Immunophenotypic Panel for Comprehensive Characterization of Aggressive Thyroid Carcinomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection and Study Batch Formation
2.2. Immunohistochemistry
2.3. Molecular Assessment
2.4. Statistical Analysis
3. Results
3.1. Clinicopathologic Assessment
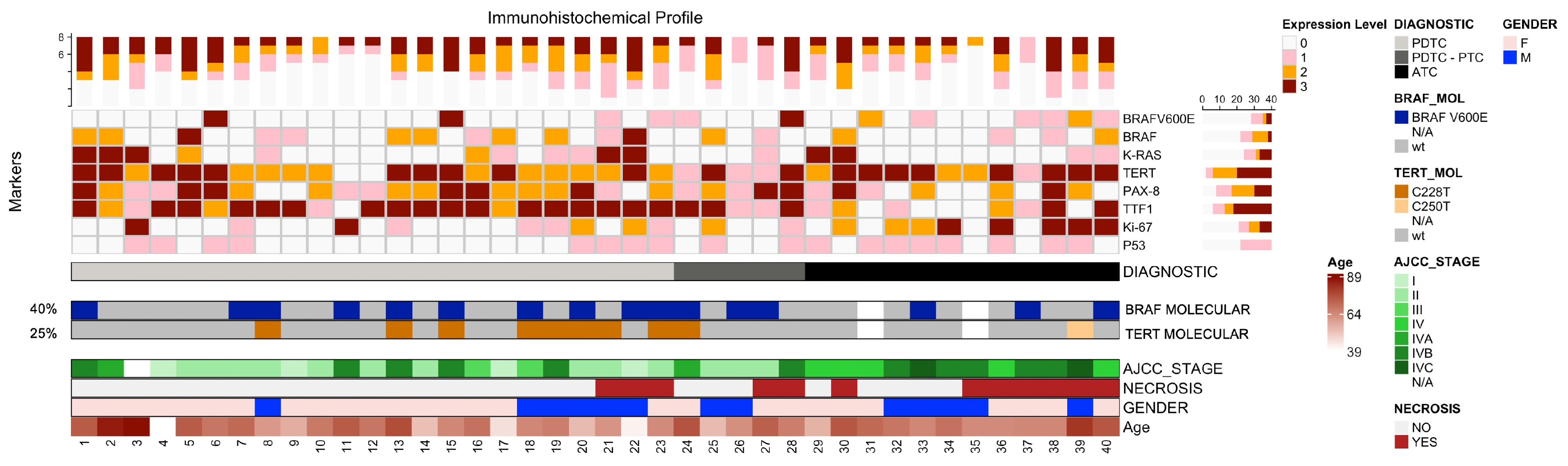
3.2. Immunohistochemical Analysis
3.3. Molecular Assessment
3.4. Correlations Between Clinical, Molecular and Pathologic Data
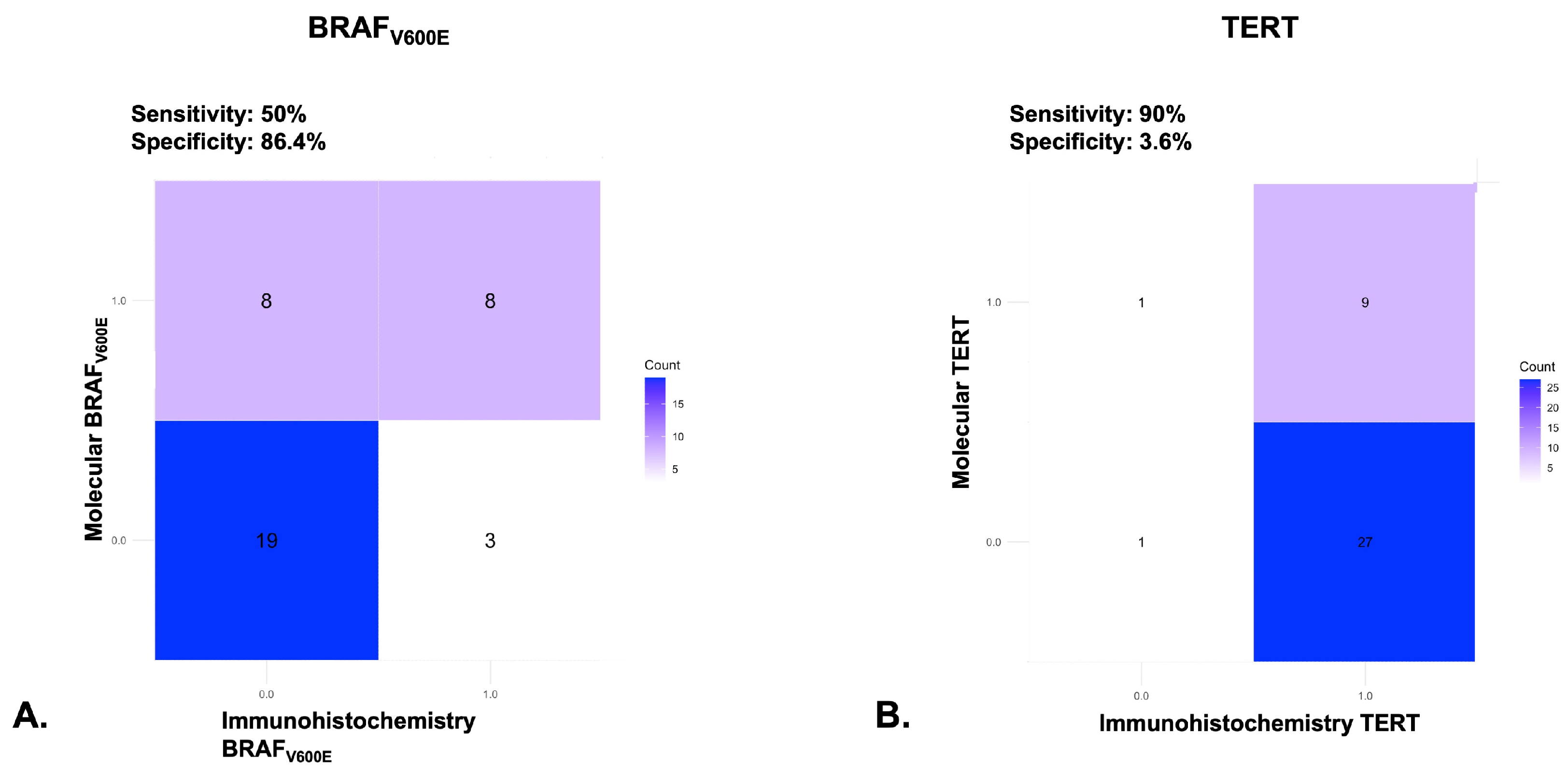
3.5. Agreement Between Molecular Techniques and Immunohistochemistry
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Gao, M.Z.; Omer, T.M.; Miller, K.M.; Simpson, M.C.; Bukatko, A.R.; Gedion, K.; Boakye, E.A.; Kost, K.M.; Dickinson, J.A.; Varvares, M.A.; et al. Thyroid Cancer Incidence and Trends in United States and Canadian Pediatric, Adolescent, and Young Adults. Cancers 2025, 17, 1429. [Google Scholar] [CrossRef]
- Lebbink, C.A.; Links, T.P.; Czarniecka, A.; Dias, R.P.; Elisei, R.; Izatt, L.; Krude, H.; Lorenz, K.; Luster, M.; Newbold, K.; et al. 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur. Thyroid. J. 2022, 11, e220146. [Google Scholar] [CrossRef]
- Bernier, M.-O.; Withrow, D.R.; de Gonzalez, A.B.; Lam, C.J.K.; Linet, M.S.; Kitahara, C.M.; Shiels, M.S. Trends in pediatric thyroid cancer incidence in the United States, 1998–2013. Cancer 2019, 125, 2497–2505. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef]
- Rodrigues, R.F.; Roque, L.; Krug, T.; Leite, V. Poorly differentiated and anaplastic thyroid carcinomas: Chromosomal and oligo-array profile of five new cell lines. Br. J. Cancer 2007, 96, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mahato, R.K.; Singh, S.; Bhatti, G.K.; Mastana, S.S.; Bhatti, J.S. Advances and challenges in thyroid cancer: The interplay of genetic modulators, targeted therapies, and AI-driven approaches. Life Sci. 2023, 332, 122110. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Endocrine and Neuroendocrine Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Schipor, S.; Publik, M.A.; Manda, D.; Ceausu, M. Aggressive Thyroid Carcinomas Clinical and Molecular Features: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 5535. [Google Scholar] [CrossRef]
- Hsu, K.-T.; Yu, X.-M.; Audhya, A.W.; Jaume, J.C.; Lloyd, R.V.; Miyamoto, S.; Prolla, T.A.; Chen, H. Novel Approaches in Anaplastic Thyroid Cancer Therapy. Oncologist 2014, 19, 1148–1155. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Al-Rasheed, U.; Mashhadani, N.; Abdlkadir, A.S.; Al-Adhami, D.A.; Ruzzeh, S.; Istatieh, F.; Mansour, A.; Hamdan, B.; Kheetan, R.; et al. Long-Term Survival Analysis and Prognostic Factors of Arabic Patients with Differentiated Thyroid Carcinoma: A 20-Year Observational Study at the King Hussein Cancer Center (KHCC) Involving 528 Patients. Cancers 2023, 15, 4102. [Google Scholar] [CrossRef]
- Patel, J.; Klopper, J.; Cottrill, E.E. Molecular diagnostics in the evaluation of thyroid nodules: Current use and prospective opportunities. Front. Endocrinol. 2023, 14, 1101410. [Google Scholar] [CrossRef]
- Guo, Y.; Zhong, Q.; Xie, L.; Wang, X.; Guo, L.; Zhuang, S.; Jia, C.; Wu, L.; Peng, J.; Pang, F.; et al. Development of a new TNM staging system for poorly differentiated thyroid carcinoma: A multicenter cohort study. Front. Endocrinol. 2025, 16, 1586542. [Google Scholar] [CrossRef]
- Cracolici, V. No Longer Well-Differentiated: Diagnostic Criteria and Clinical Importance of Poorly Differentiated/High-Grade Thyroid Carcinoma. Surg. Pathol. Clin. 2023, 16, 45–56. [Google Scholar] [CrossRef]
- BlueBooksOnline. Available online: https://tumourclassification.iarc.who.int/chaptercontent/53/47 (accessed on 12 August 2025).
- Reilly, J.; Faridmoayer, E.; Lapkus, M.; Pastewski, J.; Sun, F.; Elassar, H.; Studzinski, D.M.; Callahan, R.E.; Czako, P.; Nagar, S. Vascular invasion predicts advanced tumor characteristics in papillary thyroid carcinoma. Am. J. Surg. 2022, 223, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Bertol, B.C.; Massaro, J.D.; Debortoli, G.; Santos, A.L.P.; de Araújo, J.N.G.; Giorgenon, T.M.V.; e Silva, M.C.; de Figueiredo-Feitosa, N.L.; Collares, C.V.A.; de Freitas, L.C.C.; et al. BRAF, TERT and HLA-G Status in the Papillary Thyroid Carcinoma: A Clinicopathological Association Study. Int. J. Mol. Sci. 2023, 24, 12459. [Google Scholar] [CrossRef]
- de Biase, D.; Torricelli, F.; Ragazzi, M.; Donati, B.; Kuhn, E.; Visani, M.; Acquaviva, G.; Pession, A.; Tallini, G.; Piana, S.; et al. Not the same thing: Metastatic PTCs have a different background than ATCs. Endocr. Connect. 2018, 7, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Asioli, S.; A Erickson, L.; Righi, A.; Jin, L.; Volante, M.; Jenkins, S.; Papotti, M.; Bussolati, G.; Lloyd, R.V. Poorly differentiated carcinoma of the thyroid: Validation of the Turin proposal and analysis of IMP3 expression. Mod. Pathol. 2010, 23, 1269–1278. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fuchs, T.L.; Dogan, S.; Landa, I.; Katabi, N.; Fagin, J.A.; Tuttle, R.M.; Sherman, E.J.; Gill, A.J.; Ghossein, R. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid 2020, 30, 1505–1517. [Google Scholar] [CrossRef]
- Lam, K.Y.; Lo, C.Y.; Chan, K.W.; Wan, K.Y. Insular and anaplastic carcinoma of the thyroid: A 45-year comparative study at a single institution and a review of the significance of p53 and p21. Ann. Surg. 2000, 231, 329–338. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R. Genomic Landscape of poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr. Pathol. 2016, 27, 205–212. [Google Scholar] [CrossRef]
- Kondo, T.; Ezzat, S.; Asa, S.L. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat. Rev. Cancer 2006, 6, 292–306. [Google Scholar] [CrossRef] [PubMed]
- De Leo, S.; Trevisan, M.; Fugazzola, L. Recent advances in the management of anaplastic thyroid cancer. Thyroid. Res. 2020, 13, 17. [Google Scholar] [CrossRef]
- Masui, T.; Yane, K.; Ota, I.; Kakudo, K.; Wakasa, T.; Koike, S.; Kinugawa, H.; Yasumatsu, R.; Kitahara, T. Low Ki-67 labeling index is a clinically useful predictive factor for recurrence-free survival in patients with papillary thyroid carcinoma. J. Pathol. Transl. Med. 2025, 59, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol. Med. Rep. 2014, 11, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R.; Asa, S.L.; Bullock, M.J.; Carty, S.E.; Hodak, S.P.; McHugh, J.B.; Nikiforov, Y.E.; Pettus, J.; Richardson, M.S.; Shah, J.; et al. Protocol for the Examination of Specimens from Patients with Carcinomas of the Thyroid Gland; American College of Parthologists: Northfield, IL, USA, 2017. [Google Scholar]
- Talbott, I.; Wakely, P.E. Undifferentiated (anaplastic) thyroid carcinoma: Practical immunohistochemistry and cytologic look-alikes. Semin. Diagn. Pathol. 2015, 32, 305–310. [Google Scholar] [CrossRef]
- Crescenzi, A.; Baloch, Z. Immunohistochemistry in the pathologic diagnosis and management of thyroid neoplasms. Front. Endocrinol. 2023, 14, 1198099. [Google Scholar] [CrossRef]
- Dettmer, M.S.; Schmitt, A.; Komminoth, P.; Perren, A. Gering differenzierte Schilddrüsenkarzinome: Eine unterdiagnostizierte Entität. Der Pathol. 2020, 41, 1–8. [Google Scholar] [CrossRef]
- Walczyk, A.; Kopczyński, J.; Gąsior-Perczak, D.; Pałyga, I.; Kowalik, A.; Chrapek, M.; Hejnold, M.; Góźdź, S.; Kowalska, A. Histopathology and immunohistochemistry as prognostic factors for poorly differentiated thyroid cancer in a series of Polish patients. PLoS ONE 2020, 15, e0229264. [Google Scholar] [CrossRef]
- Bishop, J.A.; Sharma, R.; Westra, W.H. PAX8 immunostaining of anaplastic thyroid carcinoma: A reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum. Pathol. 2011, 42, 1873–1877. [Google Scholar] [CrossRef]
- Induction of TTF-1 or PAX-8 Expression on Proliferation and Tumorigenicity in Thyroid Carcinomas. Available online: https://www.spandidos-publications.com/ijo/49/3/1248 (accessed on 24 July 2025).
- Lacka, K.; Maciejewski, A.; Tyburski, P.; Manuszewska-Jopek, E.; Majewski, P.; Więckowska, B. Rationale for Testing TP53 Mutations in Thyroid Cancer—Original Data and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 1035. [Google Scholar] [CrossRef] [PubMed]
- Harahap, W.A.; Tofrizal, T.; Oktahermoniza, O. Relationship between the Expression of BRAF V600E and Ki-67 with the Recurrence of Well-Differentiated Thyroid Cancer. Asian Pac. J. Cancer Prev. 2022, 23, 3617–3622. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Collini, P.; Nikiforov, Y.E.; Sakamoto, A.; Kakudo, K.; Katoh, R.; Lloyd, R.V.; LiVolsi, V.A.; Papotti, M.; Sobrinho-Simoes, M.; et al. Poorly differentiated thyroid carcinoma: The Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am. J. Surg. Pathol. 2007, 31, 1256–1264. [Google Scholar] [CrossRef]
- Szymonek, M.; Kowalik, A.; Kopczyński, J.; Gąsior-Perczak, D.; Pałyga, I.; Walczyk, A.; Gadawska-Juszczyk, K.; Płusa, A.; Mężyk, R.; Chrapek, M.; et al. Immunohistochemistry cannot replace DNA analysis for evaluation of BRAFV600E mutations in papillary thyroid carcinoma. Oncotarget 2017, 8, 74897–74909. [Google Scholar] [CrossRef] [PubMed]
- Bedekovics, J.; Madarász, K.; Mokánszki, A.; Molnár, S.; Mester, Á.; Miltényi, Z.; Méhes, G. Exploring p53 protein expression and its link to TP53 mutation in myelodysplasia-related malignancies—Interpretive challenges and potential field of applications. Histopathology 2024, 85, 143–154. [Google Scholar] [CrossRef]
- Hwang, H.J.; Nam, S.K.; Park, H.; Park, Y.; Koh, J.; Na, H.Y.; Kwak, Y.; Kim, W.H.; Lee, H.S. Prediction of TP53 mutations by p53 immunohistochemistry and their prognostic significance in gastric cancer. J. Pathol. Transl. Med. 2020, 54, 378–386. [Google Scholar] [CrossRef]
- Ouh, Q.-Y.; Grossman, R.F. Thyroid Growth Factors, Signal Transduction Pathways, and Oncogenes. Surg. Clin. N. Am. 1995, 75, 421–437. [Google Scholar] [CrossRef]
- Smith, T.J. Insulin-Like Growth Factor Pathway and the Thyroid. Front. Endocrinol. 2021, 12, 653627. [Google Scholar] [CrossRef]
- ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Available online: https://www.spandidos-publications.com/10.3892/etm.2020.8454 (accessed on 24 July 2025).
- Burotto, M.; Chiou, V.L.; Lee, J.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Maik-Rachline, G.; Hacohen-Lev-Ran, A.; Seger, R. Nuclear ERK: Mechanism of Translocation, Substrates, and Role in Cancer. Int. J. Mol. Sci. 2019, 20, 1194. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Hu, P.; Gao, J.; Ying, J.; Xu, W.; Zhao, D.; Wang, Z.; Ye, J.; Lizaso, A.; et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology 2019, 75, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Kunisaki, C.; Sugimori, M.; Rino, Y.; Saito, A. Genetic landscape of 482 thyroid carcinomas: Analysis with the national datacenter for cancer genomic medicine in Japan. Endocrine 2024, 85, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wang, J.; Ran, W.; Li, G.; Hu, S.; Zhao, H.; Wang, X.; Wang, J. Anaplastic and poorly differentiated thyroid carcinomas: Genetic evidence of high-grade transformation from differentiated thyroid carcinoma. J. Pathol. Clin. Res. 2024, 10, e356. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.J.; Yi, J.W.; Jee, H.-G.; A Kim, Y.; Kim, J.H.; Xing, M.; Lee, K.E. Significance of the BRAF mRNA Expression Level in Papillary Thyroid Carcinoma: An Analysis of The Cancer Genome Atlas Data. PLoS ONE 2016, 11, e0159235. [Google Scholar] [CrossRef]
- Khan, S.; Bhake, A.; Sagar, S.; Yelne, S. Deciphering the Role of BRAFV600E Immunohistochemistry in Breast Lesions: A Comprehensive Review. Cureus 2024, 16, e64872. [Google Scholar] [CrossRef]
- Tsutsumi, Y. Pitfalls and Caveats in Applying Chromogenic Immunostaining to Histopathological Diagnosis. Cells 2021, 10, 1501. [Google Scholar] [CrossRef]
- Parker, K.G.; White, M.G.; Cipriani, N.A. Comparison of Molecular Methods and BRAF Immunohistochemistry (VE1 Clone) for the Detection of BRAF V600E Mutation in Papillary Thyroid Carcinoma: A Meta-Analysis. Head Neck Pathol. 2020, 14, 1067–1079. [Google Scholar] [CrossRef]
- Dvorak, K.; Aggeler, B.; Palting, J.; McKelvie, P.; Ruszkiewicz, A.; Waring, P. Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: Impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology 2014, 46, 509–517. [Google Scholar] [CrossRef]
- Kondo, T.; Nakazawa, T.; Murata, S.-I.; Kurebayashi, J.; Ezzat, S.; Asa, S.L.; Katoh, R. Enhanced B-Raf protein expression is independent of V600E mutant status in thyroid carcinomas. Hum. Pathol. 2007, 38, 1810–1818. [Google Scholar] [CrossRef]
- Khan, M.S.; Pandith, A.A.; Azad, N.; Hussain, M.U.; Masoodi, S.R.; Wani, K.A.; Andrabi, K.I.; Mudassar, S. Impact of molecular alterations of BRAF in the pathogenesis of thyroid cancer. Mutagenesis 2014, 29, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, R.; Zhu, Z.; Nikiforov, Y.E. BRAF copy number gains in thyroid tumors detected by fluorescence in situ hybridization. Endocr. Pathol. 2005, 16, 099–106. [Google Scholar] [CrossRef]
- Riesco-Eizaguirre, G.; Gutiérrez-Martínez, P.; García-Cabezas, M.A.; Nistal, M.; Santisteban, P. The oncogene BRAFV600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr.-Relat. Cancer 2006, 13, 257–269. [Google Scholar] [CrossRef]
- Romei, C.; Ciampi, R.; Faviana, P.; Agate, L.; Molinaro, E.; Bottici, V.; Basolo, F.; Miccoli, P.; Pacini, F.; Pinchera, A.; et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr.-Relat. Cancer 2008, 15, 511–520. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Mete, O.; Boucher, A.; Schrader, K.A.; Abdel-Rahman, O.; Bahig, H.; Ho, C.; Hasan, O.K.; Lemieux, B.; Winquist, E.; Wong, R.; et al. Consensus Statement: Recommendations on Actionable Biomarker Testing for Thyroid Cancer Management. Endocr. Pathol. 2024, 35, 293–308. [Google Scholar] [CrossRef]
- I Saavedra, H.; A Knauf, J.; Shirokawa, J.M.; Wang, J.; Ouyang, B.; Elisei, R.; Stambrook, P.J.; A Fagin, J. The RAS oncogene induces genomic instability in thyroid PCCL3 cells via the MAPK pathway. Oncogene 2000, 19, 3948–3954. [Google Scholar] [CrossRef]
- Garcia-Rostan, G.; Zhao, H.; Camp, R.L.; Pollan, M.; Herrero, A.; Pardo, J.; Wu, R.; Carcangiu, M.L.; Costa, J.; Tallini, G. ras Mutations Are Associated With Aggressive Tumor Phenotypes and Poor Prognosis in Thyroid Cancer. J. Clin. Oncol. 2003, 21, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, M.; Zhang, Q.-P.; Piao, Z.-A.; Wang, Z.-H.; Lv, S. Utility of BRAF protein overexpression in predicting the metastasis potential of papillary thyroid carcinoma. Oncol. Lett. 2011, 2, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: Old actors and new players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-B.; Aiba, I.; Long, Y.; Lin, H.-K.; Feun, L.; Savaraj, N.; Kuo, M.T. Activation of Ras/PI3K/ERK Pathway Induces c-Myc Stabilization to Upregulate Argininosuccinate Synthetase, Leading to Arginine Deiminase Resistance in Melanoma Cells. Cancer Res. 2012, 72, 2622–2633. [Google Scholar] [CrossRef]
- Radkay, L.A.; Chiosea, S.I.; Seethala, R.R.; Hodak, S.P.; LeBeau, S.O.; Yip, L.; McCoy, K.L.; Carty, S.E.; Schoedel, K.E.; Nikiforova, M.N.; et al. Thyroid nodules with KRAS mutations are different from nodules with NRAS and HRAS mutations with regard to cytopathologic and histopathologic outcome characteristics. Cancer Cytopathol. 2014, 122, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Tayubi, I.A.; Madar, I.H. Identification of potential inhibitor targeting KRAS mutation in Papillary Thyroid Carcinoma through molecular docking and dynamic simulation analysis. Comput. Biol. Med. 2023, 152, 106377. [Google Scholar] [CrossRef]
- Mannino, D.; Basilotta, R.; De Luca, F.; Casili, G.; Esposito, E.; Paterniti, I. KRAS–SOS-1 Inhibition as New Pharmacological Target to Counteract Anaplastic Thyroid Carcinoma (ATC). Int. J. Mol. Sci. 2025, 26, 2579. [Google Scholar] [CrossRef] [PubMed]
- Di Magliano, M.P.; Di Lauro, R.; Zannini, M. Pax8 has a key role in thyroid cell differentiation. Proc. Natl. Acad. Sci. USA 2000, 97, 13144–13149. [Google Scholar] [CrossRef]
- Nhung, N.T.; Hoang, V.D.; Mussazhanova, Z.; Kurohama, H.; Ha, L.N.; Matsuda, K.; Nguyen, V.P.T.; Hanh, N.T.M.; Nguyen, T.N.A.; Nakashima, M. BRAFV600E and TERT promoter mutations and their impact on recurrent papillary thyroid carcinoma progression. Endocr. Connect. 2025, 14, 250116. [Google Scholar] [CrossRef]
- Rusinek, D.; Pfeifer, A.; Krajewska, J.; Oczko-Wojciechowska, M.; Handkiewicz-Junak, D.; Pawlaczek, A.; Zebracka-Gala, J.; Kowalska, M.; Cyplinska, R.; Zembala-Nozynska, E.; et al. Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients. Int. J. Mol. Sci. 2018, 19, 2647. [Google Scholar] [CrossRef]
- Yu, P.; Qu, N.; Zhu, R.; Hu, J.; Han, P.; Wu, J.; Tan, L.; Gan, H.; He, C.; Fang, C.; et al. TERT accelerates BRAF mutant–induced thyroid cancer dedifferentiation and progression by regulating ribosome biogenesis. Sci. Adv. 2023, 9, eadg7125. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Zhu, G.; Xing, M. Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through FOS/GABP in human cancer. Nat. Commun. 2018, 9, 579. [Google Scholar] [CrossRef]
- Chung, J.H. BRAF and TERT promoter mutations: Clinical application in thyroid cancer. Endocr. J. 2020, 67, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.-H.; Wen, D.-Y.; Luo, Y.-H.; Chen, G.; Yang, H.; Chen, J.-Q.; He, Y. The diagnostic and prognostic values of Ki-67/MIB-1 expression in thyroid cancer: A meta-analysis with 6,051 cases. OncoTargets Ther. 2017, 10, 3261–3276. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Alsayegh, R.; Forest, V.-I.; Pusztaszeri, M.P.; da Silva, S.D.; Florianova, L.; Payne, R.J. Ki-67 Labelling Index as a Predictor of Invasive Features in Thyroid Cancer: Retrospective Analysis and Implications. Curr. Oncol. 2024, 31, 4030–4037. [Google Scholar] [CrossRef]
- Lee, S.-R.; Yim, H.; Han, J.H.; Lee, K.B.; Lee, J.; Soh, E.Y.; Kim, D.J.; Chung, Y.-S.; Jeong, S.-Y.; Sheen, S.S.; et al. VE1 Antibody Is Not Highly Specific for the BRAF V600E Mutation in Thyroid Cytology Categories With the Exception of Malignant Cases. Am. J. Clin. Pathol. 2015, 143, 437–444. [Google Scholar] [CrossRef]
- Schafroth, C.; Galván, J.A.; Centeno, I.; Koelzer, V.H.; Dawson, H.E.; Sokol, L.; Rieger, G.; Berger, M.D.; Hädrich, M.; Rosenberg, R.; et al. VE1 immunohistochemistry predicts BRAFV600E mutation status and clinical outcome in colorectal cancer. Oncotarget 2015, 6, 41453–41463. [Google Scholar] [CrossRef] [PubMed]
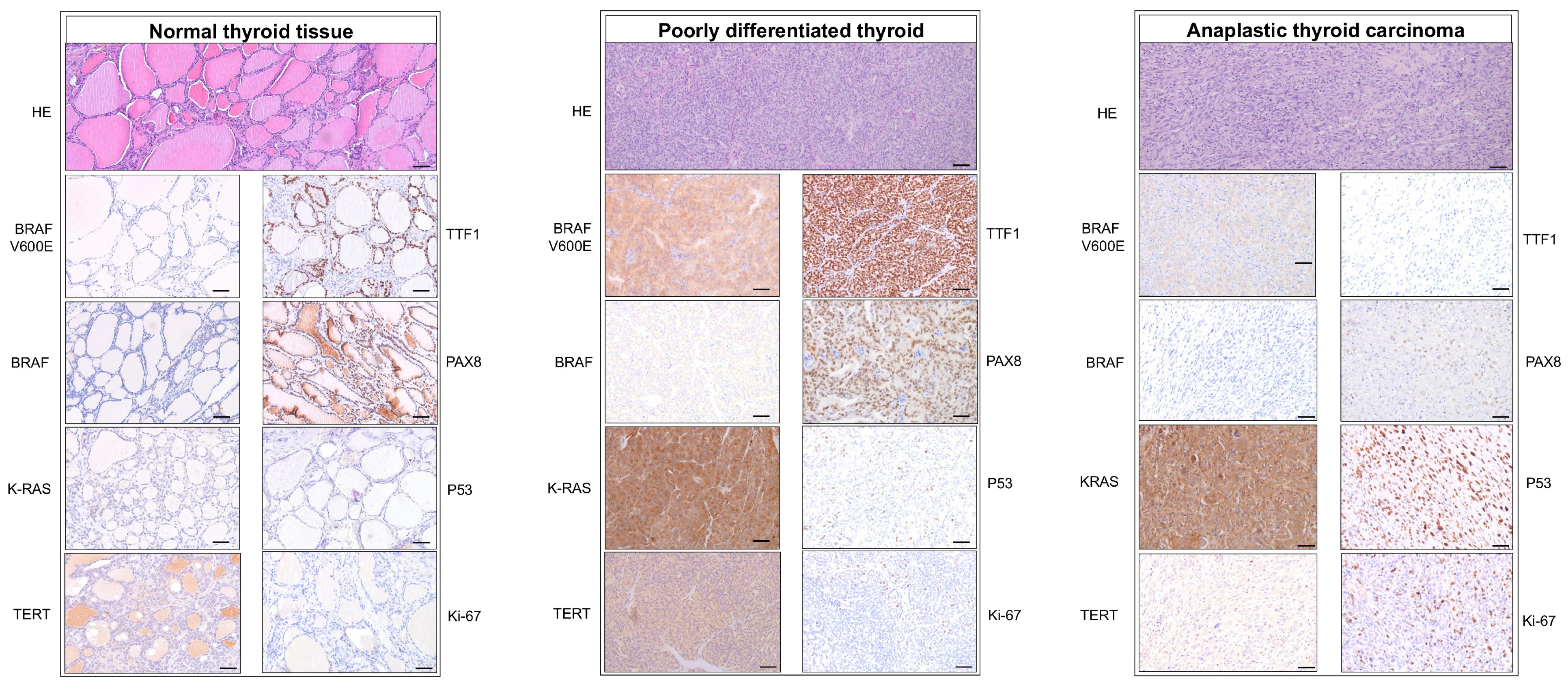


| Antibody | Clone | Dilution | Producer | Ref. Number | |
|---|---|---|---|---|---|
| 1. | BRAFv600E | VE 1 | 1:100 | Abcam, Cambridge, UK | ab228461 |
| 2. | B-RAF | EP152Y | 1:70 | Abcam, Cambridge, UK | ab33899 |
| 3. | K-RAS | EPR23474-20 | 1:70 | Abcam, Cambridge, UK | ab275875 |
| 4. | TERT | Poly | 1:70 | Abcam, Cambridge, UK | ab216625 |
| 5. | PAX-8 | PAX8R1 | 1:200 | Abcam, Cambridge, UK | ab53490 |
| 6. | TTF1 | SPT24 | RTU | Leica Biosystems, Deer Park, IL, USA | PA0364 |
| 7. | P53 | DO-7 | RTU | Leica Biosystems, Deer Park, IL, USA | PA0057 |
| 8. | Ki-67 | MM1 | RTU | Leica Biosystems, Deer Park, IL, USA | PA0118 |
| Marker | Cut off | Interpretation | Result |
|---|---|---|---|
| BRAFv600E BRAF K-RAS TERT PAX-8 | No positive cells | +0 | Qualitative |
| Rare/isolated cells | +1 | ||
| Focal/zonal positive | +2 | ||
| Diffuse positive | +3 | ||
| TTF1 | 0% | Negative | Semiquantitative |
| 0–15% | Low | ||
| 15-50% | Intermediate | ||
| >50% | High | ||
| Ki-67 | <5% | Low | |
| 5–10% | Intermediate | ||
| 10–20% | High | ||
| >20% | Very high | ||
| P53 | 0% or >10% | Mutant-type pattern | |
| >0% and <10% | Wild-type pattern |
| Type of carcinoma | Total N = 40 (%) | PDTC N = 28 (%) | ATC N = 12 (%) | p Value | |
|---|---|---|---|---|---|
| Sex | M | 13 (32.5) | 8 (28.8) | 5 (58.3) | 0.418 |
| F | 27 (67.5) | 20 (71.4) | 7 (41.7) | ||
| Mean age (years) | 63.9 (39–83) | 62.9 (39–83) | 66.4 (46–83) | 0.383 | |
| Coexistence with DTC | 5 (12.5) | 5 (17.8) | 0 (0) | ||
| Necrosis present | 12 (30) | 5 (17.8) | 7 (58.3) | 0.047 * | |
| Vascular invasion | 35 (87.5) | 25 (89.2) | 10 (83.3) | 0.884 | |
| Neural invasion | 12 (30) | 8 (28.5) | 4 (33.3) | 0.646 | |
| Extrathyroidal disease | 30 (81.1%) | 22 (78.6%) | 8 (88.9%) 1 | 0.492 | |
| AJCC stage | I | 4 (14.3) | 0 | 0.001 * | |
| II | 13 (46.4) | 0 | |||
| III | 2 (7.1) | 0 | |||
| IV IVA, IVB, IVC | 8 (28.5) 1, 6, 0 2 | 12 (100) 0 (0.00), 5 (71.4), 2 (28.6) 3 | |||
| Guo et al., 2025 [15] stage | I | 3 (10.7) | |||
| II | 7 (25.0) | ||||
| III | 7 (25.0) | ||||
| IV | 11 (39.3) | ||||
| IVA, IVB | 6, 5 | ||||
| Variable 1 | Variable 2 | Spearman’s ρ | p-Value |
|---|---|---|---|
| Immunohistochemical marker—immunohistochemical marker correlations | |||
| BRAFV600E | BRAF | −0.324 | <0.05 |
| BRAF | K-RAS | 0.711 | <0.001 |
| BRAF | TERT | 0.422 | <0.01 |
| TERT | PAX-8 | 0.323 | <0.05 |
| TTF-1 | PAX-8 | 0.711 | <0.001 |
| Ki-67 | TERT | 0.346 | <0.05 |
| Ki-67 | BRAF | 0.350 | <0.05 |
| Clinical—immunohistochemical marker correlations | |||
| Diagnostic | Ki-67 | 0.383 | <0.05 |
| Diagnostic | TTF-1 | −0.542 | <0.001 |
| Diagnostic | Necrosis | 0.405 | <0.01 |
| Ki-67 | Metastases | 0.432 | <0.05 |
| BRAFV600E | Age | 0.325 | <0.05 |
| K-RAS | Neural invasion | −0.368 | <0.05 |
| Vascular invasion | Microscopic ETE | 0.480 | <0.01 |
| Lymph node | Metastasis | 0.465 | <0.01 |
| Clinical—molecular or immunohistochemical—molecular correlations | |||
| BRAFV600E histo | BRAFV600E molecular | 0.420 | <0.01 |
| BRAFV600E histo | TERT C228T molecular | 0.328 | <0.05 |
| BRAFV600E molecular | TERT C228T molecular | 0.329 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceausu, M.; Publik, M.A.; Terzea, D.; Cristea, C.A.; Ioachim, D.; Manda, D.; Schipor, S. Immunophenotypic Panel for Comprehensive Characterization of Aggressive Thyroid Carcinomas. Cells 2025, 14, 1554. https://doi.org/10.3390/cells14191554
Ceausu M, Publik MA, Terzea D, Cristea CA, Ioachim D, Manda D, Schipor S. Immunophenotypic Panel for Comprehensive Characterization of Aggressive Thyroid Carcinomas. Cells. 2025; 14(19):1554. https://doi.org/10.3390/cells14191554
Chicago/Turabian StyleCeausu, Mihail, Mihai Alin Publik, Dana Terzea, Carmen Adina Cristea, Dumitru Ioachim, Dana Manda, and Sorina Schipor. 2025. "Immunophenotypic Panel for Comprehensive Characterization of Aggressive Thyroid Carcinomas" Cells 14, no. 19: 1554. https://doi.org/10.3390/cells14191554
APA StyleCeausu, M., Publik, M. A., Terzea, D., Cristea, C. A., Ioachim, D., Manda, D., & Schipor, S. (2025). Immunophenotypic Panel for Comprehensive Characterization of Aggressive Thyroid Carcinomas. Cells, 14(19), 1554. https://doi.org/10.3390/cells14191554







