Effect of Oral Peritumoral Tissue on Infiltration and Differentiation of Tumor-Associated Macrophages in Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
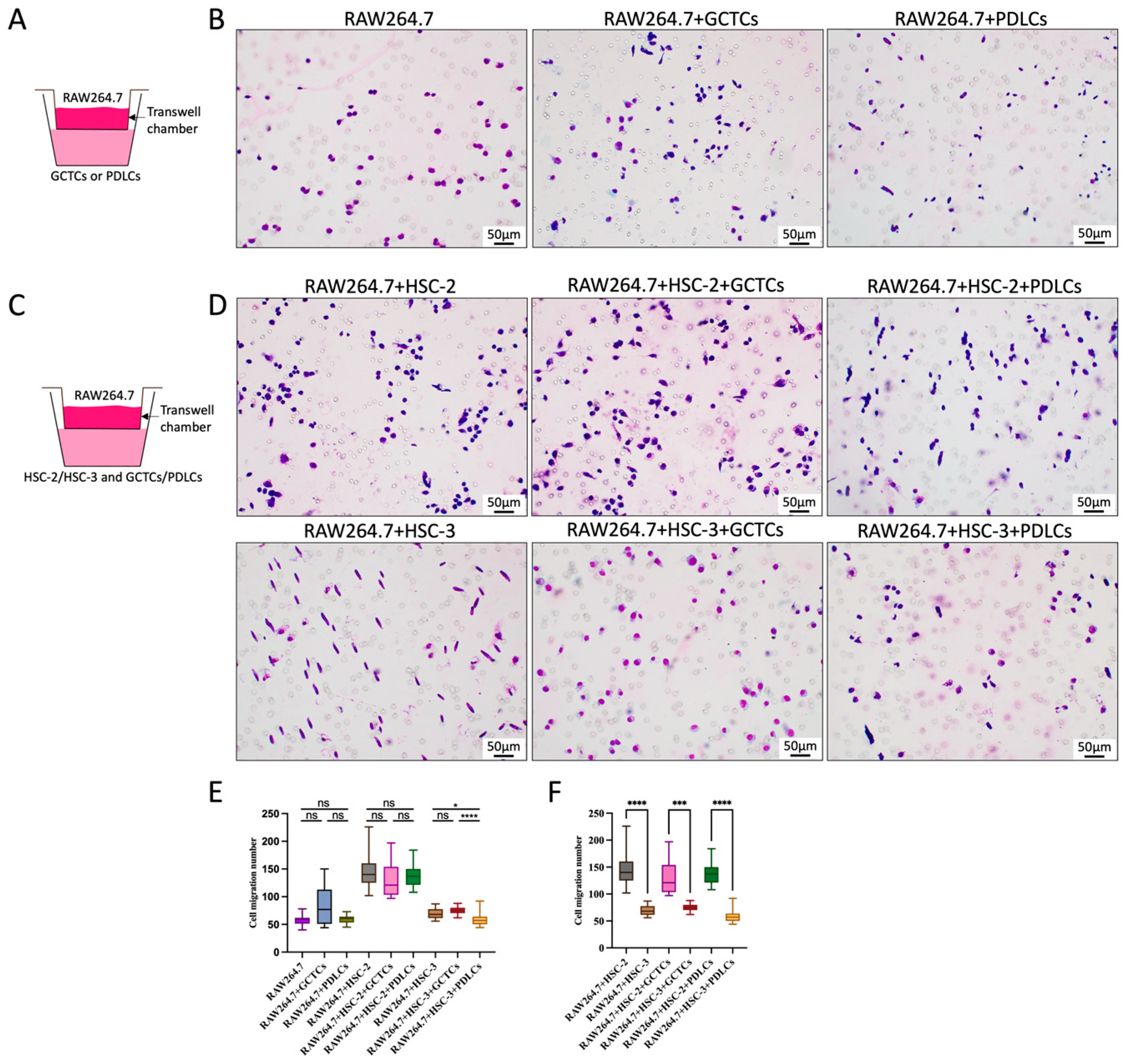
2.2. Transwell (Migration) Assay and Giemsa Staining
2.3. Indirect Co-Culture and Immunofluorescence Staining (If)
2.4. Mouse Model Construction
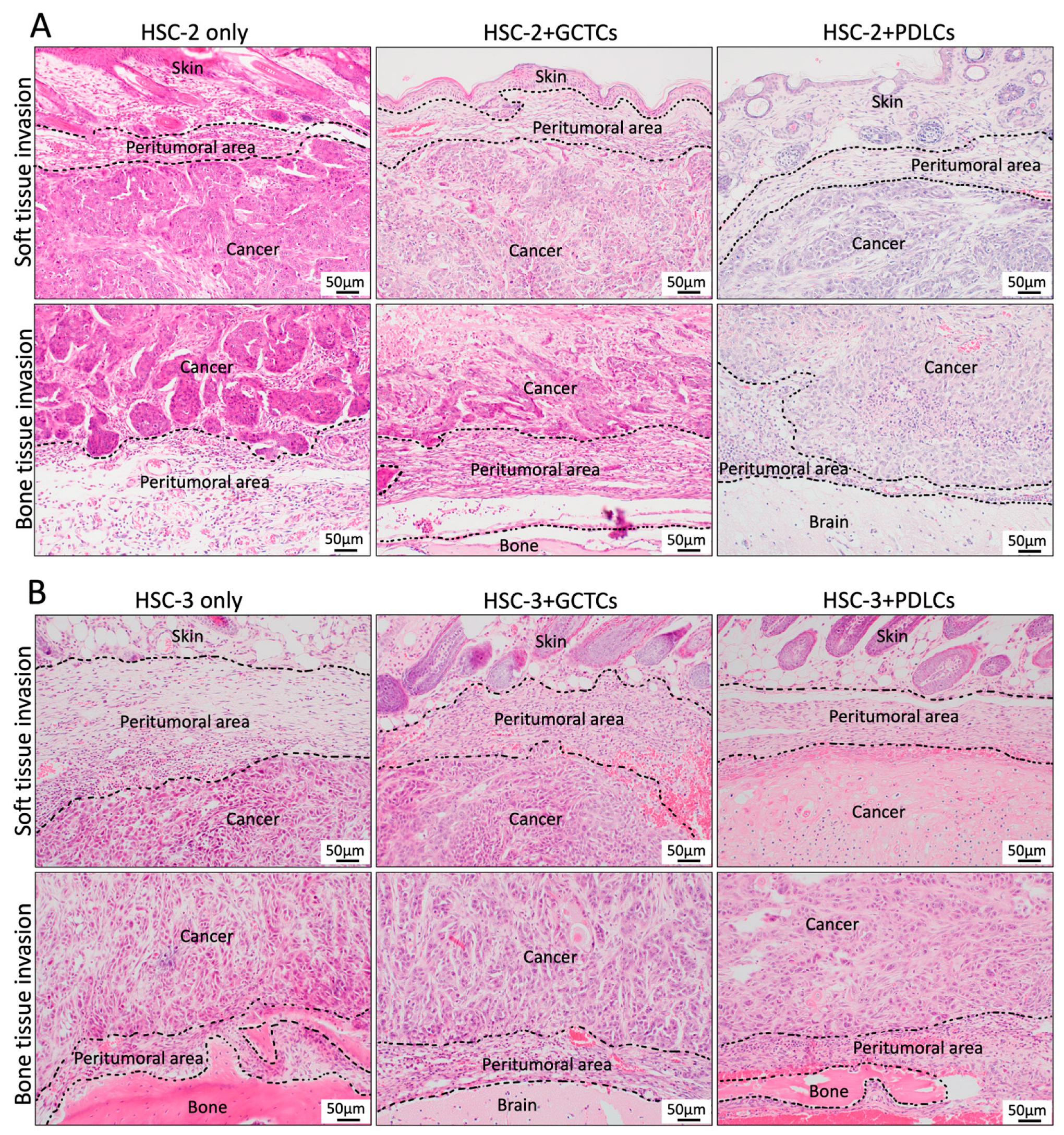
2.5. Hematoxylin and Eosin (HE) Staining
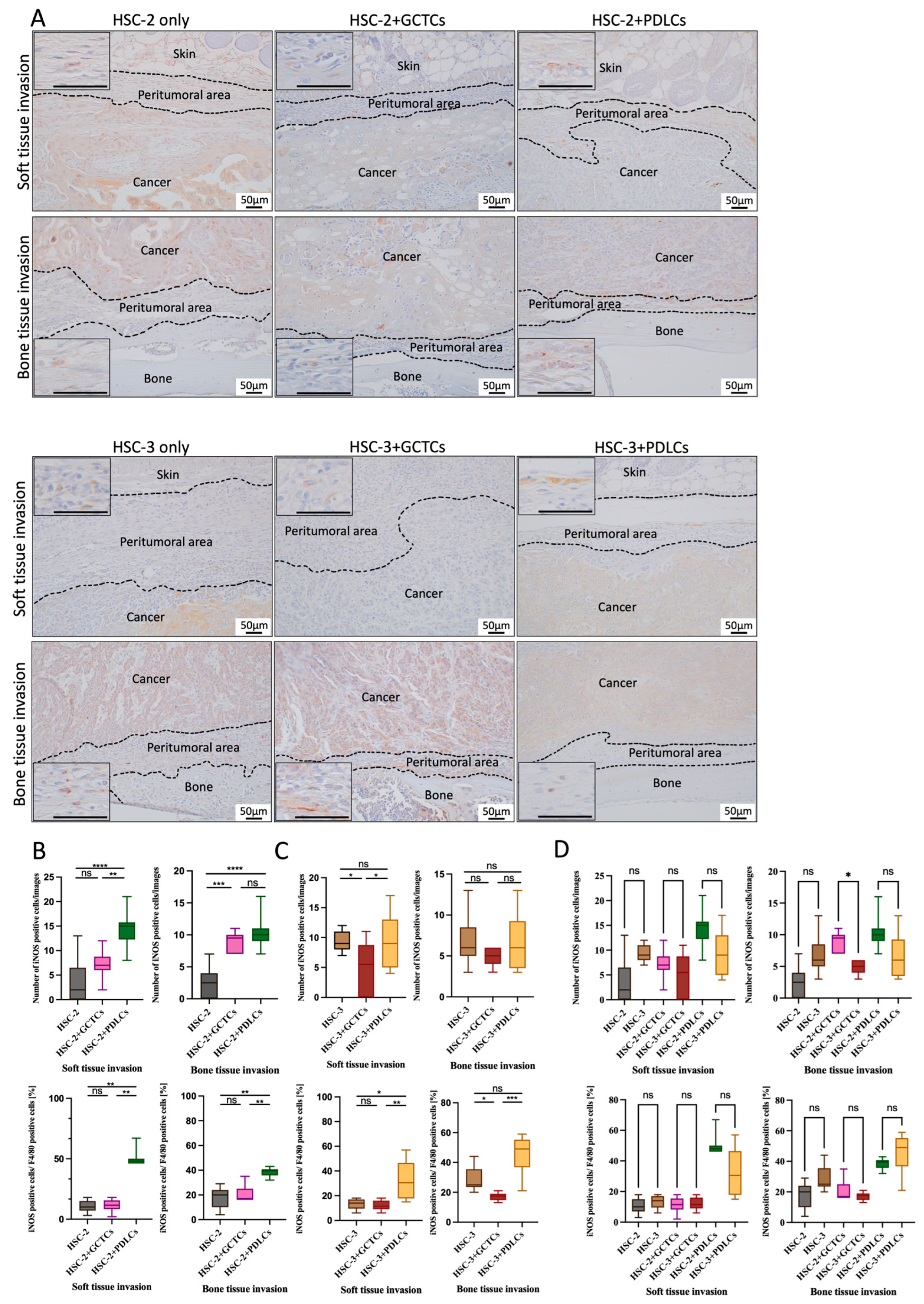
2.6. Immunohistochemistry (IHC) Staining
2.7. Statistical Analysis
3. Results
3.1. Effects of GCTCs/PDLCs and HSC-2/HSC-3 Interaction on RAW264.7 Migration
3.2. Effects of GCTCs/PDLCs and HSC-2/HSC-3 Interaction on the Differentiation of RAW264.7 into M0 or M2 Macrophages
3.3. Effects of the Interaction Between GCTCs/PDLCs and HSC-2/HSC-3 on the Differentiation of RAW264.7 to M1 Macrophages
3.4. Effects of the Interaction Between GCTCs/PDLCs and HSC-2/HSC-3 on the Histological Findings in the OSCC TME of the Animal Model
3.5. Effects of the Interaction Between GCTCs/PDLCs and HSC-2/HSC-3 on the Infiltration of CD45(+) Monocytes in OSCC TME of Animal Model
3.6. Effects of the Interaction Between GCTCs/PDLCs and HSC-2/HSC-3 on the Infiltration of F4/80(+) M0 Macrophages in OSCC TME of Animal Model
3.7. Effects of the Interaction Between GCTCs/PDLCs and HSC-2/HSC-3 on the Infiltration of CD163(+) M2 Macrophages in OSCC TME of Animal Model
3.8. Effects of the Interaction Between GCTCs/PDLCs and HSC-2/HSC-3 on the Infiltration of iNOS(+) M1 Macrophages in OSCC TME of Animal Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johanna, A.J.; Jeffrey, W.P. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Helenisa, H.O.N.; Frederico, O.G.N.; Silvia, F.S.; Cristiane, M.F.; Maria, C.F.A.; Trcilia, A.S.; Aline, C.B. A comparative study of microvessel density in squamous cell carcinoma of the oral cavity and lip. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 391–398. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, H.; Liu, X.; He, Y.; Tang, Y.; Zhu, G.; Zheng, M.; Yang, J. Effect of local hyperthermia on lymphangiogenic factors VEGF-C and -D in a nude mouse xenograft model of tongue squamous cell carcinoma. Oral Oncol. 2010, 46, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Vicki, P.; Zena, W. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Chen, L.; Yu, C.; Chen, S.; Cao, Y.; Zhong, G.; Hong, Y.; Liu, Q.; Sui, W. Non-Thermal Atmospheric Pressure Plasma-Assisted Bone Morphogenetic Protein-2 Immobilisation on Titanium Nanotube Surface Promotes Osteogenesis Differentiation by Influencing Macrophage Polarisation. J. Hard Tissue Biol. 2025, 34, 129–142. [Google Scholar] [CrossRef]
- Li, Y.; Ganesan, K.; Chen, J. Role of Biological Mediators of Tumor-Associated Macrophages in Breast Cancer Progression. Curr. Med. Chem. 2022, 29, 5420–5440. [Google Scholar] [CrossRef]
- Charles, D.M. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Wang, H.; Huang, S.; Lin, C.; Pan, Q.; Zhang, J.; Wang, C. Mechanism of 6% Hydroxyethyl Starch HES130/0.4 Regulating PGC-1α Expression and Inhibiting LPS-Induced Apoptosis of RAW264.7 Cells. J. Hard Tissue Biol. 2025, 34, 99–108. [Google Scholar] [CrossRef]
- Goswami, K.K.; Ghosh, T.; Ghosh, S.; Sarkar, M.; Bose, A.; Baral, R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Maliha, T.M.; Matthew, K.K.; Min, H.K.; Ma, M.R.; Ahmed, A.H.; Mahua, C.; Naima, M.M.; Fazle, H.; Shaikh, M.R. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bu, W.; Meng, L.; Liu, X.; Wang, S.; Jiang, L.; Ren, M.; Fan, Y.; Sun, H. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 2019, 378, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yin, P.; Su, Y.; Gao, F.; Wu, Y.; Zhang, W.; Chi, P.; Chen, J.; Zhang, X. Knockdown of HMGB1 inhibits the crosstalk between oral squamous cell carcinoma cells and tumor-associated macrophages. Int. Immunopharmacol. 2023, 119, 110259. [Google Scholar] [CrossRef]
- Haue, A.S.M.R.; Masafumi, M.; Keigo, K.; Noriko, I.; Mizuki, S.; Akira, C.; Keita, M.; Taiki, S.; Naoki, K.; Ryusuke, M.; et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef]
- Qiusheng, S.; Kiyofumi, T.; Haruka, O.; Hotaka, K.; May, W.O.; Keisuke, N.; Soichiro, I.; Akira, S.; Hitoshi, N. Stromal cells in the tumor microenvironment promote the progression of oral squamous cell carcinoma. Int. J. Oncol. 2021, 59, 72. [Google Scholar] [CrossRef]
- Omori, H.; Shan, Q.; Takabatake, K.; Nakano, K.; Kawai, H.; Sukegawa, S.; Tsujigiwa, H.; Nagatsuka, H. The Origin of Stroma Influences the Biological Characteristics of Oral Squamous Cell Carcinoma. Cancers 2021, 13, 3491. [Google Scholar] [CrossRef]
- Qiusheng, S.; Kiyofumi, T.; Haruka, O.; Hotaka, K.; May, W.O.; Shintaro, S.; Masae, F.; Yasunori, I.; Sho, S.; Keisuke, N.; et al. Investigation of bone invasion and underlying mechanisms of oral cancer using a cell line-derived xenograft model. Oncol. Lett. 2022, 24, 382. [Google Scholar] [CrossRef]
- An, Y.Z.; Cho, E.; Ling, J.; Zhang, X. The Axin2-snail axis promotes bone invasion by activating cancer-associated fibroblasts in oral squamous cell carcinoma. BMC Cancer 2020, 20, 987. [Google Scholar] [CrossRef]
- Han, T.; Guo, X.; Xie, J.; Tong, W.; Zhang, L. SUMO modified ETV1 promotes M2-polarized tumor-associated macrophage infiltration and cancer progression by facilitating CCL2 transcription in esophageal squamous cell carcinoma cells. Cancer Immunol. Immunother. 2025, 74, 87. [Google Scholar] [CrossRef]
- Siamon, G.; Philip, R.T. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, Y.; Pan, G.; Xiang, L.X.; Luo, D.C.; Shao, J.Z. Occurrences and Functions of Ly6Chi and Ly6CloMacrophages in Health and Disease. Front. Immunol. 2022, 13, 901672. [Google Scholar] [CrossRef]
- Luca, C.; Stamatina, F.; Andrew, H.S.; Agnieszka, S.; Lesley, M.F.; Hui, Z.; Daniel, Y.H.S.; Tiziana, C.; Pavana, A.; Elaine, Y.L.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e10. [Google Scholar] [CrossRef]
- Samir, D.; Tsun, K.J.T.; Ian, W.F.; Ramakrishnan, N.; Md Zahidul, A.; Minghong, L.; Yuma, T.; Konstantin, B.; Mai, T.D.; Li, Z.; et al. Tumor-Derived Retinoic Acid Regulates Intratumoral Monocyte Differentiation to Promote Immune Suppression. Cell 2020, 180, 1098–1114.e16. [Google Scholar] [CrossRef]
- Karla, M.H.; Ramon, C.C.; Jose Carlos, C.D.; Jose Avendona, O.; Elvira, M.; Roberto, L.R.; Veronica, T.A.; Marina, V.B.; Cristobal, M.; Jorge, S.A.; et al. Colorectal Cancer Stem Cells Fuse with Monocytes to Form Tumour Hybrid Cells with the Ability to Migrate and Evade the Immune System. Cancers 2022, 14, 3445. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Chen, W.; Wang, X.; Cao, M.; Han, X.; Zhang, K.; Teng, B.; Cao, J.; Wu, W.; et al. M2 Macrophage-Derived Exosomes Promote Angiogenesis and Growth of Pancreatic Ductal Adenocarcinoma by Targeting E2F2. Mol. Ther. 2006, 29, 1226–1238. [Google Scholar] [CrossRef]
- Yasuyoshi, M.; Shigeru, K.; Kojiro, O.; Koichiro, N.; Yasushi, H.; Jiro, E.; Tomayoshi, H.; Hiroshi, K. Lymphangiogenesis and angiogenesis in bladder cancer: Prognostic implications and regulation by vascular endothelial growth factors-A, -C, and -D. Clin. Cancer Res. 2006, 12 Pt 1, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Qiusheng, S.; Kiyofumi, T.; Hotaka, K.; May, W.O.; Shintaro, S.; Masae, F.; Keisuke, N.; Hitoshi, N. Crosstalk between cancer and different cancer stroma subtypes promotes the infiltration of tumor-associated macrophages into the tumor microenvironment of oral squamous cell carcinoma. Int. J. Oncol. 2022, 60, 78. [Google Scholar] [CrossRef]
- Makiko, O.; Mitomu, K.; Hideyuki, N.; Kei, S.; Kenji, M.; Ichiro, A.; Hideki, T.; Iwai, T. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci. Rep. 2016, 6, 27548. [Google Scholar] [CrossRef] [PubMed]
- Stefan, T.; Sofia, M.E.W.; Teng, W.; Carsten, S.; Carilina, D.L.T.; Marcell, T.; Fabian, R.; Yingyue, T.; Thomas, R.; Claudia, B.; et al. YAP-induced Ccl2 expression is associated with a switch in hepatic macrophage identity and vascular remodelling in liver cancer. Liver Int. 2021, 41, 3011–3023. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Wang, Y.; Zhang, W.; Liu, L.; Cheng, J. Prognostic role of CD11b+ myeloid-derived suppressor cells in oral squamous cell carcinoma. Arch. Med. Sci. 2021, 19, 171–179. [Google Scholar] [CrossRef]
- Barbora, P.; Martina, R.; Michal, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zeng, H.; Jin, K.; Liu, Z.; Hu, B.; Liu, C.; Yan, S.; Yu, Y.; You, R.; Zhang, H.; et al. Infiltration and Polarization of Tumor-associated Macrophages Predict Prognosis and Therapeutic Benefit in Muscle-Invasive Bladder Cancer. Cancer Immunol. Immunother. 2022, 71, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Li, Y.; Long, Y.; Zhao, Q.; Ouyang, Y.; Bao, W.; Gong, K. Tumor-associated macrophage infiltration and prognosis in colorectal cancer: Systematic review and meta-analysis. Int. J. Colorectal. Dis. 2020, 35, 1203–1210. [Google Scholar] [CrossRef]
- Feng, Q.; Chang, W.; Mao, Y.; He, G.; Zheng, P.; Tang, W.; Wei, Y.; Ren, L.; Zhu, D.; Ji, M.; et al. Tumor-associated Macrophages as Prognostic and Predictive Biomarkers for Postoperative Adjuvant Chemotherapy in Patients with Stage II Colon Cancer. Clin. Cancer Res. 2019, 25, 896–3907. [Google Scholar] [CrossRef]
- Kazumasa, M.; Miki, H.; Jun, S.; Yoshihiro, O. Infiltration of m2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers 2011, 3, 3726–3739. [Google Scholar] [CrossRef]
- Nadia, L.C.; Marize, C.V.; Pedro, P.C.S.; Elismauro, F.M.; Jose, C.O.; Tarcila, A.S.; Aline, C.B. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 2013, 49, 216–223. [Google Scholar] [CrossRef]
- Chiu, K.-C.; Lee, C.-H.; Liu, S.-Y.; Chou, Y.-T.; Huang, R.-Y.; Huang, S.-M.; Shieh, Y.-S. Polarization of tumor-associated macrophages and Gas6/Axl signaling in oral squamous cell carcinoma. Oral Oncol. 2015, 51, 683–689. [Google Scholar] [CrossRef]
- Manuel, W.; Christos, I.; Patrik, M.; Maike, B.-H.; Kerstin, A.; Jutta, R.; Raimund, P.; Friedrich, W.N.; Falk, W. Prognostic significance of macrophage polarization in early stage oral squamous cell carcinomas. Oral Oncol. 2016, 52, 75–84. [Google Scholar] [CrossRef]
- Weber, M.; Moebius, P.; Buttner-Herold, M.; Amann, K.; Preidl, R.; Neukam, F.W.; Wehrhan, F. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas—An immunohistochemical study. Br. J. Cancer 2015, 113, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Yuko, Y.; Hirofumi, T.; Kei, S.; Kiyoshi, S.; Hiroyuki, H.; Tohru, I.; Kou, K. CD163-Positive Macrophages Within the Tumor Stroma Are Associated With Lymphangiogenesis and Lymph Node Metastasis in Oral Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2017, 75, 2144–2153. [Google Scholar] [CrossRef]
- He, Y.; Dong, Y.; Zhang, X.; Ding, Z.; Song, Y.; Huang, X.; Chen, S.; Wang, Z.; Ni, Y.; Ding, L. Lipid Droplet-Related PLIN2 in CD68+ Tumor-Associated Macrophage of Oral Squamous Cell Carcinoma: Implications for Cancer Prognosis and Immunotherapy. Front. Oncol. 2022, 12, 824235. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Bao, J.; Zhang, Y.; Lei, L.; Yan, F. Macrophage polarization in periodontal ligament stem cells enhanced periodontal regeneration. Stem. Cell Res. Ther. 2019, 10, 320. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Zhang, L.; Li, X.; Ding, X.; Ding, G.; Wei, F. Periodontal ligament stem cells promote polarization of M2 macrophages. J. Leukoc. Biol. 2022, 111, 1185–1197. [Google Scholar] [CrossRef]
- Mizuki, N.; Kengo, I.; Keiko, A.; Motohiro, K.; Naoki, Y.; Yuichi, I.; Ikuo, M. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng. Part A 2017, 23, 367–377. [Google Scholar] [CrossRef]
- Dorrin, N.; Morris, F.M. Expression of nitric oxide synthases in orthodontic tooth movement. Angle Orthod. 2009, 79, 502–508. [Google Scholar] [CrossRef]
- Fei, L.; Ren, X.; Yu, H.; Zhan, Y. Targeting the CCL2/CCR2 Axis in Cancer Immunotherapy: One Stone, Three Birds? Front. Immunol. 2021, 12, 771210. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization of Tumor-Associated Macrophages in Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef]
- Fernando, O.M.; Siamon, G.; Massimo, L.; Alberto, M. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef]
- Antonio, S.; Paola, L.; Alessandra, M.; Luca, R.; Chiara, P.; Maria, G.T.; Monica, R.; Subhra, K.B.; Paola, A.; Alberto, M. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Yoshihiro, K.; Masahisa, J.; Motohiro, T. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2013, 105, 1–8. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, T.; Takabatake, K.; Arashima, T.; Zhao, Y.; Kawai, H.; Eain, H.S.; Soe, Y.; Min, Z.Z.; Nakano, K.; Nagatsuka, H. Effect of Oral Peritumoral Tissue on Infiltration and Differentiation of Tumor-Associated Macrophages in Oral Squamous Cell Carcinoma. Cells 2025, 14, 1481. https://doi.org/10.3390/cells14181481
Piao T, Takabatake K, Arashima T, Zhao Y, Kawai H, Eain HS, Soe Y, Min ZZ, Nakano K, Nagatsuka H. Effect of Oral Peritumoral Tissue on Infiltration and Differentiation of Tumor-Associated Macrophages in Oral Squamous Cell Carcinoma. Cells. 2025; 14(18):1481. https://doi.org/10.3390/cells14181481
Chicago/Turabian StylePiao, Tianyan, Kiyofumi Takabatake, Takuma Arashima, Yulu Zhao, Hotaka Kawai, Htoo Shwe Eain, Yamin Soe, Zin Zin Min, Keisuke Nakano, and Hitoshi Nagatsuka. 2025. "Effect of Oral Peritumoral Tissue on Infiltration and Differentiation of Tumor-Associated Macrophages in Oral Squamous Cell Carcinoma" Cells 14, no. 18: 1481. https://doi.org/10.3390/cells14181481
APA StylePiao, T., Takabatake, K., Arashima, T., Zhao, Y., Kawai, H., Eain, H. S., Soe, Y., Min, Z. Z., Nakano, K., & Nagatsuka, H. (2025). Effect of Oral Peritumoral Tissue on Infiltration and Differentiation of Tumor-Associated Macrophages in Oral Squamous Cell Carcinoma. Cells, 14(18), 1481. https://doi.org/10.3390/cells14181481




