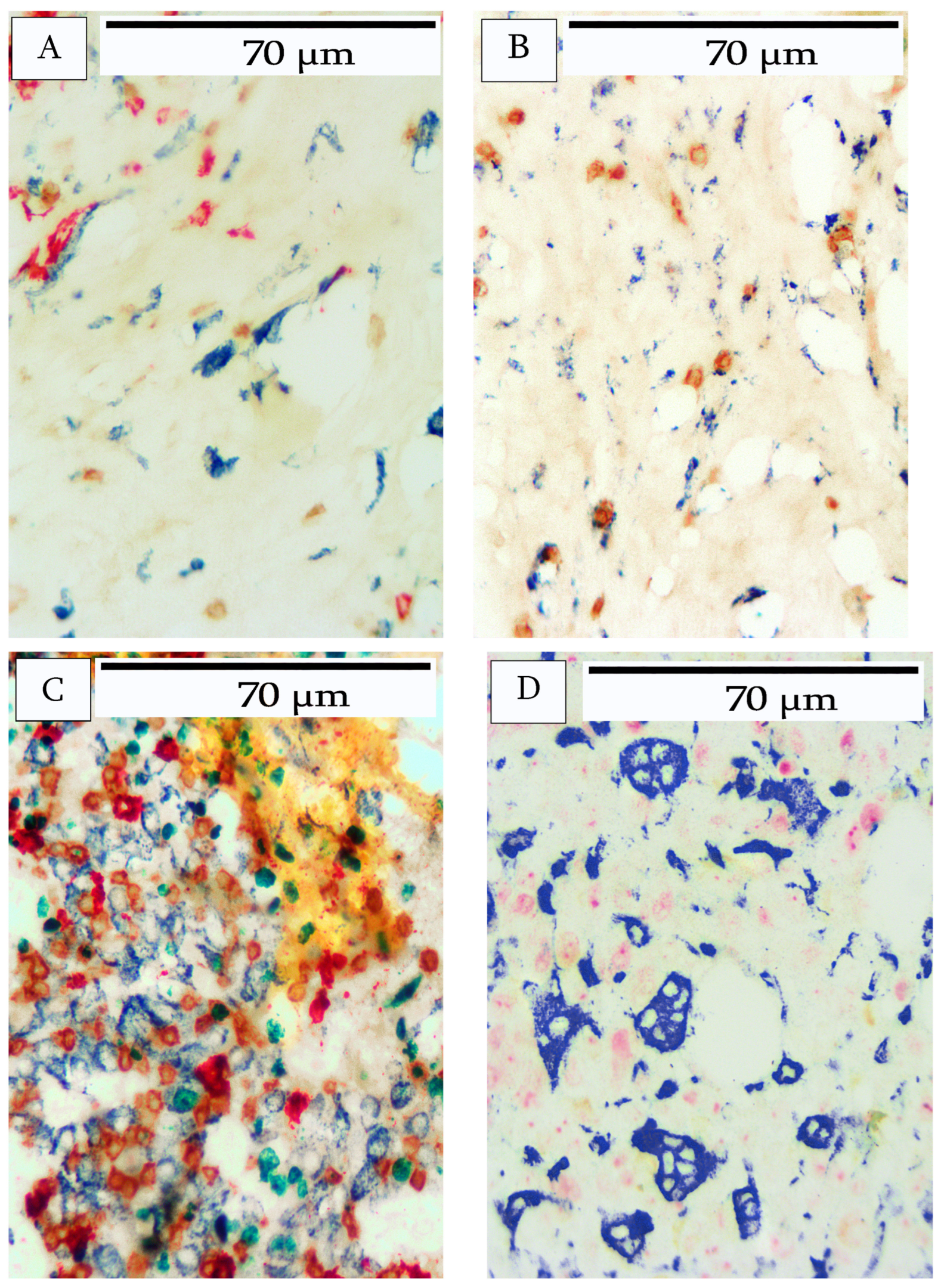
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Li, J.; Bai, X.; Huang, X.; Wang, Q. Tumor microenvironment as a complex milieu driving cancer progression: A mini review. Clin. Transl. Oncol. 2025, 27, 1943–1952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, M.Z.; Jin, W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Lee, J.; Hyeon, D.Y.; Hwang, D. Single-Cell Multiomics: Technologies and Data Analysis Methods. Exp. Mol. Med. 2020, 52, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T.; Satija, R. Integrative Single-Cell Analysis. Nat. Rev. Genet. 2019, 20, 257–272. [Google Scholar] [CrossRef]
- Galon, J.; Angell, H.K.; Bedognetti, D.; Marincola, F.M. The continuum of cancer immunosurveillance: Prognostic, predictive, and mechanistic signatures. Immunity 2013, 39, 11–26. [Google Scholar] [CrossRef]
- Angell, H.; Galon, J. From the immune contexture to the Immunoscore: The role of prognostic and predictive immune markers in cancer. Curr. Opin. Immunol. 2013, 25, 261–267. [Google Scholar] [CrossRef]
- Demaria, S.; Pikarsky, E.; Karin, M.; Coussens, L.M.; Chen, Y.C.; El-Omar, E.M.; Trinchieri, G.; Dubinett, S.M.; Mao, J.T.; Szabo, E.; et al. Cancer and inflammation: Promise for biologic therapy. J. Immunother. 2010, 33, 335–351. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Abousamra, S.; Hou, L.; Gupta, R.; Chen, C.; Samaras, D.; Kurc, T.; Batiste, R.; Zhao, T.; Kenneth, S.; Saltz, J. Learning from Thresholds: Fully Automated Classification of Tumor Infiltrating Lymphocytes for Multiple Cancer Types. arXiv 2019, arXiv:1907.03960. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Amgad, M.; Salgado, R.; Cooper, L.A.D. MuTILs: Explainable, multiresolution computational scoring of Tumor-Infiltrating Lymphocytes in breast carcinomas using clinical guidelines. medRxiv 2022. [Google Scholar] [CrossRef]
- Amgad, M.; Stovgaard, E.S.; Balslev, E.; Thagaard, J.; Chen, W.; Dudgeon, S.; Sharma, A.; Kerner, J.K.; Denkert, C.; Yuan, Y.; et al. Report on computational assessment of Tumor Infiltrating Lymphocytes from the International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2020, 6, 16. [Google Scholar] [CrossRef]
- Le, H.; Gupta, R.; Hou, L.; Abousamra, S.; Fassler, D.; Torre-Healy, L.; Moffitt, R.A.; Kurc, T.; Samaras, D.; Batiste, R.; et al. Utilizing Automated Breast Cancer Detection to Identify Spatial Distributions of Tumor-Infiltrating Lymphocytes in Invasive Breast Cancer. Am. J. Pathol. 2020, 190, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y. Modelling the spatial heterogeneity and molecular correlates of lymphocytic infiltration in triple-negative breast cancer. J. R. Soc. Interface 2014, 12, 1153. [Google Scholar] [CrossRef]
- Melichar, B.; ŠTudentovÁ, H.; KalÁBovÁ, H.; VitÁSkovÁ, D.; ÁKovÁ, P.; HornychovÁ, H.; RyŠKa, A. Predictive and Prognostic Significance of Tumor-infiltrating Lymphocytes in Patients with Breast Cancer Treated with Neoadjuvant Systemic Therapy. Anticancer. Res. 2014, 34, 1115. [Google Scholar] [PubMed]
- Basavanhally, A.N.; Ganesan, S.; Agner, S.; Monaco, J.P.; Feldman, M.D.; Tomaszewski, J.E.; Bhanot, G.; Madabhushi, A. Computerized image-based detection and grading of lymphocytic infiltration in HER2+ breast cancer histopathology. IEEE Trans. Biomed. Eng. 2010, 57, 642–653. [Google Scholar] [CrossRef]
- Gupta, B.; Yang, G.; Key, M. Novel Chromogens for Immunohistochemistry in Spatial Biology. Cells 2024, 13, 936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, S.; Shao, W.; Wu, Y.; Zhang, J.; Han, Z.; Feng, Q.; Huang, K. Deep-Learning-Based Characterization of Tumor-Infiltrating Lymphocytes in Breast Cancers From Histopathology Images and Multiomics Data. JCO Clin. Cancer Inform. 2020, 4, 480–490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruz-Roa, A.; Gilmore, H.; Basavanhally, A.; Feldman, M.; Ganesan, S.; Shih, N.N.; Tomaszewski, J.; Gonzalez, F.A.; Madabhushi, A. Accurate and reproducible invasive breast cancer detection in whole-slide images: A deep learning approach for quantifying tumor extent. Sci. Rep. 2017, 7, 46450. [Google Scholar] [CrossRef]
- Lorsakul, A.; Bredno, J.; Ochs, R.L.; Morrison, L.; Day, W. Validation of multiplex immunohistochemistry assays using automated image analysis. In Proceedings of the Medical Imaging 2018: Digital Pathology, Houston, TX, USA, 10–15 February 2018; Volume 10581, p. 105810J. [Google Scholar]
- Burugu, S.; Asleh-Aburaya, K.; Nielsen, T.O. Immune infiltrates in the breast cancer microenvironment: Detection, characterization and clinical implication. Breast Cancer 2017, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- García-Aranda, M.; Redondo, M. Immunotherapy: A Challenge of Breast Cancer Treatment. Cancers 2019, 11, 1822. [Google Scholar] [CrossRef]
- Won, H.K.; MuLan, L.I.; Wonshik, H.; Han, S.R.; Woo, K.M. The Spatial Relationship of Malignant and Benign Breast Lesions with Respect to the Fat-Gland Interface on Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 39085. [Google Scholar] [CrossRef]
- Fassler, D.J.; Torre-Healy, L.A.; Gupta, R.; Hamilton, A.M.; Kobayashi, S. Spatial Characterization of Tumor-Infiltrating Lymphocytes and Breast Cancer Progression. Cancers 2022, 14, 2148. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: Recent advances in cancer nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef] [PubMed]
- Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 2017, 17, 281–294. [Google Scholar] [CrossRef]
- Laumont, C.M.; Nelson, B.H. B cells in the tumor microenvironment: Multi-faceted organizers, regulators, and effectors of anti-tumor immunity. Cancer Cell 2023, 41, 466–489. [Google Scholar] [CrossRef]
- Guo, F.F.; Cui, J.W. The Role of Tumor-Infiltrating B Cells in Tumor Immunity. J. Oncol. 2019, 2019, 2592419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willemsen, M.; Krebbers, G.; Bekkenk, M.W.; Teunissen, M.B.M.; Luiten, R.M. Improvement of Opal Multiplex Immunofluorescence Workflow for Human Tissue Sections. J. Histochem. Cytochem. 2021, 69, 339–346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoyt, C.C. Multiplex Immunofluorescence and Multispectral Imaging: Forming the Basis of a Clinical Test Platform for Immuno-Oncology. Front. Mol. Biosci. 2021, 8, 674747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, R.; Chen, G.; Ren, Y.; Zhang, Y.; Wang, J.; Shi, Y.; Yin, J.C.; Qin, L.; Zhang, G.; Zhao, M.; et al. Prognostic value of genetic aberrations and tumor immune microenvironment in primary acral melanoma. J. Transl. Med. 2023, 21, 78. [Google Scholar] [CrossRef]
- Engelhard, V.; Conejo-Garcia, J.R.; Ahmed, R.; Nelson, B.H.; Willard-Gallo, K.; Bruno, T.C.; Fridman, W.H. B cells and cancer. Cancer Cell 2021, 39, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Dobson, L.; Conway, C.; Hanley, A.; Johnson, A.; Costello, S.; O’Grady, A.; Connolly, Y.; Magee, H.; O’Shea, D.; Jeffers, M.; et al. Image analysis as an adjunct to manual HER-2 immunohistochemical review: A diagnostic tool to standardize interpretation. Histopathology 2010, 57, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Berckelaer, C.V.; Colpaert, C.G.; Rypens, C.; Marien, K.; Kockx, M.; Waumans, Y.; Vermeulen, P.; Dirix, L.Y.; Van Laere, S.J.; Van Dam, P. The spatial relations among immune cells are prognostic in inflammatory breast cancer. Ann. Oncol. 2019, 30, mdz095.030. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Morrison, L.E.; Lefever, M.R.; Behman, L.J.; Leibold, T.; Roberts, E.A.; Horchner, U.B.; Bauer, D.R. Brightfield multiplex immunohistochemistry with multispectral imaging. Lab. Investig. 2020, 100, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Carragher, D.M.; Rangel-Moreno, J.; Randall, T.D. Ectopic lymphoid tissues and local immunity. Semin. Immunol. 2008, 20, 26–42. [Google Scholar] [CrossRef]
- van de Pavert, S.A.; Mebius, R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010, 10, 664–674. [Google Scholar] [CrossRef]
- Hiraoka, N.; Ino, Y.; Yamazaki-Itoh, R. Tertiary Lymphoid Organs in Cancer Tissues. Front. Immunol. 2016, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Lawand, M.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Dieu-Nosjean, M.C. Tertiary lymphoid structures in cancers: Prognostic value, regulation, and manipulation for therapeutic intervention. Front. Immunol. 2016, 7, 20161. [Google Scholar] [CrossRef] [PubMed]
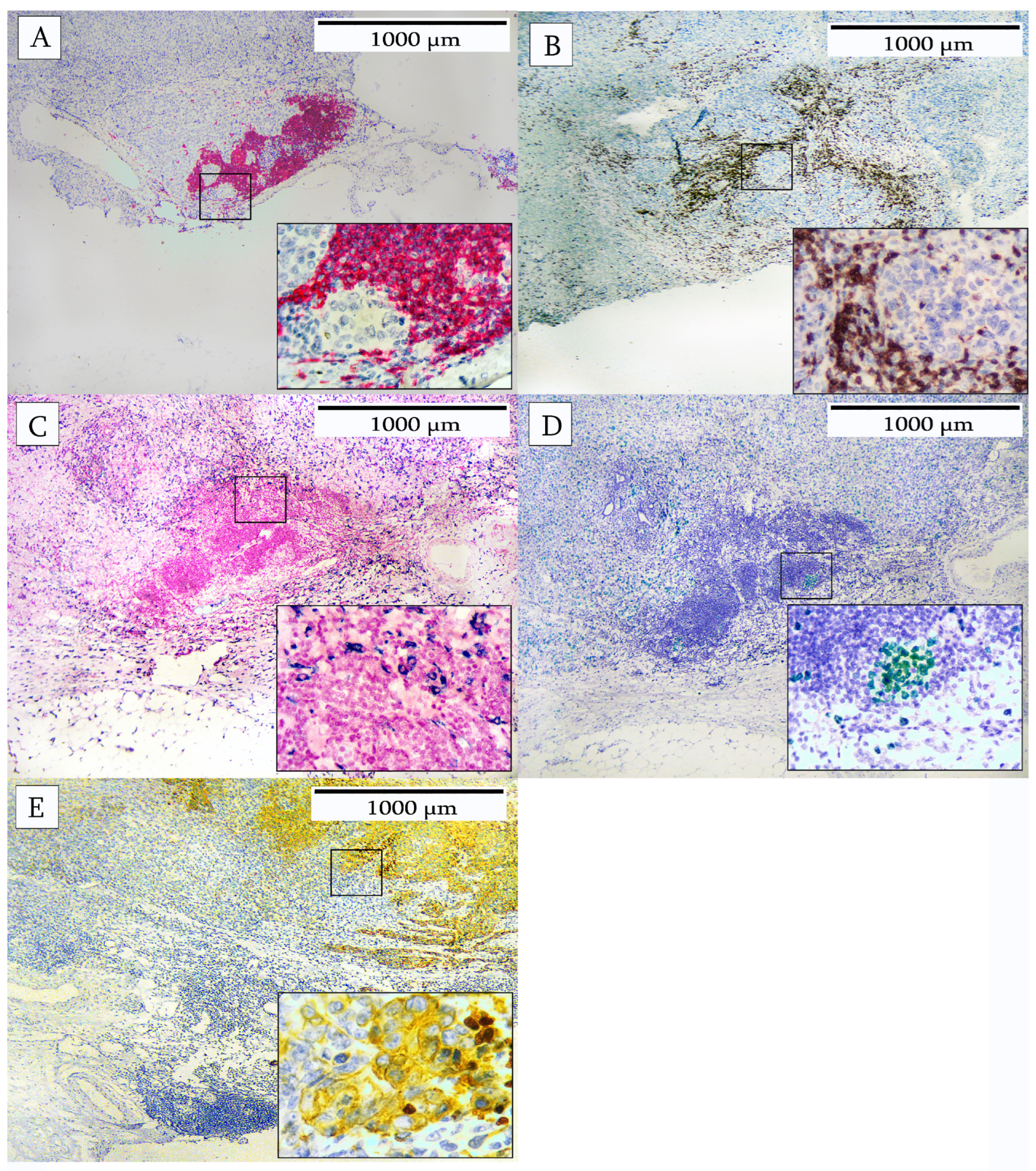
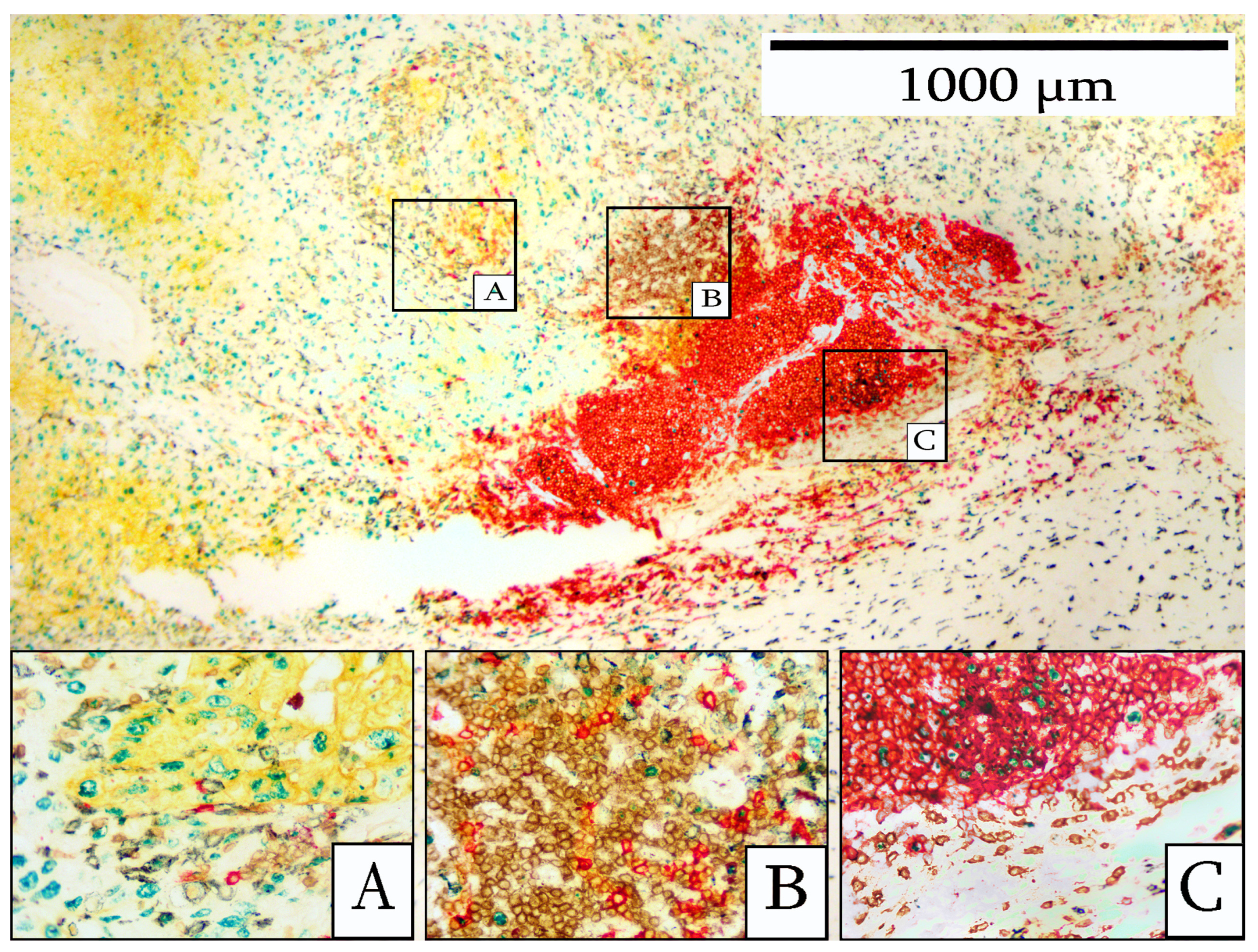
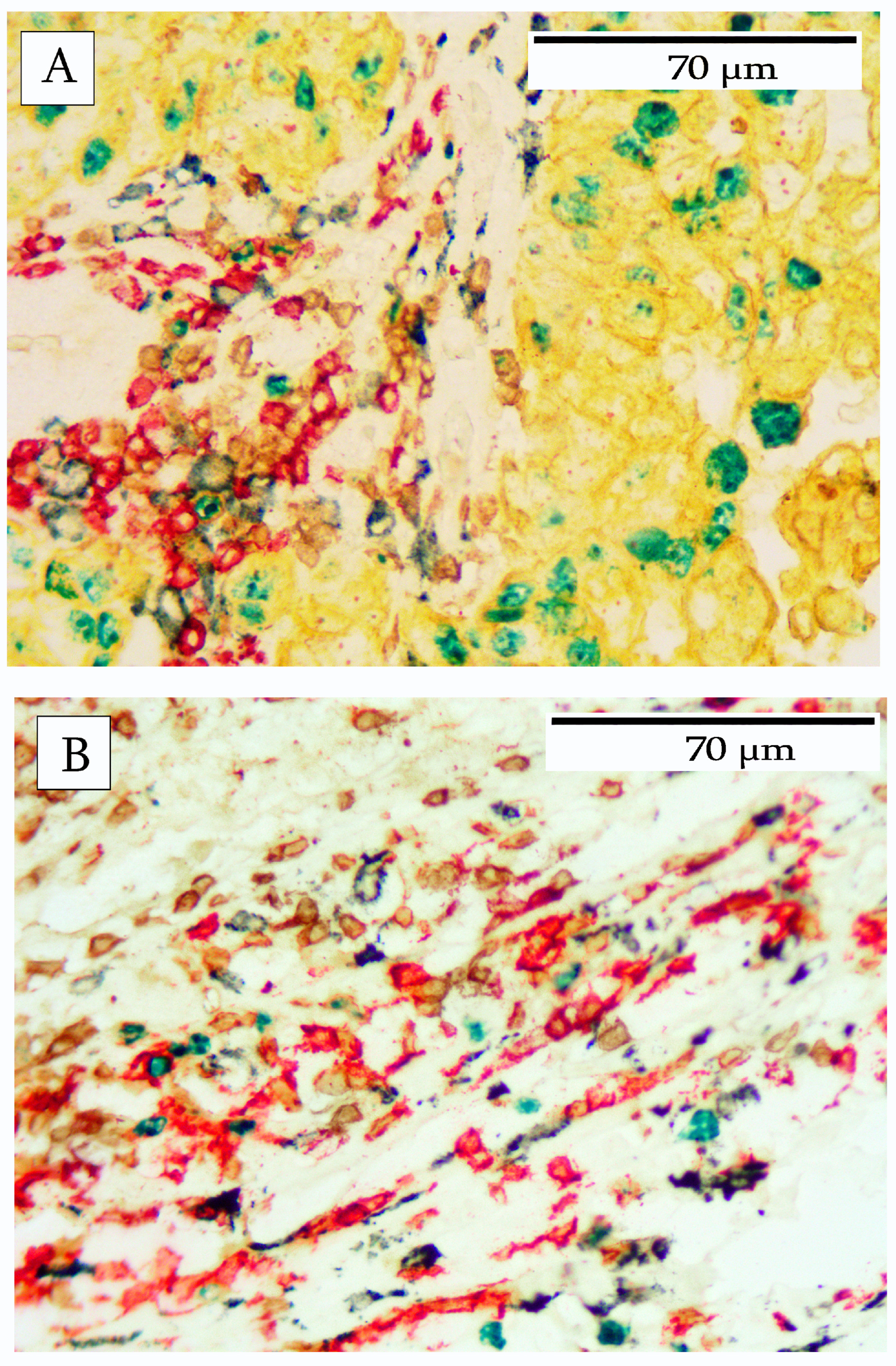
| Part | |||||
|---|---|---|---|---|---|
| Antibody | Species | Clone | Number | Dilution | Multiplex Stain |
| CD3 | Mouse | LN10 | Mob474 | 1:50 | Brown (DAB) |
| CD20 | Mouse | L26 | Mob004 | 1:80 | Red (AP-Red) |
| CD163 | Mouse | 10D6 | Mob460 | 1:50 | Blue (HRP-Blue) |
| Ki67 | Rabbit | SP6 | RMAB004 | 1:80 | Green (HRP-Green) |
| Cytokeratin | Mouse | AE1/AE3 | Mob190 | 1:40 | Yellow (HRP-Yellow) |
| Chromogen | Part Number | Enzyme System |
|---|---|---|
| PermaBlue-HRP | K063 | Peroxidase |
| PermaGreen-HRP | K074 | Peroxidase |
| PermaYellow-HRP | K060 | Peroxidase |
| Stable DAB | K047 | Peroxidase |
| PermaRed-AP | K049 | Alkaline Phosphatase |
| Breast Cancer | Morphology | Immune Cell Infiltrate | Ki67 Index |
|---|---|---|---|
| 1 | Moderately differentiated adenocarcinoma | Moderate | 5% |
| 2 | Moderately differentiated adenocarcinoma | Heavy | 75% |
| 3 | Moderately differentiated adenocarcinoma | Heavy | 50% |
| 4 | Moderately differentiated adenocarcinoma | Minimal | <1% |
| Breast Cancer | B-Cells | T-cells | Macrophages |
|---|---|---|---|
| 1 | Predominantly peripheral location. Occasional lymphoid aggregates lacking follicular structure. | Uniformly distributed. Infiltration into tumor nests. | Slight infiltration. Predominantly peripheral location in stroma and adipose tissue. |
| 2 | Infiltration throughout tumor. Tertiary lymphoid structures comprise B- and T-cells and occasional Ki67-positive B-cells at the periphery. | Heavy infiltration throughout the tumor. | Slight infiltration. Predominantly peripheral location in stroma and adipose tissue. |
| 3 | Tertiary lymphoid structures at the periphery comprise B- and T-cells. Occasional Ki67-positive B-cells. Heavy B-cell infiltrate throughout tumor. | Heavy T-cell infiltrate throughout tumor. | Moderate macrophage infiltration in the periphery of the stroma and adipose tissue. Slight infiltrate into interior tumor stroma. |
| 4 | Peripheral location and within stromal areas of the tumor. Occasional large CD20+ cells within tumor nests. | Peripheral location and within stromal areas of tumor. | Peripheral location and within stromal areas of tumor. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, B.; Yang, G.; Key, M. Unlocking the Tumor Microenvironment: Innovations in Multiplex Immunohistochemistry. Cells 2025, 14, 1819. https://doi.org/10.3390/cells14221819
Gupta B, Yang G, Key M. Unlocking the Tumor Microenvironment: Innovations in Multiplex Immunohistochemistry. Cells. 2025; 14(22):1819. https://doi.org/10.3390/cells14221819
Chicago/Turabian StyleGupta, Bipin, George Yang, and Marc Key. 2025. "Unlocking the Tumor Microenvironment: Innovations in Multiplex Immunohistochemistry" Cells 14, no. 22: 1819. https://doi.org/10.3390/cells14221819
APA StyleGupta, B., Yang, G., & Key, M. (2025). Unlocking the Tumor Microenvironment: Innovations in Multiplex Immunohistochemistry. Cells, 14(22), 1819. https://doi.org/10.3390/cells14221819





