The COX Pathway Alters Hematopoiesis in Hashimoto’s Thyroiditis
Highlights
- The association was noted between the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) with prostaglandin E2 (PGE2) and thromboxane B2 (TXB2).
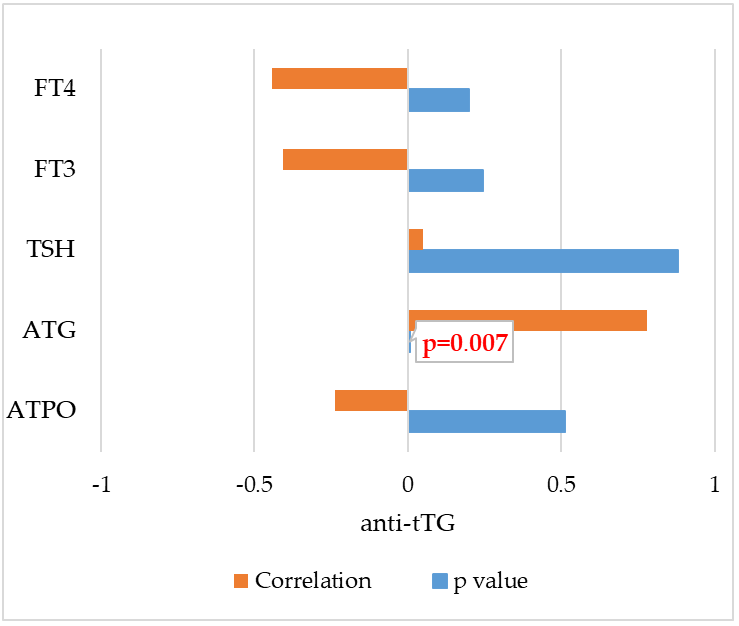
- Furthermore, a very strong correlation was demonstrated for the first time between antibodies against tissue transglutaminase (anti-tTG) and antibodies against thyroglobulin (ATG) (r = 0.781 and p = 0.007).
- The results suggest the involvement of cyclooxygenase (COX) products in the pathogenesis of Hashimoto’s Thyroiditis (HT) and hematopoiesis.
- This study may contribute to developing new guidelines for diagnosing and treating autoimmune diseases.
Abstract
1. Introduction
1.1. Hashimoto’s Thyroiditis—Symptoms, Diagnosis, Oxidative Stress and Comorbidity
1.2. The Role of COX Pathway in the Inflammatory Process
2. Materials and Methods
2.1. Characteristics of the Study Group
2.2. Sample Collection
2.3. Eicosanoid Extraction
2.4. HPLC Operating Parameters
2.5. Statistical Analysis
3. Results
3.1. Study Group
3.2. Analysis of Blood Count and C-Reactive Protein (CRP)
3.3. Analysis of Total IgA and Anti–tTG
3.4. Characteristics of Analyzed Metabolites
3.5. Correlations Between Blood Count and CRP with AA Derivatives in HT
- -
- Eosinophils and TXB2 (r = 0.401; p = 0.012);
- -
- Basophils and TXB2 (r = 0.233; p = 0.158);
- -
- Lymphocytes and TXB2 (r = 0.214; p = 0.198);
- -
- Haematocrit and TXB2 (r = 0.189; p = 0.254);
- -
- Erythrocytes and TXB2 (r = 0.167; p = 0.315);
- -
- Hemoglobin and PGE2 (r = 0.259; p = 0.117);
- -
- Basophils and PGE2 (r = 0.211; p = 0.203);
- -
- Mean corpuscular volume and PGE2 (r = 0.197; p = 0.236);
- -
- pPatelets and PGE2 (r = 0.124; p = 0.458).
4. Discussion
4.1. Prostaglandins and Thromboxanes in the Course of Hashimoto’s Thyroiditis
4.2. The Association Between Hashimoto’s Thyroiditis, Blood Count and Celiac Disease
4.3. Correlation of the COX Products with Blood Count and CRP in Hashimoto’s Thyroiditis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATG | Anti-Thyroglobulin Antibodies |
| ATPO | Anti-Thyroid Peroxidase Antibodies |
| COX | Cyclooxygenase Pathway |
| HT | Hashimoto’s Thyroiditis |
| PG | Prostaglandins |
| TX | Thromboxanes |
References
- Uhliarova, B.; Hajtman, A. Hashimoto’s thyroiditis—An independent risk factor for papillary carcinoma. Braz. J. Otorhinolaryngol. 2017, 84, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, K.; Hałasa, M.; Szczuko, M. The Role of the Immune System in the Course of Hashimoto’s Thyroiditis: The Current State of Knowledge. Int. J. Mol. Sci. 2024, 25, 6883. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Wagdy, M.; Soliman, N. Chronic anemia and thyroid function. Acta Biomed. 2017, 88, 119–127. [Google Scholar] [CrossRef]
- Kotak, P.S.; Kadam, A.; Acharya, S.; Kumar, S.; Varma, A. Beyond the Thyroid: A Narrative Review of Extra-thyroidal Manifestations in Hashimoto’s Disease. Cureus 2024, 16, e71126. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’AGuanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Pan, Y.; Sun, L.; Li, J.; Qiao, Y.; Zhao, J.; Wang, X.; Feng, Y.; Zhao, Y.; et al. Deep learning to diagnose Hashimoto’s thyroiditis from sonographic images. Nat. Commun. 2022, 13, 3759. [Google Scholar] [CrossRef]
- Kravchenko, V.; Zakharchenko, T. Thyroid hormones and minerals in immunocorrection of disorders in autoimmune thyroid diseases. Front. Endocrinol. 2023, 14, 1225494. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Ates, I.; Yilmaz, F.M.; Altay, M.; Yilmaz, N.; Berker, D.; Güler, S. The relationship between oxidative stress and autoimmunity in Hashimoto’s thyroiditis. Eur. J. Endocrinol. 2015, 173, 791–799. [Google Scholar] [CrossRef]
- Pham-Dobor, G.; Hanák, L.; Hegyi, P.; Márta, K.; Párniczky, A.; Gergics, M.; Sarlós, P.; Erőss, B.; Mezősi, E. Prevalence of other autoimmune diseases in polyglandular autoimmune syndromes type II and III. J. Endocrinol. Investig. 2020, 43, 1–9. [Google Scholar] [CrossRef]
- Szczuko, M.; Syrenicz, A.; Szymkowiak, K.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Kulpa, D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients 2022, 14, 1727. [Google Scholar] [CrossRef]
- Le, Y.; Geng, C.; Gao, X.; Zhang, P. The risk of thyroid cancer and sex differences in Hashimoto’s thyroiditis, a meta-analysis. BMC Endocr. Disord. 2024, 24, 151. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Liang, Y.; Chen, X.; Zhou, S.; Fei, W.; Yang, Y.; Que, H. Cancer Risk in Hashimoto’s Thyroiditis: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 937871. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Wang, Q.; Qiao, Y.; Xu, Z.; Zhang, L.; Xiao, H.; Lin, Z.; Wu, M.; Xia, W.; Yang, H.; et al. Arachidonic acid in aging: New roles for old players. J. Adv. Res. 2024, 70, 79–101. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- D’orazio, S.; Mattoscio, D. Dysregulation of the Arachidonic Acid Pathway in Cystic Fibrosis: Implications for Chronic Inflammation and Disease Progression. Pharmaceuticals 2024, 17, 1185. [Google Scholar] [CrossRef]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. Medcomm 2023, 4, e363. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Picado, C.; Roca-Ferrer, J. Role of the Cyclooxygenase Pathway in the Association of Obstructive Sleep Apnea and Cancer. J. Clin. Med. 2020, 9, 3237. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Ichihashi, M. Prostaglandins: Biological Action, Therapeutic Aspects, and Pathophysiology of Autism Spectrum Disorders. Curr. Issues Mol. Biol. 2025, 47, 71. [Google Scholar] [CrossRef]
- Harris, W.S. The Omega-6:Omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 34–40. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, J.; Zheng, X.; Hu, X.; He, Y. Prostaglandin and prostaglandin receptors: Present and future promising therapeutic targets for pulmonary arterial hypertension. Respir. Res. 2023, 24, 263. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.-L.; Wautier, M.-P. Pro- and Anti-Inflammatory Prostaglandins and Cytokines in Humans: A Mini Review. Int. J. Mol. Sci. 2023, 24, 9647. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Qian, C.; Lin, J.; Liu, B. Cyclooxygenase-2-Prostaglandin E2 pathway: A key player in tumor-associated immune cells. Front. Oncol. 2023, 13, 1099811. [Google Scholar] [CrossRef]
- Al-Maghrabi, J.; Gomaa, W. High COX-2 immunostaining in papillary thyroid carcinoma is associated with adverse survival outcomes. Ann. Saudi Med. 2022, 42, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Santilli, F.; Vazzana, N. Thromboxane Receptors Antagonists and/or Synthase Inhibitors. In Antiplatelet Agents; Gresele, P., Born, G., Patrono, C., Page, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 261–286. [Google Scholar] [CrossRef]
- Szczuko, M.; Kozioł, I.; Kotlęga, D.; Brodowski, J.; Drozd, A. The Role of Thromboxane in the Course and Treatment of Ischemic Stroke: Review. Int. J. Mol. Sci. 2021, 22, 11644. [Google Scholar] [CrossRef]
- Serhan, K.; Gartung, A.; Panigrahy, D. Drawing a link between the thromboxane A2 pathway and the role of platelets and tumor cells in ovarian cancer. Prostaglandins Other Lipid Mediat. 2018, 137, 40–45. [Google Scholar] [CrossRef]
- Broos, J.Y.; van der Burgt, R.T.M.; Konings, J.; Rijnsburger, M.; Werz, O.; de Vries, H.E.; Giera, M.; Kooij, G. Arachidonic acid-derived lipid mediators in multiple sclerosis pathogenesis: Fueling or dampening disease progression? J. Neuroinflamm. 2024, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.J.; Mirshafiey, A. Prostaglandins and Rheumatoid Arthritis. Arthritis 2012, 2012, 239310. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswaran, S.; Dasari, S.K.; Yang, P.; Lutgendorf, S.K.; Sood, A.K. Stress, inflammation, and eicosanoids: An emerging perspective. Cancer Metastasis Rev. 2018, 37, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kacprzak, J.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Syrenicz, A.; Drozd, A. The Influence of an Anti-Inflammatory Gluten-Free Diet with EPA and DHA on the Involvement of Maresin and Resolvins in Hashimoto’s Disease. Int. J. Mol. Sci. 2024, 25, 11692. [Google Scholar] [CrossRef]
- Szczuko, M.; Zawadzka, K.; Szczuko, U.; Rudak, L.; Pobłocki, J. The Significance and Process of Inflammation Involving Eicosapentaenoic and Docosahexaenoic Derivatives in Hashimoto’s Disease. Nutrients 2025, 17, 1715. [Google Scholar] [CrossRef]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef]
- Honda, T.; Segi-Nishida, E.; Miyachi, Y.; Narumiya, S. Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J. Exp. Med. 2006, 203, 325–335. [Google Scholar] [CrossRef]
- Wang, W.; Huang, M.; Ge, W.; Feng, J.; Zhang, X.; Li, C.; Wang, L. Identifying serum metabolite biomarkers for autoimmune diseases: A two-sample mendelian randomization and meta-analysis. Front. Immunol. 2024, 15, 1300457. [Google Scholar] [CrossRef]
- Robinson, G.; Pineda-Torra, I.; Ciurtin, C.; Jury, E.C. Lipid metabolism in autoimmune rheumatic disease: Implications for modern and conventional therapies. J. Clin. Investig. 2022, 132, e148552. [Google Scholar] [CrossRef]
- Cagiltay, E.; Bukrek, C.; Duygu, K.; Hacımustafaoğlu, F. Researching of the relationship between the numberof t regulatory cells and serum arachidonic acid level in autoimmune thyroiditis patients. Endocr. Abstr. 2024, 99, EP1352. [Google Scholar] [CrossRef]
- Krawczyk-Rusiecka, K.; Wojciechowska-Durczyńska, K.; Cyniak-Magierska, A.; Adamczewski, Z.; Gałecka, E.; Lewiński, A. COX-2 expression in papillary thyroid carcinoma (PTC) in cytological material obtained by fine needle aspiration biopsy (FNAB). Thyroid. Res. 2011, 4, 3. [Google Scholar] [CrossRef][Green Version]
- Cornetta, A.J.; Russell, J.P.; Cunnane, M.; Keane, W.M.; Rothstein, J.L. Cyclooxygenase-2 expression in human thyroid carcinoma and hashimoto’s thyroiditis. Laryngoscope 2002, 112, 238–242. [Google Scholar] [CrossRef]
- Hellmann, A.; Zwara, A.; Weryszko, O.; Czapiewska, M.; Korczynska, J.; Sztendel, A.; Śledziński, T.; Mika, A. Evaluation of the effect of Hashimoto’s Thyroiditis on fatty acids involved in inflammation in the thyroid tissue. Biomed. Pharmacother. 2025, 184, 117894. [Google Scholar] [CrossRef]
- Burkett, J.B.; Doran, A.C.; Gannon, M. Harnessing prostaglandin E2 signaling to ameliorate autoimmunity. Trends Immunol. 2023, 44, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Sa, R.; Ye, C.; Zhang, D.; Zhang, S.; Xia, H.; Wang, Y.-C.; Jiang, J.; Yin, H.; Ying, H. Effects of thyroid hormone status on metabolic pathways of arachidonic acid in mice and humans: A targeted metabolomic approach. Prostaglandins Other Lipid Mediat. 2015, 118–119, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, B.-C.; Xu, J.; Zhao, M.; Wang, Z.; Song, Y.-F.; Zhang, H.-Q.; Gao, L.; Zhang, Q.-Y.; Zhao, J.-J. Thyroxine therapy ameliorates serum levels of eicosanoids in Chinese subclinical hypothyroidism patients. Acta Pharmacol. Sin. 2016, 37, 656–663. [Google Scholar] [CrossRef]
- Chandel, R.S.; Chatterjee, G.; Abichandani, L. Impact of subclinical hypothyroidism on iron status and hematological pa-rameters. Ann. Pathol. Lab. Med. 2015, 2, A21–A25. [Google Scholar]
- Chowdappa, V.; Shetty, A. Cytomorphological Spectrum of Hashimoto’s Thyroiditis and Its Correlation with Hormonal Profile and Hematological Parameters. J. Cytol. 2019, 36, 137–141. [Google Scholar] [CrossRef]
- Mandefro, B.; Kelem, A.; Gebreegziabher, Z.A.; Gelaw, Y.; Shiferaw, E.; Adane, T. Hematological abnormalities and associated factors among patients with thyroid hormone dysfunction at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS ONE 2025, 20, e0322748. [Google Scholar] [CrossRef]
- Xue, H.; Xu, R. The lymphocyte levels of Hashimoto thyroiditis patients were significantly lower than that of healthy population. Front. Endocrinol. 2025, 16, 1472856. [Google Scholar] [CrossRef] [PubMed]
- Tomczyńska, M.; Salata, I.; Bijak, M.; Saluk-Bijak, J. The potential contribution and role of a blood platelets in autoimmune thyroid diseases. J. Cell. Mol. Med. 2018, 22, 6386–6390. [Google Scholar] [CrossRef]
- Tomczyńska, M.; Saluk-Bijak, J. The mutual cooperation of blood platelets and lymphocytes in the development of autoimmune thyroid diseases. Acta Biochim. Pol. 2018, 65, 17–24. [Google Scholar] [CrossRef]
- Gorar, S.; Alioglu, B.; Dellal, F.; Ademoglu, E.; Alphan-Uç, Z.; Bekdemir, H.; Saglam, B.; Culha, C.; Aral, Y. Evaluation of Platelet Functions in Patients with Hashimoto’s Thyroiditis Versus Healthy Controls: A Cross-Sectional Analysis. Clin. Lab. 2019, 65, 953–958. [Google Scholar] [CrossRef]
- Demir, A.D. Relationship of the platelet distribution width/platelet count ratio with thyroid antibody levels in patients with Hashimoto’s thyroiditis. J. Int. Med. Res. 2021, 49, 03000605211043241. [Google Scholar] [CrossRef]
- Elkholy, A.; Efat, A.; Shoeib, S.; Salah, A.; Tahoon, M.; Badr, H.R. Platelet Indices and RDW to Assess Inflammatory Milieu in Subclinical Hashimoto’s Thyroiditis. Clin. Med. Insights Endocrinol. Diabetes 2025, 18, 11795514251349337. [Google Scholar] [CrossRef]
- Szczepanek-Parulska, E.; Hernik, A.; Ruchała, M. Anemia in thyroid diseases. Pol. Arch. Intern. Med. 2017, 127, 352–360. [Google Scholar] [CrossRef]
- Keskin, H.; Kaya, Y.; Cadirci, K.; Kucur, C.; Ziypak, E.; Simsek, E.; Gozcu, H.; Arikan, S.; Carlioglu, A. Elevated neutrophil-lymphocyte ratio in patients with euthyroid chronic autoimmune thyreotidis. Endocr. Regul. 2016, 50, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Önalan, E.; Dönder, E. Neutrophil and platelet to lymphocyte ratio in patients with hypothyroid Hashimoto’s thyroiditis. Acta Bio-Medica Atenei Parm. 2020, 91, 310–314. [Google Scholar] [CrossRef]
- Aktas, G.; Sit, M.; Dikbas, O.; Erkol, H.; Altinordu, R.; Erkus, E.; Savli, H. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto’s thyroiditis. Front. Public Health 2017, 63, 1065–1068. [Google Scholar] [CrossRef]
- Balta, S.; Ozturk, C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets 2014, 26, 680–681. [Google Scholar] [CrossRef]
- Murad, R.; Alfeel, A.H.; Shemote, Z.; Kumar, P.; Babker, A.; Osman, A.L.; Altoum, A.A. The role of the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio in assessing hypothyroidism Hashimoto’s thyroiditis. Ital. J. Med. 2025, 19, 1953. [Google Scholar] [CrossRef]
- Arpaci, D.; Gurol, G.; Ergenc, H.; Yazar, H.; Tocoglu, A.; Ciftci, I.; Tamer, A. A Controversial New Approach to Address Hematological Parameters in Hashimoto’s Thyroiditis. Clin. Lab. 2016, 62, 1225–1231. [Google Scholar] [CrossRef]
- Erge, E.; Kiziltunc, C.; Balci, S.B.; Tel, B.M.A.; Bilgin, S.; Duman, T.T.; Aktas, G. A Novel Inflammatory Marker for the Diagnosis of Hashimoto’s Thyroiditis: Platelet-Count-to-Lymphocyte-Count Ratio. Diseases 2023, 11, 15. [Google Scholar] [CrossRef]
- Pekgör, S.; Eryılmaz, M.A.; Kaya, İ.F.K. Evaluation of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio and Mean Platelet Volume in Patients with Hypothyroidism. Eurasian J. Fam. Med. 2020, 9, 139–146. [Google Scholar] [CrossRef]
- Twito, O.; Shapiro, Y.; Golan-Cohen, A.; Dickstein, Y.; Ness-Abramof, R.; Shapiro, M. Anti-thyroid antibodies, parietal cell antibodies and tissue transglutaminase antibodies in patients with autoimmune thyroid disease. Arch. Med. Sci. 2018, 14, 516–520. [Google Scholar] [CrossRef]
- Szczuko, M.; Kwiatkowska, L.; Szczuko, U.; Rudak, L.; Ryterska, K.; Syrenicz, A.; Pobłocki, J.; Drozd, A. Dietary Gluten-Free Regimen Does Not Affect the Suppression of the Inflammatory Response in the Arachidonic Acid Cascade in Hashimoto’s Disease. Int. J. Mol. Sci. 2025, 26, 6507. [Google Scholar] [CrossRef] [PubMed]
- Ozen, G.; Boumiza, S.; Deschildre, C.; Topal, G.; Longrois, D.; Jakobsson, P.; Michel, J.; Jacob, M.; Chahed, K.; Norel, X. Inflammation increases MMP levels via PGE2 in human vascular wall and plasma of obese women. Int. J. Obes. 2018, 43, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, M.C.; Kim, B.S.; Spergel, J.M.; Artis, D. Basophils and allergic inflammation. J. Allergy Clin. Immunol. 2013, 132, 789–801. [Google Scholar] [CrossRef]
- Böhm, E.; Sturm, G.J.; Weiglhofer, I.; Sandig, H.; Shichijo, M.; McNamee, A.; Pease, J.E.; Kollroser, M.; Peskar, B.A.; Heinemann, A. 11-Dehydro-thromboxane B2, a stable thromboxane metabolite, is a full agonist of chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) in human eosinophils and basophils. J. Biol. Chem. 2004, 279, 7663–7670. [Google Scholar] [CrossRef] [PubMed]
- Droździk, A.; Barczak, K.; Bosiacki, M.; Kupnicka, P.; Cenariu, D.; Uriciuc, W.A.; Chlubek, D.; Lipski, M.; Droździk, M.; Baranowska-Bosiacka, I. Analysis of the Expression and Activity of Cyclooxygenases COX-1 and COX-2 in THP-1 Monocytes and Macrophages Cultured with Xenogenic Collagen Matrices Biofunctionalized with the Injectable Platelet-Rich Fibrin. Int. J. Mol. Sci. 2025, 26, 4386. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Küpper, J.-H.; Jung, F. Effect of Prostanoids on Human Platelet Function: An Overview. Int. J. Mol. Sci. 2020, 21, 9020. [Google Scholar] [CrossRef] [PubMed]


| Parameters | M ± SD [Min–Max] |
|---|---|
| Age [years] | 37.395 ± 8.959 [22–53] |
| Height [cm] | 166.615 ± 5.628 [150–178] |
| Body weight [kg] | 71.521 ± 13.117 [51.6–110.2] |
| BMI [kg/m2] | 25.739 ± 4.417 [18.28–37.25] |
| Fat tissue mass [g] | 26,391.41 ± 9421.325 [11,954–54,316] |
| % body fat content | 35.888 ± 6.972 [20.12–49.30] |
| Soft Lean Mass [g] | 42,686.051 ± 4749.222 [34,546–55,415] |
| ATPO [0–34 IU/mL] | 228.581 ± 290.014 [9.34–1767] |
| ATG [0–115 IU/mL] | 319.631 ± 546.504 [16.79–3423] |
| TSH [0.270–4.200 µIU/mL] | 3.041 ± 2.748 [0.01–13.92] |
| FT3 [2.00–4.40 pg/mL] | 2.985 ± 0.565 [1.78–4.87] |
| FT4 [0.93–1.70 ng/dL] | 1.284 ± 0.196 [0.8–1.72] |
| Levothyroxine dose [µg] | 68.792 ± 33.938 [25–150] |
| Parameters | M ± SD [Min–Max] |
|---|---|
| CRP [0.0–5.0 mg/L] | 1.449 ± 1.439 [<1–6.7] |
| WBC [3.8–10.00 tys/µL] | 5.87 ± 1.691 [2.86–9.89] |
| NEUT [2.5–5.4 tys/µL] | 3.153 ± 1.337 [1.05–6.74] |
| LYM [1.5–3.5 tys/µL] | 2.007 ± 0.434 [1.02–2.83] |
| MONO [0.2–1.00 tys/µL] | 0.528 ± 0.14 [0.27–0.87] |
| EOS [0.04–0.40 tys/µL] | 0.173 ± 0.112 [0.03–0.52] |
| BASO [0.02– 0.10 tys/µL] | 0.028 ± 0.015 [0.01–0.07] |
| RBC [3.7–5.10 mln/µL] | 4.558 ± 0.207 [3.99–5.03] |
| HGB [12.0–16.0 g/dL] | 13.346 ± 0.897 [10.3–15.1] |
| HCT [37.0–47.0%] | 38.987 ± 2.264 [32.7–44] |
| MCV [80.0–90.0 fL] | 85.564 ± 4.208 [71.9–92.7] |
| PLT [150–450 tys/µL] | 245.769 ± 49.794 [143–353] |
| Parameters | M ± SD; HT Patients with Normal TSH Level (n = 33) | M ± SD; HT Patients with Elevated TSH Level (n = 6) | p Value |
|---|---|---|---|
| CRP [mg/L] | 1.538 ± 1.566 | 0.862 ± 0.341 | 0.882 |
| WBC [tys/µL] | 5.693 ± 1.643 | 6.438 ± 1.608 | 0.347 |
| NEUT [tys/µL] | 3.049 ± 1.295 | 3.368 ± 1.468 | 0.673 |
| LYM [tys/µL] | 1.926 ± 0.406 | 2.362 ± 0.298 | 0.058 |
| MONO [tys/µL] | 0.505 ± 0.131 | 0.646 ± 0.146 | 0.041 |
| EOS [tys/µL] | 0.173 ± 0.116 | 0.14 ± 0.077 | 0.577 |
| BASO [tys/µL] | 0.027 ± 0.017 | 0.032 ± 0.01 | 0.337 |
| RBC [mln/µL] | 4.546 ± 0.198 | 4.688 ± 0.256 | 0.674 |
| HGB [g/dL] | 13.359 ± 0.841 | 13.22 ± 1.409 | 0.399 |
| HCT [%] | 39.088 ± 2.089 | 38.74 ± 3.603 | 0.298 |
| MCV [fL] | 86.022 ± 4.146 | 82.56 ± 4.603 | 0.085 |
| PLT [tys/µL] | 240.563 ± 50.213 | 275.20 ± 49.358 | 0.167 |
| Parameters | M ± SD; HT Patients with Normal TSH Level [n = 31] | M ± SD; HT Patients with Elevated TSH Level [n = 6] | p Value |
|---|---|---|---|
| PLR | 130.856 ± 43.422 | 115.943 ± 21.408 | 0.497 |
| NLR | 1.589 ± 0.631 | 1.574 ± 0.731 | 0.885 |
| Lipid Mediator [μg/mL] | M ± SD [Min–Max] |
|---|---|
| TXB2 | 1.417 ± 2.193 [0.019–12.621] |
| PGE2 | 8.4 ± 9.901 [0.412–41.203] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrońska, K.; Ziętek, M.; Marciniak, M.; Szczuko, M. The COX Pathway Alters Hematopoiesis in Hashimoto’s Thyroiditis. Cells 2025, 14, 1796. https://doi.org/10.3390/cells14221796
Wrońska K, Ziętek M, Marciniak M, Szczuko M. The COX Pathway Alters Hematopoiesis in Hashimoto’s Thyroiditis. Cells. 2025; 14(22):1796. https://doi.org/10.3390/cells14221796
Chicago/Turabian StyleWrońska, Karolina, Maciej Ziętek, Magdalena Marciniak, and Małgorzata Szczuko. 2025. "The COX Pathway Alters Hematopoiesis in Hashimoto’s Thyroiditis" Cells 14, no. 22: 1796. https://doi.org/10.3390/cells14221796
APA StyleWrońska, K., Ziętek, M., Marciniak, M., & Szczuko, M. (2025). The COX Pathway Alters Hematopoiesis in Hashimoto’s Thyroiditis. Cells, 14(22), 1796. https://doi.org/10.3390/cells14221796







