Abstract
Adenosine receptor (AR) antagonists have attracted considerable interest due to their therapeutic potential in a wide range of pathological conditions, including neurological, cardiovascular, and inflammatory disorders. Although a large number of AR antagonists have been developed worldwide, the interest in new derivatives remains high, and achieving subtype selectivity continue to be a major challenge. This review summarizes our research on adenosine receptor antagonists, highlighting the discovery of potent and selective compounds for the diverse AR subtypes across various chemical classes. Specifically, the paper focuses on the study of the triazolo[4,3-a]quinoxalin-1-one (TQX) and pyrazolo[3,4-c]quinoline (PQ) series, along with their simplified analogues, which have yielded highly potent and selective AR antagonists. An overview of the structure–activity relationship (SAR) studies and molecular docking investigations is provided, emphasizing the structural requirements for A2A and A3 receptor–ligand interaction. In addition, we present pharmacological studies of selected AR antagonists, in various in vitro and in vivo models of pain, depression, neuroinflammation-related diseases, and cancer.
1. Introduction
Adenosine is a ubiquitous endogenous signaling molecule that modulates a wide variety of physiological functions [1].
Adenosine biosynthesis, metabolism, and transport are summarized in Figure 1. The nucleoside can be synthesized both in the intracellular and extracellular space. The major extracellular biosynthetic mechanism involves the degradation of ATP, ADP, and AMP, due to the activation of the ecto-nucleoside triphosphate diphosphohydrolase 1 (CD39) and the ecto-5’-nucleotidase (CD73) [2]. Nevertheless, under physiological conditions adenosine is primarily generated intracellularly through the hydrolysis of S-adenosylhomocysteine (SAH) by SAH hydrolase [3], and of AMP by endo-5’-nucleotidase [4].
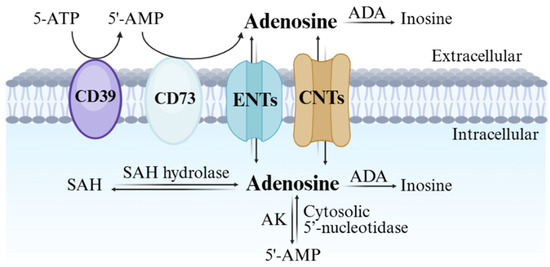
Figure 1.
Biosynthesis and metabolism of adenosine.
Adenosine uptake or release from cells is regulated by the different concentrations on the two sides of the cell membrane and involves nucleoside transporters, which can be classified into equilibrative (ENT) and concentrative (CNT) nucleoside transporters [5]. While the four subtypes of ENTs (1–4) act through passive transport and facilitated diffusion, the Na+-dependent CNTs promote adenosine transport against its concentration gradient, by using the sodium ion gradient as an energy source. Normally, adenosine transport occurs from the extracellular to the intracellular milieu; however, the flow can be reversed under pathological conditions such as inflammation, hypoxia, and ischemia [6]. After intracellular uptake, adenosine is converted into inosine by adenosine deaminase (ADA) or phosphorylated to 5’-AMP through adenosine kinase (AK), providing a physiological half-life of less than 1s. The Michaelis constant (Km) values of these enzymes are 17–45 μM (ADA) and 2 μM (AK), suggesting AK is the major enzyme responsible for adenosine clearance, while deamination takes place preferably under pathological conditions [1].
1.1. Adenosine Receptors
Adenosine mediates its physiological effects by interacting with G protein-coupled receptors (GPCRs), classified into four subtypes: A1, A2A AR, A2B AR, and A3 AR adenosine receptors (ARs). All four ARs have been cloned and pharmacologically characterized [1]. All ARs modulate adenylyl cyclase (AC), thereby affecting cyclic AMP (cAMP) levels, but other effector systems have been observed [7]. Signal transduction pathways of A1 and A3 ARs are summarized in Figure 2. These AR subtypes are activated by nanomolar concentrations of adenosine [1] and preferentially couple to Gi and G0 proteins, thus decreasing AC activity and intracellular cAMP production. Additionally, they induce phospholipase C (PLC)-β activation, leading to an increase in inositol 1,4,5-trisphosphate (IP3), diacylglycerol (DAG), and intracellular Ca2+ levels, and activation of phosphokinase C (PKC) [1]. Activation of A1 and A3 ARs induces the intracellular phosphorylative cascade of the mitogen-activated protein kinase (MAPK) family, including MAPK p38, extracellular signal-regulated kinase 1/2 (ERK1/2), and Jun NH2-terminal kinase (JNK) [8,9,10]. A3 AR activation is also correlated to a different pathway from GPCR signaling which involves Ras homolog family member A (RhoA) and phospholipase D [11].
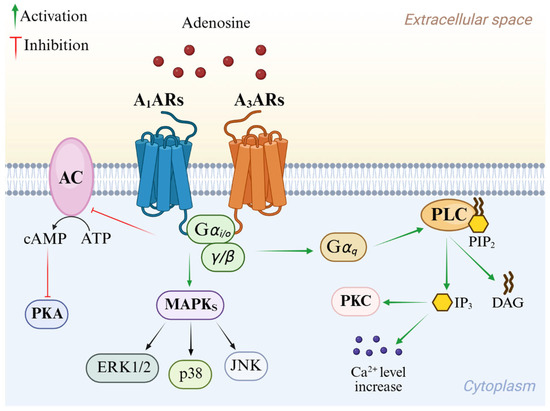
Figure 2.
Overview of A1 AR and A3 AR intracellular signaling pathways.
Signal transduction pathways of the A2A AR and A2B AR are reported in Figure 3. Although they share almost identical binding sites, A2A AR is activated by nanomolar concentrations of adenosine, while A2B AR requires micromolar levels [12]. Both subtypes couple to Gs proteins, thereby activating AC and consequently leading to the enrolment of different cAMP-dependent effectors including protein kinase A (PKA). Through the activation of PKA and cross-talk with protein kinase B (AKT), these pathways modulate cell growth and proliferation via the element-binding protein (CREB) [1]. Moreover, A2A and A2B ARs are involved in the activation of MAPK signaling [4,13,14]. In addition, A2B AR enrolment stimulates PLC through the Gq protein and regulates ion channels through its βγ subunits [4,14].
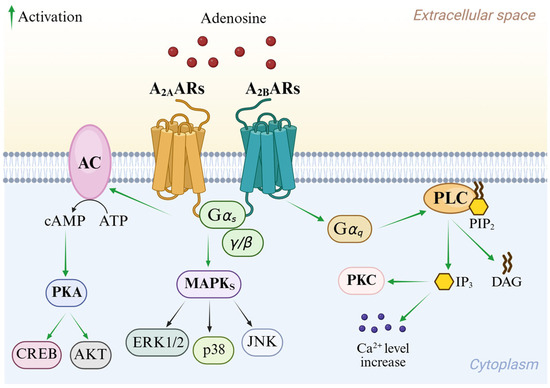
Figure 3.
Overview of A2A AR and A2B AR intracellular signaling pathways.
ARs are widely distributed throughout the body and their activation modulates several processes in different tissues and organs [15].
1.1.1. A1 Adenosine Receptor
A1 AR is highly expressed in the central nervous system (CNS), taking part in the control of physiological synaptic transmission. Particularly, its activation reduces excitatory transmission through the enhancement of potassium current [16] and the inhibition of N-type calcium channels [17], thus leading to beneficial effects in several central disorders, including epilepsy and cerebral ischemia [18,19]. Additionally, several studies reported neuroprotective effects following A1 AR activation in various models of inflammatory and neuropathic pain, due to the involvement of the nitric oxide/cGMP/protein kinase G/KATP channel [20], as well as modulation of Ca2+ and K+ channels and inhibition of excitatory amino acid release [21]. A1 AR has been detected in β-amyloid plaques and neurons with tau deposition and its modulation ameliorates β-amyloid and tau neurotoxicity, thus improving cognition and memory. Thus, this receptor has been recognized to play a central role in the pathogenesis of Alzheimer’s Disease (AD) and its dysfunction may be responsible for some of the AD clinical manifestations [22]. A1 AR is also expressed in smooth muscle cells and cardiomyocytes in atria and ventricular tissues, taking part in the regulation of heart rate and blood pressure [23]. A1 AR activation appears to play a crucial role in myocardial ischemic preconditioning, a protective mechanism involving brief episodes of sub-lethal ischemia alternated with short periods of reperfusion prior to a prolonged ischemic insult [24]. In the kidney, it modulates glomerular filtration rate [25], while in adipose tissue it suppresses lipolysis [26]. Additionally, A1 AR activation inhibits glucose-stimulated insulin secretion from pancreatic β-cells [27].
1.1.2. A2A Adenosine Receptor
A2A AR is the main AR subtype expressed in the striatum, where it co-localizes with dopamine D2 receptor (D2 R) to form A2A AR/D2 R heteromers. These complexes play a critical role in motor function regulation. Notably, stimulation of A2A AR/D2 R heteromers counteracts the D2 R-mediated inhibition of N-methyl-D-aspartate (NMDA) receptor activity [28], a mechanism implicated in the motor dysfunction characteristic of Parkinson’s disease (PD) [29]. Additionally, A2A R has been also found in both pre- and post-synaptic neurons and in glial cells where it stimulates pro-inflammatory functions, particularly by inducing activation of both microglia and astrocytes in pro-inflammatory phenotypes [30,31]. Under physiological conditions, the A2A AR expression in microglia and astrocytes is usually low, while it increases after brain insults, nerve injury, and inflammatory signals, taking part in an important feed-forward mechanism to locally control neuroinflammatory responses in the brain [32,33,34]. Therefore, modulation of A2A AR could represent an innovative therapeutic strategy to counteract neurodegenerative disorders with neuroinflammatory background. A2A AR is also involved in the development of neuropathic pain and its blockade confers protection [35]. Indeed, the changes in spinal cord excitability, which have been proposed to drive neuropathic pain, are closely linked to alterations in microglial functions [36]. A2A AR is also located presynaptically on cortico-striatal glutamatergic afferents [37], where it modulates glutamate release [38,39]. Considering that several studies support the hypothesis that cortico-striatal glutamatergic deregulation could be involved in the pathogenesis of Huntington’s disease (HD) [40], A2A AR has emerged as potentially druggable target in HD [41].
Peripherally, A2A AR controls coronary vascular due to its expression in the smooth muscle and endothelium, where it induces vasodilation, thus providing a cardioprotective action [42]. Cardioprotective properties of A2A AR also appear to be related to a reduction in neutrophil accumulation, due to its potent anti-inflammatory activity [43]. Furthermore, A2A AR is largely expressed in immune system cells and its activation exerts opposite effects to the central ones, inhibiting T and natural killer lymphocytes, dendritic cells, monocytes, and macrophages [31].
1.1.3. A2B Adenosine Receptor
In the CNS, A2B AR is mostly located in astrocytes, where its expression is upregulated in response to lipopolysaccharide (LPS) and hypoxic stimulation [44]. At this level, A2B AR stimulation induces astrogliosis [45], which is strongly linked to neurodegenerative processes [46]. Moreover, A2B AR contributes to the modulation of the inflammatory cascade and neuronal injury following global cerebral ischemia [47].
At the peripheral level, A2B AR is widely expressed in the bladder [48], intestine [49], and fibroblasts [50]. Several lines of evidence have highlighted a pronociceptive and proinflammatory role for this receptor, making it a promising therapeutic target for a range of inflammatory diseases, including multiple sclerosis (MS) [47], wound healing disorders, fibrosis, asthma, colitis, and diabetes [51]. Furthermore, A2B AR activation enhances the growth of multiple cancer cell lines and contributes to solid tumor development, by inhibiting anti-tumor immune responses. Due to its low affinity for adenosine, the receptor is primarily activated in pathological conditions where extracellular adenosine levels are elevated, such as within tumors [52].
1.1.4. A3 Adenosine Receptor
A3 AR is widely distributed in the human body. Low levels have been reported in several brain areas, including the cortex, hippocampus, thalamus, hypothalamus, retinal ganglion cells, and motor nerve terminals [53]. A3 AR is expressed in astrocytes and microglia [54], where it affects, respectively, the expression of proinflammatory genes, including those for inducible nitric oxide synthase [44], and regulates TNF-α production [55,56,57]. This suggests an anti-inflammatory role of this AR subtype in the CNS. Moreover, several studies highlighted A3 AR as an antinociceptive drug target [58], especially in neuropathic pain. At this level, A3 AR stimulation provided inhibition of the mechanoallodynia induced by the chronic constriction injury of the sciatic nerve, increasing at the same time the potency of classical analgesic drugs [59,60].
At the peripheral level, A3 AR is located in epithelial cells, respiratory apparatus, and cardiovascular system [1]. In the latter, A3 AR is greatly expressed in the coronary and carotid arteries [61]. However, it is well known that A3 AR activation provides cardioprotective effects through the reduction of inflammatory responses and the resultant apoptotic signals [62,63]. Furthermore, A3 AR has been found in inflammatory cells, such as macrophages, neutrophils, eosinophils, lymphocytes, mast cells, and osteoblasts, where it regulates anti-inflammatory processes [7]. Interestingly, A3 AR is overexpressed in several cancer cells and tissues, and both its blockade or activation produce an antitumor effect, suggesting that it might be a theranostic target in different type of cancer [64,65,66].
1.1.5. Adenosine Receptor Heteromers
AR subtypes can form supramolecular complexes—heteromers—through interactions with other GPCRs, often acquiring distinct functional properties. These complexes are relevant when considering ligand binding and downstream signaling [67,68].
The A2A AR is highly expressed in the striatum, where it co-localizes with the dopamine D2 R to form A2A AR/D2 R heteromers [28,29]. A2A AR also interacts with the cannabinoid type 1 receptor (CB1 R), forming A2A AR/CB1 R heteromers in the striatum and hippocampus. In the striatum, this complex modulates cannabinoid-induced psychomotor effects, with A2A AR overexpression leading to a downregulation of cannabinoid signaling [69]. However, under certain conditions, presynaptic A2A AR blockade may facilitate CB1 R signaling underscoring the need for context-specific evaluation [70]. In microglia, A2A AR forms heteromers with cannabinoid type 2 receptor (CB2 R), where A2A AR antagonists enhance CB2 R signaling and exert anti-inflammatory effects [71,72]. Another relevant complex is the heteromer of A1 AR with dopamine D1 receptor (D1 R) which is expressed in spinal motoneurons and reduces excitability through inhibition of adenylyl cyclase [73]. The A1/A2A AR heteromer in astrocytes acts as a molecular switch, favoring activation of one receptor subtype over the other depending on extracellular adenosine levels [68]. The formation of A2A/A2B receptor heteromers has been reported in various tissues, where it markedly diminishes A2A receptor-mediated signaling and decreases its affinity for agonists [68]. These findings have important implications for drug research, as compounds with high in vitro affinity for the A2A AR may became ineffective in the presence of A2A/A2B AR heteromers. A2A AR antagonists, in particular, may exhibit altered behavior when targeting these heteromeric complexes. In some cases, their use has shown therapeutic benefits by modulating partner receptor activity, such as enhancing D2 R-mediated effects [28,29].
1.2. Therapeutic Potential of AR Agonists
As previously discussed, AR signaling plays a pivotal role in numerous physiological and pathological processes. Consequently, its therapeutic modulation has emerged as a promising strategy to counteract a variety of diseases, especially those characterized by inflammation, immune dysfunction, and tissue injury.
Selective AR agonists, particularly those targeting the A2A and A3 ARs, are currently under active clinical investigation for conditions marked by excessive inflammation, immune dysregulation, or tissue injury [74]. Among them, A2A AR agonists have attracted significant attention due to their potent anti-inflammatory and vasodilatory effects. The A2A agonist regadenoson, already approved for pharmacologic cardiac stress testing, is being repurposed in early-phase clinical trials for COVID-19, myocardial ischemia, lung transplantation, and high-grade gliomas [75,76,77,78,79,80]. These conditions share common pathophysiological features, including hypoxia-driven inflammation and immune activation, in which A2A AR stimulation may help limit tissue damage and promote homeostatic resolution.
In parallel, A3 AR agonists have demonstrated therapeutic potential in various immune-mediated and proliferative disorders, such as plaque psoriasis, hepatocellular carcinoma (HCC), and non-alcoholic steatohepatitis (NASH). For instance, piclidenoson (CF101), currently in phase III clinical trials for psoriasis, has shown anti-inflammatory activity by downregulating pro-inflammatory cytokines, including TNF-α and IL-17 [81]. Another A3 AR agonist, namodenoson (CF102), has reached phase III trials for HCC in patients with cirrhosis and is also being investigated for NASH [82,83]. Additionally, the A3 AR agonist FM-101 is under evaluation in early-phase studies for ocular hypertension and NASH [84], further illustrating the pharmacological versatility of this receptor subtype as a therapeutic target.
1.3. Therapeutic Potential of AR Antagonists
Multiple lines of evidence have shown that blockade of AR signaling by AR antagonists can have a favorable impact on the onset and progression of various pathological conditions. In fact, AR antagonists have demonstrated significant therapeutic potential, driving the development of numerous such compounds worldwide offering promising avenues for the treatment of a range of diseases including respiratory and neurodegenerative disorders, neuropathic pain, and cancer [85,86].
The A1 AR antagonist PBF-680 is under clinical investigation for asthma and chronic obstructive pulmonary disease (COPD), based on its capacity to reduce adenosine-induced bronchoconstriction and inflammation [87].
A2A AR antagonists have been primarily investigated in neurodegenerative disorders and oncology [72]. The selective A2A AR antagonist istradefylline (Figure 4), already approved in Japan (since 2013) and the United States (since 2019) for the treatment of PD [88], is currently being investigated for additional neurological indications, such as cognitive impairment and apathy in PD patients, as well as neuroprotective effect in an animal model of experimental autoimmune encephalomyelitis (EAE) [89,90]. Among A2A AR antagonists, preladenant (Figure 4) emerged as one of the most promising compounds for PD. It demonstrated favorable outcomes in both preclinical and early-phase clinical studies, improving motor symptoms without exacerbating dyskinesias when administered in combination with L-DOPA. However, despite this initial promise, phase III trials failed to meet their primary efficacy endpoints, ultimately resulting in the withdrawal of the compound from further development [91,92,93,94]. Vipadenant (Figure 4) was one of the earliest selective A2A AR antagonists investigated in clinical trials for PD. Nerveless, its development was halted after phase II due to insufficient efficacy and safety concerns [95]. Similarly, tozadenant demonstrated effectiveness in reducing “off” time in PD patients when used as an adjunct to L-DOPA. However, clinical development was discontinued following reports of severe adverse events, including agranulocytosis [96,97,98].
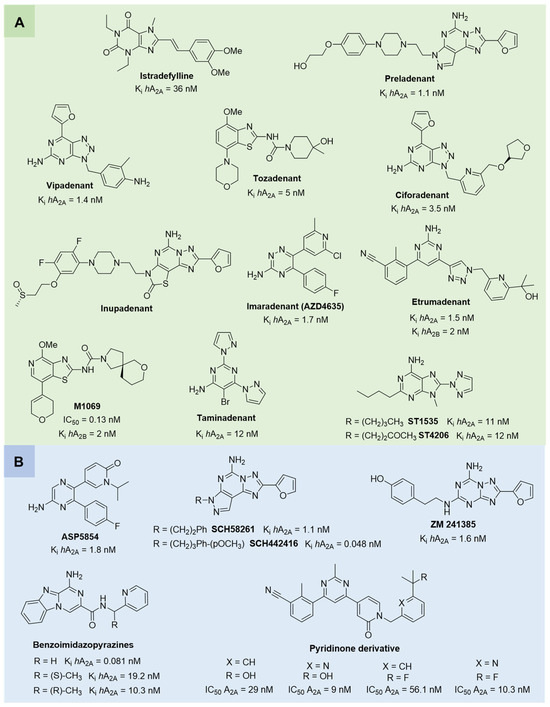
Figure 4.
(A) Structures of A2A AR antagonists and dual A2A/A2B AR antagonists undergoing clinical trials. (B) Structures of A2A AR antagonists used as pharmacological tools or showing promising activity in preclinical models of neurodegenerative diseases and cancer.
Another noteworthy class of A2A AR antagonists includes compound ST1535 and its metabolite ST4206 (Figure 4), which exhibited anti-Parkinsonian activity in rodent models. Notably, ST1535 counteracted dopaminergic neuron degeneration and glial activation, while concurrently improving motor deficits when administered alongside low doses of L-DOPA. These promising preclinical findings prompted the advance of ST1535 and its metabolites into a phase I clinical trial, where the compound demonstrated a favorable safety and tolerability profile [99,100,101,102]. In addition, the dual A1/A2A receptor antagonist ASP5854 (Figure 4) has been evaluated in preclinical models of PD, showing neuroprotective and motor symptom-improving effects [103,104].
In parallel, novel A2A AR antagonists, including ciforadenant, inupadenant, imaradenant (AZD4635), and taminadenat (Figure 4), have been evaluated in early-phase trials for various solid tumors. These agents aim to restore anti-tumor immunity by blocking adenosine-mediated immunosuppression within the tumor microenvironment [86,105,106,107,108,109,110,111]. Aligned with this immunotherapeutic strategy, dual A2A/A2B AR antagonists, etrumadenant and M1069 (Figure 4), have being investigated across multiple cancer types, including prostate, colorectal, urothelial, and head and neck tumors, progressing to phase II trials. The simultaneous blockade of A2A and A2B receptors is intended to enhance immune cell responsiveness and function, thereby counteracting the immunosuppressive effects driven by hypoxic and adenosine-rich tumor environments [112,113]. Several compounds, including SCH58261 and SCH442416 (Figure 4), have been widely used in experimental studies to elucidate A2A AR pharmacology and downstream signaling pathways. They have been instrumental in clarifying the functional role of A2A AR across various pathological conditions. In particular, SCH58261 was shown to improve cognitive function in Alzheimer’s disease model mice by activating Nrf2 via autophagy and to attenuate neuroinflammation in a mouse model of EAE by reducing microglia/macrophage activation and T cell infiltration [114,115,116,117,118]. Additional antagonists, including the potent and selective A2A AR antagonist ZM241385 (Figure 4), have primarily been employed in research settings to investigate the roles of adenosine signaling in neurodegeneration and cancer immunology [119,120,121,122]. Recently, two novel classes of A2A AR antagonists have been identified for cancer immunotherapy. The first one includes benzo[4,5]imidazo[1,2-a]pyrazin-1-amine derivatives (Figure 4), which display subnanomolar affinity for the A2A AR and exhibit potent immunostimulatory activity. Compared to the clinical candidate AZD4635, these compounds more effectively reversed the immunosuppressive tumor microenvironment, thereby inhibiting tumor growth in preclinical models, without signs of toxicity at the tested doses [123,124]. The second class includes pyridinone-based A2A AR antagonists, identified through high-throughput screening and structure–activity relationship (SAR) optimization. These compounds demonstrated potent receptor antagonism, favorable pharmacokinetic properties, and the ability to enhance T cell activation by modulating key immunoregulatory pathways. In preclinical tumor models, they showed significant antitumor activity, supporting their development as potential candidates for cancer immunotherapy [125].
Due to the critical role of A2B AR in regulating immune responses, inflammation, and tissue remodeling within the tumor microenvironment, recently A2B AR selective antagonists have emerged as promising therapeutic agents in oncology. These antagonists are particularly valued for their ability to modulate the tumor microenvironment by suppressing immunosuppressive signaling pathways, inhibiting tumor growth, and reducing angiogenesis. However, to date, the number of highly selective A2B AR antagonists remains limited. Furthermore, the evaluation of A2B AR signaling in specific tumor types is challenging due to the receptor’s low affinity for endogenous adenosine under physiological conditions. Additionally, its expression is highly context-dependent, varying significantly with physiological or pathological states such as hypoxia and inflammation, which further complicates preclinical characterization and therapeutic targeting [126,127].
Although still in early phases, A3 AR antagonists PBF-1650 and PBF-677, whose structures have not, to the best of our knowledge, been publicly disclosed [128], are under investigation for immune-mediated inflammatory conditions, including psoriasis and ulcerative colitis, expanding the therapeutic potential of this class [74]. Their clinical relevance is further supported by preclinical studies in additional pathological contexts such as neurodegenerative diseases, traumatic brain injury, and cancer, where A3 AR signaling has been implicated [11,58,108,110]. Among the selective A3 AR antagonists, compounds such as VUF5574, MRE3008-F20, and LJ1251 (Figure 5) have been extensively characterized for their pharmacological profiles and potential clinical applications. VUF5574 and MRE3008-F20 exhibited high affinity and selectivity for the human A3 AR, effectively modulating receptor activity in preclinical models, making it a valuable tool in dissecting receptor function and signaling dynamics [129,130,131,132]. Moreover, the human A3 AR antagonist LJ1251 demonstrated a remarkable neuroprotective effect in a cellular model of ischemia, preventing ischemic neuronal injury induced by severe OGD [133].
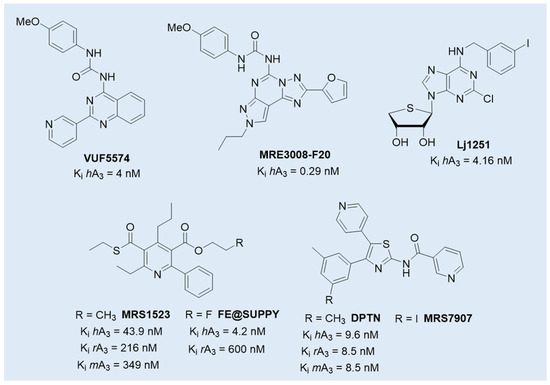
Figure 5.
Structures of selected A3 AR antagonists with preclinical efficacy or cross-species activity.
However, despite this promising therapeutic potential, significant challenges remain in translating preclinical findings for A3 AR antagonists from animal models to humans. One major obstacle lies in the notable interspecies differences in receptor sequence and structure. The amino acid sequence identity between rat and human A3 AR is approximately 72–75%, a relatively low value compared to other adenosine receptor subtypes [134]. Crucially, these differences affect specific receptor domains involved in antagonist binding, while the regions critical for agonist interaction are more conserved across species. As a result, most A3 AR antagonists at the human receptor exhibit reduced or no activity in rodent models, leading to potentially misleading preclinical data [134,135,136]. Only few compounds preserve activity across species, including DPTN (and its 3-iodo analogue MRS7907), MRS1523, and FE@SUPPY (Figure 5). DPTN displayed nanomolar affinity for human (Ki = 1.6 nM), mouse (Ki = 9.6 nM), and rat (Ki = 8.5 nM) A3 ARs, but moderate selectivity against other AR subtypes [136,137]. MRS1523 maintained cross-species antagonism but with significantly lower affinity at rodent receptor (Ki = 43.9 nM in human, 349 nM in mouse, and 216 nM in rat) and limited subtype selectivity, especially at higher concentrations [138,139]. The PET tracer FE@SUPPY (unlabeled form), structurally related to MRS1523 [138], was used to label rat brain A3 ARs. The autoradiography was performed on rat brain slices in the presence or absence of Cl-IB-MECA, suggesting that it has the potential to serve as the first positron emission tomography (PET) tracer for the A3 AR, even if the outcomes did not yield definitive conclusions [140].
The ongoing trials underscore the versatility of AR antagonists as potential drugs, indicating that development of novel more selective, potent, and pharmacokinetically optimized compounds remains a critical challenge.
2. Structural Simplification from Tricyclic to Bicyclic Scaffolds in the Design of Adenosine Receptor Antagonists
This review presents a comprehensive overview of about twenty-five years of our research in the field of adenosine receptor antagonists, which has led to the discovery of numerous potent compounds across various chemical classes, characterized by high selectivity for the different AR subtypes. The new derivatives were designed following general principles of medicinal chemistry, with the most recent work focusing on molecular simplification to obtain compounds with improved physicochemical properties.
Specifically, this review focuses on different heterocyclic classes of AR antagonists that were designed starting from two tricyclic series, the 1,2,4-triazolo[4,3-a]quinoxalin-1-ones (TQX) and the pyrazolo[3,4-c]quinolines (PQ). Both the series were deeply investigated and from each of them simplified analogues were designed leading to bicyclic and monocyclic ligands (Figure 6). For each class of ligands, structure–activity relationships (SARs) are discussed, highlighting the structural features required for affinity and selectivity toward the different AR subtypes. The results of molecular docking investigation are reported to describe the hypothetical binding pose of the ligands at the AR recognition sites. Additionally, the results of in vitro and in vivo pharmacological studies are presented, emphasizing the neuroprotective, antinociceptive, antiproliferative, and antidepressive effects of AR antagonists.
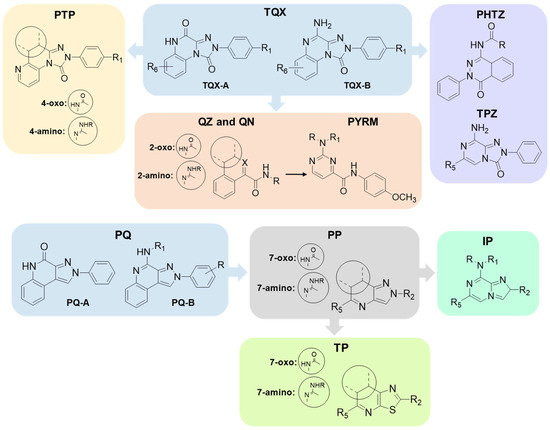
Figure 6.
The herein reported tricyclic AR antagonists and their simplified analogues.
2.1. 2-Aryl-1,2,4-triazolo[4,3-a]quinoxalin-1-one Derivatives and Their Simplified Analogues
2.1.1. 2-Aryl-1,2,4-triazolo[4,3-a]quinoxalin-1-one Derivatives
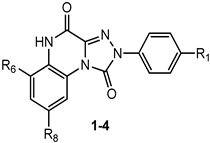
This section presents the study of 2-aryl-1,2,4-triazolo[4,3-a]quinoxalin-1-one derivatives, classified into the 1,4-dione and the 4-amino-1-one series (TQX-A and TQX-B, respectively, Figure 7). Notably, the triazoloquinoxalin-1,4-dione scaffold (TQX-A) emerged as a valuable framework for the development of potent and selective A3 AR antagonists. In contrast, the 4-amino-triazoloquinoxalin-1-one core (TQX-B) gave rise to potent and selective antagonists of A1, A2A, or A3 AR subtypes, depending on the substitution pattern on the aromatic rings and the 4-amino group [141,142]. Finding new potent antagonists for A3 AR within the TQX-A series was of high interest, as the involvement of this AR subtype in regulating several pathological processes, including neuronal inflammation, immune responses, and cardiac function, was becoming increasingly evident. Therefore, selective A3 AR antagonists were sought both as pharmacological probes to elucidate receptor-mediated pathophysiological mechanisms and as candidates for therapeutic intervention in autoimmune diseases, cancer, brain injury, and neurodegenerative disorders [13,56,74,143,144].
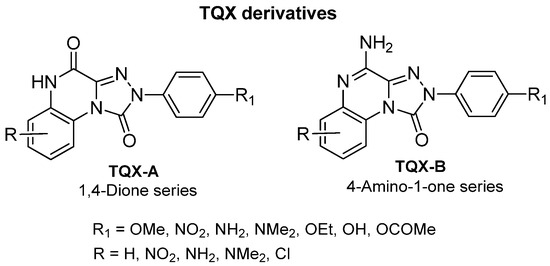
Figure 7.
2-Aryl-1,2,4-triazolo[4,3-a]quinoxalin-1-one (TQX) derivatives.
2-Aryl-1,2,4-triazolo[4,3-a]quinoxalin-1,4-dione Derivatives
The study of the TQX-A series (Figure 7) was encouraged by the interesting profile of the unsubstituted compound 1 (Table 1), which showed nanomolar A3 AR affinity (Ki = 80 nM) and high selectivity versus the A1 and A2A ARs. Thus, various substituents were inserted on the 2-phenyl ring (R1) and/or on the fused benzo moiety (R6, R8) to improve A3 affinity and selectivity of derivative 1 [141,145,146].

Table 1.
Binding affinity (Ki) of TQX-A derivatives at bovine (b) A1 and A2A ARs, and at human (h) A1 and A3 ARs a.
Among the R1 substituents, characterized by varying electronic properties, lipophilicity, and steric bulk (OMe, OH, OEt, OCOMe, NO2), the most effective were the methoxy (compound 2, Ki = 16 nM) [141] and the nitro (compound 3, Ki = 0.6 nM) [146]. Compound 3 displayed antagonist activity in [35S]GTPγS binding assays at the hA3 AR (EC50 = 5.4 nM), making it one of the most potent hA3 AR antagonists reported to date, with high selectivity versus hA1 and hA2A AR subtypes. Notably, since the most active derivatives bear either electron-donating (e.g., OMe) or electron-withdrawing (e.g., NO2) substituents, it was inferred that the electronic nature of the R1 group does not critically influence receptor binding.
Docking studies at a homology model of the hA3 AR depicted the putative binding poses of these derivatives [146], which presented the fused benzo ring located in a tiny hydrophobic pocket delimited by transmembrane (TM) domains 5 and 6, and the 2-aryl ring positioned in a small hydrophobic cavity delimited by extracellular loop 2 (EL2), and by non-polar amino acids residues, such as Leu90 (TM3), Leu246 (TM6), and Ile268 (TM7). At least two H-bonding interactions were evidenced, the first between the carbonyl group at the 1-position and the NH of the Gln167–Phe168 amide bond. The 1-carbonyl group could also interact with the amide moiety of Asn250 side chain, a residue conserved among all AR subtypes, and important for ligand binding. Steric factors and the ability of the R1 group to act as a hydrogen bond acceptor were relevant for receptor interaction [146]. In particular, the para-nitro group of derivative 3 strongly achieved two hydrogen-bonding interactions with Ser165 (EL2) and His272 (TM7) which could be responsible for the observed subnanomolar activity at the hA3 AR. Lipophilic chlorine atom(s) and polar or hydrophilic groups (NH2, NO2), at the 6,8-positions of the fused benzo ring, were not well tolerated by the A3 AR, and also negatively affected affinity for the A2A AR, while increasing the A1 AR binding [145]. When R1 = OMe was combined with R6 = NO2 (derivative 4), higher A3 AR affinity was found with respect to that of the p-methoxy derivative 2.
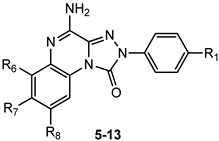
4-Amino-2-aryl-1,2,4-triazolo[4,3-a]quinoxalin-1-one Derivatives
The structural modification performed on TQX-A were applied also to the 2-aryl-1,2,4-triazolo[4,3-a]quinoxalin-4-amino-1-one series (TQX-B, Figure 7) [141,145,146]. Overall, these 4-amino derivatives demonstrated the ability to bind A1, A2A, and A3 ARs with affinities ranging from subnanomolar to high nanomolar values (Table 2). The unsubstituted derivative 5 showed high affinity for A1 and A2A ARs, and more than 10-fold lower affinity for the A3 AR subtype. Compounds bearing a substituent on the 2-phenyl group (R1) or on the fused benzo ring (R6, R7, and R8) generally displayed reduced affinity for A1 and A2A ARs, with respect to compound 5, with the exception of derivatives 12, 13, which showed, respectively, similar and better affinity for the bA1 AR, compared to 5, compound 13 being the most active (Ki = 0.2 nM). The ability of the R1 substituent to engage in a hydrogen bond, as well as its steric properties, play a role in hA3 receptor recognition. The most beneficial group for A3 AR affinity and selectivity was R1 = OMe (compound 6) while R1 = NO2 was deleterious (compound 7), in contrast to the effect elicited in the corresponding 1,4-dione derivative 3 (Table 1).

Table 2.
Binding affinity (Ki) of TQX-B derivatives at bovine (b) A1 and A2A ARs, and at hA1 and hA3 ARs a.
Lipophilic chlorine atom(s) and polar or hydrophilic groups (NH2, NO2) at the 6-, 7- and 8-positions (derivatives 8–13, Table 2) [145] significantly enhanced hA3 AR affinity, compared to the lead 5. In particular, the 6-NO2 group afforded the highest hA3 AR affinity (compound 10, Ki = 4.75 nM). Combination of R6 = NO2 or NH2 with R1 = OMe (derivatives 9 and 10) maintain a high A3 AR affinity (Ki = 47 nM, and 22 nM, respectively). Insertion of a chloro substituent at positions 7 or 8 was also favorable (derivatives 11 and 12), The combination of an 8-Cl with a 6-NO2 significantly enhanced A3 AR affinity, yielding derivative 13, the most active of this set (Ki = 0.112 nM).
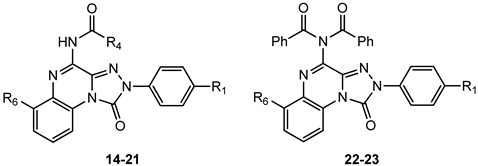
4-Amido-2-aryl-1,2,4-triazolo[4,3-a]quinoxalin-1-one Derivatives
The presence of acyl substituents on the 4-amino group yielded TQX derivatives exhibiting nanomolar affinity and high selectivity for the hA3 AR (compounds 14–23, Table 3), in accordance with the results observed in other series of antagonists of similar size and shape [147,148]. Acyl substituents with varying steric bulk were investigated either alone or in combination with the beneficial substituents on the 2-phenyl ring (R1 = OMe), and the fused benzo ring (R6 = NO2, NH2) [149]. The presence of R1 = OMe significantly increased hA3 versus hA1 selectivity, while maintaining high hA3 AR affinity (compare 17, 20, and 23 to 15, 16, and 22, respectively). The contemporary presence of R1 = OMe and R6 = NO2 dropped hA3 AR affinity to the micromolar range with the sole exception of the 2-diphenylacetamido derivative 21, highly active (Ki = 0.8 nM) and selective. Interestingly, double substitution with R1 = OMe and R6 = NH2 yielded compounds with high hA3 AR affinities (Ki = 1–5.5 nM) and selectivity (e.g., 19), regardless of the acyl group at R4.

Table 3.
Binding affinity (Ki) of TQX-B derivatives at bA1 and bA2A ARs, and at hA1 and hA3 ARs.
Derivatives 19, 20, 22, and 23, tested in cAMP functional assays, proved to be potent antagonists at the hA3 AR (IC50 = 8.6–31.8 nM), and inactive at the hA2B AR (IC50 > 1000 nM) [149].
Summarizing, the 4-methoxy group on the 2-phenyl ring, acyl moieties on the 4-amino group or a 6-nitro substituent on the benzo-fused ring provided derivatives endowed with nanomolar affinity and high selectivity for hA3 AR [141,145,146,149]. Molecular modeling studies at a homology model of the hA3 AR evidenced hydrogen-bonding interactions, involving the 1-carbonyl, the R1 = OMe, the R6 = NO2, and the 4-acylamino chain [149]. In particular, the 4-NH–CO moiety was surrounded by the polar amino acids Thr94 (TM3), His95 (TM3), and Ser247 (TM6). The hydroxyl residue of the latter was appropriately oriented to form an H-bond with the carbonyl oxygen of the 4-NHCO group, which in turn played a key role in orienting the R4 substituent within a hydrophobic pocket.
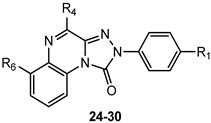
Given the critical role of the R4 group for hA3 AR affinity, other acyl substituents and sulfonyl (SO2Me, SO2Ph) or carbamoyl (CONHCOR) groups were introduced on the 4-amino residue. Additionally, 4-OCH2Ph-substituted derivatives were synthesized to assess the importance of the 4-NHCOR group for the anchoring at the hA3 AR [150]. The 4-pyridylcarbonyl-substituted compound 24 (Table 4) was one of the most active derivatives, exhibiting a nanomolar hA3 AR affinity and an enhanced selectivity, with respect to the benzoyl derivative 15 (Table 2). The 4-NHSO2Ph derivative 25 also showed high hA3 AR affinity and selectivity. Insertion of two SO2Me groups on the 4-amino moiety gave compound 27 which displayed nanomolar hA3 AR affinity (Ki = 5.5 nM), high hA3 AR antagonistic potency (IC50 = 12 nM, cAMP assay), as well as high selectivity versus the other hARs. Benzylureido group in R4 yielded good affinity for the hA3 AR (compound 28) and even better for the hA1 AR. It is notable that the 4-benzyloxy derivative 29 possessed high affinity and selectivity for hA3 AR (Ki = 21 nM), although lacking the 4-NH–CO–group. The beneficial effect of the R1 = OMe was confirmed for derivatives 26 and 30 showing higher hA3 AR affinities compared to their unsubstituted counterparts 25 and 29. These new derivatives served as molecular probes to construct refined models of the hA3 AR developed through a ligand-based homology modeling (LBHM) approach. Docking of derivatives 27 and 28 induced a different reorganization of the binding site resulting in molecular volumes of the TM binding cavity of 1120 Å3 and 980 Å3, respectively [150]. The network of interactions observed was consistent with that above described for derivatives 14–23.

Table 4.
Binding affinity (Ki) of TQX-B derivatives at hA1, hA2A, and hA3 ARs a.
Derivative 27 was tested in an in vitro electrophysiological rat model of cerebral ischemia, obtained by oxygen and glucose deprivation (OGD). During an ischemic stroke, the levels of adenosine increase, activating the harmful A3 AR subtype both in vitro and in vivo [151,152]. Thus, targeting this AR subtype could be useful for the treatment of the pathology. Derivative 27, at 10 nM concentration, delayed the onset of anoxic depolarization, a sign of brain damage, demonstrating its effectiveness in enhancing the resistance of the CA1 region of the hippocampus to ischemic injury. This result contrasted with the very low affinity of 27 at the rat A3 AR (% inhibition (I%) @ 1 µM = 5%) but was consistent with the results obtained with other hA3 AR antagonists belonging to diverse chemical series which showed high affinity for the hA3 AR and very low affinity for the rat A3 AR [133,153,154], due to the well-known low homology between the two receptors [135,136].
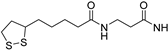
The robustness and versatility of the 4-amino-triazoloquinoxalinon-1-one scaffold to develop hA3 AR antagonists were recognized by Vernall et al. [155] who selected derivative 6 (Table 2) as the lead for the development of fluorescent antagonists to be used as molecular probes for the study of the A3 AR. Various fluorophores were introduced at the 8-amino position of 6 through linkers of different natures. The most promising compound was the BODIPY-X-630/650-conjugated derivative 31 (Figure 8) exhibiting high affinity for the hA3 AR (pKD = 9.36 ± 0.16) and over 650-fold selectivity versus other ARs. The compound possessed excellent imaging properties, making it a valuable tool for investigating A3 AR.
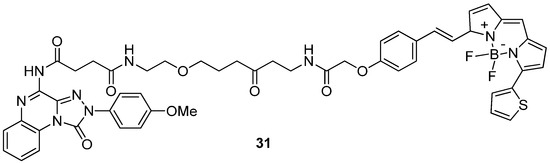
Figure 8.
Structure of the fluorescent highly potent and selective hA3 AR antagonist 31.
6-(Hetero)arylalkylamino-1,2,4-triazolo[4,3-a]quinoxalin-1-one Derivatives
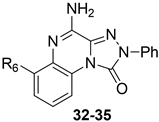
Some investigations on the TQX-B series focused on the development of hA2A AR antagonists, which were designed taking the well-known A2A AR antagonists ZM241385 [156] and derivatives belonging to SCH series as lead compounds (Figure 9) [157,158]. The 4-amino-6-benzylamino-2-phenyl-substituted TQX derivative 32 was the first to be synthesized (Figure 9), resulting in a potent and selective hA2A AR antagonist [141,142]. Polar substituents capable of forming hydrogen bonds (F, OCH3, OH, COOH) were introduced on the 6-benzylamino moiety since they might enhance both hA2A AR affinity and the physicochemical properties of the compounds, such as water solubility [142]. The carboxy group afforded the best hA2A AR affinity (Table 5, derivative 33). Even if some of the new compounds exhibited nanomolar hA2A AR affinity, none of them exceeded the hA2A AR affinity and selectivity of the parent compound 32. The replacement of the phenyl moiety of 32 with less hindered bioisostere furyl or thienyl rings gave the best binding affinity results (compounds 34, 35).
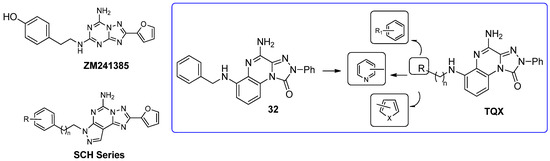
Figure 9.
Structures of known A2A AR antagonists and 6-(hetero)arylalkylamino-TQX derivatives.

Table 5.
Binding affinity (Ki) of 6-(hetero)arylalkylamino-substituted TQX-B derivatives at bovine (b) A1 and A2A ARs, and at hA3 ARs a.
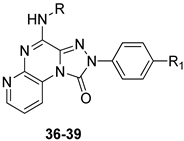
2.1.2. 2-Arylpyrido[2,3-e]-1,2,4-triazolo[4,3-a]pyrazin-1-one Derivatives
As discussed above, the presence of R6 = NO2 in the TQX series often favors anchoring to the hA3 AR through H-bond formation but in some 6-nitro-4-amido-substituted derivatives unfavorable steric interactions between the nitro group and the receptor site could occur, leading to a reduced affinity. To overcome this drawback, a series of 2-arylpyrido[2,3-e]-1,2,4-triazolo[4,3-a]pyrazin-1-one (PTP) was designed (Table 6), in which the endocyclic nitrogen atom intended to serve as an H-bond acceptor, in the place of the 6-nitro group, without causing steric hindrance [159].

Table 6.
Binding affinity (Ki) of PTP derivatives at hA1, hA2A, and hA3 ARs.
The unsubstituted compound 36 exhibited quite low affinity for the hA3 AR (Ki = 656 nM), while it was active at the bA1 and bA2A ARs. As in the TQZ lead, the presence of R1 = OMe enhanced hA3 AR affinity and selectivity (Table 6, derivative 37). The same applies to the effect of acyl residues on the 4-amino group (R = COMe, COPh, COCH2Ph), especially in combination with R1 = OMe, as demonstrated by compound 39, which showed the highest hA3 AR affinity (Ki = 4.54 nM) and selectivity versus both hA1 and hA2A ARs. The compound was tested in cAMP assays, proving to be a potent antagonist at the hA3 AR (EC50 = 36.3 nM) and inactive at the hA2B subtype (IC50 > 10,000 nM).
Compound 39 resulted to be effective in an in vitro rat model of cerebral ischemia, since it prevented the failure of synaptic activity induced by OGD in the hippocampus. Notably, this compound exhibited nanomolar affinities for the hA3 receptor, but showed no binding activity at the rA3 AR, in accordance with the findings above discussed for derivative 27 [150].
2.1.3. From the Tricyclic TQX to the 2-Oxo/2-Aminoquinazoline-4-carboxamide Scaffold
An in silico molecular simplification approach was employed to design three series of TQX analogues as new hA3 AR antagonists [160] (Figure 10).
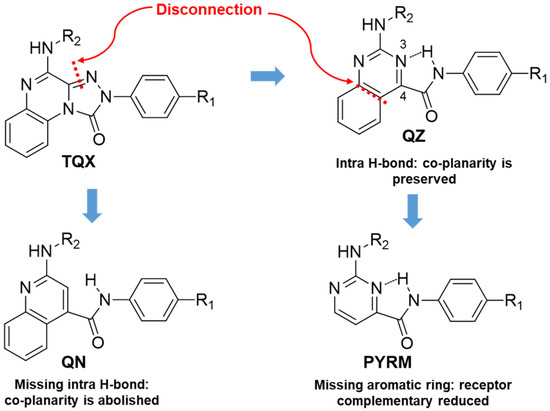
Figure 10.
Design of the TQX analogues through molecular simplification approach.
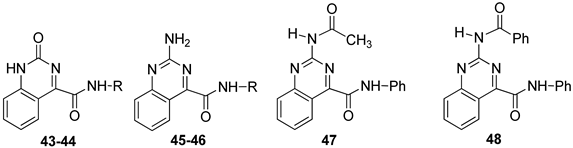
The quinazoline-4-carboxamide (QZ) series, including 2-oxo- and 2-amino-substituted, maintain all key interactions evidenced for the TQX derivatives [146,149], i.e., π-π stacking interactions with both side chains of Phe168 (EL2) and Phe182 (TM5), two H-bonds with Gln167 and Asn250, and an H-bond with His95. The intramolecular H-bond between N3 of the quinazoline system and the NH of the 4-amide moiety preserved the planarity and similar steric properties to the TQX analogues. The QZ derivatives resulted in potent hA3 AR antagonists (Table 7), and exhibited very high selectivity for the hA3 AR. As observed in the TQX series, the 2-oxo-derivatives displayed higher hA3 AR affinity compared to the corresponding 2-amino analogues (compare 43, 44 to 45, 46) likely due to a stronger H-bonding interaction of the 2-carbonyl group with His95, compared to the 2-amino group [141,142,146,149]. The para-OMe substituent on the phenyl moiety (R) was confirmed to be the most favorable (compounds 44 and 46) among those evaluated (OMe, Me, Br). The presence of acyl groups on the 2-amino moiety (derivatives 47 and 48) enhanced hA3 AR affinity, in comparison to the unsubstituted compound 45.

Table 7.
Binding affinity (Ki) of QZ derivatives at hA1, hA2A, hA3 ARs and potency (IC50) at hA2B and hA3 ARs.
The quinoline-4-carboxamide (QN) derivatives (Figure 10) exhibited no affinity for the hA3 AR because the lack of the intramolecular H-bond does not allow the system to adopt a planar conformation, thus losing some of the most important interactions with the receptor binding site. In the pyrimidine-4-carboxamide derivatives (PYRM series, Figure 10), the intramolecular H-bond allowed a simulation of the planar bicycle, therefore maintaining the π-π stacking interactions with both Phe168 and Phe182. Nevertheless, these derivatives exhibited no hA3 AR affinity, likely due to a shift in their positioning within the binding cleft which prevents the formation of H-bonding interactions with His59, Gln167, and Asn250.
2.1.4. From TQX and QZ Series to the 2-Phenylphthalazin-1(2H)-one Derivatives
To continue pursuing a molecular simplification approach for the design of novel hA3 AR antagonists, 2-phenylphthalazin-1(2H)-one derivatives (PHTZ, Figure 11) [161], featuring amido and ureido moieties at the 4-position, were synthesized due to their structural similarity with both TQX [141] and QZ series [160].
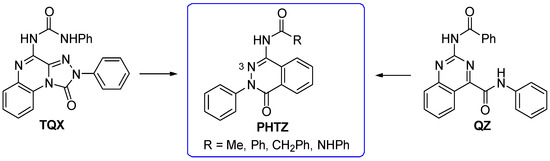
Figure 11.
Design of the 2-phenylphthalazin-1(2H)-one derivatives as analogues of TQX and QZ series.
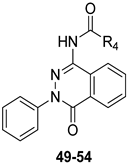
Some of the PHTZ compounds displayed both high affinity and selectivity for hA3 AR (derivatives 49–54 Table 8); the most active compounds were the 4-arylureido-substituted bearing methoxy group(s) on the phenyl pendant (derivatives 51–53), which displayed Ki values in the low nanomolar range, the best being derivative 53 (Ki = 0.776 nM).

Table 8.
Binding affinity (Ki) of PHTZ derivatives at hA1, hA2A, hA3 ARs and potency (IC50) at hA2B and hA3 ARs.
The benzylureido derivative 54 also exhibited high hA3 AR affinity and selectivity. Surprisingly, acylamino substituents at position 4 were detrimental, resulting in weakly active (e.g., 49), or completely inactive compounds, in contrast with the positive effect of these moieties in other series of hA3 AR antagonists, including the TQX and QZ series. Compounds 51–54 acted as potent antagonists by reversing NECA-mediated inhibition of cAMP levels in hA3-CHO cells, while were inactive in cAMP assay in hA2B-CHO cells. The new PHTZ derivatives were docked at the first published homology model of the hA3 AR based on the crystal structure of the A2A AR in complex with the antagonist ZM241385 (PDB code: 3EML) [162]. Derivative 50 was accommodated into the binding cavity with the 4-phenylureido group oriented toward the EL region, while maintaining all the key interactions observed for the TQX and QZ derivatives. The compound forms three H-bonds with Asn250 (TM6) side chain, involving the N3 atom of the pthalazinone core and the two NH groups of the ureido moiety. Additionally, the PHTZ core engages in π-π-stacking interactions with Phe168 (EL2).
2.1.5. From TQX Series to the 1,2,4-Triazolo[4,3-a]pyrazin-3-one Derivatives
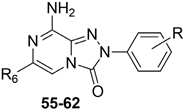
With the aim of identifying a novel bicyclic chemotype, the 2-aryl-8-amino-1,2,4-triazolo[4,3-a]pyrazin-3-one (TPZ) series was designed through structural simplification of the TQX series (Figure 12). Notably, the synthetic accessibility of the TPZ scaffold allowed the introduction of various substituents with different lipophilic and steric properties at the 6-position (Me, aryl) and on the 2-phenyl ring (OMe, OH, NO2, NH2) [163]. Moreover, replacement of the fused benzo ring of TQZ with a flexible 6-phenyl pendant might enhance both the molecular fit within the receptor site and the solubility of the compounds in the assay media.
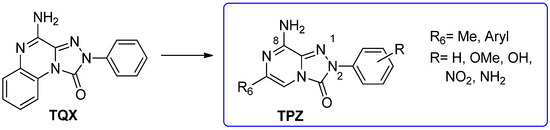
Figure 12.
Design of the 1,2,4-triazolo[4,3-a]pyrazin-3-one series as simplified analogues of TQX series.
All derivatives were totally inactive at the hA2B AR, as they exhibited IC50 values > 30,000 nM in the cAMP assays. The 2,6-diphenyl-substituted derivative 56 displayed nanomolar affinity for hA1, A2A, and hA3 ARs, while its analogue 6-methyl-substituted 55 was significantly less active. The presence of a substituent on the 2-phenyl ring (R) enhanced affinity for the hA1 AR while reducing that for hA2A and hA3 ARs (57, 58) (Table 9).

Table 9.
Binding affinity (Ki) of TPZ derivatives at hA1, hA2A, hA3 ARs.
To enhance hA2A AR affinity and selectivity, various substituents were introduced on the 6-phenyl ring, while maintaining R = H. The interest in identifying new hA2A AR antagonists was due to their significant therapeutic potential in various diseases, including cancer, neurodegenerative disorders, and neuropathic pain. The new 6-aryl-triazolopyrazines 59–62 featuring a methoxy or other small alkoxy residues, on the para position of the 6-phenyl ring, exhibited high affinity (Ki = 2.9–10.6 nM) and complete selectivity for the hA2A AR subtype. Compound 59 exhibited an antagonistic behavior (cAMP assay) and neuroprotective effect against MPP+-induced neurotoxicity in SH-SY5Y cell cultures, an in vitro model of Parkinson’s disease.
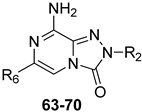
Additional triazolopyrazine derivatives featured a phenyl ring at position 2 (R2) and different aryl groups (R = NO2, Br, Cl, substituted piperazine) at position 6 (R6) [164]. To improve compound solubility, heteroaryl rings were also introduced in R6, and a flexible benzyl chain was incorporated in R2 (Table 10). All these triazolopyrazines were inactive at the hA2B AR. Several compounds possessed nanomolar affinity for both hA1 and hA2A ARs, and different degrees of selectivity versus the hA3 AR. Among the 2-phenyl-substituted derivatives, 63 (R6 = 2-furyl) and 66, 67 (R = 3-Cl and 4-Cl on the 6-aryl) were the most active at both A1 and A2A ARs. The same dual profile was exhibited by the 2-benzyl derivatives 68–70. Derivatives 64 and 65, bearing, respectively, a para nitro and bromine on the 6-phenyl ring, displayed high affinity (Ki = 7.2 and 10.6 nM, respectively) and a complete selectivity for the hA2A AR.

Table 10.
Binding affinity (Ki) of TPZ derivatives at hA1, hA2A, and hA3 ARs.
In cAMP assays, derivative 65 acted as an antagonist at the hA2A AR whereas derivatives 66 and 70 behaved as dual hA1/hA2A AR antagonists (66, 70). The three compounds were tested for their ability to counteract β-amyloid peptide (Aβ)-induced toxicity in SH-SY5Y cell lines, since data in the literature reported that selective hA2A AR antagonists mitigated cognitive impairments induced by β-amyloid peptide administration in various in vivo rodent models [119,165,166] and that dual hA1/hA2A AR antagonists were neuroprotective against β-amyloid-induced toxicity in neuroblastoma cells treated with aluminum chloride [167]. Derivative 66 did not exhibit a protective effect on cell viability, while compounds 65 and 70 demonstrated neuroprotective activity, by counteracting the neurotoxicity induced by Aβ aggregates exposure.
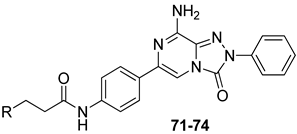
Finally, 2-phenyl-substituted TPZ derivatives were decorated on the para position of the 6-phenyl ring with amide moieties bearing terminal basic rings (pyrrolidine, piperidine, substituted piperazines, and morpholine) (Table 11) [168]. All compounds 71–74 exhibited one-digit nanomolar affinity for the hA2A AR and high selectivity versus hA1 and hA3 ARs and also the hA2B AR, being totally inactive in the cAMP assays in hA2B AR-CHO cells (IC50 > 10,000 nM).

Table 11.
Binding affinity (Ki) at hA1, hA2A, and hA3 ARs of TPZ derivatives featuring basic substituents in the lateral chain.
Docking studies at the crystal structure of the hA2A AR showed that steric hindrance of the substituents at the 2- and 6-positions of the TPZ bicyclic core influenced the ligand orientation within the binding cavity. Most of the triazolopyrazine derivatives (55–62, 64–67) [163,164] preferentially bind to the receptor site with the 2-phenyl substituent positioned in the depth of the cavity, and the R6 group pointing toward the entrance of the binding site (type one conformation, Figure 13), mimicking the binding mode of ZM241385 in the A2A AR. The bicyclic core engages in π-π interaction with Phe168 (EL2) and the 8-NH2 group forms H-bonds with Asn253 (TM6) and Glu169 (EL2). Asn253 residue seems to interact also with the endocyclic N1 atom. The presence of a substituent on the 2-phenyl ring decreases A2A AR affinity as it causes a slight shift of the molecule that reduces ability to establish some key interactions, such as those with Asn253 and Glu169.
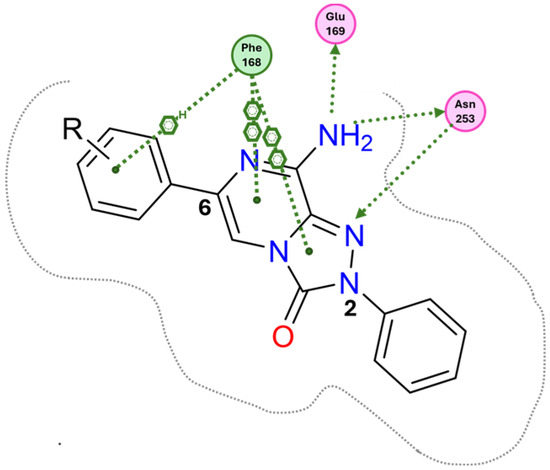
Figure 13.
Schematic representation of the type one docking conformation of TPZ derivatives at the hA2A AR.
Compounds bearing a heterocyclic moiety at position 6 and a phenyl ring at position 2 (e.g., derivative 63) [164] can adopt two docking conformations: type one and type two. A slight preference is observed for type two in which the orientation is reversed: the smaller 6-substituent penetrates the binding cavity, and the bulkier 2-substituent points outward. Derivatives 64–67 preferentially adopt the type one conformation, whereas compounds 68–70, bearing a benzyl group at position 2 and a heterocycle at position 6, favor the type two pose. This preference is driven by steric hindrance, positioning the bulkier 2-substituent toward the extracellular space.
Derivatives 71–74 assumed similar docking conformations to those observed for the lead compound 56, presenting the bulky 6-aryl substituent oriented toward the extracellular environment. The lateral basic rings, which may carry a positive charge at pH = 7.4, were involved in polar interaction with the backbone carbonyl group of the nearby amino acids, as well as with the side chains of Ser67 and Ser6, at the entrance of the cavity. The carbon residue of the amide linker (X = NHCO) was capable of forming an H-bond with the OH group of Tyr271 (TM7). A similar interaction can also occur with the oxygen atom of the ether linker (X = O), but it is associated with lower binding energy.
1,2,4-Triazolo[4,3-a]pyrazin-3-ones as Multi-Functional A2A AR Antagonists
Traditional pharmacological approaches rely on the use of drugs that are as selective as possible for a specific target, with the aim of minimizing side effects arising from interactions with off-target biomolecules. However, it is well known that certain disorders, including neurodegenerative diseases, neuropathic pain, and cancer, involve complex pathways associated with the dysfunction of multiple biological targets. In this context, traditional therapies have revealed the limitations of the one-drug-one-target paradigm. Thus, in the recent years, the multifunctional approach has gained increasing relevance in the treatment of the aforementioned diseases. This approach can be pursued by two different strategies: (1) a combination of monofunctional drugs, each active on a specific target and administrated either in association or within the same formulation; or (2) the use of a single multifunctional compound capable of modulating multiple biological targets [169,170].
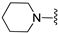
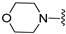
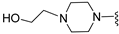
- Antioxidant-hybridized 1,2,4-triazolo[4,3-a]pyrazin-3-ones
A2A AR signaling plays a key role in modulating neuroinflammatory pathways, which are involved in various disorders, including neuropathic pain. Although the role of the A2A AR in pain is still a matter of debate, since both pro-nociceptive and antinociceptive effects have been reported, A2A AR blockade appears to confer protection [35]. Neuropathic pain is a condition characterized by a complex pathophysiology involving both central and peripheral mechanisms [35]. Although the molecular basis is not fully understood, oxidative stress might contribute to its development prompting growing interest in antioxidants as potential therapeutic agents [171,172,173]. Therefore, we focused on the development of dual-acting compounds exhibiting both A2A AR antagonism and antioxidant activity, as they may offer enhanced therapeutic efficacy against neuropathic pain. To this end, two sets of novel triazolopyrazine derivatives were designed and synthesized, incorporating various antioxidant moieties (Figure 14) [174].
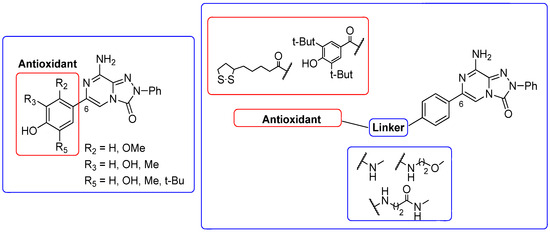
Figure 14.
Antioxidant-based triazolopyrazin-3-one derivatives.
The new compounds exhibited nanomolar activity at the hA2A AR and different degrees of selectivity versus hA1 and hA3 ARs (Table 12), while they were inactive in the cAMP assay in hA2B-CHO cells.

Table 12.
Binding affinity (Ki) of the antioxidant-based TPZ derivatives at hA1, hA2A, and hA3 ARs.
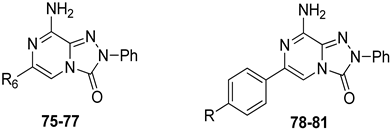
In the first set, phenolic residues at the 6-position yielded derivatives that showed nanomolar affinity for both hA1 and hA2A ARs and high selectivity versus hA3 AR (derivatives 75, 76). Notably, the compound featuring the 3-terbut-4-phenol ring (derivative 77) exhibited high hA2A AR affinity (Ki = 8.5 nM) and high selectivity versus all the other AR subtypes.
The second set of triazolopyrazines contained α-lipoyl and 3,5-di-tert-butyl-4-hydroxybenzoyl residues as antioxidant portions which were appended via either an ether linkage or an amide bond to the para position of the 6-phenyl ring (Figure 14). The choice of this position was guided by molecular docking studies at the hA2A AR, which indicated that bulky substituents on the 6-phenyl ring favor a binding pose in which this moiety is oriented toward the extracellular side of the receptor. As expected, these new derivatives showed nanomolar A2A AR affinity and high selectivity. The most potent compounds were the lipoyl derivatives 78 and 79 (Ki = 2.4 and 36.4 nM, respectively) and compound 80, featuring the 3,5-di-tert-butyl-4-hydroxyphenyl residue.
Compounds 77, 78, and 80 were evaluated in vitro for their neuroprotective activity against oxaliplatin-induced toxicity in microglial cells. To assess the specific contribution of A2A AR antagonism, compound 81, lacking the antioxidant moiety, was also tested. All compounds exhibited protective effects, with compounds 78 and 80 additionally reducing ROS levels, suggesting a direct antioxidant mechanism. Compounds 78, 80, and 81 were further evaluated in a mouse model of oxaliplatin-induced neuropathic pain, where they demonstrated efficacy in alleviating pain-related symptoms. The lipoyl derivative 78 emerged as the most potent, completely reversing oxaliplatin-induced pain. The efficacy of compound 81, despite lacking an antioxidant moiety, underscores the contribution of A2A AR antagonism to the reduction of oxaliplatin-induced neurotoxicity, both in vitro and in vivo.
- 2.
- First-in-Class Multi-Target Adenosine A2A AR Antagonists–Carbonic Anhydrase IX and XII Inhibitors
Another study employing a multi-target approach within the triazolopyrazine series led to the development of the first-in-class multi-target agents acting as both hA2A AR antagonists and human carbonic anhydrase (hCA) IX and XII inhibitors, representing promising new candidates for anticancer therapy [175]. In recent years, the A2A AR and hCAs IX and XII have emerged as promising targets for anticancer therapy. A2A AR is frequently upregulated in the tumor microenvironment, where its activation suppresses immune cell function and promotes tumor cell proliferation, thereby facilitating immune evasion, tumor progression, and metastasis [105,106,107,108,109,110,176,177].
CAs are a family of ubiquitous metalloenzymes characterized by the presence of a zinc ion in their catalytic site. Although they exhibit distinct catalytic efficiencies and tissue-specific expression patterns, their most prominent physiological roles include the regulation of pH homeostasis and the facilitation of ion transport [178,179,180]. In particular, the membrane-bound isoforms hCAs IX and XII are markedly upregulated in hypoxic tumors. Their overexpression contributes to the maintenance of an alkaline intracellular pH, preventing acidification within tumor cells while simultaneously promoting extracellular acidosis. These pH imbalances are known to favor the initiation and progression of solid tumors. Consequently, tumor-associated hCAs IX and XII represent promising therapeutic targets to counteract tumor growth and metastasis [179].
On this basis, our efforts were devoted toward the development of dual-acting compounds capable of simultaneously blocking both hA2A AR and hCAs IX and XII, to achieve a synergistic anticancer effect. Thus, the potent A2A AR antagonist triazolopyrazine 56 was decorated with various zinc-binding groups (ZBGs) (Figure 15).
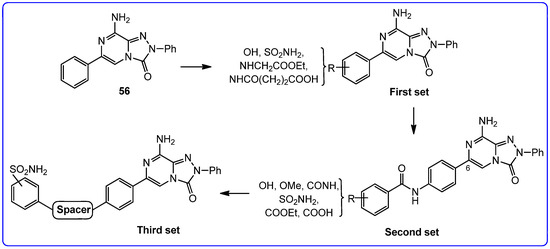
Figure 15.
Design of triazolopyrazines as multitarget human A2A AR antagonists–carbonic anhydrase IX and XII inhibitors.
The new compounds exhibited binding activity at hA1, hA2A, and hA3 ARs at concentrations ranging from micromolar to nanomolar, while they were inactive in the cAMP assay in hA2B-CHO cells (Table 13).

Table 13.
Biological activity of multitarget TPZ derivatives at hARs and inhibition data against hCA I, II, IX, and XII isoforms as determined by a stopped-flow CO2 hydrase assay.
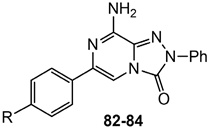
In the first set, simple substituents able to coordinate the zinc ion either directly or via a water molecule (-OH, –COOH, –CONHOH, and –SO2NH2), were introduced on the 6-phenyl ring of compound 56. Among them, the sulfonamide moiety proved to be the most effective, yielding derivative 82, which showed some affinity for the hA2A AR and good inhibitory activity against hCA IX/XII isoforms. In contrast, compounds bearing a hydroxy group exhibited high hA2A AR affinity but weak inhibition of hCA isoforms.
In the second set, the ZBGs were introduced on a lateral phenyl ring, attached to the 6-phenyl group through an amide linker. Due to the presence of the longer tail, several compounds exhibited nanomolar affinity and enhanced selectivity for the hA2A AR, but limited or no inhibitory activity against hCA isoforms. The only notable exception was compound 83, which demonstrated potent dual-target activity, confirming benzenesulfonamide as the most effective ZBG for simultaneously targeting hA2A AR and hCA XII (Ki = 6.4 and 6 nM, respectively, Table 13).
In the third set, the benzenesulfonamide moiety was connected to the 6-phenyl ring via spacers of varying length and flexibility. The new triazolopyrazines showed nanomolar affinity for hA2A AR and inhibited hCAs with different degrees of selectivity. Among them, derivatives 84 exhibited the best profile, as it showed good affinity for hA2A AR (Ki = 108 nM) and inhibited both the tumor-associated hCA IX and XII isozymes at nanomolar concentration (Ki = 5.0 and 27.0 nM, respectively).
Docking studies showed that these compounds bind to the hA2A AR similarly to the previously reported triazolopyrazines (type one conformation). In hCA II, IX, and XII isoforms, the benzenesulfonamide moiety coordinated the catalytic Zn(II) ion and established two hydrogen bonds with Thr199. An increased distance between the sulfonamide and the scaffold facilitated better accommodation within the enzyme active sites, with spacer length and flexibility critically influencing binding efficiency.
2.2. 2-Arylpyrazolo[3,4-c]quinolone Derivatives and Their Simplified Analogs
2.2.1. 2-Arylpyrazolo[3,4-c]quinoline Derivatives
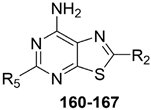
In addition to the TQX derivatives (Section 2.1), compounds with 2-arylpyrazolo[3,4-c]quinoline structure, differently substituted at position 4 to give, respectively, the 4-one (PQ-A) and 4-amino (PQ-B) series, were deeply investigated (Figure 16). Indeed, the pyrazolo[3,4-c]quinoline core proved to be a versatile scaffold for the development of potent and selective hA3 AR antagonists.
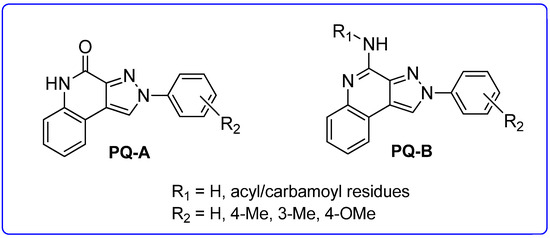
Figure 16.
2-Arylpyrazolo[3,4-c]quinoline derivatives PQ-A and PQ-B.
Pyrazolo[3,4-c]quinolin-4-one Derivatives
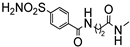
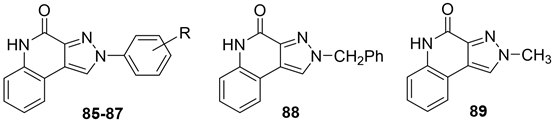
In the PQ-A series, the 2-phenyl substitution pattern strongly influenced AR binding (Table 14). While all PQ-A compounds did not bind to the hA2A receptor, they exhibited good hA1 and hA3 binding affinity, but limited hA3 versus hA1 selectivity. The 4-methoxy substitution on the 2-phenyl ring (87) afforded the best selectivity (hA3/hA1 ratio = 55), followed by 4-methyl (86, hA3/hA1 ratio = 9). Substitution of the 2-phenyl moiety with a benzyl one (88) preserved hA3 AR affinity but eliminated hA1 binding, indicating the hA3 pocket can accommodate bulkier groups. In contrast, replacing the 2-phenyl moiety with the smaller 2-methyl group (89) abolished binding activity, highlighting the need for more hindering, lipophilic substituents at this position [181,182].

Table 14.
Binding affinity (Ki) of PQ-A derivatives at hA1, hA2A, and hA3 ARs a.
Ligand-based homology modeling (LBHM) supported these findings. Ligand recognition occurred in the upper region of the TM bundle, with the pyrazoloquinoline core near TMs 3, 5, 6, and 7. Key hydrogen bonds involved Thr94, His95, and Ser247 interacting with the 4-carbonyl group. Additionally, the 4-methoxy group on the 2-phenyl moiety (87) likely engaged in a weak hydrogen bond with Ser165 in the second extracellular loop (EL2) contributing to enhanced affinity and selectivity [154].
To improve affinity for A1 and/or A2A ARs, PQ-A derivatives bearing bulky, lipophilic groups on the benzo-fused moiety were synthesized, as suggested by the literature data [13,142,183]. However, all compounds proved inactive at the targeted receptors [182].
Pyrazolo[3,4-c]quinolin-4-amino Derivatives
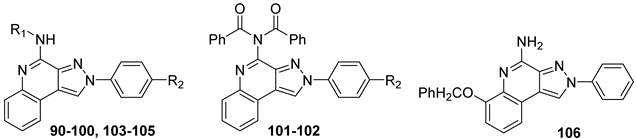
Several structural modifications on the PQ-B series (Figure 14) were carried out to explore SARs [154,181,182,184]. Accordingly, 4-amino-, 4-amido-, and 4-benzylureido-pyrazoloquinoline derivatives were synthesized (90–106, Table 15) [154,181]. The amino derivatives (R1 = H, compound 90) generally showed good hA1, hA2A, and hA3 AR affinities. In contrast, 4-acylamino, 4-benzylureido, and 4-diacylamino compounds (91–102) displayed significantly higher affinity and selectivity for the hA3 receptor subtype.

Table 15.
Binding affinities (Ki) of PQ-B derivatives at hA1, hA2A, and hA3 ARs a.
Docking studies showed that PQ-B compounds interact with the upper part of the binding pocket (TMs 3, 5, 6, 7) with the 4-substituent oriented toward the intracellular environment and the 2-phenyl ring positioned near to TMs 3, 6, and 7. Polar amino acid residues such as Thr94, His95, and especially Ser247 in the hA3 AR are key for receptor selectivity, forming hydrogen bonds with the 4-amido/ureido groups. In particular, the hydroxyl group of Ser247 engaged in hydrogen-bonding interaction with the carbonyl oxygen of the 4-amido/ureido moieties. Ser247 was absent in hA1 and hA2 receptors, where a bulkier histidine residue prevents access for large 4-acylamino and 4-benzylureido substituents explaining the low affinity of compounds 91–102 for those receptor subtypes. In general, the hA3 AR affinity of 4-N-acylated derivatives increased with the bulkiness of the N4 substituent (compare 91, 92 to 93–98) that accommodated in the hydrophobic region delimitated by five nonpolar amino acids (Ile98, Ile186, Leu190, Phe239, and Leu244). Hydrophobic substituents (Me, OMe) on the 2-phenyl ring did not influence binding affinity. This was particularly evident for 4-N-acylated derivatives. As the size of the N4 substituent increases, the position of the 2-phenyl group moves away from EL2 leading to the loss of the hydrogen-bonding interaction between the methoxy group and the Ser165 residue [154].
Further SAR studies introduced various heteroaroyl or aroyl groups at the 4-amino position [184]. Compounds bearing the 2-furyl or 4-pyridyl ring on the 4-amido group (103, 104, Table 15) showed nanomolar hA3 AR affinity and high selectivity. Substituents on the 4-benzoylamino group produced variable effects: methyl groups preserved activity (105), while trifluoromethyl groups reduced affinity. Finally, introducing bulky and lipophilic groups at position 6 of the benzo-fused ring (106) shifted affinity towards the hA1 receptor subtype [182].
Compounds 97 and 102 showed potent inhibition of NECA-modulated adenylyl cyclase activity in CHO cells, with EC50 values of 3.8 nM and 11.2 nM, respectively. As expected, their affinity at the rA3 AR was lower than that at the hA3 AR.
In a rat model of cerebral ischemia, both compounds protected against OGD-induced neuronal damage and delayed or prevented anoxic depolarization, confirming their neuroprotective effects. As mentioned for derivatives 27 and 39, these results are consistent with those previously observed in the same brain region using other potent hA3 AR antagonists, exhibiting low binding affinity at the rA3 AR [150,159].
2.2.2. From PQ series to Pyrazolo[4,3-d]pyrimidine Compounds
To develop new hA3 AR-targeting compounds with improved physicochemical properties, a molecular simplification of the PQ-A and PQ-B scaffolds led to the 2-arylpyrazolo[4,3-d]pyrimidin-7-one (PP-7-oxo) and -7-amino (PP-7-amino) series, respectively (Figure 17) [162,185,186,187,188,189].
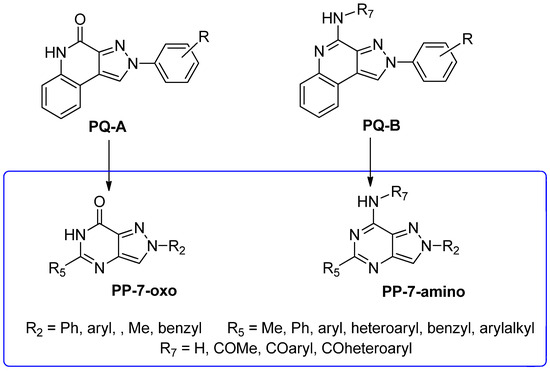
Figure 17.
Design of pyrazolo[4,3-d]pyrimidine.
This strategy improved hA3 versus hA1 selectivity, while maintaining or enhancing affinity for the target receptor. To explore SARs, substituents with varying steric bulk, flexibility, and lipophilicity (Figure 17) were introduced at the 5- and 2-positions of the bicyclic scaffold.
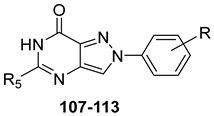
Pyrazolo[4,3-d]pyrimidin-7-one Derivatives
Notably, PP-7-oxo derivatives (107–113, Table 16) showed nanomolar affinity and high selectivity at the hA3 AR. Introduction of small R5 substituents such as methyl (108) was preferred over bulkier groups like phenyl (109) or benzyl (110), likely due to a better accommodation within a lipophilic receptor pocket of limited size. Among the modifications on the 2-phenyl ring, the 4-OMe group (111) conferred the highest hA3 AR affinity (Ki = 1.2 nM, Table 16), outperforming other substitutions such as 3-Me and 4-Me (112, 113) [162,185]. To depict the binding mode of these new potent and selective hA3 AR antagonists, a new homology model of the hA3 AR using the crystal structure of the hA2A receptor (PDB code: 3EML) as a template was built [162]. Molecular docking studies indicated that the pyrazolo[4,3-d]pyrimidin-7-one scaffold is embedded within TMs 3, 5, 6, and 7, engaging in aromatic π-π stacking interactions with Phe168. The 2-phenyl ring is oriented toward EL2, while the R5 substituent is directed toward the intracellular environment. Small R5 substituents enabled the formation of two stabilizing hydrogen-bonds—one involving the carbonyl group at position 7 and the other the nitrogen atom of the pyrazole ring—both interacting with the amide moiety of Asn250. In contrast, derivatives bearing bulkier R5 groups (phenyl (109), benzyl (110)) adopted an altered binding orientation, redirecting the 2-phenyl ring away from EL2 and positioning R5 toward TM2. This rearrangement disrupted the π-π stacking interaction with Phe168 and eliminated one of the hydrogen bonds with Asn250 potentially accounting for the absent or diminished hA3 AR binding affinity of compounds 109 and 110 [162].

Table 16.
Binding affinity (Ki or I%) of PP-7-oxo derivatives at h A1, hA2A, and hA3 ARs and potency (cAMP assays I%) at hA2B AR.
Pyrazolo[4,3-d]pyrimidin-7-amino Derivatives
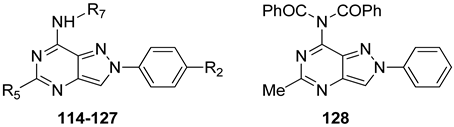
Substitution of the 7-oxo group of the pyrazolo[4,3-d]pyrimidine scaffold with an amino function yielded the PP-7-amino series (114–118, Table 17).

Table 17.
Binding affinity (Ki or I%) of PP-7-amino derivatives at hA1, hA2A, and hA3 ARs and potency (cAMP assays, IC50 or I%) at hA2B AR.
The free 7-amino function conferred good affinity for the hA1 and hA2A ARs (compounds 114, 116, and 117), while it affected hA3 affinity based on R5 substituents [185,186]. Substituents with varying steric bulk, flexibility, and lipophilicity (R5 = Me, Ph, C6H4-4-OMe, 2-thienyl) were well tolerated at hA1 and hA2A subtypes, while for hA3 affinity heteroaryl or aryl substituents (117, 118) were necessary. Introduction of a 4-OMe group on the 2-phenyl ring did not consistently enhance hA3 AR affinity and selectivity.
To optimize hA3 AR affinity, acyl groups were added to the 7-amino moiety (119–127, Table 17) [185,186]. These 7-amido derivatives displayed nanomolar affinity for the hA3 AR, with minimal activity at the other AR subtypes, the most active being 125 (Ki = 0.027 nM). Even compounds with two acyl groups (129, Table 17) retained high affinity, suggesting the existence of a spacious receptor pocket. Molecular modeling showed that ligand recognition took place in the upper portion of the transmembrane domain, stabilized by hydrogen bonds with Asn250 and π-π stacking interaction with Phe168. Due to this specific binding pose, the R7 group was positioned facing outward from the binding cleft.
Compounds 119 (Ki = 5.6 nM), 121 (Ki = 18 nM), 122 (Ki = 18 nM), and 123 (Ki = 24 nM) improved cell viability in rat astrocyte cultures exposed to oxaliplatin with 123 being the most effective [186]. Importantly, compound 123 did not interfere with the antineoplastic activity of oxaliplatin in HT-29 cancer cells, indicating distinct mechanisms of oxaliplatin toxicity in normal neurons versus tumor cells. However, these compounds lacked affinity for the rat A3 AR, confirming known species differences between rat and human [190]. Therefore, these results support an A3 receptor-independent mechanism.
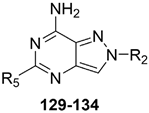
Introduction of arylalkyl and heteroaryl substituents at positions 2 and 5 of the PP-7-amino scaffold led to potent hA2A or balanced hA2A/hA1 antagonists (129–134, Table 18) [187,188,189]. The free 7-amino group was crucial for hA1 and hA2A selectivity, while the 2- and 5-substituents modulated receptor preference. Specifically, a phenyl group at position 2 and an arylalkyl chain at position 5 (129, 130) yielded dual-acting hA1/hA2A antagonists with higher affinity toward the A1 AR subtype. In contrast, a heteroaryl moiety at position 5 (e.g., 2-furyl or 2-(5-methylfuryl)) and a (substituted) benzyl group at position 2 (131–134) favored A2A AR selectivity.

Table 18.
Binding affinity (Ki or I%) of PP-7-amino derivatives at h A1, hA2A, and hA3 ARs and potency (cAMP assays, IC50 or I%) at hA2B AR.
2.2.3. Imidazo[1,2-a]pyrazin-8-amino Derivatives
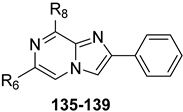
Building on the success of PP-based hA3 AR antagonists, a series of 2-aryl-imidazo[1,2-a]pyrazin-8-amino derivatives (IP series, Table 19) was developed [191]. These compounds also possess features typical of fluorescent heteroaromatic systems [192,193]. The aim was to optimize both hA3 AR affinity and fluorescence emission properties. In particular, the N8-(hetero)arylcarboxamido-substituted IP compounds, either with or without a 6-phenyl group (135–139, Table 19) showed good hA3 AR affinity and selectivity, but lacked activity at the rat A3 AR, confirming species-specific differences. Some derivatives exhibited moderate fluorescence, though not sufficient for use as biological probes.

Table 19.
Binding affinity (Ki or I%) of IP derivatives at hA1, hA2A, and hA3 ARs and potency (cAMP assays, I%) at hA2B AR.
Compound 139 was selected for further investigation. In a rat model of cerebral ischemia, it significantly restored impaired neurotransmission, likely through a mechanism independent of A3 receptor antagonism and possibly involving an intracellular target.
2.2.4. Thiazolo[5,4-d]pyrimidine Derivatives
Thiazolo[5,4-d]pyrimidin-7-one Derivatives
Starting from 2-arylpyrazolo[4,3-d]pyrimidin-7-one (PP-7-oxo series) derivatives [162], a novel class of thiazolo[5,4-d]pyrimidin-7-one (TP-7-oxo series) compounds was rationally designed (Figure 18) [194].
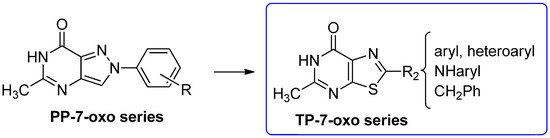
Figure 18.
Design of thiazolo[5,4-d]pyrimidin-7-one derivatives.
The thiazolopyrimidine scaffold maintains key structural features, such as the 7-oxo function and the nitrogen at position 1, that are important for hA3 receptor binding, and incorporates a methyl group at position 5, in line with previous findings in the lead series for enhancing hA3 AR antagonist activity [162].
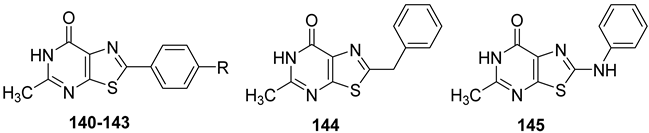
SAR studies showed that a 2-phenyl group bearing a para-hydrophobic substituent (e.g., Cl, OMe, Me, 140–142) enhanced hA3 AR affinity, while a hydrophilic group (e.g., OH, 143) reduced it (Table 20). Increasing the distance between the 2-phenyl and the bicyclic core using linkers (e.g., methylene in 144 or the amino group in 145) also led to decreased affinity.

Table 20.
Binding affinity (Ki or I%) of TP-7-oxo derivatives at h A1, hA2A, and hA3 ARs and potency (cAMP assays, I%) at hA2B AR.
Thiazolo[5,4-d]pyrimidin-7-amino Derivatives
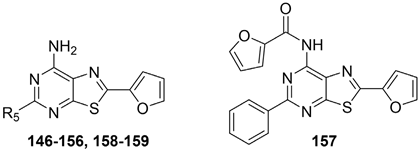
The thiazolo-pyrimidine (TP) core resulted a versatile scaffold for the development of hA2A AR antagonists. Starting from the hA2A AR inverse agonist ZM241385 (Figure 19) [156], a bioisosteric replacement of its triazolotriazine core with a thiazolopyrimidine led to compound 146 [195].
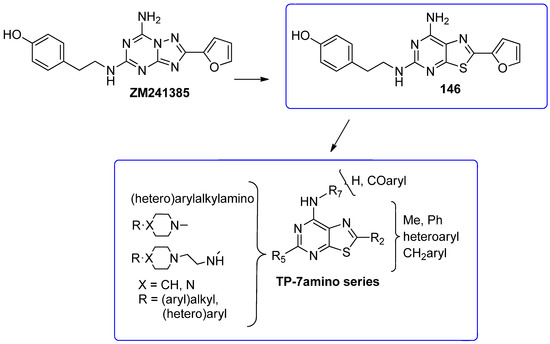
Figure 19.
Design of new TP-based hA2A AR antagonists/inverse agonists.
While 146 (Table 21) showed affinity for all hARs, it lacked selectivity for hA2A over hA1 AR. To improve selectivity, many other TP derivatives were synthesized, taking advantage of the ease of modifying the TP core at positions 2, 5, and 7 (Figure 19).

Table 21.
Binding affinity (Ki) of 2-furyl-TP-7-amino derivatives at hA1, hA2A, and hA3 ARs and potency (cAMP assays, IC50) at hA2B AR.
Keeping the 2-furane ring and the 7-amino group constant, substitutions at position 5 were explored [195,196,197,198]. Compounds 147–152 (Table 21) [195,196], featuring a (hetero)arylalkylamino chain at position 5, showed two affinity values (KH in the femtomolar, KL in the nanomolar range) for the hA2A AR and potent inverse agonist activity, significantly inhibiting basal cAMP accumulation (IC50 in the picomolar range). Despite moderate to good/high affinities for hA1, hA3, and hA2B ARs, compounds 147–152 are among the most potent and selective hA2A AR inverse agonists reported, leading to their patenting [199].
Given the known role of A2A AR signaling in pain modulation [200,201], compounds 147 and 148 (Table 21) were tested in vivo alongside ZM241385 and morphine [195]. In both the writhing and hot water tail immersion tests, they showed anti-nociceptive effects comparable or superior to morphine and greater than those of ZM241385.
Additionally, 147 was evaluated for its anti-cancer potential [202]. In A375 (melanoma), A549 (lung), and MRMT-1 (breast) cancer cell lines, selective A2A AR activation promoted tumour cell proliferation through ERK1/2, JNK1/2, and AKT pathways. These effects were reversed by both ZM241385 and compound 147, supporting the role of A2A AR in tumorigenesis and highlighting 147 as a promising anticancer agent.
To further investigate the role of substituents at position 5 in hA2A receptor binding, 7-amino-2-(furan-2-yl)thiazolopyrimidines bearing a piperazine chain at position 5 were synthesized (compounds 153, 154, Table 21). These compounds showed potent inverse agonist activity and good selectivity toward the hA2A AR, whether the piperazine was directly attached to the bicyclic core (153) or linked via an ethylamino spacer (154) [197]. Additionally, bioinformatics prediction revealed that they possessed good drug-likeness profiles [197]. Similar results were achieved when an aryl or heteroaryl group was directly attached at position 5 of the bicyclic scaffold (155, 156), with compound 156, featuring furan-2-yl groups at both positions 2 and 5, exhibiting the most favorable combination of hA2A AR affinity and selectivity [203].
To enhance affinity and selectivity toward the hA3 AR subtype, various acyl groups were introduced on the 7-amino function. This led to compound 157 (Table 21), which showed high affinity and selectivity for the hA3 AR. As expected, this modification reduced hA2A binding activity [203] confirming that a free 7-amino group is crucial for effective anchoring of ligands at the hA2A AR subtype—a trend observed in other classes of similar AR antagonists [186,204,205].
Further SAR studies involved simultaneous modifications at positions 2 and 5, keeping the 7-amino group free. Replacing the 2-furan-2-yl group in compound 147 with phenyl, pyrazin-2-yl, methyl, or 2-thienyl group led to compounds 160–163 (Table 22), which displayed dual A1 AR antagonism/A2A AR inverse agonism [196,198].

Table 22.
Binding affinity (Ki) of TP-7-amino derivatives at hA1, hA2A, and hA3 ARs and potency (cAMP assays, IC50) at hA2B AR.
Additional dual-acting ligands (164, 165, Table 22) were obtained by coupling a 2-phenyl group with (hetero)aryl substituents at position 5 [203]. Similarly, potent hA1/A2A AR dual antagonists (166, 167, Table 22) were obtained by combining 5-aryl or heteroaryl groups with 2-unsubstituted or ortho-substituted benzyl moieties [206].
Adenosine signaling has emerged as a promising target for depression treatment [207,208]. Caffeine, a weak non-selective A1 and A2A AR antagonist, has shown behavioral effects in classical animal depression models [209]. Based on these findings, compound 166 (hA1 Ki = 1.9 nM; hA2A Ki = 0.06 nM, Table 22) was evaluated for antidepressant-like activity. In the forced swim test (FST) and tail suspension test (TST), it showed efficacy comparable to amitriptyline. Additionally, in the sucrose preference test, compound 166 demonstrated significant anti-anhedonic effects also matching those of amitriptyline [206].
Docking studies using the high-resolution crystal structure of the hA2A AR-ZM241385 complex revealed that TP ligands align similarly to ZM241385, preserving key interactions: a double hydrogen bond with Asn253 (via N1 and the 7-amino group), polar contact with Glu169, π-π stacking with Phe168, and hydrophobic interaction with Leu249. The 2-substituent penetrates deep into the receptor cavity near TM3, TM5, and TM6, while the 5-substituent, oriented toward the extracellular space, can exhibit diverse steric and electronic properties and may be exploited for the design of multi-target agents.
Recent studies indicate that simultaneous inhibition of adenosine biosynthesis and A2A AR activity can suppress tumor growth [210]. Based on this, new TP derivatives were developed to co-target CD73, the main enzyme in adenosine biosynthesis, and A2A AR [211]. These multi-target compounds incorporate a 7-amino-2-(furan-2-yl)thiazolo[5,4-d]pyrimidine core with a 5-substituent linked to a benzenesulfonamide group, typical of CD73 inhibitors [212]. While most of these compounds showed strong inverse agonist activity at hA2A AR (158 Table 21), their CD73 inhibition was weak [211]. However, compound 159 (Table 21) emerged as an intriguing dual hA2A/hA2B AR inverse agonist/antagonist with high selectivity over the hA1 and hA3 AR subtypes. Given the A2B AR role in promoting tumor growth [213], compounds able to block A2B ARs, or both A2A and A2B ARs, like 159, hold promise as anticancer agents.
3. Conclusions
This review summarizes some research conducted by our group which has led to the discovery of some of the most potent and selective antagonists for the hA2A and hA3 AR subtypes reported to date, characterized by diverse molecular chemotypes. Preliminary pharmacological investigations carried out with selected hA2A AR and hA3 AR antagonists are briefly summarized in Figure 20.
Figure 20.
Summary of the pharmacological activity of selected AR antagonists in in vitro and in vivo models.
The structural simplification from tricyclic to bicyclic scaffolds was partly aimed at addressing the frequently limited solubility of the compounds in assay media, which often hindered both in vitro and in vivo testing. This strategy proved successful, including in terms of AR affinity, particularly for the thiazolopyrimidine series, which yielded extremely potent hA2A AR antagonists as well as dual hA2A/hA2B AR antagonists with predicted favorable drug-like properties. Ongoing studies of this series aim to identify selective hA2B AR antagonists as growing evidence highlights the significant therapeutic potential of compounds blocking the hA2B AR. This is due to their ability to modulate immune responses, inhibit tumor growth, and regulate inflammatory signaling pathways. Antagonists of the hA2B AR warrant further investigation also in neuroinflammatory diseases, such as cerebral ischemia and multiple sclerosis, in which they have demonstrated neuroprotective effect.
Our research has also led to the development of several highly potent and selective hA3 AR antagonists. However, the lack of affinity for the rat A3 receptor has limited their pharmacological characterization in both in vitro and in vivo models. To date, only a limited number of A3 AR antagonists have been shown to retain activity across both human and rodent receptors. Therefore, the discovery of pan-species A3 AR antagonists is highly desirable to facilitate more robust and predictive preclinical studies and to advance the development of A3 AR-targeted therapies. This need is particularly relevant given that A3 AR antagonists have demonstrated antitumor efficacy in preclinical models. Moreover, further studies are essential to unravel the dual and context-dependent role of A3 AR, promoting either cell proliferation or cell death.
Our research has also contributed to the development of multifunctional compounds as innovative potential drugs, e.g., dual-acting antioxidant–hA2A AR antagonists as neuroprotective agents and human A2A AR antagonists–carbonic anhydrase inhibitors, as potential antitumor agents. These multifunctional strategies may offer a more comprehensive and effective approach to address the multifactorial nature of complex disorders such as tumors and neurodegenerative diseases.
To conclude, we believe that the findings presented herein will be of interest to researchers working in the field of adenosine. The diversity of chemotypes and the therapeutic potential of AR antagonists may pave the way for future studies aimed at identifying novel compounds for the treatment of neuroinflammatory, neurological, and oncological diseases.
Funding
The work was supported by NEXT-GENERATION-EU (NGEU)-National Recovery and Resilience Plan (NRRP), Mission 4—Component 2. Investment 1.5-Ministry of University and Research (MUR) Call n° 3277, Project Code ECS_00000017—MUR Directoral Decree no. 1055, 23 June 2022, CUP B83C22003920001. Project title Tuscany Health Ecosystem-THE, Spoke 8.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Zimmermann, H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 299–309. [Google Scholar] [CrossRef]
- Kloor, D.; Osswald, H. S-Adenosylhomocysteine hydrolase as a target for intracellular adenosine action. Trends Pharmacol. Sci. 2004, 25, 294–297. [Google Scholar] [CrossRef]
- Chen, J.F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets—What are the challenges? Nat. Rev. Drug. Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef]
- Kong, W.; Engel, K.; Wang, J. Mammalian Nucleoside Transporters. Curr. Drug. Metab. 2004, 5, 63–84. [Google Scholar] [CrossRef]
- Podgorska, M.; Kocbuch, K.; Pawelczyk, T. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta. Biochim. Pol. 2005, 52, 749–758. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Robin, E.; Sabourin, J.; Benoit, R.; Pedretti, S.; Raddatz, E. Adenosine A1 receptor activation is arrhythmogenic in the developing heart through NADPH oxidase/ERK- and PLC/PKC-dependent mechanisms. J. Mol. Cell. Cardiol. 2011, 51, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Borse, V.; Sheth, S.; Sheehan, K.; Ghosh, S.; Tupal, S.; Jajoo, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine A1 Receptor Protects Against Cisplatin Ototoxicity by Suppressing the NOX3/STAT1 Inflammatory Pathway in the Cochlea. J. Neurosci. 2016, 36, 3962–3977. [Google Scholar] [CrossRef]
- Ye, W.; Sun, J.; Li, C.; Fan, X.; Gong, F.; Huang, X.; Deng, M.; Chu, J.Q. Adenosine A3 Receptor Mediates ERK1/2- and JNK-Dependent TNF-α Production in Toxoplasma gondii-Infected HTR8/SVneo Human Extravillous Trophoblast Cells. Korean J. Parasitol. 2020, 58, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Varani, K.; Vincenzi, F.; Baraldi, P.G.; Tabrizi, M.A.; Merighi, S.; Gessi, S. The A3 Adenosine Receptor: History and Perspectives. Pharmacol. Rev. 2015, 67, 74–102. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, E.; Hinz, S.; Pellizzari, V.; Deganutti, G.; El-Tayeb, A.; Navarro, G.; Franco, R.; Moro, S.; Schiedel, A.C.; Müller, C.E. A2A and A2B adenosine receptors: The extracellular loop 2 determines high (A2A) or low affinity (A2B) for adenosine. Biochem. Pharmacol. 2020, 172, 113718. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Tabrizi, M.A.; Gessi, S.; Borea, P.A. Adenosine Receptor Antagonists: Translating Medicinal Chemistry and Pharmacology into Clinical Utility. Chem. Rev. 2008, 108, 238–263. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, P. Adenosine A2B Receptor: From Cell Biology to Human Diseases. Front. Chem. 2016, 24, 4–37. [Google Scholar] [CrossRef]
- Al-Qattan, M.N.M.; Mordi, M.N. Molecular Basis of Modulating Adenosine Receptors Activities. Curr. Pharm. Des. 2019, 25, 817–831. [Google Scholar] [CrossRef]
- Ma, W.X.; Yuan, P.C.; Zhang, H.; Kong, L.X.; Lazarus, M.; Qu, W.M.; Wang, Y.Q.; Huang, Z.L. Adenosine and P1 receptors: Key targets in the regulation of sleep, torpor, and hibernation. Front. Pharmacol. 2023, 14, 1098976. [Google Scholar] [CrossRef]
- Spanoghe, J.; Larsen, L.E.; Craey, E.; Manzella, S.; Van Dycke, A.; Boon, P.; Raedt, R. The Signaling Pathways Involved in the Anticonvulsive Effects of the Adenosine A1 Receptor. Int. J. Mol. Sci. 2020, 22, 320. [Google Scholar] [CrossRef] [PubMed]
- Persike, D.S.; Puccinelli, R.P.A.; da Silva Fernandes, M.J. Adenosine A1 Receptor Agonist (R-PIA) before Pilocarpine Modulates Pro- and Anti-Apoptotic Factors in an Animal Model of Epilepsy. Pharmaceuticals 2021, 14, 376. [Google Scholar] [CrossRef]
- Deng, P.; Pang, Z.P.; Lei, Z.; Shikano, S.; Xiong, Q.; Harvey, B.K.; London, B.; Wang, Y.; Li, M.; Xu, Z.C. Up-Regulation of A-Type Potassium Currents Protects Neurons Against Cerebral Ischemia. J. Cereb. Blood Flow. Metab. 2011, 31, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Sawynok, J. Adenosine receptor targets for pain. Neuroscience 2016, 338, 1–18. [Google Scholar] [CrossRef]
- Kashfi, S.; Ghaedi, K.; Baharvand, H.; Nasr-Esfahani, M.H.; Javan, M. A1 Adenosine Receptor Activation Modulates Central Nervous System Development and Repair. Mol. Neurobiol. 2017, 54, 8128–8139. [Google Scholar] [CrossRef]
- Trinh, P.N.H.; Baltos, J.A.; Hellyer, S.D.; May, L.T.; Gregory, K.J. Adenosine receptor signalling in Alzheimer’s disease. Purinergic Signal. 2022, 18, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Grossfeld, D.; Kasselman, L.J.; Renna, H.A.; Vernice, N.A.; Drewes, W.; Konig, J.; Carsons, S.E.; DeLeon, J. Adenosine and the Cardiovascular System. Am. J. Cardiovasc. Drugs 2019, 19, 449–464. [Google Scholar] [CrossRef]
- Wölkart, G.; Gissing, S.; Stessel, H.; Fassett, E.K.; Klösch, B.; Greene, R.W.; Mayer, B.; Fassett, J.T. An adenosinergic positive feedback loop extends pharmacological cardioprotection duration. Br. J. Pharmacol. 2024, 181, 4920–4936. [Google Scholar] [CrossRef]
- Vallon, V.; Mühlbauer, B.; Osswald, H. Adenosine and Kidney Function. Physiol. Rev. 2006, 86, 901–940. [Google Scholar] [CrossRef]
- Pardo, F.; Villalobos-Labra, R.; Chiarello, D.I.; Salsoso, R.; Toledo, F.; Gutierrez, J.; Leiva, A.; Sobrevia, L. Molecular implications of adenosine in obesity. Mol. Aspects Med. 2017, 55, 90–101. [Google Scholar] [CrossRef]
- Silva, L.; Subiabre, M.; Araos, J.; Sáez, T.; Salsoso, R.; Pardo, F.; Leiva, A.; San Martín, R.; Toledo, F.; Sobrevia, L. Insulin/adenosine axis linked signalling. Mol. Aspects Med. 2017, 55, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Azdad, K.; Gall, D.; Woods, A.S.; Ledent, C.; Ferré, S.; Schiffmann, S.N. Dopamine D2 and Adenosine A2A Receptors Regulate NMDA-Mediated Excitation in Accumbens Neurons Through A2A–D2 Receptor Heteromerization. Neuropsychopharmacology 2009, 34, 972–986. [Google Scholar] [CrossRef] [PubMed]
- Romero-Fernandez, W.; Taura, J.J.; Crans, R.A.J.; Lopez-Cano, M.; Fores-Pons, R.; Narváez, M.; Carlsson, J.; Ciruela, F.; Fuxe, K.; Borroto-Escuela, D.O. The mGlu5 Receptor Protomer-Mediated Dopamine D2 Receptor Trans-Inhibition Is Dependent on the Adenosine A2A Receptor Protomer: Implications for Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 5955–5969. [Google Scholar] [CrossRef]
- Stockwell, J.; Jakova, E.; Cayabyab, F. Adenosine A1 and A2A Receptors in the Brain: Current Research and Their Role in Neurodegeneration. Molecules 2017, 22, 676. [Google Scholar] [CrossRef]
- Van Waarde, A.; Dierckx, R.A.J.O.; Zhou, X.; Khanapur, S.; Tsukada, H.; Ishiwata, K.; Luurtsema, G.; de Vries, E.F.J.; Elsinga, P.H. Potential Therapeutic Applications of Adenosine A2A Receptor Ligands and Opportunities for A2A Receptor Imaging. Med. Res. Rev. 2018, 38, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.R.; Baptista, F.I.; Santos, P.F.; Cristóvão, G.; Ambrósio, A.F.; Cunha, R.A.; Gomes, C.A. Role of Microglia Adenosine A2A Receptors in Retinal and Brain Neurodegenerative Diseases. Mediators Inflamm. 2014, 2014, 465694. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Zinni, M.; Pansiot, J.; Cassanello, M.; Mairesse, J.; Ramenghi, L.; Baud, O. Modulation of Microglial Activation by Adenosine A2A Receptor in Animal Models of Perinatal Brain Injury. Front. Neurol. 2018, 9, 605. [Google Scholar] [CrossRef]
- Du, H.; Li, C.; Gao, R.; Tan, Y.; Wang, B.; Peng, Y.; Yang, N.; Ning, Y.; Li, P.; Zhao, Y.; et al. Inhibition of the Interaction between Microglial Adenosine A2A Receptor and NLRP3 Inflammasome Attenuates Neuroinflammation Posttraumatic Brain Injury. CNS Neurosci. Ther. 2024, 30, e14408. [Google Scholar] [CrossRef]
- Luongo, L.; Salvemini, D. Targeting Metabotropic Adenosine Receptors for Neuropathic Pain: Focus on A2A. Brain Behav. Immun. 2018, 69, 60–61. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in Neuropathic Pain: Cellular and Molecular Mechanisms and Therapeutic Potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Hettinger, B.D.; Lee, A.; Linden, J.; Rosin, D.L. Ultrastructural Localization of Adenosine A2A Receptors Suggests Multiple Cellular Sites for Modulation of GABAergic Neurons in Rat Striatum. J. Comp. Neurol. 2001, 431, 331–346. [Google Scholar] [CrossRef]
- Ciruela, F.; Casadó, V.; Rodrigues, R.J.; Luján, R.; Burgueño, J.; Canals, M.; Borycz, J.; Rebola, N.; Goldberg, S.R.; Mallol, J.; et al. Presynaptic Control of Striatal Glutamatergic Neurotransmission by Adenosine A1–A2A Receptor Heteromers. J. Neurosci. 2006, 26, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.J.; Alfaro, T.M.; Rebola, N.; Oliveira, C.R.; Cunha, R.A. Co-localization and Functional Interaction between Adenosine A2A and Metabotropic Group 5 Receptors in Glutamatergic Nerve Terminals of the Rat Striatum. J. Neurochem. 2005, 92, 433–441. [Google Scholar] [CrossRef]
- Wright, D.J.; Gray, L.J.; Finkelstein, D.I.; Crouch, P.J.; Pow, D.; Pang, T.Y.; Li, S.; Smith, Z.M.; Francis, P.S.; Renoir, T.; et al. N-acetylcysteine modulates glutamatergic dysfunction and depressive behavior in Huntington’s disease. Hum. Mol. Genet. 2016, 25, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G. Adenosine A2A Receptor Antagonists in Neurodegenerative Diseases: Huge Potential and Huge Challenges. Front. Psychiatry 2018, 9, 68. [Google Scholar] [CrossRef]
- Zhang, Y.; Wernly, B.; Cao, X.; Mustafa, S.J.; Tang, Y.; Zhou, Z. Adenosine and Adenosine Receptor-Mediated Action in Coronary Microcirculation. Basic Res. Cardiol. 2021, 116, 22. [Google Scholar] [CrossRef]
- Lovászi, M.; Németh, Z.H.; Pacher, P.; Gause, W.C.; Wagener, G.; Haskó, G. A2A Adenosine Receptor Activation Prevents Neutrophil Aging and Promotes Polarization from N1 towards N2 Phenotype. Purinergic Signal. 2022, 18, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Gessi, S.; Merighi, S.; Stefanelli, A.; Fazzi, D.; Varani, K.; Borea, P.A. A1 and A3 Adenosine Receptors Inhibit LPS-Induced Hypoxia-Inducible Factor-1 Accumulation in Murine Astrocytes. Pharmacol. Res. 2013, 76, 157–170. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Kopach, O.; Braga, A.; Nizari, S.; Hosford, P.S.; Sagi-Kiss, V.; Hadjihambi, A.; Konstantinou, C.; Esteras, N.; Gutierrez Del Arroyo, A.; et al. Adenosine signalling to astrocytes coordinates brain metabolism and function. Nature 2024, 632, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, A.M.; Alberghina, L.; Papa, M. Astrogliosis as a Therapeutic Target for Neurodegenerative Diseases. Neurosci. Lett. 2014, 565, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Coppi, E.; Dettori, I.; Cherchi, F.; Bulli, I.; Venturini, M.; Lana, D.; Giovannini, M.G.; Pedata, F.; Pugliese, A.M. A2B Adenosine Receptors: When Outsiders May Become an Attractive Target to Treat Brain Ischemia or Demyelination. Int. J. Mol. Sci. 2020, 21, 9697. [Google Scholar] [CrossRef]
- Yu, W.; Zacharia, L.C.; Jackson, E.K.; Apodaca, G. Adenosine Receptor Expression and Function in Bladder Uroepithelium. Am. J. Physiol. Cell Physiol. 2006, 291, C254–C265. [Google Scholar] [CrossRef]
- Colgan, S.P.; Fennimore, B.; Ehrentraut, S.F. Adenosine and Gastrointestinal Inflammation. J. Mol. Med. 2013, 91, 157–164. [Google Scholar] [CrossRef]
- Haskó, G.; Csóka, B.; Németh, Z.H.; Vizi, E.S.; Pacher, P. A2B Adenosine Receptors in Immunity and Inflammation. Trends. Immunol. 2009, 30, 263–270. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pathological Overproduction: The Bad Side of Adenosine. Br. J. Pharmacol. 2017, 174, 1945–1960. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Jacobson, K.A. A2B Adenosine Receptor and Cancer. Int. J. Mol. Sci. 2019, 20, 5139. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A.; Thevananther, S.; Hao, H. Are A3 Adenosine Receptors Expressed in the Brain? NeuroReport 2000, 11, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.; Esposito, E.; Doyle, T.; Cuzzocrea, S.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. A3 Adenosine Receptor Agonist Prevents the Development of Paclitaxel-Induced Neuropathic Pain by Modulating Spinal Glial-Restricted Redox-Dependent Signaling Pathways. Pain 2014, 155, 2560–2567. [Google Scholar] [CrossRef]
- Choi, I.-Y.; Lee, J.-C.; Ju, C.; Hwang, S.; Cho, G.-S.; Lee, H.W.; Choi, W.J.; Jeong, L.S.; Kim, W.-K. A3 Adenosine Receptor Agonist Reduces Brain Ischemic Injury and Inhibits Inflammatory Cell Migration in Rats. Am. J. Pathol. 2011, 179, 2042–2052. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jhun, B.S.; Oh, Y.T.; Lee, J.H.; Choe, W.; Baik, H.H.; Ha, J.; Yoon, K.-S.; Kim, S.S.; Kang, I. Activation of Adenosine A3 Receptor Suppresses Lipopolysaccharide-Induced TNF-α Production through Inhibition of PI 3-Kinase/Akt and NF-κB Activation in Murine BV2 Microglial Cells. Neurosci. Lett. 2006, 396, 1–6. [Google Scholar] [CrossRef]
- Ohsawa, K.; Sanagi, T.; Nakamura, Y.; Suzuki, E.; Inoue, K.; Kohsaka, S. Adenosine A3 Receptor Is Involved in ADP-Induced Microglial Process Extension and Migration. J. Neurochem. 2012, 121, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Coppi, E.; Cherchi, F.; Venturini, M.; Lucarini, E.; Corradetti, R.; Di Cesare Mannelli, L.; Ghelardini, C.; Pedata, F.; Pugliese, A. Therapeutic Potential of Highly Selective A3 Adenosine Receptor Ligands in the Central and Peripheral Nervous System. Molecules 2022, 27, 1890. [Google Scholar] [CrossRef]
- Chen, Z.; Janes, K.; Chen, C.; Doyle, T.; Bryant, L.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. Controlling Murine and Rat Chronic Pain Through A3 Adenosine Receptor Activation. FASEB J. 2012, 26, 1855–1865. [Google Scholar] [CrossRef]
- Little, J.W.; Ford, A.; Symons-Liguori, A.M.; Chen, Z.; Janes, K.; Doyle, T.; Xie, J.; Luongo, L.; Tosh, D.K.; Maione, S.; et al. Endogenous Adenosine A3 Receptor Activation Selectively Alleviates Persistent Pain States. Brain 2015, 138, 28–35. [Google Scholar] [CrossRef]
- Ho, M.-F.; Low, L.M.; Meyer, R.B. Pharmacology of the Adenosine A3 Receptor in the Vasculature and Essential Hypertension. PLoS ONE 2016, 11, e0150021. [Google Scholar] [CrossRef]
- Galal, A.; El-Bakly, W.M.; Al Haleem, E.N.A.; El-Demerdash, E. Selective A3 Adenosine Receptor Agonist Protects Against Doxorubicin-Induced Cardiotoxicity. Cancer Chemother. Pharmacol. 2016, 77, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Gharanei, A.M.; Nagra, A.S.; Maddock, H.L. Caspase Inhibition via A3 Adenosine Receptors: A New Cardioprotective Mechanism Against Myocardial Infarction. Cardiovasc. Drugs Ther. 2014, 28, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Fishman, P.; Stemmer, S.M.; Bareket-Samish, A.; Silverman, M.H.; Kerns, W.D. Targeting the A3 Adenosine Receptor to Treat Hepatocellular Carcinoma: Anti-Cancer and Hepatoprotective Effects. Purinergic Signal. 2023, 19, 513–522. [Google Scholar] [CrossRef]
- Morelli, M.B.; Spinaci, A.; Chang, C.; Volpini, R.; Lambertucci, C.; Landriscina, M.; Conteduca, V.; Amantini, C.; Aguzzi, C.; Zeppa, L.; et al. Adenosine A3 Receptor Antagonists as Anti-Tumor Treatment in Human Prostate Cancer: An In Vitro Study. FEBS Open Bio. 2025, 11, 70024. [Google Scholar] [CrossRef]
- Marucci, G.; Santinelli, C.; Buccioni, M.; Navia, A.M.; Lambertucci, C.; Zhurina, A.; Yli-Harja, O.; Volpini, R.; Kandhavelu, M. Anticancer Activity Study of A3 Adenosine Receptor Agonists. Life Sci. 2018, 205, 155–163. [Google Scholar] [CrossRef]
- Dale, N.C.; Johnstone, E.K.M.; Pfleger, K.D.G. GPCR heteromers: An overview of their classification, function and physiological relevance. Front. Endocrinol. 2022, 13, 931573. [Google Scholar] [CrossRef]
- Franco, R.; Cordomí, A.; Llinas del Torrent, C.; Lillo, A.; Serrano-Marín, J.; Navarro, G.; Pardo, L. Structure and function of adenosine receptor heteromers. Cell. Mol. Life Sci. 2021, 78, 3957–3968. [Google Scholar] [CrossRef]
- Ferré, S.; Sebastião, A.M. Dissecting striatal adenosine–cannabinoid receptor interactions. New clues from rats over-expressing adenosine A2A receptors. J. Neurochem. 2016, 136, 897–899. [Google Scholar] [CrossRef]
- Aso, E.; Fernández-Dueñas, V.; López-Cano, M.; Taura, J.; Watanabe, M.; Ferrer, I.; Luján, R.; Ciruela, F. Adenosine A2A–cannabinoid CB1 receptor heteromers in the hippocampus: Cannabidiol blunts Δ9-tetrahydrocannabinol-induced cognitive impairment. Mol. Neurobiol. 2019, 56, 5382–5391. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Reyes-Resina, I.; Aguinaga, D.; Lillo, A.; Jiménez, J.; Raïch, I.; Borroto-Escuela, D.O.; Ferreiro-Vera, C.; Canela, E.I.; Sánchez de Medina, V.; et al. Potentiation of cannabinoid signaling in microglia by adenosine A2A receptor antagonists. Glia 2019, 67, 2410–2423. [Google Scholar] [CrossRef]
- Linas del Torrent, C.; Iu Raïch, I.; Gonzalez, A.; Lillo, J.; Casajuana-Martin, N.; Franco, F.; Pardo, L.; Navarro, G. Allosterism in the adenosine A2A and cannabinoid CB2 heteromer. Br. J. Pharmacol. 2025, 182, 3371–3384. [Google Scholar] [CrossRef]
- Rivera-Oliver, M.; Moreno, E.; Álvarez-Bagnarol, Y.; Ayala-Santiago, C.; Cruz-Reyes, N.; Molina-Castro, G.C.; Clemens, S.; Canela, E.I.; Ferré, S.; Casadó, V.; et al. Adenosine A1–dopamine D1 receptor heteromers control the excitability of the spinal motoneuron. Mol. Neurobiol. 2019, 56, 797–811. [Google Scholar] [CrossRef]
- Vincenzi, F.; Pasquini, S.; Contri, C.; Cappello, M.; Nigro, M.; Travagli, A.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K. Pharmacology of Adenosine Receptors: Recent Advancements. Biomolecules 2023, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Rabin, J.; Zhao, Y.; Mostafa, E.; Al-Suqi, M.; Fleischmann, E.; Conaway, M.R.; Mann, B.J.; Chhabra, P.; Brayman, K.L.; Krupnick, A.; et al. Regadenoson for the treatment of COVID-19: A five case clinical series and mouse studies. PLoS ONE 2023, 18, e0288920. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zając, B.; Kawecka, A.; Nasadiuk, K.; Braczko, A.; Stawarska, K.; Caiazzo, E.; Koszałka, P.; Cicala, C. Drugs targeting adenosine signaling pathways: A current view. Biomed. Pharmacother. 2023, 165, 115184. [Google Scholar] [CrossRef]
- Reyes, E. Regadenoson stress for myocardial perfusion imaging. Future Cardiol. 2016, 12, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Beller, J.P.; Boys, J.A.; Zhao, Y.; Phillips, J.; Cosner, M.; Conaway, M.R.; Petroni, G.; Charles, E.J.; Mehaffey, J.H.; et al. Adenosine A2A receptor agonist (regadenoson) in human lung transplantation. J. Heart Lung Transplant. 2020, 39, 563–570. [Google Scholar] [CrossRef]
- Zhao, Y.; Dhru, U.; Fleischmann, E.; Mostafa, E.; Al-Suqi, M.; Conaway, M.R.; Krupnick, A.S.; Linden, J.; Rabin, J.; Lau, C.L. Regadenoson reduces soluble receptor for advanced glycation end-products in lung recipients. Ann. Thorac. Surg. 2023, 116, 1150–1158. [Google Scholar] [CrossRef]
- Grossman, S.A.; Romo, C.G.; Ye, X.; Kral, B.; Strowd, R.E.; Lesser, G.; Raymond, C.; Iacoboni, M.; Desideri, S.; Fisher, J.; et al. Assessing the dose of regadenoson required to transiently alter blood–brain barrier integrity in patients with infiltrating gliomas. Neurooncol. Adv. 2025, 7, vdaf041. [Google Scholar] [CrossRef]
- Papp, K.A.; Beyska-Rizova, S.; Gantcheva, M.L.; Slavcheva Simeonova, E.; Brezoev, P.; Celic, M.; Groppa, L.; Blicharski, T.; Selmanagic, A.; Kalicka-Dudzik, M.; et al. Efficacy and safety of piclidenoson in plaque psoriasis: Results from a randomized phase 3 clinical trial (COMFORT-1). J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1112–1120. [Google Scholar] [CrossRef]
- Ciurescu, I.A.; Lencioni, R.; Stemmer, S.M.; Farbstein, M.; Harpaz, Z.; Bareket-Samish, A.; Silverman, M.H.; Fishman, P. A long-term complete response to namodenoson in liver cancer with Child-Pugh B cirrhosis: A case report. Exp. Ther. Med. 2024, 27, 263. [Google Scholar] [CrossRef]
- Etzion, O.; Bareket-Samish, A.; Yardeni, D.; Fishman, P. Namodenoson at the crossroad of metabolic dysfunction-associated steatohepatitis and hepatocellular carcinoma. Biomedicines 2024, 12, 848. [Google Scholar] [CrossRef]
- Park, C.-W.; Han, C.-T.; Sakaguchi, Y.; Lee, J.; Youn, H.-Y. Safety evaluation of FM101, an A3 adenosine receptor modulator, in rat, for developing as therapeutics of glaucoma and hepatitis. EXCLI J. 2020, 19, 187–200. [Google Scholar]
- Chang, C.-P.; Wu, K.-C.; Lin, C.-Y.; Chern, Y. Emerging Roles of Dysregulated Adenosine Homeostasis in Brain Disorders with a Specific Focus on Neurodegenerative Diseases. J. Biomed. Sci. 2021, 28, 70. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, L.; Xu, Y.; Liang, L.; Liu, L.; Chen, X.; Li, H.; Liu, H. The Progress and Prospects of Targeting the Adenosine Pathway in Cancer Immunotherapy. Biomark. Res. 2025, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Barbón, D.; Brienza, N.S.; Bigorra Rodríguez, T.; Gich Saladich, I.; Puntes Rodríguez, M.; Antonijoan Arbos, R.M.; Castro Palomino Laria, N.; Castro Palomino Laria, J. PBF-680, an oral A1 adenosine receptor antagonist, inhibits adenosine monophosphate (AMP) airway hyperresponsiveness (AHR) in mild-to-moderate asthma: A Phase-IIa proof-of-concept trial. Eur. Respir. J. 2020, 56 (Suppl. S64), 2279. [Google Scholar]
- Mori, A.; Chen, J.-F.; Uchida, S.; Durlach, C.; King, S.M.; Jenner, P. The pharmacological potential of adenosine A2A receptor antagonists for treating Parkinson’s disease. Molecules 2022, 27, 2366. [Google Scholar] [CrossRef]
- Nagayama, H.; Kano, O.; Murakami, H.; Ono, K.; Hamada, M.; Toda, T.; Sengoku, R.; Shimo, Y.; Hattori, N. Effect of istradefylline on mood disorders in Parkinson’s disease. J. Neurol. Sci. 2019, 396, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, Y.; Zeng, Z.; Ye, M.; Wang, M.; Liu, X.; Tang, P.; Shang, H.; Sun, X.; Lin, X.; et al. Choroid plexus-selective inactivation of adenosine A2A receptors protects against T cell infiltration and experimental autoimmune encephalomyelitis. J. Neuroinflammation 2022, 19, 52. [Google Scholar] [CrossRef]
- Salamone, J.D. Preladenant, a novel adenosine A(2A) receptor antagonist for the potential treatment of parkinsonism and other disorders. IDrugs 2010, 13, 723–731. [Google Scholar]
- Hauser, R.A.; Cantillon, M.; Pourcher, E.; Micheli, F.; Mok, V.; Onofrj, M.; Huyck, S.; Wolski, K. Preladenant in patients with Parkinson’s disease and motor fluctuations: A phase 2, double-blind, randomised trial. Lancet Neurol. 2011, 10, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Stocchi, F.; Rascol, O.; Huyck, S.B.; Capece, R.; Ho, T.W.; Sklar, P.; Lines, C.; Michelson, D.; Hewitt, D. Preladenant as an adjunctive therapy with levodopa in Parkinson disease: Two randomized clinical trials and lessons learned. JAMA Neurol. 2015, 72, 1491–1500. [Google Scholar] [CrossRef]
- Karimi, M.A.; Ghajari, A.; Khademi, R.; Etemadi, M.H.; Safar Firouz, N.; Mohammadvand, B.; Janeshin, K.; Darvishi, A.; Asgarzadeh, S.; Sadat-Madani, S.-F.; et al. Efficacy of preladenant in improving motor symptoms in Parkinson’s disease: A systematic review and meta-analysis. IBRO Neurosci. Rep. 2024, 17, 207–219. [Google Scholar] [CrossRef]
- Brooks, D.J.; Papapetropoulos, S.; Vandenhende, F.; Tomic, D.; He, P.; Coppell, A.; O’Neill, G. An open-label, positron emission tomography study to assess adenosine A2A brain receptor occupancy of vipadenant (BIIB014) at steady-state levels in healthy male volunteers. Clin. Neuropharmacol. 2010, 33, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Olanow, C.W.; Kieburtz, K.D.; Pourcher, E.; Docu-Axelerad, A.; Lew, M.; Kozyolkin, O.; Neale, A.; Resburg, C.; Meya, U.; et al. Tozadenant (SYN115) in patients with Parkinson’s disease who have motor fluctuations on levodopa: A phase 2b, double-blind, randomised trial. Lancet Neurol. 2014, 13, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Mancel, V.; Mathy, F.-X.; Boulanger, P.; English, S.; Croft, M.; Kenney, C.; Knott, T.; Stockis, A.; Bani, M. Pharmacokinetics and metabolism of [14C]-tozadenant (SYN-115), a novel A2A receptor antagonist ligand, in healthy volunteers. Xenobiotica 2017, 47, 705–718. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Aradi, S.D.; Hauser, R.A.; Rascol, O. The challenge of developing adenosine A2A antagonists for Parkinson disease: Istradefylline, preladenant, and tozadenant. Parkinsonism Relat. Disord. 2020, 80, S54–S63. [Google Scholar] [CrossRef]
- Frau, L.; Borsini, F.; Wardas, J.; Khairnar, A.S.; Schintu, N.; Morelli, M. Neuroprotective and anti-inflammatory effects of the adenosine A(2A) receptor antagonist ST1535 in a MPTP mouse model of Parkinson’s disease. Synapse 2011, 65, 181–188. [Google Scholar] [CrossRef]
- Pinna, A. Adenosine A2A receptor antagonists in Parkinson’s disease: Progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued. CNS Drugs 2014, 28, 455–474. [Google Scholar] [CrossRef]
- Stasi, M.A.; Minetti, P.; Lombardo, K.; Riccioni, T.; Caprioli, A.; Vertechy, M.; Di Serio, S.; Pace, S.; Borsini, F. Animal models of Parkinson’s disease: Effects of two adenosine A2A receptor antagonists ST4206 and ST3932, metabolites of 2-n-Butyl-9-methyl-8-[1–3]triazol-2-yl-9H-purin-6-ylamine (ST1535). Eur. J. Pharmacol. 2015, 761, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Pourcher, E.; Huot, P. Adenosine 2A Receptor Antagonists for the Treatment of Motor Symptoms in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2015, 2, 331–340. [Google Scholar] [CrossRef]
- Mihara, T.; Iwashita, A.; Matsuoka, N. A novel adenosine A1 and A2A receptor antagonist, ASP5854, ameliorates motor impairment in MPTP-treated marmosets: Comparison with existing anti-Parkinson’s disease drugs. Behav. Brain Res. 2008, 194, 152–161. [Google Scholar] [CrossRef]
- Mihara, T.; Noda, A.; Arai, H.; Mihara, K.; Iwashita, A.; Murakami, Y.; Matsuya, T.; Miyoshi, S.; Nishimura, S.; Matsuoka, N. Brain adenosine A2A receptor occupancy by a novel A1/A2A receptor antagonist, ASP5854, in rhesus monkeys: Relationship to anticataleptic effect. J. Nucl. Med. 2008, 49, 1183–1188. [Google Scholar] [CrossRef]
- Remley, V.A.; Linden, J.; Bauer, T.W.; Dimastromatteo, J. Unlocking antitumor immunity with adenosine receptor blockers. Cancer Drug Resist. 2023, 6, 748–767. [Google Scholar] [CrossRef]
- Deb, P.K.; Maity, P.; Sarkar, B.; Venugopala, K.N.; Tekade, R.K.; Batra, S. Insights from Clinical Trials on A2A Adenosine Receptor Antagonists for Cancer Treatment. ACS Pharmacol. Transl. Sci. 2025, 8, 1498–1512. [Google Scholar] [CrossRef]
- Sek, K.; Mølck, C.; Stewart, G.D.; Kats, L.; Darcy, P.K.; Beavis, P.A. Targeting adenosine receptor signaling in cancer immunotherapy. Int. J. Mol. Sci. 2018, 19, 3837. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Buccioni, M. Current understanding of the role of adenosine receptors in cancer. Molecules 2024, 29, 3501. [Google Scholar] [CrossRef]
- Franco, R.; Lillo, A.; Navarro, G.; Reyes-Resina, I. The adenosine A2A receptor is a therapeutic target in neurological, heart and oncogenic diseases. Expert Opin. Ther. Targets 2022, 26, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Ganguly, S.; Deb, P.K. Therapeutic potential of adenosine receptor modulators in cancer treatment. RSC Adv. 2025, 15, 20418. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.A.; Creelan, B.; Tanvetyanon, T.; Gray, J.E.; Haura, E.B.; Thapa, R.; Barlow, M.L.; Chen, Z.; Chen, D.T.; Beg, A.A.; et al. Phase I study of taminadenant (PBF509/NIR178), an adenosine 2A receptor antagonist, with or without spartalizumab, in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2022, 28, 2313–2320. [Google Scholar] [CrossRef]
- Seifert, M.; Benmebarek, M.-R.; Briukhovetska, D.; Märkl, F.; Dörr, J.; Cadilha, B.L.; Jobst, J.; Stock, S.; Andreu-Sanz, D.; Lorenzini, T.; et al. Impact of the selective A2AR and A2BR dual antagonist AB928/etrumadenant on CAR T cell function. Br. J. Cancer 2022, 127, 2175–2185. [Google Scholar] [CrossRef]
- Schiemann, K.; Belousova, N.; Matevossian, A.; Nallaparaju, K.C.; Kradjian, G.; Pandya, M.; Chen, Z.; Aral, E.; Krauel, E.-M.; Petrova, E.; et al. Dual A2A/A2B adenosine receptor antagonist M1069 counteracts immunosuppressive mechanisms of adenosine and reduces tumor growth in vivo. Mol. Cancer Ther. 2024, 23, 1517–1529. [Google Scholar] [CrossRef]
- Yang, M.; Soohoo, D.; Soelaiman, S.; Kalla, R.; Zablocki, J.; Chu, N.; Leung, K.; Yao, L.; Diamond, I.; Belardinelli, L.; et al. Characterization of the potency, selectivity, and pharmacokinetic profile for six adenosine A2A receptor antagonists. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 375, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Khanapur, S.; Paul, S.; Shah, A.; Vatakuti, S.; Koole, M.J.B.; Zijlma, R.; Dierckx, R.A.J.O.; Luurtsema, G.; Garg, P.; van Waarde, A.; et al. Development of [18F]-labeled pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (SCH442416) analogs for the imaging of cerebral adenosine A2A receptors with positron emission tomography. J. Med. Chem. 2014, 57, 6765–6780. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; He, L. Adenosine A2A Receptor Antagonist Sch58261 Improves the Cognitive Function in Alzheimer’s Disease Model Mice Through Activation of Nrf2 via an Autophagy-Dependent Pathway. Antioxid. Redox Signal. 2024, 41, 1117–1133. [Google Scholar] [CrossRef]
- Moresco, R.M.; Todde, S.; Belloli, S.; Simonelli, P.; Panzacchi, A.; Rigamonti, M.; Galli-Kienle, M.; Fazio, F. In vivo imaging of adenosine A2A receptors in rat and primate brain using [11C]SCH442416. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 405–413. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.-X.; Zheng, L.-P.; Wang, L.; Liu, Y.-F.; Yin, W.-Y.; Chen, Y.-Y.; Wang, X.-S.; Hou, S.-T.; Chen, J.-F.; et al. The adenosine A2A receptor antagonist SCH58261 reduces macrophage/microglia activation and protects against experimental autoimmune encephalomyelitis in mice. Neurochem. Int. 2019, 129, 104490. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Porciúncula, L.O.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br. J. Pharmacol. 2003, 138, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Gorelik, E.; Prasad, S.J.; Ronchese, F.; Lukashev, D.; Wong, M.K.K.; Huang, X.; Caldwell, S.; Liu, K.; Smith, P.; et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13132–13137. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.M.; Traini, C.; Cipriani, S.; Gianfriddo, M.; Mello, T.; Giovannini, M.G.; Galli, A.; Pedata, F. The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices. Br. J. Pharmacol. 2009, 157, 818–830. [Google Scholar] [CrossRef]
- Fathalla, A.M.; Soliman, A.M.; Moustafa, A.A. Selective A2A receptors blockade reduces degeneration of substantia nigra dopamine neurons in a rotenone-induced rat model of Parkinson’s disease: A histological study. Neurosci. Lett. 2017, 643, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ding, W.; Huang, W.; Zhang, Z.; Guo, Y.; Zhang, Q.; Wu, L.; Li, Y.; Qin, R.; Li, J.; et al. Discovery of novel benzo[4,5]imidazo[1,2-a]pyrazin-1-amine-3-amide-one derivatives as anticancer human A2A adenosine receptor antagonists. J. Med. Chem. 2022, 65, 8933–8947. [Google Scholar] [CrossRef]
- Ding, W.; Liu, S.; Liu, W.; Zhang, Z.; Zhao, J.; Zhang, X.; Shi, T.; Hu, W. Chirality-guided optimization of A2A adenosine receptor antagonists for enhanced metabolic stability and antitumor efficacy. J. Med. Chem. 2025, 68, 14962–14980. [Google Scholar] [CrossRef]
- Zhu, C.; Ze, S.; Zhou, R.; Yang, X.; Wang, H.; Chai, X.; Fang, M.; Liu, M.; Wang, Y.; Lu, W.; et al. Discovery of pyridinone derivatives as potent, selective, and orally bioavailable adenosine A2A receptor antagonists for cancer immunotherapy. J. Med. Chem. 2023, 66, 4734–4754. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.H.M.; Prieto-Díaz, R.; Neo, S.; Tong, L.; Chen, X.; Carannante, V.; Önfelt, B.; Hartman, J.; Haglund, F.; Majellaro, M.; et al. A2B adenosine receptor antagonists rescue lymphocyte activity in adenosine-producing patient-derived cancer models. J. Immunother. Cancer 2022, 10, e004592. [Google Scholar] [CrossRef]
- Lan, J.; Wei, G.; Liu, J.; Yang, F.; Sun, R.; Lu, H. Chemotherapy-induced adenosine A2B receptor expression mediates epigenetic regulation of pluripotency factors and promotes breast cancer stemness. Theranostics 2022, 12, 2598–2612. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lei, M.M.L.; Xie, L.; Shou, Y.; Lee, T.K.W. Deciphering adenosine signaling in hepatocellular carcinoma: Pathways, prognostic models, and therapeutic implications. Clin. Mol. Hepatol. 2025, 31, 706–729. [Google Scholar] [CrossRef]
- Van Muijlwijk-Koezen, J.E.; Timmerman, H.; van der Goot, H.; Menge, W.M.; Frijtag Von Drabbe Künzel, J.; de Groote, M.; IJzerman, A.P. Isoquinoline and quinazoline urea analogues as antagonists for the human adenosine A3 receptor. J. Med. Chem. 2000, 43, 2227–2238. [Google Scholar] [CrossRef]
- Gessi, S.; Varani, K.; Merighi, S.; Morelli, A.; Ferrari, D.; Leung, E.; Baraldi, P.G.; Spalluto, G.; Borea, P.A. Pharmacological and biochemical characterization of A3 adenosine receptors in Jurkat T cells. Br. J. Pharmacol. 2001, 134, 116–126. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Klutz, A.M.; Tosh, D.K.; Ivanov, A.A.; Preti, D.; Baraldi, P.G. Medicinal chemistry of the A3 adenosine receptor: Agonists, antagonists, and receptor engineering. Handb. Exp. Pharmacol. 2009, 193, 123–159. [Google Scholar]
- IJzerman, A.P.; Jacobson, K.A.; Müller, C.E.; Cronstein, B.N.; Cunha, R.A. International Union of Basic and Clinical Pharmacology. CXII: Adenosine receptors: A further update. Pharmacol. Rev. 2022, 74, 340–372. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.M.; Coppi, E.; Volpini, R.; Cristalli, G.; Corradetti, R.; Jeong, L.S.; Jacobson, K.A.; Pedata, F. Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen-glucose deprivation episodes of different duration. Biochem. Pharmacol. 2007, 74, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-G.; Auchampach, J.A.; Jacobson, K.A. Species dependence of A3 adenosine receptor pharmacology and function. Purinergic Signal. 2023, 19, 523–550. [Google Scholar] [CrossRef]
- Ji, X.-D.; von Lubitz, D.; Olah, M.E.; Stiles, G.L.; Jacobson, K.A. Species differences in ligand affinity at central A3-adenosine receptors. Drug Dev. Res. 1994, 33, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-G.; Suresh, R.R.; Jacobson, K.A. Pharmacological characterization of DPTN and other selective A3 adenosine receptor antagonists. Purinergic Signal. 2021, 17, 737–746. [Google Scholar] [CrossRef]
- Miwatashi, S.; Arikawa, Y.; Matsumoto, T.; Uga, K.; Kanzaki, N.; Imai, Y.N.; Ohkawa, S. Synthesis and biological activities of 4-phenyl-5-pyridyl-1,3-thiazole derivatives as selective adenosine A3 antagonists. Chem. Pharm. Bull. 2008, 56, 1126–1137. [Google Scholar] [CrossRef]
- Li, A.-H.; Moro, S.; Melman, N.; Ji, X.-D.; Jacobson, K.A. Structure−Activity Relationships and Molecular Modeling of 3,5-Diacyl-2,4-Dialkylpyridine Derivatives as Selective A3 Adenosine Receptor Antagonists. J. Med. Chem. 1998, 41, 3186–3201. [Google Scholar] [CrossRef]
- Alnouri, M.W.; Jepards, S.; Casari, A.; Schiedel, A.C.; Hinz, S.; Müller, C.E. Selectivity is species-dependent: Characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal. 2015, 11, 389–407. [Google Scholar] [CrossRef]
- Wadsak, W.; Mien, L.-K.; Shanab, K.; Ettlinger, D.E.; Haeusler, D.; Sindelar, K.; Lanzenberger, R.R.; Spreitzer, H.; Viernstein, H.; Keppler, B.K.; et al. Preparation and first evaluation of [18F]FE@SUPPY: A new PET tracer for the adenosine A3 receptor. Nucl. Med. Biol. 2008, 35, 61–66. [Google Scholar] [CrossRef]
- Colotta, V.; Catarzi, D.; Varano, F.; Cecchi, L.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Lucacchini, A. 1,2,4-Triazolo[4,3-a]quinoxalin-1-one: A Versatile Tool for the Synthesis of Potent and Selective Adenosine Receptor Antagonists. J. Med. Chem. 2000, 43, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Colotta, V.; Catarzi, D.; Varano, F.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Lucacchini, A. Synthesis of 4-Amino-6-(Hetero)arylalkylamino-1,2,4-triazolo[4,3-a]quinoxalin-1-one Derivatives as Potent A2A Adenosine Receptor Antagonists. Bioorg. Med. Chem. 2003, 11, 5509–5518. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.E. Medicinal chemistry of adenosine A3 receptor ligands. Curr. Topics Med. Chem. 2003, 3, 445–462. [Google Scholar] [CrossRef]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on adenosine receptors as potential targeted therapy in human diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Colotta, V.; Catarzi, D.; Varano, F.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Lucacchini, A. Synthesis and structure-activity relationships of a new set of 1,2,4-triazolo[4,3-a]quinoxalin-1-one derivatives as adenosine receptor antagonists. Bioorg. Med. Chem. 2003, 11, 3541–3550. [Google Scholar] [CrossRef]
- Colotta, V.; Catarzi, D.; Varano, F.; Calabri, F.R.; Lenzi, O.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Deflorian, F.; Moro, S. 1,2,4-Triazolo[4,3-a]quinoxalin-1-one moiety as an attractive scaffold to develop new potent and selective human A3 adenosine receptor antagonists: Synthesis, pharmacological, and ligand−receptor modeling Studies. J. Med. Chem. 2004, 47, 3580–3590. [Google Scholar] [CrossRef]
- Kim, Y.-C.; de Zwart, M.; Chang, L.; Moro, S.; von Frijtag Drabbe Kunzel, J.K.; Melman, N.; IJzerman, A.P.; Jacobson, K.A. Derivatives of the Triazoloquinazoline Adenosine Antagonist (CGS 15943) Having High Potency at the Human A2B and A3 receptor Subtypes. J. Med. Chem. 1998, 41, 2835–2845. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Cacciari, B.; Moro, S.; Spalluto, G.; Pastorin, G.; Da Ros, T.; Klotz, K.-N.; Varani, K.; Gessi, S.; Borea, P.A. Synthesis, Biological Activity, and Molecular Modeling Investigation of New Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine Derivatives as Human A3 Adenosine Receptor Antagonists. J. Med. Chem. 2002, 45, 770–780. [Google Scholar] [CrossRef]
- Lenzi, O.; Colotta, V.; Catarzi, D.; Varano, F.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Ciampi, O.; Varani, K.; Marighetti, F.; et al. 4-Amido-2-aryl-1,2,4-triazolo[4,3-a]quinoxalin-1-ones as new Potent and Selective Human A3 adenosine receptor antagonists. Synthesis, pharmacological evaluation, and ligand–receptor modeling studies. J. Med. Chem. 2006, 49, 3916–3925. [Google Scholar] [CrossRef]
- Colotta, V.; Catarzi, D.; Varano, F.; Lenzi, O.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Ciampi, O.; Traini, C.; Pugliese, A.M.; et al. Synthesis, ligand–receptor modeling studies and Pharmacological Evaluation of Novel 4-Modified-2-Aryl-1,2,4-Triazolo[4,3-a]quinoxalin-1-One Derivatives as Potent and Selective Human A3 Adenosine Receptor Antagonists. Bioorg. Med. Chem. 2008, 16, 6086–6102. [Google Scholar] [CrossRef] [PubMed]
- Latini, F.; Pedata, F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef]
- Latini, S.; Bordoni, F.; Corradetti, R.; Pepeu, G.; Pedata, F. Temporal Correlation between Adenosine Outflow and Synaptic Potential Inhibition in Rat Hippocampal Slices During Ischemia-like Conditions. Brain Res. 1998, 794, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.M.; Coppi, E.; Spalluto, G.; Corradetti, R.; Pedata, F. A3 Adenosine Receptor Antagonists Delay Irreversible Synaptic Failure Caused by Oxygen and Glucose Deprivation in the Rat CA1 Hippocampus In Vitro. Br. J. Pharmacol. 2006, 147, 524–532. [Google Scholar] [CrossRef]
- Colotta, V.; Catarzi, D.; Varano, F.; Capelli, F.; Lenzi, O.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Ciampi, O.; Pugliese, A.M.; et al. New 2-Arylpyrazolo[3,4-c]quinoline Derivatives as Potent and Selective Human A3 Adenosine Receptor Antagonists. Synthesis, Pharmacological Evaluation, and Ligand–Receptor Modeling Studies. J. Med. Chem. 2007, 50, 4061–4074. [Google Scholar] [CrossRef]
- Vernall, A.J.; Stoddart, L.A.; Briddon, S.J.; Hill, S.J.; Kellam, B. Highly Potent and Selective Fluorescent Antagonists of the Human Adenosine A3 Receptor Based on the 1,2,4-Triazolo [4,3-a]quinoxalin-1-one Scaffold. J. Med. Chem. 2012, 55, 1771–1782. [Google Scholar] [CrossRef]
- Poucher, S.M.; Keddie, J.R.; Singh, P.; Stoggall, S.M.; Caulkett, P.W.; Jones, G.; Coll, M.G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2A selective adenosine receptor antagonist. Br. J. Pharmacol. 1995, 115, 1096–1102. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Cacciari, B.; Spalluto, G.; Bergonzoni, M.; Dionisotti, S.; Ongini, E.; Varani, K.; Borea, P.A. Design, synthesis, and biological evaluation of a second generation of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines as potent and selective A2A adenosine receptor antagonists. J. Med. Chem. 1998, 41, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Cacciari, B.; Romagnoli, R.; Spalluto, G.; Monopoli, A.; Ongini, E.; Varani, K.; Borea, P.A. 7-Substituted 5-amino-2-(2-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines as A2A adenosine receptor antagonists: A study on the importance of modifications at the side chain on the activity and solubility. J. Med. Chem. 2002, 45, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Colotta, V.; Lenzi, O.; Catarzi, D.; Varano, F.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Ciampi, O.; Pugliese, A.M.; Traini, C.; et al. Pyrido[2,3-e]-1,2,4-triazolo[4,3-a]pyrazin-1-one as a new scaffold to develop potent and selective human A3 adenosine receptor antagonists. Synthesis, pharmacological evaluation, and ligand-receptor modeling studies. J. Med. Chem. 2009, 52, 2407–2419. [Google Scholar] [CrossRef]
- Morizzo, E.; Capelli, F.; Lenzi, O.; Catarzi, D.; Varano, F.; Filacchioni, G.; Vincenzi, F.; Varani, K.; Borea, P.A.; Colotta, V.; et al. Scouting human A3 adenosine receptor antagonist binding mode using a molecular simplification approach: From triazoloquinoxaline to a pyrimidine skeleton as a key study. J. Med. Chem. 2007, 50, 6596–6606. [Google Scholar] [CrossRef]
- Poli, D.; Catarzi, D.; Colotta, V.; Varano, F.; Filacchioni, G.; Daniele, S.; Trincavelli, L.; Martini, C.; Paoletta, S.; Moro, S. The identification of the 2-phenylphthalazin-1 (2 H)-one scaffold as a new decorable core skeleton for the design of potent and selective human A3 adenosine receptor antagonists. J. Med. Chem. 2011, 54, 2102–2113. [Google Scholar] [CrossRef]
- Lenzi, O.; Colotta, V.; Catarzi, D.; Varano, F.; Poli, D.; Filacchioni, G.; Varani, K.; Vincenzi, F.; Borea, P.A.; Paoletta, S.; et al. 2-Phenylpyrazolo[4,3-d]pyrimidin-7-one as a new scaffold to obtain potent and selective human A3 adenosine receptor antagonists: New insights into the receptor-antagonist recognition. J. Med. Chem. 2009, 52, 7640–7652. [Google Scholar] [CrossRef]
- Falsini, M.; Squarcialupi, L.; Catarzi, D.; Varano, F.; Betti, M.; Dal Ben, D.; Marucci, G.; Buccioni, M.; Volpini, R.; De Vita, T.; et al. The 1, 2, 4-triazolo [4,3-a] pyrazin-3-one as a versatile scaffold for the design of potent adenosine human receptor antagonists. Structural investigations to target the A2A receptor subtype. J. Med. Chem. 2017, 60, 5772–5790. [Google Scholar] [CrossRef]
- Falsini, M.; Catarzi, D.; Varano, F.; Dal Ben, D.; Marucci, G.; Buccioni, M.; Volpini, R.; Di Cesare Mannelli, L.; Ghelardini, C.; Colotta, V. Novel 8-amino-1, 2, 4-triazolo [4,3-a] pyrazin-3-one derivatives as potent human adenosine A1 and A2A receptor antagonists. Evaluation of their protective effect against β-amyloid-induced neurotoxicity in SH-SY5Y cells. Bioorg. Chem. 2019, 87, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Canas, P.M.; Porciúncula, L.O.; Cunha, G.M.A.; Silva, C.G.; Machado, N.J.; Oliveira, J.M.A.; Oliveira, C.R.; Cunha, R.A. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J. Neurosci. 2009, 29, 14741–14751. [Google Scholar] [CrossRef] [PubMed]
- Faivre, E.; Coelho, J.E.; Zornbach, K.; Malik, E.; Baqi, Y.; Schneider, M.; Cellai, L.; Carvalho, K.; Sebda, S.; Figeac, M.; et al. Beneficial Effect of a Selective Adenosine A2A Receptor Antagonist in the APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 235. [Google Scholar] [CrossRef]
- Giunta, S.; Andriolo, V.; Castorina, A. Dual blockade of the A1 and A2A adenosine receptor prevents amyloid beta toxicity in neuroblastoma cells exposed to aluminium chloride. Int. J. Biochem. Cell Biol. 2014, 54, 122–136. [Google Scholar] [CrossRef]
- Falsini, M.; Ceni, C.; Catarzi, D.; Varano, F.; Dal Ben, D.; Marucci, G.; Buccioni, M.; Navia, A.M.; Volpini, R.; Colotta, V. New 8-amino-1,2,4-triazolo[4,3-a]pyrazin-3-one derivatives. Evaluation of different moieties on the 6-aryl ring to obtain potent and selective human A2A adenosine receptor antagonists. Bioorg. Med. Chem. Lett. 2020, 30, 127126. [Google Scholar] [CrossRef]
- Jana, A.; Bhattacharjee, A.; Das, S.S.; Srivastava, A.; Choudhury, A.; Bhattacharjee, R.; De, S.; Perveen, A.; Iqbal, D.; Gupta, P.K.; et al. Molecular Insights into Therapeutic Potentials of Hybrid Compounds Targeting Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 3512–3528. [Google Scholar] [CrossRef] [PubMed]
- García-Beltrán, O.; Urrutia, p.J.; Núñez, M.T. On the Chemical and Biological Characteristics of Multifunctional Compounds for the Treatment of Parkinson’s Disease. Antioxidants 2023, 12, 214. [Google Scholar] [CrossRef]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef]
- Tu, C.; Wang, S.C.; Dai, M.X.; Lai, S.Q.; Huang, Z.W.; Yu, Y.P.; Chen, Y.B.; Zeng, J.H.; Wang, L.; Zhong, Z.M. Accumulation of advanced oxidative protein products exacerbate satellite glial cells activation and neuropathic pain. Mol. Med. 2025, 31, 25. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Dong, Y.; Chen, Y.; Li, M.; Song, B.; Zhang, Y.; Jiang, M.; Zhang, X. The role of antioxidant therapy in modulating neuropathic pain: A systematic review of mechanistic insights and research trends (2003–2024). Brain Circ. 2025, 11, 113–126. [Google Scholar] [CrossRef]
- Falsini, M.; Catarzi, D.; Varano, F.; Ceni, C.; Dal Ben, D.; Marucci, G.; Buccioni, M.; Volpini, R.; Di Cesare Mannelli, L.; Lucarini, E.; et al. Antioxidant-Conjugated 1,2,4-Triazolo[4,3-a]pyrazin-3-one Derivatives: Highly Potent and Selective Human A2A Adenosine Receptor Antagonists Possessing Protective Efficacy in Neuropathic Pain. J. Med. Chem. 2019, 62, 8511–8531. [Google Scholar] [CrossRef]
- Ceni, C.; Catarzi, D.; Varano, F.; Dal Ben, D.; Marucci, G.; Buccioni, M.; Volpini, R.; Angeli, A.; Nocentini, A.; Gratteri, P.; et al. Discovery of first-in-class multi-target adenosine A2A receptor antagonists-carbonic anhydrase IX and XII inhibitors. 8-Amino-6-aryl-2-phenyl-1,2,4-triazolo[4,3-a]pyrazin-3-one derivatives as new potential antitumor agents. Eur. J. Med. Chem. 2020, 201, 112478. [Google Scholar] [CrossRef] [PubMed]
- Steingold, J.M.; Hatfield, S.M. Targeting Hypoxia-A2A Adenosinergic Immunosuppression of Antitumor T Cells During Cancer Immunotherapy. Front. Immunol. 2020, 11, 570041. [Google Scholar] [CrossRef] [PubMed]
- Arruga, F.; Serra, S.; Vitale, N.; Guerra, G.; Papait, A.; Gyau, B.B.; Tito, F.; Efremov, D.; Vaisitti, T.; Deaglio, S. Targeting of the A2A adenosine receptor counteracts immunosuppression in vivo in a mouse model of chronic lymphocytic leukemia. Haematologica 2021, 106, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017, 7, 48. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging hypoxic tumors. Expert Opin. Investig. Drugs 2018, 27, 963–970. [Google Scholar] [CrossRef]
- Chen, F.; Licarete, E.; Wu, X.; Petrusca, D.; Maguire, C.; Jacobsen, M.; Colter, A.; Sandusky, G.E.; Czader, M.; Capitano, M.L.; et al. Pharmacological inhibition of Carbonic Anhydrase IX and XII to enhance targeting of acute myeloid leukaemia cells under hypoxic conditions. J. Cell. Mol. Med. 2021, 25, 11039–11052. [Google Scholar] [CrossRef]
- Colotta, V.; Catarzi, D.; Varano, F.; Cecchi, L.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Lucacchini, A. Synthesis and structure-activity relationships of a new set of 2-arylpyrazolo[3,4-c]quinoline derivative sas adenosine receptor antagonists. J. Med. Chem. 2000, 43, 3118–3124. [Google Scholar] [CrossRef]
- Lenzi, O.; Colotta, V.; Catarzi, D.; Varano, F.; Squarcialupi, L.; Filacchioni, G.; Varani, K.; Vincenzi, F.; Borea, P.A.; Dal Ben, D.; et al. Synthesis, structure–affinity relationships, and molecular modeling studies of novel pyrazolo[3,4-c]quinoline derivatives as adenosine receptor antagonists. Bioorg. Med. Chem. 2011, 19, 3757–3768. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Brussee, J.; IJzerman, A.P. Non-xanthine antagonists for the adenosine A1 receptor. Chem. Biodivers. 2004, 1, 1591–1626. [Google Scholar] [CrossRef]
- Colotta, V.; Capelli, F.; Lenzi, O.; Catarzi, D.; Varano, F.; Poli, D.; Vincenzi, F.; Varani, K.; Borea, P.A.; Dal Ben, D.; et al. Novel potent and highly selective human A3 adenosine receptor antagonists belonging to the 4-amido-2-arylpyrazolo[3,4-c]quinoline series: Molecular docking analysis and pharmacological studies. Bioorg. Med. Chem. 2009, 17, 401–410. [Google Scholar] [CrossRef]
- Squarcialupi, L.; Catarzi, D.; Varano, F.; Betti, M.; Falsini, M.; Vincenzi, F.; Ravani, A.; Ciancetta, A.; Varani, K.; Moro, S.; et al. Structural refinement of pyrazolo[4,3-d]pyrimidine derivatives to obtain highly potent and selective antagonists for the human A3 adenosine receptor. Eur. J. Med. Chem. 2016, 108, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Squarcialupi, L.; Colotta, V.; Catarzi, D.; Varano, F.; Filacchioni, G.; Varani, K.; Corciulo, C.; Vincenzi, F.; Borea, P.A.; Ghelardini, C.; et al. 2-Arylpyrazolo[4,3-d]pyrimidin-7-amino derivatives as new potent and selective human A3 adenosine receptor antagonists: Molecular modeling studies and pharmacological evaluation. J. Med. Chem. 2013, 56, 2256–2269. [Google Scholar] [CrossRef] [PubMed]
- Squarcialupi, L.; Colotta, V.; Catarzi, D.; Varano, F.; Betti, M.; Varani, K.; Vincenzi, F.; Borea, P.A.; Porta, N.; Ciancetta, A.; et al. 7-Amino-2-phenylpyrazolo[4,3-d]pyrimidine derivatives: Structural investigations at the 5-position to target human A1 and A2A adenosine receptors. Molecular modeling and pharmacological studies. Eur. J. Med. Chem. 2014, 84, 614–627. [Google Scholar] [CrossRef]
- Squarcialupi, L.; Falsini, M.; Catarzi, D.; Varano, F.; Betti, M.; Varani, K.; Vincenzi, F.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; et al. Exploring the 2- and 5-positions of the pyrazolo[4,3-d]pyrimidin-7-amino scaffold to target human A1 and A2A adenosine receptors. Bioorg. Med. Chem. 2016, 24, 2794–2808. [Google Scholar] [CrossRef]
- Squarcialupi, L.; Betti, M.; Catarzi, D.; Varano, F.; Falsini, M.; Ravani, A.; Pasquini, S.; Vincenzi, F.; Salmaso, V.; Sturlese, M.; et al. The role of 5-arylalkylamino- and 5-piperazino-moieties on the 7-aminopyrazolo[4,3-d]pyrimidine core in affecting adenosine A1 and A2A receptor affinity and selectivity profiles. J. Enzyme Inhib. Med. Chem. 2017, 32, 248–263. [Google Scholar] [CrossRef]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Poli, D.; Falsini, M.; Varano, F.; Betti, M.; Varani, K.; Vincenzi, F.; Pugliese, A.M.; Pedata, F.; Dal Ben, D.; Thomas, A.; et al. Imidazo[1,2-a]pyrazine-8-amine core for the design of new adenosine receptor antagonists: Structural exploration to target the A3 and A2A subtypes. Eur. J. Med. Chem. 2017, 125, 611–628. [Google Scholar] [CrossRef]
- Velázquez-Olvera, S.; Salgado-Zamora, H.; Velázquez-Ponce, M.; Campos-Aldrete, E.; Reyes-Arellano, A.; Pérez-González, C. Fluorescent property of 3-hydroxymethyl imidazo[1,2-a]pyridine and pyrimidine derivatives. Chem. Cent. J. 2012, 6, 83. [Google Scholar] [CrossRef]
- Leopoldo, M.; Lacivita, E.; Passafiume, E.; Contino, M.; Colabufo, N.A.; Berardi, F.; Perrone, R. 4-[ω-[4-Arylpiperazin-1-yl]alkoxy]phenyl)imidazo[1,2-a]pyridine derivatives: Fluorescent high-affinity dopamine D3 receptor ligands as potential probes for receptor visualization. J. Med. Chem. 2007, 50, 5043–5047. [Google Scholar] [CrossRef] [PubMed]
- Varano, F.; Catarzi, D.; Squarcialupi, L.; Betti, M.; Vincenzi, F.; Ravani, A.; Varani, K.; Dal Ben, D.; Thomas, A.; Volpini, R.; et al. Exploring the 7-oxo-thiazolo[5,4-d]pyrimidine core for the design of new human adenosine A3 receptor antagonists: Synthesis, molecular modeling studies and pharmacological evaluation. Eur. J. Med. Chem. 2015, 96, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Varano, F.; Catarzi, D.; Vincenzi, F.; Betti, M.; Falsini, M.; Ravani, A.; Borea, P.A.; Colotta, V.; Varani, K. Design, synthesis, and pharmacological characterization of 2-(2-furanyl)thiazolo[5,4-d]pyrimidine-5,7-diamine derivatives: New highly potent A2A adenosine receptor inverse agonists with antinociceptive activity. J. Med. Chem. 2016, 59, 10564–10576. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Vincenzi, F.; Falsini, M.; Pasquini, S.; Borea, P.A.; Colotta, V.; Varani, K. Structure-activity relationship studies and pharmacological characterization of N5-heteroarylalkyl-substituted-2-(2-furanyl)thiazolo[5,4-d]pyrimidine-5,7-diamine-based derivatives as inverse agonists at human A2A adenosine receptor. Eur. J. Med. Chem. 2018, 155, 552–561. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Vigiani, E.; Vincenzi, F.; Pasquini, S.; Varani, K.; Colotta, V. Piperazine- and piperidine-containing thiazolo[5,4-d]pyrimidine derivatives as new potent and selective adenosine A2A receptor inverse agonists. Pharmaceuticals 2020, 13, 161. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Falsini, M.; Vincenzi, F.; Pasquini, S.; Varani, K.; Colotta, V. Identification of novel thiazolo[5,4-d]pyrimidine derivatives as human A1 and A2A adenosine receptor antagonists/inverse agonists. Bioorg. Med. Chem. 2018, 26, 3688–3695. [Google Scholar] [CrossRef]
- Varano, F.; Colotta, V.; Catarzi, D.; Varani, K.; Borea, P.A.; Vincenzi, F. Novel thiazolo[5,4-d]pyrimidine derivatives as inverse agonists of A2A adenosine receptors. WO Patent 2018/007957 A1, 11 January 2018. [Google Scholar]
- Li, L.; Hao, J.X.; Fredholm, B.B.; Schulte, G.; Wiesenfeld-Hallin, Z.; Xu, X.J. Peripheral adenosine A2A receptors are involved in carrageenan-induced mechanical hyperalgesia in mice. Neuroscience 2010, 170, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Derry, C.J.; Derry, S.; Moore, R.A. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst. Rev. 2014, 2014, CD009281. [Google Scholar] [CrossRef]
- Gessi, S.; Merighi, S.; Varani, K.; Leung, E.; Maclennan, S.; Baraldi, P.G.; Borea, P.A. Inhibition of A2A adenosine receptor signaling in cancer cells proliferation by the novel antagonist TP455. Front. Pharmacol. 2017, 8, 310596. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Falsini, M.; Dal Ben, D.; Buccioni, M.; Marucci, G.; Volpini, R.; Colotta, V. Novel human adenosine receptor antagonists based on the 7-amino-thiazolo[5,4-d]pyrimidine scaffold. Structural investigations at the 2-, 5- and 7-positions to enhance affinity and tune selectivity. Bioorg. Med. Chem. Lett. 2019, 29, 563–569. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Preti, D.; Borea, P.A.; Varani, K. Medicinal chemistry of A3 adenosine receptor modulators: Pharmacological activities and therapeutic implications. J. Med. Chem. 2012, 55, 5676–5703. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.-G. A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy. Med. Res. Rev. 2018, 38, 1031–1072. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Vigiani, E.; Dal Ben, D.; Buccioni, M.; Marucci, G.; Di Cesare Mannelli, L.; Lucarini, E.; Ghelardini, C.; Volpini, R.; et al. Design and synthesis of novel thiazolo[5,4-d]pyrimidine derivatives with high affinity for both the adenosine A1 and A2A receptors, and efficacy in animal models of depression. Pharmaceuticals 2021, 14, 657. [Google Scholar] [CrossRef]
- Szopa, A.; Socała, K.; Serefko, A.; Doboszewska, U.; Wróbel, A.; Poleszak, E.; Wlaź, P. Purinergic transmission in depressive disorders. Pharmacol. Ther. 2021, 224, 107821. [Google Scholar] [CrossRef]
- Bartoli, F.; Burnstock, G.; Crocamo, C.; Carrà, G. Purinergic signaling and related biomarkers in depression. Brain Sci. 2020, 10, 160. [Google Scholar] [CrossRef]
- López-Cruz, L.; Salamone, J.D.; Correa, M. Caffeine and selective adenosine receptor antagonists as new therapeutic tools for the motivational symptoms of depression. Front. Pharmacol. 2018, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, D.; Young, A.; Teng, M.W.L.; Smyth, M.J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 2017, 17, 709–724. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Vincenzi, F.; Pasquini, S.; Pelletier, J.; Fietto, J.L.R.; Gelsleichter, N.E.; Sarlandie, M.; Guilbaud, A.; Sévigny, J.; et al. Structural investigation on thiazolo[5,4-d]pyrimidines to obtain dual-acting blockers of CD73 and adenosine A2A receptor as potential antitumor agents. Bioorg. Med. Chem. Lett. 2020, 30, 127067. [Google Scholar] [CrossRef] [PubMed]
- Ripphausen, P.; Freundlieb, M.; Brunschweiger, A.; Zimmermann, H.; Müller, C.E.; Bajorath, J. Virtual screening identifies novel sulfonamide inhibitors of ecto-5’-nucleotidase. J. Med. Chem. 2012, 55, 6576–6581. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Costanzi, S.; Balasubramanian, R.; Gao, Z.-G.; Jacobson, K.A. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal. 2013, 9, 271–280. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).