Checkpoint Imbalance in Primary Glomerulopathies: Comparative Insights into IgA Nephropathy and Membranoproliferative Glomerulonephritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Study Participants, Eligibility Criteria, and Study Material
2.2. Isolation of Peripheral Blood Mononuclear Cells (PBMC)
2.3. RNA Isolation, cDNA Synthesis, and qPCR Analysis
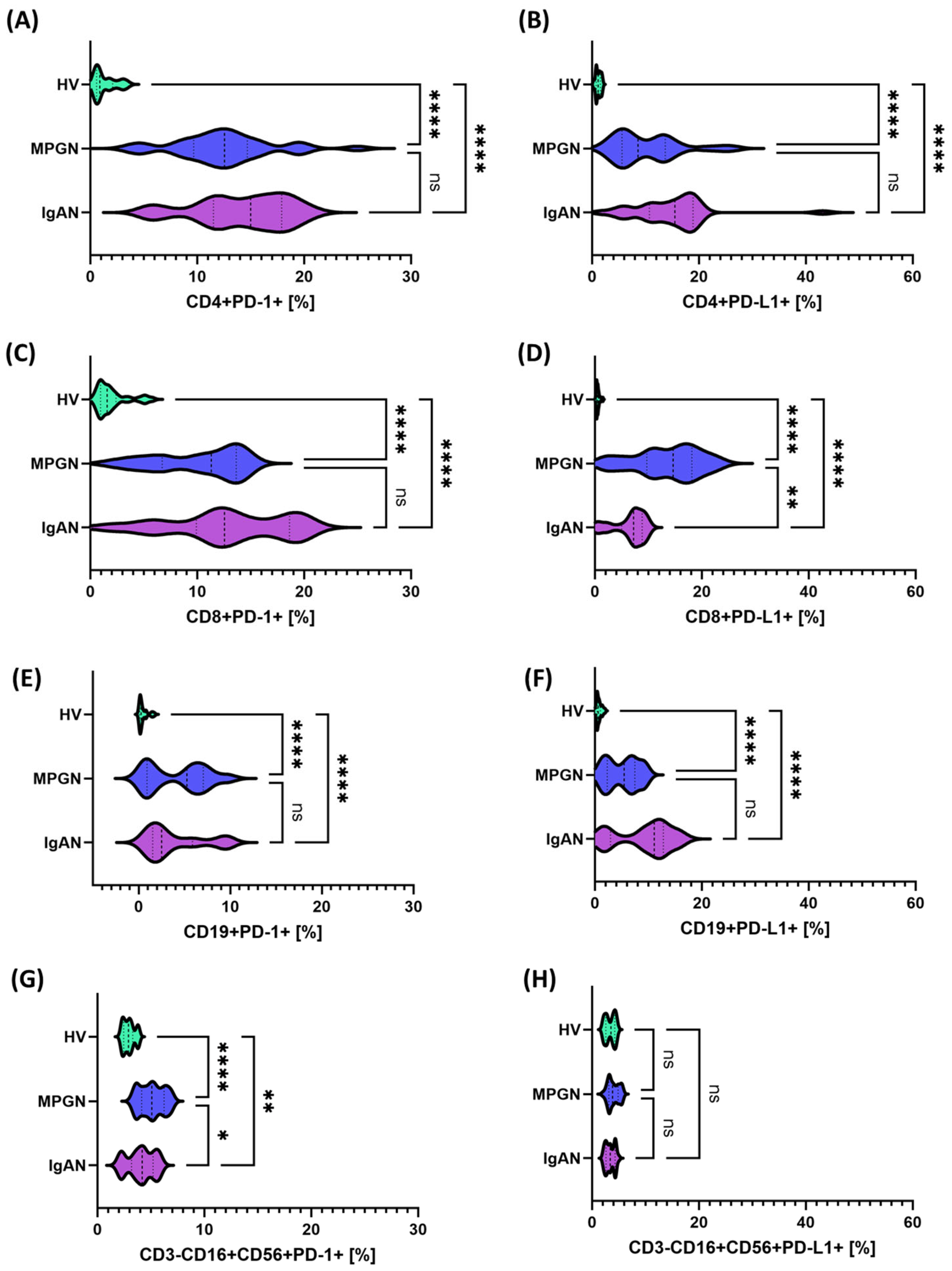
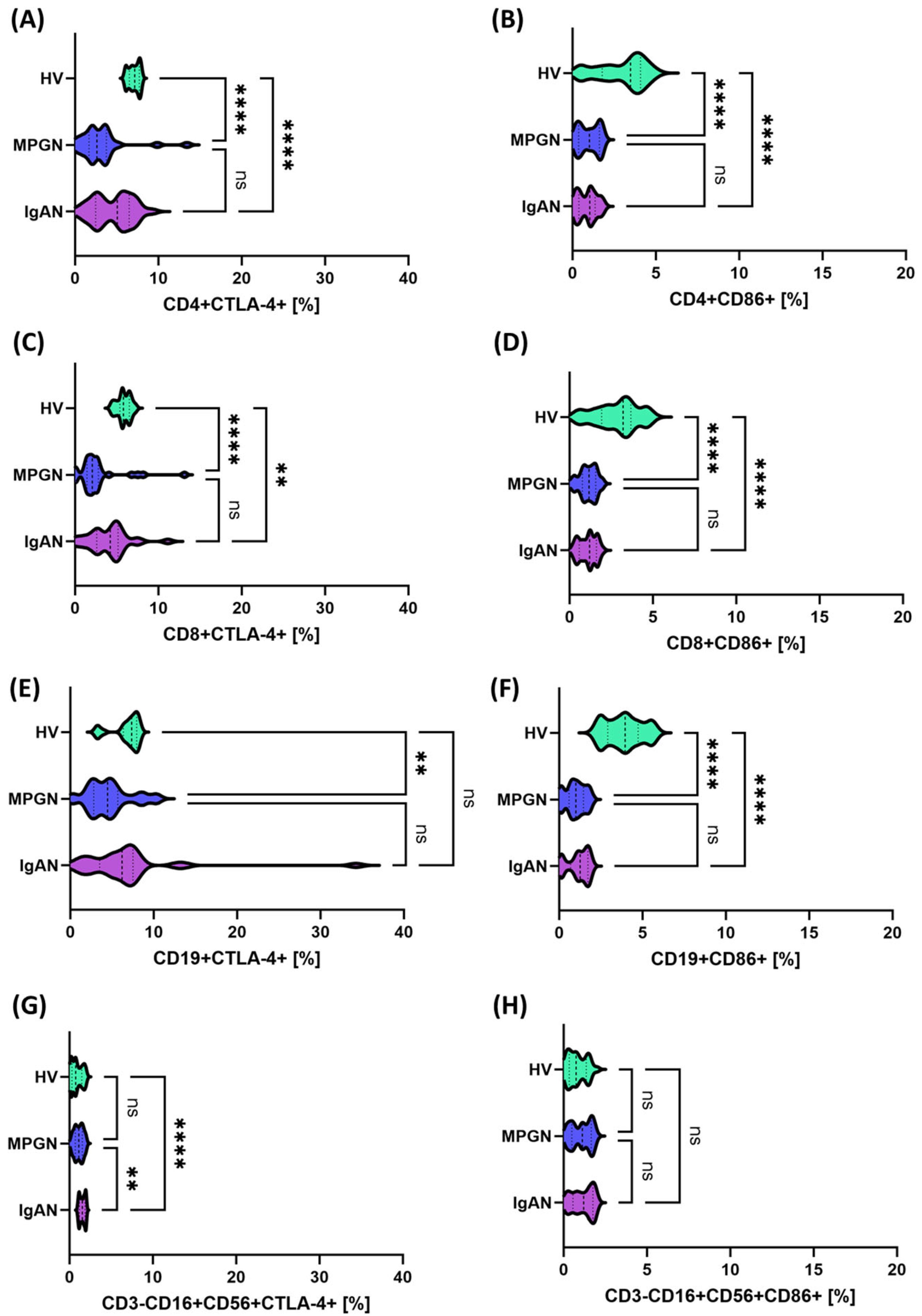
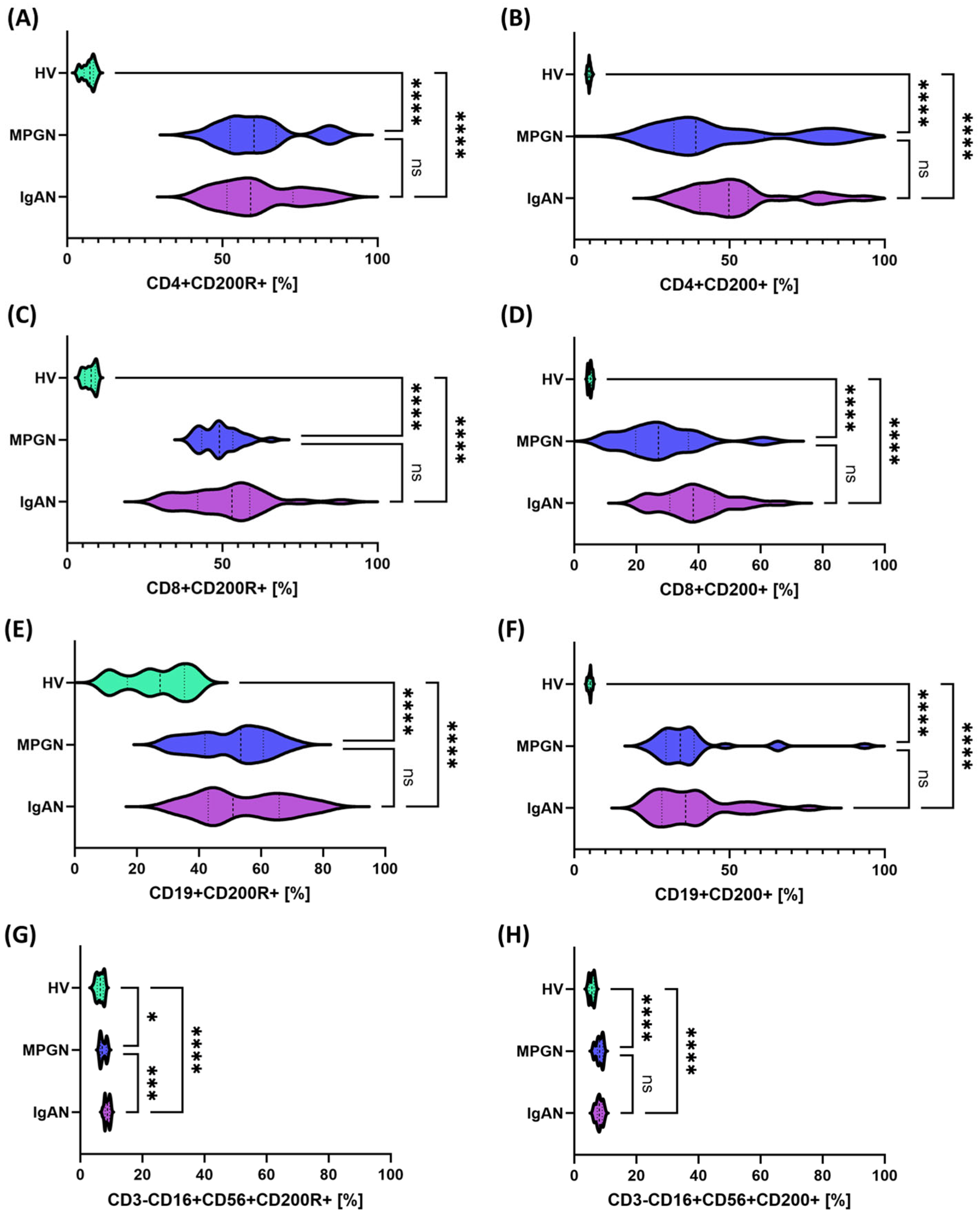
2.4. Immunophenotyping and Analysis of PD-1, PD-L1, CTLA-4, CD86, CD200 and CD200R Expression on Lymphocyte Subpopulations
2.5. Determination of Serum Control Point Concentrations by ELISA
2.6. Statistical Analysis
3. Results
3.1. Analysis of Basic Hematological, Biochemical, and Immunological Parameters of Patients with IgAN, MPGN, and Healthy Volunteers Recruited to the Study
3.2. Expression of Immune Checkpoints on the Cell Surface, Concentrations of Their Serum Soluble Forms, and Transcript Levels in PBMCs from Patients with IgAN, MPGN, and Controls
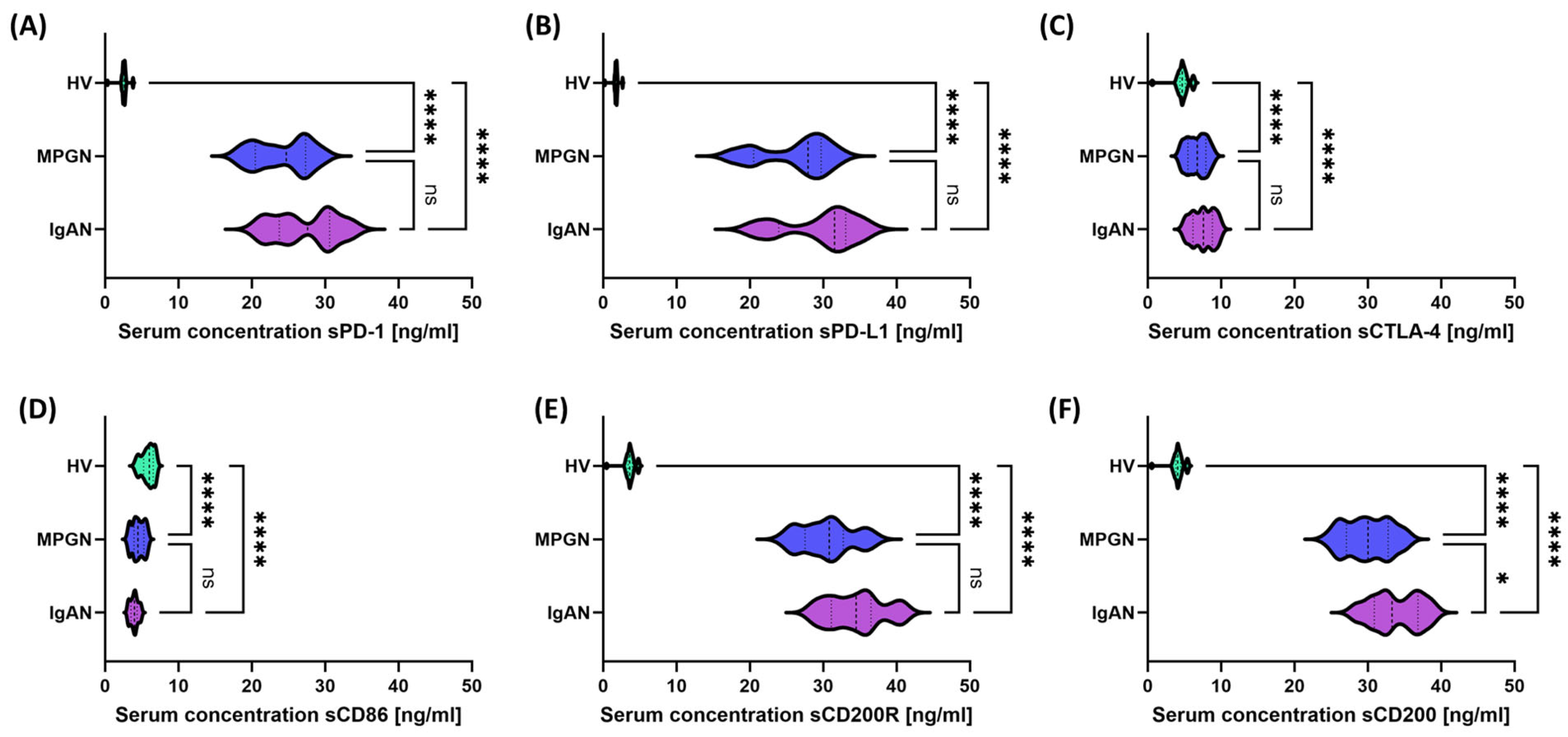
3.3. Comparison of Serum Concentrations and Gene Expression of Selected Immune Checkpoints and Their Ligands in Patients with IgAN, MPGN, and HV
3.4. Similarities and Differences in the Assessment of Immunological Control Parameters Depending on the Gender of Patients with IgAN, MPGN, and HV
3.5. Common Biochemical-Immunological Correlations in IgAN and MPGN as a Potential Reflection of Similar Pathogenetic Mechanisms
3.6. ROC-Based Assessment of Similarities and Differences in Immune Checkpoint Profiles Between IgAN, MPGN, and Healthy Controls
4. Discussion
4.1. The Role of the PD-1/PD-L1, CTLA-4/CD86 and CD200/CD200R Axes in the Pathogenesis of IgAN and MPGN
4.2. Therapeutic Potential of Immune Checkpoints in GN
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| IgAN | IgA nephropathy |
| MPGN | Membranoproliferative glomerulonephritis |
| HV | Healthy volunteers |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| CTLA-4 | Cytotoxic T-lymphocyte–associated protein 4 |
| CD86 | Cluster of differentiation 86 |
| CD200R | CD200 receptor |
| CD200 | Cluster of differentiation 200 |
| PBMC | Peripheral blood mononuclear cells |
| ROC | Receiver operating characteristic |
| sPD-1 | Soluble PD-1 |
| sPD-L1 | Soluble PD-L1 |
| sCTLA-4 | Soluble CTLA-4 |
| sCD86 | Soluble CD86 |
| sCD200 | Soluble CD200 |
| sCD200R | Soluble CD200R |
| GN | Glomerulonephritis |
| NK | Natural killer (cells) |
| ELISA | Enzyme-linked immunosorbent assay |
| CD4+/CD8+ T cells | T lymphocyte subsets expressing CD4 or CD8 |
| CD19+ B cells | B lymphocytes expressing CD19 |
References
- Faucon, A.; Lando, S.; Chrysostomou, C.; Wijkström, J.; Lundberg, S.; Bellocco, R.; Segelmark, M.; Evans, M.; Carrero, J. Primary Glomerular Diseases and Long-term Adverse Health Outcomes: A Nationwide Cohort Study. J. Intern. Med. 2025, 297, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Kitching, A.R.; Leung, N.; Romagnani, P. Glomerulonephritis: Immunopathogenesis and Immunotherapy. Nat. Rev. Immunol. 2023, 23, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Windpessl, M.; Odler, B.; Bajema, I.M.; Geetha, D.; Säemann, M.; Lee, J.M.; Vaglio, A.; Kronbichler, A. Glomerular Diseases Across Lifespan: Key Differences in Diagnostic and Therapeutic Approaches. Semin. Nephrol. 2023, 43, 151435. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, K.; Safa, A.; Kotha, S.; Ratti, G.; Meri, S. Complement Dysregulation in Glomerulonephritis. Semin. Immunol. 2019, 45, 101331. [Google Scholar] [CrossRef]
- Couser, W.G.; Johnson, R.J. The Etiology of Glomerulonephritis: Roles of Infection and Autoimmunity. Kidney Int. 2014, 86, 905–914. [Google Scholar] [CrossRef]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The Role of the Immune System in Kidney Disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef]
- Kazi, A.M.; Hashmi, M.F. Glomerulonephritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tan, L.; Jin, L.-H.; Wang, Y.-Q.; Chen, W.; Wen, Q. Clinical Features, Pathological Characteristics, and Prognosis of Patients with IgA Nephropathy Complicated with Nephrotic Syndrome. Sci. Rep. 2025, 15, 1804. [Google Scholar] [CrossRef]
- Petrou, D.; Kalogeropoulos, P.; Liapis, G.; Lionaki, S. IgA Nephropathy: Current Treatment and New Insights. Antibodies 2023, 12, 40. [Google Scholar] [CrossRef]
- Wendt, R.; Sobhani, A.; Diefenhardt, P.; Trappe, M.; Völker, L.A. An Updated Comprehensive Review on Diseases Associated with Nephrotic Syndromes. Biomedicines 2024, 12, 2259. [Google Scholar] [CrossRef]
- Sethi, S.; Nester, C.M.; Smith, R.J.H. Membranoproliferative Glomerulonephritis and C3 Glomerulopathy: Resolving the Confusion. Kidney Int. 2012, 81, 434–441. [Google Scholar] [CrossRef]
- Alchi, B.; Jayne, D. Membranoproliferative Glomerulonephritis. Pediatr. Nephrol. 2010, 25, 1409–1418. [Google Scholar] [CrossRef]
- Yu, S.M.-W.; Deoliveira, M.; Chung, M.; Lafayette, R. Membranoproliferative Glomerulonephritis Pattern of Injury. Adv. Kidney Dis. Health 2024, 31, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Fervenza, F.C.; Sethi, S. Membranoproliferative Glomerulonephritis: Classification, Clinical Features, and Diagnosis. Available online: https://www.uptodate.com/contents/membranoproliferative-glomerulonephritis-classification-clinical-features-and-diagnosis (accessed on 4 September 2025).
- Knoppova, B.; Reily, C.; Maillard, N.; Rizk, D.V.; Moldoveanu, Z.; Mestecky, J.; Raska, M.; Renfrow, M.B.; Julian, B.A.; Novak, J. The Origin and Activities of IgA1-Containing Immune Complexes in IgA Nephropathy. Front. Immunol. 2016, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.C.; Cheung, C.K.; Barratt, J. New Insights into the Pathogenesis of IgA Nephropathy. Pediatr. Nephrol. 2018, 33, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; Gharavi, A.G.; et al. The Pathophysiology of IgA Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795. [Google Scholar] [CrossRef]
- Such-Gruchot, A.; Mizerska-Wasiak, M.; Płatos, E.; Pańczyk-Tomaszewska, M. Modern Biomarkers in IgA Nephropathy and Their Potential in Paediatric Research. J. Clin. Med. 2025, 14, 3263. [Google Scholar] [CrossRef]
- Ponticelli, C.; Calatroni, M.; Moroni, G. C3 Glomerulopathies: Dense Deposit Disease and C3 Glomerulonephritis. Front. Med. 2023, 10, 1289812. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Serafinelli, J.; Giani, M.; Montini, G. Clinical and Pathophysiological Insights into Immunological Mediated Glomerular Diseases in Childhood. Front. Pediatr. 2020, 8, 205. [Google Scholar] [CrossRef]
- Noris, M.; Donadelli, R.; Remuzzi, G. Autoimmune Abnormalities of the Alternative Complement Pathway in Membranoproliferative Glomerulonephritis and C3 Glomerulopathy. Pediatr. Nephrol. 2019, 34, 1311–1323. [Google Scholar] [CrossRef]
- Michels, M.A.H.M.; van de Kar, N.C.A.J.; van Kraaij, S.A.W.; Sarlea, S.A.; Gracchi, V.; Engels, F.A.P.T.; Dorresteijn, E.M.; van der Deure, J.; Duineveld, C.; Wetzels, J.F.M.; et al. Different Aspects of Classical Pathway Overactivation in Patients with C3 Glomerulopathy and Immune Complex-Mediated Membranoproliferative Glomerulonephritis. Front. Immunol. 2021, 12, 715704. [Google Scholar] [CrossRef]
- Ibis, B.; Aliazis, K.; Cao, C.; Yenyuwadee, S.; Boussiotis, V.A. Immune-Related Adverse Effects of Checkpoint Immunotherapy and Implications for the Treatment of Patients with Cancer and Autoimmune Diseases. Front. Immunol. 2023, 14, 1197364. [Google Scholar] [CrossRef] [PubMed]
- Paluch, C.; Santos, A.M.; Anzilotti, C.; Cornall, R.J.; Davis, S.J. Immune Checkpoints as Therapeutic Targets in Autoimmunity. Front. Immunol. 2018, 9, 2306. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, H.; Safira, A.; Fetarayani, D. From Tumor to Tolerance: A Comprehensive Review of Immune Checkpoint Inhibitors and Immune-Related Adverse Events. Asia Pac. Allergy 2024, 14, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Cheng, Z.; Jiang, Z.; Gan, L.; Zhang, Z.; Xie, Z. Immunomodulatory Precision: A Narrative Review Exploring the Critical Role of Immune Checkpoint Inhibitors in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5490. [Google Scholar] [CrossRef]
- Śledzińska, A.; Menger, L.; Bergerhoff, K.; Peggs, K.S.; Quezada, S.A. Negative Immune Checkpoints on T Lymphocytes and Their Relevance to Cancer Immunotherapy. Mol. Oncol. 2015, 9, 1936–1965. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune Checkpoint Molecules in Natural Killer Cells as Potential Targets for Cancer Immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Brusa, D.; Serra, S.; Coscia, M.; Rossi, D.; D’Arena, G.; Laurenti, L.; Jaksic, O.; Fedele, G.; Inghirami, G.; Gaidano, G.; et al. The PD-1/PD-L1 Axis Contributes to T-Cell Dysfunction in Chronic Lymphocytic Leukemia. Haematologica 2013, 98, 953–963. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- López-Novoa, J.M.; Rodríguez-Peña, A.B.; Ortiz, A.; Martínez-Salgado, C.; López Hernández, F.J. Etiopathology of Chronic Tubular, Glomerular and Renovascular Nephropathies: Clinical Implications. J. Transl. Med. 2011, 9, 13. [Google Scholar] [CrossRef]
- Sansom, D.M. CD28, CTLA-4 and Their Ligands: Who Does What and to Whom? Immunology 2000, 101, 169–177. [Google Scholar] [CrossRef]
- Soskic, B.; Jeffery, L.E.; Kennedy, A.; Gardner, D.H.; Hou, T.Z.; Halliday, N.; Williams, C.; Janman, D.; Rowshanravan, B.; Hirschfield, G.M.; et al. CD80 on Human T Cells Is Associated with FoxP3 Expression and Supports Treg Homeostasis. Front. Immunol. 2021, 11, 577655. [Google Scholar] [CrossRef] [PubMed]
- Babamohamadi, M.; Mohammadi, N.; Faryadi, E.; Haddadi, M.; Merati, A.; Ghobadinezhad, F.; Amirian, R.; Izadi, Z.; Hadjati, J. Anti-CTLA-4 Nanobody as a Promising Approach in Cancer Immunotherapy. Cell Death Dis. 2024, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, W.; Jin, S.; Wan, S.; Wu, S. Inflammation in Glomerular Diseases. Front. Immunol. 2025, 16, 1526285. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Barratt, J.; Beck, L.H.; Fakhouri, F.; Gale, D.P.; Goicoechea de Jorge, E.; Mosca, M.; Noris, M.; Pickering, M.C.; Susztak, K.; et al. The Role of Complement in Kidney Disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2024, 106, 369–391. [Google Scholar] [CrossRef]
- Grywalska, E.; Smarz-Widelska, I.; Mertowski, S.; Gosik, K.; Mielnik, M.; Podgajna, M.; Abramiuk, M.; Drop, B.; Roliński, J.; Załuska, W. CTLA-4 Expression Inversely Correlates with Kidney Function and Serum Immunoglobulin Concentration in Patients with Primary Glomerulonephritides. Arch. Immunol. Ther. Exp. 2019, 67, 335–349. [Google Scholar] [CrossRef]
- Kitching, A.R.; Huang, X.R.; Ruth, A.-J.; Tipping, P.G.; Holdsworth, S.R. Effects of CTLA4-Fc on Glomerular Injury in Humorally-Mediated Glomerulonephritis in BALB/c Mice. Clin. Exp. Immunol. 2002, 128, 429–435. [Google Scholar] [CrossRef]
- Broka, A.; Young, B.Y.; Hamdan, H.; Dimitriades, V.R.; Jen, K.-Y.; Wiegley, N. CTLA-4 Haploinsufficiency Presenting with IgA Nephropathy: TH-PO708. J. Am. Soc. Nephrol. 2024, 35. [Google Scholar] [CrossRef]
- Oweira, H.; Khajeh, E.; Mohammadi, S.; Ghamarnejad, O.; Daniel, V.; Schnitzler, P.; Golriz, M.; Mieth, M.; Morath, C.; Zeier, M.; et al. Pre-Transplant CD200 and CD200R1 Concentrations Are Associated with Post-Transplant Events in Kidney Transplant Recipients. Medicine 2019, 98, e17006. [Google Scholar] [CrossRef]
- Jenmalm, M.C.; Cherwinski, H.; Bowman, E.P.; Phillips, J.H.; Sedgwick, J.D. Regulation of Myeloid Cell Function through the CD200 Receptor. J. Immunol. 2006, 176, 191–199. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Hu, A.; Zhu, J.; Yu, J.; Talebian, F.; Bai, X.-F. CD200-CD200R Pathway in the Regulation of Tumor Immune Microenvironment and Immunotherapy. Adv. Exp. Med. Biol. 2020, 1223, 155–165. [Google Scholar] [CrossRef]
- Nip, C.; Wang, L.; Liu, C. CD200/CD200R: Bidirectional Role in Cancer Progression and Immunotherapy. Biomedicines 2023, 11, 3326. [Google Scholar] [CrossRef]
- Shao, A.; Owens, D.M. The Immunoregulatory Protein CD200 as a Potentially Lucrative yet Elusive Target for Cancer Therapy. Oncotarget 2023, 14, 96–103. [Google Scholar] [CrossRef]
- Kushlinskii, N.E.; Kovaleva, O.V.; Gratchev, A.N.; Alferov, A.A.; Kuzmin, Y.B.; Sokolov, N.Y.; Tsekatunov, D.A.; Ryzhavskaya, I.B.; Kuznetsov, I.N.; Kushlinskii, D.N.; et al. Assessing the Clinical Relevance of Soluble PD-1 and PD-L1: A Multi-Cohort Study Across Diverse Tumor Types and Prognostic Implications. Biomedicines 2025, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Liu, Y.; Yi, M.; Jiao, D.; Wu, K. Biological Characteristics and Clinical Significance of Soluble PD-1/PD-L1 and Exosomal PD-L1 in Cancer. Front. Immunol. 2022, 13, 827921. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chao, Y.; Li, W.; Wu, Z.; Wang, Q. Soluble Immune Checkpoint Molecules in Cancer Risk, Outcomes Prediction, and Therapeutic Applications. Biomark. Res. 2024, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Uemura, K.; Ito, N.; Sakai, Y.; Ohnishi, S.; Suekane, H.; Kurose, H.; Hiroshige, T.; Chikui, K.; Nishihara, K.; et al. Soluble Immune Checkpoint Molecules as Predictors of Efficacy in Immuno-Oncology Combination Therapy in Advanced Renal Cell Carcinoma. Curr. Oncol. 2024, 31, 1701–1712. [Google Scholar] [CrossRef]
- Righi, I.; Trabattoni, D.; Rosso, L.; Vaira, V.; Clerici, M. Immune Checkpoint Molecules in Solid Organ Transplantation: A Promising Way to Prevent Rejection. Immunol. Lett. 2024, 267, 106860. [Google Scholar] [CrossRef]
- Hayashi, A.; Ishihara, H.; Kawabe, M.; Kato, K.; Nakashima, A.; Yamamoto, I.; Sakano, T.; Kobashi, H.; Morita, M.; Yokoo, T.; et al. Increased Serum Soluble PD-L1 Levels in Patients with Advanced Stages of Chronic Kidney Disease. Front. Med. 2025, 12, 1530804. [Google Scholar] [CrossRef]
- Gomez-Preciado, F.; Martinez-Valenzuela, L.; Anton-Pampols, P.; Fulladosa, X.; Tena, M.G.; Gomà, M.; Jove, M.; Nadal, E.; Merino-Ribas, A.; Martin-Alemany, N.; et al. Urinary Soluble PD-1 as a Biomarker of Checkpoint Inhibitor-Induced Acute Tubulointerstitial Nephritis. Clin. Kidney J. 2024, 17, sfae200. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, J.; Jiang, X.; Yao, C.; Chen, H.; Ding, Y.; Niu, P.; Chen, Q.; Ding, H.; Shen, N. PD-1 Activation Mitigates Lupus Nephritis by Suppressing Hyperactive and Heterogeneous PD-1+CD8+ T Cells. Theranostics 2025, 15, 5029–5044. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-L.; Jiang, H.; Feng, W.; Liu, H.; Han, L.; Chen, Y.; Zhang, Q.; Zheng, F.; Lu, C.-J.; Dai, Z. Total Glucosides of Paeony Ameliorate Pristane-Induced Lupus Nephritis by Inducing PD-1 Ligands+ Macrophages via Activating IL-4/STAT6/PD-L2 Signaling. Front. Immunol. 2021, 12, 683249. [Google Scholar] [CrossRef] [PubMed]
- Grywalska, E.; Smarz-Widelska, I.; Korona-Głowniak, I.; Mertowski, S.; Gosik, K.; Hymos, A.; Ludian, J.; Niedźwiedzka-Rystwej, P.; Roliński, J.; Załuska, W. PD-1 and PD-L1 Expression on Circulating Lymphocytes as a Marker of Epstein-Barr Virus Reactivation-Associated Proliferative Glomerulonephritis. Int. J. Mol. Sci. 2020, 21, 8001. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Ge, Y.; Wu, Y. Causal Role of Immune Cells in IgA Nephropathy: A Mendelian Randomization Study. Ren. Fail. 2024, 46, 2381593. [Google Scholar] [CrossRef]
- Fehres, C.M.; van Uden, N.O.; Yeremenko, N.G.; Fernandez, L.; Franco Salinas, G.; van Duivenvoorde, L.M.; Huard, B.; Morel, J.; Spits, H.; Hahne, M.; et al. APRIL Induces a Novel Subset of IgA+ Regulatory B Cells That Suppress Inflammation via Expression of IL-10 and PD-L1. Front. Immunol. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Si, R.; Chen, J.; Qu, Z.; Jiang, Y. Increased Frequency of PD-1hiCXCR5- T Cells and B Cells in Patients with Newly Diagnosed IgA Nephropathy. Sci. Rep. 2020, 10, 492. [Google Scholar] [CrossRef]
- Kiryluk, K.; Sanchez-Rodriguez, E.; Zhou, X.J.; Zanoni, F.; Liu, L.; Mladkova, N.; Khan, A.; Marasa, M.; Zhang, J.Y.; Balderes, O.; et al. Genome-Wide Association Analyses Define Pathogenic Signaling Pathways and Prioritize Drug Targets for IgA Nephropathy. Nat. Genet. 2023, 55, 1091–1105. [Google Scholar] [CrossRef]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-Expressed CTLA-4 Depletes CD80/CD86 by Trogocytosis, Releasing Free PD-L1 on Antigen-Presenting Cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef]
- Zhao, Y.; Lee, C.K.; Lin, C.-H.; Gassen, R.B.; Xu, X.; Huang, Z.; Xiao, C.; Bonorino, C.; Lu, L.-F.; Bui, J.D.; et al. PD-L1:CD80 Cis-Heterodimer Triggers the Co-Stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways. Immunity 2019, 51, 1059–1073.e9. [Google Scholar] [CrossRef]
- Edner, N.M.; Carlesso, G.; Rush, J.S.; Walker, L.S.K. Targeting Co-Stimulatory Molecules in Autoimmune Disease. Nat. Rev. Drug Discov. 2020, 19, 860–883. [Google Scholar] [CrossRef]
- Bomback, A.S.; Charu, V.; Fakhouri, F. Challenges in the Diagnosis and Management of Immune Complex-Mediated Membranoproliferative Glomerulonephritis and Complement 3 Glomerulopathy. Kidney Int. Rep. 2025, 10, 17–28. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, F.; Jin, Y.; Fu, H.; Mao, J. Glomerular Diseases after Immune Checkpoint Inhibitors Use: What Do We Know so Far? Ren. Fail. 2022, 44, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Kitchlu, A.; Jhaveri, K.D.; Wadhwani, S.; Deshpande, P.; Harel, Z.; Kishibe, T.; Henriksen, K.; Wanchoo, R. A Systematic Review of Immune Checkpoint Inhibitor–Associated Glomerular Disease. Kidney Int. Rep. 2020, 6, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Yamani, N.E.; Cote, G.; Riopel, J.; Marcoux, N.; Mac-Way, F.; Philibert, D.; Agharazii, M. Pembrolizumab-Induced Anti-GBM Glomerulonephritis: A Case Report. Kidney Med. 2023, 5, 100682. [Google Scholar] [CrossRef]
- Abudayyeh, A.; Abdelrahim, M.; Selamet, U.; Workeneh, B.; Machado, S.; Lahoti, A.; Glass, W.F.; Tchakarov, A.; Tannir, N.M.; Abdel-Wahab, N.; et al. Checkpoint Inhibitor Induced Glomerulonephritis. J. Clin. Oncol. 2018, 36, e15083. [Google Scholar] [CrossRef]
- Miao, J.; Sise, M.E.; Herrmann, S.M. Immune Checkpoint Inhibitor Related Nephrotoxicity: Advances in Clinicopathologic Features, Noninvasive Approaches, and Therapeutic Strategy and Rechallenge. Front. Nephrol. 2022, 2, 1017921. [Google Scholar] [CrossRef]
- Alasadi, Y.; Arani, N.; Tchakarov, A.; Abudayyeh, A. Rituximab in Treatment of Glomerulonephritis Induced after Immune Checkpoint Inhibitors: FR-PO206. J. Am. Soc. Nephrol. 2024, 35. [Google Scholar] [CrossRef]
- Longhitano, E.; Muscolino, P.; Lo Re, C.; Ferrara, S.A.; Cernaro, V.; Gembillo, G.; Tessitore, D.; Speranza, D.; Figura, F.; Santarpia, M.; et al. Immune Checkpoint Inhibitors and the Kidney: A Focus on Diagnosis and Management for Personalised Medicine. Cancers 2023, 15, 1891. [Google Scholar] [CrossRef]
- Askanase, A.; Byron, M.; Keyes-Elstein, L.; Cagnoli, P.; McCune, W.J.; Chatham, W.W.; Contreras, G.; Daikh, D.; Dall’Era, M.; Wofsy, D.; et al. Treatment of Lupus Nephritis with Abatacept: The Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol. 2014, 66, 3096–3104. [Google Scholar] [CrossRef]
- Furie, R.; Nicholls, K.; Cheng, T.-T.; Houssiau, F.; Burgos-Vargas, R.; Chen, S.-L.; Hillson, J.L.; Meadows-Shropshire, S.; Kinaszczuk, M.; Merrill, J.T. Efficacy and Safety of Abatacept in Lupus Nephritis: A Twelve-Month, Randomized, Double-Blind Study. Arthritis Rheumatol. 2014, 66, 379–389. [Google Scholar] [CrossRef]
- Hanif, I.H.; Abdelrahim, S.; Shaarani, M.A.; Lu, H.; Ahmad, R.; Goswami, S.; Abudayyeh, A. Immune Checkpoint Therapy–Induced Lupus Nephritis. Kidney Int. Rep. 2024, 9, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Prodeus, A.; Sparkes, A.; Fischer, N.W.; Cydzik, M.; Huang, E.; Khatri, I.; Young, A.; Woo, L.; Chow, C.W.; Gorczynski, R.; et al. A Synthetic Cross-Species CD200R1 Agonist Suppresses Inflammatory Immune Responses In Vivo. Mol. Ther. Nucleic Acids 2018, 12, 350–358. [Google Scholar] [CrossRef]
- Addissouky, T.A.; Sayed, I.E.T.E.; Ali, M.M.A.; Alubiady, M.H.S.; Wang, Y. Transforming Glomerulonephritis Care through Emerging Diagnostics and Therapeutics. J. Biomed. Res. 2024, 5, 41–52. [Google Scholar] [CrossRef]
- Zhu, W.; He, H.; Pai, P. The Use of Rituximab in Glomerulonephritis: What Is the Evidence? Biomedicines 2025, 13, 2157. [Google Scholar] [CrossRef]
- Mallat, S.G.; Itani, H.S.; Abou-Mrad, R.M.; Abou Arkoub, R.; Tanios, B.Y. Rituximab Use in Adult Primary Glomerulopathy: Where Is the Evidence? Ther. Clin. Risk Manag. 2016, 12, 1317–1327. [Google Scholar] [CrossRef]
- Fabrizi, F.; Cresseri, D.; Fogazzi, G.B.; Moroni, G.; Passerini, P.; Martin, P.; Messa, P. Rituximab Therapy for Primary Glomerulonephritis: Report on Two Cases. World J. Clin. Cases 2015, 3, 736–742. [Google Scholar] [CrossRef]
- Hendra, H.; Salama, A.D. Steroids as Treatment for Glomerulonephritis: Time for a Rethink. Nephrol. Dial. Transplant. 2022, 37, 1212–1217. [Google Scholar] [CrossRef]
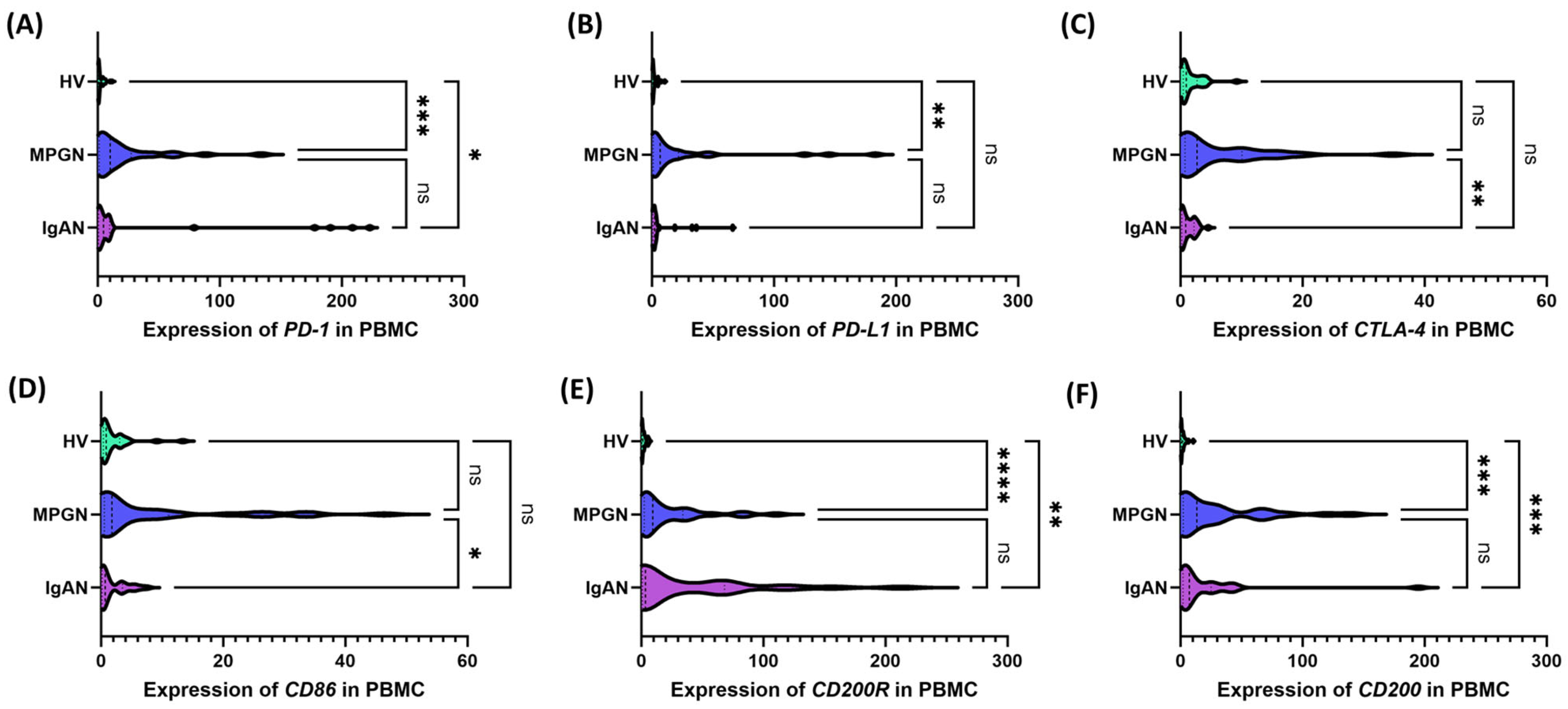
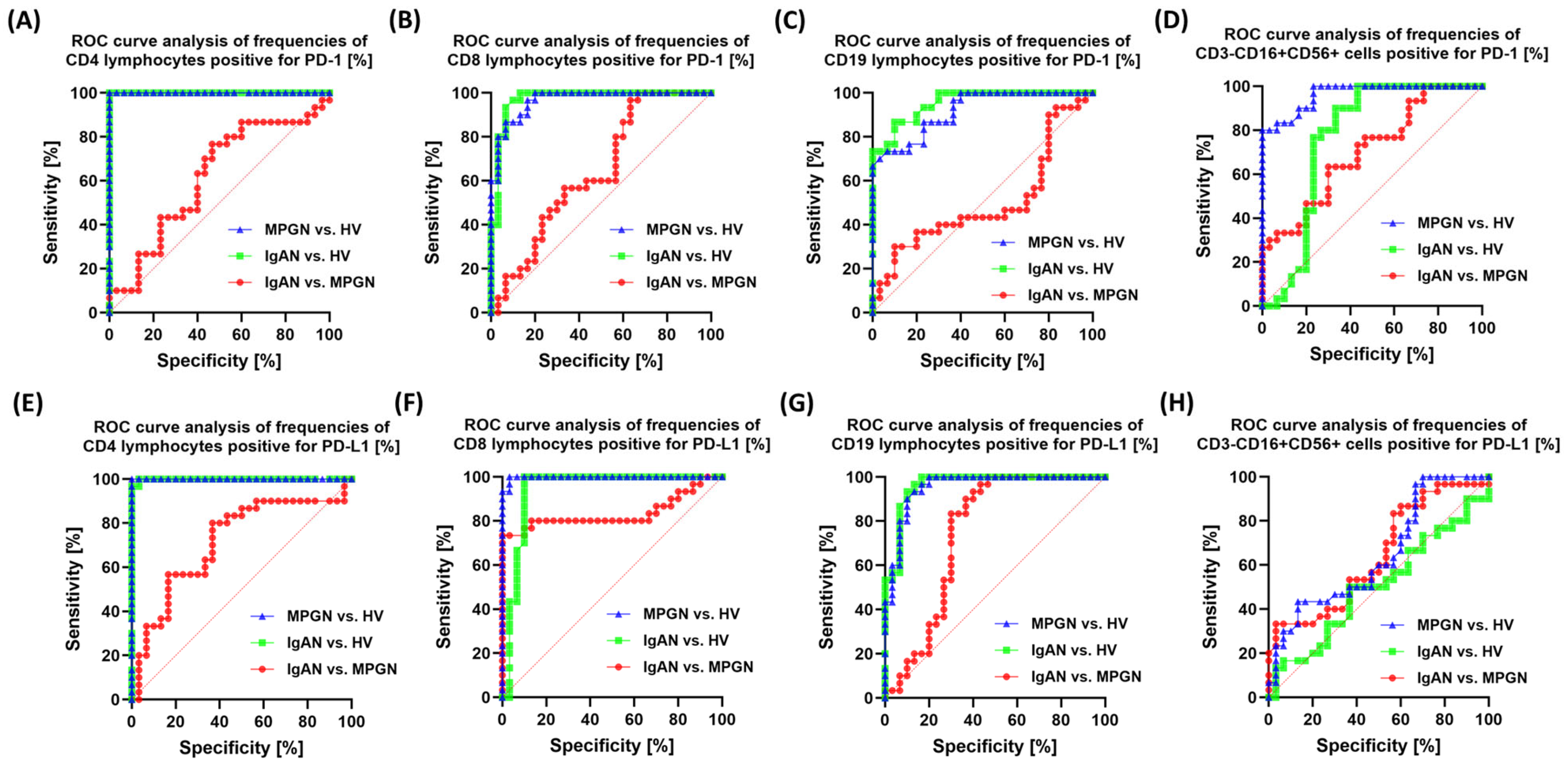
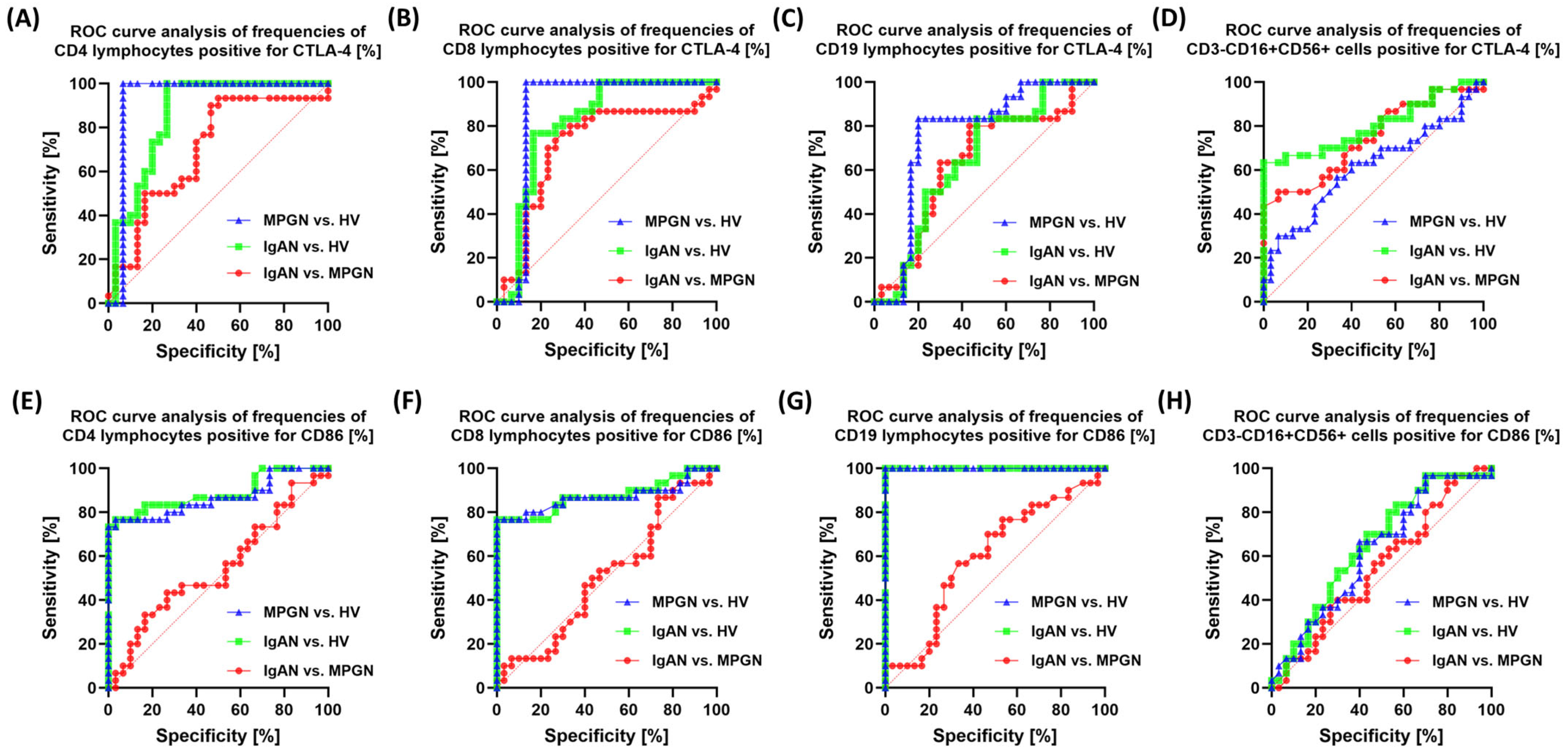
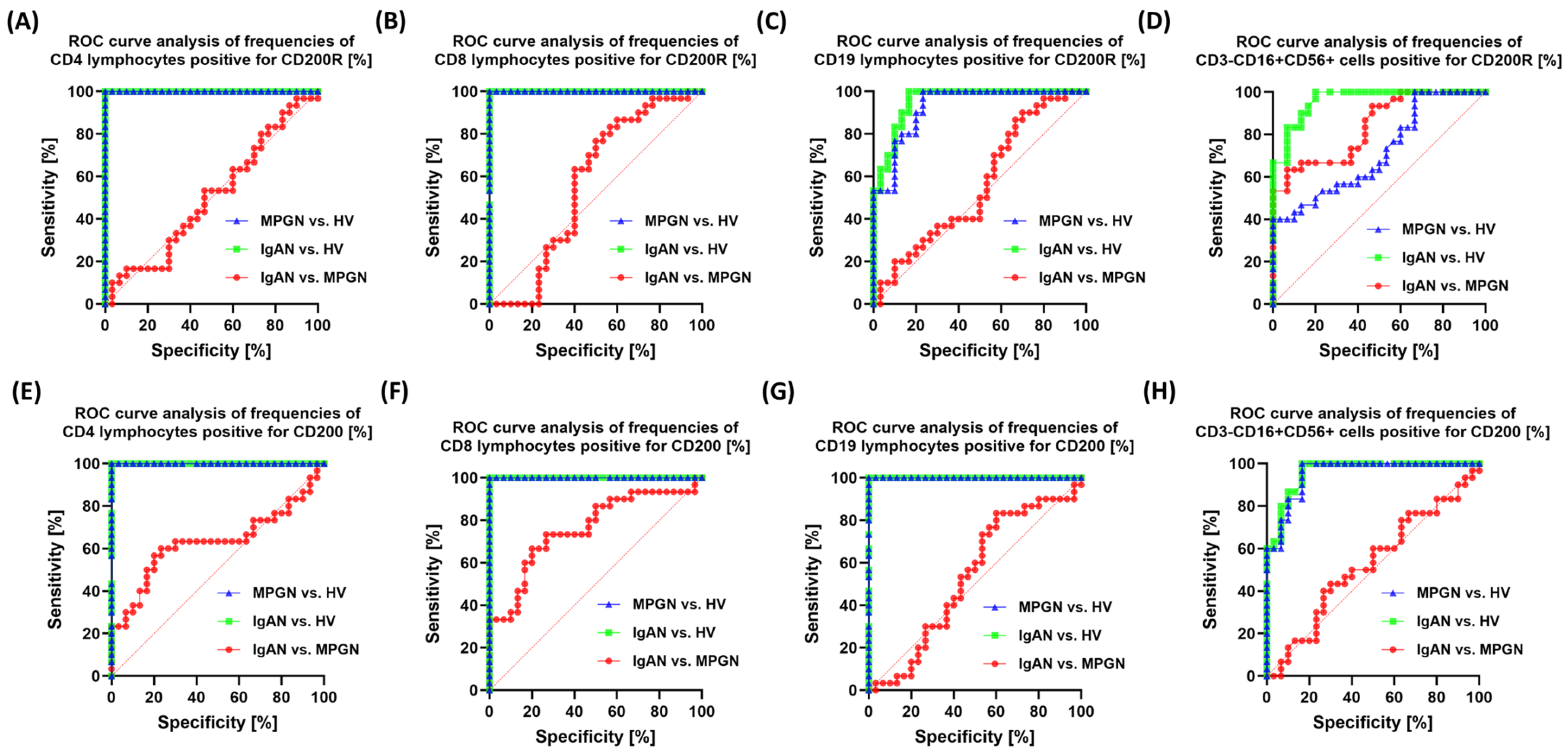
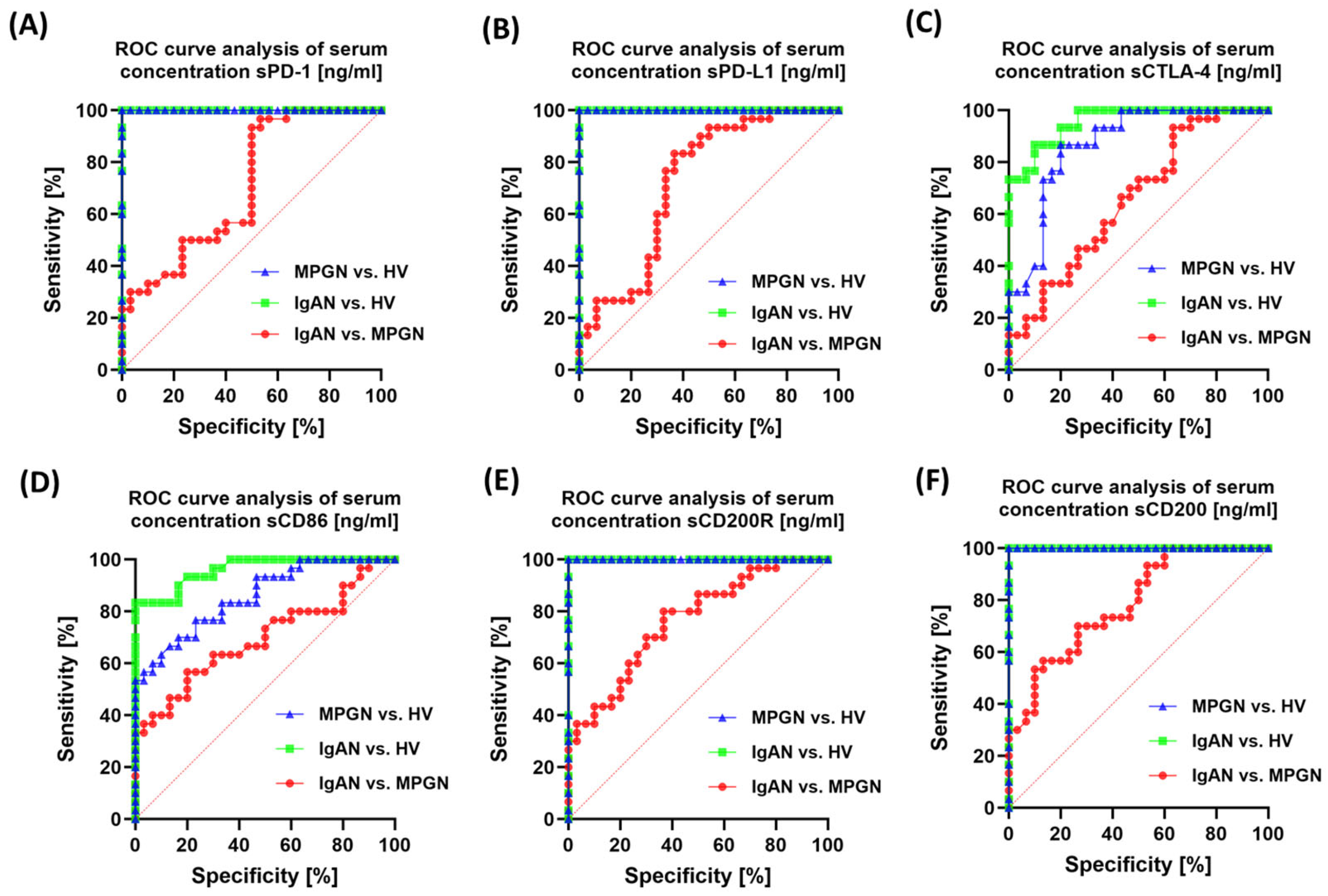
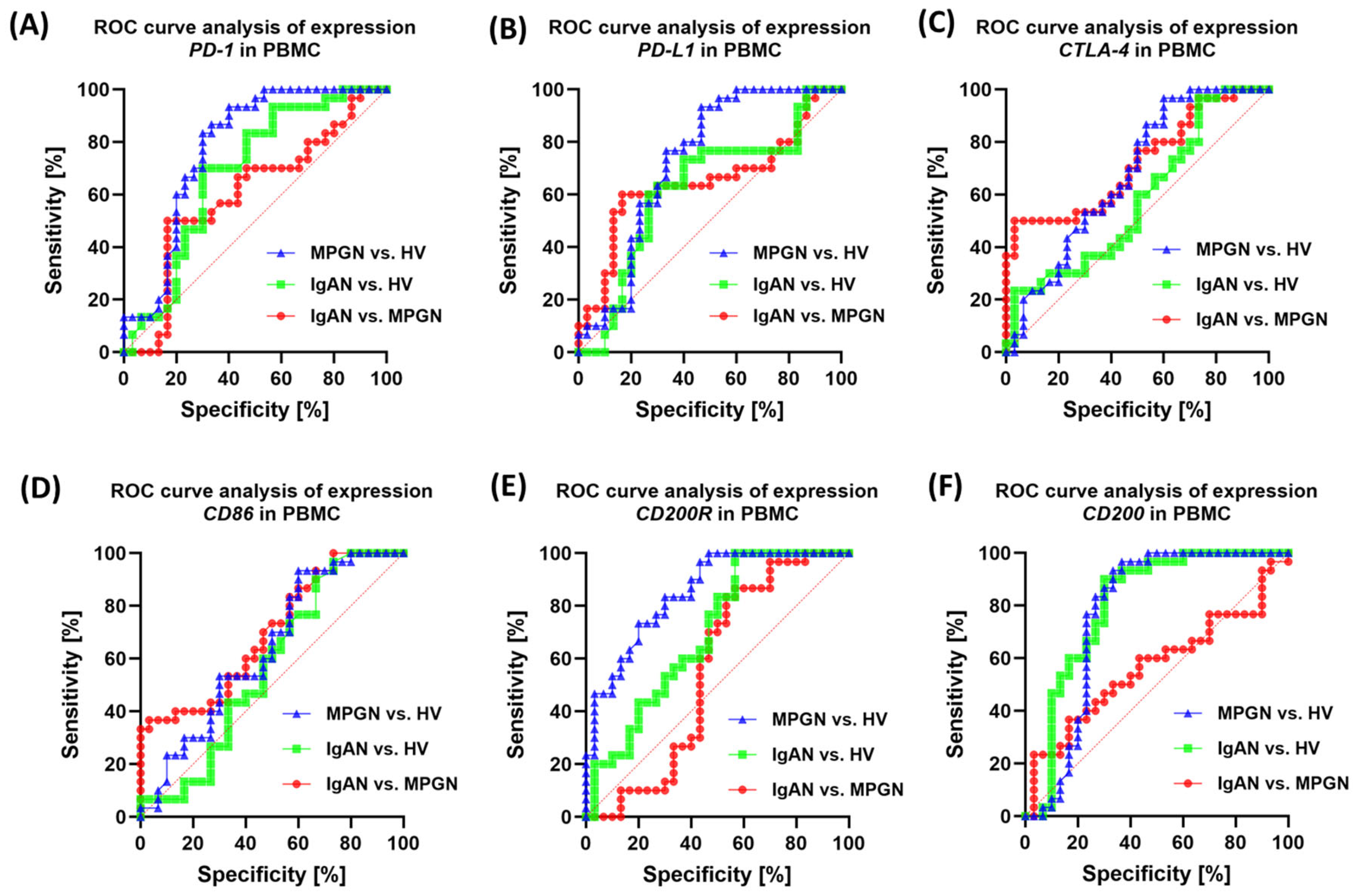
| Parameters | IgAN (n = 30) | MPGN (n = 30) | HV (n = 30) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Mediana (Q1–Q3) | Mediana (Q1–Q3) | Mediana (Q1–Q3) | All | IgAN vs. MPGN | IgAN vs. HV | MPGN vs. HV | ||
| Hematological parameters | WBC [103/mm3] | 7.12 (5.88–9.57) | 7.50 (7.00–9.49) | 6.30 (5.91–6.80) | 0.0003 | 0.3083 | 0.0478 | 0.0002 |
| NEU [103/mm3] | 4.79 (4.51–5.04) | 6.50 (5.92–7.08) | 6.65 (4.82–6.98) | 0.0011 | 0.0013 | 0.02 | >0.9999 | |
| MON [103/mm3] | 0.59 (0.50–0.60) | 0.50 (0.46–0.60) | 0.57 (0.43–0.77) | 0.2795 | 0.4564 | >0.9999 | 0.5529 | |
| LYM [103/mm3] | 2.11 (1.57–2.63) | 1.75 (1.47–2.10) | 1.95 (1.84–2.21) | 0.1634 | 0.2699 | >0.9999 | 0.331 | |
| EOS [103/mm3] | 0.19 (0.18–0.20) | 0.27 (0.19–0.36) | 0.17 (0.12–0.19) | 0.0016 | 0.1614 | 0.2954 | 0.001 | |
| BAS [103/mm3] | 0.10 (0.09–0.10) | 0.09 (0.00–0.10) | 0.02 (0.00–0.02) | <0.0001 | 0.0036 | <0.0001 | 0.0058 | |
| RBC [106/mm3] | 4.23 (3.60–4.55) | 4.55 (4.20–5.00) | 4.51 (4.24–4.90) | 0.0028 | 0.0055 | 0.0156 | >0.9999 | |
| HGB [g/dL] | 12.32 (10.90–13.63) | 13.87 (12.90–14.43) | 14.76 (13.03–15.70) | 0.0002 | 0.02 | 0.0002 | 0.5911 | |
| PLT [103/mm3] | 213.50 (196.19–273.47) | 226.21 (202.77–254.00) | 247.00 (212.54–306.00) | 0.1381 | >0.9999 | 0.1713 | 0.4367 | |
| Kidney function and protein metabolism | UREA [mg/dL] | 44.00 (31.64–51.56) | 33.55 (31.93–35.85) | 21.00 (18.80–26.75) | <0.0001 | 0.561 | <0.0001 | 0.0001 |
| CREATININE [mg/dL] | 1.25 (0.90–1.65) | 0.68 (0.59–0.85) | 0.70 (0.70–0.80) | <0.0001 | <0.0001 | 0.0002 | >0.9999 | |
| URIC ACID [mg/dL] | 6.27 (4.61–7.47) | 7.53 (6.90–7.88) | 4.20 (3.60–4.70) | <0.0001 | 0.0247 | <0.0001 | <0.0001 | |
| eGFR | 47.98 (41.55–56.75) | 52.50 (50.00–57.00) | 129.00 (126.00–137.00) | <.0001 | 0.5315 | <0.0001 | <0.0001 | |
| TOTAL PROTEIN [g/dL] | 5.70 (5.33–6.20) | 4.62 (4.12–5.15) | 7.40 (7.09–7.80) | <0.0001 | 0.0007 | 0.0004 | <0.0001 | |
| ALBUMIN [g/L] | 3.50 (2.98–3.64) | 2.93 (2.31–3.39) | 4.28 (3.99–4.47) | <0.0001 | 0.2342 | 0.0003 | <0.0001 | |
| PROTEINURIA [g/24 h] | 1.80 (1.27–2.94) | 5.00 (4.14–6.40) | 0.00 (0.00–0.00) | <0.0001 | 0.0029 | <0.0001 | <0.0001 | |
| Lipid profile | CHOLESTEROL [mg/dL] | 185.50 (170.43–207.74) | 209.61 (189.78–289.93) | 149.20 (134.25–161.97) | <0.0001 | 0.0179 | 0.0003 | <0.0001 |
| TRIGLYCERIDES [mg/dL] | 121.44 (98.58–187.75) | 159.50 (147.66–175.25) | 115.00 (96.75–134.00) | <0.0001 | 0.1732 | 0.0433 | <0.0001 | |
| HDL [mg/dL] | 46.74 (38.54–57.78) | 52.84 (50.16–59.13) | 60.00 (50.00–70.00) | 0.007 | 0.3464 | 0.0049 | 0.3464 | |
| LDL [mg/dL] | 103.00 (93.38–122.59) | 117.90 (109.78–166.95) | 102.00 (93.00–119.00) | 0.0006 | 0.0039 | >0.9999 | 0.0019 | |
| Immunoglobulins | IgG [g/L] | 7.98 (6.34–9.13) | 5.25 (4.08–6.02) | 5.20 (4.87–6.15) | <0.0001 | <0.0001 | <0.0001 | >0.9999 |
| IgM [g/L] | 1.10 (0.80–1.37) | 1.36 (1.18–1.60) | 1.80 (1.13–2.30) | 0.0009 | 0.0499 | 0.0007 | 0.577 | |
| IgA [g/L] | 3.25 (2.49–4.16) | 2.81 (1.76–3.07) | 2.42 (1.82–2.94) | 0.0081 | 0.0342 | 0.0143 | >0.9999 | |
| Parameters | IgAN (n = 30) | MPGN (n = 30) | HV (n = 30) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mediana (Q1–Q3) | Mediana (Q1–Q3) | Mediana (Q1–Q3) | All | IgAN vs. MPGN | IgAN vs. HV | MPGN vs. HV | |
| CD45+ [%] | 98.86 (97.96–99.51) | 97.34 (96.12–99.14) | 98.65 (91.25–99.68) | 0.079 | 0.073 | 0.6948 | 0.8737 |
| CD3+ [%] | 74.10 (71.33–78.28) | 74.64 (71.60–76.47) | 73.16 (59.72–87.98) | 0.9489 | >0.9999 | >0.9999 | >0.9999 |
| CD4+ [%] | 44.66 (38.85–47.39) | 42.31 (35.95–48.31) | 42.31 (24.42–63.35) | 0.565 | >0.9999 | >0.9999 | >0.9999 |
| CD8+ [%] | 24.96 (19.82–29.06) | 26.55 (22.36–34.42) | 27.11 (17.89–48.68) | 0.2697 | 0.4323 | 0.5459 | >0.9999 |
| CD19+ [%] | 11.77 (7.87–14.84) | 10.69 (6.42–16.06) | 12.36 (1.66–70.30) | 0.6345 | >0.9999 | >0.9999 | >0.9999 |
| CD3-CD16+CD56+ [%] | 11.61 (8.83–13.23) | 11.11 (9.36–16.55) | 9.96 (6.63–36.79) | 0.4064 | 0.581 | >0.9999 | >0.9999 |
| Ratio CD4+/CD8+ | 1.74 (1.42–2.35) | 1.57 (1.08–2.11) | 1.58 (0.71–3.24) | 0.3492 | 0.5412 | 0.7483 | >0.9999 |
| Para Zmiennych | R (MPGN) | R (IgAN) |
|---|---|---|
| Albumin & IgG | 0.509 | 0.673 |
| Albumin & Total protein | 0.603 | 0.718 |
| Albumin & Urea | 0.566 | 0.384 |
| CD19+CD86+ & CD19+PD-L1+ | −0.562 | 0.370 |
| CD19+CTLA-4+ & CD4+CTLA-4+ | 0.496 | 0.601 |
| CD19+CTLA-4+ & CD8+CTLA-4+ | 0.570 | 0.374 |
| CD19+PD-1+ & CD19+PD-L1+ | 0.715 | 0.475 |
| CD19+PD-L1+ & CD8+PD-1+ | 0.538 | 0.362 |
| CD3-CD16+CD56+CTLA-4+ & CD4+CD86+ | −0.546 | 0.430 |
| CD3-CD16+CD56+PD-L1+ & Total protein | 0.532 | −0.370 |
| CD3-CD16+CD56+PD-L1+ & WBC | −0.365 | 0.385 |
| CD4+CD200R+ & CD8+PD-1+ | 0.485 | −0.539 |
| CD4+CTLA-4+ & CD8+CTLA-4+ | 0.466 | 0.452 |
| CHOLESTEROL & LDL | 0.534 | 0.839 |
| CHOLESTEROL & Proteinuria | 0.459 | 0.379 |
| Creatine & Urea | 0.510 | 0.871 |
| Creatine & Uric acid | 0.413 | 0.609 |
| IgG & Total protein | 0.638 | 0.793 |
| IgM & RBC | −0.427 | −0.397 |
| LYM & RBC | −0.495 | 0.445 |
| PLT & sCD200R | −0.390 | 0.363 |
| qCD200R & qPD-1 | 0.439 | 0.455 |
| qCD86 & qPD-L1 | 0.383 | 0.466 |
| sPD-1 & sPD-L1 | 0.380 | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertowski, S.; Mertowska, P.; Czosnek, M.; Smarz-Widelska, I.; Załuska, W.; Grywalska, E. Checkpoint Imbalance in Primary Glomerulopathies: Comparative Insights into IgA Nephropathy and Membranoproliferative Glomerulonephritis. Cells 2025, 14, 1551. https://doi.org/10.3390/cells14191551
Mertowski S, Mertowska P, Czosnek M, Smarz-Widelska I, Załuska W, Grywalska E. Checkpoint Imbalance in Primary Glomerulopathies: Comparative Insights into IgA Nephropathy and Membranoproliferative Glomerulonephritis. Cells. 2025; 14(19):1551. https://doi.org/10.3390/cells14191551
Chicago/Turabian StyleMertowski, Sebastian, Paulina Mertowska, Milena Czosnek, Iwona Smarz-Widelska, Wojciech Załuska, and Ewelina Grywalska. 2025. "Checkpoint Imbalance in Primary Glomerulopathies: Comparative Insights into IgA Nephropathy and Membranoproliferative Glomerulonephritis" Cells 14, no. 19: 1551. https://doi.org/10.3390/cells14191551
APA StyleMertowski, S., Mertowska, P., Czosnek, M., Smarz-Widelska, I., Załuska, W., & Grywalska, E. (2025). Checkpoint Imbalance in Primary Glomerulopathies: Comparative Insights into IgA Nephropathy and Membranoproliferative Glomerulonephritis. Cells, 14(19), 1551. https://doi.org/10.3390/cells14191551






