Impact of Tobacco Smoke Exposure on Male Fertility: An In Vivo Study Using Drosophila melanogaster
Highlights
- Paternal tobacco smoke exposure significantly impairs the fertility, prolificacy, and longevity of offspring of two strains of Drosophila melanogaster: Oregon K (a wild-type strain proficient in all major DNA repair pathways) and mus308 (a DNA repair-deficient strain).
- The DNA repair-deficient mus308 strain showed higher vulnerability to spermatozoa cytotoxicity and a greater magnitude of reduction in spermatozoa count.
- The reproductive toxicity of tobacco smoke is genotype-dependent, suggesting that compromised DNA repair pathways exacerbate the transgenerational impact of environmental toxicants.
- D. melanogaster is a robust and sensitive in vivo model to mechanistically evaluate the paternal reproductive risks and transgenerational effects of complex smoke-related compounds.
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
- -
- -
- mus308: This line is homozygous for the mus308 mutation, which results in a non-functional dmPolQ protein (the functional equivalent of human DNA polymerase θ, or PolQ). The mus308 mutation imparts severe deficits in the repair of DNA interstrand cross-links and complex, persistent DNA damage. Its principal role is recognized in alternative end-joining pathways, particularly MMEJ, in addition to its involvement in translesion DNA synthesis and damage bypass [20,21].
2.2. Medium Conditions
2.3. Fertility, Prolificacy, and Toxicity Assessment
2.4. Longevity Assay
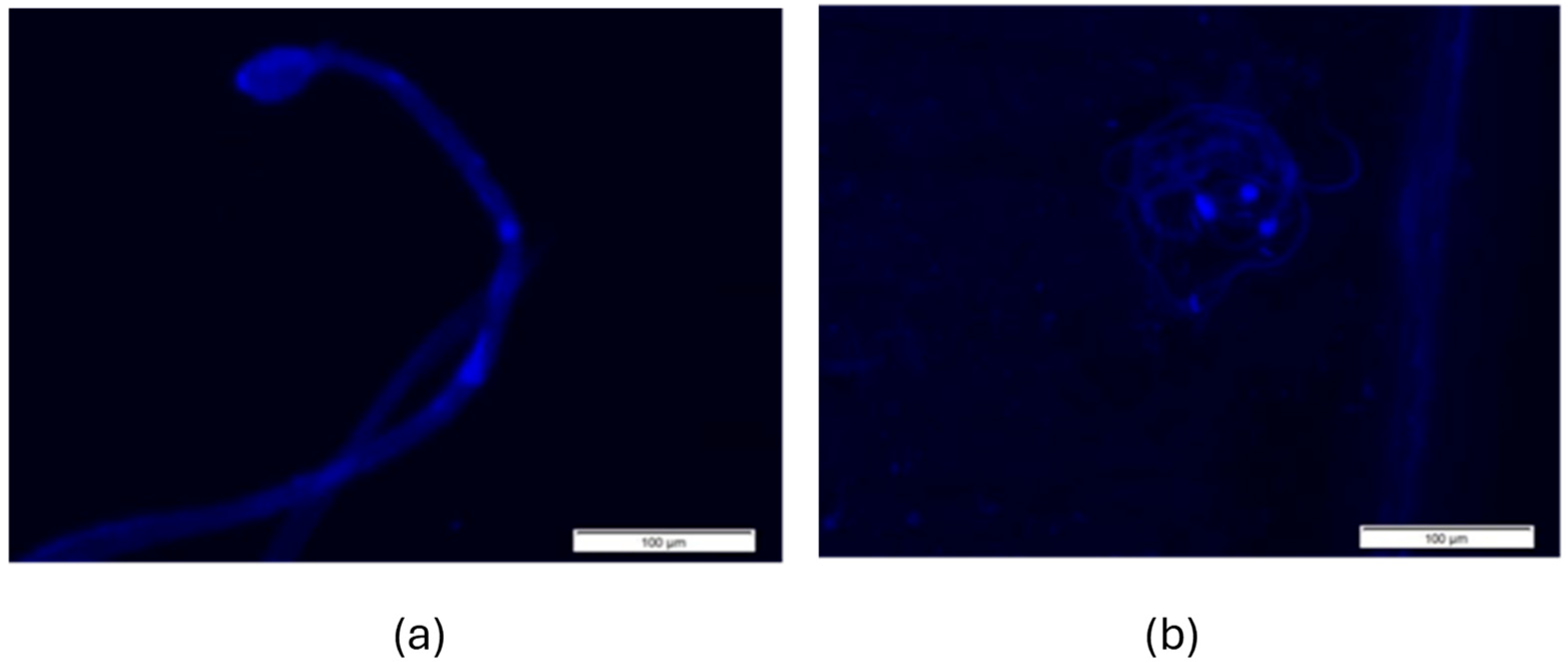
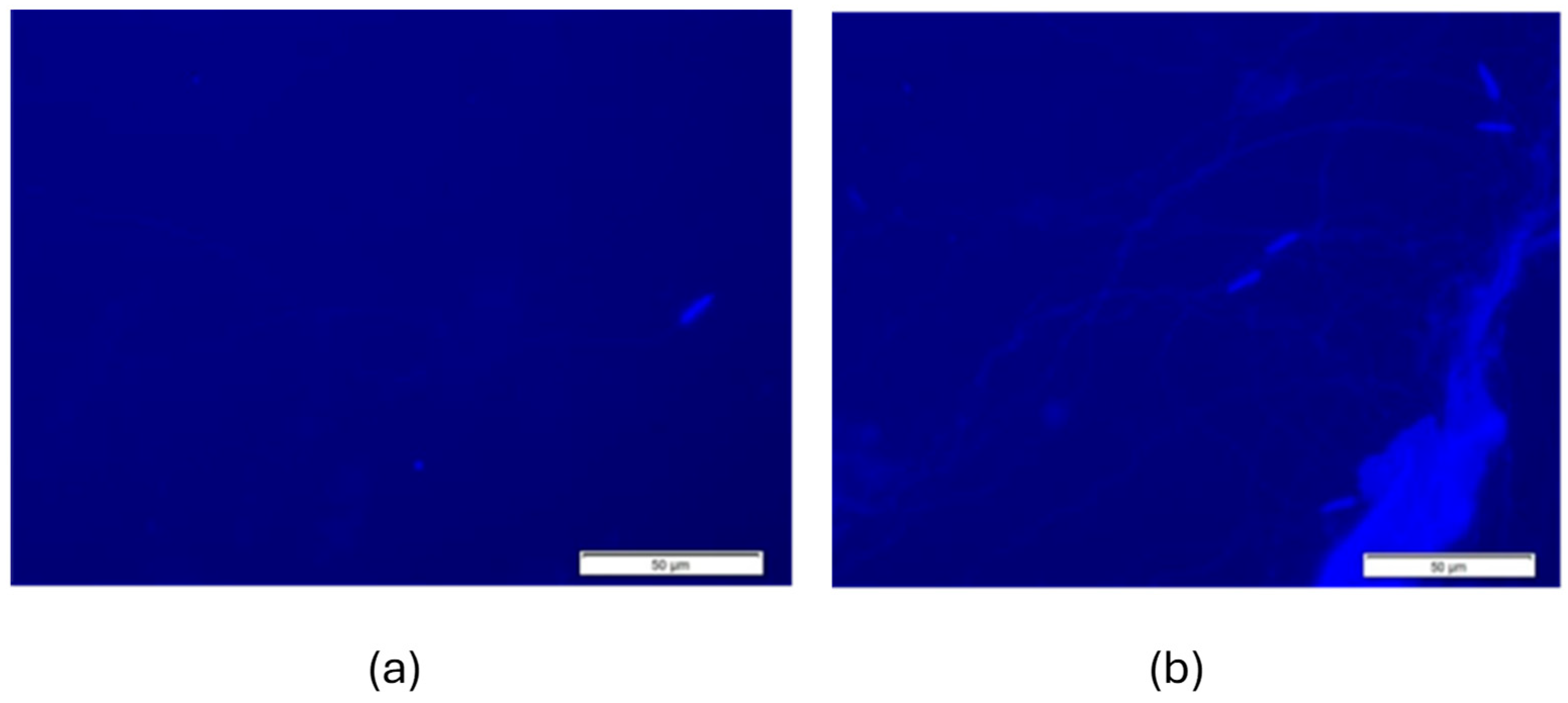
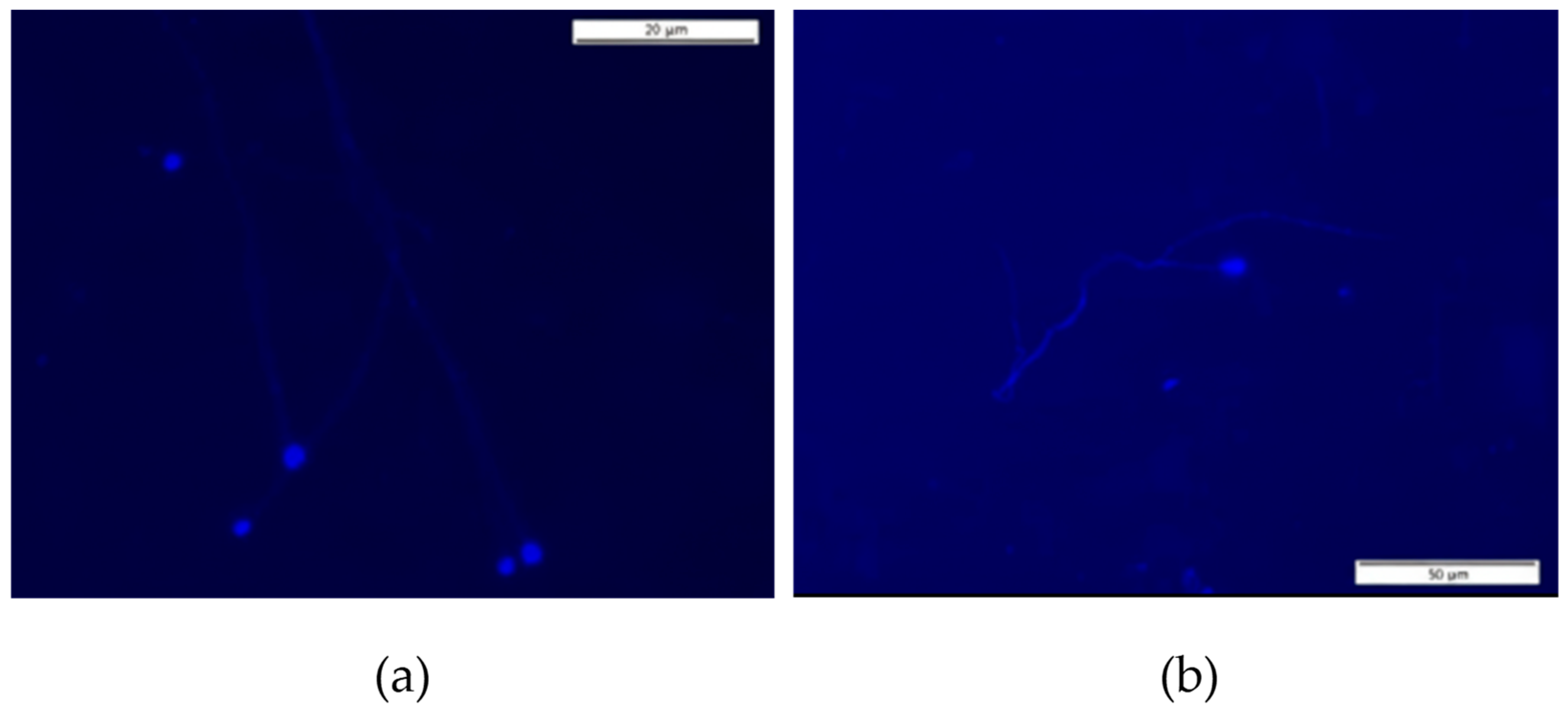
2.5. Morphological Examination of Spermatozoa
2.6. Statistical Analysis
3. Results
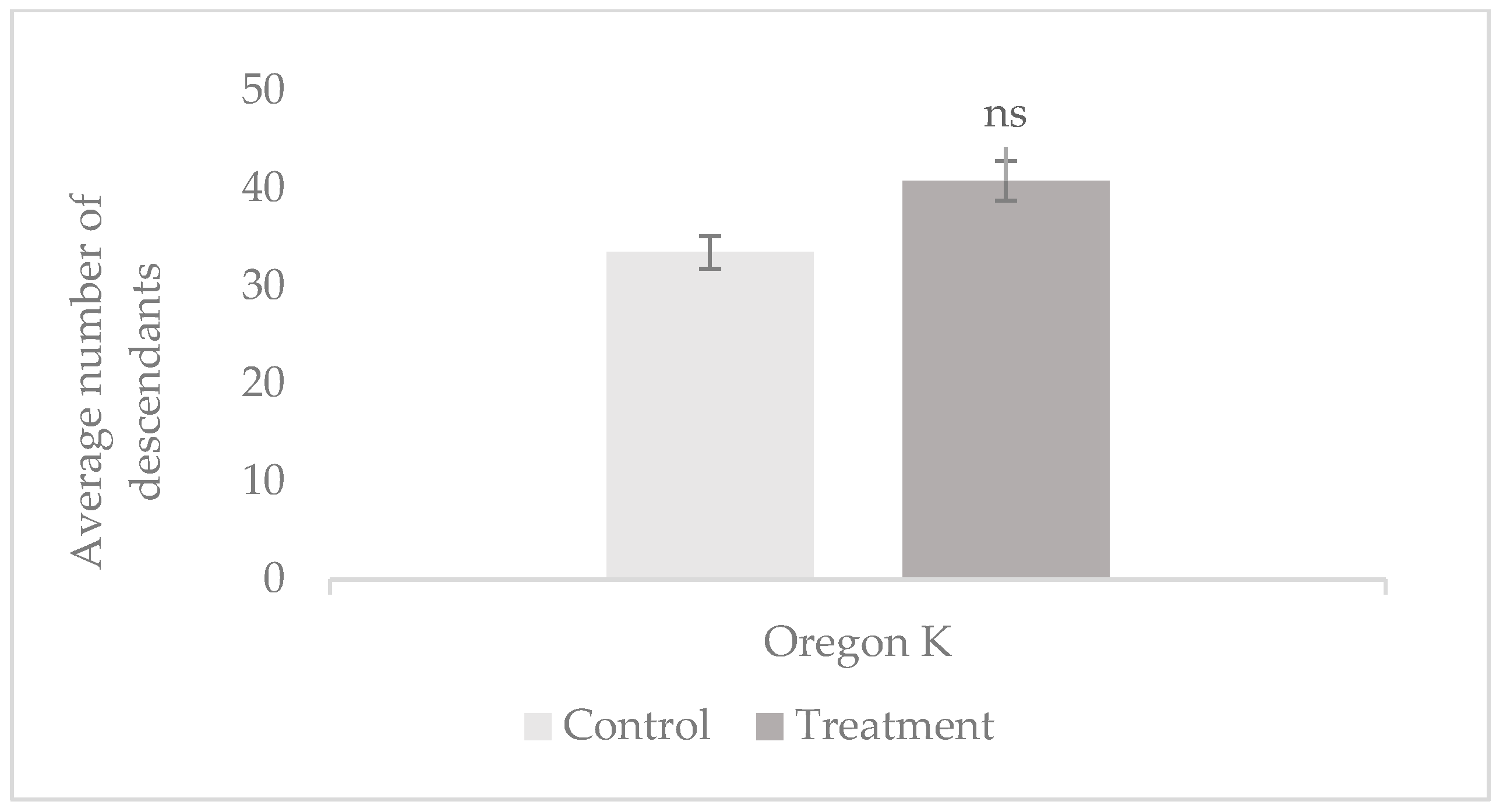
3.1. Male Fertility
3.1.1. Prolificacy
3.1.2. Male Toxicity
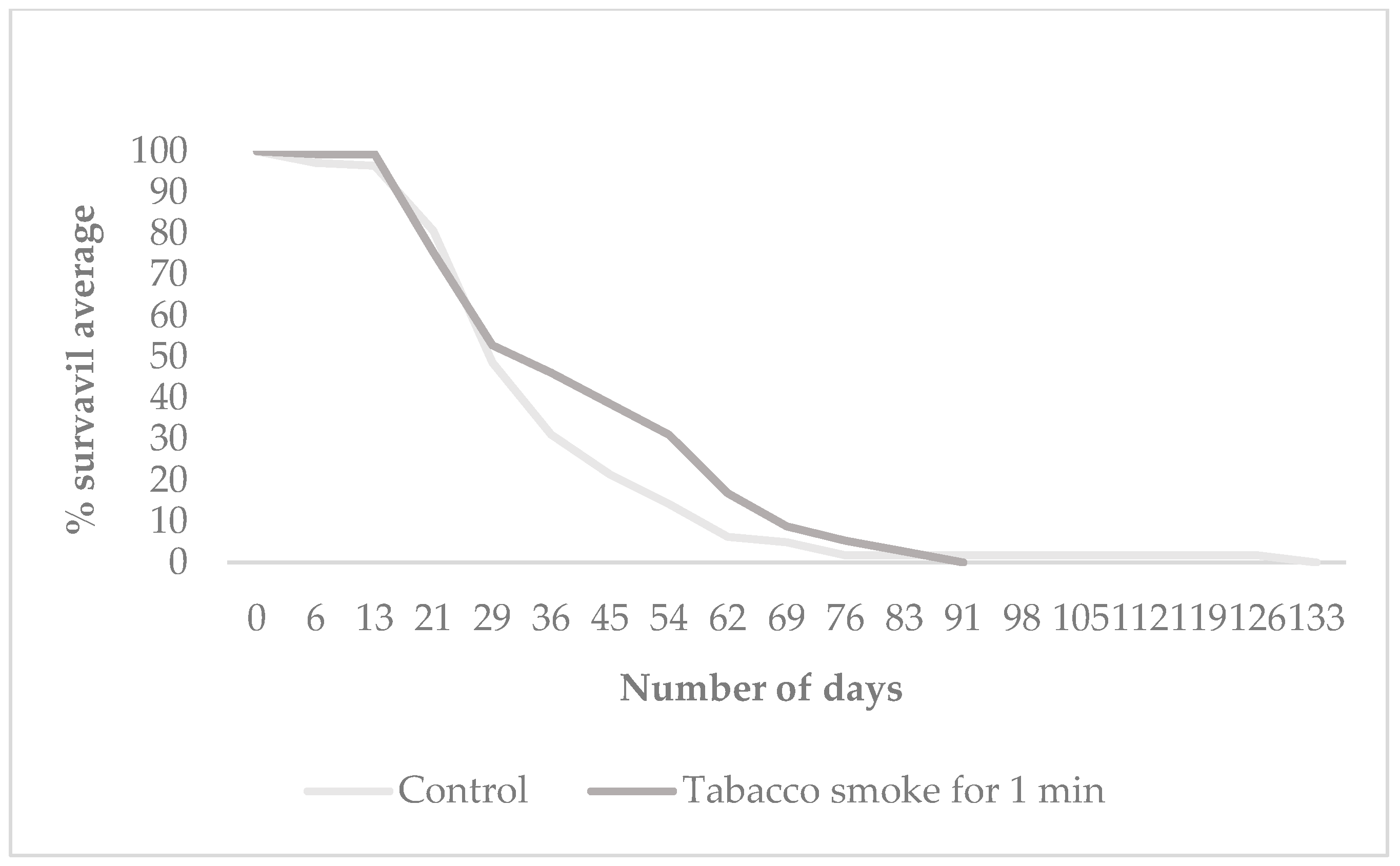
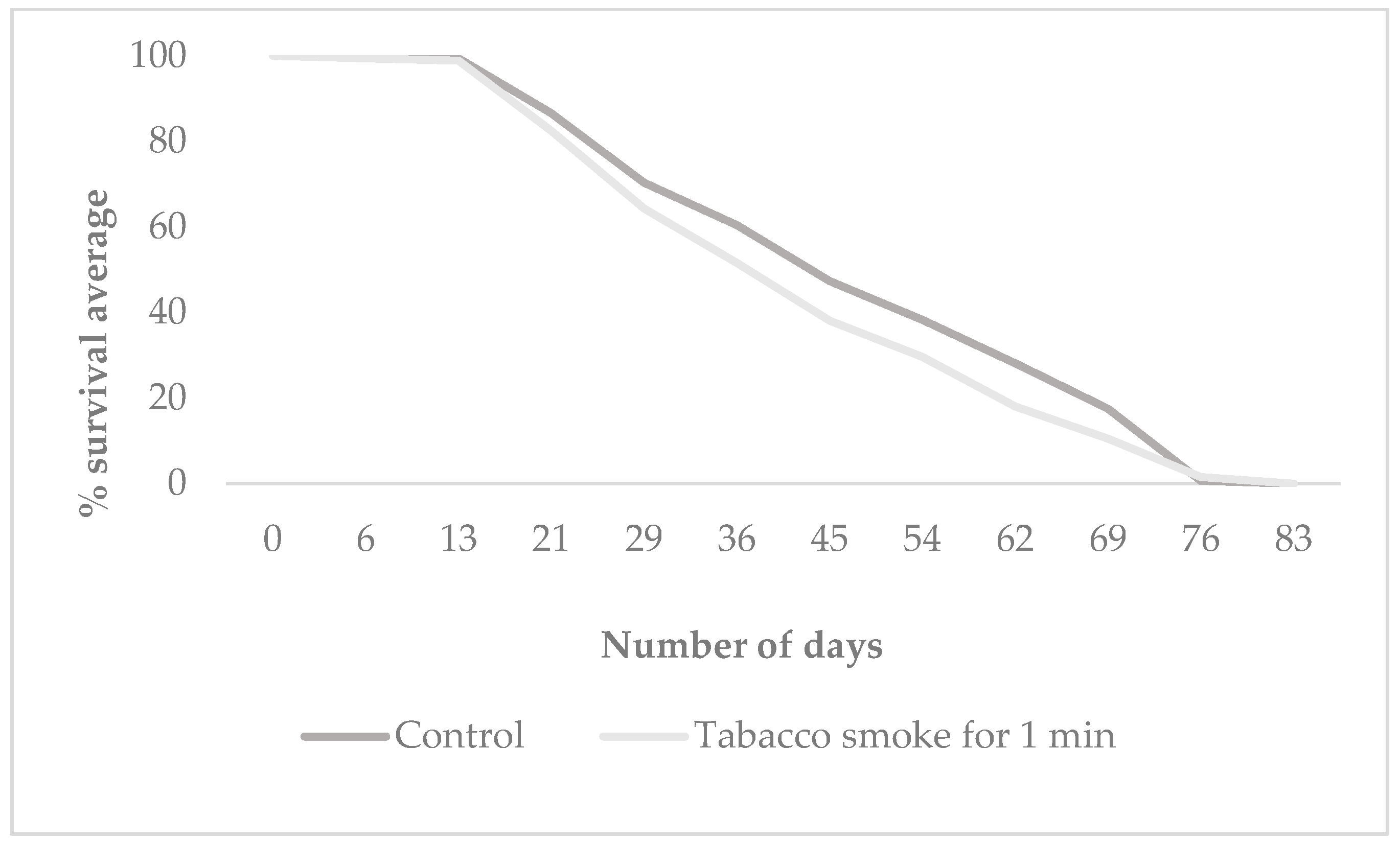
3.2. Offspring Longevity
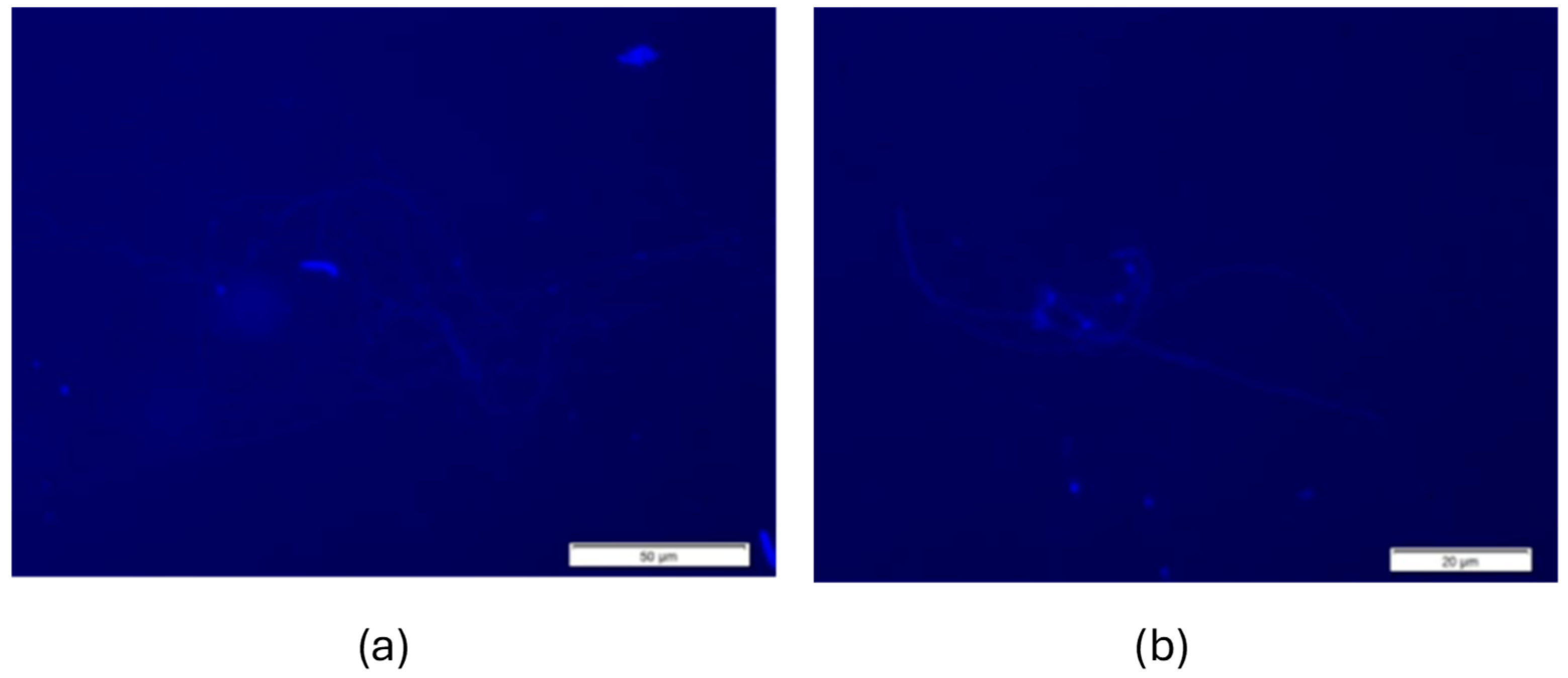
3.3. Spermatozoa Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OK | Oregon K |
| DSBs | DNA double-strand breaks |
| HR | Homologous recombination |
| c-NHEJ | Classical non-homologous end joining |
| MMEJ | Microhomology-mediated end joining |
| NMP | Normal melting point |
| LMP | Low melting point |
| SIM | Inclusion sperm in microgels |
| DAPI | 4′,6-diamidino-2-phenylindole |
References
- Zenzes, M.T. Smoking and reproduction: Gene damage to human gametes and embryos. Hum. Reprod. Update 2000, 6, 122–131. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A.B. Male Infertility. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK562258/ (accessed on 10 October 2024).
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner, S.M.K.; Shah, R. Male infertility. Nat. Rev. Dis. Prim. 2021, 7, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Belcheva, A.; Ivanova-Kicheva, M.; Tzvetkova, P.; Marinov, M. Effects of cigarette smoking on sperm plasma membrane integrity and DNA fragmentation. Int. J. Androl. 2004, 27, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Capitani, S.; Pammolli, A.; Giannerini, V.; Geminiani, M.; Moretti, E. Semen quality of male idiopathic infertile smokers and nonsmokers: An ultrastructural study. J. Androl. 2010, 31, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Künzle, R.; Mueller, M.D.; Hänggi, W.; Birkhäuser, M.H.; Drescher, H.; Bersinger, N.A. Semen quality of male smokers and nonsmokers in infertile couples. Fertil. Steril. 2003, 79, 287–291. [Google Scholar] [CrossRef]
- Colagar, A.H.; Jorsaraee, G.A.; Marzony, E.T. Cigarette smoking and the risk if male infertility. Pak. J. Biol. Sci. 2007, 10, 3870–3874. [Google Scholar] [CrossRef]
- Rau-Hansen, C.H.; Thulstrup, A.M.; Aggerholm, A.S.; Jensen, M.S.; Toft, G.; Bonde, J.P. Is smoking a risk factor for decreased semen quality? A cross—Sectional analysis. Hum. Reprod. 2007, 22, 188–196. Available online: https://pubmed.ncbi.nlm.nih.gov/16966350/ (accessed on 10 October 2024). [CrossRef]
- Gaur, D.S.; Talekar, M.; Pathak, V.P. Effect of cigarette smoking on semen quality of infertile men. Med. J. Armed Forces India 2007, 23, 119–123. [Google Scholar] [PubMed]
- Chia, S.E.; Ong, C.N.; Tsakok, F.M. Effects of cigarette smoking on human semen quality. Arch. Androl. 1994, 33, 163–168. [Google Scholar] [CrossRef]
- Kumosani, T.A.; Elshal, M.F.; Al-Jonaid, A.A.; Abduljabar, H.S. The influence of smoking on semen quality, seminal microelements and Ca2+-ATPase activity among infertile and fertile men. Clin. Biochem. 2008, 41, 14–15. [Google Scholar] [CrossRef]
- Pantaleão, S.M.; Spano, M.A.; Morelli, S.; Nepomoceno, J.C.; Takahashi, C.S. Impacto Genotóxico de Poluentes Químicos Presentes na Água e Sedimento do Rio Japaratuba (Sergipe). Ph.D. Thesis, Universidade Federal de Uberlândia, Uberlândia, Brazil, 2006. [Google Scholar]
- Ouchi, R.Y.; Manzato, A.J.; Ceron, C.R.; Bonilla-Rodriguez, G.O. Evaluation of the effects of a single exposure to ethidium bromide in Drosophila melanogaster (Diptera-Drosophilidae). Bull. Environ. Contam. Toxicol. 2007, 78, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Pragya, P.; Ravi Ram, K.; Chowdhuri, D.K. Environmental chemical mediated male reproductive toxicity: Drosophila melanogaster as an alternate animal model. Theriogenology 2011, 76, 197–216. [Google Scholar] [CrossRef]
- Kanippayoor, R.L.; Alpern, J.H.M.; Moehring, A.J. Protamines and spermatogenesis in Drosophila and Homo sapiens: A comparative analysis. Spermatogenesis 2013, 3, e24376. [Google Scholar] [CrossRef] [PubMed]
- Rand, M.D. Drosophotoxicology: The growing potential for Drosophila in neurotoxicology. Neurotoxicology Teratol. 2010, 32, 74–83. [Google Scholar] [CrossRef]
- Papanikolopoulou, K.; Skoulakis, E.M. The power and richness of modelling tauopathies in Drosophila. Mol. Neurobiol. 2011, 44, 122–133. [Google Scholar] [CrossRef]
- Rand, M.D.; Tennessen, J.M.; Mackay, T.F.C.; Anholt, R.R.H. Perspectives on the Drosophila melanogaster Model for Advances in Toxicological Science. Curr. Protoc. 2023, 3, e870. [Google Scholar] [CrossRef]
- Trizzino, M.; Kapusta, A.; Brown, C.D. Transposable elements generate regulatory novelty in a tissue-specific fashion. BMC Genomics 2018, 19, 468. [Google Scholar] [CrossRef]
- Rodríguez, R.; Gaivão, I.; Aguado, L.; Espina, M.; Garcia, J.; Martínez-Camblor, P.; Sierra, L.M. The Comet Assay in Drosophila: A Tool to Study Interactions between DNA Repair Systems in DNA Damage Responses In Vivo and Ex Vivo. Cells 2023, 12, 1979. [Google Scholar] [CrossRef]
- Alexander, J.; Beagan, K.; Kelly, J.; Orr-Weaver, T.L.; McVey, M. Multiple mechanisms contribute to double-strand break repair at rereplication forks in Drosophila follicle cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13809–13814. [Google Scholar] [CrossRef]
- Miranda, N.; Tkach, V.V.; Barros, A.N.; Martins-Bessa, A.; Gaivão, I. Biological and Behavioral Responses of Drosophila melanogaster to Dietary Sugar and Sucralose. Int. J. Mol. Sci. 2025, 26, 8951. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Henke, A.L.; Reinhardt, K. Sperm viability varies with buffer and genotype in Drosophila melanogaster. Fly 2021, 15, 1–7. [Google Scholar] [CrossRef]
- Dalton, J.E.; Kacheria, T.S.; Knott, S.R.; Lebo, M.S.; Nishitani, A.; Sanders, L.E.; Stirling, E.J.; Winbush, A.; Arbeitman, M.N. Dynamic, mating-induced gene expression changes in female head and brain tissues of Drosophila melanogaster. BMC Genom. 2010, 11, 541. [Google Scholar] [CrossRef]
- Sepaniak, S.; Forges, T.; Gerard, H.; Foliguet, B.; Bene, M.C.; Monnier-Barbarino, P. The influence of cigarette smoking on human sperm quality and DNA fragmentation. Toxicology 2006, 223, 54–60. [Google Scholar] [CrossRef]
- Evenson, D.P.; Jost, L.K.; Corzett, M.; Balhorn, R. Characteristics of human sperm chromatin structure following an episode of influenza and high fever: A case study. J. Androl. 2000, 21, 739–746. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N.; Thomson, L.K.; Mitchell, L.A.; Finnie, J.M.; Koppers, A.J.; Hedges, A.; Nixon, B. DNA Damage in Human Spermatozoa Is Highly Correlated with the Efficiency of Chromatin Remodeling and the Formation of 8-Hydroxy-2′-Deoxyguanosine, a Marker of Oxidative Stress. Biol. Reprod. 2009, 81, 517–524. [Google Scholar] [CrossRef]
- Boyd, J.B.; Golino, M.D.; Nguyen, T.D.; Green, M.M. Isolation and characterization of X-linked mutants of Drosophila melanogaster which are sensitive to mutagens. Genetics 1976, 84, 485–506. [Google Scholar] [CrossRef]
- Graf, U.; Würgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Moratore, C. Use of Drosophila melanogaster as bioindicators in the evaluation of the lethality of extracts of nicotiana tabacum. Arq. Inst. Biol. 2009, 76, 471–474. [Google Scholar] [CrossRef]
- Linford, N.J.; Bilgir, C.; Ro, J.; Pletcher, S.D. Measurement of Lifespan in Drosophila melanogaster. J. Vis. Exp. 2013, 71, e50068. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yolitz, J.; Wang, C.; Spangler, E.; Zhan, M.; Zou, S. Aging Studies in Drosophila melanogaster. Methods Mol. Biol. 2013, 1048, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.D.W.; Partridge, L. Protocols to Study Aging in Drosophila. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1478, pp. 291–302. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis is central to toxicology, pharmacology and risk assessment. Hum. Exp. Toxicol. 2010, 29, 249–261. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis: A Revolution in Toxicology, Risk Assessment and Medicine. EMBO Rep. 2013, 14, 497–498. [Google Scholar] [CrossRef]
- López-Fernández, C.; Baltanás, A.; De La Torre, J.; Gosálvez, J. Drosophila melanogaster and Eucypris virens giant spermatozoa as visualized by cell inclusion in microgels. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2007, 307, 140–144. [Google Scholar] [CrossRef]
- Piperakis, S.; Visvardis, E.E.; Sagnou, M.; Tassiou, A. Effects of smoking and aging on oxidative DNA damage of human lymphocytes. Carcinogenesis 1998, 19, 695–698. [Google Scholar] [CrossRef][Green Version]
- Kleinsasser, N.H.; Sassen, A.W.; Semmler, M.P.; Harréus, U.A.; Licht, A.K.; Richter, E. The Tobacco Alkaloid Nicotine Demonstrates Genotoxicity in Human Tonsillar Tissue and Lymphocytes. Toxicol. Sci. 2005, 86, 342–353. [Google Scholar] [CrossRef]
- La Maestra, S.; Bonomo, P.; Martone, F.; Calandra, A.; De Lisa, M.; Ferraro, M.; De Flora, S.; Micale, R.T. Effect of cigarette smoke on DNA damage, oxidative stress, and morphological alterations in mouse testis and spermatozoa. Int. J. Hyg. Environ. Health 2015, 218, 117–122. [Google Scholar] [CrossRef]
- Moktar, A.; Singh, R.; Vadhanam, M.V.; Ravoori, S.; Lillard, J.W., Jr.; Gairola, C.G.; Gupta, R.C. Cigarette smoke condensate-induced oxidative DNA damage and its removal in human cervical cancer cells. Int. J. Oncol. 2011, 39, 941–947. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; El Shahawy, O.; Skosana, B.T.; Boillat, T.; Loney, T.; du Plessis, S.S. The mutagenic effect of tobacco smoke on male fertility. Environ. Sci. Pollut. Res. Int. 2022, 29, 62055–62066. [Google Scholar] [CrossRef]
- Russo, M.; Cocco, S.; Secondo, A.; Adornetto, A.; Bassi, A.; Nunziata, A.; Polichetti, G.; De Felice, B.; Damiano, S.; Serù, R.; et al. Cigarette smoke condensate causes a decrease of the gene expression of Cu-Zn superoxide dismutase, Mn superoxide dismutase, glutathione peroxidase, catalase, and free radical-induced cell injury in SH-SY5Y human neuroblastoma cells. Neurotox Res. 2011, 19, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Ande, A.; Sinha, N.; Kumar, A.; Kumar, S. Effects of Cigarette Smoke Condensate on Oxidative Stress, Apoptotic Cell Death, and HIV Replication in Human Monocytic Cells. PLoS ONE 2016, 11, e0155791. [Google Scholar] [CrossRef]
- Xu, W.-J.; Fang, P.; Zhu, Z.-J.; Xu, D.-X.; Wang, T.-T.; Dai, J.-B. The alteration of protein profile induced by cigarette smoking via oxidative stress in mice epididymis. Int. J. Biochem. Cell Biol. 2013, 45, 571–582. [Google Scholar] [CrossRef]
- Dai, J.-B.; Wang, Z.-X.; Qiao, Z.-D. The hazardous effects of tobacco smoking on male fertility. Asian J. Androl. 2015, 17, 954–960. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J., Jr. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: A prospective study. Fertil. Steril. 2002, 78, 491–499. [Google Scholar] [CrossRef]
- Pasqualotto, F.F.; Grempel, R.G.; Pasqualotto, E.B. Effect of cryopreservation on sperm DNA integrity: A review. Int. Braz. J. Urol. 2011, 37, 592–598. [Google Scholar] [CrossRef]
- Fraga, C.G.; Motchnik, P.A.; Wyrobek, A.J.; Rempel, D.M.; Ames, B.N. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat. Res. 1996, 351, 199–203. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Amount (per Litre Distilled Water) |
|---|---|
| Sugar | 100 g |
| Agar-agar | 12 g |
| Inactive yeast | 100 g |
| Propionic acid | 5 mL |
| Groups | Exposure Time | % Fertile Males | IC 95% |
|---|---|---|---|
| Control O.K | 0 min | 60 | [45.7, 74.3] |
| Treatment O.K | 1 min | 87 | [77.2, 96.8] |
| Treatment O.K | 7 min | 100 | [79.0, 100.0] |
| Control mus308 | 0 min | 100 | [92.0, 100.0] |
| Treatment mus308 | 1 min | 93 | [85.5, 100.0] |
| Treatment mus308 | 7 min | 60 | [35.2, 84.8] |
| Strain | % of Male Mortality | IC 95% |
|---|---|---|
| Oregon K | 40 | [28.9%, 51.1%] |
| mus 308 | 51 | [39.7%, 62.3%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, N.G.d.; Gajeiro, A.; Martins-Bessa, A.; Gaivão, I. Impact of Tobacco Smoke Exposure on Male Fertility: An In Vivo Study Using Drosophila melanogaster. Cells 2025, 14, 1689. https://doi.org/10.3390/cells14211689
Miranda NGd, Gajeiro A, Martins-Bessa A, Gaivão I. Impact of Tobacco Smoke Exposure on Male Fertility: An In Vivo Study Using Drosophila melanogaster. Cells. 2025; 14(21):1689. https://doi.org/10.3390/cells14211689
Chicago/Turabian StyleMiranda, Natasha Gomes de, Ana Gajeiro, Ana Martins-Bessa, and Isabel Gaivão. 2025. "Impact of Tobacco Smoke Exposure on Male Fertility: An In Vivo Study Using Drosophila melanogaster" Cells 14, no. 21: 1689. https://doi.org/10.3390/cells14211689
APA StyleMiranda, N. G. d., Gajeiro, A., Martins-Bessa, A., & Gaivão, I. (2025). Impact of Tobacco Smoke Exposure on Male Fertility: An In Vivo Study Using Drosophila melanogaster. Cells, 14(21), 1689. https://doi.org/10.3390/cells14211689







