Highlights
What are the main findings?
- m6A modification exerts cell-type-specific regulatory roles in central nervous system.
- Dysregulation of m6A modification is closely involved in the pathological progression of neurodegenerative diseases.
What is the implication of the main finding?
- The cell-type-specific molecular mechanisms of m6A provide novel potential therapeutic targets for neurodegenerative diseases.
Abstract
N6-methyladenosine (m6A) is the most abundant internal RNA modification in eukaryotes and plays a critical role in gene expression regulation by influencing RNA stability, splicing, nuclear export, and translation. Emerging evidence suggests that dysregulation of m6A contributes to neuroinflammation, neurotoxicity, and synaptic dysfunction—key features of neurodegenerative diseases. This review aims to examine the role of m6A modification in neurodegenerative diseases from a cell-type-specific perspective. We systematically reviewed recent studies investigating m6A modifications in neurons and glial cells. Data from transcriptomic, epitranscriptomic, and functional studies were analyzed to understand how m6A dynamics influence disease-related processes. Findings indicate that m6A modifications regulate neuroinflammation and immune responses in microglia, modulate astrocytic support functions, affect myelination through oligodendrocytes, and alter m6A patterns in neurons, impacting synaptic plasticity, stress responses, and neuronal survival. These cell-type-specific roles of m6A contribute to the progression of neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Amyotrophic lateral sclerosis (ALS). Understanding m6A-modulated mechanisms in specific neural cell types may facilitate the development of targeted interventions for neurodegenerative diseases.
1. Introduction
N6-methyladenosine (m6A), the most common chemical epigenetic modification among mRNA post-transcriptional modifications, is a dynamic and reversible methylation modification that occurs at the N6 position of adenine, including methylation, demethylation, and recognition [1,2]. Regulators of m6A are primarily classified into three categories, including writers, erasers, and readers, which are responsible for methylation, demethylation, and recognition, respectively, and collaborate to dynamically control m6A modification and its biological functions. Writers, including methyltransferase-like enzyme 3 (METTL3), methyltransferase-like enzyme 14 (METTL14), WT1-associated protein (WTAP), and Vir-like m6A methyltransferase-associated protein (VIRMA), mediate the process of methylation, which can be reversed with erasers such as fat mass and obesity-associated protein (FTO) and AlkB homolog H5 (ALKBH5). Moreover, the recognition of m6A is mediated by readers, notably YTH structural domain family protein 1/2/3 (YTHDF1/2/3), Insulin-like growth factor-binding proteins (IGFBPs), and Fragile X Mental Retardation Protein 1 (FMR1) [3,4,5]. m6A modification, which is mainly enriched in the 5′untranslated region (5′UTR) and 3′untranslated region (3′UTR) [6], influences RNA splicing and mRNA stability [7,8]. Additionally, m6A can promote the transport of mRNA from the nucleus to the cytoplasm, enhance translation efficiency, and increase protein expression [9,10]. However, dysregulation of m6A modification, including aberrant activity of writers, erasers, and readers, plays a significant role in the pathogenesis of diseases, such as vascular diseases, malignant tumors, and neurodegenerative diseases [1,11,12,13].
Neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), Multiple Sclerosis (MS), and Amyotrophic lateral sclerosis (ALS), which are characterized by neuroinflammation, progressive neurological dysfunction, and neuronal loss in the nervous system, result in cognitive decline and increased disability. Increasing studies have elucidated, m6A affects aging and brain cell development in AD [14]. Enhanced m6A methylation in the 5′UTR of Acyl-CoA synthetase long-chain family member 4 (ACSL4) has been shown to upregulate ACSL4 protein expression, thereby accelerating ferroptosis in dopaminergic neurons, which exacerbates both the onset and progression of PD [15]. It should be noted that resident cells in the central nervous system (CNS), including microglia, astrocytes, oligodendrocytes, and neurons, play different roles in the CNS and are involved in the development of neurodegenerative diseases (Figure 1).
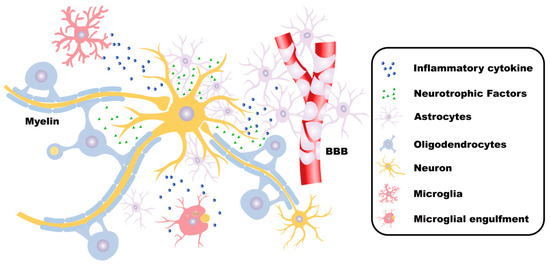
Figure 1.
The functions of neurons and glial cells in the CNS. In the central nervous system (CNS), neurons receive, integrate, and transmit information. Astrocytes fulfill critical roles in maintaining CNS homeostasis, supporting neuronal survival, regulating synaptic transmission, forming the blood–brain barrier (BBB), and facilitating tissue repair processes. Microglia sense environmental changes and maintain physiological homeostasis. Under pathological conditions, microglia also secrete inflammatory mediators, phagocytize foreign substances, and participate in immune defense. Oligodendrocytes form myelin sheaths, which insulate and protect the axons of neurons. Moreover, oligodendrocytes provide nutritional support and metabolic maintenance for the axons, contributing to maintaining the integrity and normal function of the axons. These cells collaborate with each other to jointly maintain the normal structure and function of the CNS.
Here, we summarize recent advances in understanding the dynamic regulation of m6A modifications within CNS-resident cells and their pivotal roles in inflammation and neurodegenerative diseases, highlighting the molecular mechanisms driving pathogenesis and the challenges impeding the development of targeted therapeutic strategies.
2. Microglia
2.1. Roles of Microglia in CNS
Microglia, the intrinsic immune cells of the CNS, are distributed extensively throughout the brain. The primary function of microglia is to sense environmental changes, sustain physiological homeostasis, and defend against harmful agents [16,17].
In the resting state, unstimulated microglia (M0 phenotype) contribute significantly to neuronal development and maintenance. Upon activation, microglia are differentiated into two distinct functional phenotypes: pro-inflammatory M1-like (M1) and anti-inflammatory M2-like (M2) [18,19]. M1 microglia produce inflammatory cytokines and drive neuroinflammation, which is implicated in the progression of neurodegenerative diseases [20]. In contrast, M2 microglia generally facilitate tissue repair and resolution of inflammation [21].
2.2. m6A Modifications in Microglia
Recent studies have identified that m6A RNA modifications undergo dynamic regulation across M0, M1, and M2 microglial phenotypes during neuroinflammation processes, along with numerous transcripts exhibiting significant upregulation or downregulation in m6A levels [19]. Notably, the m6A writer, METTL3, enhances the expression of the basic leucine zipper transcriptional factor ATF-like (BATF) via an IGF2BP2-dependent pathway in microglia-mediated neuroinflammation [22]. These observations highlight the potential importance of m6A modifications in regulating microglial function and suggest their involvement in the pathogenesis of neurodegenerative diseases.
2.3. m6A Modifications of Microglia in AD
AD, the most prevalent neurodegenerative disorder, is characterized by cognitive impairments, including deficits in memory and language expression. Its hallmark pathological features include intracellular neurofibrillary tangles (NFTs) due to abnormal tau protein aggregation and extracellular amyloid plaques formed by amyloid-beta (Aβ) protein accumulation [23,24,25,26].
Recent studies demonstrate significant alterations of m6A RNA methylation regulators in cortical tissues of AD patients. Compared to healthy controls, AD brains show substantial downregulation of m6A writers METTL3 (66.7%), METTL14 (74.0%), and WTAP (76.0%), as well as the eraser FTO (60%) and reader YTHDF1 (73.5%). These changes correlate with reduced m6A modifications in large pyramidal neurons and increased levels in GFAP-positive astrocytes and Iba-1-positive microglia [27]. Long-term circadian rhythm disruption regulates Hif3α m6A methylation at site 3632 and accelerates the progression of AD through the Hif3α/Lysine demethylase 3A (KDM3A)/TGF-β1 axis [28]. Furthermore, downregulation of FTO activates the Notch1–HES1 pathway, thereby triggering an immune response and promoting the progression of AD [29].
The genetic risk factor apolipoprotein E (APOE4), abundantly expressed in microglia, contributes significantly to AD pathogenesis. APOE4 expression in microglia correlates with the downregulation of YTHDC2 and the upregulation of METTL3 and METTL16, facilitating tau-associated neurodegeneration independently of triggering receptor expressed on myeloid cells 2 (TREM2). Notably, conditional deletion of APOE4 from microglia ameliorates plaque pathology [30,31,32,33], indicating a crucial role for m6A regulators in modulating APOE4 expression and AD progression. Moreover, m6A-YTHDF2 inhibits microglial NLRP3/caspase-1/GSDMD signaling, potentially mitigating AD-related CNS damage [34]. In microglia, the METTL3/IGF2BP2/IκBα axis modulates microglia M1/M2 polarization, thus contributing to inflammation and neuronal damage in AD [35]. Furthermore, METTL3-mediated activation of the TRAF6/NF-κB pathway via m6A promotes neuroinflammation in microglia, highlighting a novel target for therapeutic strategies in AD [36,37,38,39] (Figure 2).
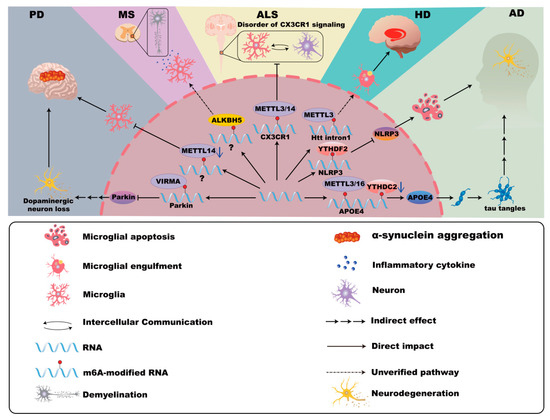
Figure 2.
m6A modifications in microglia in neurodegenerative disease. In Alzheimer’s disease (AD), the expression of APOE4 is associated with YTHDC2, METTL3, and METTL16, promoting neurodegeneration. Furthermore, YTHDF2 inhibits microglial NLRP3/caspase-1/GSDMD signaling, potentially mitigating the damage associated with AD in the central nervous system. In Parkinson’s disease (PD), VIRMA-mediated m6A methylation suppresses Parkin translation, enhancing microglial activation, dopaminergic neuron loss, and neuroinflammation. Mettl14 deletion in the substantia nigra intensifies microglial activation and exacerbates PD pathology. In Huntington’s disease (HD), the abundant m6A modifications in mHTT transcripts may be related to the pathogenic mechanism of microglia. In Multiple Sclerosis (MS), the m6A modification mediated by ALKBH5 may have the possibility of influencing the microglial pyroptosis and inflammation. In Amyotrophic lateral sclerosis (ALS), m6A modifications in CX3CR1 of microglia disrupt the communication between neurons and glial cells.
2.4. m6A Modifications of Microglia in PD
PD, the second-most common neurodegenerative disorder, is characterized by dopaminergic neuron loss and pathological α-synuclein aggregation, resulting in motor dysfunctions such as tremors, bradykinesia, rigidity, and gait disturbances [40,41,42,43,44,45,46]. Studies show that reduced m6A modification levels and decreased expressions of METTL3, METTL14 and YTHDF2 in peripheral blood mononuclear cells (PBMCs) from PD patients emphasize m6A dysregulation’s role in PD pathogenesis [47].
Microglial activation contributes significantly to α-synuclein pathology and its propagation. For instance, VIRMA-mediated m6A methylation suppresses Parkin translation, thereby enhancing microglial activation and neuroinflammation [3,48,49]. Furthermore, Mettl14 deletion in the substantia nigra intensifies microglial activation and exacerbates PD pathology [50,51]. These findings underscore the involvement of m6A modification in microglia-driven neuroinflammation in PD (Figure 2).
2.5. m6A Modifications of Microglia in HD
HD, caused by an expanded CAG repeat within the Htt gene, leads to mutant huntingtin (mHTT) protein formation, neuronal toxicity, and widespread neurodegeneration, notably affecting the functions of GABAergic medium spiny neurons (MSNs) and white matter [52,53,54,55,56]. Studies from Hippocamps of HD mice reveal hypermethylation of m6A in synaptic function–related genes and altered expression of m6A modulators. Additionally, altered TAR DNA-binding protein 43 (TDP-43) function associated with m6A dysregulation may exacerbate HD pathology [57,58]. mHTT is associated with microglia-mediated inflammation and neuronal death [59]. Notably, abundant m6A modifications in mHTT transcripts suggest microglia-related pathogenic mechanisms linked to m6A RNA methylation [59,60,61] (Figure 2).
2.6. m6A Modifications of Microglia in MS
MS is an autoimmune neurodegenerative disease characterized by immune-mediated damage to the CNS, leading to inflammatory cell infiltration, demyelination, axonal injury, and reactive gliosis [62,63,64]. Cerebrospinal fluid from MS patients shows elevated expression of multiple m6A regulators (e.g., METTL3, METTL14, ALKBH5), though methylation levels are notably decreased in progressive MS compared to relapsing-remitting forms [65]. METTL3 deficiency in Th17 cells significantly reduces disease severity in experimental autoimmune encephalomyelitis (EAE), an animal model for MS, highlighting m6A’s role in disease progression [66].
Microglial activation contributes critically to MS pathogenesis through myelin debris clearance, cytokine release, and remyelination. Activation of TREM2 in microglia or antagonizing Gasdermin D (GSDMD)-mediated inflammation provides therapeutic potential for MS management. Notably, ALKBH5-mediated m6A modifications may influence microglial pyroptosis and inflammation through GSDMD, opening avenues for future MS research [67,68,69,70,71] (Figure 2).
2.7. m6A Modifications of Microglia in ALS
ALS involves extensive motor neuron degeneration, causing muscle atrophy, impaired mobility, and cognitive disturbances [72,73,74]. Studies report increased m6A methylation in ALS spinal cord tissue, with mutations in Fused in sarcoma (FUS) and TDP-43 linked to altered m6A signaling pathways contributing to disease pathology [75,76,77].
Microglial dysfunction significantly impacts ALS progression, transitioning from early neuroprotective roles to chronic neurotoxic and pro-inflammatory states [78,79,80,81]. C9orf72 gene repeat expansions lead to microglial activation deficits and increased ALS susceptibility [82]. Differential m6A modifications in immune-related genes, such as CX3CR1, further disrupt neuron–glia communication and exacerbate ALS pathology, highlighting the potential for future studies targeting microglial m6A regulation [83] (Figure 2).
Collectively, these findings underscore the importance of m6A RNA methylation in microglial function across inflammation in multiple neurodegenerative diseases, providing new insights and therapeutic targets for future research.
3. Astrocyte
3.1. Roles of Astrocytes in the CNS
Astrocytes fulfill critical roles in maintaining CNS homeostasis, supporting neuronal survival, regulating synaptic transmission, forming the blood–brain barrier (BBB), and facilitating tissue repair processes [84,85,86,87,88]. Although historically categorized into neurotoxic (pro-inflammatory A1) and neuroprotective (anti-inflammatory A2) phenotypes [89], emerging evidence indicates that astrocyte activation exhibits substantial heterogeneity beyond this binary classification, such as pro-inflammatory astrocytes differentiate into ACLY+EP300+ memory-like cells with an epigenetic phenotype, maintaining the persistent activation of inflammation-associated genes and enabling rapid and robust secondary inflammatory responses in MS [90], so necessitating deeper investigation into their diverse functions in neurological disorders.
3.2. m6A Modifications in Astrocyte
m6A RNA methylation is increasingly recognized as a critical regulator of astrocyte functions. For example, circHECW2-mediated inhibition of m6A methylation via downregulated WTAP contributes to astrocytic dysfunction in major depressive disorder (MDD) by decreasing guanine nucleotide binding-protein gamma subunit-4 (GNG4) mRNA levels [91]. ALKBH5 modulates glutamatergic transmission under chronic stress conditions, and its translocation blockade by circSTAG1 enhances FAAH mRNA degradation in astrocytes, reducing depressive behaviors in mice [92,93]. Additionally, ALKBH5-mediated suppression of LncRNA SNHG3 in astrocytes alleviates ischemic damage and inflammation induced by cerebral ischemia–reperfusion injury [94]. Furthermore, METTL3 enhances the stability of SOX2 mRNA, protecting glioma stem-like cells from radiation cytotoxicity [95], and METTL3-induced stabilization of nuclear paraspeckle assembly transcript 1 (NEAT1) and the activation of the miR-3773p/Nampt axis confer neuroprotection in ischemic injury [96]. METTL3 also mediates anti-inflammatory and anti-pyroptotic responses via NLRP3 downregulation in emodin-treated astrocytes [97]. Under neurotoxic stress, the epitranscriptomic reader YTHDF2 is responsible for the regulation of SEK1(MAP2K4)-JNK-c-JUN inflammatory signaling in astrocytes [98].
Moreover, m6A also facilitates astrocyte-associated tumorigenesis. It is reported that YTHDF2-mediated translation of circMET produces the MET404 variant, promoting glioblastoma tumorigenesis [99].
Reactive astrocytes play an important role in neurodegenerative diseases, contributing to both neuroinflammation and neuroprotection [100,101,102,103]. Alterations in m6A RNA modifications significantly influence these processes.
3.3. m6A Modifications of Astrocytes in AD
Astrocytic dysfunction in AD, including impaired calcium signaling, disrupted glutamate buffering, and enhanced synapse uptake via MFG-E8, exacerbates neuronal damage [104,105]. It has been found that ApoE4 expression correlates with enhanced neurotoxicity in AD, which is associated with elevated METTL3/METTL16 and decreased YTHDC2 levels [30,31,32,33,106]. Increased METTL3 and reduced ALKBH5 also promote astrocyte activation, intracellular aggregation of Microtubule-associated protein tau (MAPT), and neuroinflammation [107]. Moreover, elevated FTO and YTHDF1 in astrocytes induce mitochondrial dysfunction and oxidative stress, which can be mitigated by the FTO inhibitor MO-I-500 in AD [108] (Figure 3).
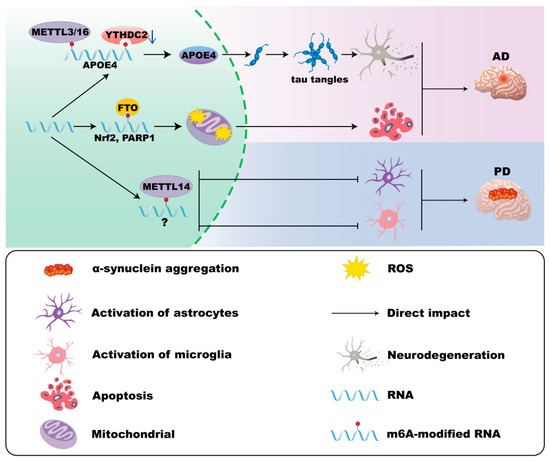
Figure 3.
m6A modifications in astrocytes in AD and PD. In Alzheimer’s disease (AD), ApoE4 expression is associated with elevated METTL3/METTL16 and decreased YTHDC2 levels in astrocytes, which correlates with enhanced neurotoxicity. Moreover, FTO and YTHDF1 in astrocytes induce mitochondrial dysfunction and oxidative stress. In Parkinson’s disease (PD), the loss of Mettl14 in the substantia nigra increases astrocyte activation.
3.4. m6A Modifications of Astrocytes in PD
Astrocytes in PD demonstrate both protective roles, such as debris phagocytosis and inflammation inhibition [109,110], and detrimental roles, including pathogenic activation by α-synuclein and impaired protein clearance due to LRRK2 mutations [111,112]. Although direct evidence remains limited, loss of Mettl14 in the substantia nigra increases astrocyte activation, and manganese-induced downregulation of YTHDF2 exacerbates inflammatory responses, suggesting a potential role for m6A regulation in PD pathology [50,98] (Figure 3).
3.5. m6A Modifications of Astrocytes in HD
Although direct evidence linking m6A modifications in astrocytes to HD is absent, astrocytes significantly influence HD progression. They exhibit enhanced clearance of mutant huntingtin (mHTT), release protective cytokines like brain-derived neurotrophic factor (BDNF) and metallothionein-3 (MT3), but progressively lose normal functions due to mHTT aggregates [113,114,115,116,117,118,119,120]. Dysfunctional astrocytic cholesterol metabolism and impaired K+ buffering exacerbate neuronal vulnerability [121,122,123,124]. Gene expression changes, including reduced µ-crystallin (Crym), further reflect astrocytic dysfunction in HD [125].
3.6. m6A Modifications of Astrocytes in MS
In MS, astrocytes significantly contribute to neuroinflammation, demyelination, and impaired oligodendrocyte regeneration, exacerbating disease severity [126,127,128]. Current studies directly exploring astrocytic m6A in MS are scarce. Further support is found in spinal cord injury models, where METTL3-driven m6A modifications enhance astrocyte-mediated neuroprotection [129].
3.7. m6A Modifications of Astrocytes in ALS
Astrocytes in ALS exhibit significant molecular and functional heterogeneity, becoming toxic to motor neurons through mechanisms involving C9orf72-induced metabolic dysfunction, mutant SOD1 toxicity, and disrupted tripartite synapse plasticity [126,130,131,132]. Although no direct evidence currently links astrocytic m6A modifications to ALS pathology, mutations in RNA-binding proteins such as Fused in sarcoma (FUS) and TAR DNA-binding protein-43 (TDP-43), which are associated with altered m6A modifications, imply a potential epitranscriptomic mechanism that warrants further investigation [76,77,133,134]. Astrocytic m6A RNA modifications are crucial regulators of CNS homeostasis and pathology.
Notwithstanding the growing body of evidence, substantial knowledge gaps remain, especially regarding the direct roles of m6A modifications in astrocytes across various neurodegenerative diseases. Future investigations to clarify these epitranscriptomic mechanisms could potentially unveil novel therapeutic targets for CNS disorders.
4. Oligodendrocyte
4.1. Roles of Oligodendrocytes in CNS
Oligodendrocytes, derived from oligodendrocyte precursor cells (OPCs), are critical myelinating cells within the CNS [135]. Beyond myelination, they support axonal integrity and functionality [136], provide metabolic support [137], and modulate immune responses [138]. Dysfunctional oligodendrocytes are significantly implicated in various neurodegenerative diseases, including AD, PD, HD, MS, and ALS.
4.2. m6A Modifications in Oligodendrocyte
m6A RNA methylation is crucial for oligodendrocyte differentiation, maturation, and myelination processes. Mettl14 deficiency leads to a reduction in the number of mature oligodendrocytes and a decrease in myelin in the CNS [139]. In detail, loss of Mettl14 in oligodendrocytes causes aberrant splicing of transcripts such as neurofascin 155 (NF155), disrupting paranodal junctions and promoting demyelination [139,140]. Inflammatory cytokines, particularly IFN-γ, induce dysfunction in m6A reader heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1), leading to altered expression of myelin-related genes (Plp, Mag, and Mbp) and promoting oligodendrocyte dysfunction and death [141]. Dysregulated m6A modifications in oligodendrocytes have been increasingly associated with neurodegenerative diseases, suggesting a potential therapeutic target (Figure 4).
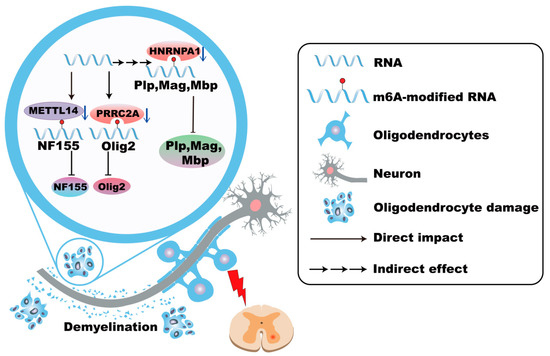
Figure 4.
m6A modifications in oligodendrocytes in MS. In Multiple Sclerosis (MS), the absence of METTL14 in oligodendrocytes leads to abnormal splicing of transcripts such as NF155, disrupts paranodal junctions of myelin sheaths, and promotes demyelination. Genetic variations in PRRC2A are associated with increased MS susceptibility, which is related to the downregulation of Olig2. The dysfunction of hnRNP A1 leads to alterations in the expression of myelin-related genes and promotes the dysfunction and death of oligodendrocytes.
4.3. m6A Modifications of Oligodendrocytes in AD
Oligodendrocytes significantly contribute to AD pathology involving myelin dysfunction, demyelination, and amyloid-beta (Aβ) peptide production [142,143]. Disease-associated oligodendrocytes, primarily located near Aβ plaques in AD patients, influence disease progression via ERK signaling [144]. Although direct evidence linking m6A modifications in oligodendrocytes and AD pathology remains limited, existing data suggest its important roles. Deletion of the m6A methyltransferase Mettl14 impairs oligodendrocyte differentiation, resulting in hypomyelination [139]. Furthermore, m6A readers such as Prrc2a stabilize Olig2 mRNA, a transcription factor critical for oligodendrocyte differentiation, and downregulation of Olig2 is linked to neuronal death in AD [145,146]. Additionally, Prrc2b deficiency destabilizes Sox2 mRNA, which is implicated in AD progression [147,148].
4.4. m6A Modifications of Oligodendrocytes in PD
Oligodendrocytes exhibit disease-specific molecular signatures in PD, characterized by inflammatory reprogramming and myelination abnormalities [149]. White matter abnormalities driven by myelin defects significantly contribute to PD pathology [150,151]. Oligodendrocytes prominently express leucine-rich repeat kinase 2 (LRRK2), a gene closely related to idiopathic and familial PD, highlighting their significance in PD pathogenesis [152,153,154]. Loss of oligodendrocyte-specific β-glucocerebrosidase triggers axonal degeneration, α-synuclein accumulation, astrogliosis, and lipid dyshomeostasis, exacerbating PD pathology [155]. Meanwhile, α-synuclein aggregates impair OPC differentiation and disrupt cellular energetics, possibly affecting age-related remyelination processes [156]. Although direct research into m6A modifications in oligodendrocytes in PD is limited, these pathways highlight oligodendrocytes as a potential target for future PD research.
4.5. m6A Modifications of Oligodendrocytes in HD
Oligodendrocyte dysfunction and associated white matter abnormalities constitute early pathological events in HD [55]. Mutant huntingtin protein enhances the activity of the polycomb repressive complex 2 (PRC2), causing delayed oligodendrocyte maturation and myelination deficits [157]. Metabolic disruptions involving glucose, thiamine, and lipid metabolism further impair oligodendrocyte maturation through pathways involving diacylglycerol (DAG) and protein kinase C epsilon (PRKCE). Dysfunction in thiamine metabolism, particularly thiamine pyrophosphokinase 1 (TPK1), is linked to behavioral deficits in HD mouse models [158]. Despite these findings, the direct role of m6A modifications in HD remains poorly understood, marking an important direction for future studies.
4.6. m6A Modifications of Oligodendrocytes in MS
Demyelination is a hallmark of MS, characterized by initial myelin sheath damage progressing toward oligodendrocyte cell body deterioration [159]. Oligodendrocyte pathology in MS is exacerbated by physiological aging and inflammation-induced cellular senescence [160]. Specific pathological events, including Gasdermin D (GSDMD) activation and Fas-mediated apoptosis, significantly contribute to oligodendrocyte damage and demyelination [70,161]. Genetic variations in m6A reader, PRRC2A, are associated with increased MS susceptibility, which is related to the downregulation of Olig2 [145,162]. Elevated levels of various m6A regulatory proteins (METTL3, METTL14, ALKBH5, FTO, WTAP, etc.) are observed in cerebrospinal fluid from MS patients, suggesting their potential roles in MS pathogenesis [65] (Figure 4).
4.7. m6A Modifications of Oligodendrocytes in ALS
ALS pathology involves motor neuron degeneration accompanied by oligodendrocyte dysfunction and impaired myelination [163]. Reduced numbers of m6A-positive oligodendrocytes in ALS patient brains suggest that impaired m6A methylation may contribute to disease pathology [164]. Additionally, the RNA-binding protein TDP-43, crucial for oligodendrocyte survival and myelin integrity, is regulated by m6A modifications. Loss of TDP-43 leads to progressive myelin degeneration, highlighting a key connection between m6A modification, TDP-43 dysfunction, and ALS pathogenesis [75,165]. This interaction presents a promising target for novel therapeutic strategies in ALS.
5. Neuron
5.1. Roles of Neurons in CNS
Neurons serve as the fundamental units of the nervous system. They establish intricate networks via synaptic connections, which are essential for mediating perception, cognition, motor function, and memory processes [166,167]. Proper neuronal function is vital for the overall health and operation of the nervous system.
5.2. m6A Modifications in Neuron
m6A RNA modification is crucially involved in neuronal health, influencing learning, memory, aging, and neurodegeneration. Notably, neuronal m6A-modified transcripts exhibit lower translational efficiency compared to glial cells, underscoring distinct roles of m6A across cell types [168,169]. This section reviews recent insights into neuronal m6A modifications in neurodegenerative diseases.
5.3. m6A Modifications of Neurons in AD
In AD, neuronal dysfunction manifests through amyloid plaques, neurofibrillary tangles, synaptic loss, and neuronal death, causing cognitive decline [170,171,172,173,174]. Several studies have elucidated the significant roles of m6A modifications in AD pathogenesis. METTL3-mediated m6A modification activates the circRIMS2/miR-3968 signal, resulting in aberrant Ubiquitin-conjugating enzyme E2K (UBE2K) activation, subsequent GluN2B degradation, and synaptic impairment associated with memory deficits [175]. Additionally, dysregulation of METTL3 induced by Aβ42 disrupts mitochondrial proteostasis via the Lon protease homolog 1 (LONP1) complex, exacerbating mitochondrial dysfunction [176]. METTL13 deficiency has also been reported to diminish postsynaptic density protein 95 (PSD95) expression, further contributing to synaptic defects and neurodegeneration [27]. However, METTL3 mediates the m6A modification of Leucine-rich repeat and immunoglobulin containing nogo receptor 2 (Lingo2), which promotes the degradation of Lingo2 mRNA and facilitates the production of Aβ in AD mice [177]. Furthermore, increased VIRMA activity leads to heightened m6A modification of PRKN mRNA, reducing its stability and consequently impairing mitophagy, which promotes neuronal death [3]. Moreover, exposure to 27-Hydroxycholesterol (27-OHC) decreases m6A methylation levels by downregulating key regulators (METTL14, YTHDF1, FTO), thereby reducing synapse-associated molecules and impairing cognition. Interestingly, the intestinal commensal bacterium Roseburia intestinalis can reverse these detrimental effects, suggesting potential therapeutic approaches [178] (Figure 5).
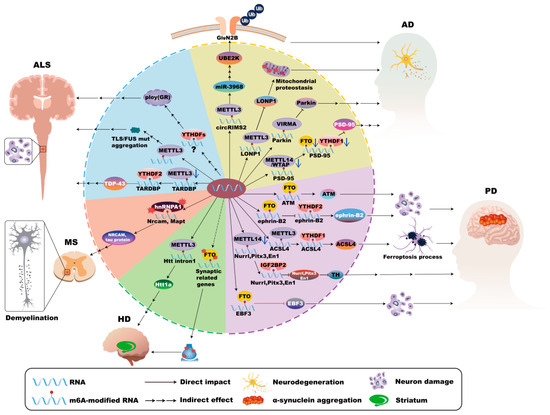
Figure 5.
m6A modifications in neurons in neurodegenerative disease. In Alzheimer’s disease (AD), METTL3-mediated m6A modification activates the circRIMS2/miR-3968 pathway, causing abnormal activation of UBE2K, subsequent degradation of GluN2B, and synaptic impairment. Dysregulation of METTL3 disrupts mitochondrial proteostasis via the LONP1 complex. Moreover, increased VIRMA activity leads to heightened m6A modification of PRKN RNA, reducing its stability and consequently impairing mitophagy and neuronal death. METTL13 deficiency diminishes PSD95 expression, contributing to synaptic defects. In Parkinson’s disease (PD), FTO expression reduces m6A levels, stabilizing ATM mRNA and consequently promoting dopaminergic neuron death. Manganese exposure disrupts neuronal projections through the FTO/ephrin-B2/YTHDF2 pathway, which underscores the sensitivity of neuronal m6A machinery to external insults. Furthermore, Soot nanoparticles increase METTL3 and YTHDF1 expression, amplifying ACSL4-mediated ferroptosis. However, METTL14 deficiency reduces the expression of essential transcription factors (Nurr1, Pitx3, En1) and impacts the Tyrosine hydroxylase (TH) expression and dopaminergic functions. Increased m6A methylation of Early B-cell factor 3 (EBF3) mRNA stabilizes its expression, ameliorating motor deficits and inhibiting apoptosis in PD. In Huntington’s Disease (HD), METTL3-mediated m6A methylation of the Huntingtin gene (Htt1a) correlates with HD progression. Impairment of cognitive-training-induced changes in FTO distribution disrupts synaptic gene expression and memory functions. In Multiple Sclerosis (MS), dysfunctional hnRNP A1 disrupts RNA splicing of critical neuronal genes (Mapt and Nrcam), resulting in functional damage of neurons. In Amyotrophic lateral sclerosis (ALS), the loss of METTL3 activity elevates TDP-43 expression by reducing m6A modification of TARDBP, disrupting neuronal homeostasis. However, Mutations in the RNA-binding protein FUS are associated with elevated neuronal m6A levels. m6A-modified RNAs and YTHDF proteins promote poly (GR) inclusions, intensifying neuronal toxicity and disease progression.
5.4. m6A Modifications of Neurons in PD
PD is characterized by the degeneration of dopaminergic neurons in the substantia nigra, which is closely associated with altered neuronal m6A modifications. Elevated FTO expression reduces m6A levels, stabilizes Ataxia telangiectasia mutated (ATM) mRNA, and consequently promotes dopaminergic neuron death [179]. However, in another study, Salsolinol, a catechol isoquinoline that causes neurotoxicity, increases the level of m6A modification by inhibiting the expression of FTO and ALKBH5 in neurons, which led to the downregulation of Yes-associated protein 1 (YAP1), promoting autophagy and the death of dopaminergic neurons [180]. Environmental factors such as manganese exposure disrupt neuronal projections through the Foxo3a/FTO/ephrin-B2/YTHDF2 signaling, which underscores the sensitivity of neuronal m6A machinery to external stress [181]. Additionally, soot nanoparticles have been shown to increase METTL3 and YTHDF1 expression, amplifying ACSL4-mediated ferroptosis and exacerbating neuronal loss in PD [15]. FTO mediates the m6A demethylation of BRCA1-associated protein 1 (BAP1) and promotes its upregulation, resulting in the death of dopaminergic neurons by inducing ferroptosis [182]. Therapeutically, FTO inhibitors exhibit promising neuroprotective effects in PD models [183]. Furthermore, Mettl14-mediated m6A modification acts on transcription factors Nurr1, Pitx3, and EN1, thereby regulating the expression of tyrosine hydroxylase (TH) and its related functions. METTL14 deficiency reduces the expression of these essential transcription factors and impairs dopaminergic neuron function [50,184,185]. METTL14 binds to and modifies the m6A motif in the coding region of α-synuclein (α-syn) mRNA, while the reader protein YTHDF2 participates in recognizing m6A-modified α-syn mRNA and impairs its stability. However, the mRNA levels of m6A, METTL3, METTL14, and YTHDF2 are significantly reduced in PD patients, which may be associated with the pathogenesis of PD [47]. Conversely, NRF1-mediated upregulation of METTL3 elevates glutaredoxin (GLRX) expression, supporting neuronal survival through enhanced RNA stability regulated by IGF2BP2 [186,187]. Similarly, increased m6A methylation of EBF3 mRNA stabilizes its expression, ameliorating motor deficits and inhibiting apoptosis, thus providing symptomatic relief in PD [188] (Figure 5).
5.5. m6A Modifications of Neurons in HD
HD primarily refers to medium-sized spiny neurons in the striatum [189], and altered m6A modifications contribute significantly to its pathology. Research using Hdh+/Q111 mouse models of HD demonstrates that impaired cognitive-training-induced alterations in nuclear METTL14 and FTO distribution disrupts synaptic gene expression and memory functions. Pharmacological inhibition of m6A demethylation ameliorates these cognitive deficits [57]. Additionally, increased m6A methylation of the Huntingtin gene (Htt1a) correlates with HD progression, which is influenced by METTL3 enzymatic activity [61]. Furthermore, TDP-43 dysfunction in conjunction with altered m6A modification patterns affects RNA splicing and gene regulation, contributing to neuronal dysregulation and the pathogenic expansion of CAG repeats characteristic of HD [58,190] (Figure 5).
5.6. m6A Modifications of Neurons in MS
MS involves inflammation-driven neuronal injury, where alterations in m6A modifications significantly impact disease progression. Dysfunctional hnRNP A1, an important m6A reader, disrupts RNA splicing of critical neuronal genes, including Mapt and Nrcam, resulting in neurite damage and impaired neuronal functionality [191,192]. Environmental aluminum exposure further exacerbates neuronal injury by downregulating key m6A regulators (METTL3, METTL14, FTO, and YTHDF2), causing global reductions in m6A RNA methylation and subsequent neuronal dysfunction [193,194,195] (Figure 5).
5.7. m6A Modifications of Neurons in ALS
ALS, characterized by motor neuron degeneration, is closely linked to dysregulated neuronal m6A modifications. The loss of METTL3 activity in cholinergic neurons elevates TDP-43 expression by reducing m6A modification of TARDBP, then disrupts neuronal homeostasis [77]. YTHDF2 is also implicated in mediating TDP-43 toxicity, and knockout of YTHDF2 alleviates neurodegeneration [75]. Additionally, mutations in the RNA-binding protein FUS are associated with elevated neuronal m6A levels, suggesting pathogenic interactions that can be mitigated by inhibiting METTL3 in ALS [76,133]. ALS is associated with C9orf72 gene repeat expansions, which involve downregulated METTL3 and METTL14 expression and result in global m6A hypomethylation. This disrupts RNA metabolism and impairs neuronal function, primarily through dysregulated glutamate synapses and calcium signaling pathways [196,197]. Furthermore, YTHDFs promote poly(GR) inclusion formation and intensify neuronal toxicity and disease progression [198]. Moreover, downregulation of the m6A reader RNA binding motif protein X-linked (RBMX) can induce the activation of the p53 pathway, resulting in neuronal defects and the progression of ALS [199] (Figure 5).
Neuronal m6A modifications significantly influence neurodegenerative diseases by modulating essential biological processes, including RNA metabolism, synaptic integrity, mitochondrial homeostasis, and neuronal survival. Continued investigation into these pathways could reveal novel therapeutic strategies for neurodegenerative disorders.
6. Conclusions and Perspectives
Research on neurodegenerative diseases currently spans multiple domains, focusing on the misfolding and aggregation of proteins such as Tau, α-synuclein, and TDP-43, along with neurotoxic effects, as well as neuroinflammation involving glial cells. Recently, RNA modifications, specifically m6A, have emerged as critical regulators in glial cells and neurons in neurodegenerative diseases (Table 1 and Table 2).

Table 1.
The role of m6A modification in glial cells in neurodegenerative diseases.

Table 2.
The role of m6A modification in neurons in neurodegenerative diseases.
However, research into m6A modifications in neurodegenerative diseases is still in the early stages, and most studies focus primarily on AD and PD. Investigations into other neurodegenerative diseases remain exploratory. Neuronal populations have been the primary focus of research on m6A modification dynamics, while studies of glial cells remain limited despite their critical roles in neurodegeneration.
In AD, research on m6A modifications has advanced significantly, spanning both neurons and glial cells populations. By contrast, in MS, initial studies focusing on oligodendrocytes have emerged, but substantial knowledge gaps remain for other glial cell types. A further critical limitation is the lack of specificity in m6A modification site mapping, and precise cellular localization in CNS. Given the distinct biological functions of neurons and glial cells, future research must prioritize the detailed identification of cell-specific m6A sites.
Additionally, current studies have demonstrated alterations in m6A modification in individual neurodegenerative diseases, but fail to identify the initiating factors driving this cascade of changes. In this review, we summarize m6A modifications from the perspective of distinct cells. It is evident that further investigations are needed to elucidate how m6A modifications mediate crosstalk between different cell populations.
Furthermore, the mapping of cell-type-specific m6A landscapes in the brain, the crosstalk between m6A and other epigenetic modifications (e.g., 5-methylcytosine [m5C] and histone lactylation), as well as the temporal dynamics of m6A modifications, remain to be fully elucidated.
Currently, the discovery of various m6A modification-related protein modulators has led to the development of chemical agents that show therapeutic promise, particularly in cancer. Nevertheless, the role of m6A in neurodegenerative diseases requires further elucidation. Apelin-13, a neuropeptide, upregulates the expression of METTL3 through an m6A-dependent mechanism, so it downregulates the lncRNA BDNF-AS, thereby suppressing neuroinflammation and activating the BDNF/TrkB pathway to ameliorate Alzheimer’s disease in rats [200]. Therapeutic strategies targeting m6A modification have opened up a new potential direction for the treatment of neurodegenerative diseases. By regulating RNA methylation status to interfere with disease-related molecular pathways, such as leveraging CRISPR-dCas13 to target and recruit m6A regulators to specific RNA loci of disease-related genes. These strategies have demonstrated promising application prospects in alleviating neuroinflammation, protecting neuronal survival, and improving synaptic function. However, these strategies currently face key challenges, including insufficient selectivity and off-target effects, which may impair therapeutic efficacy and pose potential safety risks. Therefore, future research needs to closely integrate cell-type-specific m6A mechanisms to further optimize therapeutic tools. For example, developing cell-type-targeted delivery systems for m6A modulators to enable their precise action on specific cells in the CNS. This will avoid damage to other tissues and cells, thereby reducing side effects in clinical practice.
In summary, research on m6A RNA modification holds significant promise for uncovering novel mechanisms and therapeutic strategies in neurodegenerative diseases. Despite existing limitations, including the need for enhanced cellular localization specificity and broader disease coverage, advancing our understanding of m6A modifications could substantially improve therapeutic outcomes for neurodegenerative diseases.
Author Contributions
S.W. (Shuowei Wang) prepared the related literature and was the major contributor in writing the manuscript. Z.F. and H.W. were responsible for preparing the relevant literature. S.W. (Shen Wang), S.Q., X.W., and F.Z. are responsible for the production of Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5. X.L., K.Z., and X.H. provided guidance throughout the preparation of the manuscript and made significant revisions to it. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81971179 to Liu), the Natural Science Foundation of Jiangsu Province (BK20231347 to Liu), Jiangsu Commission of Health (Z2019035 to Zhou), Jiangsu Provincial Department of Education (20KJA320004 to Zhou), and the Technology Innovation Foundation of Xuzhou City (KC23242 to Qin), Open Competition Grant of Xuzhou Medical University (JBGS202202 to Team).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| AD | Alzheimer’s disease |
| ALKBH5 | AlkB homolog H5 |
| ALS | Amyotrophic lateral sclerosis |
| APOE4 | Apolipoprotein E4 |
| ATM | Ataxia telangiectasia mutated |
| BAP1 | BRCA1-associated protein 1 |
| EBF3 | Early B-cell factor 3 |
| En1 | Engrailed 1 |
| FTO | Fat mass and obesity-associated protein |
| FUS | Fused in sarcoma |
| GLRX | Glutaredoxin |
| HD | Huntington’s disease |
| hnRNP A1 | Heterogeneous nuclear ribonucleoprotein A1 |
| Htt intron1 | Huntingtin gene |
| IGF2BP2 | Insulin-like growth factor 2 mRNA-binding protein 2 |
| KDM3A | Lysine demethylase 3A |
| Lingo2 | Leucine-rich repeat and immunoglobulin-containing |
| LONP1 | Lon protease 1 |
| Mag | Myelin-associated glycoprotein |
| MAPT | Microtubule-associated protein tau |
| Mbp | Myelin basic protein |
| METTL3/14/16 | Methyltransferase-like enzyme 3/14/16 |
| MS | Multiple Sclerosis |
| NF155 | Neurofascin 155 |
| NLRP3 | NLR family pyrin domain containing 3 |
| NRCAM | Neuronal cell adhesion molecule |
| Nrf2 | NF-E2-related factor 2 |
| Olig2 | Oligodendrocyte transcription factor 2 |
| PARP1 | Poly (ADP-ribose) polymerase 1 |
| PD | Parkinson’s disease |
| Pitx3 | Paired-like homeodomain transcription factor 3 |
| Plp | Proteolipid protein |
| PRRC2A | Proline-rich Coiled-coil 2a |
| PSD-95 | Postsynaptic density protein-95 |
| RBMX | RNA-binding motif protein X-linked |
| Salsolinol | 1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline |
| TARDBP | Transactive Response DNA Binding Protein |
| TDP-43 | TAR DNA-binding protein-43 |
| TH | Tyrosine hydroxylase |
| UBE2K | Ubiquitin-conjugating enzyme E2K |
| VIRMA | Vir-like N6-methyladenosine Methyltransferase-associated protein |
| WTAP | WT1-associated protein |
| YAP1 | Yes-associated protein 1 |
| YTHDF1/2/3 | YTH structural domain family protein 1/2/3 |
References
- Zhang, N.; Ding, C.; Zuo, Y.; Peng, Y.; Zuo, L. N6-methyladenosine and Neurological Diseases. Mol. Neurobiol. 2022, 59, 1925–1937. [Google Scholar] [CrossRef]
- Ries, R.J.; Zaccara, S.; Klein, P.; Olarerin-George, A.; Namkoong, S.; Pickering, B.F.; Patil, D.P.; Kwak, H.; Lee, J.H.; Jaffrey, S.R. m6A enhances the phase separation potential of mRNA. Nature 2019, 571, 424–428. [Google Scholar] [CrossRef]
- Zou, D.; Huang, X.; Lan, Y.; Pan, M.; Xie, J.; Huang, Q.; Zeng, J.; Zou, C.; Pei, Z.; Zou, C.; et al. Single-cell and spatial transcriptomics reveals that PTPRG activates the m6A methyltransferase VIRMA to block mitophagy-mediated neuronal death in Alzheimer’s disease. Pharmacol. Res. 2024, 201, 107098. [Google Scholar] [CrossRef]
- Knight, H.M.; Demirbugen Öz, M.; PerezGrovas-Saltijeral, A. Dysregulation of RNA modification systems in clinical populations with neurocognitive disorders. Neural Regen. Res. 2024, 19, 1256–1261. [Google Scholar] [CrossRef]
- Liu, S.; Xiu, J.; Zhu, C.; Meng, K.; Li, C.; Han, R.; Du, T.; Li, L.; Xu, L.; Liu, R.; et al. Fat mass and obesity-associated protein regulates RNA methylation associated with depression-like behavior in mice. Nat. Commun. 2021, 12, 6937. [Google Scholar] [CrossRef]
- Wen, D.; Xiao, H.; Gao, Y.; Zeng, H.; Deng, J. N6-methyladenosine-modified SENP1, identified by IGF2BP3, is a novel molecular marker in acute myeloid leukemia and aggravates progression by activating AKT signal via de-SUMOylating HDAC2. Mol. Cancer 2024, 23, 116. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.S.; Hao, Y.J.; Sun, B.F.; Sun, H.Y.; Li, A.; Ping, X.L.; Lai, W.Y.; et al. Nuclear m6A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2013, 505, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.B.; Jaffrey, S.R. 5′ UTR m6A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Luo, G.Z.; Zhang, Z.; Wang, X.; Zhou, T.; Cui, Y.; Sha, J.; Huang, X.; Guerrero, L.; Xie, P.; et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. eLife 2017, 6, e31311. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, G.; Li, G.; Zhang, W.; Wang, Y.; Lin, X.; Han, C.; Chen, H.; Shi, L.; Reheman, A.; et al. m6A writer WTAP targets NRF2 to accelerate bladder cancer malignancy via m6A-dependent ferroptosis regulation. Apoptosis 2023, 28, 627–638. [Google Scholar] [CrossRef]
- Yang, K.; Zhong, Z.; Zou, J.; Liao, J.Y.; Chen, S.; Zhou, S.; Zhao, Y.; Li, J.; Yin, D.; Huang, K.; et al. Glycolysis and tumor progression promoted by the m6A writer VIRMA via m6A-dependent upregulation of STRA6 in pancreatic ductal adenocarcinoma. Cancer Lett. 2024, 590, 216840. [Google Scholar] [CrossRef]
- Chen, K.; Li, W.-D.; Li, X.-Q. The role of m6A in angiogenesis and vascular diseases. iScience 2024, 27, 110082. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Q.; Zhao, X.; Zheng, F.; Xiao, J.; Ge, F.; Wang, D.; Gao, X. The Landscape of m6A Regulators in Multiple Brain Regions of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 5184–5198. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, P.; Chen, K.; Huang, P.; Liang, X.; Dong, J.; Zhu, B.; Fu, Z.; Deng, T.; Zhu, L.; et al. Soot nanoparticles promote ferroptosis in dopaminergic neurons via alteration of m6A RNA methylation in Parkinson’s disease. J. Hazard. Mater. 2024, 473, 134691. [Google Scholar] [CrossRef]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodeg. 2020, 9, 11368. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, S.; Ye, W.; Zhao, S.; Liu, X. The potential roles of m6A modification in regulating the inflammatory response in microglia. J. Neuroinflamm. 2021, 18, 149. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Song, Y.; Pan, J.-j.; Jiang, Y.; Shi, X.; Liu, C.; Ma, Y.; Luo, L.; Mamtilahun, M.; et al. M2 microglia-derived extracellular vesicles promote white matter repair and functional recovery via miR-23a-5p after cerebral ischemia in mice. Theranostics 2022, 12, 3553–3573. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Wang, J.; Zhang, S.; Hu, Q.; Wang, T.; Cui, W.; Shi, Y.; Bai, H.; Zhou, J.; et al. The m6A methyltransferase METTL3 drives neuroinflammation and neurotoxicity through stabilizing BATF mRNA in microglia. Cell Death Differ. 2024, 32, 100–117. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Duyckaerts, C.; Delatour, B.; Potier, M.-C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, Y.; Gao, S.; Qin, L.; Austria, Q.; Siedlak, S.L.; Pajdzik, K.; Dai, Q.; He, C.; Wang, W.; et al. METTL3-dependent RNA m6A dysregulation contributes to neurodegeneration in Alzheimer’s disease through aberrant cell cycle events. Mol. Neuro Degener. 2021, 16, 70. [Google Scholar] [CrossRef]
- Li, X.; Han, Z.; Li, H. Hif3α Plays Key Roles in the Progression of Alzheimer’s Disease Caused by Circadian Rhythm Disruption through Regulating the m6A/KDM3A/TGF-β1 Axis. Biology 2024, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Mei, Y.; Xia, M.; Luo, D.; Gao, L. The role of m6A modification in the risk prediction and Notch1 pathway of Alzheimer’s disease. iScience 2024, 27, 110235. [Google Scholar] [CrossRef]
- Yin, Z.; Rosenzweig, N.; Kleemann, K.L.; Zhang, X.; Brandão, W.; Margeta, M.A.; Schroeder, C.; Sivanathan, K.N.; Silveira, S.; Gauthier, C.; et al. APOE4 impairs the microglial response in Alzheimer’s disease by inducing TGFβ-mediated checkpoints. Nat. Immunol. 2023, 24, 1839–1853. [Google Scholar] [CrossRef]
- Kleemann, K.; Rosenzweig, N.; Yin, Z.; Zhang, X.; Brandao, W.N.; Margeta, M.; Schroeder, C.M.; Silveira, S.; Gauthier, C.; Mallah, D.; et al. APOE4 impairs Microglia—Astrocyte Crosstalk in Alzheimer Disease. Alzheimers Dement 2023, 19, e071852. [Google Scholar] [CrossRef]
- Du, B.Z.Y.; Liang, M.; Du, Z.; Li, H.; Fan, C.; Zhang, H.; Jiang, Y.; Bi, X. N6-methyladenosine (m6A) modification and its clinical relevance in cognitive dysfunctions. Aging 2021, 13, 20716–20737. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T. ApoE4 makes microglia trem2bling. Neuron 2023, 111, 142–144. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Huang, S.-Y.; Zhong, K.-Y.; Huang, W.-G.; Huang, Z.-H.; He, T.-T.; Yang, M.-T.; Wusiman, M.; Zhou, D.-D.; Chen, S.; et al. Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m6A-YTHDF2-dependent manner. Redox Biol. 2024, 69, 103026. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, C.; Wang, Y.; Zhu, X.; Wu, L.; Chen, L.; Zhou, J.; Wang, F. METTL3/IGF2BP2/IκBα axis participates in neuroinflammation in Alzheimer’s disease by regulating M1/M2 polarization of microglia. Neurochem. Int. 2025, 186, 105964. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2020, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, J.; Ma, Y.; Ye, K.; Zhao, X.; Ge, F.; Zheng, F.; Liu, L.; Gao, X.; Wang, D.; et al. The complex roles of m6A modifications in neural stem cell proliferation, differentiation, and self-renewal and implications for memory and neurodegenerative diseases. Neural Regen. Res. 2025, 20, 1582–1598. [Google Scholar] [CrossRef] [PubMed]
- Cuní-López, C.; Stewart, R.; Quek, H.; White, A.R. Recent Advances in Microglia Modelling to Address Translational Outcomes in Neurodegenerative Diseases. Cells 2022, 11, 1662. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Wang, F.; Xie, G.; Liu, S.; Li, Z.; Wang, P.; Liu, J.; Lin, L. Gastrodin regulates the TLR4/TRAF6/NF-κB pathway to reduce neuroinflammation and microglial activation in an AD model. Phytomedicine 2024, 128, 155518. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Dirkx, M.F.; Shine, J.M.; Helmich, R.C. Integrative Brain States Facilitate the Expression of Parkinson’s Tremor. Mov. Disord. 2023, 38, 1615–1624. [Google Scholar] [CrossRef]
- Herz, D.M.; Brown, P. Moving, fast and slow: Behavioural insights into bradykinesia in Parkinson’s disease. Brain 2023, 146, 3576–3586. [Google Scholar] [CrossRef]
- Asci, F.; Falletti, M.; Zampogna, A.; Patera, M.; Hallett, M.; Rothwell, J.; Suppa, A. Rigidity in Parkinson’s disease: Evidence from biomechanical and neurophysiological measures. Brain 2023, 146, 3705–3718. [Google Scholar] [CrossRef]
- Burtscher, J.; Moraud, E.M.; Malatesta, D.; Millet, G.P.; Bally, J.F.; Patoz, A. Exercise and gait/movement analyses in treatment and diagnosis of Parkinson’s Disease. Ageing Res. Rev. 2024, 93, 102147. [Google Scholar] [CrossRef]
- Maggi, G.; Vitale, C.; Cerciello, F.; Santangelo, G. Sleep and wakefulness disturbances in Parkinson’s disease: A meta-analysis on prevalence and clinical aspects of REM sleep behavior disorder, excessive daytime sleepiness and insomnia. Sleep Med. Rev. 2023, 68, 101759. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, Q.; Liao, J.; Lei, J.; Luo, M.; Huang, J.; Chen, M.; Shen, Y.; Wang, J.; Xu, P.; et al. METTL14 is decreased and regulates m6A modification of α-synuclein in Parkinson’s disease. J. Neurochem. 2023, 166, 609–622. [Google Scholar] [CrossRef]
- Olanow, C.W.; Savolainen, M.; Chu, Y.; Halliday, G.M.; Kordower, J.H. Temporal evolution of microglia and α-synuclein accumulation following foetal grafting in Parkinson’s disease. Brain 2019, 142, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Q.; Zheng, R.; Liu, Y.; Ruan, Y.; Lin, Z.H.; Xue, N.J.; Chen, Y.; Zhang, B.R.; Pu, J.L. Parkin regulates microglial NLRP3 and represses neurodegeneration in Parkinson’s disease. Aging Cell 2023, 22, e13834. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, Z.; Chen, X.; Liu, Y.; Geng, F.; Le, W.; Jiang, H.; Yang, L. Conditional deficiency of m6A methyltransferase Mettl14 in substantia nigra alters dopaminergic neuron function. J. Cell. Mol. Med. 2021, 25, 8567–8572. [Google Scholar] [CrossRef] [PubMed]
- Isik, S.; Yeman Kiyak, B.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef]
- Schultz, J.L.; Neema, M.; Nopoulos, P.C. Unravelling the role of huntingtin: From neurodevelopment to neurodegeneration. Brain 2023, 146, 4408–4410. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N.; et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Jiang, A.; Handley, R.R.; Lehnert, K.; Snell, R.G. From Pathogenesis to Therapeutics: A Review of 150 Years of Huntington’s Disease Research. Int. J. Mol. Sci. 2023, 24, 13021. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tong, H.; Yang, T.; Liu, L.; Li, X.-J.; Li, S. Insights into White Matter Defect in Huntington’s Disease. Cells 2022, 11, 3381. [Google Scholar] [CrossRef]
- Snell, R.G.; MacMillan, J.C.; Cheadle, J.P.; Fenton, I.; Lazarou, L.P.; Davies, P.; MacDonald, M.E.; Gusella, J.F.; Harper, P.S.; Shaw, D.J. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington’s disease. Nat. Genet. 1993, 4, 393–397. [Google Scholar] [CrossRef]
- Pupak, A.; Singh, A.; Sancho-Balsells, A.; Alcalá-Vida, R.; Espina, M.; Giralt, A.; Martí, E.; Ørom, U.A.V.; Ginés, S.; Brito, V. Altered m6A RNA methylation contributes to hippocampal memory deficits in Huntington’s disease mice. Cell. Mol. Life Sci. 2022, 79, 416. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Miramontes, R.; Chillon-Marinas, C.; Maimon, R.; Vazquez-Sanchez, S.; Lau, A.L.; McClure, N.R.; Wu, Z.; Wang, K.Q.; England, W.E.; et al. Aberrant splicing in Huntington’s disease accompanies disrupted TDP-43 activity and altered m6A RNA modification. Nat. Neurosci. 2025, 28, 280–292. [Google Scholar] [CrossRef]
- Crotti, A.; Benner, C.; Kerman, B.E.; Gosselin, D.; Lagier-Tourenne, C.; Zuccato, C.; Cattaneo, E.; Gage, F.H.; Cleveland, D.W.; Glass, C.K. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat. Neurosci. 2014, 17, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Wilton, D.K.; Mastro, K.; Heller, M.D.; Gergits, F.W.; Willing, C.R.; Fahey, J.B.; Frouin, A.; Daggett, A.; Gu, X.; Kim, Y.A.; et al. Microglia and complement mediate early corticostriatal synapse loss and cognitive dysfunction in Huntington’s disease. Nat. Med. 2023, 29, 2866–2884. [Google Scholar] [CrossRef]
- Pupak, A.R.-N.I.; Sathasivam, K.; Singh, A.; Essmann, A.; Del Toro, D.; Ginés, S.; Mouro Pinto, R.; Bates, G.P.; Vang Ørom, U.A.; Martí, E.; et al. m6A modification of mutant huntingtin RNA promotes the biogenesis of pathogenic huntingtin transcripts. EMBO Rep. 2024, 24, 5026–5052. [Google Scholar] [CrossRef]
- Brody, H. Multiple sclerosis. Nature 2012, 484, S1. [Google Scholar] [CrossRef]
- Rodríguez Murúa, S.; Farez, M.F.; Quintana, F.J. The Immune Response in Multiple Sclerosis. Annu. Rev. Pathol. 2022, 17, 121–139. [Google Scholar] [CrossRef]
- Filippi, M.; Preziosa, P.; Langdon, D.; Lassmann, H.; Paul, F.; Rovira, À.; Schoonheim, M.M.; Solari, A.; Stankoff, B.; Rocca, M.A. Identifying Progression in Multiple Sclerosis: New Perspectives. Ann. Neurol. 2020, 88, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wang, T.; Wu, X.; Liang, J.; Li, J.; Sheng, W. N6-Methyladenosine RNA modification in cerebrospinal fluid as a novel potential diagnostic biomarker for progressive multiple sclerosis. J. Transl. Med. 2021, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, C.; Sun, H.; Mao, K.; Yao, J.; Zhang, W.; Zhan, M.; Li, H.-B.; Zhang, Z.; Zhu, S.; et al. m6A mRNA modification potentiates Th17 functions to inflame autoimmunity. Sci. China Life Sci. 2023, 66, 2543–2552. [Google Scholar] [CrossRef]
- Zrzavy, T.; Hametner, S.; Wimmer, I.; Butovsky, O.; Weiner, H.L.; Lassmann, H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 2017, 140, 1900–1913. [Google Scholar] [CrossRef]
- Singh, S.; Metz, I.; Amor, S.; van der Valk, P.; Stadelmann, C.; Brück, W. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol. 2013, 125, 595–608. [Google Scholar] [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef]
- Pollock, N.M.; Fernandes, J.P.; Woodfield, J.; Moussa, E.; Hlavay, B.; Branton, W.G.; Wuest, M.; Mohammadzadeh, N.; Schmitt, L.; Plemel, J.R.; et al. Activation in oligodendrocytes and microglia drives inflammatory demyelination in progressive multiple sclerosis. Brain Behav. Immun. 2024, 115, 374–393. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, L.; Xu, D.; Tu, Y.; Yang, C.; Zhang, M.; Wang, H.; Nong, X. Deficiency of N6-Methyladenosine Demethylase ALKBH5 Alleviates Ultraviolet B Radiation-Induced Chronic Actinic Dermatitis via Regulating Pyroptosis. Inflammation 2023, 47, 159–172. [Google Scholar] [CrossRef]
- Brown, R.H.; Longo, D.L.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- McMillan, M.; Gomez, N.; Hsieh, C.; Bekier, M.; Li, X.; Miguez, R.; Tank, E.M.H.; Barmada, S.J. RNA methylation influences TDP43 binding and disease pathogenesis in models of amyotrophic lateral sclerosis and frontotemporal dementia. Mol. Cell 2023, 83, 219–236.e7. [Google Scholar] [CrossRef] [PubMed]
- Di Timoteo, G.; Giuliani, A.; Setti, A.; Biagi, M.C.; Lisi, M.; Santini, T.; Grandioso, A.; Mariani, D.; Castagnetti, F.; Perego, E.; et al. M6A reduction relieves FUS-associated ALS granules. Nat. Commun. 2024, 15, 5033. [Google Scholar] [CrossRef]
- Dermentzaki, G.; Furlan, M.; Tanaka, I.; Leonardi, T.; Rinchetti, P.; Passos, P.M.S.; Bastos, A.; Ayala, Y.M.; Hanna, J.H.; Przedborski, S.; et al. Depletion of Mettl3 in cholinergic neurons causes adult-onset neuromuscular degeneration. Cell Rep. 2024, 43, 113999. [Google Scholar] [CrossRef]
- Dols-Icardo, O.; Montal, V.; Sirisi, S.; López-Pernas, G.; Cervera-Carles, L.; Querol-Vilaseca, M.; Muñoz, L.; Belbin, O.; Alcolea, D.; Molina-Porcel, L.; et al. Motor cortex transcriptome reveals microglial key events in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e829. [Google Scholar] [CrossRef]
- Barreto-Núñez, R.; Béland, L.C.; Boutej, H.; Picher-Martel, V.; Dupré, N.; Barbeito, L.; Kriz, J. Chronically activated microglia in ALS gradually lose their immune functions and develop unconventional proteome. Glia 2024, 72, 1319–1339. [Google Scholar] [CrossRef] [PubMed]
- Volonté, C.; Amadio, S.; Fabbrizio, P.; Apolloni, S. Functional microglia neurotransmitters in amyotrophic lateral sclerosis. Semin. Cell Dev. Biol. 2019, 94, 121–128. [Google Scholar] [CrossRef]
- Hooten, K.G.; Beers, D.R.; Zhao, W.; Appel, S.H. Protective and Toxic Neuroinflammation in Amyotrophic Lateral Sclerosis. Neurotherapeutics 2015, 12, 364–375. [Google Scholar] [CrossRef]
- Masrori, P.; Bijnens, B.; Fumagalli, L.; Davie, K.; Poovathingal, S.K.; Meese, T.; Storm, A.; Hersmus, N.; Fazal, R.; van den Biggelaar, D.; et al. C9orf72 hexanucleotide repeat expansions impair microglial response in ALS. Nat. Neurosci. 2025, 28, 2217–2230. [Google Scholar] [CrossRef]
- He, D.; Yang, X.; Liu, L.; Shen, D.; Liu, Q.; Liu, M.; Zhang, X.; Cui, L. Dysregulated N6-methyladenosine modification in peripheral immune cells contributes to the pathogenesis of amyotrophic lateral sclerosis. Front. Med. 2024, 18, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-G.; Quintana, F.J. Astrocytes at the border of repair. Nat. Neurosci. 2024, 27, 1445–1446. [Google Scholar] [CrossRef]
- de Rus Jacquet, A.; Alpaugh, M.; Denis, H.L.; Tancredi, J.L.; Boutin, M.; Decaestecker, J.; Beauparlant, C.; Herrmann, L.; Saint-Pierre, M.; Parent, M.; et al. The contribution of inflammatory astrocytes to BBB impairments in a brain-chip model of Parkinson’s disease. Nat. Commun. 2023, 14, 3651. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.N.; Ali, H.M.; Lopez, M.R.; Kang, S.; Choi, D.-S. Astrocytic GABAergic Regulation in Alcohol Use and Major Depressive Disorders. Cells 2024, 13, 318. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, Z. Swell1 channel-mediated tonic GABA release from astrocytes modulates cocaine reward. Neuropsychopharmacology 2023, 49, 327–328. [Google Scholar] [CrossRef]
- Sun, M.; You, H.; Hu, X.; Luo, Y.; Zhang, Z.; Song, Y.; An, J.; Lu, H. Microglia–Astrocyte Interaction in Neural Development and Neural Pathogenesis. Cells 2023, 12, 1942. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wen, J. Metabolic reprogramming and astrocytes polarization following ischemic stroke. Free. Radic. Biol. Med. 2025, 228, 197–206. [Google Scholar] [CrossRef]
- Lee, H.-G.; Rone, J.M.; Li, Z.; Akl, C.F.; Shin, S.W.; Lee, J.-H.; Flausino, L.E.; Pernin, F.; Chao, C.-C.; Kleemann, K.L.; et al. Disease-associated astrocyte epigenetic memory promotes CNS pathology. Nature 2024, 627, 865–872. [Google Scholar] [CrossRef]
- Bai, Y.; Chang, D.; Ren, H.; Ju, M.; Wang, Y.; Chen, B.; Li, H.; Liu, X.; Li, D.; Huo, X.; et al. Engagement of N6-methyladenisine methylation of Gng4 mRNA in astrocyte dysfunction regulated by CircHECW2. Acta Pharm. Sin. B 2024, 14, 1644–1660. [Google Scholar] [CrossRef]
- Guo, F.; Fan, J.; Liu, J.-M.; Kong, P.-L.; Ren, J.; Mo, J.-W.; Lu, C.-L.; Zhong, Q.-L.; Chen, L.-Y.; Jiang, H.-T.; et al. Astrocytic ALKBH5 in stress response contributes to depressive-like behaviors in mice. Nat. Commun. 2024, 15, 4347. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Bai, Y.; Han, B.; Ju, M.; Chen, B.; Yang, L.; Wang, Y.; Zhang, H.; Zhang, H.; et al. N6-Methyladenosine Modification of Fatty Acid Amide Hydrolase Messenger RNA in Circular RNA STAG1–Regulated Astrocyte Dysfunction and Depressive-like Behaviors. Biol. Psychiatry 2020, 88, 392–404. [Google Scholar] [CrossRef]
- Qiu, Y.; Fan, Y.; Huang, G.; Liu, J. N6-methyladenosine demethylase ALKBH5 homologous protein protects against cerebral I/R injury though suppressing SNHG3-mediated neural PANoptosis: Involvement of m6A-related macromolecules in the diseases of nervous system. Int. J. Biol. Macromol. 2024, 274, 133815. [Google Scholar] [CrossRef]
- Visvanathan, A.; Patil, V.; Arora, A.; Hegde, A.S.; Arivazhagan, A.; Santosh, V.; Somasundaram, K. Essential role of METTL3-mediated m6A modification in glioma stem-like cells maintenance and radioresistance. Oncogene 2017, 37, 522–533. [Google Scholar] [CrossRef]
- Hu, J.D.H.; Zou, J.; Ding, W.; Wei, Z.; Peng, Q.; Li, Z.; Duan, R.; Sun, J.; Zhu, J. METTL3-dependent N6-methyladenosine modification is involved in berberine-mediated neuroprotection in ischemic stroke by enhancing the stability of NEAT1 in astrocytes. Aging 2024, 16, 299–321. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Jiang, R.; Liu, Z.; Gao, H.; Chen, F.; Mei, J. Emodin relieves the inflammation and pyroptosis of lipopolysaccharide-treated 1321N1 cells by regulating methyltransferase-like 3 -mediated NLR family pyrin domain containing 3 expression. Bioengineered 2022, 13, 6739–6748. [Google Scholar] [CrossRef]
- Malovic, E.; Ealy, A.; Miller, C.; Jang, A.; Hsu, P.J.; Sarkar, S.; Rokad, D.; Goeser, C.; Hartman, A.K.; Zhu, A.; et al. Epitranscriptomic reader YTHDF2 regulates SEK1(MAP2K4)-JNK-cJUN inflammatory signaling in astrocytes during neurotoxic stress. iScience 2024, 27, 110619. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wu, X.; Gao, Y.; Chen, J.; Zhang, M.; Zhou, H.; Yang, J.; Xiao, F.; Yang, X.; Huang, N.; et al. Circular RNA encoded MET variant promotes glioblastoma tumorigenesis. Nat. Commun. 2023, 14, 4467. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.W.; Nadarajah, C.J.; Kanan, M.F.; Patterson, J.N.; Novotny, B.; Lawrence, J.H.; King, M.W.; Brase, L.; Inman, C.E.; Yuede, C.M.; et al. An astrocyte BMAL1-BAG3 axis protects against alpha-synuclein and tau pathology. Neuron 2023, 111, 2383–2398.e7. [Google Scholar] [CrossRef] [PubMed]
- Lee HG, L.J.; Flausino, L.E.; Quintana, F.J. Neuroinflammation: An astrocyte perspective. Sci. Transl. Med. 2023, 15, eadi7828. [Google Scholar] [CrossRef]
- Rostami, J.; Mothes, T.; Kolahdouzan, M.; Eriksson, O.; Moslem, M.; Bergström, J.; Ingelsson, M.; O’Callaghan, P.; Healy, L.M.; Falk, A.; et al. Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J. Neuroinflamm. 2021, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.H.; Demir, S.; Wiese, S.; Kruse, N.; Siglienti, I.; Gerhardt, E.; Neumann, H.; Sendtner, M.; Luhder, F.; et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: Therapeutic implications in a model of multiple sclerosis. Brain 2010, 133, 2248–2263. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoo, I.D.; Lim, J.; Moon, J.-S. Pathological phenotypes of astrocytes in Alzheimer’s disease. Exp. Mol. Med. 2024, 56, 95–99. [Google Scholar] [CrossRef]
- Tzioras, M.; Daniels, M.J.D.; Davies, C.; Baxter, P.; King, D.; McKay, S.; Varga, B.; Popovic, K.; Hernandez, M.; Stevenson, A.J.; et al. Human astrocytes and microglia show augmented ingestion of synapses in Alzheimer’s disease via MFG-E8. Cell Rep. Med. 2023, 4, 101175. [Google Scholar] [CrossRef]
- Thorwald, M.A.; Godoy-Lugo, J.A.; Forman, H.J.; Finch, C.E. ApoE4 is associated with higher lipid peroxidation but not protein nitration in AD brains. Alzheimers Dement 2023, 19, e079300. [Google Scholar] [CrossRef]
- Jiang, L.; Roberts, R.; Wong, M.; Zhang, L.; Webber, C.J.; Kilci, A.; Jenkins, M.; Sun, J.; Sun, G.; Rashad, S.; et al. Accumulation of m6A exhibits stronger correlation with MAPT than β-amyloid pathology in an APPNLG-F/MAPTP301S mouse model of Alzheimer’s disease. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cockova, Z.; Honc, O.; Telensky, P.; Olsen, M.J.; Novotny, J. Streptozotocin-Induced Astrocyte Mitochondrial Dysfunction Is Ameliorated by FTO Inhibitor MO-I-500. ACS Chem. Neurosci. 2021, 12, 3818–3828. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Puertas-Avendaño, R.; Sanchez, A.; Perez-Barreto, A.; Rodriguez-Sabate, C.; Rodriguez, M. Astrocytes and retrograde degeneration of nigrostriatal dopaminergic neurons in Parkinson’s disease: Removing axonal debris. Transl. Neurodeg. 2021, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.L.; Boyle, A.M.; Budge, K.M.; Safadi, F.F.; Richardson, J.R. The glycoprotein GPNMB attenuates astrocyte inflammatory responses through the CD44 receptor. J. Neuroinflamm. 2018, 15, 73. [Google Scholar] [CrossRef]
- Chou, T.-W.; Chang, N.P.; Krishnagiri, M.; Patel, A.P.; Lindman, M.; Angel, J.P.; Kung, P.-L.; Atkins, C.; Daniels, B.P. Fibrillar α-synuclein induces neurotoxic astrocyte activation via RIP kinase signaling and NF-κB. Cell Death Dis. 2021, 12, 756. [Google Scholar] [CrossRef]
- Streubel-Gallasch, L.; Giusti, V.; Sandre, M.; Tessari, I.; Plotegher, N.; Giusto, E.; Masato, A.; Iovino, L.; Battisti, I.; Arrigoni, G.; et al. Parkinson’s Disease–Associated LRRK2 Interferes with Astrocyte-Mediated Alpha-Synuclein Clearance. Mol. Neurobiol. 2021, 58, 3119–3140. [Google Scholar] [CrossRef]
- Yu, X.; Nagai, J.; Marti-Solano, M.; Soto, J.S.; Coppola, G.; Babu, M.M.; Khakh, B.S. Context-Specific Striatal Astrocyte Molecular Responses Are Phenotypically Exploitable. Neuron 2020, 108, 1146–1162.e10. [Google Scholar] [CrossRef]
- Saba, J.; López Couselo, F.; Turati, J.; Carniglia, L.; Durand, D.; de Laurentiis, A.; Lasaga, M.; Caruso, C. Astrocytes from cortex and striatum show differential responses to mitochondrial toxin and BDNF: Implications for protection of striatal neurons expressing mutant huntingtin. J. Neuroinflamm. 2020, 17, 290. [Google Scholar] [CrossRef]
- Paryani, F.; Kwon, J.-S.; Ng, C.W.; Jakubiak, K.; Madden, N.; Ofori, K.; Tang, A.; Lu, H.; Xia, S.; Li, J.; et al. Multi-omic analysis of Huntington’s disease reveals a compensatory astrocyte state. Nat. Commun. 2024, 15, 6742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Hong, Y.; Li, S.; Li, X.J. Compartment-Dependent Degradation of Mutant Huntingtin Accounts for Its Preferential Accumulation in Neuronal Processes. J. Neurosci. 2016, 36, 8317–8328. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, B.G.M.; Yu, X.; Coppola, G.; Khakh, B.S. Astrocyte molecular signatures in Huntington’s disease. Sci. Transl. Med. 2019, 11, eaaw8546. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Fraga, C.; Altmann, A.; Parker, C.S.; Scahill, R.I.; Costa, B.; Chen, Z.; Manzoni, C.; Zarkali, A.; Durr, A.; Roos, R.A.C.; et al. Genetic topography and cortical cell loss in Huntington’s disease link development and neurodegeneration. Brain 2023, 146, 4532–4546. [Google Scholar] [CrossRef]
- Abjean, L.; Ben Haim, L.; Riquelme-Perez, M.; Gipchtein, P.; Derbois, C.; Palomares, M.-A.; Petit, F.; Hérard, A.-S.; Gaillard, M.-C.; Guillermier, M.; et al. Reactive astrocytes promote proteostasis in Huntington’s disease through the JAK2-STAT3 pathway. Brain 2023, 146, 149–166. [Google Scholar] [CrossRef]
- Palpagama, T.; Mills, A.R.; Ferguson, M.W.; Vikas Ankeal, P.; Turner, C.; Tippett, L.; van der Werf, B.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Microglial and Astrocytic Responses in the Human Midcingulate Cortex in Huntington’s Disease. Ann. Neurol. 2023, 94, 895–910. [Google Scholar] [CrossRef]
- González-Guevara, E.; Cárdenas, G.; Pérez-Severiano, F.; Martínez-Lazcano, J.C. Dysregulated Brain Cholesterol Metabolism Is Linked to Neuroinflammation in Huntington’s Disease. Mov. Disord. 2020, 35, 1113–1127. [Google Scholar] [CrossRef]
- Villanueva, C.B.; Stephensen, H.J.T.; Mokso, R.; Benraiss, A.; Sporring, J.; Goldman, S.A. Astrocytic engagement of the corticostriatal synaptic cleft is disrupted in a mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2210719120. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Ao, Y.; Faas, G.C.; Nwaobi, S.E.; Xu, J.; Haustein, M.D.; Anderson, M.A.; Mody, I.; Olsen, M.L.; Sofroniew, M.V.; et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 2014, 17, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Sun, Q.; Long, C.; Rasmussen, R.N.; Peng, S.; Xu, Q.; Kang, N.; Song, W.; Weikop, P.; Goldman, S.A.; et al. Dysregulation of extracellular potassium distinguishes healthy ageing from neurodegeneration. Brain 2024, 147, 1726–1739. [Google Scholar] [CrossRef]
- Kruyer, A. Non-neuronal brain cells modulate behaviour. Nature 2024, 627, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019, 137, 757–783. [Google Scholar] [CrossRef]
- Colombo, E.; Triolo, D.; Bassani, C.; Bedogni, F.; Di Dario, M.; Dina, G.; Fredrickx, E.; Fermo, I.; Martinelli, V.; Newcombe, J.; et al. Dysregulated copper transport in multiple sclerosis may cause demyelination via astrocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2025804118. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Ye, W.; Zhu, Y.; Cui, M.; Zhou, J.; Xiao, C.; Jiang, D.; Tang, P.; Wang, J.; Wang, Z.; et al. USP1/UAF1-Stabilized METTL3 Promotes Reactive Astrogliosis and Improves Functional Recovery after Spinal Cord Injury through m6A Modification of YAP1 mRNA. J. Neurosci. 2023, 43, 1456–1474. [Google Scholar] [CrossRef]
- Allen, S.P.; Hall, B.; Woof, R.; Francis, L.; Gatto, N.; Shaw, A.C.; Myszczynska, M.; Hemingway, J.; Coldicott, I.; Willcock, A.; et al. C9orf72 expansion within astrocytes reduces metabolic flexibility in amyotrophic lateral sclerosis. Brain 2019, 142, 3771–3790. [Google Scholar] [CrossRef]
- Broadhead, M.J.; Bonthron, C.; Waddington, J.; Smith, W.V.; Lopez, M.F.; Burley, S.; Valli, J.; Zhu, F.; Komiyama, N.H.; Smith, C.; et al. Selective vulnerability of tripartite synapses in amyotrophic lateral sclerosis. Acta Neuropathol. 2022, 143, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Yoneda, R.; Ueda, N.; Kurokawa, R. m6A Modified Short RNA Fragments Inhibit Cytoplasmic TLS/FUS Aggregation Induced by Hyperosmotic Stress. Int. J. Mol. Sci. 2021, 22, 11014. [Google Scholar] [CrossRef]
- Kia, A.; McAvoy, K.; Krishnamurthy, K.; Trotti, D.; Pasinelli, P. Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha. Glia 2018, 66, 1016–1033. [Google Scholar] [CrossRef]
- Emery, B.; Wood, T.L. Regulators of Oligodendrocyte Differentiation. CSH Perspect. Biol. 2024, 16, a041358. [Google Scholar] [CrossRef]
- Krämer-Albers, E.-M.; Werner, H.B. Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles. Nat. Rev. Neurosci. 2023, 24, 474–486. [Google Scholar] [CrossRef]
- Looser, Z.J.; Faik, Z.; Ravotto, L.; Zanker, H.S.; Jung, R.B.; Werner, H.B.; Ruhwedel, T.; Möbius, W.; Bergles, D.E.; Barros, L.F.; et al. Oligodendrocyte-axon metabolic coupling is mediated by extracellular K+ and maintains axonal health. Nat. Neurosci. 2024, 27, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Falcão, A.M.; Bruggen, D.V.; Marques, S.; Meijer, M.; Jäkel, S.; Agirre, E.; Samudyata; Floriddia, E.M.; Vanichkina, D.P.; Ffrench-Constant, C.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018, 24, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dzhashiashvili, Y.; Shah, A.; Kunjamma, R.B.; Weng, Y.-l.; Elbaz, B.; Fei, Q.; Jones, J.S.; Li, Y.I.; Zhuang, X.; et al. m6A mRNA Methylation Is Essential for Oligodendrocyte Maturation and CNS Myelination. Neuron 2020, 105, 293–309.e5. [Google Scholar] [CrossRef]
- Maier, O.; Baron, W.; Hoekstra, D. Reduced raft-association of NF155 in active MS-lesions is accompanied by the disruption of the paranodal junction. Glia 2007, 55, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Jahanbazi Jahan-Abad, A.; Salapa, H.E.; Libner, C.D.; Thibault, P.A.; Levin, M.C. hnRNP A1 dysfunction in oligodendrocytes contributes to the pathogenesis of multiple sclerosis. Glia 2022, 71, 633–647. [Google Scholar] [CrossRef]
- Depp, C.; Sun, T.; Sasmita, A.O.; Spieth, L.; Berghoff, S.A.; Nazarenko, T.; Overhoff, K.; Steixner-Kumar, A.A.; Subramanian, S.; Arinrad, S.; et al. Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature 2023, 618, 349–357. [Google Scholar] [CrossRef]
- Gazestani, V.; Kamath, T.; Nadaf, N.M.; Dougalis, A.; Burris, S.J.; Rooney, B.; Junkkari, A.; Vanderburg, C.; Pelkonen, A.; Gomez-Budia, M.; et al. Early Alzheimer’s disease pathology in human cortex involves transient cell states. Cell 2023, 186, 4438–4453.e23. [Google Scholar] [CrossRef]
- Park, H.; Cho, B.; Kim, H.; Saito, T.; Saido, T.C.; Won, K.-J.; Kim, J. Single-cell RNA-sequencing identifies disease-associated oligodendrocytes in male APP NL-G-F and 5XFAD mice. Nat. Commun. 2023, 14, 802. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, A.; Sun, B.; Sun, J.-G.; Zhang, J.; Zhang, T.; Chen, Y.; Xiao, Y.; Gao, Y.; Zhang, Q.; et al. A novel m6A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2018, 29, 23–41. [Google Scholar] [CrossRef]
- Uchida, Y.; Nakano, S.-i.; Gomi, F.; Takahashi, H. Differential Regulation of Basic Helix-Loop-Helix Factors Mash1 and Olig2 by β-Amyloid Accelerates Both Differentiation and Death of Cultured Neural Stem/Progenitor Cells. J. Biol. Chem. 2007, 282, 19700–19709. [Google Scholar] [CrossRef]
- Sarlak, G.; Vincent, B. The Roles of the Stem Cell-Controlling Sox2 Transcription Factor: From Neuroectoderm Development to Alzheimer’s Disease? Mol. Neurobiol. 2015, 53, 1679–1698. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Z.; Wu, R.; Kong, X.; Zhang, H.; Li, S.; Gong, X.; Gong, S.; Cheng, J.; Yuan, F.; et al. PRRC2B modulates oligodendrocyte progenitor cell development and myelination by stabilizing Sox2 mRNA. Cell Rep. 2024, 43, 113930. [Google Scholar] [CrossRef]
- Bae, E.-J.; Pérez-Acuña, D.; Rhee, K.H.; Lee, S.-J. Changes in oligodendroglial subpopulations in Parkinson’s disease. Mol. Brain 2023, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Long, J.; Li, W.; Wang, X.; Hu, N.; Zhao, X.; Sun, T. White matter changes in Parkinson’s disease. Npj Park. Dis. 2023, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Kim, C.; Park, S.; Joo, J.; Choi, B.; Yang, D.; Jun, K.; Eom, J.; Lee, S.-J.; Chung, S.J.; et al. Characterization of altered molecular mechanisms in Parkinson’s disease through cell type-resolved multiomics analyses. Sci. Adv. 2023, 14, eabo2467. [Google Scholar] [CrossRef]
- Rocha, E.M.; Keeney, M.T.; Di Maio, R.; De Miranda, B.R.; Greenamyre, J.T. LRRK2 and idiopathic Parkinson’s disease. Trends Neurosci. 2022, 45, 224–236. [Google Scholar] [CrossRef]
- Jeong, G.R.; Lee, B.D. Pathological Functions of LRRK2 in Parkinson’s Disease. Cells 2020, 9, 2565. [Google Scholar] [CrossRef]
- Agarwal, D.; Sandor, C.; Volpato, V.; Caffrey, T.M.; Monzón-Sandoval, J.; Bowden, R.; Alegre-Abarrategui, J.; Wade-Martins, R.; Webber, C. A single-cell atlas of the human substantia nigra reveals cell-specific pathways associated with neurological disorders. Nat. Commun. 2020, 11, 4183. [Google Scholar] [CrossRef]
- Gregorio, I.; Russo, L.; Torretta, E.; Barbacini, P.; Contarini, G.; Pacinelli, G.; Bizzotto, D.; Moriggi, M.; Braghetta, P.; Papaleo, F.; et al. GBA1 inactivation in oligodendrocytes affects myelination and induces neurodegenerative hallmarks and lipid dyshomeostasis in mice. Mol. Neuro Degener. 2024, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, A.G.; Queiroz, R.M.L.; Ghosh, T.; McMurran, C.E.; Cubillos, J.F.; Bergles, D.E.; Fitzgerald, D.C.; Jones, C.A.; Lilley, K.S.; Glover, C.P.; et al. Changes in the Oligodendrocyte Progenitor Cell Proteome with Ageing. Mol. Cell. Proteom. 2020, 19, 1281–1302. [Google Scholar] [CrossRef]
- Ferrari Bardile, C.; Garcia-Miralles, M.; Caron, N.S.; Rayan, N.A.; Langley, S.R.; Harmston, N.; Rondelli, A.M.; Teo, R.T.Y.; Waltl, S.; Anderson, L.M.; et al. Intrinsic mutant HTT-mediated defects in oligodendroglia cause myelination deficits and behavioral abnormalities in Huntington disease. Proc. Natl. Acad. Sci. USA 2019, 116, 9622–9627. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.G.; Al-Dalahmah, O.; Wu, J.; Gold, M.P.; Reidling, J.C.; Tang, G.; Adam, M.; Dansu, D.K.; Park, H.-J.; Casaccia, P.; et al. Huntington disease oligodendrocyte maturation deficits revealed by single-nucleus RNAseq are rescued by thiamine-biotin supplementation. Nat. Commun. 2022, 13, 7791. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, E.; Merkler, D.; Mezydlo, A.; Weil, M.-T.; Weber, M.S.; Nikić, I.; Potz, S.; Meinl, E.; Matznick, F.E.H.; Kreutzfeldt, M.; et al. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat. Commun. 2016, 7, 13275. [Google Scholar] [CrossRef]
- Windener, F.; Grewing, L.; Thomas, C.; Dorion, M.-F.; Otteken, M.; Kular, L.; Jagodic, M.; Antel, J.; Albrecht, S.; Kuhlmann, T. Physiological aging and inflammation-induced cellular senescence may contribute to oligodendroglial dysfunction in MS. Acta Neuropathol. 2024, 147, 82. [Google Scholar] [CrossRef]
- D’Souza, S.D.; Bonetti, B.; Balasingam, V.; Cashman, N.R.; Barker, P.A.; Troutt, A.B.; Raine, C.S.; Antel, J.P. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J. Exp. Med. 1996, 184, 2361–2370. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.-J.; Zhao, G.-X.; Li, H.-F.; Wu, L.; Xu, Y.-F.; Liao, Y.; Yuan, Z.; Wu, Z.-Y. Common genetic variants in PRRC2A are associated with both neuromyelitis optica spectrum disorder and multiple sclerosis in Han Chinese population. J. Neurol. 2020, 268, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef]
- Martin, L.J.; Adams, D.A.; Niedzwiecki, M.V.; Wong, M. Aberrant DNA and RNA Methylation Occur in Spinal Cord and Skeletal Muscle of Human SOD1 Mouse Models of ALS and in Human ALS: Targeting DNA Methylation Is Therapeutic. Cells 2022, 11, 3448. [Google Scholar] [CrossRef]
- Wang, J.; Ho, W.Y.; Lim, K.; Feng, J.; Tucker-Kellogg, G.; Nave, K.-A.; Ling, S.-C. Cell-autonomous requirement of TDP-43, an ALS/FTD signature protein, for oligodendrocyte survival and myelination. Proc. Natl. Acad. Sci. USA 2018, 115, E10941–E10950. [Google Scholar] [CrossRef]
- Carrillo-Reid, L. Neuronal ensembles in memory processes. Semin. Cell Dev. Biol. 2022, 125, 136–143. [Google Scholar] [CrossRef]
- Francis, N.A.; Mukherjee, S.; Koçillari, L.; Panzeri, S.; Babadi, B.; Kanold, P.O. Sequential transmission of task-relevant information in cortical neuronal networks. Cell Rep. 2022, 39, 110878. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Geng, X.; Han, P.; Che, P.; Liang, F.; Liu, C.; Yang, L.; Yu, J.; Zhang, Z.; Dong, W.; et al. YTHDF2 in dentate gyrus is the m6A reader mediating m6A modification in hippocampus-dependent learning and memory. Mol. Psychiatry 2023, 28, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Perlegos, A.E.; Byrns, C.N.; Bonini, N.M. Cell type-specific regulation of m6A modified RNAs in the aging Drosophilabrain. Aging Cell 2024, 23, e14076. [Google Scholar] [CrossRef]
- Huang, Y.-R.; Xie, X.-X.; Yang, J.; Sun, X.-Y.; Niu, X.-Y.; Yang, C.-G.; Li, L.-J.; Zhang, L.; Wang, D.; Liu, C.-Y.; et al. ArhGAP11A mediates amyloid-β generation and neuropathology in an Alzheimer’s disease-like mouse model. Cell Rep. 2023, 42, 112624. [Google Scholar] [CrossRef]
- Otero-Garcia, M.; Mahajani, S.U.; Wakhloo, D.; Tang, W.; Xue, Y.-Q.; Morabito, S.; Pan, J.; Oberhauser, J.; Madira, A.E.; Shakouri, T.; et al. Molecular signatures underlying neurofibrillary tangle susceptibility in Alzheimer’s disease. Neuron 2022, 110, 2929–2948.e8. [Google Scholar] [CrossRef]
- Plascencia-Villa, G.; Perry, G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Wen, L.; Bi, D.; Shen, Y. Complement-mediated synapse loss in Alzheimer’s disease: Mechanisms and involvement of risk factors. Trends Neurosci. 2024, 47, 135–149. [Google Scholar] [CrossRef]
- Tang, Y.; Yan, Y.; Mao, J.; Ni, J.; Qing, H. The hippocampus associated GABAergic neural network impairment in early-stage of Alzheimer’s disease. Ageing Res. Rev. 2023, 86, 101865. [Google Scholar] [CrossRef]
- Wang, X.; Xie, J.; Tan, L.; Lu, Y.; Shen, N.; Li, J.; Hu, H.; Li, H.; Li, X.; Cheng, L. N6-methyladenosine-modified circRIMS2 mediates synaptic and memory impairments by activating GluN2B ubiquitination in Alzheimer’s disease. Transl. Neurodeg. 2023, 12, 53. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Bhatt, S.; Shao, C.; Zhao, F.; Fujiokab, H.; Torresa, S.; Wua, F.; Zhu, X. Intraneuronal β-amyloid impaired mitochondrial proteostasis through the impact on LONP1. Proc. Natl. Acad. Sci. USA 2023, 120, e2316823120. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, C.; Sun, Q.; Huang, X.; Qu, W.; Chen, Y.; Liu, Z.; Bao, A.; Sun, B.; Yang, Y.; et al. Mettl3 regulates the pathogenesis of Alzheimer’s disease via fine-tuning Lingo2. Mol. Psychiatry 2025, 30, 4047–4063. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, C.; Ju, M.; Feng, W.; Guo, Z.; Qi, C.; Yang, K.; Xiao, R. Roseburia intestinalis Supplementation Could Reverse the Learning and Memory Impairment and m6A Methylation Modification Decrease Caused by 27-Hydroxycholesterol in Mice. Nutrients 2024, 16, 1288. [Google Scholar] [CrossRef]
- Geng, Y.; Long, X.; Zhang, Y.; Wang, Y.; You, G.; Guo, W.; Zhuang, G.; Zhang, Y.; Cheng, X.; Yuan, Z.; et al. FTO-targeted siRNA delivery by MSC-derived exosomes synergistically alleviates dopaminergic neuronal death in Parkinson’s disease via m6A-dependent regulation of ATM mRNA. J. Transl. Med. 2023, 21, 652. [Google Scholar] [CrossRef]
- Wang, J.; Ran, Y.; Li, Z.; Zhao, T.; Zhang, F.; Wang, J.; Liu, Z.; Chen, X. Salsolinol as an RNA m6A methylation inducer mediates dopaminergic neuronal death by regulating YAP1 and autophagy. Neural Regen. Res. 2025, 20, 887–899. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, S.; Li, J.; Wen, Y.; Cui, R.; Zhang, K.; Liu, Y.; Yang, X.; Zhang, L.; Xu, B.; et al. Protective role of mRNA demethylase FTO on axon guidance molecules of nigro-striatal projection system in manganese-induced parkinsonism. J. Hazard. Mater. 2022, 426, 128099. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Xiang, W.; Tang, T.; Gan, L. m6A Demethylase FTO-Mediated Upregulation of BAP1 Induces Neuronal Ferroptosis via the p53/SLC7A11 Axis in the MPP+/MPTP-Induced Parkinson’s Disease Model. ACS Chem. Neurosci. 2025, 16, 405–416. [Google Scholar] [CrossRef]
- Selberg, S.; Yu, L.-Y.; Bondarenko, O.; Kankuri, E.; Seli, N.; Kovaleva, V.; Herodes, K.; Saarma, M.; Karelson, M. Small-Molecule Inhibitors of the RNA M6A Demethylases FTO Potently Support the Survival of Dopamine Neurons. Int. J. Mol. Sci. 2021, 22, 4537. [Google Scholar] [CrossRef]
- Smidt, M.P.; Smits, S.M.; Burbach, J.P.H. Molecular mechanisms underlying midbrain dopamine neuron development and function. Eur. J. Pharmacol. 2003, 480, 75–88. [Google Scholar] [CrossRef]
- Reddy, S.D.N.; Rayala, S.K.; Ohshiro, K.; Pakala, S.B.; Kobori, N.; Dash, P.; Yun, S.; Qin, J.; O’Malley, B.W.; Kumar, R. Multiple coregulatory control of tyrosine hydroxylase gene transcription. Proc. Natl. Acad. Sci. USA 2011, 108, 4200–4205. [Google Scholar] [CrossRef]
- Gong, X.; Huang, M.; Chen, L. NRF1 mitigates motor dysfunction and dopamine neuron degeneration in mice with Parkinson’s disease by promoting GLRX m6A methylation through upregulation of METTL3 transcription. CNS Neurosci. Ther. 2023, 30, e14441. [Google Scholar] [CrossRef]
- Hu, X.; Peng, W.-X.; Zhou, H.; Jiang, J.; Zhou, X.; Huang, D.; Mo, Y.-Y.; Yang, L. IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. 2019, 27, 1782–1794. [Google Scholar] [CrossRef]
- Hu, W.; Wang, M.; Sun, G.; Zhang, L.; Lu, H. Early B Cell Factor 3 (EBF3) attenuates Parkinson’s disease through directly regulating contactin-associated protein-like 4 (CNTNAP4) transcription: An experimental study. Cell Signal. 2024, 118, 111139. [Google Scholar] [CrossRef]
- Ehrlich, M.E. Huntington’s Disease and the Striatal Medium Spiny Neuron: Cell-Autonomous and Non-Cell-Autonomous Mechanisms of Disease. Neurotherapeutics 2012, 9, 270–284. [Google Scholar] [CrossRef]
- Bai, D.; Zhu, L.; Jia, Q.; Duan, X.; Chen, L.; Wang, X.; Hou, J.; Jiang, G.; Yang, S.; Li, S.; et al. Loss of TDP-43 promotes somatic CAG repeat expansion in Huntington’s disease knock-in mice. Prog. Neurobiol. 2023, 227, 102484. [Google Scholar] [CrossRef]
- Kumar, R.; Khandelwal, N.; Chander, Y.; Nagori, H.; Verma, A.; Barua, A.; Godara, B.; Pal, Y.; Gulati, B.R.; Tripathi, B.N.; et al. S-adenosylmethionine-dependent methyltransferase inhibitor DZNep blocks transcription and translation of SARS-CoV-2 genome with a low tendency to select for drug-resistant viral variants. Antivir. Res. 2022, 197, 105232. [Google Scholar] [CrossRef]
- Salapa, H.E.; Thibault, P.A.; Libner, C.D.; Ding, Y.; Clarke, J.-P.W.E.; Denomy, C.; Hutchinson, C.; Abidullah, H.M.; Austin Hammond, S.; Pastushok, L.; et al. hnRNP A1 dysfunction alters RNA splicing and drives neurodegeneration in multiple sclerosis (MS). Nat. Commun. 2024, 15, 356. [Google Scholar] [CrossRef]
- Exley, C.; Mamutse, G.; Korchazhkina, O.; Pye, E.; Strekopytov, S.; Polwart, A.; Hawkins, C. Elevated urinary excretion of aluminium and iron in multiple sclerosis. Mult. Scler. J. 2006, 12, 533–540. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Li, W.; Zhang, Y.; Yang, G.; Huang, B.; Cen, Y.; Wang, H.; Yang, X.; Lin, F.; et al. METTL3-mediated m6A RNA methylation was involved in aluminum-induced neurotoxicity. Ecotoxicol. Environ. Saf. 2024, 270, 115878. [Google Scholar] [CrossRef]
- Song, J.; Hao, J.; Lu, Y.; Ding, X.; Li, M.; Xin, Y. Increased m6A modification of BDNF mRNA via FTO promotes neuronal apoptosis following aluminum-induced oxidative stress. Environ. Pollut. 2024, 349, 123848. [Google Scholar] [CrossRef]
- Hendricks, E.; Quihuis, A.M.; Hung, S.-T.; Chang, J.; Dorjsuren, N.; Der, B.; Staats, K.A.; Shi, Y.; Sta Maria, N.S.; Jacobs, R.E.; et al. The C9ORF72 repeat expansion alters neurodevelopment. Cell Rep. 2023, 42, 112983. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dou, X.; Liu, J.; Xiao, Y.; Zhang, Z.; Hayes, L.; Wu, R.; Fu, X.; Ye, Y.; Yang, B.; et al. Globally reduced N6-methyladenosine (m6A) in C9ORF72-ALS/FTD dysregulates RNA metabolism and contributes to neurodegeneration. Nat. Neurosci. 2023, 26, 1328–1338. [Google Scholar] [CrossRef]
- Park, J.; Wu, Y.; Shao, W.; Gendron, T.F.; van der Spek, S.J.F.; Sultanakhmetov, G.; Basu, A.; Castellanos Otero, P.; Jones, C.J.; Jansen-West, K.; et al. Poly(GR) interacts with key stress granule factors promoting its assembly into cytoplasmic inclusions. Cell Rep. 2023, 42, 112822. [Google Scholar] [CrossRef] [PubMed]
- He, D.; He, X.; Shen, D.; Liu, L.; Yang, X.; Hao, M.; Wang, Y.; Li, Y.; Liu, Q.; Liu, M.; et al. Loss-of-function variants in RNA binding motif protein X-linked induce neuronal defects contributing to amyotrophic lateral sclerosis pathogenesis. MedComm 2024, 5, e712. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wei, S.; Wang, R.; Liu, Y.; Zhong, Y.; Fu, J.; Luo, H.; Bao, M. Apelin-13-Mediated Upregulation of METTL3 Ameliorates Alzheimer’s Disease via Inhibiting Neuroinflammation Through m6A-Dependent Regulation of lncRNA BDNF-AS. Biomolecules 2025, 15, 1188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).