The Role of Neutrophil Extracellular Networks in Cardiovascular Pathology
Abstract
1. Introduction
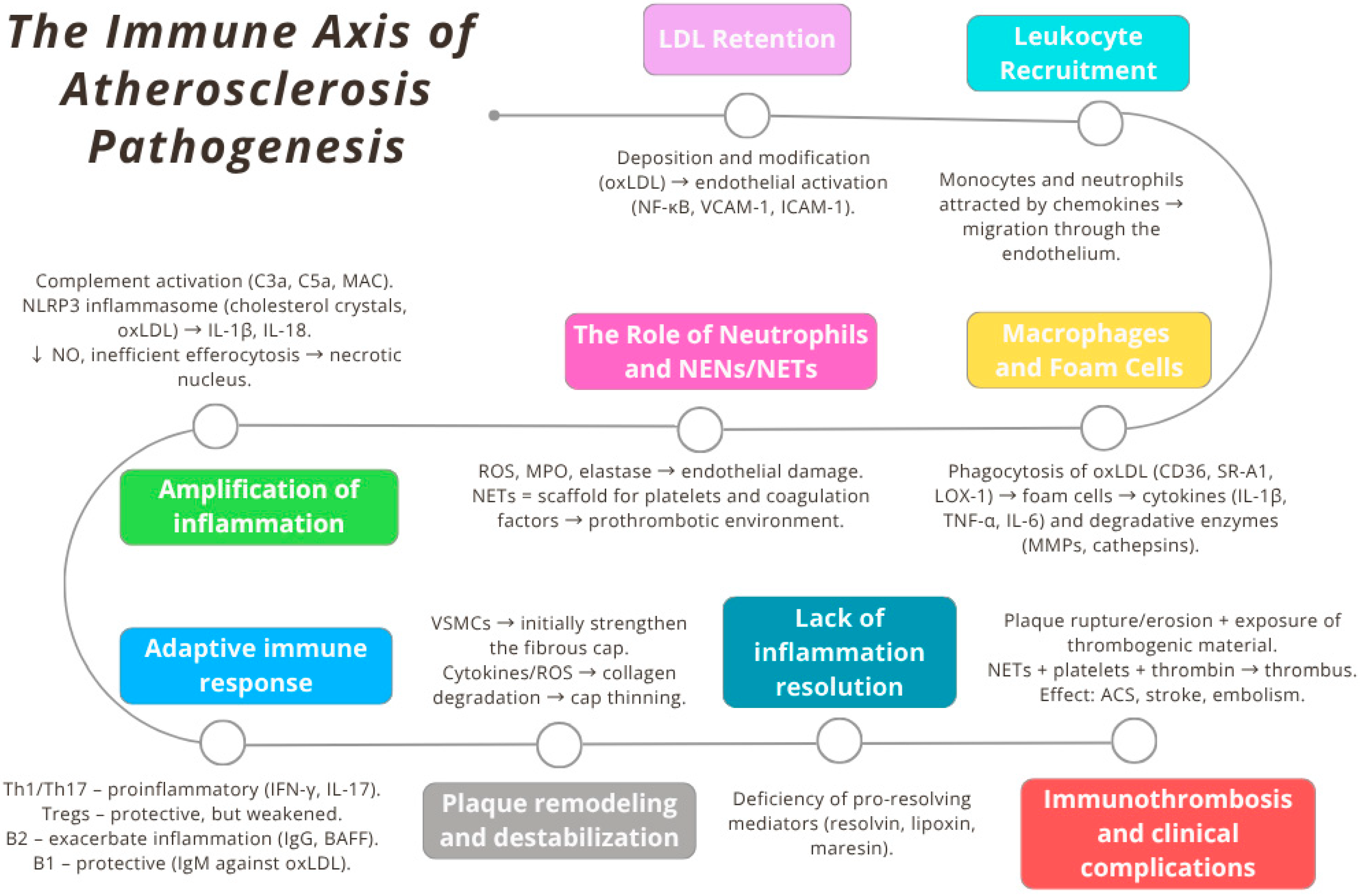
2. Immunological Basis of Atherosclerosis Pathogenesis: From Lipoprotein Retention to Immunothrombosis
3. Neutrophils and the Mechanisms of Formation of Neutrophil Extracellular Networks (NENs)
- Suicidal NETosis—a form of programmed cell death alternative to apoptosis, in which, within a few hours, massive nuclear envelope disintegration, chromatin decondensation, and cell lysis occur, with the release of DNA lattice. In this variant, the key source of ROS is NADPH oxidase (NOX2) [39,40,41].
- Additionally, mitochondrial NETosis (mtNETosis) has been described—a usually nonlytic variant in which oxidized mtDNA is exocytosed in vesicles; the process is essentially NOX2-independent, dependent on mROS and Ca2+ influx/mPTP opening, and often overlaps with the viable form [43,44,45] (Figure 3).
- ROS generation (NOX2 vs. mitochondria),
- NE and MPO translocation to the nucleus,
- histone modifications—including PAD4-catalyzed citrullination—leading to chromatin loosening,
- cytoskeletal rearrangement and disruption of the nuclear envelope integrity,
3.1. The oxLDL–NET Axis in the Initiation, Progression, and Destabilization of Atherosclerotic Plaque
3.2. NETs as Mediators of Endothelial Dysfunction and Immunothrombosis
4. NETs as a Bridge Between Pathophysiological Conditions and the Cardiovascular Phenotype
5. NET and Response to Applied Therapies
5.1. Pharmacotherapy
5.2. Procedural Interventions and the Ischemia–Reperfusion Context
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT (Akt) | serine/threonine protein kinase B |
| AMPK | AMP-activated protein kinase |
| Ang II | angiotensin II |
| CAD | coronary artery disease |
| CDKN1B (p27^Kip1) | cyclin-dependent kinase inhibitor 1B |
| cfDNA | cell-free DNA |
| CitH3 | citrullinated histone H3 |
| COX (COX-1) | cyclooxygenase (cyclooxygenase-1) |
| cGAS | cyclic GMP–AMP synthase |
| CVD | cardiovascular disease |
| DAMP(s) | damage-associated molecular pattern(s) |
| DNase | deoxyribonuclease |
| DNase I (dornase alfa) | deoxyribonuclease I |
| EC | endothelial cells |
| HDL | high-density lipoprotein |
| IL-1β | interleukin-1 beta |
| IL-1β/IL-18 | interleukin-1 beta/interleukin-18 |
| IsoLGs | isolevuglandins |
| LDL | low-density lipoprotein |
| Lp(a) | lipoprotein(a) |
| MPO | myeloperoxidase |
| NAC | N-acetylcysteine |
| NE | neutrophil elastase |
| NET | neutrophil extracellular trap(s) |
| NET/NETosis | neutrophil extracellular trap formation |
| NF-κB (NF-kappa B) | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 (inflammasome) |
| NO | nitric oxide |
| oxLDL | oxidized low-density lipoprotein |
| oxPL (OxPL) | oxidized phospholipids |
| P2Y12 | P2Y12 adenosine diphosphate receptor |
| PAD | peripheral artery disease |
| PAD4 | peptidyl arginine deiminase 4 |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PF4 | platelet factor 4 |
| P-selectin | platelet selectin |
| PSGL-1 | P-selectin glycoprotein ligand-1 |
| ROS | reactive oxygen species |
| TK1 | thymidine kinase 1 |
| TLR9 | Toll-like receptor 9 |
| TNF-α | tumor necrosis factor alpha |
| tPA | tissue plasminogen activator |
| TXA2 | thromboxane A2 |
| VSMC | vascular smooth muscle cells |
References
- Murray, C.J.L. The Global Burden of Disease Study at 30 Years. Nat. Med. 2022, 28, 2019–2026. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010-2022. Am. J. Prev. Med. 2024, 66, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in Atherosclerosis: Pathophysiology and Mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Wei, J.; Zheng, H.; Zhou, N.; Xu, X.; Deng, X.; Liu, T.; Zou, Y. The Immune System in Cardiovascular Diseases: From Basic Mechanisms to Therapeutic Implications. Signal Transduct. Target. Ther. 2025, 10, 166. [Google Scholar] [CrossRef]
- Romeo, M.; Silvestrin, A.; Senese, G.; Di Nardo, F.; Napolitano, C.; Vaia, P.; Coppola, A.; Federico, P.; Dallio, M.; Federico, A. From “Traditional” to “Trained” Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomedicines 2025, 13, 2004. [Google Scholar] [CrossRef]
- Riksen, N.P.; Bekkering, S.; Mulder, W.J.M.; Netea, M.G. Trained Immunity in Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 799–811. [Google Scholar] [CrossRef]
- Wang, Y.; Du, C.; Zhang, Y.; Zhu, L. Composition and Function of Neutrophil Extracellular Traps. Biomolecules 2024, 14, 416. [Google Scholar] [CrossRef]
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil Extracellular Traps: From Physiology to Pathology. Cardiovasc. Res. 2021, 118, 2737–2753. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Shetty, S.; Subramanian, M. Neutrophil Extracellular Traps (NETs) as Drivers of Atherosclerosis: Pathogenic Mechanisms and Therapeutic Opportunities. Pharmacol. Ther. 2025, 274, 108917. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and Atherosclerosis: Signaling Pathways and Therapeutic Intervention. Sig Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Gurgoglione, F.L.; Russo, A.; Rinaldi, R.; Torlai Triglia, L.; Foschi, M.; Vigna, C.; Vergallo, R.; Montone, R.A.; Benedetto, U.; et al. Coronary Artery Disease and Atherosclerosis in Other Vascular Districts: Epidemiology, Risk Factors and Atherosclerotic Plaque Features. Life 2025, 15, 1226. [Google Scholar] [CrossRef]
- Nappi, F.; Bellomo, F.; Avtaar Singh, S.S. Worsening Thrombotic Complication of Atherosclerotic Plaques Due to Neutrophils Extracellular Traps: A Systematic Review. Biomedicines 2023, 11, 113. [Google Scholar] [CrossRef]
- Pérez-Olivares, L.; Soehnlein, O. Contemporary Lifestyle and Neutrophil Extracellular Traps: An Emerging Link in Atherosclerosis Disease. Cells 2021, 10, 1985. [Google Scholar] [CrossRef]
- Mambo, A.; Yang, Y.; Mahulu, E.; Zihua, Z. Investigating the Interplay of Smoking, Cardiovascular Risk Factors, and Overall Cardiovascular Disease Risk: NHANES Analysis 2011–2018. BMC Cardiovasc. Disord. 2024, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Orecchioni, M.; Ley, K. How the Immune System Shapes Atherosclerosis: Roles of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; van der Vorst, E.P.C.; Weber, C. Targeting Immune Cell Recruitment in Atherosclerosis. Nat. Rev. Cardiol. 2024, 21, 824–840. [Google Scholar] [CrossRef] [PubMed]
- Clancy, D.M.; Henry, C.M.; Sullivan, G.P.; Martin, S.J. Neutrophil Extracellular Traps Can Serve as Platforms for Processing and Activation of IL-1 Family Cytokines. FEBS J. 2017, 284, 1712–1725. [Google Scholar] [CrossRef]
- Pi, H.; Wang, G.; Wang, Y.; Zhang, M.; He, Q.; Zheng, X.; Yin, K.; Zhao, G.; Jiang, T. Immunological Perspectives on Atherosclerotic Plaque Formation and Progression. Front. Immunol. 2024, 15, 1437821. [Google Scholar] [CrossRef]
- Maffia, P.; Mauro, C.; Case, A.; Kemper, C. Canonical and Non-Canonical Roles of Complement in Atherosclerosis. Nat. Rev. Cardiol. 2024, 21, 743–761. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial Dysfunction Due to eNOS Uncoupling: Molecular Mechanisms as Potential Therapeutic Targets. Cell Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Howangyin, K.-Y.; Zlatanova, I.; Pinto, C.; Ngkelo, A.; Cochain, C.; Rouanet, M.; Vilar, J.; Lemitre, M.; Stockmann, C.; Fleischmann, B.K.; et al. Myeloid-Epithelial-Reproductive Receptor Tyrosine Kinase and Milk Fat Globule Epidermal Growth Factor 8 Coordinately Improve Remodeling After Myocardial Infarction via Local Delivery of Vascular Endothelial Growth Factor. Circulation 2016, 133, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.; Grimaudo, S.; Pipitone, R.M.; Lori, G.; Raggi, C.; Petta, S.; Marra, F. Role of Myeloid-Epithelial-Reproductive Tyrosine Kinase and Macrophage Polarization in the Progression of Atherosclerotic Lesions Associated With Nonalcoholic Fatty Liver Disease. Front. Pharmacol. 2019, 10, 604. [Google Scholar] [CrossRef]
- Barcia Durán, J.G.; Das, D.; Gildea, M.; Amadori, L.; Gourvest, M.; Kaur, R.; Eberhardt, N.; Smyrnis, P.; Cilhoroz, B.; Sajja, S.; et al. Immune Checkpoint Landscape of Human Atherosclerosis and Influence of Cardiometabolic Factors. Nat. Cardiovasc. Res. 2024, 3, 1482–1502. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; Serhan, C.N. Specialized Pro-Resolving Mediators in Vascular Inflammation and Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2024, 21, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Hernanz, R.; García-Redondo, A.B.; Rodríguez, C.; Blanco-Colio, L.M.; Val-Blasco, A.; Alonso, M.J.; Salaices, M. Role of Inflammatory and Proresolving Mediators in Endothelial Dysfunction. Basic Clin. Pharmacol. Toxicol. 2025, 136, e70026. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, R.; Zhao, L.; Wang, Y.; Liu, G. Mechanisms of Neutrophil Extracellular Trap Formation and Regulation in Cancers. Int. J. Mol. Sci. 2023, 24, 10265. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil Extracellular Traps in Homeostasis and Disease. Sig Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Folco, E.J.; Mawson, T.L.; Vromman, A.; Bernardes-Souza, B.; Franck, G.; Persson, O.; Nakamura, M.; Newton, G.; Luscinskas, F.W.; Libby, P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1α and Cathepsin G. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1901–1912. [Google Scholar] [CrossRef]
- Mozzini, C.; Pagani, M. Cardiovascular Diseases: Consider Netosis. Curr. Probl. Cardiol. 2022, 47, 100929. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida Albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, F.; Fortier, M.; Portes, M.; Demattei, C.; Mousty, E.; Nouvellon, E.; Mercier, E.; Chea, M.; Letouzey, V.; Gris, J.-C.; et al. Vital NETosis vs. Suicidal NETosis during Normal Pregnancy and Preeclampsia. Front. Cell Dev. Biol. 2022, 10, 1099038. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular Mechanisms and Therapeutic Target of NETosis in Diseases. MedComm 2022, 3, e162. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Aziz, M.; Wang, P. The Vitals of NETs. J. Leukoc. Biol. 2021, 110, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Takishita, Y.; Yasuda, H.; Shimizu, M.; Matsuo, A.; Morita, A.; Tsutsumi, T.; Tsuchiya, M.; Sato, E.F. Formation of Neutrophil Extracellular Traps in Mitochondrial DNA-Deficient Cells. J. Clin. Biochem. Nutr. 2020, 66, 15–23. [Google Scholar] [CrossRef]
- Drury, B.; Chuah, C.S.; Hall, R.; Hardisty, G.R.; Rossi, A.G.; Ho, G.-T. Neutrophil-Dependent Mitochondrial DNA Release Associated With Extracellular Trap Formation in Inflammatory Bowel Disease. Gastro Hep Adv. 2023, 2, 788–798. [Google Scholar] [CrossRef]
- Azzouz, D.; Palaniyar, N. Mitochondrial ROS and Base Excision Repair Steps Leading to DNA Nick Formation Drive Ultraviolet Induced-NETosis. Front. Immunol. 2023, 14, 1198716. [Google Scholar] [CrossRef]
- Berger-Achituv, S.; Elhasid, R. Reduced Neutrophil Elastase Activity and Neutrophil Extracellular Traps in Pediatric Acute Myeloid Leukemia May Increase the Rate of Infections. J. Pediatr. Hematol. Oncol. 2018, 40, e248–e252. [Google Scholar] [CrossRef]
- Natorska, J.; Ząbczyk, M.; Undas, A. Neutrophil Extracellular Traps (NETs) in Cardiovascular Diseases: From Molecular Mechanisms to Therapeutic Interventions. Pol. Heart J. 2023, 81, 1205–1216. [Google Scholar] [CrossRef]
- Gu, C.; Pang, B.; Sun, S.; An, C.; Wu, M.; Wang, N.; Yuan, Y.; Liu, G. Neutrophil Extracellular Traps Contributing to Atherosclerosis: From Pathophysiology to Clinical Implications. Exp. Biol. Med. 2023, 248, 1302–1312. [Google Scholar] [CrossRef]
- Tang, Y.; Jiao, Y.; An, X.; Tu, Q.; Jiang, Q. Neutrophil Extracellular Traps and Cardiovascular Disease: Associations and Potential Therapeutic Approaches. Biomed. Pharmacother. 2024, 180, 117476. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Neutrophils and Neutrophil Extracellular Traps in Cardiovascular Disease: An Overview and Potential Therapeutic Approaches. Biomedicines 2022, 10, 1850. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, Y.; Yang, X.; Yan, C.; Feng, Y. Recent Insights into Neutrophil Extracellular Traps in Cardiovascular Diseases. J. Clin. Med. 2022, 11, 6662. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Bukrinsky, M.I.; Utkina, A.S.; Ravani, A.L.; Sukhorukov, V.N.; Orekhov, A.N. Exploring Neutrophil Extracellular Traps in Cardiovascular Pathologies: The Impact of Lipid Profiles, PAD4, and Radiation. Biocell 2025, 49, 931–959. [Google Scholar] [CrossRef]
- Gao, F.; Peng, H.; Gou, R.; Zhou, Y.; Ren, S.; Li, F. Exploring Neutrophil Extracellular Traps: Mechanisms of Immune Regulation and Future Therapeutic Potential. Exp. Hematol. Oncol. 2025, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Boyalla, V.; Gallego-Colon, E.; Spartalis, M. Immunity and Inflammation in Cardiovascular Disorders. BMC Cardiovasc. Disord. 2023, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Hu, T.; Wang, J.; Xiao, R.; Liao, X.; Liu, M.; Sun, Z. Systemic Immune-Inflammation Index as a Potential Biomarker of Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 933913. [Google Scholar] [CrossRef]
- Corriere, T.; Di Marca, S.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-Lymphocyte Ratio Is a Strong Predictor of Atherosclerotic Carotid Plaques in Older Adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 23–27. [Google Scholar] [CrossRef]
- Regolo, M.; Sorce, A.; Vaccaro, M.; Colaci, M.; Stancanelli, B.; Natoli, G.; Motta, M.; Isaia, I.; Castelletti, F.; Giangreco, F.; et al. Assessing Humoral Immuno-Inflammatory Pathways Associated with Respiratory Failure in COVID-19 Patients. J. Clin. Med. 2023, 12, 4057. [Google Scholar] [CrossRef]
- Obama, T.; Ohinata, H.; Takaki, T.; Iwamoto, S.; Sawada, N.; Aiuchi, T.; Kato, R.; Itabe, H. Cooperative Action of Oxidized Low-Density Lipoproteins and Neutrophils on Endothelial Inflammatory Responses Through Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 1899. [Google Scholar] [CrossRef] [PubMed]
- Ohinata, H.; Obama, T.; Makiyama, T.; Watanabe, Y.; Itabe, H. High-Density Lipoprotein Suppresses Neutrophil Extracellular Traps Enhanced by Oxidized Low-Density Lipoprotein or Oxidized Phospholipids. Int. J. Mol. Sci. 2022, 23, 13992. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL Induced Extracellular Trap Formation in Human Neutrophils via TLR-PKC-IRAK-MAPK and NADPH-Oxidase Activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef]
- Polacek, D.; Byrne, R.E.; Fless, G.M.; Scanu, A.M. In Vitro Proteolysis of Human Plasma Low Density Lipoproteins by an Elastase Released from Human Blood Polymorphonuclear Cells. J. Biol. Chem. 1986, 261, 2057–2063. [Google Scholar] [CrossRef]
- Mobilia, M.; Karakashian, A.; Neupane, K.R.; Hage, O.; Whitus, C.; Carter, A.; Voy, C.; Johnson, L.A.; Graf, G.A.; Gordon, S.M. Enhancement of High-Density Lipoprotein-Associated Protease Inhibitor Activity Prevents Atherosclerosis Progress. Atheroscler. 2024, 396, 118544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carmona-Rivera, C.; Moore, E.; Seto, N.L.; Knight, J.S.; Pryor, M.; Yang, Z.-H.; Hemmers, S.; Remaley, A.T.; Mowen, K.A.; et al. Myeloid-Specific Deletion of Peptidylarginine Deiminase 4 Mitigates Atherosclerosis. Front. Immunol. 2018, 9, 1680. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil Extracellular Traps License Macrophages for Cytokine Production in Atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Li, J.; Yu, J.; Wang, X.; Gao, H.; Zhang, W.; Wei, Z.; Zhang, J.; Zhang, Y.; Zhao, J.; et al. Neutrophil Extracellular Traps Induced by IL-8 Aggravate Atherosclerosis via Activation NF-κB Signaling in Macrophages. Cell Cycle 2019, 18, 2928–2938. [Google Scholar] [CrossRef]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of Interleukin-1beta Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef]
- Rao, V.H.; Kansal, V.; Stoupa, S.; Agrawal, D.K. MMP-1 and MMP-9 Regulate Epidermal Growth Factor-dependent Collagen Loss in Human Carotid Plaque Smooth Muscle Cells. Physiol. Rep. 2014, 2, e00224. [Google Scholar] [CrossRef]
- Tavora, F.R.; Ripple, M.; Li, L.; Burke, A.P. Monocytes and Neutrophils Expressing Myeloperoxidase Occur in Fibrous Caps and Thrombi in Unstable Coronary Plaques. BMC Cardiovasc. Disord. 2009, 9, 27. [Google Scholar] [CrossRef]
- Kawakami, R.; Finn, A.V.; Virmani, R. Can Myeloperoxidase Identify High-Risk Plaques and Subjects Harboring Them? JACC Adv. 2023, 2, 100313. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Wichapong, K.; Lee, E.Y.; Teulon, J.M.; Berrebeh, N.; Winter, J.; Adrover, J.M.; Santos, G.S.; Froese, A.; et al. Externalized Histone H4 Orchestrates Chronic Inflammation by Inducing Lytic Cell Death. Nature 2019, 569, 236–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, W.; He, L.; Wu, J. Externalized Histone H4: A Novel Target That Orchestrates Chronic Inflammation by Inducing Lytic Cell Death. Acta Biochim. Biophys. Sin. 2020, 52, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Meegan, J.E.; Yang, X.; Beard, R.S.; Jannaway, M.; Chatterjee, V.; Taylor-Clark, T.E.; Yuan, S.Y. Citrullinated Histone 3 Causes Endothelial Barrier Dysfunction. Biochem. Biophys. Res. Commun. 2018, 503, 1498–1502. [Google Scholar] [CrossRef]
- Blotnick, E.; Sol, A.; Muhlrad, A. Histones Bundle F-Actin Filaments and Affect Actin Structure. PLoS ONE 2017, 12, e0183760. [Google Scholar] [CrossRef] [PubMed]
- Giannotta, M.; Trani, M.; Dejana, E. VE-Cadherin and Endothelial Adherens Junctions: Active Guardians of Vascular Integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA Traps Promote Thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular Histones Promote Thrombin Generation through Platelet-Dependent Mechanisms: Involvement of Platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef]
- Eustes, A.S.; Ahmed, A.; Swamy, J.; Patil, G.; Jensen, M.; Wilson, K.M.; Kudchadkar, S.; Wahab, A.; Perepu, U.; Miller, F.J.; et al. Extracellular Histones: A Unifying Mechanism Driving Platelet-Dependent Extracellular Vesicle Release and Thrombus Formation in COVID-19. J. Thromb. Haemost. 2024, 22, 2514–2530. [Google Scholar] [CrossRef]
- Foltan, M.; Dinh, D.; Gruber, M.; Müller, T.; Hart, C.; Krenkel, L.; Schmid, C.; Lehle, K. Incidence of Neutrophil Extracellular Traps (NETs) in Different Membrane Oxygenators: Pilot in Vitro Experiments in Commercially Available Coated Membranes. J. Artif. Organs 2025, 28, 374–382. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Networks That Stop the Flow: A Fresh Look at Fibrin and Neutrophil Extracellular Traps—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0049384819303263 (accessed on 10 September 2025).
- von Brühl, M.-L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, Neutrophils, and Platelets Cooperate to Initiate and Propagate Venous Thrombosis in Mice in Vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of Functional Tissue Factor by Neutrophil Extracellular Traps in Culprit Artery of Acute Myocardial Infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Wienkamp, A.-K.; Erpenbeck, L.; Rossaint, J. Platelets in the NETworks Interweaving Inflammation and Thrombosis. Front. Immunol. 2022, 13, 953129. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shan, W.; Zhao, X.; Sun, W. Neutrophils: Linking Inflammation to Thrombosis and Unlocking New Treatment Horizons. Int. J. Mol. Sci. 2025, 26, 1965. [Google Scholar] [CrossRef]
- Sennett, C.; Pula, G. Trapped in the NETs: Multiple Roles of Platelets in the Vascular Complications Associated with Neutrophil Extracellular Traps. Cells 2025, 14, 335. [Google Scholar] [CrossRef]
- Peetermans, M.; Meyers, S.; Liesenborghs, L.; Vanhoorelbeke, K.; De Meyer, S.F.; Vandenbriele, C.; Lox, M.; Hoylaerts, M.F.; Martinod, K.; Jacquemin, M.; et al. Von Willebrand Factor and ADAMTS13 Impact on the Outcome of Staphylococcus Aureus Sepsis. J. Thromb. Haemost. 2020, 18, 722–731. [Google Scholar] [CrossRef]
- Sorvillo, N.; Mizurini, D.M.; Coxon, C.; Martinod, K.; Tilvawala, R.; Cherpokova, D.; Salinger, A.J.; Seward, R.J.; Staudinger, C.; Weerapana, E.; et al. Plasma Peptidylarginine Deiminase IV Promotes VWF-Platelet String Formation and Accelerates Thrombosis After Vessel Injury. Circ. Res. 2019, 125, 507–519. [Google Scholar] [CrossRef]
- Carestia, A.; Kaufman, T.; Rivadeneyra, L.; Landoni, V.I.; Pozner, R.G.; Negrotto, S.; D’Atri, L.P.; Gómez, R.M.; Schattner, M. Mediators and Molecular Pathways Involved in the Regulation of Neutrophil Extracellular Trap Formation Mediated by Activated Platelets. J. Leukoc. Biol. 2016, 99, 153–162. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Migliari Branco, L.; Franklin, B.S. Regulation of Innate Immune Responses by Platelets. Front. Immunol. 2019, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Grahl, L.; von Bruehl, M.-L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal Coupling of Coagulation and Innate Immunity via Neutrophil Serine Proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef]
- Ammollo, C.T.; Semeraro, F.; Xu, J.; Esmon, N.L.; Esmon, C.T. Extracellular Histones Increase Plasma Thrombin Generation by Impairing Thrombomodulin-Dependent Protein C Activation. J. Thromb. Haemost. 2011, 9, 1795–1803. [Google Scholar] [CrossRef]
- Barranco-Medina, S.; Pozzi, N.; Vogt, A.D.; Di Cera, E. Histone H4 Promotes Prothrombin Autoactivation. J. Biol. Chem. 2013, 288, 35749–35757. [Google Scholar] [CrossRef]
- Schoen, J.; Euler, M.; Schauer, C.; Schett, G.; Herrmann, M.; Knopf, J.; Yaykasli, K.O. Neutrophils’ Extracellular Trap Mechanisms: From Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 12855. [Google Scholar] [CrossRef]
- Malliora, A.; Lazaridis, A.; Natsi, A.-M.; Papadopoulos, V.; Antoniadou, C.; Gavriilidis, E.; Tsilingiris, D.; Kotsis, V.; Skendros, P.; Gkaliagkousi, E. Increasing Blood Pressure Predicts Levels of Circulating Neutrophil Extracellular Traps in Untreated Hypertensive Patients. Hypertens. Res. 2025, 48, 2688–2700. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, J.; Hennen, E.M.; Ao, M.; Kirabo, A.; Ahmad, T.; de la Visitación, N.; Patrick, D.M. NETosis Drives Blood Pressure Elevation and Vascular Dysfunction in Hypertension. Circ. Res. 2024, 134, 1483–1494. [Google Scholar] [CrossRef]
- Fang, X.; Ma, L.; Wang, Y.; Ren, F.; Yu, Y.; Yuan, Z.; Wei, H.; Zhang, H.; Sun, Y. Neutrophil Extracellular Traps Accelerate Vascular Smooth Muscle Cell Proliferation via Akt/CDKN1b/TK1 Accompanying with the Occurrence of Hypertension. J. Hypertens. 2022, 40, 2045–2057. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Chen, J.; Zhang, X.; Ma, Z.; Wang, J.; Wu, Q. Role of Neutrophil Extracellular Traps in Hypertension and Their Impact on Target Organs. J. Clin. Hypertens. 2025, 27, e14942. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, U.K.; Bhattacharya, P.; Narayanan, S.; Manickam, V.; Aggarwal, A.; Subramanian, M. Hypercholesterolemia Impairs Clearance of Neutrophil Extracellular Traps and Promotes Inflammation and Atherosclerotic Plaque Progression. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2598–2615. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; Fotakis, P.; Liu, W.; Endo-Umeda, K.; Dou, H.; Abramowicz, S.; Xiao, T.; Libby, P.; Wang, N.; Tall, A.R.; et al. Cholesterol Accumulation in Macrophages Drives NETosis in Atherosclerotic Plaques via IL-1β Secretion. Cardiovasc. Res. 2023, 119, 969–981. [Google Scholar] [CrossRef]
- Chen, J.; Wang, T.; Li, X.; Gao, L.; Wang, K.; Cheng, M.; Zeng, Z.; Chen, L.; Shen, Y.; Wen, F. DNA of Neutrophil Extracellular Traps Promote NF-κB-Dependent Autoimmunity via cGAS/TLR9 in Chronic Obstructive Pulmonary Disease. Sig Transduct. Target. Ther. 2024, 9, 163. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; He, B.; Xiao, J.; Yu, Q.; Xie, B.; Yang, S.; Dai, L.; Dai, Z.; Chen, Q. Neutrophil Extracellular Traps Induced by Cigarette Smoke Contribute to Airway Inflammation in Mice. Exp. Cell Res. 2020, 389, 111888. [Google Scholar] [CrossRef]
- Webster, D.A.; Orii, Y. Biphasic Recombination of Photodissociated CO Compound of Cytochrome o(s) from Vitreoscilla. J. Biol. Chem. 1985, 260, 15526–15529. [Google Scholar] [CrossRef]
- Qiu, S.-L.; Zhang, H.; Tang, Q.-Y.; Bai, J.; He, Z.-Y.; Zhang, J.-Q.; Li, M.-H.; Deng, J.-M.; Liu, G.-N.; Zhong, X.-N. Neutrophil Extracellular Traps Induced by Cigarette Smoke Activate Plasmacytoid Dendritic Cells. Thorax 2017, 72, 1084–1093. [Google Scholar] [CrossRef]
- Marino, F.; Tozzi, M.; Schembri, L.; Ferraro, S.; Tarallo, A.; Scanzano, A.; Legnaro, M.; Castelli, P.; Cosentino, M. Production of IL-8, VEGF and Elastase by Circulating and Intraplaque Neutrophils in Patients with Carotid Atherosclerosis. PLoS ONE 2015, 10, e0124565. [Google Scholar] [CrossRef]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, T.; Jakowitsch, J.; Panzenböck, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary Neutrophil Extracellular Trap Burden and Deoxyribonuclease Activity in ST-Elevation Acute Coronary Syndrome Are Predictors of ST-Segment Resolution and Infarct Size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Langseth, M.S.; Andersen, G.Ø.; Husebye, T.; Arnesen, H.; Zucknick, M.; Solheim, S.; Eritsland, J.; Seljeflot, I.; Opstad, T.B.; Helseth, R. Neutrophil Extracellular Trap Components and Myocardial Recovery in Post-Ischemic Acute Heart Failure. PLoS ONE 2020, 15, e0241333. [Google Scholar] [CrossRef]
- He, L.; Liu, R.; Yue, H.; Zhang, X.; Pan, X.; Sun, Y.; Shi, J.; Zhu, G.; Qin, C.; Guo, Y. Interaction between Neutrophil Extracellular Traps and Cardiomyocytes Contributes to Atrial Fibrillation Progression. Sig Transduct. Target. Ther. 2023, 8, 279. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, M.; Jiao, X.; Li, S.; Wang, D.; Zhan, Y.; Li, J.; Hao, Z.; Li, Q.; Liu, Y.; et al. Neutrophil Extracellular Traps Mediate the Crosstalk between Plaque Microenvironment and Unstable Carotid Plaque Formation. Exp. Mol. Med. 2024, 56, 1717–1735. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, D.; Bai, J.; Wen, J.; Shen, X. Neutrophil Extracellular Traps-Mediated Upregulation of HIF-1α Promotes Corneal Neovascularization. Tissue Cell 2025, 95, 102891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, M.; Liu, Q.; Liu, J.; Cui, Y. Neutrophil Extracellular Traps Induce Thrombogenicity in Severe Carotid Stenosis. Immun. Inflamm. Dis. 2021, 9, 1025–1036. [Google Scholar] [CrossRef]
- Celebi, S.; Berkalp, B.; Amasyali, B. The Association between Thrombotic and Inflammatory Biomarkers and Lower-Extremity Peripheral Artery Disease. Int. Wound J. 2020, 17, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, T.; Malinowski, K.; Natorska, J.; Undas, A. Neutrophil Extracellular Traps-Related Markers in Chronic Limb Threatening Ischemia—A Relation with Progression and Prognosis. Thromb. Res. 2025, 252, 109378. [Google Scholar] [CrossRef]
- Farkas, Á.Z.; Farkas, V.J.; Gubucz, I.; Szabó, L.; Bálint, K.; Tenekedjiev, K.; Nagy, A.I.; Sótonyi, P.; Hidi, L.; Nagy, Z.; et al. Neutrophil Extracellular Traps in Thrombi Retrieved during Interventional Treatment of Ischemic Arterial Diseases. Thromb. Res. 2019, 175, 46–52. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Tang, Y.; Wen, Z.; Peng, L.; Ci, X. Long-Term Cigarette Smoke Exposure Promotes Neutrophil Ferroptosis Resistance, Inducing Neutrophil Extracellular Trap Formation and Driving Glucocorticoid Resistance in Chronic Obstructive Pulmonary Disease. Research 2025, 8, 0751. [Google Scholar] [CrossRef]
- Espiritu, A.; O’Sullivan, K.M. A Web of Challenges: The Therapeutic Struggle to Target NETs in Disease. Int. J. Mol. Sci. 2025, 26, 4773. [Google Scholar] [CrossRef]
- Smith, E.R. Prevention of Cardiovascular Risk Factors: Moving Upstream. Can. J. Cardiol. 2010, 26, 7C. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Kirshenbaum, L.A. Circadian Regulated Control of Myocardial Ischemia-Reperfusion Injury. Trends Cardiovasc. Med. 2024, 34, 1–7. [Google Scholar] [CrossRef]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial Ischemia Reperfusion Injury—From Basic Science to Clinical Bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, W.; Shen, F.; Du, W.; Xu, Z.; Liu, Z. The Emerging Role of Neutrophil Extracellular Traps in Arterial, Venous and Cancer-Associated Thrombosis. Front. Cardiovasc. Med. 2021, 8, 786387. [Google Scholar] [CrossRef]
- Grajek, S.; Michalak, M.; Urbanowicz, T.; Olasińska-Wiśniewska, A. A Meta-Analysis Evaluating the Colchicine Therapy in Patients With Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 740896. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C. Low-Dose Aspirin for the Prevention of Atherosclerotic Cardiovascular Disease. Eur. Heart J. 2024, 45, 2362–2376. [Google Scholar] [CrossRef]
- Caudrillier, A.; Kessenbrock, K.; Gilliss, B.M.; Nguyen, J.X.; Marques, M.B.; Monestier, M.; Toy, P.; Werb, Z.; Looney, M.R. Platelets Induce Neutrophil Extracellular Traps in Transfusion-Related Acute Lung Injury. J. Clin. Investig. 2012, 122, 2661–2671. [Google Scholar] [CrossRef]
- Tilgner, J.; von Trotha, K.T.; Gombert, A.; Jacobs, M.J.; Drechsler, M.; Döring, Y.; Soehnlein, O.; Grommes, J. Aspirin, but Not Tirofiban Displays Protective Effects in Endotoxin Induced Lung Injury. PLoS ONE 2016, 11, e0161218. [Google Scholar] [CrossRef]
- Baldetti, L.; Melillo, F.; Moroni, F.; Gallone, G.; Pagnesi, M.; Venuti, A.; Beneduce, A.; Calvo, F.; Gramegna, M.; Godino, C.; et al. Meta-Analysis Comparing P2Y12 Inhibitors in Acute Coronary Syndrome. Am. J. Cardiol. 2020, 125, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Nassif, R.M.; Chalhoub, E.; Chedid, P.; Hurtado-Nedelec, M.; Raya, E.; Dang, P.M.-C.; Marie, J.-C.; El-Benna, J. Metformin Inhibits ROS Production by Human M2 Macrophages via the Activation of AMPK. Biomedicines 2022, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.-P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5’-Monophosphate-Activated Protein Kinase Promotes Macrophage Polarization to an Anti-Inflammatory Functional Phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef]
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; Vigili de Kreutzeberg, S.; Avogaro, A.; et al. The Antidiabetic Drug Metformin Blunts NETosis in Vitro and Reduces Circulating NETosis Biomarkers in Vivo. Acta Diabetol. 2018, 55, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Menegazzo, L.; Ciciliot, S.; Poncina, N.; Mazzucato, M.; Persano, M.; Bonora, B.; Albiero, M.; Vigili de Kreutzenberg, S.; Avogaro, A.; Fadini, G.P. NETosis Is Induced by High Glucose and Associated with Type 2 Diabetes. Acta Diabetol. 2015, 52, 497–503. [Google Scholar] [CrossRef]
- CIULLA, M.; MEAZZA, R.; ROBERTS, N.; BRANZI, G.; MAGRINI, F. A Percutaneous Approach to Cardiac Haemodynamics in Anaesthetised Rats. Cardiovasc. Res. 1989, 23, 21–24. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Kovanen, P.T.; Sahebkar, A. Regulating NETosis: An Emerging Facet of Statin Pleiotropy. Drug Discov. Today 2022, 27, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.; Natorska, J.; Zabczyk, M.; Zalewski, J.; Jawien, J.; Undas, A. The High-Dose Statin Treatment Reduces Neutrophil Extracellular Traps Formation in Patients with Coronary Artery Disease. Eur. Heart J. 2024, 45, ehae666.3293. [Google Scholar] [CrossRef]
- Adamidis, P.S.; Pantazi, D.; Moschonas, I.C.; Liberopoulos, E.; Tselepis, A.D. Neutrophil Extracellular Traps (NETs) and Atherosclerosis: Does Hypolipidemic Treatment Have an Effect? J. Cardiovasc. Dev. Dis. 2024, 11, 72. [Google Scholar] [CrossRef]
- Luquero, A.; Badimon, L.; Borrell-Pages, M. PCSK9 Functions in Atherosclerosis Are Not Limited to Plasmatic LDL-Cholesterol Regulation. Front. Cardiovasc. Med. 2021, 8, 639727. [Google Scholar] [CrossRef]
- Adcock, I.M.; Ito, K. Molecular Mechanisms of Corticosteroid Actions. Monaldi Arch. Chest Dis. 2000, 55, 256–266. [Google Scholar]
- Hayashi, R.; Wada, H.; Ito, K.; Adcock, I.M. Effects of Glucocorticoids on Gene Transcription. Eur. J. Pharmacol. 2004, 500, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Boivin, R.; Cano, P.; Murcia, Y.; Bazin, I.; Lavoie, J.-P. Neutrophil Extracellular Traps Are Downregulated by Glucocorticosteroids in Lungs in an Equine Model of Asthma. Respir. Res. 2017, 18, 207. [Google Scholar] [CrossRef]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 Activity Is Sufficient to Disrupt Mouse and Human NET Formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Jia, Y.; Jia, R.; Taledaohan, A.; Wang, Y.; Wang, Y. Structure–Activity Relationship of PAD4 Inhibitors and Their Role in Tumor Immunotherapy. Pharmaceutics 2024, 16, 335. [Google Scholar] [CrossRef]
- Martinod, K.; Witsch, T.; Farley, K.; Gallant, M.; Remold-O’Donnell, E.; Wagner, D.D. Neutrophil Elastase-Deficient Mice Form Neutrophil Extracellular Traps in an Experimental Model of Deep Vein Thrombosis. J. Thromb. Haemost. 2016, 14, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, P.; Mellhammar, L.; Rasmussen, M.; Inghammar, M.; Jesperson, S.; Månsson, F.; Economou Lundeberg, E.; Walles, J.; Wallberg, M.; Frigyesi, A.; et al. Aerosolized Dornase Alfa (DNase I) for the Treatment of Severe Respiratory Failure in COVID-19: A Randomized Controlled Trial. Open Forum Infect. Dis. 2025, 12, ofaf246. [Google Scholar] [CrossRef]
- Elrod, J.; Heuer, A.; Knopf, J.; Schoen, J.; Schönfeld, L.; Trochimiuk, M.; Stiel, C.; Appl, B.; Raluy, L.P.; Saygi, C.; et al. Neutrophil Extracellular Traps and DNases Orchestrate Formation of Peritoneal Adhesions. iScience 2023, 26, 108289. [Google Scholar] [CrossRef]
- Sainglers, W.; Khamwong, M.; Chareonsudjai, S. N-Acetylcysteine Inhibits NETs, Exhibits Antibacterial and Antibiofilm Properties and Enhances Neutrophil Function against Burkholderia Pseudomallei. Sci. Rep. 2025, 15, 29943. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sánchez, G.; Godínez-Méndez, L.A.; Fafutis-Morris, M.; Delgado-Rizo, V. Effect of Antioxidant Supplementation on NET Formation Induced by LPS In Vitro; the Roles of Vitamins E and C, Glutathione, and N-Acetyl Cysteine. Int. J. Mol. Sci. 2023, 24, 13162. [Google Scholar] [CrossRef]
- Khan, S.A.; Campbell, A.M.; Lu, Y.; An, L.; Alpert, J.S.; Chen, Q.M. N-Acetylcysteine for Cardiac Protection During Coronary Artery Reperfusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 752939. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Abbate, A.; Montecucco, F. Neutrophil Extracellular Traps and Cardiovascular Diseases: An Update. Cells 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Helseth, R.; Shetelig, C.; Andersen, G.Ø.; Langseth, M.S.; Limalanathan, S.; Opstad, T.B.; Arnesen, H.; Hoffmann, P.; Eritsland, J.; Seljeflot, I. Neutrophil Extracellular Trap Components Associate with Infarct Size, Ventricular Function, and Clinical Outcome in STEMI. Mediat. Inflamm. 2019, 2019, 7816491. [Google Scholar] [CrossRef] [PubMed]
- Ferré-Vallverdú, M.; Latorre, A.M.; Fuset, M.P.; Sánchez, E.; Madrid, I.; Ten, F.; Vallés, J.; Santos, M.T.; Bonanad, S.; Moscardó, A. Neutrophil Extracellular Traps (NETs) in Patients with STEMI. Association with Percutaneous Coronary Intervention and Antithrombotic Treatments. Thromb. Res. 2022, 213, 78–83. [Google Scholar] [CrossRef]
- Vaidya, K.; Tucker, B.; Kurup, R.; Khandkar, C.; Pandzic, E.; Barraclough, J.; Machet, J.; Misra, A.; Kavurma, M.; Martinez, G.; et al. Colchicine Inhibits Neutrophil Extracellular Trap Formation in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2021, 10, e018993. [Google Scholar] [CrossRef]
- Farhan, A.; Hassan, G.; Ali, S.H.L.; Yousaf, Z.; Shafique, K.; Faisal, A.; Younis, B.B.; Mirza, S. Spontaneous NETosis in Diabetes: A Role of Hyperglycemia Mediated ROS and Autophagy. Front. Med. 2023, 10, 1076690. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Neagoe, P.-E.; Gagnon, D.; Denault, A.Y.; Sirois, M.G. Increased Circulating Levels of Neutrophil Extracellular Traps During Cardiopulmonary Bypass. CJC Open 2019, 2, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Aldemir, M.; Adalı, F.; Çarşanba, G.; Tecer, E.; Bakı, E.D.; Taş, H.U. Comparison of Neutrophil:Lymphocyte Ratios Following Coronary Artery Bypass Surgery with or without Cardiopulmonary Bypass. Cardiovasc. J. Afr. 2015, 26, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.R.; Stuckey, T.D.; Wall, T.C.; Kissling, G.; Hansen, C.J.; Muncy, D.B.; Weintraub, R.A.; Kelly, T.A. Importance of Time to Reperfusion for 30-Day and Late Survival and Recovery of Left Ventricular Function after Primary Angioplasty for Acute Myocardial Infarction. J. Am. Coll. Cardiol. 1998, 32, 1312–1319. [Google Scholar] [CrossRef]
- Liu, Y.; Suarez-Arnedo, A.; Shetty, S.; Wu, Y.; Schneider, M.; Collier, J.H.; Segura, T. A Balance between Pro-Inflammatory and Pro-Reparative Macrophages Is Observed in Regenerative D-MAPS. Adv. Sci. 2023, 10, 2204882. [Google Scholar] [CrossRef]
- Vignoli, A.; Sticchi, E.; Piccardi, B.; Palumbo, V.; Sarti, C.; Sodero, A.; Arba, F.; Fainardi, E.; Gori, A.M.; Giusti, B.; et al. Predicting Reperfusion Injury and Functional Status after Stroke Using Blood Biomarkers: The STROKELABED Study. J. Transl. Med. 2025, 23, 491. [Google Scholar] [CrossRef]
- Helseth, R.; Solheim, S.; Arnesen, H.; Seljeflot, I.; Opstad, T.B. The Time Course of Markers of Neutrophil Extracellular Traps in Patients Undergoing Revascularisation for Acute Myocardial Infarction or Stable Angina Pectoris. Mediat. Inflamm. 2016, 2016, 2182358. [Google Scholar] [CrossRef] [PubMed]




| Feature/Characteristic | NETs (Classic Neutrophil Extracellular Traps) | NENs (Broader Definition of Neutrophil Extracellular Networks) |
|---|---|---|
| Basic Material | Decondensed nuclear chromatin (DNA, histones H2A/H2B/H3/H4) and granule proteins (MPO, NE, cathepsin G, LL-37) | Similar molecular composition (DNA, histones, proteolytic and antimicrobial proteins), but this concept also encompasses potentially diverse network variants with distinct architecture and composition |
| Release mechanism | NETosis in its classic variants: suicidal, vital, and mitochondrial, involving ROS, PAD4, and cell membrane disintegration. | The term encompasses both classic NET forms and other, network-like extracellular structures of neutrophils, the formation of which does not necessarily correspond to the full mechanisms of NETosis. |
| Immunological consequences | High pro-inflammatory properties, generation of immuno-inflammatory signals, presentation of autoantigens, initiation and maintenance of thrombosis | Similar immunological effects, but the network connection with immune cells and matrix elements is emphasized, indicating the integrative nature of the inflammatory response. |
| Role in pathophysiology | Well-documented role in autoimmune diseases, cancer, thrombotic processes, and in the pathogenesis of cardiovascular diseases | Conceptually useful for describing the spatial organization and multifunctionality of neutrophil structures, but less clearly characterized mechanistically |
| Removal/Degradation | Degradation by DNases (DNase I, DNase1L3), phagocytosis of fragments by macrophages, and other cellular “clearing” mechanisms. | The degradation mechanisms are analogous, but in the context of more extensive networks, removal failure (defect in NEN clearing) may potentially be more important. |
| Research and clinical applications | Used as a source of biomarkers: cfDNA, MPO–DNA complexes, citrullinated histones (CitH3), neutrophil elastase (NE) | Potential for expanding analyses to a holistic approach, encompassing network organization as an integral element of immunopathology and new translational strategies |
| Unit/State | Main Mechanisms Related to NET | Biomarkers/Laboratory Changes | Vascular/Clinical Consequences | References |
|---|---|---|---|---|
| Arterial hypertension | EC activation and impairment of vascular function by histones; VSMC proliferation (Akt/CDKN1b/TK1); enhancement of neutropenia by Ang II, isolevuglandins | ↓ pressure and better relaxation | Increased peripheral resistance; enhancement of the inflammatory–prothrombotic axis | [94,95,96,97] |
| Dyslipidemia | ↑ NET creation by oxLDL/OxPL; ↓ clearance (DNase); IL-1β from NLRP3 induces netosis; HDL inhibits netosis | ↑ NET accumulation; pro-inflammatory profile (IL-1β) | Perpetuation of intima inflammation; facilitation of LDL modification and plaque progression | [59,98,99] |
| Exposure to tobacco smoke | ROS-dependent NETosis; cGAS/TLR9 → NF-KB; neutrophil reprogramming (ferroptosis, “vital” NETosis); ↓DNase | ↑ NET and pro-inflammatory mediators; ↓ DNase activity | Chronic inflammation, endothelial dysfunction, and increased susceptibility to immunothrombosis | [100,101,102,103] |
| CAD | NETs in plaques and thrombosis; antithrombolytic properties; potential induction of cardiomyocyte autophagy/apoptosis | High NET content in thrombi; presence of NE | Worse prognosis with high NET burden; difficult clot lysis; myocardial damage | [104,105,106,107] |
| Carotid artery disease | NETs stimulate angiogenesis, shifting the coagulation/thrombolysis balance toward coagulation. | — | Plaque destabilization (neovascularization); increased risk of thrombotic events | [108,109,110] |
| PAD | Atherogenic and prothrombotic effects of NETs; presence of NETs in thrombotic material | ↑ MPO, CitH3, cfDNA; DNA in clots | Increasing the severity of PAD promotes thrombosis in the micro- and macrocirculation. | [111,112,113] |
| Class/Drug | NET Axis | Primary Grip Point/Mechanism | Expected Effect on NET | Key Risks/Considerations | References |
|---|---|---|---|---|---|
| Colchicine | Upstream | Microtubule stabilization; ↓ inflammasome (IL-1β/IL-18); ↓ neutrophil recruitment | ↓ frequency of episodes of netosis | Gastrointestinal intolerance | [120] |
| Aspirin | Upstream | ↓ COX (mainly COX-1) →↓ TXA2; attenuation of the P-selectin–PSGL-1 axis and platelet signals (PF4) | ↓ platelet–neutrophil interaction→ ↓ NET | Bleeding (esp. long-term/focused therapy) | [121,122,123] |
| P2Y12 inhibitors (ticagrelor/prasugrel/clopidogrel) | Upstream | Block P2Y12 → ↓ platelet activation; (ticagrelor: effect on the adenosine system) | ↓ NET triggering signals from the plates | Bleeding; differences between molecules | [124] |
| Metformin | Upstream | AMPK → ↓ ROS (mitochondria); ↓ pro-inflammatory cytokines; ↓ glycemia | ↓ ROS-dependent neutropenia and hyperglycemia | Gastrointestinal complaints; rarely lactic acidosis | [125,126,127,128,129,130,131] |
| Statins | Upstream | ↓ LDL/oxLDL; pleiotropy: antioxidation, NO improvement | ↓ DAMP stimuli; indirectly ↓ NET | Myopathy; ↑ liver enzymes (rare) | [132,133] |
| PCSK9 inhibitors | Upstream | Deep reduction in LDL/Lp(a) → ↓ oxPL/DAMP | Potentially ↓ NET (indirectly) | Cost; injections | [134,135] |
| Dexamethasone | Upstream | Inhibition of TNF-α/IL-6; enhancement of anti-inflammatory signals | ↓ NETosis in models | Immunosuppression (infections, sepsis) | [136,137,138] |
| PAD4 inhibitors (e.g., cl-amidine) | Upstream/Midstream | Block histone citrullination → inhibition of chromatin decondensation | ↓ The formation of NET | No clinical data on CVD | [139,140] |
| NE/MPO inhibitors (e.g., sivelestat/MPO candidate) | Midstream | ↓ cytotoxicity of NET-related proteases/oxidases | ↓ EC/matrix damage; ↓ prothrombotic | Limited CVD data; particle safety | [13,32,141] |
| Heparin/heparinoids | Midstream | Histone binding; neutralization of toxicity | ↓ endothelial damage; partially “anti-NET” | Bleeding; HIT (heparin) | [47] |
| DNase I (dornase alfa) ± tPA | Downstream | Degradation of NET DNA-scaffolds; synergism with fibrinolysis | ↑ NET clearance; ↑ NET-rich clot lysis | No routine vascular use | [142,143] |
| N-acetylcysteine (NAC) | Upstream/Downstream | Antioxidant; disruption of NET protein disulfide bonds | ↓ ROS-NET; easier clearance | Good tolerability; side effects rare | [144,145,146] |
| Treatment Phase | Intervention (Examples) | Effect on Neutrophil/Platelet Activation | Effect on NET Dynamics | Expected Clinical Signal | Notes/Limitations |
|---|---|---|---|---|---|
| Before (PCI/CABG) | Rapid platelet inhibition (full P2Y12 saturation); statin loading dose; glycemia, oxygenation control | ↓ initial activation of neutrophils/platelets → smaller IR-burst | cfDNA, MPO–DNA, CitH3, NE; troponins | Lower risk of no-reflow/distal embolization | Risk of bleeding with intense antiaggregation |
| Before (selected cases) | Colchicine (if compatible with CVD indications) | ↓ inflammatory priming → ↓ NET releases | jcfDNA, MPO–DNA, CitH3, NE; troponins and CRP/IL-6 (inflammatory background) | Potentially milder IR peak | Individual qualification: GI tolerance |
| Intraprocedural PCI | Adequate heparinization; gentle work on the clot, minimizing embolization; no-reflow treatment (microvasodilators) | ↓ platelet activation and neutrophil adhesion; EC protection | ACT/anti-Xa; perfusion parameters (TIMI/MBG/IMR) | Better microcirculation perfusion | Heparin: + histone binding effect (partial neutralization) |
| Intraprocedural CABG | Biocompatible circuits; shortening CPB time; considering off-pump | ↓ generalized neutrophil/complement activation | hemodynamics; hemolysis/complement markers | Smaller NET system burst | Requires experience of the center/team |
| After treatment (0–48 h) | Continue DAPT; maintain NO/endothelial protection; tight glycemic control | Suppression of secondary neutropenia stimuli; protection of the EC barrier | cfDNA, MPO–DNA, CitH3 at 0–3 h and 24–48 h; troponins | IR peak declines; perfusion stabilizes | Bleeding risk balance |
| After treatment (7–14 days) | Supporting clearance: circulatory rehabilitation, lipid optimization, NO; (research: DNase I, histone neutralization, NE/MPO inhibitors) | ↑ network breakdown/clearance; inflammation quenching | cfDNA/MPO–DNA/CitH3/NE | Decrease in NET markers (resolution) | Mainly translational approaches |
| Long term (1–4 months) | Lipid optimization (statins/PCSK9), glycemic control; lifestyle modification | ↓ DAMP/ROS stimuli → stable NET reduction | periodically: NET panel + lipid profile/glycemia | Sustained decrease in NET; plaque stabilization | The continuing decline in NET |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, Z.; Staniewski, A.; Karpiński, M.; Zalewska, K.; Kalus, O.; Gramala, Z.; Maćkowiak, J.; Mertowski, S.; Filipiak, K.J.; Rahnama-Hezavah, M.; et al. The Role of Neutrophil Extracellular Networks in Cardiovascular Pathology. Cells 2025, 14, 1562. https://doi.org/10.3390/cells14191562
Szymańska Z, Staniewski A, Karpiński M, Zalewska K, Kalus O, Gramala Z, Maćkowiak J, Mertowski S, Filipiak KJ, Rahnama-Hezavah M, et al. The Role of Neutrophil Extracellular Networks in Cardiovascular Pathology. Cells. 2025; 14(19):1562. https://doi.org/10.3390/cells14191562
Chicago/Turabian StyleSzymańska, Zofia, Antoni Staniewski, Michał Karpiński, Katarzyna Zalewska, Oliwia Kalus, Zofia Gramala, Joanna Maćkowiak, Sebastian Mertowski, Krzysztof J. Filipiak, Mansur Rahnama-Hezavah, and et al. 2025. "The Role of Neutrophil Extracellular Networks in Cardiovascular Pathology" Cells 14, no. 19: 1562. https://doi.org/10.3390/cells14191562
APA StyleSzymańska, Z., Staniewski, A., Karpiński, M., Zalewska, K., Kalus, O., Gramala, Z., Maćkowiak, J., Mertowski, S., Filipiak, K. J., Rahnama-Hezavah, M., Grywalska, E., & Urbanowicz, T. (2025). The Role of Neutrophil Extracellular Networks in Cardiovascular Pathology. Cells, 14(19), 1562. https://doi.org/10.3390/cells14191562







