1. Introduction
Leprosy, or Hansen’s disease, is a chronic granulomatous infectious condition caused by
Mycobacterium leprae and
Mycobacterium lepromatosis. It is characterized by slow progression and primarily affects the mucosal surfaces of the respiratory tract, skin, eyes, and peripheral nervous system (PNS) [
1,
2] Schwann cells, the main glial cells of the PNS, are key targets of
M. leprae and play a critical role in maintaining nerve integrity and facilitating repair. Infection of the Schwann cells can significantly impair their metabolic activity and viability, ultimately leading to nerve dysfunction and peripheral neuropathy [
3,
4].
The invasion of peripheral nerves by
M. leprae has long been recognized as a major contributor to neural damage in leprosy, regardless of the host’s immunological response or the presence of leprosy reactions [
5].
M. leprae modulates Schwann cell function by inducing lipid droplet formation, which facilitates bacterial survival through inhibition of phagosome maturation and disruption of host energy metabolism [
6]. Additionally, the bacilli can reprogram Schwann cells into progenitor-like states, impairing their roles in nerve maintenance and regeneration [
7].
One of the key mechanisms implicated in immune modulation in leprosy is the induction of Indoleamine 2,3-dioxygenase (IDO). The indoleamine 2,3-dioxygenase enzyme exists in two isoforms, IDO1 and IDO2, which catalyze the initial and rate-limiting step of tryptophan degradation along the kynurenine pathway (KP); while IDO1 is well characterized and broadly expressed in immune and non-immune cells, IDO2 shares partial functional overlap but displays more restricted tissue distribution and lower enzymatic activity [
8].
M. leprae induces IDO1 expression in human monocytes, with elevated levels observed in patients with the lepromatous (LL) form of leprosy compared to the tuberculoid (TT) form [
9,
10]. Patients with the LL form may develop a condition known as silent neuropathy, characterized by progressive nerve damage in the absence of overt inflammation or clinical symptoms [
11,
12,
13]. Upregulation of IDO in infected tissues, particularly in macrophages and dendritic cells, promotes bacterial persistence by shaping an immunosuppressive environment [
14,
15,
16]. Furthermore, IDO influences the balance between effector and regulatory T cells, contributing to immune dysregulation in leprosy [
9,
16].
Cells isolated from lepromatous lesions exhibited a CD68
+IDO
+ phenotype, and higher soluble IL-10 levels in sera positively correlated with IDO activity. In vitro, stimulation of healthy monocytes with increasing
M. leprae concentrations induced IDO expression alongside a regulatory cytokine profile including IL-10, TNF, and TGF-β. Importantly, IL-10 blockades significantly reduced
M. leprae-induced IDO expression, highlighting IL-10 as a key mediator of IDO induction. These findings indicate that IL-10 plays a central role in modulating IDO expression and function, contributing to the establishment of a regulatory environment that favors bacterial persistence [
10].
At the tuberculoid end of the disease spectrum, where IFN-γ expression is dominant, IDO-1 is likely induced primarily by this cytokine and may be linked to microbicidal activity [
17], suggesting that in leprosy, IDO-1 can play a dual role, functioning either as a tolerogenic or microbicidal mediator depending on the local environment and the cytokines driving its expression. In leprosy skin lesions, besides intact bacilli, mycobacterial components from the cell membrane and cytosol released following bacterial death—a process driven by both the host immune response and treatment—are expected to trigger immune responses [
18]. IDO plays dual roles in immune regulation and neurotoxicity by producing several biologically active metabolites. Among these, kynurenine (KYN) serves as a central intermediate, which can be further converted into either neuroprotective or neurotoxic compounds [
19,
20,
21].
Disruption of tryptophan metabolism through KP activation has been extensively studied in central nervous system (CNS) disorders, but its role in peripheral neuropathy, particularly in the context of leprosy, remains poorly understood.
The mechanisms underlying the progression of nerve damage in this immunosuppressive environment remain unclear. It is not yet known whether metabolic modulation of Schwann cells by the bacillus itself may disrupt this suppressive milieu, thereby permitting nerve damage to occur. In this context, IDO1 emerges as a potential mediator, given its ability to generate metabolites capable of directly affecting the viability and functionality of neural cells [
19,
20,
21]. Given that Schwann cells are the primary targets of
M. leprae in the peripheral nervous system, assessing IDO1 activity in these cells may provide crucial insights into the local immunometabolic mechanisms contributing to nerve damage in leprosy. Despite these insights, the role of IDO in non-immune cells such as Schwann cells during
M. leprae infection is not well characterized. Clarifying the impact of IDO activity on Schwann cell metabolism, viability, and the local inflammatory environment could reveal new mechanisms involved in leprosy-associated neuropathy. Targeting IDO and the kynurenine pathway may offer novel therapeutic strategies to mitigate nerve damage, restore immune homeostasis, and improve clinical outcomes in leprosy. The present study aims to explore the role of IDO1 in Schwann cell responses to
M. leprae, with a focus on metabolic activity.
2. Materials and Methods
2.1. Cell Culture and Stimulation
For in vitro assays, the human Schwann cell line ST88-14, derived from a malignant peripheral nerve sheath tumor in a patient with neurofibromatosis type 1, was used (provided by Dr. J.A. Fletcher, Dana Farber Cancer Institute, Boston, MA, USA). Prior to experiments, cells were cultured in RPMI medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco Invitrogen, Waltham, MA, USA), 2 mM L-glutamine (Gibco Invitrogen, USA), and 100 U/mL penicillin plus 100 μg/mL streptomycin (Pen/Strep, Gibco Invitrogen, USA). Additionally, routine screening of ST88-14 cells line was conducted for any mycoplasma contamination through polymerase chain reaction (PCR) to ensure the cell culture reliability and integrity. All the experiments were performed after the third passage of the cell culture to ensure consistent cellular characteristics according to the laboratory protocol. Unless otherwise specified, cells were incubated with irradiated M. leprae at 10, 50, or 100 μg/mL, with or without 1 mM 1-methyl-L-tryptophan (1-MT—cat. Number: 447439 Sigma-Aldrich, St. Louis, MO, USA), an IDO1 inhibitor, for 24 h at 37 °C in a 5% CO2 atmosphere. Irradiated M. leprae (strain NHDP, gamma-irradiated whole cells, lyophilized, NR-19326) was obtained from BEI Resources, NIAID, NIH, Manassas, VA, USA), and stored at −20 °C in 1 mg/mL aliquots.
2.2. Microarray Analysis
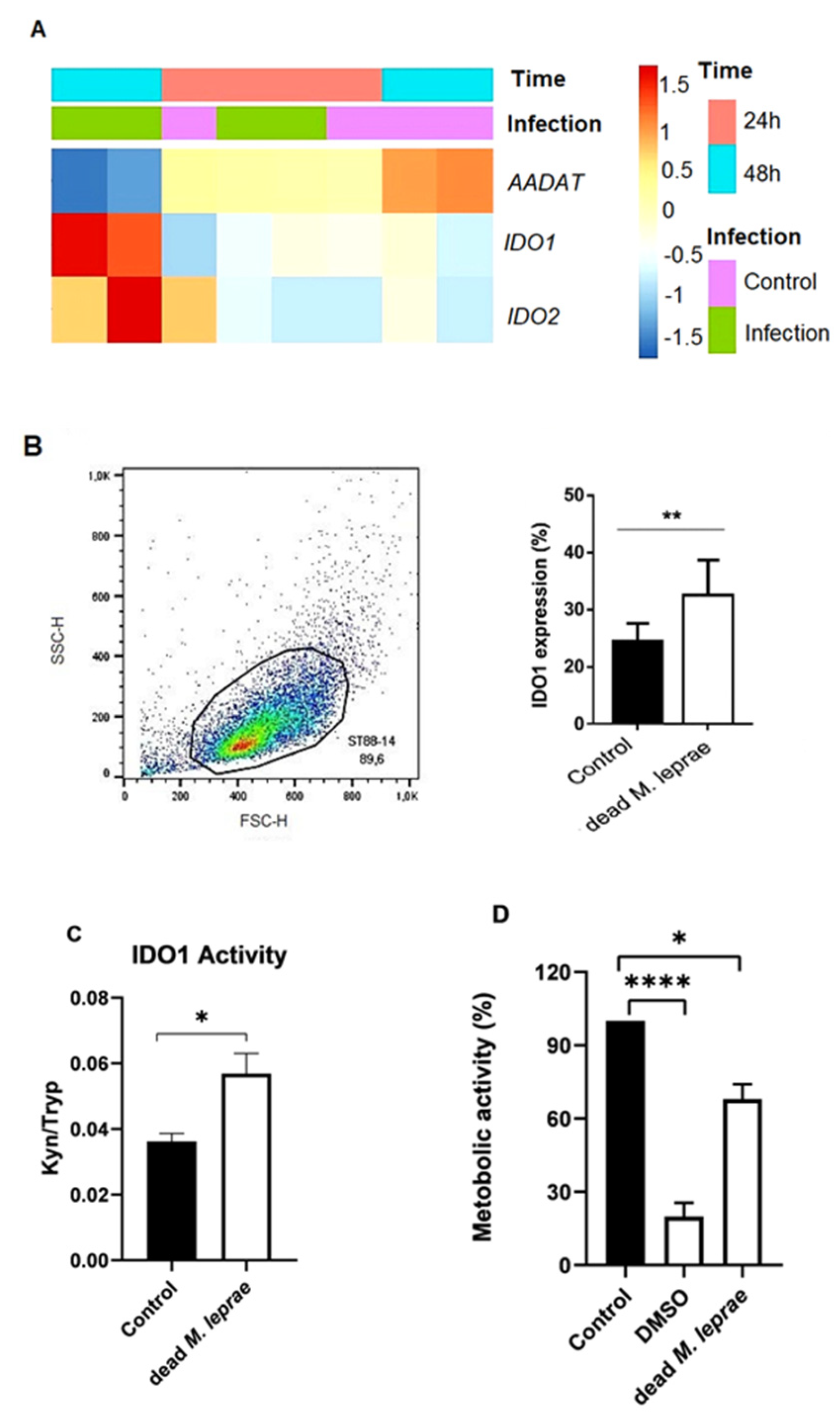
Microarray analysis was used to evaluate gene expressions related to the kynurenine pathway in Schwann cells. Data from infected primary human Schwann cells (
M. leprae Thai-53, MOI 100:1, 24 or 48 h at 33 °C; NCBI Gene Expression Omnibus accession number GSE40950) were analyzed using RStudio (v3.4.1) and the Pretty Heatmap package (v1.0.12). Heatmaps were generated from log2-transformed, normalized expression values. R computer source code (v. 3.4.1) and data used in the analyzes are readily available at
https://doi.org/10.5281/zenodo.3840319.
2.3. Flow Cytometry Assay
IDO1 expression was quantified in ST88-14 Schwann cells using flow cytometry. A total of 1 × 105 cells were seeded in 6-well plates and treated as indicated. After treatment, cells were harvested using 0.25% trypsin/1 mM EDTA, washed with FACS buffer (PBS + 0.1% BSA), and fixed with 4% paraformaldehyde for 30 min at 4 °C. Permeabilization was performed using PBS containing 0.1% saponin and 1% BSA. Cells were then blocked with 2% normal goat serum for 15 min at room temperature. Intracellular staining was conducted using a PE-conjugated anti-IDO1 antibody (R&D Systems, Minneapolis, MN, USA; 1:50 dilution, cat. IC6030P) for 30 min at room temperature in the dark. A PE-conjugated isotype control antibody (R&D Systems, cat. IC002P) was used to assess non-specific staining. Samples were filtered through 40 μm cell strainers to minimize cell aggregates and analyzed on a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Data were acquired using standard instrument settings that collect only pulse area (integrated signal) parameters. As FACSCalibur does not provide pulse height or width data, conventional singlet gating (e.g., FSC-A vs. FSC-H) could not be performed. To reduce the inclusion of doublets or aggregates, we applied tight forward scatter (FSC) vs. side scatter (SSC) gating to select the main cell population. A minimum of 10,000 events was collected per sample. Data were analyzed using FlowJo software v10 (BD Biosciences, Ashland, OR, USA).
2.4. High-Performance Liquid Chromatography (HPLC)
HPLC was used to quantify kynurenine (Kyn) and tryptophan (Tryp) levels in supernatants. Samples of supernatants were mixed with 5 μL of 10 mM tyrosine and 25 μL trichloroacetic acid in 400 μL supernatant, vortexed, and centrifuged at 14,000×
g for 10 min. The supernatant was injected into a Shim-pack CLC-ODS(M)
® C18 column using a Shimadzu LC-20AT system (Shimadzu Corporation, Kyoto, Japan). The mobile phase consisted of 15 mM sodium acetate buffer and acetonitrile with 0.1% trifluoroacetic acid. Absorbance was read at 365 nm (Kyn) and 285 nm (Tryp), with tyrosine as the internal standard. IDO1 activity was expressed as the Kyn/Tryp peak area ratio [
22].
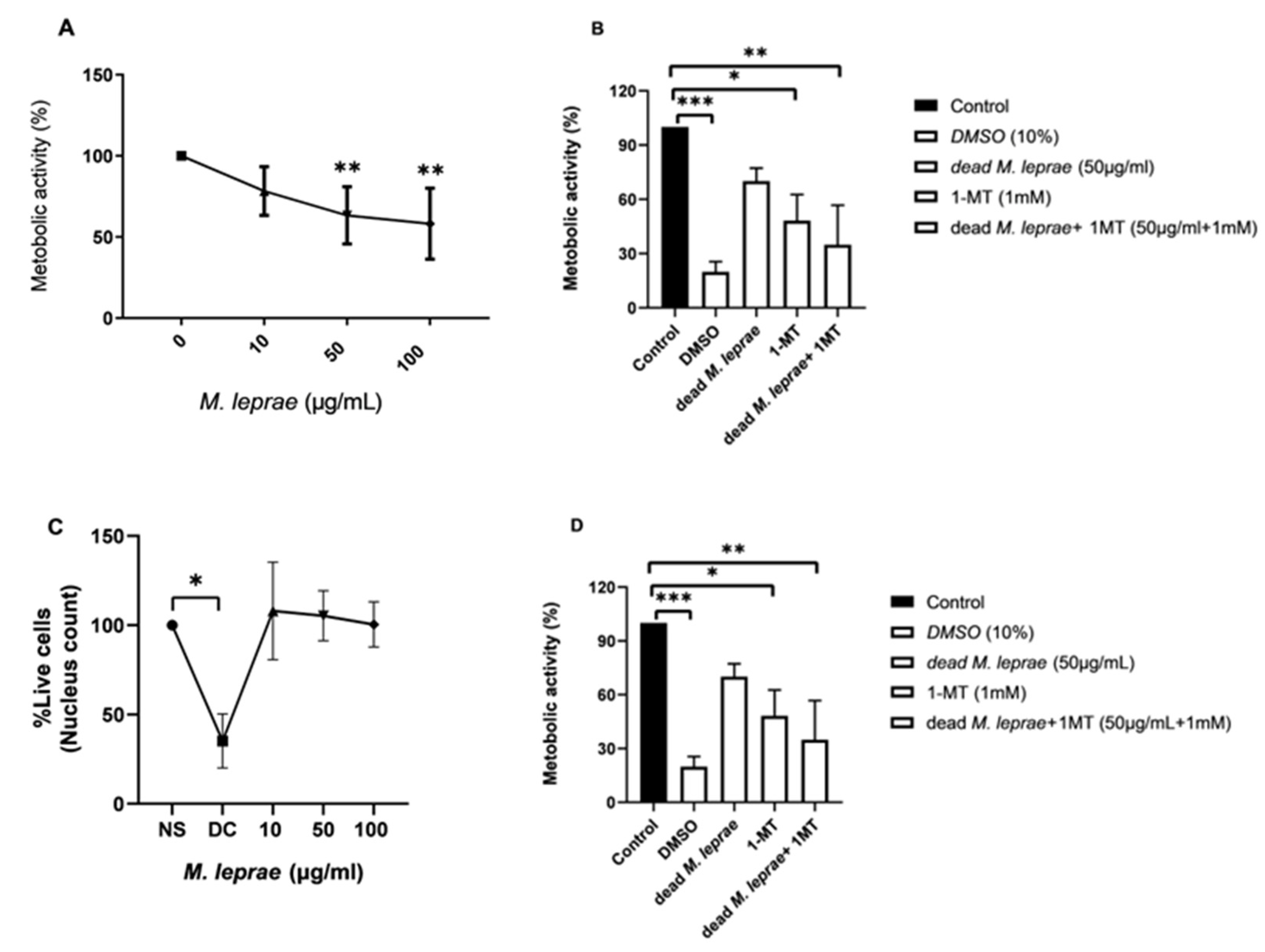
2.5. Evaluation of Schwann Cell Metabolic Activity
Schwann cell line ST88-14 (8000/well) were plated in 96-well plates and treated with irradiated
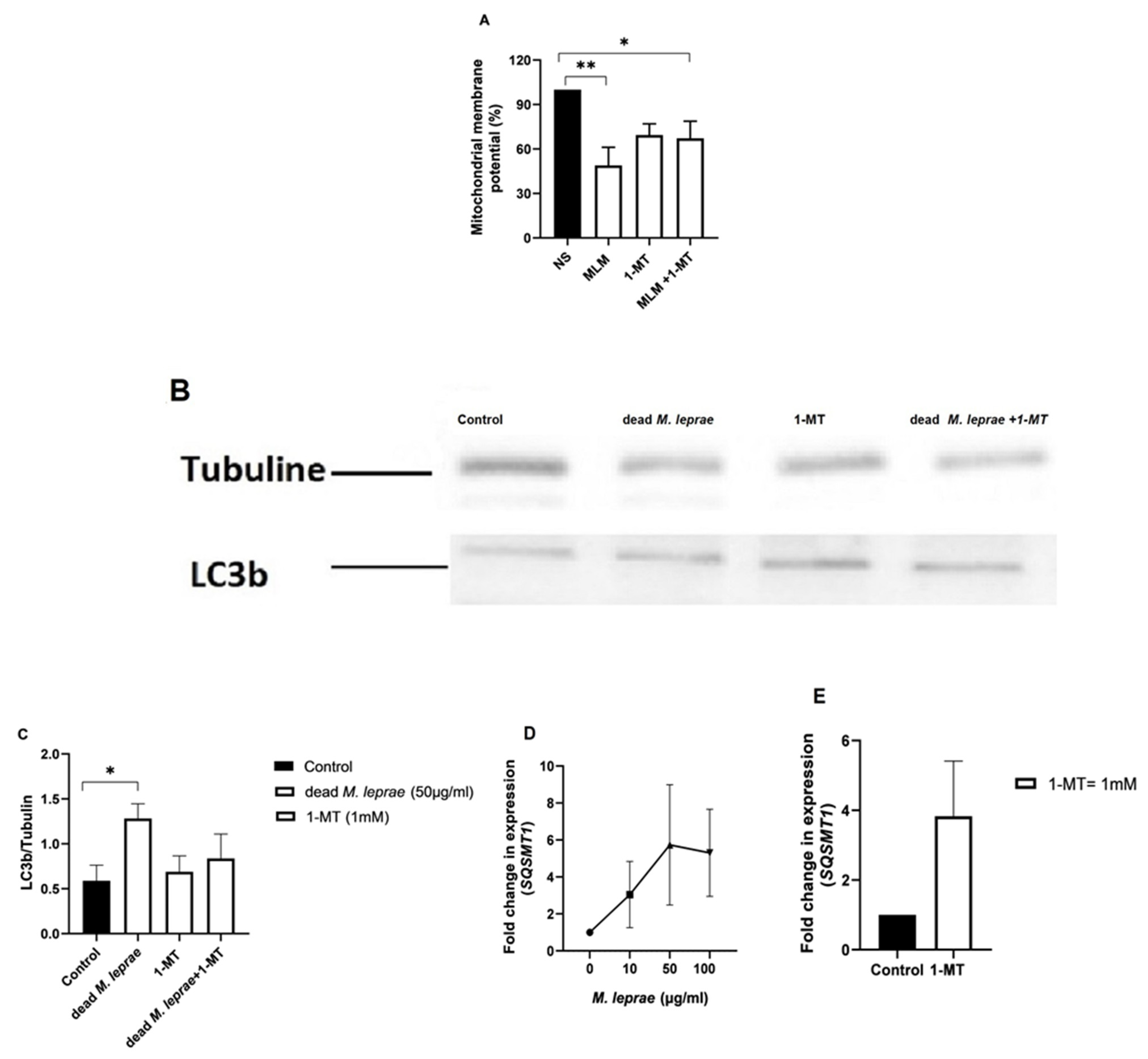
M. leprae, 1-MT, or their combinations for 24 h. Metabolic activity was assessed using the MTT assay (Thermo Fisher). MTT solution (10 μL, 5 mg/mL) was added to each well; reduced formazan crystals were solubilized in DMSO and read at 590 nm using a SpectraMax 190 spectrophotometer (San Jose, CA, USA. Mitochondrial membrane potential (Δψm) was assessed using 1 nM TMRM (Sigma Aldrich–Cat. T5428, Burlington, MA, USA) staining. After 24 h incubation with irradiated
M. leprae (with or without 1-MT), Schwann cells (10,000/well) were stained for 20 min and imaged using the Cytell™ Cell Imaging System (GE Healthcare, Chicago, IL, USA), measuring changes in mitochondrial polarization [
3]. The results were shown by the ratio of the TMRM signal to the same signal from a culture preincubated with the proton ionophore CCCP (15 μM) (Sigma) for 10 min.
2.6. Cell Viability Assay
Cell viability was evaluated using the Differential Nuclear Staining (DNS) assay. Schwann cell line ST88-14 (10,000/well) were stained with Hoechst 33,342 (Thermo Fisher Scientific, Waltham, MA, USA) for 30 min and Propidium Iodide (PI) (Life Technologies Corporation, Eugene, OR, USA) for 5 min. Hoechst stains all nuclei, while PI selectively stains dead cells. Fluorescence was quantified using the Cytell system [
23].
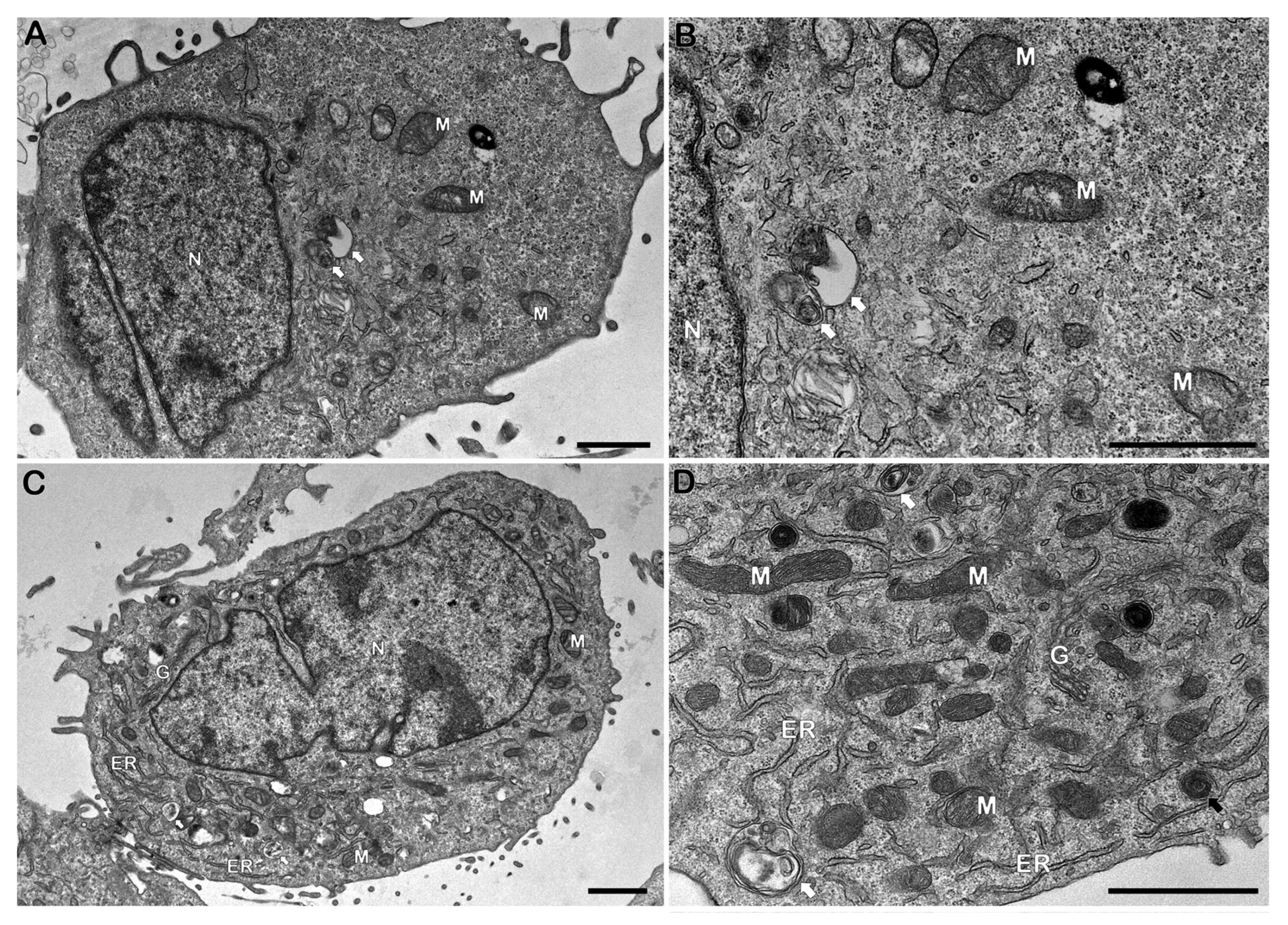
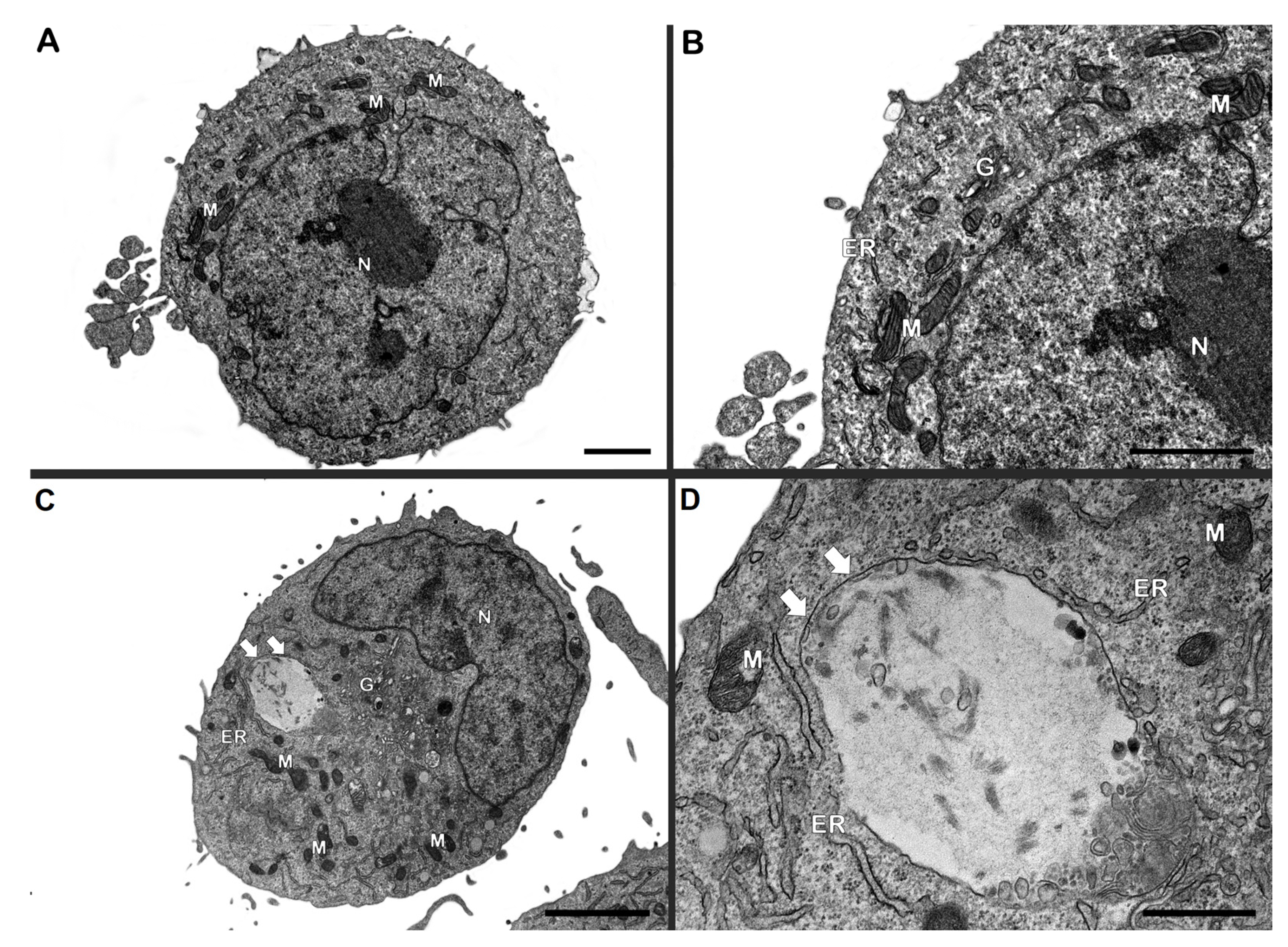
2.7. Transmission Electron Microscopy (TEM) Analysis
Schwann cell line ST88-14 (5 × 105/well) were incubated with irradiated M. leprae (50 μg/mL), with or without 1-MT, for 24 h. Cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, post-fixed with 1% OsO4, dehydrated in graded acetone, and embedded in PolyBed 812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate and imaged with a JEM1011 (Jeol, Akishima, Tokyo, Japan) or HT7800 (Hitachi, Tokyo, Japan) microscope at the Electron Microscopy Platform, Instituto Oswaldo Cruz (Fiocruz).
2.8. Western Blotting
To assess autophagy, LC3B expression was evaluated. Schwann cell line ST88-14 (4 × 105/well) were lysed after 24 h incubation. Proteins (20 μg/sample) were separated by 12% SDS-PAGE, transferred to nitrocellulose membranes, and blocked with 5% BSA or milk. Membranes were incubated overnight at 4 °C with anti-LC3B (1:1000) and anti-Tubulin (1:4000), followed by HRP-conjugated secondary antibodies (1:1000). Detection was performed using ECL and visualized on an iBright CL750 system. Densitometric analysis was performed in ImageJ 1.53t.
2.9. RNA Extraction and RT-qPCR
Total RNA was extracted with TRIzol (Invitrogen) following the manufacturer’s instructions, treated with DNase, and quantified using NanoDrop (ThermoFisher Scientific). cDNA synthesis (1500 ng RNA) was performed according to the manufacturer instruction with the Superscript III kit (Invitrogen). RT-qPCR was carried out with SYBR Green Master Mix (Applied Biosystems) on a StepOnePlus™ system using a Schwann cell-targeted primer array (GenOne, Brazil). SQSTM1 was evaluated and GAPDH and RPL13A served as reference genes for normalization. Data was analyzed with LinRegPCR (2021.2.0.0). While for genes GPX4 (Hs00989766_g1), NFE2L2 (Hs00232352_m1), NCOA4 (Hs00428331_g1), HMOX (Hs01110250_m1), and ACSL4 (Hs00244871_m1), TaqMan assays (Thermo Fisher Scientific) with FAM probe were used for expression analysis. The TaqMan data was analyzed using 2−ΔΔCT method and normalized using the ACTB (Hs01060665_g1) and GAPDH (Hs99999905_m1) as internal control.
2.10. Statistical Analysis
All data were analyzed using GraphPad Prism version 8.0.2 (GraphPad Software, San Diego, CA, USA). Data distribution was assessed for normality using the Shapiro–Wilk test, and homogeneity of variances was evaluated using Levene’s test. For datasets that met assumptions of normality and equal variance, parametric tests (e.g., one-way ANOVA followed by Tukey’s multiple comparison test) were applied. For non-normally distributed data or data with unequal variances, non-parametric tests were used, including the Kruskal–Wallis test followed by Dunn’s multiple comparison test.
Results are presented as mean ± SD, as indicated in the figure legends. The number of independent biological replicates (n) is reported in each figure panel. Statistical significance was defined as p ≤ 0.05. Where possible, exact p values and effect sizes are provided in the figure legends. Multiple comparisons were adjusted using appropriate post hoc tests, as specified above.
4. Discussion
Despite significant advances in molecular and cellular techniques in recent years—enabling a deeper understanding of the immunopathogenic mechanisms underlying various infectious diseases—many aspects of leprosy pathogenesis remain poorly understood. A major obstacle to progress in this field is the lack of a robust experimental model. Moreover, Mycobacterium leprae, the primary etiological agent, cannot be cultured in conventional laboratory media, further limiting research. One of the most pressing challenges is unraveling the mechanisms of leprosy-associated neuropathy.
It is well established that
M. leprae infects macrophages and adipocytes in the dermis, as well as Schwann cells in peripheral nerves [
28]. Nerve damage may result from both the direct interaction of the bacillus with infected cells and from inflammatory mediators produced by immune infiltrates. Although multidrug therapy (MDT) remains a cornerstone for controlling disease transmission, it is not effective in preventing or managing nerve damage. Therefore, elucidating the immunopathogenic mechanisms involved in neural injury is critical for the development of adjunctive therapies aimed at reducing both infection and tissue damage.
In this study, we demonstrate for the first time that M. leprae can modulate indoleamine 2,3-dioxygenase (IDO1) expression and activity in Schwann cells. Our results show a significant increase in IDO1 expression in M. leprae-stimulated ST88-14 Schwann cells compared to controls. Notably, primary Schwann cells also exhibited elevated IDO1 expression, supporting a potential link between IDO1 activity and nerve damage. While these results were derived from distinct experimental models—ST88-14 cells in vitro and primary Schwann cells in the GSE40950 transcriptomic dataset—we observed consistent upregulation of IDO1 across both, reinforcing the robustness of the finding.
In the inflammatory milieu typical of leprosy neuropathy, bacillary death is common, and antigenic stimulation of resident cells occurs through both viable and dead bacilli. Thus, the immune response induced by irradiated M. leprae in vitro likely mimics key antigenic stimuli present in infected tissues. This supports the notion that, at least with regard to IDO1 modulation, the use of irradiated bacilli serves as a valid and practical experimental surrogate. Despite differences in cell type, temperature, and bacterial viability, the convergence of findings across models underscores the biological relevance of IDO1 induction in Schwann cell responses to M. leprae.
In the present study, we employed irradiated, non-viable
M. leprae, which cannot actively transcribe mRNA and therefore does not fully engage pathways dependent on live bacilli, such as type I IFN induction via STING/TBK1/IRF3 signaling [
29]. It may represent a limitation when considering responses that require transcriptionally active bacteria. However, in the context of IDO1 induction and metabolic modulation in Schwann cells, the contribution of IL-10, a downstream gene of type I IFN pathway, appears as pivotal as type I IFNs in shaping the regulatory response.
IL-10 produced by Schwann cells or surrounding immune cells can robustly induce and sustain IDO expression, promoting a tolerogenic and immunomodulatory environment even in the absence of live bacilli. Therefore, while the use of irradiated M. leprae does not capture the full spectrum of type I IFN–dependent responses, it remains a valid and informative model for interrogating pathways where IL-10–driven regulation of IDO is central, reflecting a physiologically relevant component of the inflammatory milieu observed in leprosy lesions. Although we recognize the inherent limitations of using non-viable bacilli, this approach allows mechanistic insights into IDO-mediated metabolic modulation, highlighting pathways that are operative in vivo and complementing studies that focus on live bacilli–dependent immune responses.
It is well documented that
M. leprae disrupts Schwann cell metabolism [
3]. Mitochondria—central organelles for energy production and immune signaling—regulate redox balance, apoptosis, inflammasome activation, and xenophagy. Schwann cells infected by
M. leprae show a reduction in mitochondrial membrane potential (Δψm), increased glucose uptake, decreased mitochondrial activity, and reduced lactate production. These metabolic alterations reflect a shift toward glycolysis, accompanied by diminished pyruvate dehydrogenase activity and HIF-1α stabilization. These changes contribute to mitochondrial dysfunction, reduced β-oxidation of lipids, accumulation of lipid bodies, loss of axonal metabolic support, and ultimately nerve damage [
3,
30,
31]. Our findings are consistent with these observations, demonstrating that
M. leprae antigens impair Schwann cell metabolic function.
This mitochondrial dysfunction may reflect an adaptive cellular response aimed at limiting oxidative stress. Transcriptomic data have shown a downregulation of genes involved in the mitochondrial respiratory chain during
M. leprae infection [
24]. Mitochondria are major sources of reactive oxygen species (ROS), particularly superoxide anions produced at complexes I and III of the electron transport chain. These ROS can be converted into peroxynitrite and hydrogen peroxide, leading to damage of
M. leprae lipids, proteins, and DNA [
32,
33].
In our study, Schwann cells exhibited decreased mitochondrial membrane potential (Δψm) and reduced mitochondrial activity following exposure to M. leprae, suggesting mitochondrial dysfunction in the absence of cell death. This is likely due to the activation of survival-promoting mechanisms.
Our results further show that inhibition of IDO1 using 1-methyl-L-tryptophan (1-MT) reduced Schwann cell viability and metabolic activity in the presence of M. leprae, as measured by the MTT assay. This finding suggests that IDO1 plays a supportive role in maintaining metabolic homeostasis during infection-induced stress. IDO1 catalyzes the first and rate-limiting step in the degradation of tryptophan via the kynurenine pathway, generating metabolites that influence redox balance, mitochondrial function, and NAD+ biosynthesis. Inhibition of this pathway may disrupt these processes, leading to impaired mitochondrial activity and increased cellular vulnerability. Since the MTT assay relies on the activity of mitochondrial dehydrogenases, it is sensitive to changes in metabolic and redox status. The observed reduction in MTT activity following IDO1 inhibition likely reflects compromised mitochondrial function. Additionally, reduced kynurenine levels may impair immunometabolic signaling, further contributing to the observed effects. These findings support the hypothesis that IDO1 contributes not only to immune modulation but also to the metabolic adaptation of Schwann cells during M. leprae infection.
Although this study did not comprehensively assess the autophagic process, we evaluated key markers, including LC3-II and p62, to obtain preliminary evidence of potential modulation. Our primary aim was not to fully characterize the autophagy pathway but rather to lay the groundwork for future studies exploring how M. leprae and IDO1 activity may influence autophagic flux in Schwann cells. Previous work from our group has shown that IDO1 may play either immunoregulatory or effector roles, depending on the inflammatory context. It is therefore plausible that the effect of IDO1 on autophagy is similarly context dependent.
To support the hypothesis that inflammatory mediators can modulate autophagy in Schwann cells, we showed that IFN-γ treatment increases autophagic flux in ST88-14 cells (
Supplementary Materials Figure S1). This was visualized using monodansylcadaverine (MDC), a fluorescent marker of autophagic vacuoles, in combination with LysoTracker, confirming autolysosome formation under inflammatory stimulation. These findings reinforce the concept that Schwann cells have functional autophagy machinery that is responsive to inflammatory cues. Although we did not observe marked ultrastructural or molecular evidence of autophagy following
M. leprae stimulation in this model, our results—together with the IFN-γ data and previous studies demonstrating
M. leprae-induced myelinophagy in murine Schwann cells [
34]—highlight the potential relevance of this pathway.
Future studies will focus on dissecting the mechanisms through which M. leprae and IDO1 influence autophagy, and how this interaction affects bacterial persistence and nerve damage in the context of leprosy neuropathy. Notably, our findings demonstrate that inhibition of IDO1 with 1-MT significantly reduces both metabolic activity and viability in human Schwann cells, independent of M. leprae infection. This underscores the central role of IDO1 in maintaining Schwann cell homeostasis. Through its contribution to NAD+ biosynthesis—an essential cofactor for mitochondrial energy metabolism—IDO1 activation appears critical for sustaining mitochondrial function and overall cell viability. Inhibition of this pathway may impair ATP production and lead to metabolic failure.
Furthermore, IDO1 inhibition led to the accumulation of p62 (
SQSTM1), a key adaptor protein involved in autophagy. Under physiological conditions, p62 is continuously degraded through autophagic flux; its accumulation indicates impaired autophagy [
35]. This suggests that IDO1 activity is necessary to maintain effective autophagic degradation in Schwann cells. Given the importance of autophagy in clearing damaged organelles and maintaining proteostasis [
36,
37], impaired autophagic flux could contribute to cellular stress and functional decline.
Previous studies from our group have shown that in skin lesion cells from multibacillary patients, there is an increase in IDO1 expression [
9,
10], accompanied by elevated levels of CD163—a scavenger receptor that binds haptoglobin-hemoglobin complexes and contributes to increased intracellular iron stores [
10]. This accumulation of intracellular iron is associated with upregulation of the ferritin receptor and downregulation of ferroportin expression in macrophages from multibacillary patients [
38].
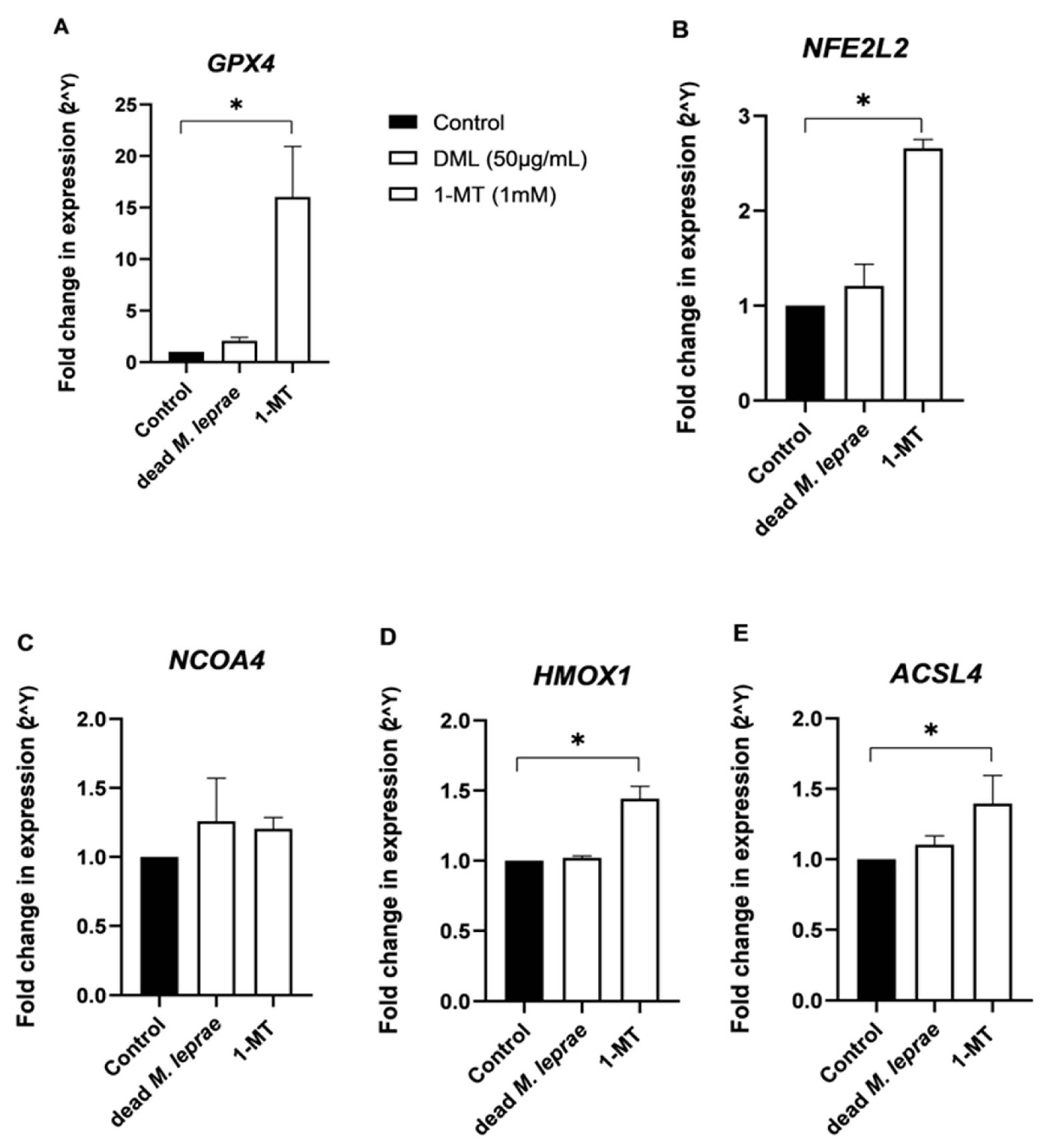
Iron homeostasis plays a significant role in the regulation of ferroptosis, a form of iron-dependent lipid peroxidation that is negatively regulated by glutathione peroxidase 4 (
GPX4) [
39,
40]. Elevated intracellular iron is a key factor in triggering this form of programmed cell death [
41]. These observations support a link between increased IDO1 expression, altered iron metabolism, and enhanced susceptibility to ferroptosis in the context of leprosy.
Interestingly, despite evidence of reduced metabolic activity, mitochondrial morphology remained largely unaltered under electron microscopy. Although the MTT assay and mitochondrial membrane potential measurements showed a reduction in metabolic activity and mitochondrial function following stimulation with dead M. leprae, no significant alterations in mitochondrial or host cell morphology were observed at the 24 h time point.
This apparent discrepancy may be explained by the sensitivity and nature of the assays used. Functional assays like MTT and membrane potential assessments can detect early or subtle metabolic impairments that precede visible structural changes. Mitochondrial dysfunction can occur without immediate morphological damage, particularly during the early stages of stress or cellular adaptation [
42,
43]. Additionally, mitochondrial morphology is highly dynamic and regulated by fission–fusion processes, which may temporarily preserve organelle structure despite functional compromise. It is also possible that longer exposure times are required for ultrastructural alterations to become apparent, or that compensatory cellular mechanisms—such as antioxidant responses or autophagy—transiently maintain mitochondrial and cellular integrity under stress conditions induced by dead
M. leprae.
M. leprae also induced the upregulation of the NFE2L2 (NRF2) → GPX4/HMOX1 antioxidant response pathway. NRF2 is a master regulator of antioxidant defense; GPX4 inhibits lipid peroxidation and ferroptosis, while HMOX1 degrades heme into cytoprotective byproducts. Activation of this antioxidant axis suggests a compensatory response to oxidative stress triggered by mitochondrial dysfunction and impaired autophagy.
Taken together, our findings suggest that M. leprae modulates Schwann cell homeostasis through multiple, interconnected mechanisms involving immunometabolic reprogramming, mitochondrial dysfunction, autophagy modulation, and redox imbalance. The upregulation of IDO1 in both established and primary Schwann cell models reinforces its central role in cellular metabolism and immune regulation during infection. The observed reduction in cell viability and metabolic activity following IDO1 inhibition further underscores the enzyme’s importance for Schwann cell survival, even in the absence of bacilli. By contributing to NAD+ biosynthesis, IDO1 may support mitochondrial function and energy production, particularly under stress conditions. Moreover, its influence on autophagic flux—evidenced by p62 accumulation—indicates that IDO1 is also essential for maintaining cellular proteostasis, which is critical in metabolically active cells such as Schwann cells.
The mitochondrial and autophagic alterations observed following exposure to M. leprae antigens appear to reflect a complex adaptive response. Despite measurable declines in metabolic activity and mitochondrial membrane potential, the lack of marked ultrastructural changes at early time points suggests activation of compensatory mechanisms. The induction of the NRF2–GPX4–HMOX1 antioxidant pathway further supports the idea that Schwann cells mount a cytoprotective response to infection-related oxidative stress. Importantly, this pathway also intersects with ferroptosis regulation, suggesting that M. leprae may actively modulate the redox environment of host cells to prevent cell death and promote persistence.
Together, these findings provide new insights into how metabolic and redox signaling pathways contribute to Schwann cell vulnerability and resilience during M. leprae infection and highlight IDO1 as a potential therapeutic target for mitigating nerve damage in leprosy.