GV1001, an hTERT-Derived Peptide, Prevents Cisplatin-Induced Nephrotoxicity by Preserving Mitochondrial Function
Highlights
- GV1001 prevents cisplatin-induced nephrotoxicity in mice, as evidenced by the reversal of cisplatin-induced histopathological abnormalities, inflammatory responses, apoptotic cell death, and elevations in serum and renal injury markers.
- GV1001 preserves mitochondrial integrity and function against cisplatin-induced damages.
- GV1001 can serve as a novel protective agent against cisplatin-induced nephrotoxicity.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animal Experiments and Animal Welfare
- Control Group: Received phosphate-buffered saline (PBS) via intraperitoneal injection (i.p. inj.) three times per week for 6 weeks.
- GV1001 Group: Received GV1001 (2 mg/kg) i.p. inj. three times per week for 6 weeks. This dosage has been shown to effectively suppress inflammation without inducing toxicity in mice [14].
- Cisplatin + PBS Group: Received cisplatin (2.5 mg/kg) i.p. inj. twice per week for 6 weeks, along with PBS i.p. inj. three times per week for 6 weeks. This dosage was reported to demonstrate anti-cancer activity without mortality in mice, like the dose for cisplatin treatment in cancer patients [21].
- Cisplatin + GV1001 Group: Received cisplatin (2.5 mg/kg) i.p. inj. twice per week for 6 weeks, in combination with GV1001 (2 mg/kg) i.p. inj. three times per week for 6 weeks.
2.3. Tissue Collection and Determination of Kidney Damages
2.4. Immunofluorescence (IF) Staining
2.5. Apoptosis Assessment
2.6. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.7. Western Blotting
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. ATP Detection Assay
2.10. Mitochondrial Membrane Potential (MMP) Detection Assay
2.11. Labeling GV1001 with Fluorescein Isothiocyanate (FITC)
2.12. Lipid Binding Assay
2.13. Isolation of Mitochondria and Protein Analysis from the Mitochondria and Cytosol
2.14. Statistical Analyses
3. Results
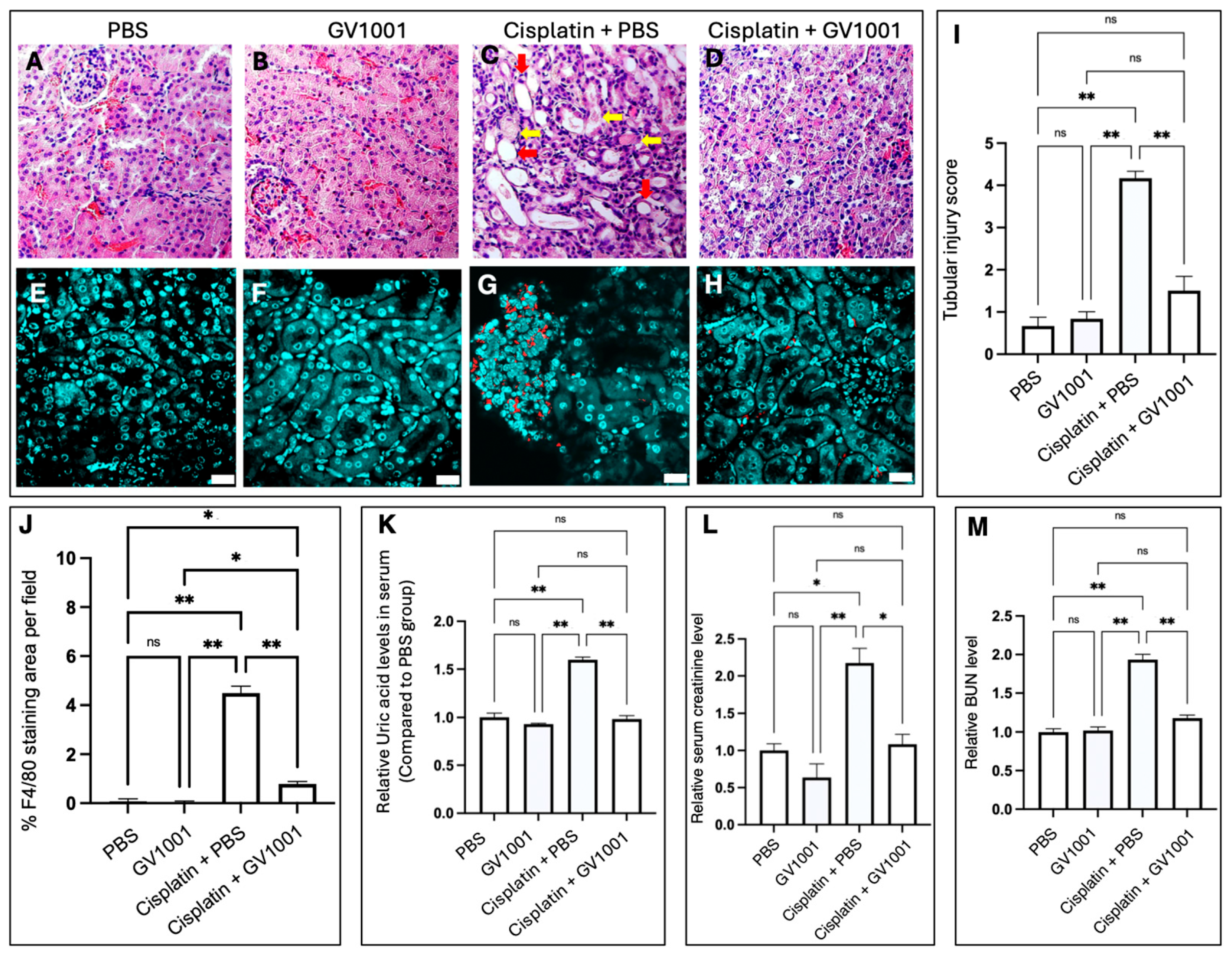
3.1. Effect of GV1001 on Cisplatin-Induced Nephrotoxicity
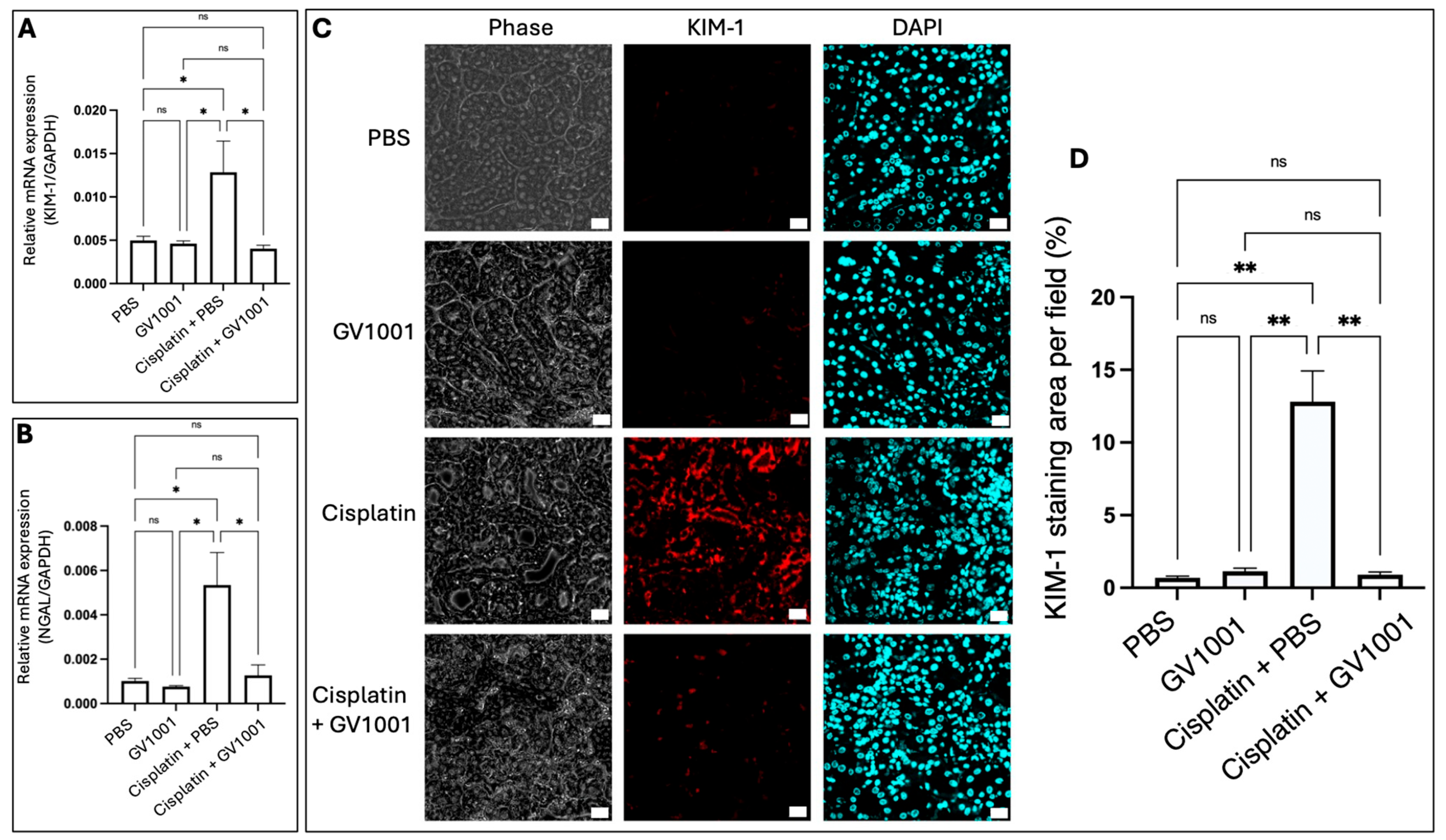
3.2. Effect of GV1001 on the Expression of Genes Associated with the Cisplatin-Induced Kidney Injury
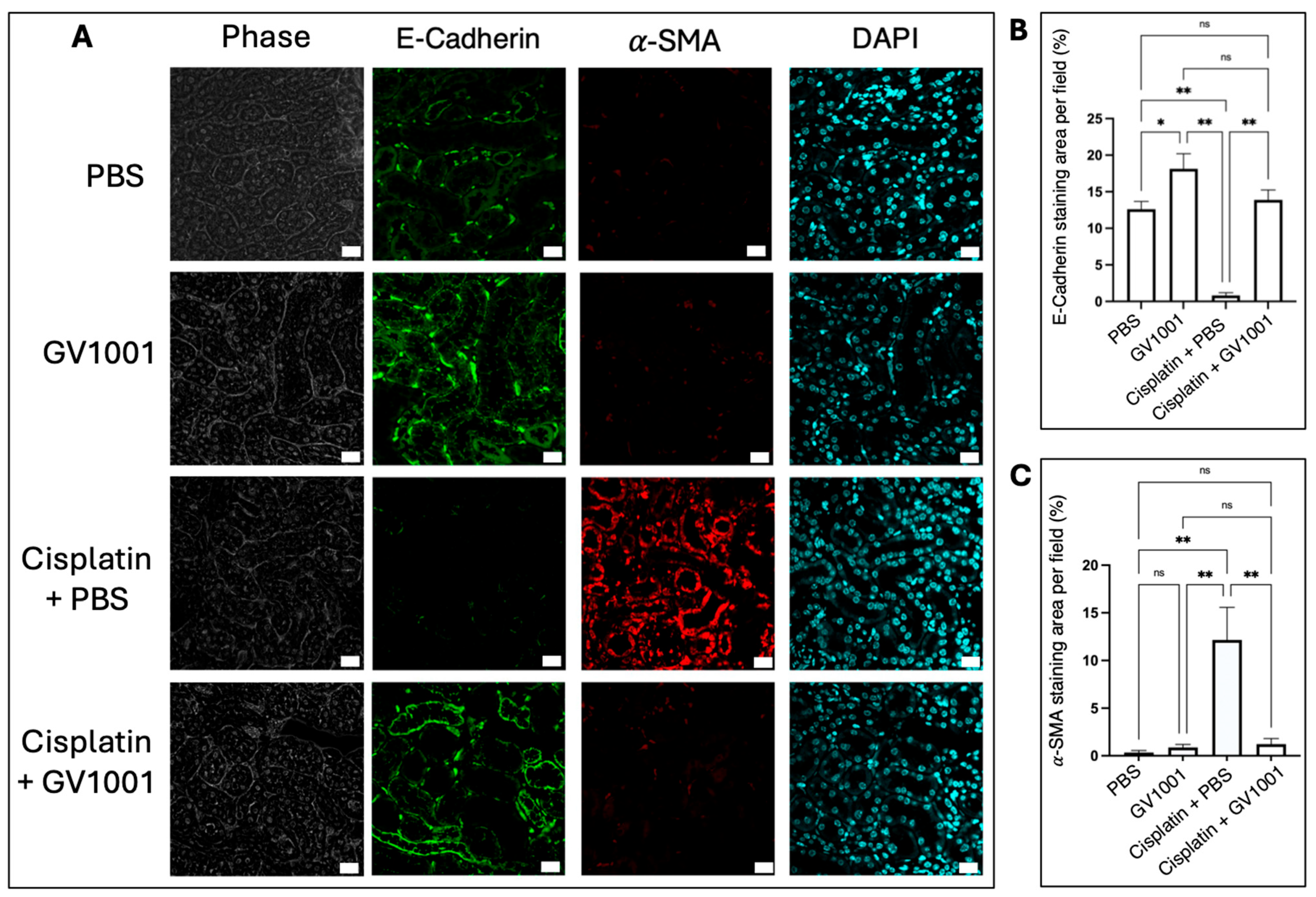
3.3. Effect of GV1001 on the Cisplatin-Induced Epithelial-to-Mesenchymal Transition (EMT) of Mouse Kidney Epithelial Cells
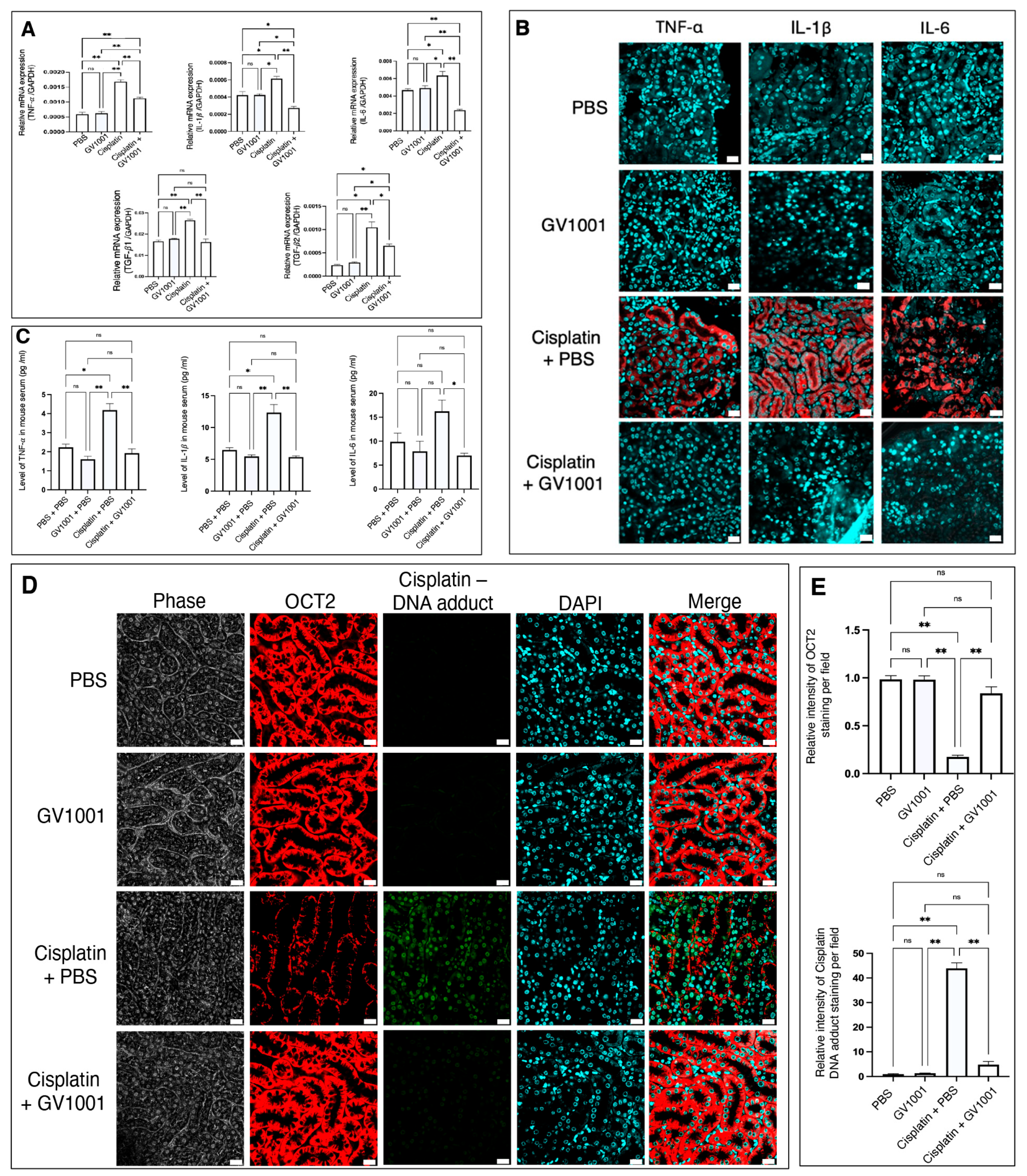
3.4. Effect of GV1001 on the Cisplatin-Induced Systemic and Renal Inflammation in Mice
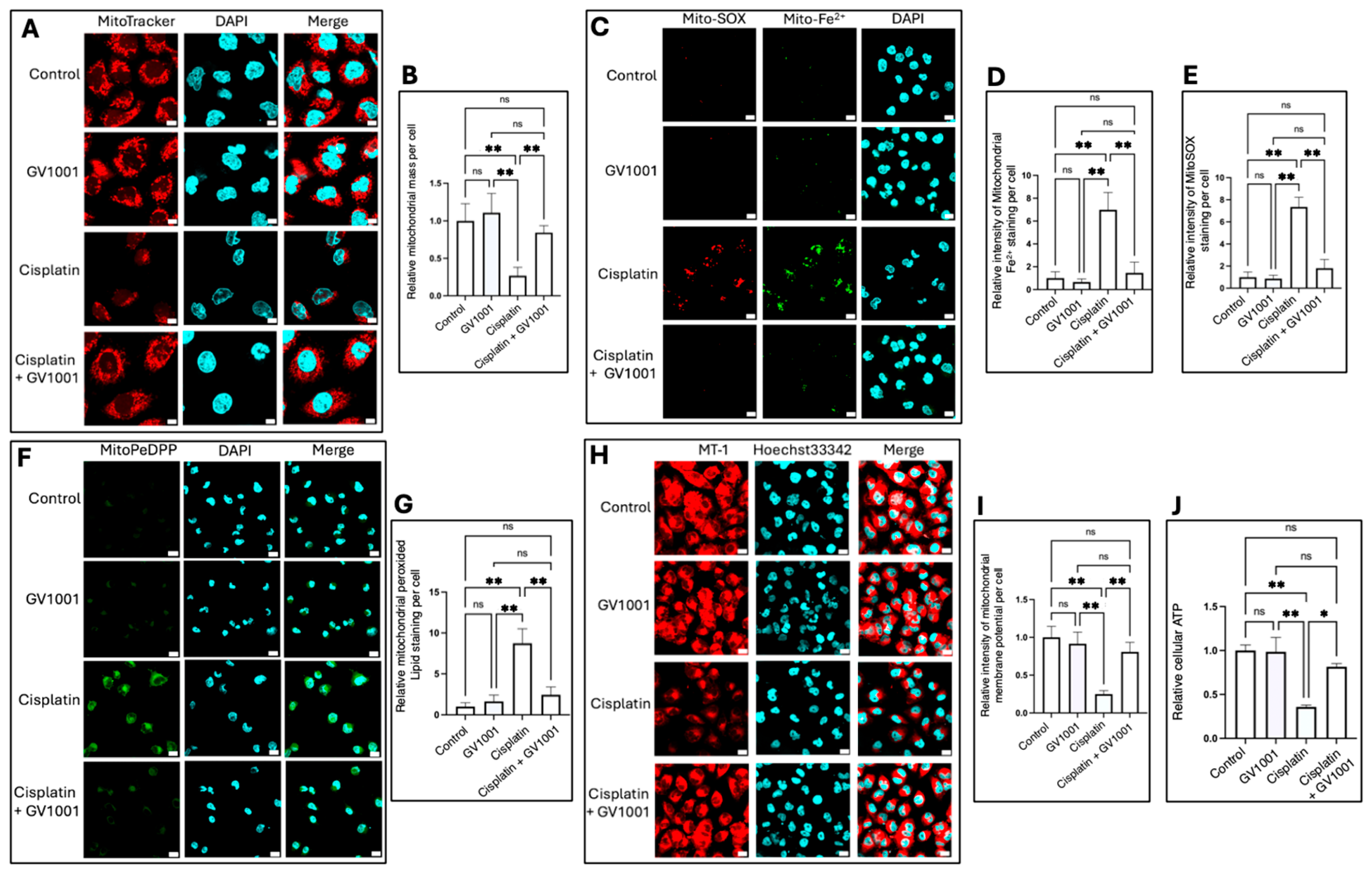
3.5. Effects of Cisplatin and GV1001 on Mitochondrial Mass and Function
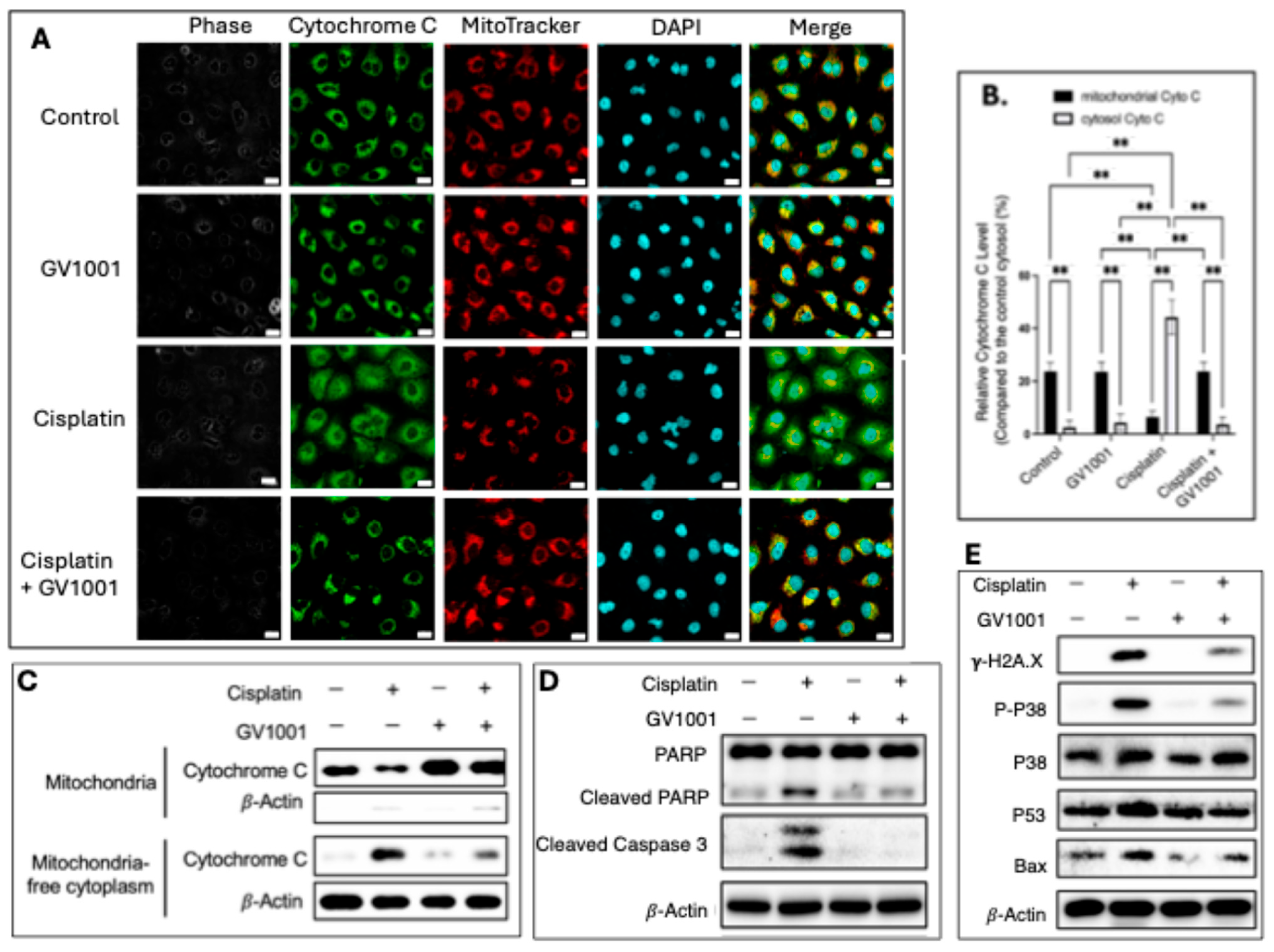
3.6. Effect of GV1001 on Cisplatin-Caused Apoptosis in Human Renal Epithelial Cells
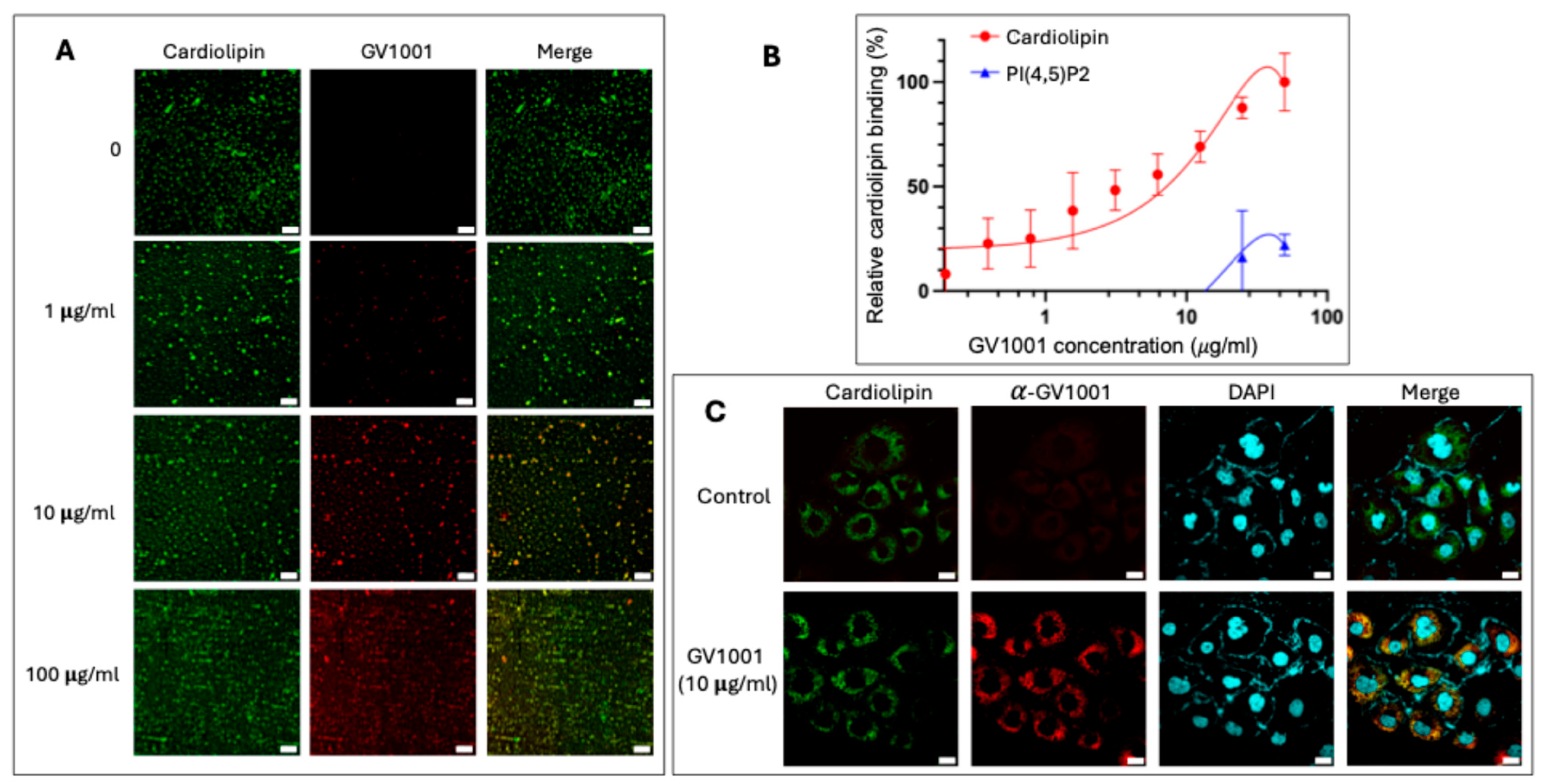
3.7. GV1001 Binds to Mitochondrial Cardiolipin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIN | Cisplatin-Induced Nephrotoxicity |
| FBS | Fetal Bovine Serum |
| PBS | Phosphate-Buffered Saline (PBS) |
| H&E | Hematoxylin and Eosin |
| PARP | Poly (ADP-Ribose) Polymerase |
| γ-H2AX | Gamma-H2A histone family member X |
| DAPI | 4′,6-diamidino-2-phenylindole |
| cDNA | Complementary DNA |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| DMSO | Dimethyl Sulfoxide |
| hTERT | Human Telomerase Reverse Transcriptase |
| KIM-1 | Kidney Injury Molecule-1 |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| TUNEL | Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling |
| IF | Immunofluorescence |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor Necrosis Factor-alpha |
| MMP | Mitochondrial Membrane Potential |
| FITC | Fluorescein Isothiocyanate |
| BUN | Blood Urea Nitrogen |
| α-SMA | Alpha Smooth Muscle Actin |
| EMT | Epithelial-to-Mesenchymal Transition |
| Oct-02 | Organic Cation Transporter 2 |
| NHREC | Normal Human Renal Epithelial Cell |
| ETC | Electron Transport Chain |
| COX-4 | Cytochrome c Oxidase Subunit IV |
| PI(4,5)P2 | Phosphatidylinositol 4,5-Bisphosphate |
| NAO | 10-N-nonyl Acridine Orange |
| NDUFS1 | NADH Dehydrogenase (ubiquinone) Fe-S Protein 1 |
| SDHB | Succinate Dehydrogenase Complex Iron–Sulfur Subunit |
| UQCRFS1 | Ubiquinol–Cytochrome C Reductase Rieske Iron–Sulfur Polypeptide 1 |
| p-p65 | Phosphorylated NF-kB p65 |
| ROS | Reactive Oxygen Species |
| MitoSox | Mitochondrial Superoxide Indicator |
| CMSRos | Chromomethy-X-Rosamine |
| dUTP | deoxyuridine triphosphate |
| RT-qPCR | Reverse Transcription quantitative polymerase Chain Reaction |
| DMEM | Dulbecco’s Modified Eagle Medium |
| HEPES | 4-(2-Hydroxuethyl)-1-piperazineethanesulfonic acid |
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Davoudi, M.; Jadidi, Y.; Moayedi, K.; Farrokhi, V.; Afrisham, R. Ameliorative impacts of polymeric and metallic nanoparticles on cisplatin-induced nephrotoxicity: A 2011–2022 review. J. Nanobiotechnol. 2022, 20, 504. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem. Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef]
- Sikking, C.; Niggebrugge-Mentink, K.L.; van der Sman, A.S.E.; Smit, R.H.P.; Bouman-Wammes, E.W.; Beex-Oosterhuis, M.M.; van Kesteren, C. Hydration Methods for Cisplatin Containing Chemotherapy: A Systematic Review. Oncologist 2024, 29, e173–e186. [Google Scholar] [CrossRef] [PubMed]
- Kleih, M.; Bopple, K.; Dong, M.; Gaissler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.J.; Kim, I.; Kim, J.H.; Kim, H.K.; Ryu, H.; Choi, D.R.; Hwang, I.G.; Song, H.; Kwon, J.H.; et al. A phase II study of chemotherapy in combination with telomerase peptide vaccine (GV1001) as second-line treatment in patients with metastatic colorectal cancer. J. Cancer 2022, 13, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shin, K.H.; Kim, S.; Shon, W.J.; Kim, R.H.; Park, N.H.; Kang, M.K. hTERT peptide fragment GV1001 demonstrates radioprotective and antifibrotic effects through suppression of TGF-beta signaling. Int. J. Mol. Med. 2018, 41, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Lee, K.Y.; Kim, S.; Lee, J.W.; Choi, N.Y.; Lee, E.H.; Lee, Y.J.; Lee, S.H.; Koh, S.H. Novel vaccine peptide GV1001 effectively blocks beta-amyloid toxicity by mimicking the extra-telomeric functions of human telomerase reverse transcriptase. Neurobiol. Aging 2014, 35, 1255–1274. [Google Scholar] [CrossRef]
- Chen, W.; Kim, S.Y.; Lee, A.; Kim, Y.J.; Chang, C.; Ton-That, H.; Kim, R.; Kim, S.; Park, N.H. hTERT Peptide Fragment GV1001 Prevents the Development of Porphyromonas gingivalis-Induced Periodontal Disease and Systemic Disorders in ApoE-Deficient Mice. Int. J. Mol. Sci. 2024, 25, 6126. [Google Scholar] [CrossRef]
- Chen, W.; Kim, S.; Kim, S.Y.; Beheshtian, C.; Kim, N.; Shin, K.H.; Kim, R.H.; Kim, S.; Park, N.H. GV1001, hTERT Peptide Fragment, Prevents Doxorubicin-Induced Endothelial-to-Mesenchymal Transition in Human Endothelial Cells and Atherosclerosis in Mice. Cells 2025, 14, 98. [Google Scholar] [CrossRef]
- Choi, I.A.; Choi, J.Y.; Jung, S.; Basri, F.; Park, S.; Lee, E.Y. GV1001 immunotherapy ameliorates joint inflammation in a murine model of rheumatoid arthritis by modifying collagen-specific T-cell responses and downregulating antigen-presenting cells. Int. Immunopharmacol. 2017, 46, 186–193. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Sun, F.; Lei, G.; Cheng, J.; Tian, C.; Yu, H.; Deng, Z.; Lu, S.; Wang, L.; et al. A Recombinant Oncolytic Influenza Virus Carrying GV1001 Triggers an Antitumor Immune Response. Hum. Gene Ther. 2024, 35, 48–58. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, Y.J.; Kim, S.; Momeni, M.; Lee, A.; Treanor, A.; Kim, S.; Kim, R.H.; Park, N.H. GV1001 Inhibits the Severity of the Ligature-Induced Periodontitis and the Vascular Lipid Deposition Associated with the Periodontitis in Mice. Int. J. Mol. Sci. 2023, 24, 12566. [Google Scholar] [CrossRef]
- Park, H.H.; Yu, H.J.; Kim, S.; Kim, G.; Choi, N.Y.; Lee, E.H.; Lee, Y.J.; Yoon, M.Y.; Lee, K.Y.; Koh, S.H. Neural stem cells injured by oxidative stress can be rejuvenated by GV1001, a novel peptide, through scavenging free radicals and enhancing survival signals. Neurotoxicology 2016, 55, 131–141. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, J.; Sim, J.; Kim, S.G.; Kook, Y.H.; Park, C.G.; Kim, H.R.; Kim, B.J. A telomerase-derived peptide regulates reactive oxygen species and hepatitis C virus RNA replication in HCV-infected cells via heat shock protein 90. Biochem. Biophys. Res. Commun. 2016, 471, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Perse, M. Cisplatin Mouse Models: Treatment, Toxicity and Translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef]
- Ma, N.; Wei, Z.; Hu, J.; Gu, W.; Ci, X. Farrerol Ameliorated Cisplatin-Induced Chronic Kidney Disease Through Mitophagy Induction via Nrf2/PINK1 Pathway. Front. Pharmacol. 2021, 12, 768700. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.K.; Conway, E.M.; Harris, R.C. Survivin mediates renal proximal tubule recovery from AKI. J. Am. Soc. Nephrol. 2013, 24, 2023–2033. [Google Scholar] [CrossRef]
- Jang, S.K.; Ahn, S.H.; Kim, G.; Kim, S.; Hong, J.; Park, K.S.; Park, I.C.; Jin, H.O. Inhibition of VDAC1 oligomerization blocks cysteine deprivation-induced ferroptosis via mitochondrial ROS suppression. Cell Death Dis. 2024, 15, 811. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Suh, J.S.; Lee, S.H.; Fouladian, Z.; Lee, J.Y.; Kim, T.; Kang, M.K.; Lusis, A.J.; Bostrom, K.I.; Kim, R.H.; Park, N.H. Rosuvastatin Prevents the Exacerbation of Atherosclerosis in Ligature-Induced Periodontal Disease Mouse Model. Sci. Rep. 2020, 10, 6383. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.S.; Kim, S.Y.J.; Lee, S.H.; Kim, R.H.; Park, N.H. Hyperlipidemia is necessary for the initiation and progression of atherosclerosis by severe periodontitis in mice. Mol. Med. Rep. 2022, 26, 273. [Google Scholar] [CrossRef]
- Lin, H.Y.; Liang, C.J.; Yang, M.Y.; Chen, P.L.; Wang, T.M.; Chen, Y.H.; Shih, Y.H.; Liu, W.; Chiu, C.C.; Chiang, C.K.; et al. Critical roles of tubular mitochondrial ATP synthase dysfunction in maleic acid-induced acute kidney injury. Apoptosis 2024, 29, 620–634. [Google Scholar] [CrossRef]
- Sun, Y.; Preiss, N.K.; Valenteros, K.B.; Kamal, Y.; Usherwood, Y.K.; Frost, H.R.; Usherwood, E.J. Zbtb20 Restrains CD8 T Cell Immunometabolism and Restricts Memory Differentiation and Antitumor Immunity. J. Immunol. 2020, 205, 2649–2666. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Malicka, J.; Huang, J.; Gryczynski, Z.; Gryczynski, I. Ultrabright fluorescein-labeled antibodies near silver metallic surfaces. Biopolymers 2004, 74, 467–475. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Choi, Y.B. Modulation of Mitochondrial Antiviral Signaling by Human Herpesvirus 8 Interferon Regulatory Factor 1. J. Virol. 2016, 90, 506–520. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Roncal, C.A.; Mu, W.; Croker, B.; Reungjui, S.; Ouyang, X.; Tabah-Fisch, I.; Johnson, R.J.; Ejaz, A.A. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am. J. Physiol. Ren. Physiol. 2007, 292, F116–F122. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Strader, M.; Friedman, G.; Benain, X.; Camerlingo, N.; Sultana, S.; Shapira, S.; Aber, N.; Murray, P.T. Early and Sensitive Detection of Cisplatin-Induced Kidney Injury Using Novel Biomarkers. Kidney Int. Rep. 2025, 10, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Morisi, N.; Oliveira Paulo, C.; Clementi, A.; Ronco, C.; Zanella, M. Neutrophil Gelatinase-Associated Lipocalin: Biological Aspects and Potential Diagnostic Use in Acute Kidney Injury. J. Clin. Med. 2025, 14, 1570. [Google Scholar] [CrossRef]
- Chen, S.; Xue, K.; Zhao, R.; Chai, J.; Zhu, X.; Kong, X.; Ding, Y.; Xu, L.; Wang, W. Insufficient S-sulfhydration of cAMP-response element binding protein 1 participates in hyperhomocysteinemia or cisplatin induced kidney fibrosis via promoting epithelial-mesenchymal transition. Free Radic. Biol. Med. 2025, 237, 312–325. [Google Scholar] [CrossRef]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef]
- Hadpech, S.; Thongboonkerd, V. Epithelial-mesenchymal plasticity in kidney fibrosis. Genesis 2024, 62, e23529. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.A.; Saber, S.; Hamad, R.S.; Abdel-Reheim, M.A.; El-Kott, A.F.; AlShehri, M.A.; Morsy, K.; Salama, S.A.; Youssef, M.E. Advances in understanding cisplatin-induced toxicity: Molecular mechanisms and protective strategies. Eur. J. Pharm. Sci. 2024, 203, 106939. [Google Scholar] [CrossRef]
- Han, C.; Zheng, J.; Wang, F.; Lu, Q.; Chen, Q.; Hu, A.; Visentin, M.; Kullak-Ublick, G.A.; Gai, Z.; Chu, L. The Role of NF-kB in the Downregulation of Organic Cation Transporter 2 Expression and Renal Cation Secretion in Kidney Disease. Front. Med. 2021, 8, 800421. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Verissimo, T.; de Seigneux, S. New evidence of the impact of mitochondria on kidney health and disease. Nat. Rev. Nephrol. 2024, 20, 81–82. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Tang, C.; Dong, Z. Mitophagy in Acute Kidney Injury and Kidney Repair. Cells 2020, 9, 338. [Google Scholar] [CrossRef]
- Pham, L.; Arroum, T.; Wan, J.; Pavelich, L.; Bell, J.; Morse, P.T.; Lee, I.; Grossman, L.I.; Sanderson, T.H.; Malek, M.H.; et al. Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease—Implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer. Redox Biol. 2024, 78, 103426. [Google Scholar] [CrossRef]
- Li, J.Y.; Sun, X.A.; Wang, X.; Yang, N.H.; Xie, H.Y.; Guo, H.J.; Lu, L.; Xie, X.; Zhou, L.; Liu, J.; et al. PGAM5 exacerbates acute renal injury by initiating mitochondria-dependent apoptosis by facilitating mitochondrial cytochrome c release. Acta Pharmacol. Sin. 2024, 45, 125–136. [Google Scholar] [CrossRef]
- Liu, P.F.; Hu, Y.C.; Kang, B.H.; Tseng, Y.K.; Wu, P.C.; Liang, C.C.; Hou, Y.Y.; Fu, T.Y.; Liou, H.H.; Hsieh, I.C.; et al. Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. PLoS ONE 2017, 12, e0180620. [Google Scholar] [CrossRef]
- Gorbunova, A.S.; Denisenko, T.V.; Yapryntseva, M.A.; Pivnyuk, A.D.; Prikazchikova, T.A.; Gogvadze, V.G.; Zhivotovsky, B. BNIP3 as a Regulator of Cisplatin-Induced Apoptosis. Biochemistry 2020, 85, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Takahashi, M.; Yamada, Y.; Matsui, R.; Inoue, A.; Ashida, R.; Noguchi, T.; Matsuzawa, A. trans-Fatty acids promote p53-dependent apoptosis triggered by cisplatin-induced DNA interstrand crosslinks via the Nox-RIP1-ASK1-MAPK pathway. Sci. Rep. 2021, 11, 10350. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Jiang, Z.; Shen, T.; Huynh, H.; Fang, X.; Han, Z.; Ouyang, K. Cardiolipin Regulates Mitochondrial Ultrastructure and Function in Mammalian Cells. Genes 2022, 13, 1889. [Google Scholar] [CrossRef]
- Fuentes, J.M.; Morcillo, P. The Role of Cardiolipin in Mitochondrial Function and Neurodegenerative Diseases. Cells 2024, 13, 609. [Google Scholar] [CrossRef]
- Onuigbo, M. Renoprotection and the Bardoxolone Methyl Story—Is This the Right Way Forward? A Novel View of Renoprotection in CKD Trials: A New Classification Scheme for Renoprotective Agents. Nephron Extra 2013, 3, 36–49. [Google Scholar] [CrossRef]
- Louvis, N.; Coulson, J. Renoprotection by Direct Renin Inhibition: A Systematic Review and Meta- Analysis. Curr. Vasc. Pharmacol. 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.; Guo, W.; Chen, J.; Wu, X.; Gu, S.; Xu, L.; Wu, Z.; Wang, Y. Renoprotection of Microcystin-RR in Unilateral Ureteral Obstruction-Induced Renal Fibrosis: Targeting the PKM2-HIF-1alpha Pathway. Front. Pharmacol. 2022, 13, 830312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Beheshtian, C.; Kim, S.; Kim, R.; Kim, S.; Park, N.-H. GV1001, an hTERT-Derived Peptide, Prevents Cisplatin-Induced Nephrotoxicity by Preserving Mitochondrial Function. Cells 2025, 14, 1818. https://doi.org/10.3390/cells14221818
Chen W, Beheshtian C, Kim S, Kim R, Kim S, Park N-H. GV1001, an hTERT-Derived Peptide, Prevents Cisplatin-Induced Nephrotoxicity by Preserving Mitochondrial Function. Cells. 2025; 14(22):1818. https://doi.org/10.3390/cells14221818
Chicago/Turabian StyleChen, Wei, Cheyenne Beheshtian, Seojin Kim, Reuben Kim, Sangjae Kim, and No-Hee Park. 2025. "GV1001, an hTERT-Derived Peptide, Prevents Cisplatin-Induced Nephrotoxicity by Preserving Mitochondrial Function" Cells 14, no. 22: 1818. https://doi.org/10.3390/cells14221818
APA StyleChen, W., Beheshtian, C., Kim, S., Kim, R., Kim, S., & Park, N.-H. (2025). GV1001, an hTERT-Derived Peptide, Prevents Cisplatin-Induced Nephrotoxicity by Preserving Mitochondrial Function. Cells, 14(22), 1818. https://doi.org/10.3390/cells14221818





