NuMA and Ninein: Dynein Cargo-Adaptors Without a Classical Cargo
Abstract
1. Introduction
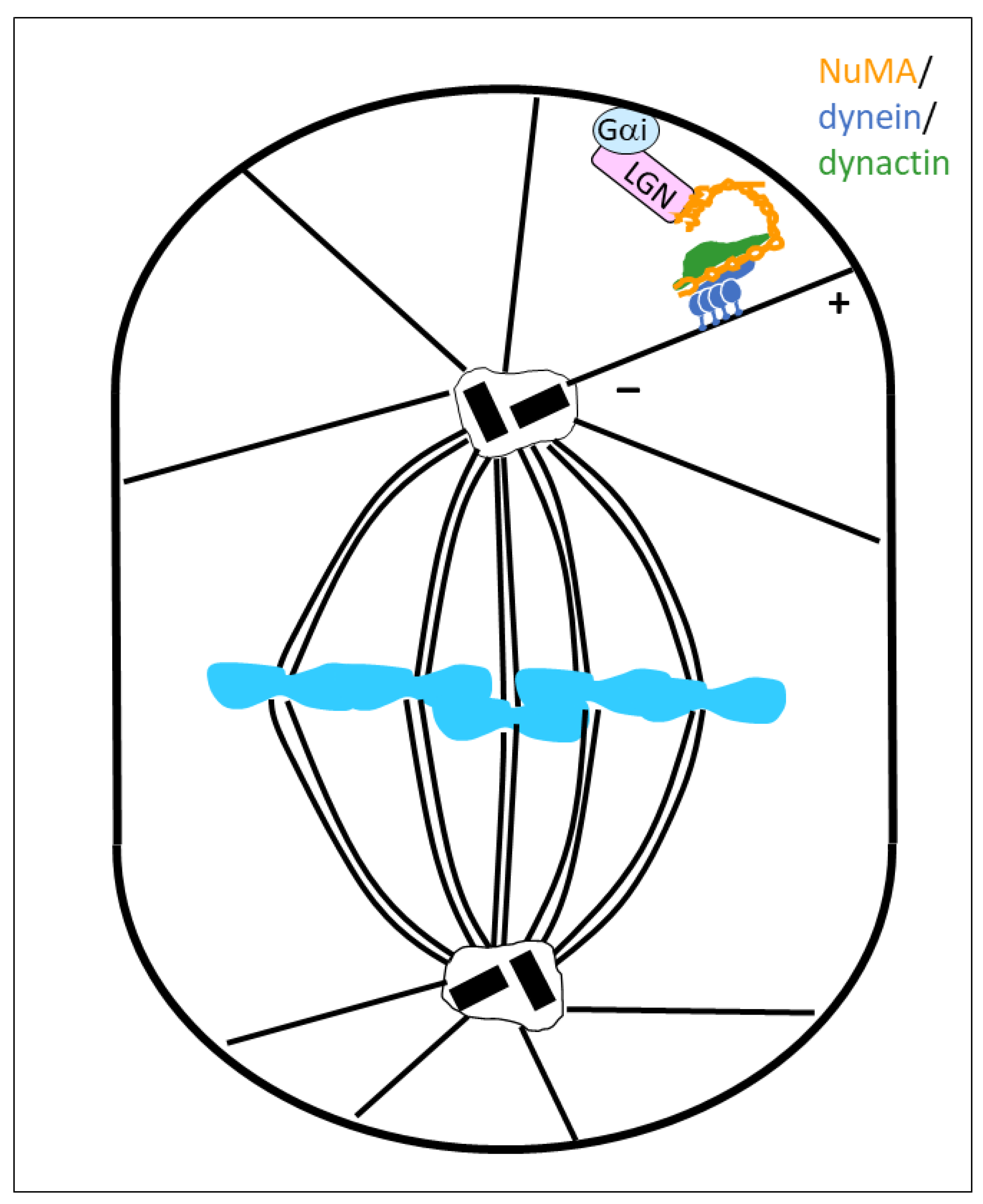
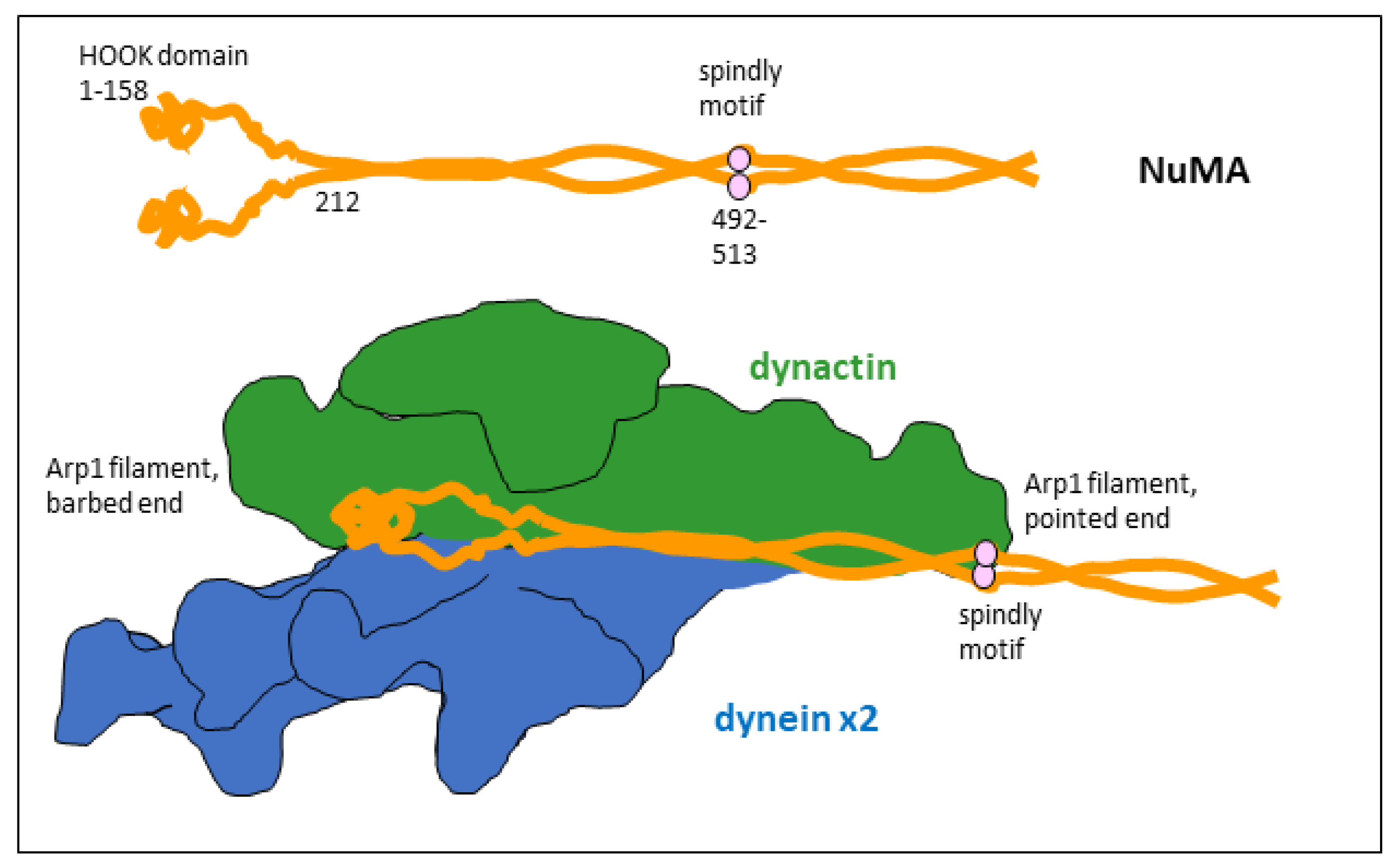
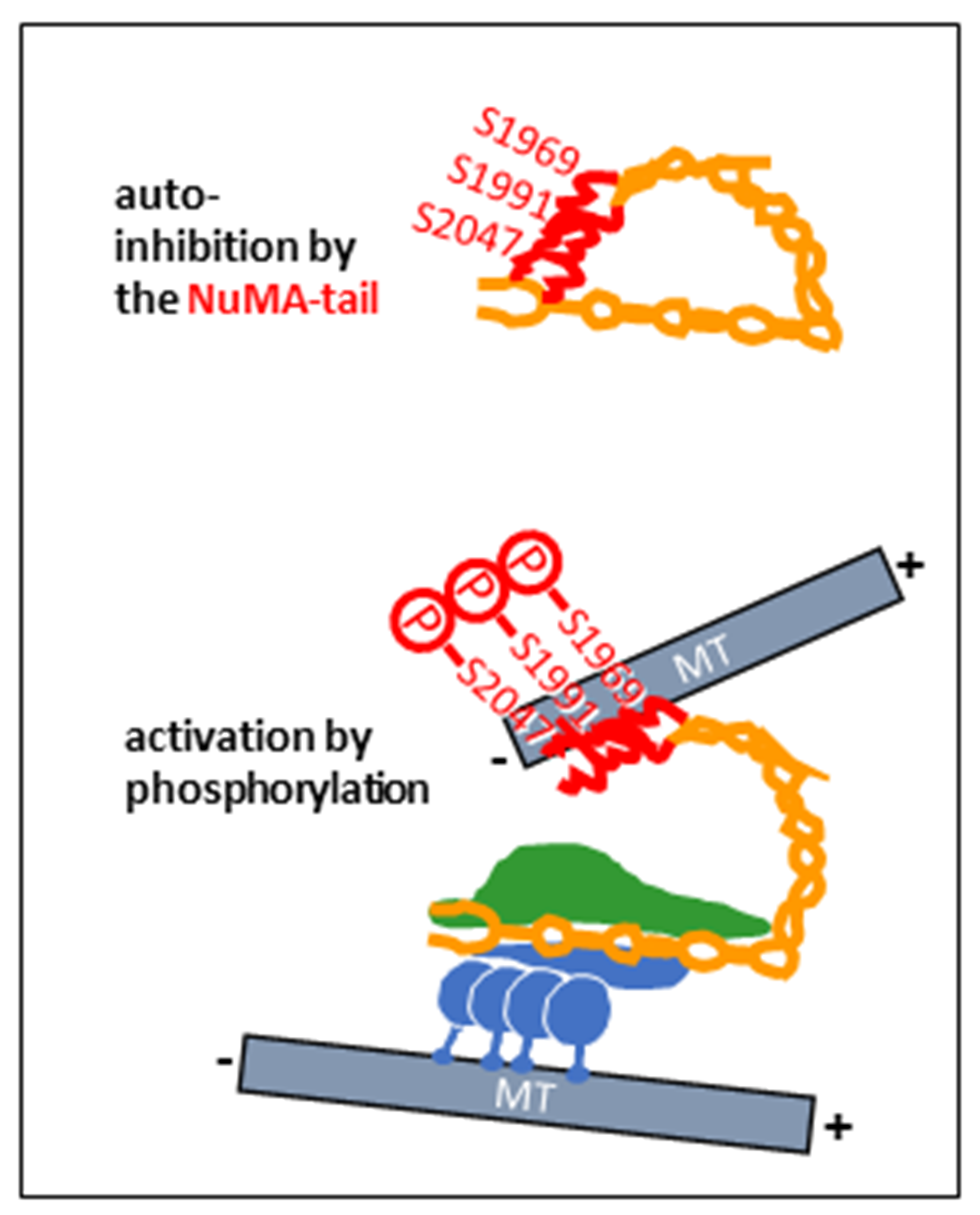
2. NuMA as a Dynein Co-Activator and Cargo-Adaptor in Mitosis
3. Ninein and NLP as a Dynein Co-Activators and Cargo-Adaptor During Interphase
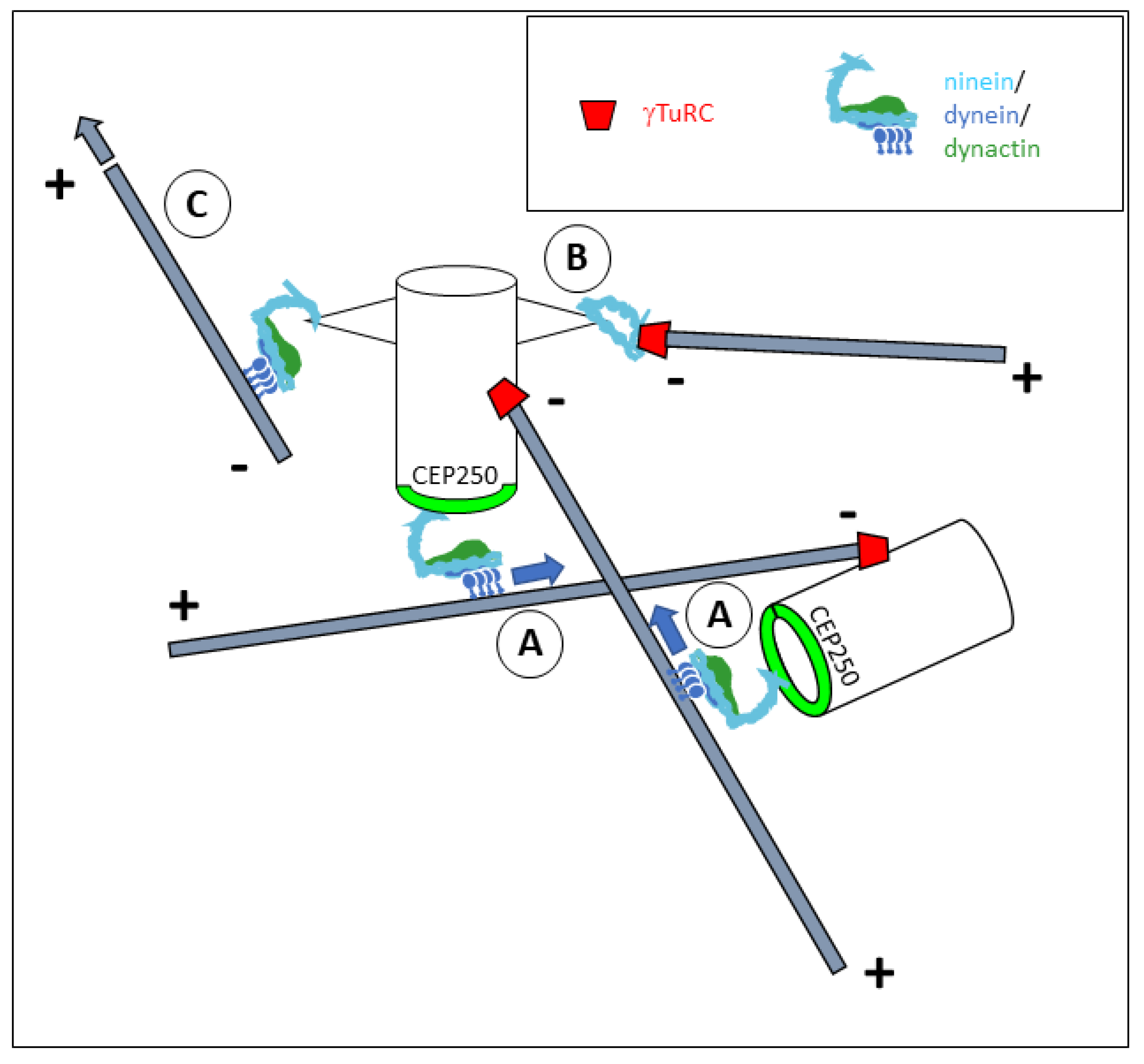
4. Mechanisms of Microtubule Organization by Ninein
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canty, J.T.; Tan, R.; Kusakci, E.; Fernandes, J.; Yildiz, A. Structure and Mechanics of Dynein Motors. Annu. Rev. Biophys. 2021, 50, 549–574. [Google Scholar] [CrossRef]
- Urnavicius, L.; Zhang, K.; Diamant, A.G.; Motz, C.; Schlager, M.A.; Yu, M.; Patel, N.A.; Robinson, C.V.; Carter, A.P. The structure of the dynactin complex and its interaction with dynein. Science 2015, 347, 1441–1446. [Google Scholar] [CrossRef]
- Schlager, M.A.; Hoang, H.T.; Urnavicius, L.; Bullock, S.L.; Carter, A.P. In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J. 2014, 33, 1855–1868. [Google Scholar] [CrossRef]
- Reck-Peterson, S.L.; Redwine, W.B.; Vale, R.D.; Carter, A.P. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 2018, 19, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.A.; Medema, R.H. Function and regulation of dynein in mitotic chromosome segregation. Chromosoma 2014, 123, 407–422. [Google Scholar] [CrossRef]
- Du, Q.; Macara, I.G. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 2004, 119, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.E.; Huang, N.N.; Cho, H.; Miki, T.; Tall, G.G.; Kehrl, J.H. Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle. Mol. Cell. Biol. 2010, 30, 3519–3530. [Google Scholar] [CrossRef]
- Kotak, S.; Busso, C.; Gönczy, P. Cortical dynein is critical for proper spindle positioning in human cells. J. Cell Biol. 2012, 199, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Siller, K.H.; Cabernard, C.; Doe, C.Q. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat. Cell Biol. 2006, 8, 594–600. [Google Scholar] [CrossRef]
- Izumi, Y.; Ohta, N.; Hisata, K.; Raabe, T.; Matsuzaki, F. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat. Cell Biol. 2006, 8, 586–593. [Google Scholar] [CrossRef]
- Bowman, S.K.; Neumüller, R.A.; Novatchkova, M.; Du, Q.; Knoblich, J.A. The Drosophila NuMA Homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell 2006, 10, 731–742. [Google Scholar] [CrossRef]
- van der Voet, M.; Berends, C.W.; Perreault, A.; Nguyen-Ngoc, T.; Gönczy, P.; Vidal, M.; Boxem, M.; van den Heuvel, S. NuMA-related LIN-5, ASPM-1, calmodulin and dynein promote meiotic spindle rotation independently of cortical LIN-5/GPR/Galpha. Nat. Cell Biol. 2009, 11, 269–277. [Google Scholar] [CrossRef]
- Lee, I.G.; Olenick, M.A.; Boczkowska, M.; Franzini-Armstrong, C.; Holzbaur, E.L.F.; Dominguez, R. A conserved interaction of the dynein light intermediate chain with dynein-dynactin effectors necessary for processivity. Nat. Commun. 2018, 9, 986. [Google Scholar] [CrossRef]
- Lee, I.G.; Cason, S.E.; Alqassim, S.S.; Holzbaur, E.L.F.; Dominguez, R. A tunable LIC1-adaptor interaction modulates dynein activity in a cargo-specific manner. Nat. Commun. 2020, 11, 5695. [Google Scholar] [CrossRef]
- Aslan, M.; d’Amico, E.A.; Cho, N.H.; Taheri, A.; Zhao, Y.; Zhong, X.; Blaauw, M.; Carter, A.P.; Dumont, S.; Yildiz, A. Structural and functional insights into activation and regulation of the dynein-dynactin-NuMA complex. bioRxiv 2024. [Google Scholar] [CrossRef]
- Merdes, A.; Ramyar, K.; Vechio, J.D.; Cleveland, D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 1996, 87, 447–458. [Google Scholar] [CrossRef]
- Haren, L.; Merdes, A. Direct binding of NuMA to tubulin is mediated by a novel sequence motif in the tail domain that bundles and stabilizes microtubules. J. Cell Sci. 2002, 115, 1815–1824. [Google Scholar] [CrossRef]
- Gallini, S.; Carminati, M.; De Mattia, F.; Pirovano, L.; Martini, E.; Oldani, A.; Asteriti, I.A.; Guarguaglini, G.; Mapelli, M. NuMA Phosphorylation by Aurora-A Orchestrates Spindle Orientation. Curr. Biol. 2016, 26, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Harborth, J.; Wang, J.; Gueth-Hallonet, C.; Weber, K.; Osborn, M. Self assembly of NuMA: Multiarm oligomers as structural units of a nuclear lattice. EMBO J. 1999, 18, 1689–7700. [Google Scholar] [CrossRef]
- Okumura, M.; Natsume, T.; Kanemaki, M.T.; Kiyomitsu, T. Dynein-Dynactin-NuMA clusters generate cortical spindle-pulling forces as a multi-arm ensemble. Elife 2018, 7, e36559. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Yamamoto, S.; Takeda, Y.; Watanabe, K.; Kuroki, K.; Hashimoto, K.; Takao, D.; Kitagawa, D. NuMA assemblies organize microtubule asters to establish spindle bipolarity in acentrosomal human cells. EMBO J. 2020, 39, e102378. [Google Scholar] [CrossRef]
- Casenghi, M.; Barr, F.A.; Nigg, E.A. Phosphorylation of Nlp by Plk1 negatively regulates its dynein-dynactin-dependent targeting to the centrosome. J. Cell Sci. 2005, 118, 5101–5108. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, D.; Quintin, S.; Green, R.A.; Cheerambathur, D.K.; Ochoa, S.D.; Desai, A.; Oegema, K. NOCA-1 functions with gamma-tubulin and in parallel to Patronin to assemble non-centrosomal microtubule arrays in C. elegans. Elife 2015, 4, e08649. [Google Scholar] [CrossRef]
- He, L.; van Beem, L.; Snel, B.; Hoogenraad, C.C.; Harterink, M. PTRN-1 (CAMSAP) and NOCA-2 (NINEIN) are required for microtubule polarity in Caenorhabditis elegans dendrites. PLoS Biol. 2022, 20, e3001855. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Mennella, V.; Marks, S.; Wildonger, J.; Elnagdi, E.; Agard, D.; Megraw, T.L. The Seckel syndrome and centrosomal protein Ninein localizes asymmetrically to stem cell centrosomes but is not required for normal development, behavior, or DNA damage response in Drosophila. Mol. Biol. Cell 2016, 27, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Kowanda, M.; Bergalet, J.; Wieczorek, M.; Brouhard, G.; Lécuyer, É.; Lasko, P. Loss of function of the Drosophila Ninein-related centrosomal protein Bsg25D causes mitotic defects and impairs embryonic development. Biol. Open 2016, 5, 1040–1051. [Google Scholar] [CrossRef]
- Redwine, W.B.; DeSantis, M.E.; Hollyer, I.; Htet, Z.M.; Tran, P.T.; Swanson, S.K.; Florens, L.; Washburn, M.P.; Reck-Peterson, S.L. The human cytoplasmic dynein interactome reveals novel activators of motility. Elife 2017, 6, e28257. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.H.; Wu, X.; Kodani, A.; Fan, J.; Doan, R.; Ozawa, M.; Ma, J.; Yoshida, N.; Reiter, J.F.; et al. Cell-Type-Specific Alternative Splicing Governs Cell Fate in the Developing Cerebral Cortex. Cell 2016, 166, 1147–1162.e15. [Google Scholar] [CrossRef]
- Delgehyr, N.; Sillibourne, J.; Bornens, M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 2005, 118, 1565–1575. [Google Scholar] [CrossRef]
- Theile, L.; Li, X.; Dang, H.; Mersch, D.; Anders, S.; Schiebel, E. Centrosome linker diversity and its function in centrosome clustering and mitotic spindle formation. EMBO J. 2023, 42, e109738. [Google Scholar] [CrossRef]
- Gilbert, T.; Gorlt, C.; Barbier, M.; Duployer, B.; Plozza, M.; Dufrancais, O.; Martet, L.E.; Dalbard, E.; Segot, L.; Tenailleau, C.; et al. Loss of ninein interferes with osteoclast formation and causes premature ossification. Elife 2024, 13, e93457. [Google Scholar] [CrossRef]
- Tassin, A.M.; Maro, B.; Bornens, M. Fate of microtubule-organizing centers during myogenesis in vitro. J. Cell Biol. 1985, 100, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Piel, M.; Meyer, P.; Khodjakov, A.; Rieder, C.L.; Bornens, M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 2000, 149, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Ibi, M.; Zou, P.; Inoko, A.; Shiromizu, T.; Matsuyama, M.; Hayashi, Y.; Enomoto, M.; Mori, D.; Hirotsune, S.; Kiyono, T.; et al. Trichoplein controls microtubule anchoring at the centrosome by binding to Odf2 and ninein. J. Cell Sci. 2011, 124, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Xia, Y.; Zhang, D.; Wang, S.; Bao, Y.; He, R.; Teng, J.; Chen, J. Hierarchical assembly of centriole subdistal appendages via centrosome binding proteins CCDC120 and CCDC68. Nat. Commun. 2017, 8, 15057. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.M.; Malik, A.; Piel, M.; Bouckson-Castaing, V.; Bornens, M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: The role of ninein. J. Cell Sci. 2000, 113, 3013–3023. [Google Scholar] [CrossRef]
- Chong, W.M.; Wang, W.J.; Lo, C.H.; Chiu, T.Y.; Chang, T.J.; Liu, Y.P.; Tanos, B.; Mazo, G.; Tsou, M.B.; Jane, W.N.; et al. Super-resolution microscopy reveals coupling between mammalian centriole subdistal appendages and distal appendages. Elife 2020, 9, e53580. [Google Scholar] [CrossRef]
- Casenghi, M.; Meraldi, P.; Weinhart, U.; Duncan, P.I.; Körner, R.; Nigg, E.A. Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev. Cell 2003, 5, 113–125. [Google Scholar] [CrossRef]
- Lin, C.C.; Cheng, T.S.; Hsu, C.M.; Wu, C.H.; Chang, L.S.; Shen, Z.S.; Yeh, H.M.; Chang, L.K.; Howng, S.L.; Hong, Y.R. Characterization and functional aspects of human ninein isoforms that regulated by centrosomal targeting signals and evidence for docking sites to direct gamma-tubulin. Cell Cycle 2006, 5, 2517–2527. [Google Scholar] [CrossRef]
- Chen, C.H.; Howng, S.L.; Cheng, T.S.; Chou, M.H.; Huang, C.Y.; Hong, Y.R. Molecular characterization of human ninein protein: Two distinct subdomains required for centrosomal targeting and regulating signals in cell cycle. Biochem. Biophys. Res. Commun. 2003, 308, 975–983. [Google Scholar] [CrossRef]
- Bugnard, E.; Zaal, K.J.; Ralston, E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil. Cytoskeleton 2005, 60, 1–13. [Google Scholar] [CrossRef]
- Tillery, M.M.L.; Zheng, C.; Zheng, Y.; Megraw, T.L. Ninein domains required for its localization, association with partners dynein and ensconsin, and microtubule organization. Mol. Biol. Cell 2024, 35, ar116. [Google Scholar] [CrossRef]
- Lechler, T.; Fuchs, E. Desmoplakin: An unexpected regulator of microtubule organization in the epidermis. J. Cell Biol. 2007, 176, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lecland, N.; Hsu, C.Y.; Chemin, C.; Merdes, A.; Bierkamp, C. Epidermal development requires ninein for spindle orientation and cortical microtubule organization. Life Sci. Alliance 2019, 2, e201900373. [Google Scholar] [CrossRef]
- Guo, K.; Merdes, A. Mechanisms of cortical microtubule organization in epidermal keratinocytes. Cell. Mol. Life Sci. 2025, 82, 193. [Google Scholar] [CrossRef] [PubMed]
- Berisha, A.M.; Pascal, A.; Guelle, M.; Bousquet, C.; Chrétien, D.; Bataillé, L.; Giet, R. Spatial activation of Kinesin-1 by Ensconsin shapes microtubule networks via ncMTOCs recruitment. bioRxiv 2025. [Google Scholar] [CrossRef]
- Omer, S.; Persaud, E.; Mohammad, S.; Ayo-Farinloye, B.; Heineman, R.E.; Wellwood, E.; Mott, G.A.; Harrison, R.E. Ninein isoform contributions to intracellular processes and macrophage immune function. J. Biol. Chem. 2025, 301, 108419. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Merdes, A. NuMA and Ninein: Dynein Cargo-Adaptors Without a Classical Cargo. Cells 2025, 14, 1797. https://doi.org/10.3390/cells14221797
Guo K, Merdes A. NuMA and Ninein: Dynein Cargo-Adaptors Without a Classical Cargo. Cells. 2025; 14(22):1797. https://doi.org/10.3390/cells14221797
Chicago/Turabian StyleGuo, Keying, and Andreas Merdes. 2025. "NuMA and Ninein: Dynein Cargo-Adaptors Without a Classical Cargo" Cells 14, no. 22: 1797. https://doi.org/10.3390/cells14221797
APA StyleGuo, K., & Merdes, A. (2025). NuMA and Ninein: Dynein Cargo-Adaptors Without a Classical Cargo. Cells, 14(22), 1797. https://doi.org/10.3390/cells14221797




