Exercise, Epigenetics, and Body Composition: Molecular Connections
Abstract
1. Introduction
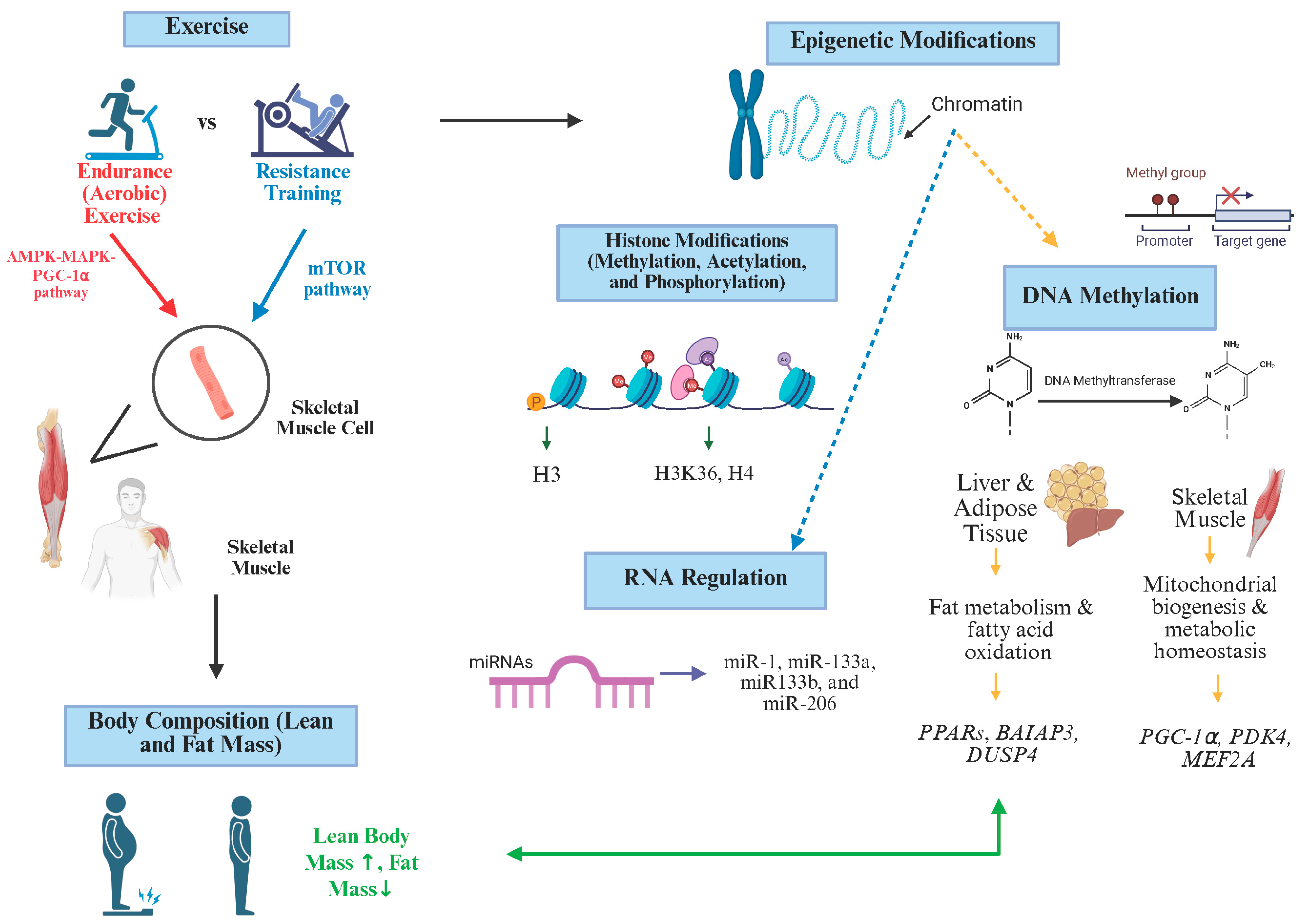
2. The Role of Exercise in Body Composition
3. Exercise and Epigenetic Regulation
3.1. DNA Methylation and Exercise
3.2. Histone Modification and Exercise
3.3. MicroRNAs (miRNAs) and Exercise
4. The Interaction Between Body Composition and Epigenetic Regulation
4.1. Fat Mass
4.2. Lean Body Mass and Skeletal Muscle
4.3. Bone and Other Components
5. Limitations, Contradictions, and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FM | Fat Mass |
| WHO | World Health Organization |
| T2DM | Type 2 diabetes |
| LBM | Lean body mass |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| PKA | Protein kinase A |
| CaMK | Calcium/calmodulin-dependent protein kinase |
| MAPK | Mitogen-activated protein kinase |
| PKC | Protein kinase C |
| mTOR | Mammalian target of rapamycin |
| PGC-1α | Peroxisome proliferator activated receptor gamma coactivator 1-alpha |
| CREB | Cyclic AMP response element-binding protein |
| MEF2-HDAC | Myocyte enhancer factor 2-histone deacetylase |
| BMI | Body mass index |
| AVG | Active video games |
| DMNT | DNA methyltransferase |
| ROS | Reactive oxygen species |
| SAM | S-adenosyl methionine |
| PDK4 | Pyruvate dehydrogenase kinase |
| TFAM | Mitochondrial transcription factor A |
| MEF2A | Myocyte enhancer factor 2A |
| FAIM2 | Fas apoptotic inhibitory molecule 2 |
| HSB11B2 | Hydroxysteroid (11-beta) dehydrogenase 2 |
| SOD | Superoxide dismutase |
| GPX | Glutathione peroxidase |
| HATs | Histone acetyl transferase |
| HDAC | Histone deacetylase |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| EWAS | Epigenome-wide association studies |
| PPARs | Peroxisome proliferator activated receptor |
| DUSP4 | Dual-specificity phosphatase 4 |
| BAIAP3 | BAI1 associated protein 3 |
References
- Widmann, M.; Nieß, A.M.; Munz, B. Physical Exercise and Epigenetic Modifications in Skeletal Muscle. Sports Med. 2019, 49, 509–523. [Google Scholar] [CrossRef]
- Hechanova, R.L.; Wegler, J.L.; Forest, C.P. Exercise. J. Am. Acad. Physician Assist. 2017, 30, 17–22. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Activity. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 26 June 2024).
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical Activity, Exercise, and Physical Fitness: Definitions and Distinctions for Health-Related Research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z. Exercise, PGC-1α, and Metabolic Adaptation in Skeletal Muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 424–427. [Google Scholar] [CrossRef]
- Petracci, I.; Gabbianelli, R.; Bordoni, L. The Role of Nutri(Epi)Genomics in Achieving the Body’s Full Potential in Physical Activity. Antioxidants 2020, 9, 498. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Bredin, S.S.D. Reflections on Physical Activity and Health: What Should We Recommend? Can. J. Cardiol. 2016, 32, 495–504. [Google Scholar] [CrossRef]
- CDC. Physical Activity for Children: An Overview. Physical Activity Basics. Available online: https://www.cdc.gov/physical-activity-basics/guidelines/children.html (accessed on 27 March 2025).
- Landry, B.W.; Driscoll, S.W. Physical Activity in Children and Adolescents. PM&R 2012, 4, 826–832. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; Rebullido, T.R.; MacDonald, J.P. Pediatric Inactivity Triad. Curr. Sports Med. Rep. 2018, 17, 45–47. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Brown, J.; Ramirez, S. Are Lean Body Mass and Fat-Free Mass the Same or Different Body Components? A Critical Perspective. Adv. Nutr. 2024, 15, 100335. [Google Scholar] [CrossRef]
- Westerterp, K.R. Changes in Physical Activity over the Lifespan: Impact on Body Composition and Sarcopenic Obesity. Obes. Rev. 2018, 19, 8–13. [Google Scholar] [CrossRef]
- Maruszczak, K.; Kasperek, W.; Kustra, K.; Baran, J.; Kochman, M. Exploring the Science of Shape: How Physical Activity, Sleep, and Stress Affect Body Composition. Healthcare 2025, 13, 949. [Google Scholar] [CrossRef]
- Pomeroy, E.; Macintosh, A.; Wells, J.C.K.; Cole, T.J.; Stock, J.T. Relationship between Body Mass, Lean Mass, Fat Mass, and Limb Bone Cross-Sectional Geometry: Implications for Estimating Body Mass and Physique from the Skeleton. Am. J. Phys. Anthropol. 2018, 166, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Rascon, J.; Trujillo, E.; Morales Acuna, F.J.; Gurovich, A.N. Differences in Determining Exercise Intensity in Males and Females. Med. Sci. Sports Exerc. 2019, 51, 765–766. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Yu, Y.; Xin, H.; Zhang, H.; Liu, J.; Zhang, Z. Associations between Body Composition and Physical Fitness among Chinese Medical Students: A Cross-Sectional Study. BMC Public Health 2022, 22, 2041. [Google Scholar] [CrossRef]
- Marriott, B.M.; Grumstrup-Scott, J.; Institute of Medicine (U.S.). Committee On Military Nutrition Research. In Body Composition and Physical Performance: Applications for the Military Services; National Academy Press: Washington, DC, USA, 1992. [Google Scholar]
- Lombardo, M.; Feraco, A.; Armani, A.; Camajani, E.; Gorini, S.; Strollo, R.; Padua, E.; Caprio, M.; Bellia, A. Gender Differences in Body Composition, Dietary Patterns, and Physical Activity: Insights from a Cross-Sectional Study. Front. Nutr. 2024, 11, 1414217. [Google Scholar] [CrossRef]
- Lee, S.J.; Arslanian, S.A. Cardiorespiratory Fitness and Abdominal Adiposity in Youth. Eur. J. Clin. Nutr. 2006, 61, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Slotte, S.; Kukkonen-Harjula, K.; Rinne, M.; Valtonen, J.; Rintala, P. Associations of Muscular Fitness and Body Composition in Children. Early Child Dev. Care 2021, 192, 2078–2086. [Google Scholar] [CrossRef]
- Kasović, M.; Oreški, A.; Vespalec, T.; Gimunová, M.; Štefan, L. Associations between Fat Mass and Fat Free Mass with Physical Fitness in Adolescent Girls: A 3-Year Longitudinal Study. Biology 2022, 11, 783. [Google Scholar] [CrossRef]
- Liao, J.; Hu, M.; Imm, K.; Holmes, C.J.; Zhu, J.; Cao, C.; Yang, L. Association of Daily Sitting Time and Leisure-Time Physical Activity with Body Fat among US Adults. J. Sport Health Sci. 2022, 13, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Artero, E.G.; España-Romero, V.; Ortega, F.B.; Jiménez-Pavón, D.; Ruiz, J.R.; Vicente-Rodríguez, G.; Bueno, M.; Marcos, A.; Gómez-Martínez, S.; Urzanqui, A.; et al. Health-Related Fitness in Adolescents: Underweight, and Not Only Overweight, as an Influencing Factor. The AVENA Study. Scand. J. Med. Sci. Sports 2009, 20, 418–427. [Google Scholar] [CrossRef]
- Moliner-Urdiales, D.; Ruiz, J.R.; Vicente-Rodriguez, G.; Ortega, F.B.; Rey-Lopez, J.P.; Espana-Romero, V.; Casajus, J.A.; Molnar, D.; Widhalm, K.; Dallongeville, J.; et al. Associations of Muscular and Cardiorespiratory Fitness with Total and Central Body Fat in Adolescents: The HELENA Study. Br. J. Sports Med. 2009, 45, 101–108. [Google Scholar] [CrossRef]
- Liu, K.; Animesh, A.; Hinz, C.; Liggi, S.; Murgia, A.; Denes, J.; Gulston, M.K.; Wang, X.; Chu, Y.; West, J.A.; et al. Consequences of Lipid Remodeling of Adipocyte Membranes Being Functionally Distinct from Lipid Storage in Obesity. J. Proteome Res. 2020, 19, 3919–3935. [Google Scholar] [CrossRef]
- Dupont, C.; Armant, D.R.; Brenner, C. Epigenetics: Definition, Mechanisms and Clinical Perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- CDC. Epigenetics, Health, and Disease. Genomics and Your Health. Available online: https://www.cdc.gov/genomics-and-health/epigenetics/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fgenomics-and-health%2Fabout%2Fepigenetic-impacts-on-health.html (accessed on 27 March 2025).
- Światowy, W.J.; Drzewiecka, H.; Kliber, M.; Sąsiadek, M.; Karpiński, P.; Pławski, A.; Jagodziński, P.P. Physical Activity and DNA Methylation in Humans. Int. J. Mol. Sci. 2021, 22, 12989. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Izquierdo, D.; Torres-Martos, Á.; Baig, A.T.; Aguilera, C.M.; Ruiz-Ojeda, F.J. Impact of Physical Activity and Exercise on the Epigenome in Skeletal Muscle and Effects on Systemic Metabolism. Biomedicines 2022, 10, 126. [Google Scholar] [CrossRef]
- Geiger, C.; Needhamsen, M.; Emanuelsson, E.B.; Norrbom, J.; Steindorf, K.; Sundberg, C.J.; Reitzner, S.M.; Lindholm, M.E. DNA Methylation of Exercise-Responsive Genes Differs between Trained and Untrained Men. BMC Biol. 2024, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Tian, H.; Qiao, H.; Han, F.; Kong, X.; Zhu, S.; Xing, F.; Duan, H.; Li, W.; Wang, W.; Zhang, D.; et al. Genome-Wide DNA Methylation Analysis of Body Composition in Chinese Monozygotic Twins. Eur. J. Clin. Investig. 2023, 53, e14055. [Google Scholar] [CrossRef]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P.; et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Brown, A.M.; Frontera, W.R. Principles of Exercise Physiology: Responses to Acute Exercise and Long-Term Adaptations to Training. PMR 2012, 4, 797–804. [Google Scholar] [CrossRef]
- Hoffman, N.J.; Parker, B.L.; Chaudhuri, R.; Fisher-Wellman, K.H.; Kleinert, M.; Humphrey, S.J.; Yang, P.; Holliday, M.; Trefely, S.; Fazakerley, D.J.; et al. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 2015, 22, 922–935. [Google Scholar] [CrossRef]
- Gowans, G.J.; Hawley, S.A.; Ross, F.A.; Hardie, D.G. AMP Is a True Physiological Regulator of AMP-Activated Protein Kinase by Both Allosteric Activation and Enhancing Net Phosphorylation. Cell Metab. 2013, 18, 556–566. [Google Scholar] [CrossRef]
- Chin, E.R. Intracellular Ca2+ Signaling in Skeletal Muscle: Decoding a Complex Message. Exerc. Sport Sci. Rev. 2010, 38, 76–85. [Google Scholar] [CrossRef]
- McKinsey, T.A.; Zhang, C.L.; Olson, E.N. Activation of the Myocyte Enhancer Factor-2 Transcription Factor by Calcium/Calmodulin-Dependent Protein Kinase-Stimulated Binding of 14-3-3 to Histone Deacetylase 5. Proc. Natl. Acad. Sci. USA 2000, 97, 14400–14405. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.B.; Richter, E.A.; Wojtaszewski, J.F.P. Role of AMPK in Skeletal Muscle Metabolic Regulation and Adaptation in Relation to Exercise. J. Physiol. 2006, 574 Pt 1, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, S.P.; Stocks, B.; Egan, B.; Zierath, J.R. Exercise Induces Tissue-Specific Adaptations to Enhance Cardiometabolic Health. Cell Metab. 2024, 36, 278–300. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C.; Akimoto, T.; Blaauw, B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J. Neuromuscul. Dis. 2020, 8, 169–183. [Google Scholar] [CrossRef]
- Jaremków, A.; Markiewicz-Górka, I.; Hajdusianek, W.; Czerwińska, K.; Gać, P. The Relationship between Body Composition and Physical Activity Level in Students of Medical Faculties. J. Clin. Med. 2023, 13, 50. [Google Scholar] [CrossRef]
- Savikangas, T.; Tirkkonen, A.; Alen, M.; Rantanen, T.; Fielding, R.A.; Rantalainen, T.; Sipilä, S. Associations of Physical Activity in Detailed Intensity Ranges with Body Composition and Physical Function. A Cross-Sectional Study among Sedentary Older Adults. Eur. Rev. Aging Phys. Act. 2020, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Dewi, R.C.; Rimawati, N.; Purbodjati, P. Body Mass Index, Physical Activity, and Physical Fitness of Adolescence. J. Public Health Res. 2021, 10, 2230. [Google Scholar] [CrossRef] [PubMed]
- Comeras-Chueca, C.; Villalba-Heredia, L.; Perez-Lasierra, J.L.; Marín-Puyalto, J.; Lozano-Berges, G.; Matute-Llorente, Á.; Vicente-Rodríguez, G.; Gonzalez-Aguero, A.; Casajús, J.A. Active Video Games Improve Muscular Fitness and Motor Skills in Children with Overweight or Obesity. Int. J. Environ. Res. Public Health 2022, 19, 2642. [Google Scholar] [CrossRef]
- Comeras-Chueca, C.; Villalba-Heredia, L.; Pérez-Llera, M.; Lozano-Berges, G.; Marín-Puyalto, J.; Vicente-Rodríguez, G.; Matute-Llorente, Á.; Casajús, J.A.; González-Agüero, A. Assessment of Active Video Games’ Energy Expenditure in Children with Overweight and Obesity and Differences by Gender. Int. J. Environ. Res. Public Health 2020, 17, 6714. [Google Scholar] [CrossRef]
- Gibney, E.; Nolan, C. Epigenetics and Gene Expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Lyko, F. The DNA Methyltransferase Family: A Versatile Toolkit for Epigenetic Regulation. Nat. Rev. Genet. 2017, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for de Novo Methylation and Mammalian Development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Jang, H.S.; Shin, W.J.; Lee, J.E.; Do, J.T. CpG and Non-CpG Methylation in Epigenetic Gene Regulation and Brain Function. Genes 2017, 8, 148. [Google Scholar] [CrossRef]
- Deaton, A.M.; Bird, A. CpG Islands and the Regulation of Transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef]
- Bagley, J.R.; Burghardt, K.J.; McManus, R.; Howlett, B.; Costa, P.B.; Coburn, J.W.; Arevalo, J.A.; Malek, M.H.; Galpin, A.J. Epigenetic Responses to Acute Resistance Exercise in Trained vs. Sedentary Men. J. Strength Cond. Res. 2020, 34, 1574–1580. [Google Scholar] [CrossRef]
- Turner, D.C.; Gorski, P.P.; Maasar, M.F.; Seaborne, R.A.; Baumert, P.; Brown, A.D.; Kitchen, M.O.; Erskine, R.M.; Dos-Remedios, I.; Voisin, S.; et al. DNA Methylation across the Genome in Aged Human Skeletal Muscle Tissue and Muscle-Derived Cells: The Role of HOX Genes and Physical Activity. Sci. Rep. 2020, 10, 15360. [Google Scholar] [CrossRef] [PubMed]
- Barrès, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute Exercise Remodels Promoter Methylation in Human Skeletal Muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.; Chen, M.-M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- Hood, D.A. Mechanisms of Exercise-Induced Mitochondrial Biogenesis in Skeletal Muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 465–472. [Google Scholar] [CrossRef]
- Rubenstein, A.; Hinkley, J.M.; Nair, V.D.; Nudelman, G.; Standley, R.A.; Yi, F.; Yu, G.; Trappe, T.A.; Bamman, M.M.; Trappe, S.; et al. Skeletal Muscle Transcriptome Response to a Bout of Endurance Exercise in Physically Active and Sedentary Older Adults. Am. J. Physiol.—Endocrinol. Metab. 2022, 322, E260–E277. [Google Scholar] [CrossRef] [PubMed]
- Amar, D.; Lindholm, M.E.; Norrbom, J.; Wheeler, M.T.; Rivas, M.A.; Ashley, E.A. Time Trajectories in the Transcriptomic Response to Exercise—A Meta-Analysis. Nat. Commun. 2021, 12, 3471. [Google Scholar] [CrossRef]
- Pillon, N.J.; Gabriel, B.M.; Dollet, L.; Smith, J.A.B.; Sardón Puig, L.; Botella, J.; Bishop, D.J.; Krook, A.; Zierath, J.R. Transcriptomic Profiling of Skeletal Muscle Adaptations to Exercise and Inactivity. Nat. Commun. 2020, 11, 470. [Google Scholar] [CrossRef]
- Zhang, F.F.; Cardarelli, R.; Carroll, J.; Zhang, S.; Fulda, K.G.; Gonzalez, K.; Vishwanatha, J.K.; Morabia, A.; Santella, R.M. Physical Activity and Global Genomic DNA Methylation in a Cancer-Free Population. Epigenetics 2011, 6, 293–299. [Google Scholar] [CrossRef]
- Willis, C.R.G.; Deane, C.S.; Ames, R.M.; Bass, J.J.; Wilkinson, D.J.; Smith, K.; Phillips, B.E.; Szewczyk, N.J.; Atherton, P.J.; Etheridge, T. Transcriptomic Adaptation during Skeletal Muscle Habituation to Eccentric or Concentric Exercise Training. Sci. Rep. 2021, 11, 23930. [Google Scholar] [CrossRef]
- Voisin, S.; Eynon, N.; Yan, X.; Bishop, D.J. Exercise Training and DNA Methylation in Humans. Acta Physiol. 2014, 213, 39–59. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Lally, J.; Holloway, G.P.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Repeated Transient MRNA Bursts Precede Increases in Transcriptional and Mitochondrial Proteins during Training in Human Skeletal Muscle. J. Physiol. 2010, 588, 4795–4810. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha Regulation by Exercise Training and Its Influences on Muscle Function and Insulin Sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sahlin, K. The Effect of Continuous and Interval Exercise on PGC-1α and PDK4 MRNA in Type I and Type II Fibres of Human Skeletal Muscle. Acta Physiol. 2011, 204, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Theilen, N.T.; Kunkel, G.H.; Tyagi, S.C. The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J. Cell. Physiol. 2017, 232, 2348–2358. [Google Scholar] [CrossRef]
- Theilen, N.T.; Jeremic, N.; Weber, G.J.; Tyagi, S.C. TFAM Overexpression Diminishes Skeletal Muscle Atrophy after Hindlimb Suspension in Mice. Arch. Biochem. Biophys. 2019, 666, 138–147. [Google Scholar] [CrossRef]
- Benite-Ribeiro, S.A.; Barbosa, H.C.; Ramadan, W.; dos Santos, J.M. Exercise-Mediated Increase in PGC1α and MEF2 Expression in Type 2 Diabetes Mellitus. Gene Rep. 2023, 31, 101758. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, X.; Shen, Y.; Huang, G.; Zhang, M.; Yan, Y.; Hou, D.; Meng, L.; Liu, J.; Cheng, H.; et al. Influence of Lifestyle on the FAIM2 Promoter Methylation between Obese and Lean Children: A Cohort Study. BMJ Open 2015, 5, e007670. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Walters, G.B.; Gudbjartsson, D.F.; Steinthorsdottir, V.; Sulem, P.; Helgadottir, A.; Styrkarsdottir, U.; Gretarsdottir, S.; Thorlacius, S.; Jonsdottir, I.; et al. Genome-Wide Association Yields New Sequence Variants at Seven Loci That Associate with Measures of Obesity. Nat. Genet. 2009, 41, 18–24. [Google Scholar] [CrossRef]
- Plaza-Florido, A.; Pérez-Prieto, I.; Molina-Garcia, P.; Radom-Aizik, S.; Ortega, F.B.; Altmäe, S. Transcriptional and Epigenetic Response to Sedentary Behavior and Physical Activity in Children and Adolescents: A Systematic Review. Front. Pediatr. 2022, 10, 917152. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Goodrich, J.M.; Dolinoy, D.C.; Sánchez, B.N.; Ruiz-Narváez, E.A.; Banker, M.; Cantoral, A.; Mercado-Garcia, A.; Téllez-Rojo, M.M.; Peterson, K.E. Accelerometer-Measured Physical Activity, Reproductive Hormones, and DNA Methylation. Med. Sci. Sports Exerc. 2019, 52, 598–607. [Google Scholar] [CrossRef]
- Dovio, A.; Roveda, E.; Sciolla, C.; Montaruli, A.; Raffaelli, A.; Saba, A.; Calogiuri, G.; De Francia, S.; Borrione, P.; Salvadori, P.; et al. Intense Physical Exercise Increases Systemic 11β-Hydroxysteroid Dehydrogenase Type 1 Activity in Healthy Adult Subjects. Eur. J. Appl. Physiol. 2009, 108, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Shin, K.O.; Yoo, J.-H.; Park, S.; Kang, S. The Effects of Detraining on Blood Adipokines and Antioxidant Enzyme in Korean Overweight Children. Eur. J. Pediatr. 2011, 171, 235–243. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Maciejczyk, M.; Kantorowicz, M.; Szygula, Z. Anaerobic Exercise-Induced Activation of Antioxidant Enzymes in the Blood of Women and Men. Front. Physiol. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Mariño-Ramírez, L.; Kann, M.G.; Shoemaker, B.A.; Landsman, D. Histone Structure and Nucleosome Stability. Expert Rev. Proteom. 2005, 2, 719–729. [Google Scholar] [CrossRef]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Kouzarides, T. Histone Post-Translational Modifications—Cause and Consequence of Genome Function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-Translational Modifications of Histones: Mechanisms, Biological Functions, and Therapeutic Targets. MedComm 2023, 4, e292. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Grant, P.A. The Role of DNA Methylation and Histone Modifications in Transcriptional Regulation in Humans. Subcell Biochem. 2013, 61, 289–317. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Fan, J.; Krautkramer, K.A.; Feldman, J.L.; Denu, J.M. Metabolic Regulation of Histone Post-Translational Modifications. ACS Chem. Biol. 2015, 10, 95–108. [Google Scholar] [CrossRef]
- Peserico, A.; Simone, C. Physical and Functional HAT/HDAC Interplay Regulates Protein Acetylation Balance. J. Biomed. Biotechnol. 2011, 2011, 371832. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Shimizu, J.; Kawano, F.; Kim, H.J.; Kim, C.K. Adaptive Responses of Histone Modifications to Resistance Exercise in Human Skeletal Muscle. PLoS ONE 2020, 15, e0231321. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Fairlie, E.; Garnham, A.P.; Hargreaves, M. Exercise-Induced Histone Modifications in Human Skeletal Muscle. J. Physiol. 2009, 587, 5951–5958. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.; Hiam, D.; Craig, J.; Barrès, R.; Eynon, N.; Voisin, S. Epigenetic Changes in Healthy Human Skeletal Muscle Following Exercise—A Systematic Review. Epigenetics 2019, 14, 633–648. [Google Scholar] [CrossRef]
- McGee, S.L.; Sparling, D.; Olson, A.-L.; Hargreaves, M. Exercise Increases MEF2- and GEF DNA-Binding Activities in Human Skeletal Muscle. FASEB J. 2005, 20, 348–349. [Google Scholar] [CrossRef]
- McGee, S.L.; Hargreaves, M. Histone Modifications and Exercise Adaptations. J. Appl. Physiol. 2011, 110, 258–263. [Google Scholar] [CrossRef]
- Bure, I.V.; Nemtsova, M.V.; Kuznetsova, E.B. Histone Modifications and Non-Coding RNAs: Mutual Epigenetic Regulation and Role in Pathogenesis. Int. J. Mol. Sci. 2022, 23, 5801. [Google Scholar] [CrossRef]
- Wang, H.; Helin, K. Roles of H3K4 Methylation in Biology and Disease. Trends Cell Biol. 2025, 35, 115–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of Histone Modification. Histone Mutat. Cancer 2020, 1283, 1–16. [Google Scholar] [CrossRef]
- Lavratti, C.; Dorneles, G.; Pochmann, D.; Peres, A.; Bard, A.; de Lima Schipper, L.; Dal Lago, P.; Wagner, L.C.; Elsner, V.R. Exercise-Induced Modulation of Histone H4 Acetylation Status and Cytokines Levels in Patients with Schizophrenia. Physiol. Behav. 2017, 168, 84–90. [Google Scholar] [CrossRef]
- Rossetto, D.; Avvakumov, N.; Côté, J. Histone Phosphorylation: A Chromatin Modification Involved in Diverse Nuclear Events. Epigenetics 2012, 7, 1098–1108. [Google Scholar] [CrossRef]
- Yu, M.; Stepto, N.K.; Chibalin, A.V.; Fryer, L.G.D.; Carling, D.; Krook, A.; Hawley, J.A.; Zierath, J.R. Metabolic and Mitogenic Signal Transduction in Human Skeletal Muscle after Intense Cycling Exercise. J. Physiol. 2003, 546, 327–335. [Google Scholar] [CrossRef]
- Schaffer, B.E.; Levin, R.S.; Hertz, N.T.; Maures, T.J.; Schoof, M.; Hollstein, P.E.; Benayoun, B.A.; Banko, M.R.; Shaw, R.J.; Shokat, K.M.; et al. Identification of AMPK Phosphorylation Sites Reveals a Network of Proteins Involved in Cell Invasion and Facilitates Large-Scale Substrate Prediction. Cell Metab. 2015, 22, 907–921. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Reik, W. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Wang, H.; Wang, W.; Liu, F. microRNAs in Action: Biogenesis, Function and Regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Ultimo, S.; Zauli, G.; Martelli, A.M.; Vitale, M.; McCubrey, J.A.; Capitani, S.; Neri, L.M. Influence of Physical Exercise on MicroRNAs in Skeletal Muscle Regeneration, Aging and Diseases. Oncotarget 2018, 9, 17220–17237. [Google Scholar] [CrossRef]
- Keller, M.; Meir, A.Y.; Bernhart, S.H.; Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Tsaban, G.; Zelicha, H.; Hopp, L.; Müller, L.; et al. DNA Methylation Signature in Blood Mirrors Successful Weight-Loss during Lifestyle Interventions: The CENTRAL Trial. Genome Med. 2020, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Malvandi, A.M.; Faraldi, M.; Sansoni, V.; Gerosa, L.; Jaworska, J.; Lombardi, G. Circulating Myo-MiRs in Physical Exercise. Adv. Exerc. Health Sci. 2024, 1, 86–98. [Google Scholar] [CrossRef]
- Sapp, R.M.; Hagberg, J.M. Circulating MicroRNAs: Advances in Exercise Physiology. Curr. Opin. Physiol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Siracusa, J.; Koulmann, N.; Banzet, S. Circulating MyomiRs: A New Class of Biomarkers to Monitor Skeletal Muscle in Physiology and Medicine. J. Cachexia Sarcopenia Muscle 2017, 9, 20–27. [Google Scholar] [CrossRef]
- Barreiro, E.; Sznajder, J.I. Epigenetic Regulation of Muscle Phenotype and Adaptation: A Potential Role in COPD Muscle Dysfunction. J. Appl. Physiol. 2013, 114, 1263–1272. [Google Scholar] [CrossRef]
- Silva, G.J.J.; Bye, A.; el Azzouzi, H.; Wisløff, U. MicroRNAs as Important Regulators of Exercise Adaptation. Prog. Cardiovasc. Dis. 2017, 60, 130–151. [Google Scholar] [CrossRef]
- Tokłowicz, M.; Żbikowska, A.; Janusz, P.; Kotwicki, T.; Andrusiewicz, M.; Kotwicka, M. MicroRNA Expression Profile Analysis in Human Skeletal Muscle Tissue: Selection of Critical Reference. Biomed. Pharmacother. 2023, 162, 114682. [Google Scholar] [CrossRef]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-Specific MicroRNAs in Skeletal Muscle Development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef]
- Davidsen, P.K.; Gallagher, I.J.; Hartman, J.W.; Tarnopolsky, M.A.; Dela, F.; Helge, J.W.; Timmons, J.A.; Phillips, S.M. High Responders to Resistance Exercise Training Demonstrate Differential Regulation of Skeletal Muscle MicroRNA Expression. J. Appl. Physiol. 2011, 110, 309–317. [Google Scholar] [CrossRef]
- Russell, A.P.; Lamon, S.; Boon, H.; Wada, S.; Güller, I.; Brown, E.L.; Chibalin, A.V.; Zierath, J.R.; Snow, R.J.; Stepto, N.; et al. Regulation of MiRNAs in Human Skeletal Muscle Following Acute Endurance Exercise and Short-Term Endurance Training. J. Physiol. 2013, 591, 4637–4653. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Scheele, C.; Yfanti, C.; Akerström, T.; Nielsen, A.R.; Pedersen, B.K.; Laye, M. Muscle Specific microRNAs Are Regulated by Endurance Exercise in Human Skeletal Muscle. J. Physiol. 2010, 588, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Vriens, A.; Provost, E.B.; Saenen, N.D.; Boever, P.D.; Vrijens, K.; Wever, O.D.; Plusquin, M.; Nawrot, T.S. Children’s Screen Time Alters the Expression of Saliva Extracellular MiR-222 and MiR-146a. Sci. Rep. 2018, 8, 8209. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.; Røsjø, H.; Aspenes, S.T.; Condorelli, G.; Omland, T.; Wisløff, U. Circulating MicroRNAs and Aerobic Fitness—The HUNT-Study. PLoS ONE 2013, 8, e57496. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism During Aerobic Exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Moleres, A.; Campión, J.; Milagro, F.I.; Marcos, A.; Campoy, C.; Garagorri, J.M.; Gómez-Martínez, S.; Martínez, J.A.; Azcona-Sanjulian, M.C.; Marti, A. Differential DNA Methylation Patterns between High and Low Responders to a Weight Loss Intervention in Overweight or Obese Adolescents: The EVASYON Study. FASEB J. 2013, 27, 2504–2512. [Google Scholar] [CrossRef]
- Luo, W.; Guo, Z.; Wu, M.; Hao, C.; Hu, X.; Zhou, Z.; Zhou, Z.; Yao, X.; Zhang, L.; Liu, J. Association of Peroxisome Proliferator-Activated Receptor α/δ/γ with Obesity, and Gene-Gene Interaction, in the Chinese Han Population. J. Epidemiol. 2013, 23, 187–194. [Google Scholar] [CrossRef]
- Fernando, S.; Sellers, J.; Smith, S.; Bhogoju, S.; Junkins, S.; Welch, M.; Willoughby, O.; Ghimire, N.; Secunda, C.; Barmanova, M.; et al. Metabolic Impact of MKP-2 Upregulation in Obesity Promotes Insulin Resistance and Fatty Liver Disease. Nutrients 2022, 14, 2475. [Google Scholar] [CrossRef] [PubMed]
- Mariman, E.C.M.; Bouwman, F.G.; Aller, E.E.J.G.; van Baak, M.A.; Wang, P. Extreme Obesity Is Associated with Variation in Genes Related to the Circadian Rhythm of Food Intake and Hypothalamic Signaling. Physiol. Genom. 2015, 47, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Rönn, T.; Volkov, P.; Davegårdh, C.; Dayeh, T.; Hall, E.; Olsson, A.H.; Nilsson, E.; Eriksson, K.F.; Jones, H.A.; Groop, L.; et al. A Six Months Exercise Intervention Influences the Genome-Wide DNA Methylation Pattern in Human Adipose Tissue. PLoS Genet. 2013, 9, e1003572. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Maasar, M.-F.; Turner, D.C.; Gorski, P.P.; Seaborne, R.A.; Strauss, J.A.; Shepherd, S.O.; Cocks, M.; Pillon, N.J.; Zierath, J.R.; Hulton, A.T.; et al. The Comparative Methylome and Transcriptome After Change of Direction Compared to Straight Line Running Exercise in Human Skeletal Muscle. Front. Physiol. 2021, 12, 619447. [Google Scholar] [CrossRef]
- Kasch, J.; Kanzleiter, I.; Saussenthaler, S.; Schürmann, A.; Keijer, J.; van Schothorst, E.; Klaus, S.; Schumann, S. Insulin Sensitivity–Linked Skeletal Muscle Nr4a1 DNA Methylation Is Programmed by the Maternal Diet and Modulated by Voluntary Exercise in Mice. J. Nutr. Biochem. 2018, 57, 86–92. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical Regulation of Bone Remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
| Human Studies | |||||||
|---|---|---|---|---|---|---|---|
| Gene/ Marker | Modification Type | Function | Tissue/ Region | Exercise Protocol & Subjects | Epigenetic & Gene Expression Changes | Physiological Consequences | References |
| PGC-1α | DNA Methylation | Regulates mitochondrial biogenesis, fatty acid oxidation, insulin sensitivity | Skeletal muscle, brown adipose tissue (BAT) | Untrained males and females completed: (1) 90 min steady-state cycling at ~60% VO2max or (2) Interval training alternating between 120% and 20% VO2max; OR 3 h acute cycling at 40% or 80% VO2max | Hypomethylation; dose-dependent ↑ mRNA expression | ↑ Mitochondrial density, improved oxidative metabolism, enhanced endurance capacity and insulin sensitivity | [57,68] |
| PDKA | DNA Methylation | Regulates skeletal muscle glucose metabolism | Oxidative skeletal muscle | Same as above (see PGC-1a entry) | Hypomethylation; ↑ gene expression at both intensities | Enhanced fat oxidation, reduced reliance on glucose during exercise, improved metabolic flexibility | [57,68] |
| MEF2A | DNA Methylation | Muscle development; regulates PGC-1α transcription | Skeletal, cardiac, smooth muscle | Male subjects (physically active) performed a 60 min cycling session at 75 ± 2% VO2peak after a 12 h overnight fast | ↑ Mitochondrial biogenesis via MEF-PGC-1α interaction; enhanced DNA binding of MEF2 due to HDAC 4/5 dissociation ↑ MEF2 binding | Enhanced mitochondrial function, muscle development, potential protection against T2DM | [89,90] |
| FAIM2 | DNA Methylation | Regulates neuronal apoptosis; linked to obesity | Hippocampus | Children categorized as obese or lean based on BMI and weekly physical activity (<150 min/week) | Differential methylation at 7 CpG sites in obese vs. lean children | Potential link to obesity via neural regulation of appetite and stress response | [72] |
| HSD11B2 | DNA Methylation | Converts cortisone to cortisol | Muscle, kidney, colon, pancreas, thyroid | Adolescents (wrist accelerometer monitoring); substitution analysis modeling 30 min of vigorous PA replaced with sedentary time | ↑ Methylation with increased sedentary time | Potential elevation in cortisol bioavailability → ↑ metabolic risk (e.g., insulin resistance) | [75] |
| SOX, GPX | DNA Methylation | Antioxidant defense, oxidative stress regulation | Liver, lung, kidney, mitochondria, extracellular | Overweight and normal-weight children underwent 24-week aerobic training, followed by 12-week detraining | ↑ Gene expression in both overweight and normal weight children | Improved antioxidant defense, reduced oxidative stress, long-term cellular protection | [77] |
| Histone H3 | Histone Modification (Acetylation / Phosphorylation) | Involved in gene activation and response to muscle activity | Skeletal muscle | (1) Healthy males did 10-week resistance training; (2) Elite endurance cyclists completed 8 × 5 min bouts at VO2peak with rest intervals | ↑ H3 acetylation and phosphorylation post-exercise | Enhanced muscle adaptation, hypertrophy, endurance capacity via gene activation | [86,96] |
| Histone H3K36 | Histone Modification (Acetylation) | Facilitates transcription elongation | Skeletal muscle | Male participants (<2h exercise/week) cycled for 60 min at ~75% VO2peak after 12 h fast | ↑ H3K36 acetylation in skeletal muscle | Enhanced transcription of exercise-responsive genes → improved muscle adaptation | [87] |
| Histone H4 | Histone Modification (Acetylation) | Regulates muscle growth, metabolism, cytokine production | Skeletal muscle | Schizophrenia patients (male/female mixed) completed aerobic + resistance training 3×/week over 3 months | ↑ Acetylation; altered cytokine production | Improved immune response regulation, muscle differentiation, and energy metabolism | [94] |
| miR-1, miR-133a/b | MicroRNA (miRNA) | Regulate muscle biogenesis, regeneration, and maintenance | Skeletal muscle (myomiRs) | Healthy males (<2h exercise/week) performed 10 days of endurance cycling at ~75% VO2peak for 45 min/day | ↑ miRNA expression after single session | Promotes muscle repair, growth, and mitochondrial biogenesis | [111] |
| miR-1, miR-133a, miR-133b, and miR-206 | MicroRNA (miRNA) | Regulate muscle biogenesis, regeneration, and maintenance | Skeletal muscle (myomiRs) | Healthy, trained males completed a cycle ergometer (chronic endurance program) 5×/week over 12 weeks | ↓ of miR-1, miR-133a, miR-133b, and miR-206 in the human vastus lateralis | Improved endurance capacity, VO2max, and insulin sensitivity | [112] |
| miR-146a, miR-222 | MicroRNA (miRNA) | Inflammatory response regulators, linked to chronic disease | Epithelium, monocytes, endothelial cells | Weekly screen time and physical activity assessed over 2 years in underweight and overweigh t children | ↑ salivary miRNA expression with increased screen time | Linked to inflammation and possibly increased risk of chronic disease, no PA or BMI correlation | [113] |
| Animal Studies | |||||||
| TFAM | DNA Methylation | Mitochondrial transcription, replication, and structure | Skeletal muscle | Mice underwent 2 weeks aerobic training (duration and intensity not specified) | ↑ TFAM expression; increase antioxidant levels | Enhanced mitochondrial function, redox balance, protection against muscle atrophy | [70] |
| MEF2A | DNA Methylation | Regulates muscle development; control PGC-1α transcription | Skeletal, cardiac, and smooth muscle | Mice completed moderate aerobic training 5×/week for 3 weeks | ↑ MEF2A and PGC-1α expression | Improved mitochondrial homeostasis, metabolic flexibility, potential T2DM prevention | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, A.; Wadsworth, D.D.; Geetha, T. Exercise, Epigenetics, and Body Composition: Molecular Connections. Cells 2025, 14, 1553. https://doi.org/10.3390/cells14191553
Williams A, Wadsworth DD, Geetha T. Exercise, Epigenetics, and Body Composition: Molecular Connections. Cells. 2025; 14(19):1553. https://doi.org/10.3390/cells14191553
Chicago/Turabian StyleWilliams, Ashley, Danielle D. Wadsworth, and Thangiah Geetha. 2025. "Exercise, Epigenetics, and Body Composition: Molecular Connections" Cells 14, no. 19: 1553. https://doi.org/10.3390/cells14191553
APA StyleWilliams, A., Wadsworth, D. D., & Geetha, T. (2025). Exercise, Epigenetics, and Body Composition: Molecular Connections. Cells, 14(19), 1553. https://doi.org/10.3390/cells14191553








