Targeting the CXCR4/CXCL12 Axis to Overcome Drug Resistance in Triple-Negative Breast Cancer
Abstract
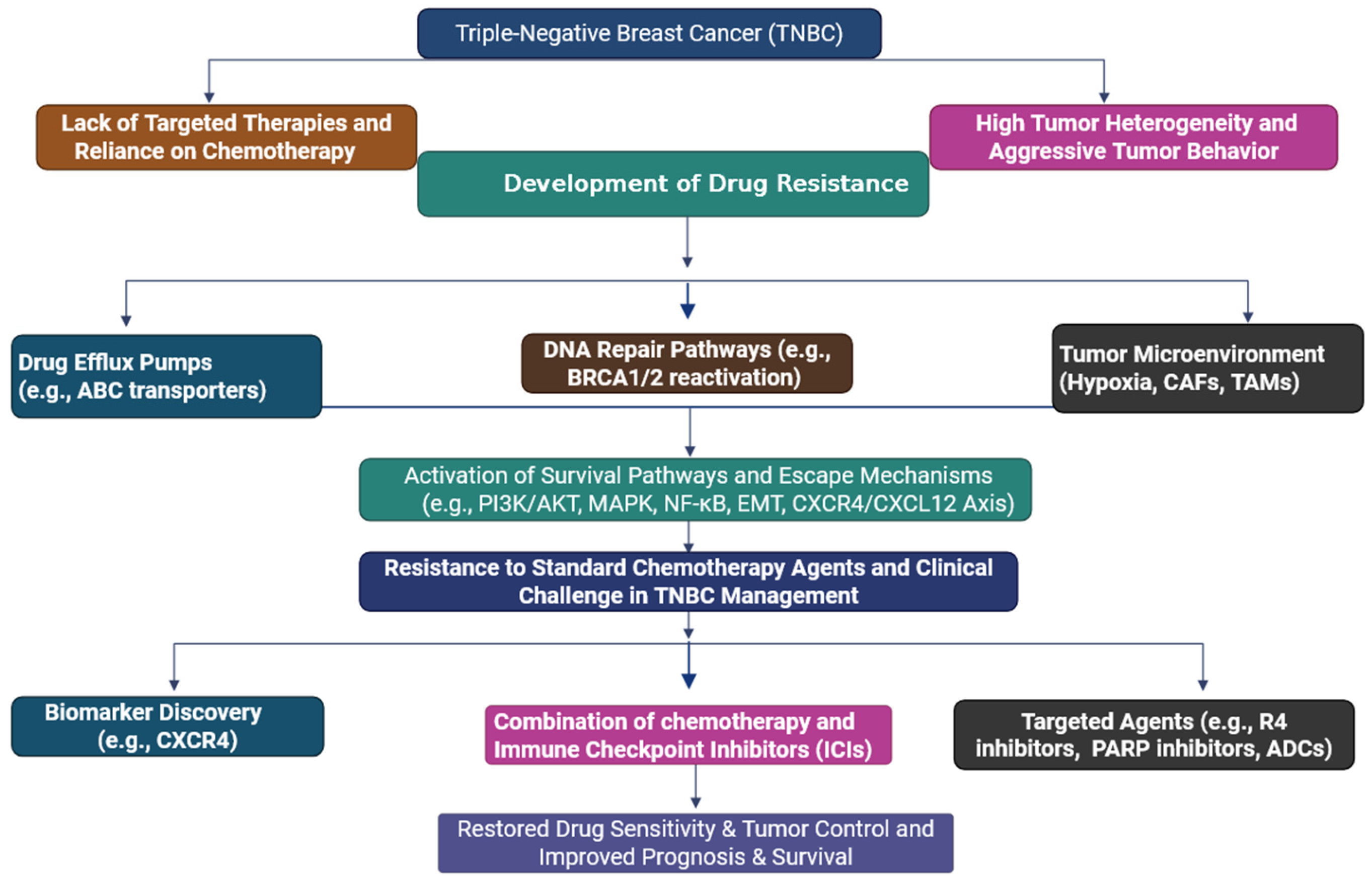
1. Introduction
2. Triple-Negative Breast Cancer and Drug Resistance: A Clinical Challenge
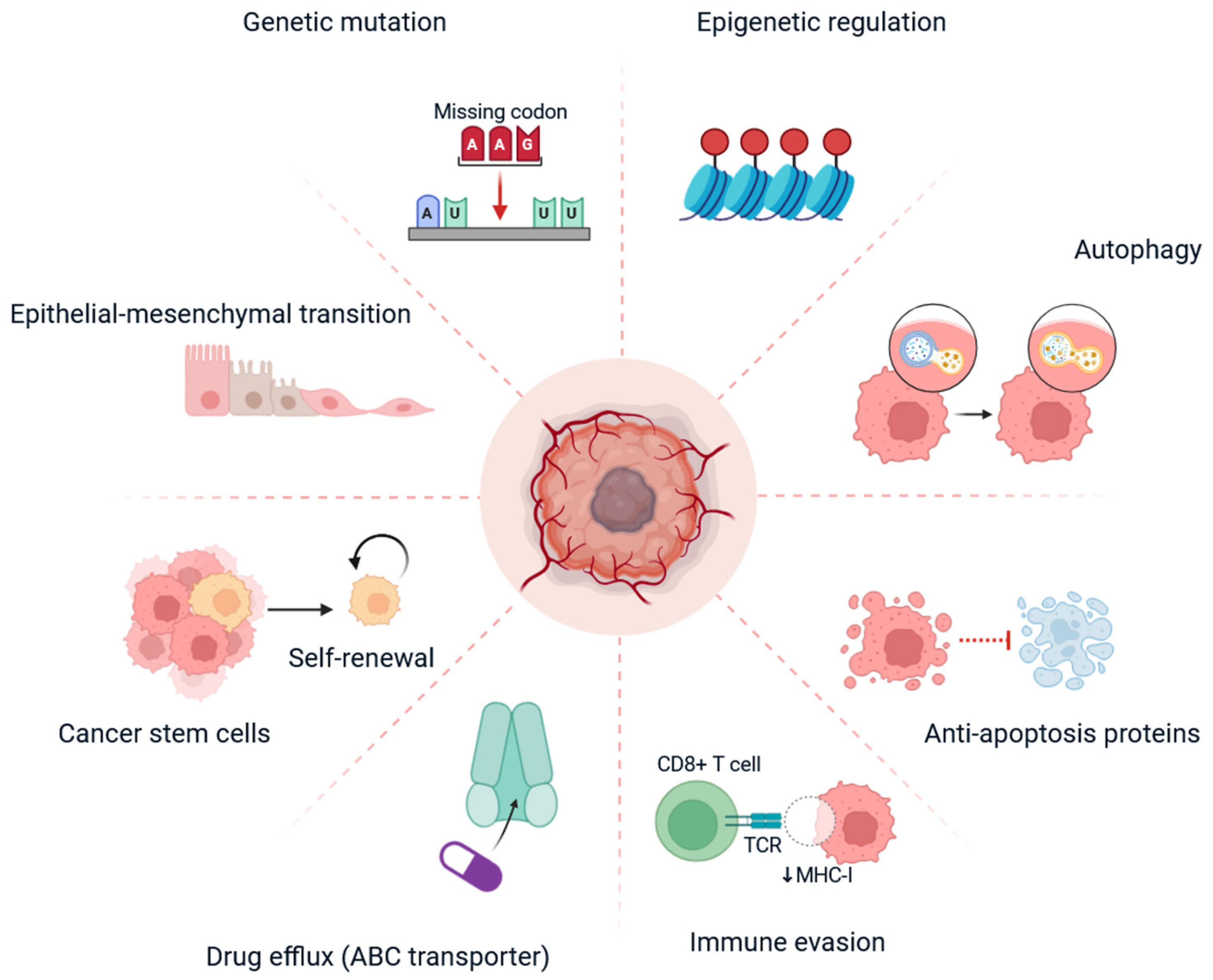
3. Mechanisms of Drug Resistance in TNBC
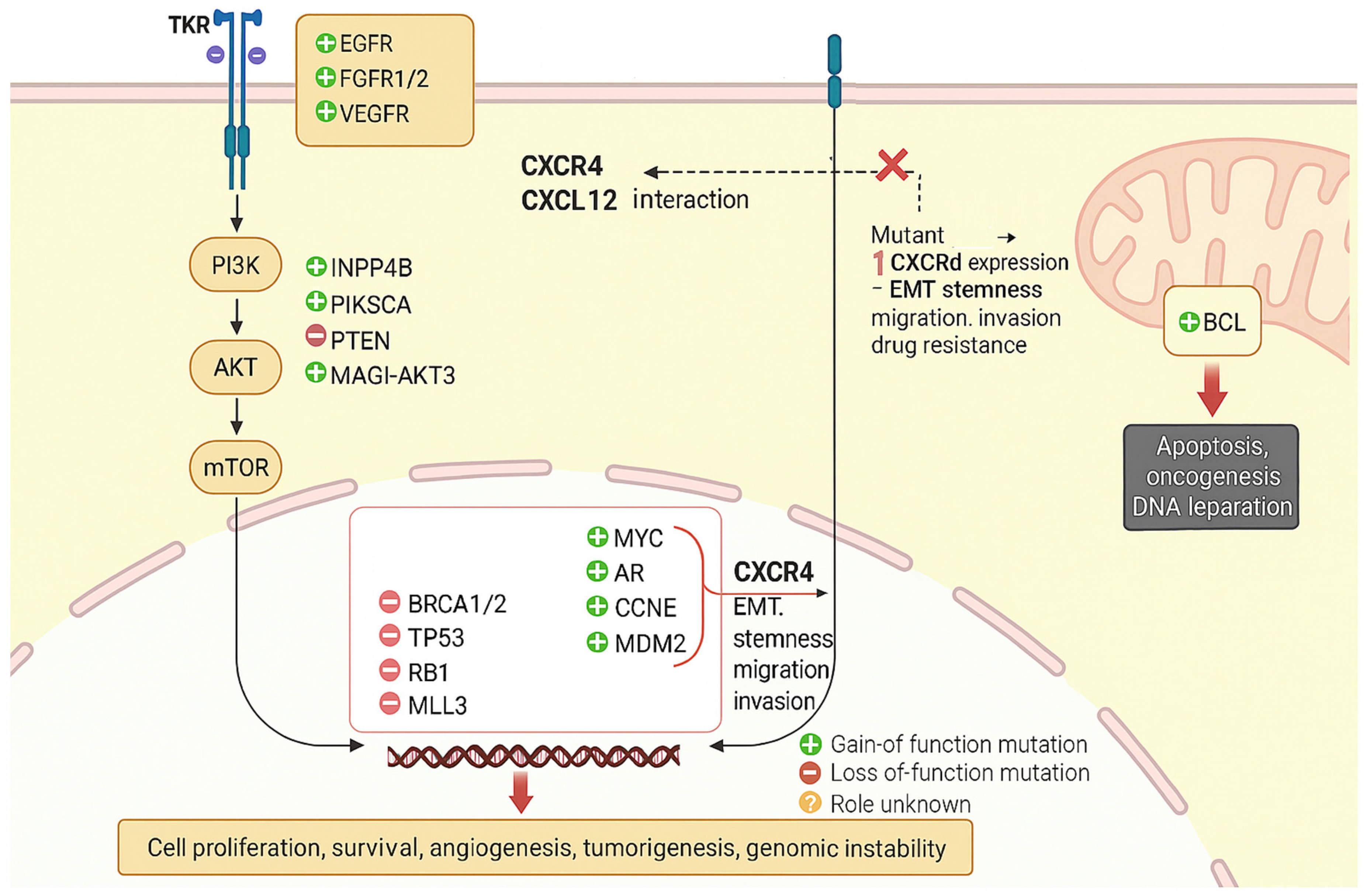
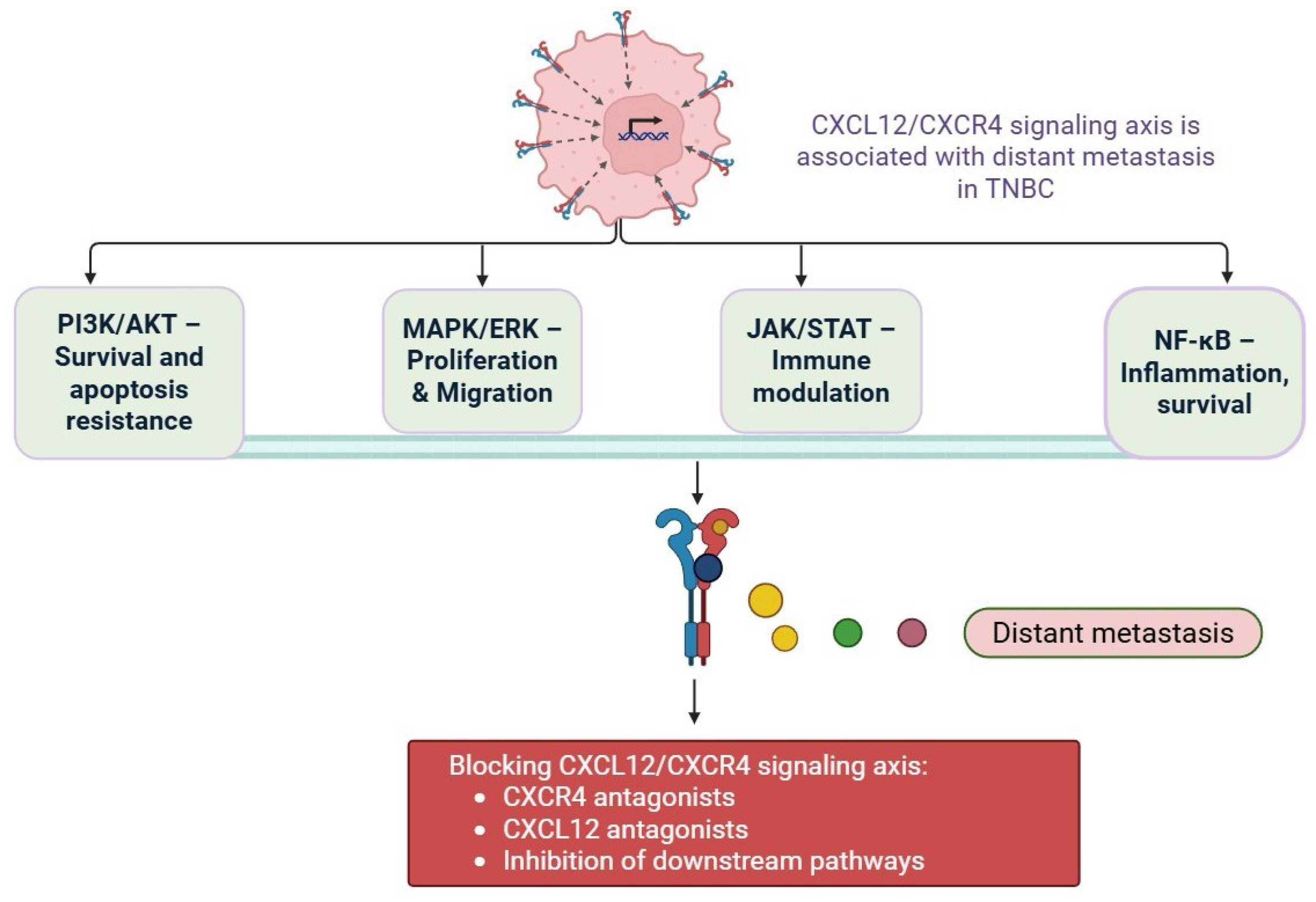
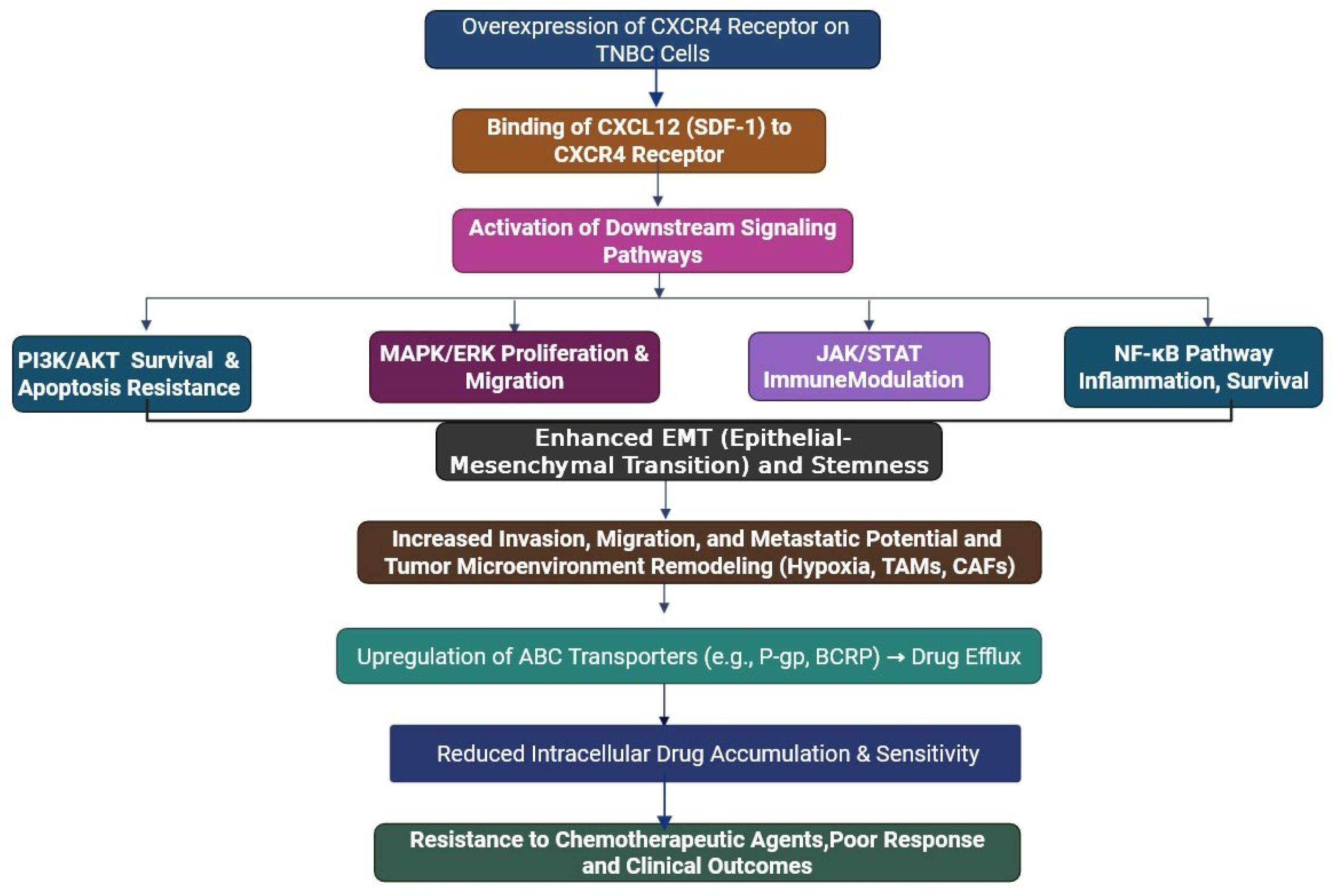
4. Mechanism of CXCR4/CXCL12 Axis in Drug Resistance in Triple-Negative Breast Cancer
5. Activation of Survival
5.1. Induction of Epithelial–Mesenchymal Transition (EMT)
5.2. Maintenance and Expansion of Cancer Stem Cells (CSCs)
5.3. Modulation of the Tumour Microenvironment and Immune Evasion
5.4. Promotion of Hypoxia-Induced Resistance
5.5. Facilitation of Tumour Cell Dormancy and Metastasis
6. Strategies to Overcome CXCR4/CXCL12-Mediated Drug Resistance in TNBC
6.1. CXCR4 Antagonists
6.2. Monoclonal Antibodies and Ligand Traps
6.3. Combination Therapies
6.4. CXCR4 Inhibitors with Chemotherapy
6.5. CXCR4 Blockade with Immune Checkpoint Inhibitors (ICIs)
6.6. CXCR4 Targeting with PARP Inhibitors
6.7. Radiotherapy or Anti-Angiogenic Agents with CXCR4 Inhibition
6.8. Novel Nanotechnological
7. Clinical Trials to Overcome Drug Resistance in Triple-Negative Breast Cancer
| S. No. | Trial/Agent | Phase | Combination | Target Population | Key Objectives | Status | References |
|---|---|---|---|---|---|---|---|
| 1 | CTCE-9908 | Phase I | Peptide-based CXCL12 inhibitor | Solid tumours, including breast cancer | Safety and pharmacokinetics | Completed (no TNBC-specific results published) | [148] |
| 2 | POL5551 + Eribulin | Phase I | CXCR4 antagonist + chemotherapy | Advanced breast cancer | Dose escalation, safety, preliminary anti-tumour activity | Initiated | [135] |
| 3 | Balixafortide (POL6326) + Eribulin | Phase III | CXCR4 antagonist + chemotherapy | HER2-negative MBC, including TNBC | Evaluate efficacy and safety vs. eribulin alone (PFS, OS) | Ongoing (FORTRESS Trial, NCT03786094) | [102] |
| 4 | NOX-A12 (Olaptesed Pegol) | Phase I/II | CXCL12 neutralisation + checkpoint inhibitor | Solid tumours and hematological cancers | Blockade of tumour-stroma interaction, immune enhancement | Completed (other cancers) | [103] |
| 5 | BL-8040 (Motixafortide) | Phase II | CXCR4 inhibitor + pembrolizumab | Pancreatic and other solid tumours | Evaluate immune activation and anti-tumour activity | Completed in other cancers, potential for TNBC | [147] |
| 6 | X4P-001 + Toripalimab | Phase I/II | CXCR4 inhibitor + PD-1 inhibitor | Solid tumours, including TNBC | Assess safety, tolerability, and preliminary efficacy | Ongoing | [146] |
| 7 | Plerixafor (AMD3100) + Radiotherapy | Preclinical | CXCR4 inhibitor + FAP-targeted radionuclide | TNBC and CAF-rich tumours | Deplete CAFs, enhance T-cell infiltration | Preclinical | [144] |
8. Limitations of CXCR4/CXCL12-Targeted Therapy in TNBC
8.1. Lack of Predictive Biomarkers
8.2. Drug Delivery and Pharmacokinetics
8.3. Off-Target Effects and Toxicity
8.4. Tumour Heterogeneity and Plasticity
8.5. Incomplete Clinical Validation
9. Future Directions for Targeting the CXCR4/CXCL12 Axis in TNBC
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNBC | Triple-Negative Breast Cancer |
| CXCR4 | C-X-C Chemokine Receptor Type 4 |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| SDF-1 | Stromal-Derived Factor-1 |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| GPCR | G-Protein-Coupled Receptor |
| TME | Tumour Microenvironment |
| EMT | Epithelial–Mesenchymal Transition |
| CSC | Cancer Stem Cell |
| ABC | ATP-Binding Cassette |
| PI3K | Phosphoinositide 3-Kinase |
| AKT | Protein Kinase B |
| MAPK/ERK | Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase |
| JAK/STAT | Janus Kinase/Signal Transducers and Activators of Transcription |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells |
| MHC-I | Major Histocompatibility Complex Class I |
| Tregs | Regulatory T Cells |
| MDSCs | Myeloid-Derived Suppressor Cells |
| TAMs | Tumour-Associated Macrophages |
| ICIs | Immune Checkpoint Inhibitors |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| HIF-1α | Hypoxia-Inducible Factor-1 Alpha |
| BRCA1/2 | Breast Cancer Gene 1/2 |
| TP53 | Tumour Protein p53 |
| PIK3CA | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha |
| PTEN | Phosphatase and Tensin Homolog |
| AR | Androgen Receptor |
| BCRP | Breast Cancer Resistance Protein |
| P-gp | P-glycoprotein |
| RTKs | Receptor Tyrosine Kinases |
| EGFR | Epidermal Growth Factor Receptor |
| FGFR1/2 | Fibroblast Growth Factor Receptor 1/2 |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
| PARP | Poly (ADP-ribose) Polymerase |
| ALDH1 | Aldehyde Dehydrogenase 1 |
| CTL | Cytotoxic T Lymphocyte |
| FAP | Fibroblast Activation Protein |
| AuNRs | Gold Nanorods |
| NIR | Near Infrared |
| mAbs | Monoclonal Antibodies |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| ORR | Objective Response Rate |
References
- Zagami, P.; Carey, L.A. Triple Negative Breast Cancer: Pitfalls and Progress. npj Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-Negative Breast Cancer: Is There a Treatment on the Horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef] [PubMed]
- Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Mahapatra, K.K.; Patra, S.; Mishra, S.R.; Behera, B.P.; Bhutia, S.K. Epigenetic Modifications of Autophagy in Cancer and Cancer Therapeutics. Semin. Cancer Biol. 2020, 66, 22–33. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Wang, W.; Pu, N.; Liu, L. Epithelial-Mesenchymal Transition Orchestrates Tumor Microenvironment: Current Perceptions and Challenges. J. Transl. Med. 2025, 23, 386. [Google Scholar] [CrossRef]
- Choi, Y.; Yu, A.-M. ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Xiong, N.; Wu, H.; Yu, Z. Advancements and Challenges in Triple-Negative Breast Cancer: A Comprehensive Review of Therapeutic and Diagnostic Strategies. Front. Oncol. 2024, 14, 1405491. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Triple-Negative Breast Cancer Therapeutic Resistance: Where Is the Achilles’ Heel? Cancer Lett. 2021, 497, 100–111. [Google Scholar] [CrossRef]
- Sabit, H.; Adel, A.; Abdelfattah, M.M.; Ramadan, R.M.; Nazih, M.; Abdel-Ghany, S.; El-hashash, A.; Arneth, B. The Role of Tumor Microenvironment and Immune Cell Crosstalk in Triple-Negative Breast Cancer (TNBC): Emerging Therapeutic Opportunities. Cancer Lett. 2025, 628, 217865. [Google Scholar] [CrossRef]
- Shi, Y.; Riese, D.J.; Shen, J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharmacol. 2020, 11, 574667. [Google Scholar] [CrossRef]
- Rueda, A.; Serna, N.; Mangues, R.; Villaverde, A.; Unzueta, U. Targeting the Chemokine Receptor CXCR4 for Cancer Therapies. Biomark. Res. 2025, 13, 68. [Google Scholar] [CrossRef]
- Mortezaee, K. CXCL12/CXCR4 Axis in the Microenvironment of Solid Tumors: A Critical Mediator of Metastasis. Life Sci. 2020, 249, 117534. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Lei, W.; Wang, H.; Ni, Y.; Liu, Y.; Yan, H.; Tian, Y.; Wang, Z.; Yang, Z.; et al. CXCL12-CXCR4/CXCR7 Axis in Cancer: From Mechanisms to Clinical Applications. Int. J. Biol. Sci. 2023, 19, 3341–3359. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, A.Q.; Altalbawy, F.M.A.; Jabir, M.S.; Hasan, T.F.; Jain, V.; Abbot, V.; Nakash, P.; Kumar, M.R.; Mustafa, Y.F.; Jawad, M.A. CXCR4/CXCL12 Blockade Therapy; a New Horizon in TNBC Therapy. Med. Oncol. 2025, 42, 161. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Peng, X.; Li, X.; Yang, P.; Xie, L.; Li, Y.; Du, C.; Zhang, G. Silencing of CXCR4 Sensitizes Triple-Negative Breast Cancer Cells to Cisplatin. Oncotarget 2015, 6, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Han, S. Targeting Cancer Stem Cell Plasticity in Triple-Negative Breast Cancer. Explor. Target. Anti-Tumor Ther. 2023, 4, 1165–1181. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Oupický, D. Potential of CXCR4/CXCL12 Chemokine Axis in Cancer Drug Delivery. Curr. Pharmacol. Rep. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- De Clercq, E. Recent Advances on the Use of the CXCR4 Antagonist Plerixafor (AMD3100, MozobilTM) and Potential of Other CXCR4 Antagonists as Stem Cell Mobilizers. Pharmacol. Ther. 2010, 128, 509–518. [Google Scholar] [CrossRef]
- Li, Z.; Lu, H.; Zhang, Y.; Lv, J.; Zhang, Y.; Xu, T.; Yang, D.; Duan, Z.; Guan, Y.; Jiang, Z.; et al. Blocking CXCR4-CARM1-YAP Axis Overcomes Osteosarcoma Doxorubicin Resistance by Suppressing Aerobic Glycolysis. Cancer Sci. 2024, 115, 3305–3319. [Google Scholar] [CrossRef]
- Mezzapelle, R.; Leo, M.; Caprioglio, F.; Colley, L.S.; Lamarca, A.; Sabatino, L.; Colantuoni, V.; Crippa, M.P.; Bianchi, M.E. CXCR4/CXCL12 Activities in the Tumor Microenvironment and Implications for Tumor Immunotherapy. Cancers 2022, 14, 2314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 Axis in Tumor Immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [CrossRef] [PubMed]
- Romain, B.; Hachet-Haas, M.; Rohr, S.; Brigand, C.; Galzi, J.-L.; Gaub, M.-P.; Pencreach, E.; Guenot, D. Hypoxia Differentially Regulated CXCR4 and CXCR7 Signaling in Colon Cancer. Mol. Cancer 2014, 13, 58. [Google Scholar] [CrossRef]
- Lefort, S.; Thuleau, A.; Kieffer, Y.; Sirven, P.; Bieche, I.; Marangoni, E.; Vincent-Salomon, A.; Mechta-Grigoriou, F. CXCR4 Inhibitors Could Benefit to HER2 but Not to Triple-Negative Breast Cancer Patients. Oncogene 2017, 36, 1211–1222. [Google Scholar] [CrossRef]
- Pradhan, R.; Dey, A.; Taliyan, R.; Puri, A.; Kharavtekar, S.; Dubey, S.K. Recent Advances in Targeted Nanocarriers for the Management of Triple Negative Breast Cancer. Pharmaceutics 2023, 15, 246. [Google Scholar] [CrossRef]
- Gupta, N.; Mohan, C.D.; Shanmugam, M.K.; Jung, Y.Y.; Chinnathambi, A.; Alharbi, S.A.; Ashrafizadeh, M.; Mahale, M.; Bender, A.; Kumar, A.P.; et al. CXCR4 Expression Is Elevated in TNBC Patient Derived Samples and Z-Guggulsterone Abrogates Tumor Progression by Targeting CXCL12/CXCR4 Signaling Axis in Preclinical Breast Cancer Model. Environ. Res. 2023, 232, 116335. [Google Scholar] [CrossRef]
- El Hejjioui, B.; Lamrabet, S.; Amrani Joutei, S.; Senhaji, N.; Bouhafa, T.; Malhouf, M.A.; Bennis, S.; Bouguenouch, L. New Biomarkers and Treatment Advances in Triple-Negative Breast Cancer. Diagnostics 2023, 13, 1949. [Google Scholar] [CrossRef]
- Santagata, S.; Ieranò, C.; Trotta, A.M.; Capiluongo, A.; Auletta, F.; Guardascione, G.; Scala, S. CXCR4 and CXCR7 Signaling Pathways: A Focus on the Cross-Talk Between Cancer Cells and Tumor Microenvironment. Front. Oncol. 2021, 11, 591386. [Google Scholar] [CrossRef]
- Smaldone, G.; Di Matteo, F.; Castelluccio, R.; Napolitano, V.; Miranda, M.R.; Manfra, M.; Campiglia, P.; Vestuto, V. Targeting the CXCR4/CXCL12 Axis in Cancer Therapy: Analysis of Recent Advances in the Development of Potential Anticancer Agents. Molecules 2025, 30, 1380. [Google Scholar] [CrossRef]
- Bao, G.; Wang, Z.; Liu, L.; Zhang, B.; Song, S.; Wang, D.; Cheng, S.; Moon, E.-S.; Roesch, F.; Zhao, J.; et al. Targeting CXCR4/CXCL12 Axis via [177Lu]Lu-DOTAGA.(SA.FAPi)2 with CXCR4 Antagonist in Triple-Negative Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2744–2757. [Google Scholar] [CrossRef]
- Toft, D.J.; Cryns, V.L. Minireview: Basal-Like Breast Cancer: From Molecular Profiles to Targeted Therapies. Mol. Endocrinol. 2011, 25, 199–211. [Google Scholar] [CrossRef]
- Sporikova, Z.; Koudelakova, V.; Trojanec, R.; Hajduch, M. Genetic Markers in Triple-Negative Breast Cancer. Clin. Breast Cancer 2018, 18, e841–e850. [Google Scholar] [CrossRef]
- Soung, Y.H.; Ju, J.; Chung, J. The Sensitization of Triple-Negative Breast Cancers to Poly ADP Ribose Polymerase Inhibition Independent of BRCA1/2 Mutation Status by Chemically Modified microRNA-489. Cells 2023, 13, 49. [Google Scholar] [CrossRef]
- Marvalim, C.; Datta, A.; Lee, S.C. Role of P53 in Breast Cancer Progression: An Insight into P53 Targeted Therapy. Theranostics 2023, 13, 1421–1442. [Google Scholar] [CrossRef]
- Garg, P.; Ramisetty, S.; Nair, M.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Strategic Advancements in Targeting the PI3K/AKT/mTOR Pathway for Breast Cancer Therapy. Biochem. Pharmacol. 2025, 236, 116850. [Google Scholar] [CrossRef]
- Chatterjee, S.; Behnam Azad, B.; Nimmagadda, S. The Intricate Role of CXCR4 in Cancer. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 124, pp. 31–82. ISBN 978-0-12-411638-2. [Google Scholar]
- Lei, Z.; Tian, Q.; Teng, Q.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z. Understanding and Targeting Resistance Mechanisms in Cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Current Treatment Landscape for Early Triple-Negative Breast Cancer (TNBC). J. Clin. Med. 2023, 12, 1524. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast Cancer Subtypes Based on ER/PR and Her2 Expression: Comparison of Clinicopathologic Features and Survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Skverchinskaya, E.; Levdarovich, N.; Ivanov, A.; Mindukshev, I.; Bukatin, A. Anticancer Drugs Paclitaxel, Carboplatin, Doxorubicin, and Cyclophosphamide Alter the Biophysical Characteristics of Red Blood Cells, In Vitro. Biology 2023, 12, 230. [Google Scholar] [CrossRef]
- Ouedraogo, S.Y.; Zoure, A.A.; Zeye, M.M.J.; Kiendrebeogo, T.I.; Zhou, X.; Sawadogo, A.Y.; Simpore, J.; Chen, H. BRCA1, BRCA2, TP53, PIK3CA, PTEN and AKT1 Genes Mutations in Burkina Faso Breast Cancer Patients: Prevalence, Spectrum and Novel Variant. Mol. Genet. Genom. 2022, 297, 1257–1268. [Google Scholar] [CrossRef]
- Gunaydin, G. CAFs Interacting With TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front. Oncol. 2021, 11, 668349. [Google Scholar] [CrossRef]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, C.R.; Long, J.; Laidler, P.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stivala, F.; Mazzarino, M.C.; et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR Pathways in Controlling Growth and Sensitivity to Therapy-Implications for Cancer and Aging. Aging 2011, 3, 192–222. [Google Scholar] [CrossRef]
- Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.-I.; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth Factors, PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, H.; Chen, P.; Tang, J.; Yang, S.; Nicot, C.; Guan, Z.; Li, X.; Tang, H. Clinical Approaches to Overcome PARP Inhibitor Resistance. Mol. Cancer 2025, 24, 156. [Google Scholar] [CrossRef] [PubMed]
- Błaszczak, E.; Miziak, P.; Odrzywolski, A.; Baran, M.; Gumbarewicz, E.; Stepulak, A. Triple-Negative Breast Cancer Progression and Drug Resistance in the Context of Epithelial–Mesenchymal Transition. Cancers 2025, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Shyanti, R.K.; Mishra, M.K. Targeted Therapy Approaches for Epithelial-Mesenchymal Transition in Triple Negative Breast Cancer. Front. Oncol. 2024, 14, 1431418. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Park, S.-Y.; Choi, J.-H.; Nam, J.-S. Targeting Cancer Stem Cells in Triple-Negative Breast Cancer. Cancers 2019, 11, 965. [Google Scholar] [CrossRef]
- Kumar, H.; Gupta, N.V.; Jain, R.; Madhunapantula, S.V.; Babu, C.S.; Kesharwani, S.S.; Dey, S.; Jain, V. A Review of Biological Targets and Therapeutic Approaches in the Management of Triple-Negative Breast Cancer. J. Adv. Res. 2023, 54, 271–292. [Google Scholar] [CrossRef]
- Sharma, P.; Barlow, W.E.; Godwin, A.K.; Pathak, H.; Isakova, K.; Williams, D.; Timms, K.M.; Hartman, A.R.; Wenstrup, R.J.; Linden, H.M.; et al. Impact of Homologous Recombination Deficiency Biomarkers on Outcomes in Patients with Triple-Negative Breast Cancer Treated with Adjuvant Doxorubicin and Cyclophosphamide (SWOG S9313). Ann. Oncol. 2018, 29, 654–660. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Khan, F.; Verma, B.; Sinha, P.; Dmello, C.C.; Jogalekar, M.P.; Gangadaran, P.; Ahn, B. Tumor Microenvironment Signaling and Therapeutics in Cancer Progression. Cancer Commun. 2023, 43, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, L.; Mahalingaiah, P.K.S.; Chang, Y.-W.; Singh, K.P. Role of Cellular Reprogramming and Epigenetic Dysregulation in Acquired Chemoresistance in Breast Cancer. Cancer Drug Resist. 2019, 2, 297. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-D.; Pang, K.; Wu, Z.-X.; Dong, Y.; Hao, L.; Qin, J.-X.; Wang, W.; Chen, Z.-S.; Han, C.-H. Tumor Cell Plasticity in Targeted Therapy-Induced Resistance: Mechanisms and New Strategies. Signal Transduct. Target. Ther. 2023, 8, 113. [Google Scholar] [CrossRef]
- Nguyen, K.T.P.; Druhan, L.J.; Avalos, B.R.; Zhai, L.; Rauova, L.; Nesmelova, I.V.; Dréau, D. CXCL12-CXCL4 Heterodimerization Prevents CXCL12-Driven Breast Cancer Cell Migration. Cell. Signal. 2020, 66, 109488. [Google Scholar] [CrossRef]
- Garg, P.; Jallepalli, V.R.; Verma, S. Unravelling the CXCL12/CXCR4 Axis in Breast Cancer: Insights into Metastasis, Microenvironment Interactions, and Therapeutic Opportunities. Hum. Gene 2024, 40, 201272. [Google Scholar] [CrossRef]
- Yang, P.; Hu, Y.; Zhou, Q. The CXCL12-CXCR4 Signaling Axis Plays a Key Role in Cancer Metastasis and Is a Potential Target for Developing Novel Therapeutics against Metastatic Cancer. Curr. Med. Chem. 2020, 27, 5543–5561. [Google Scholar] [CrossRef]
- Zielińska, K.A.; Katanaev, V.L. The Signaling Duo CXCL12 and CXCR4: Chemokine Fuel for Breast Cancer Tumorigenesis. Cancers 2020, 12, 3071. [Google Scholar] [CrossRef]
- Anastasiadou, D.P.; Quesnel, A.; Duran, C.L.; Filippou, P.S.; Karagiannis, G.S. An Emerging Paradigm of CXCL12 Involvement in the Metastatic Cascade. Cytokine Growth Factor Rev. 2024, 75, 12–30. [Google Scholar] [CrossRef]
- Jung, Y.-D.; Shim, J.-W.; Park, S.-J.; Choi, S.H.; Yang, K.; Heo, K.; Park, M.-T. Downregulation of UHRF1 Promotes EMT via Inducing CXCR4 in Human Cancer Cells. Int. J. Oncol. 2015, 46, 1232–1242. [Google Scholar] [CrossRef]
- Makena, M.R.; Ranjan, A.; Thirumala, V.; Reddy, A.P. Cancer Stem Cells: Road to Therapeutic Resistance and Strategies to Overcome Resistance. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165339. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Chen, C.; Huang, H.; Zhang, Z.; Zeng, F.; Zhang, S. Hypoxia-Inducible Factor in Breast Cancer: Role and Target for Breast Cancer Treatment. Front. Immunol. 2024, 15, 1370800. [Google Scholar] [CrossRef] [PubMed]
- Kiss, R.C.; Xia, F.; Acklin, S. Targeting DNA Damage Response and Repair to Enhance Therapeutic Index in Cisplatin-Based Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 8199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; Liu, Z.; Yang, L.; Jiang, Q.; Cheng, Q.; et al. Define Cancer-Associated Fibroblasts (CAFs) in the Tumor Microenvironment: New Opportunities in Cancer Immunotherapy and Advances in Clinical Trials. Mol. Cancer 2023, 22, 159. [Google Scholar] [CrossRef]
- Mollaei, M.; Hassan, Z.M.; Khorshidi, F.; Langroudi, L. Chemotherapeutic Drugs: Cell Death- and Resistance-Related Signaling Pathways. Are They Really as Smart as the Tumor Cells? Transl. Oncol. 2021, 14, 101056. [Google Scholar] [CrossRef]
- Yen, J.-H.; Chang, C.-C.; Hsu, H.-J.; Yang, C.-H.; Mani, H.; Liou, J.-W. C-X-C Motif Chemokine Ligand 12―C-X-C Chemokine Receptor Type 4 Signaling Axis in Cancer and the Development of Chemotherapeutic Molecules. Tzu Chi Med. J. 2024, 36, 231–239. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Kim, D.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.-H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and Triple-Negative Breast Cancer: Challenges and Treatment Options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef]
- Subramaniyan, B.; Sridharan, S.; Howard, C.M.; Tilley, A.M.C.; Basuroy, T.; De La Serna, I.; Butt, E.; Raman, D. Role of the CXCR4-LASP1 Axis in the Stabilization of Snail1 in Triple-Negative Breast Cancer. Cancers 2020, 12, 2372. [Google Scholar] [CrossRef] [PubMed]
- Liaghat, M.; Ferdousmakan, S.; Mortazavi, S.H.; Yahyazadeh, S.; Irani, A.; Banihashemi, S.; Seyedi Asl, F.S.; Akbari, A.; Farzam, F.; Aziziyan, F.; et al. The Impact of Epithelial-Mesenchymal Transition (EMT) Induced by Metabolic Processes and Intracellular Signaling Pathways on Chemo-Resistance, Metastasis, and Recurrence in Solid Tumors. Cell Commun. Signal. 2024, 22, 575. [Google Scholar] [CrossRef]
- Grasset, E.M.; Dunworth, M.; Sharma, G.; Loth, M.; Tandurella, J.; Cimino-Mathews, A.; Gentz, M.; Bracht, S.; Haynes, M.; Fertig, E.J.; et al. Triple-Negative Breast Cancer Metastasis Involves Complex Epithelial-Mesenchymal Transition Dynamics and Requires Vimentin. Sci. Transl. Med. 2022, 14, eabn7571. [Google Scholar] [CrossRef] [PubMed]
- Cojoc, M.; Peitzsch, C.; Trautmann, F.; Polishchuk, L.; Telegeev, G.D.; Dubrovska, A. Emerging Targets in Cancer Management: Role of the CXCL12/CXCR4 Axis. OncoTargets Ther. 2013, 6, 1347–1361. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, X.; Prabhu, J.S.; Pandey, V. Therapeutic Vulnerabilities in Triple Negative Breast Cancer: Stem-like Traits Explored within Molecular Classification. Biomed. Pharmacother. 2024, 174, 116584. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhou, F.; Jin, H.; Wu, X. Crosstalk between CXCL12/CXCR4/ACKR3 and the STAT3 Pathway. Cells 2024, 13, 1027. [Google Scholar] [CrossRef]
- Song, K.; Farzaneh, M. Signaling Pathways Governing Breast Cancer Stem Cells Behavior. Stem Cell Res. Ther. 2021, 12, 245. [Google Scholar] [CrossRef]
- Mengistu, B.A.; Tsegaw, T.; Demessie, Y.; Getnet, K.; Bitew, A.B.; Kinde, M.Z.; Beirhun, A.M.; Mebratu, A.S.; Mekasha, Y.T.; Feleke, M.G.; et al. Comprehensive Review of Drug Resistance in Mammalian Cancer Stem Cells: Implications for Cancer Therapy. Cancer Cell Int. 2024, 24, 406. [Google Scholar] [CrossRef]
- Sun, H.-R.; Wang, S.; Yan, S.-C.; Zhang, Y.; Nelson, P.J.; Jia, H.-L.; Qin, L.-X.; Dong, Q.-Z. Therapeutic Strategies Targeting Cancer Stem Cells and Their Microenvironment. Front. Oncol. 2019, 9, 1104. [Google Scholar] [CrossRef]
- Domanska, U.M.; Kruizinga, R.C.; Nagengast, W.B.; Timmer-Bosscha, H.; Huls, G.; De Vries, E.G.E.; Walenkamp, A.M.E. A Review on CXCR4/CXCL12 Axis in Oncology: No Place to Hide. Eur. J. Cancer 2013, 49, 219–230. [Google Scholar] [CrossRef]
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 2020, 6283796. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, D.; Tan, S.; Li, H.; Zhou, F.; Qiu, L.; Xie, X.; Wu, X. CXCL12 Alone Is Enough to Reprogram Normal Fibroblasts into Cancer-Associated Fibroblasts. Cell Death Discov. 2025, 11, 156. [Google Scholar] [CrossRef]
- Chen, Y.; Ramjiawan, R.R.; Reiberger, T.; Ng, M.R.; Hato, T.; Huang, Y.; Ochiai, H.; Kitahara, S.; Unan, E.C.; Reddy, T.P.; et al. CXCR4 Inhibition in Tumor Microenvironment Facilitates Anti-programmed Death Receptor-1 Immunotherapy in Sorafenib-treated Hepatocellular Carcinoma in Mice. Hepatology 2015, 61, 1591–1602. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, B.; Liang, Y.; Reeves, P.M.; Qu, X.; Ran, C.; Liu, Q.; Callahan, M.V.; Sluder, A.E.; Gelfand, J.A.; et al. Dual Blockade of CXCL12-CXCR4 and PD-1–PD-L1 Pathways Prolongs Survival of Ovarian Tumor–Bearing Mice by Prevention of Immunosuppression in the Tumor Microenvironment. FASEB J. 2019, 33, 6596–6608. [Google Scholar] [CrossRef]
- Chaudary, N.; Hill, R.P.; Milosevic, M. Targeting the CXCL12/CXCR4 Pathway to Reduce Radiation Treatment Side Effects. Radiother. Oncol. 2024, 194, 110194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Sun, Z.-J. Turning Cold Tumors into Hot Tumors by Improving T-Cell Infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Martin, S.K.; Diamond, P.; Williams, S.A.; To, L.B.; Peet, D.J.; Fujii, N.; Gronthos, S.; Harris, A.L.; Zannettino, A.C.W. Hypoxia-Inducible Factor-2 Is a Novel Regulator of Aberrant CXCL12 Expression in Multiple Myeloma Plasma Cells. Haematologica 2010, 95, 776–784. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and Drug Resistance: The Mechanistic Link and Clinical Implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, J.; Zhang, Y.; Guo, Q. Drug Resistance and Tumor Immune Microenvironment: An Overview of Current Understandings (Review). Int. J. Oncol. 2024, 65, 96. [Google Scholar] [CrossRef]
- Conley-LaComb, M.K.; Semaan, L.; Singareddy, R.; Li, Y.; Heath, E.I.; Kim, S.; Cher, M.L.; Chinni, S.R. Pharmacological Targeting of CXCL12/CXCR4 Signaling in Prostate Cancer Bone Metastasis. Mol. Cancer 2016, 15, 68. [Google Scholar] [CrossRef]
- Misra, A.C.; Luker, K.E.; Durmaz, H.; Luker, G.D.; Lahann, J. CXCR4-Targeted Nanocarriers for Triple Negative Breast Cancers. Biomacromolecules 2015, 16, 2412–2417. [Google Scholar] [CrossRef]
- Chittasupho, C.; Anuchapreeda, S.; Sarisuta, N. CXCR4 Targeted Dendrimer for Anti-Cancer Drug Delivery and Breast Cancer Cell Migration Inhibition. Eur. J. Pharm. Biopharm. 2017, 119, 310–321. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J.; Taichman, R.S.; Pienta, K.J.; Wang, J. CXCL12/CXCR4/CXCR7 Chemokine Axis and Cancer Progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G. Mechanisms Governing Metastatic Dormancy and Reactivation. Cell 2013, 155, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, C.; Yang, H.; Chen, G.; Wang, K.; Ji, P.; Sun, X.; Fan, X.; Ma, J.; Cui, Z.; et al. A Dual-Targeting Peptide–Drug Conjugate Based on CXCR4 and FOLR1 Inhibits Triple-Negative Breast Cancer. Acta Pharm. Sin. B, 2025; in press. [Google Scholar] [CrossRef]
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 Axis as a Mechanism of Immune Resistance in Gastrointestinal Malignancies. Semin. Cancer Biol. 2020, 65, 176–188. [Google Scholar] [CrossRef]
- Karpova, D.; Dauber, K.; Spohn, G.; Chudziak, D.; Wiercinska, E.; Schulz, M.; Pettit, A.R.; Levesque, J.P.; Romagnoli, B.; Patel, K.; et al. The Novel CXCR4 Antagonist POL5551 Mobilizes Hematopoietic Stem and Progenitor Cells with Greater Efficiency than Plerixafor. Leukemia 2013, 27, 2322–2331. [Google Scholar] [CrossRef]
- Lu, G.; Qiu, Y.; Su, X. Targeting CXCL12-CXCR4 Signaling Enhances Immune Checkpoint Blockade Therapy Against Triple Negative Breast Cancer. Eur. J. Pharm. Sci. 2021, 157, 105606. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Zhang, W.; Yu, B.; Cen, Y.; Liu, Q.; Tang, Y.; Li, S. A CXCR4-Targeted Immunomodulatory Nanomedicine for Photodynamic Amplified Immune Checkpoint Blockade Therapy against Breast Cancer. Acta Biomater. 2025, 197, 400–415. [Google Scholar] [CrossRef]
- Pernas, S.; Martin, M.; Kaufman, P.A.; Gil-Martin, M.; Gomez Pardo, P.; Lopez-Tarruella, S.; Manso, L.; Ciruelos, E.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide plus Eribulin in HER2-Negative Metastatic Breast Cancer: A Phase 1, Single-Arm, Dose-Escalation Trial. Lancet Oncol. 2018, 19, 812–824. [Google Scholar] [CrossRef]
- Steurer, M.; Montillo, M.; Scarfò, L.; Mauro, F.R.; Andel, J.; Wildner, S.; Trentin, L.; Janssens, A.; Burgstaller, S.; Frömming, A.; et al. Olaptesed Pegol (NOX-A12) with Bendamustine and Rituximab: A Phase IIa Study in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Haematologica 2019, 104, 2053–2060. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, T.; Qian, D.; Liu, X.; Xu, Y.; Hong, W.; Meng, X.; Tang, H. Z-Guggulsterone Induces Cell Cycle Arrest and Apoptosis by Targeting the P53/CCNB1/PLK1 Pathway in Triple-Negative Breast Cancer. ACS Omega 2023, 8, 2780–2792. [Google Scholar] [CrossRef]
- Gupta, A.; Nishchaya, K.; Saha, M.; Naik, G.A.R.R.; Yadav, S.; Srivastava, S.; Roy, A.A.; Moorkoth, S.; Mutalik, S.; Dhas, N. Recent Advancements in Nanoconstructs for the Theranostics Applications for Triple Negative Breast Cancer. J. Drug Deliv. Sci. Technol. 2024, 93, 105401. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Fei, X.; Chen, L.; Yao, L.; Lei, X. Potential Therapeutic Targets of the JAK2/STAT3 Signaling Pathway in Triple-Negative Breast Cancer. Front. Oncol. 2024, 14, 1381251. [Google Scholar] [CrossRef] [PubMed]
- Massihnia, D.; Galvano, A.; Fanale, D.; Perez, A.; Castiglia, M.; Incorvaia, L.; Listì, A.; Rizzo, S.; Cicero, G.; Bazan, V.; et al. Triple Negative Breast Cancer: Shedding Light onto the Role of Pi3k/Akt/Mtor Pathway. Oncotarget 2016, 7, 60712–60722. [Google Scholar] [CrossRef]
- Pusic, I.; DiPersio, J.F. Update on Clinical Experience with AMD3100, an SDF-1/CXCL12–CXCR4 Inhibitor, in Mobilization of Hematopoietic Stem and Progenitor Cells. Curr. Opin. Hematol. 2010, 17, 319–326. [Google Scholar] [CrossRef]
- Russell, N.; Douglas, K.; Ho, A.D.; Mohty, M.; Carlson, K.; Ossenkoppele, G.J.; Milone, G.; Pareja, M.O.; Shaheen, D.; Willemsen, A.; et al. Plerixafor and Granulocyte Colony-Stimulating Factor for First-Line Steady-State Autologous Peripheral Blood Stem Cell Mobilization in Lymphoma and Multiple Myeloma: Results of the Prospective PREDICT Trial. Haematologica 2013, 98, 172–178. [Google Scholar] [CrossRef]
- Ciavattone, N.G.; Bevoor, A.; Farfel, A.; Rehman, A.; Ho, K.K.Y.; Rock, E.C.; Chen, Y.-C.; Luker, K.E.; Humphries, B.A.; Luker, G.D. Inhibiting CXCR4 Reduces Immunosuppressive Effects of Myeloid Cells in Breast Cancer Immunotherapy. Sci. Rep. 2025, 15, 5204. [Google Scholar] [CrossRef]
- Gil-Martin, M.; Gomez Pardo, P.; Lopez-Tarruella, S.; Manso, L.; Perez-Fidalgo, J.A.; Ademuyiwa, F.O.; Mayer, I.A.; Pluard, T.J.; Martinez Garcia, M.; Kaufman, P.A.; et al. Phase I Study of the Combination of Balixafortide (CXCR4 Inhibitor) and Eribulin in HER2-Negative Metastatic Breast Cancer (MBC) Patients (Pts). J. Clin. Oncol. 2017, 35, 2555. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Liu, C.-J.; Redd, R.A.; Perez, R.P.; Baz, R.; Zavidij, O.; Sklavenitis-Pistofidis, R.; Richardson, P.G.; Anderson, K.C.; Laubach, J.; et al. A Phase Ib/II Trial of the First-in-Class Anti-CXCR4 Antibody Ulocuplumab in Combination with Lenalidomide or Bortezomib Plus Dexamethasone in Relapsed Multiple Myeloma. Clin. Cancer Res. 2020, 26, 344–353. [Google Scholar] [CrossRef]
- Liu, G.; Chen, T.; Zhang, X.; Hu, B.; Shi, H. Immune Checkpoint Inhibitor-Associated Cardiovascular Toxicities: A Review. Heliyon 2024, 10, e25747. [Google Scholar] [CrossRef]
- Cambier, S.; Gouwy, M.; Proost, P. The Chemokines CXCL8 and CXCL12: Molecular and Functional Properties, Role in Disease and Efforts towards Pharmacological Intervention. Cell. Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Duda, D.G.; Kozin, S.V.; Kirkpatrick, N.D.; Xu, L.; Fukumura, D.; Jain, R.K. CXCL12 (SDF1α)-CXCR4/CXCR7 Pathway Inhibition: An Emerging Sensitizer for Anticancer Therapies? Clin. Cancer Res. 2011, 17, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, L.; Huang, Y.; Wu, Y.; Xie, N. Mechanisms and Strategies to Overcome PD-1/PD-L1 Blockade Resistance in Triple-Negative Breast Cancer. Cancers 2022, 15, 104. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Breaking Barriers: The Promise and Challenges of Immune Checkpoint Inhibitors in Triple-Negative Breast Cancer. Biomedicines 2024, 12, 369. [Google Scholar] [CrossRef]
- Imani, S.; Farghadani, R.; Roozitalab, G.; Maghsoudloo, M.; Emadi, M.; Moradi, A.; Abedi, B.; Jabbarzadeh Kaboli, P. Reprogramming the Breast Tumor Immune Microenvironment: Cold-to-Hot Transition for Enhanced Immunotherapy. J. Exp. Clin. Cancer Res. 2025, 44, 131. [Google Scholar] [CrossRef]
- Brown, J.S.; O’Carrigan, B.; Jackson, S.P.; Yap, T.A. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 2017, 7, 20–37. [Google Scholar] [CrossRef]
- Khan, M.A.; Srivastava, S.K.; Zubair, H.; Patel, G.K.; Arora, S.; Khushman, M.; Carter, J.E.; Gorman, G.S.; Singh, S.; Singh, A.P. Co-Targeting of CXCR4 and Hedgehog Pathways Disrupts Tumor-Stromal Crosstalk and Improves Chemotherapeutic Efficacy in Pancreatic Cancer. J. Biol. Chem. 2020, 295, 8413–8424. [Google Scholar] [CrossRef]
- Gagner, J.-P.; Sarfraz, Y.; Ortenzi, V.; Alotaibi, F.M.; Chiriboga, L.A.; Tayyib, A.T.; Douglas, G.J.; Chevalier, E.; Romagnoli, B.; Tuffin, G.; et al. Multifaceted C-X-C Chemokine Receptor 4 (CXCR4) Inhibition Interferes with Anti–Vascular Endothelial Growth Factor Therapy–Induced Glioma Dissemination. Am. J. Pathol. 2017, 187, 2080–2094. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, J.; Li, Z.; Zhang, W.; Wang, F.; Zhang, B. Recent Advances in CXCL12/CXCR4 Antagonists and Nano-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1541. [Google Scholar] [CrossRef]
- Meng, Y.; Zhou, J.; Liu, X.; Zeng, F.; Wen, T.; Meng, J.; Liu, J.; Xu, H. CXC Chemokine Receptor Type 4 Antagonistic Gold Nanorods Induce Specific Immune Responses and Long-Term Immune Memory to Combat Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2023, 15, 18734–18746. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Anandasabapathy, N. The Role of Dendritic Cells in Cancer and Anti-Tumor Immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Yu, M.; Zhou, Q.; Wang, J.; Chen, S.; Gong, J.; Yang, M.; Huang, J.; Zhao, Y. Co-Delivery Paclitaxel and IR783 as Nanoparticles for Potentiated Chemo-Photothermal-Immunotherapy of Triple-Negative Breast Cancer. Mater. Today Bio 2025, 33, 101993. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Cui, Y.; Sun, M.; Wang, W.; Chen, J.; Gu, J.; Yang, Z. Macrophage-Centered Therapy Strategies: A Promising Weapon in Cancer Immunotherapy. Asian J. Pharm. Sci. 2025; in press. [Google Scholar] [CrossRef]
- Cortés, J.; Holgado, E.; Perez-Garcia, J. CXCR4 Antagonists for Treatment of Breast Cancer. Oncotarget 2018, 9, 33442–33443. [Google Scholar] [CrossRef]
- Barbari, C.; Fontaine, T.; Parajuli, P.; Lamichhane, N.; Jakubski, S.; Lamichhane, P.; Deshmukh, R.R. Immunotherapies and Combination Strategies for Immuno-Oncology. Int. J. Mol. Sci. 2020, 21, 5009. [Google Scholar] [CrossRef]
- He, M.; Hao, S.; Ma, L.; Xiu, B.; Yang, B.; Wang, Z.; Xue, J.; Chi, Y.; Xiong, M.; Chen, J.; et al. Neoadjuvant Anthracycline Followed by Toripalimab Combined with Nab-Paclitaxel in Patients with Early Triple-Negative Breast Cancer (NeoTENNIS): A Single-Arm, Phase II Study. eClinicalMedicine 2024, 74, 102700. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Shen, Y.; Qian, C.; Oupicky, D.; Sun, M. Targeting Pulmonary Tumor Microenvironment with CXCR4-Inhibiting Nanocomplex to Enhance Anti–PD-L1 Immunotherapy. Sci. Adv. 2020, 6, eaaz9240. [Google Scholar] [CrossRef]
- Jain, A.; Stebbing, J. The Relationship Between Response Rate and Survival Benefits in Randomized Immunotherapy Studies. Cancers 2025, 17, 495. [Google Scholar] [CrossRef]
- Ribeiro, R.; Carvalho, M.J.; Goncalves, J.; Moreira, J.N. Immunotherapy in Triple-Negative Breast Cancer: Insights into Tumor Immune Landscape and Therapeutic Opportunities. Front. Mol. Biosci. 2022, 9, 903065. [Google Scholar] [CrossRef]
- Xiang, J.; Hurchla, M.A.; Fontana, F.; Su, X.; Amend, S.R.; Esser, A.K.; Douglas, G.J.; Mudalagiriyappa, C.; Luker, K.E.; Pluard, T.; et al. CXCR4 Protein Epitope Mimetic Antagonist POL5551 Disrupts Metastasis and Enhances Chemotherapy Effect in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2015, 14, 2473–2485. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.R.J.; Dean, E.; Molife, L.R.; Lopez, J.; Ranson, M.; El-Khouly, F.; Zubairi, I.; Savulsky, C.; Reyderman, L.; Jia, Y.; et al. Phase 1 Dose-Finding and Pharmacokinetic Study of Eribulin-Liposomal Formulation in Patients with Solid Tumours. Br. J. Cancer 2019, 120, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Korn, R.L.; Crowley, J.J. Overview: Progression-Free Survival as an Endpoint in Clinical Trials with Solid Tumors. Clin. Cancer Res. 2013, 19, 2607–2612. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Song, B.; Yi, M.; Yan, Y.; Mei, Q.; Wu, K. Recent Advances in Targeted Strategies for Triple-Negative Breast Cancer. J. Hematol. Oncol. 2023, 16, 100. [Google Scholar] [CrossRef]
- Malik, J.A.; Ahmed, S.; Jan, B.; Bender, O.; Al Hagbani, T.; Alqarni, A.; Anwar, S. Drugs Repurposed: An Advanced Step towards the Treatment of Breast Cancer and Associated Challenges. Biomed. Pharmacother. 2022, 145, 112375. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, Y.; Li, D.; Wei, J.; Chen, K.; Zhang, E.; Liu, G.; Chu, X.; Liu, X.; Liu, W.; et al. Cancer Associated Fibroblasts and Metabolic Reprogramming: Unraveling the Intricate Crosstalk in Tumor Evolution. J. Hematol. Oncol. 2024, 17, 80. [Google Scholar] [CrossRef]
- Spiotto, M.; Fu, Y.-X.; Weichselbaum, R.R. The Intersection of Radiotherapy and Immunotherapy: Mechanisms and Clinical Implications. Sci. Immunol. 2016, 1, eaag1266. [Google Scholar] [CrossRef]
- Ruiz-Martínez, S.; Ribas, X.; Costas, M.; Landberg, G.; Puig, T. Characterization and Targeting of Chemoresistant Triple-Negative Breast Cancer Subtypes Using Amino-Pyridine Compounds. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2025, 1871, 167899. [Google Scholar] [CrossRef]
- Takaki, H.; Cornelis, F.H. Can the Combination of Ablation and Immunomodulation Become the Breakthrough of Cancer Treatment? Diagn. Interv. Imaging 2018, 99, 121–122. [Google Scholar] [CrossRef]
- Ziyao, L.; Jingzhe, W.; Huabiao, C. CXCR4 Antagonist AMD3100 (Plerixafor) Modulates Immune Responses in the Tumor Microenvironment. Int. J. Cancer Clin. Res. 2021, 8. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Atkins, M.B.; Rose, T.L.; Alter, R.S.; Ju, Y.; Niland, K.; Wang, Y.; Arbeit, R.; Parasuraman, S.; Gan, L.; et al. A Phase 1b Trial of the CXCR4 Inhibitor Mavorixafor and Nivolumab in Advanced Renal Cell Carcinoma Patients with No Prior Response to Nivolumab Monotherapy. Investig. New Drugs 2021, 39, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 Antagonist, in Combination with Pembrolizumab and Chemotherapy for Pancreatic Cancer: The COMBAT Trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.H.; Singh, B.; Cristofanilli, M.; Gelovani, J.; Wei, C.; Vincent, L.; Cook, K.R.; Lucci, A. A CXCR4 Antagonist CTCE-9908 Inhibits Primary Tumor Growth and Metastasis of Breast Cancer. J. Surg. Res. 2009, 155, 231–236. [Google Scholar] [CrossRef]
- Masrour, M.; Moeinafshar, A.; Poopak, A.; Razi, S.; Rezaei, N. The Role of CXC Chemokines and Receptors in Breast Cancer. Clin. Exp. Med. 2025, 25, 128. [Google Scholar] [CrossRef]
- Chuan, T.; Li, T.; Yi, C. Identification of CXCR4 and CXCL10 as Potential Predictive Biomarkers in Triple Negative Breast Cancer (TNBC). Med. Sci. Monit. 2020, 26, e918281-1. [Google Scholar] [CrossRef]
- Liu, S.; Xie, S.M.; Liu, W.; Gagea, M.; Hanker, A.B.; Nguyen, N.; Raghavendra, A.S.; Yang-Kolodji, G.; Chu, F.; Neelapu, S.S.; et al. Targeting CXCR4 Abrogates Resistance to Trastuzumab by Blocking Cell Cycle Progression and Synergizes with Docetaxel in Breast Cancer Treatment. Breast Cancer Res. 2023, 25, 62. [Google Scholar] [CrossRef]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast Cancer as an Example of Tumour Heterogeneity and Tumour Cell Plasticity during Malignant Progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef]
- Hamshaw, I.; Cominetti, M.M.D.; Lai, W.-Y.; Searcey, M.; Mueller, A. The Development of Potent, Competitive CXCR4 Antagonists for the Prevention of Cancer Metastasis. Biochem. Pharmacol. 2023, 218, 115921. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid Biopsy in Cancer: Current Status, Challenges and Future Prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Yang, J.; Tian, E.; Chen, L.; Liu, Z.; Ren, Y.; Mao, W.; Zhang, Y.; Zhang, J. Development and Therapeutic Perspectives of CXCR4 Antagonists for Disease Therapy. Eur. J. Med. Chem. 2024, 275, 116594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Luo, C.; Zhou, Z.; Li, L.; Huang, Y. Improving Anti-PD-L1 Therapy in Triple Negative Breast Cancer by Polymer-Enhanced Immunogenic Cell Death and CXCR4 Blockade. J. Control. Release 2021, 334, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-R.; Fang, L.-L.; Guo, Y.; Wang, Q.; Li, Y.-J.; Sun, H.-F.; Xie, S.-Y.; Liang, Y. Advancements in Stimulus-Responsive Co-Delivery Nanocarriers for Enhanced Cancer Immunotherapy. Int. J. Nanomed. 2024, 19, 3387–3404. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-S.; Kim, H.-J.; Konopleva, M. Targeting the CXCL12/CXCR4 Axis in Acute Myeloid Leukemia: From Bench to Bedside. Korean J. Intern. Med. 2017, 32, 248–257. [Google Scholar] [CrossRef]
- Singh, D.D.; Haque, S.; Kim, Y.; Han, I.; Yadav, D.K. Remodeling of tumour microenvironment: Strategies to overcome therapeutic resistance and innovate immunoengineering in triple-negative breast cancer. Front. Immunol. 2024, 10, 1455211. [Google Scholar] [CrossRef]
- Singh, D.D.; Lee, H.J.; Yadav, D.K. Recent Clinical Advances on Long Non-Coding RNAs in Triple-Negative Breast Cancer. Cells 2023, 20, 674. [Google Scholar] [CrossRef]
- Singh, D.D.; Lee, H.J.; Yadav, D.K. Clinical updates on tyrosine kinase inhibitors in HER2-positive breast cancer. Front. Pharmacol. 2022, 12, 1089066. [Google Scholar] [CrossRef]
- Singh, D.D.; Han, I.; Choi, E.H.; Yadav, D.K. CRISPR/Cas9 based genome editing for targeted transcriptional control in triple-negative breast cancer. Comput. Struct. Biotechnol. J. 2021, 18, 2384–2397. [Google Scholar] [CrossRef]
| S. No. | Characteristic | Particulars | Reference |
|---|---|---|---|
| 1 | Subtype Characteristics | Lacks ER, PR, and HER2 expression; highly aggressive; limited targeted therapies. | [39] |
| 2 | Emerging Resistance Pathways | PI3K/AKT/mTOR, MAPK/ERK, JAK/STAT, NF-κB. | [43] |
| 3 | Tumour Microenvironment | Hypoxia, tumour-associated macrophages (TAMs), cancer-associated fibroblasts (CAFs), cytokine signalling (e.g., CXCR4/CXCL12). | [42] |
| 4 | Clinical Consequences | Poor prognosis, high recurrence, limited response to standard therapies. | [44] |
| 5 | Major Mechanisms of Resistance | Genetic mutations: TP53, BRCA1/2, PIK3CA, PTEN Epigenetic changes: Histone modifications, DNA methylation EMT and stemness: Enhanced invasion and self-renewal Drug efflux: ABC transporters (P-gp, BCRP) Immune evasion: Downregulation of MHC-I, T-cell dysfunction | [41] |
| 6 | Primary Treatment | Cytotoxic chemotherapy (e.g., doxorubicin, paclitaxel, carboplatin). | [40] |
| 7 | Strategies to Overcome Resistance | PARP inhibitors (e.g., olaparib in BRCA-mutated TNBC) CXCR4 antagonists (e.g., AMD3100) Immunotherapy (e.g., checkpoint inhibitors) Combination therapies targeting signalling and immune pathways | [45] |
| S. No. | Mechanism | Description | Downstream Pathways/Effects | Impact on Drug Resistance | Reference |
|---|---|---|---|---|---|
| 1 | Epithelial–Mesenchymal Transition (EMT) | CXCR4 signalling upregulates EMT transcription factors, increasing cell plasticity and invasiveness. | Snail, Slug, Twist, ZEB1 | Enhances migratory capacity, reduces drug sensitivity. | [61] |
| 2 | Metastatic Niche Formation | CXCR4+ cells home to distant CXCL12-rich organs (e.g., lung, liver, bone) and enter dormancy. | CXCL12 gradients, integrins | Enables survival during systemic therapy and leads to late recurrence. | [10] |
| 3 | Cancer Stem Cell (CSC) Maintenance | Supports self-renewal and pluripotency of CSCs that are inherently resistant to therapy. | Oct4, Sox2, Nanog, Wnt/β-catenin | Sustains tumour-initiating, drug-resistant cell populations. | [62] |
| 4 | Pro-survival Signalling Activation | CXCL12 binding to CXCR4 activates anti-apoptotic signalling cascades. | PI3K/AKT, MAPK/ERK, NF-κB | Inhibits apoptosis, promotes cell survival under chemotherapeutic stress. | [57] |
| 5 | Hypoxia and Stromal Protection | Hypoxic niches with high CXCL12 expression protect tumour cells via paracrine signalling and reduced vascular access. | HIF-1α, TGF-β | Limits drug penetration; maintains resistant cell niches. | [63] |
| 6 | Immune Suppression | Recruits regulatory T cells (Tregs), MDSCs, and excludes CD8+ T cells from tumour site. | CXCR4-dependent immune exclusion | Reduces efficacy of immune-based therapies (e.g., checkpoint inhibitors). | [63] |
| S. No. | Description | Examples/Agents | Mechanism of Action | Clinical Status | References |
|---|---|---|---|---|---|
| 1 | Direct inhibition of CXCR4 receptor to block CXCL12-mediated survival and migration signalling. | Plerixafor (AMD3100), Balixafortide, POL5551 | Disrupts CXCL12/CXCR4 binding; inhibits EMT, CSC maintenance, and stromal protection | Plerixafor approved (other agents in trials) | [19] |
| 2 | Co-administration of CXCR4 inhibitors with standard chemotherapeutics. | Balixafortide + Eribulin, Plerixafor + Paclitaxel | Enhances cytotoxic efficacy by sensitising resistant cancer cells | Ongoing clinical trials (e.g., FORTRESS) | [102] |
| 3 | Binds or depletes CXCL12 to prevent receptor activation. | NOX-A12 (olaptesed pegol), CTCE-9908 | Reduces stromal-mediated resistance and immune evasion | Early-phase trials/preclinical | [103] |
| 4 | Inhibits both CXCR4 and CXCR7 receptors to overcome pathway redundancy. | Dual antagonists in development | Prevents bypass signalling and reinforces blockade of chemokine activity | Preclinical stage | [28] |
| 5 | Plant-derived agents that downregulate CXCR4 or associated pathways. | Z-Guggulsterone, Curcumin | Inhibits NF-κB/CXCR4 signalling; reduces metastasis and stemness | Preclinical | [104] |
| 6 | Targeted delivery of CXCR4 antagonists and chemotherapeutics to the tumour microenvironment. | CXCR4-targeted liposomes, AuNR-E5 nanocarriers | Enhances drug accumulation in tumour tissue; improves precision and reduces toxicity | Preclinical/experimental | [105] |
| 7 | Augments response to immune checkpoint inhibitors by improving immune infiltration. | Plerixafor + anti-PD-1 (Toripalimab), BL-8040 combos | Reverses immune exclusion; enhances CD8+ T cell infiltration | Phase I/II trials | [106] |
| 8 | Combines stromal ablation with CXCR4 inhibition to disrupt the protective tumour niche. | 177Lu-FAPi + AMD3100 | Depletes CAFs and enhances immune response within TME | Preclinical | [30] |
| 9 | Inhibition of signalling pathways downstream of CXCR4 (e.g., PI3K/AKT, STAT3). | PI3K inhibitors, JAK/STAT blockers | Prevents survival signalling even if CXCR4 is partially active | Clinical and preclinical stages | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.D.; Yadav, D.K.; Shin, D. Targeting the CXCR4/CXCL12 Axis to Overcome Drug Resistance in Triple-Negative Breast Cancer. Cells 2025, 14, 1482. https://doi.org/10.3390/cells14181482
Singh DD, Yadav DK, Shin D. Targeting the CXCR4/CXCL12 Axis to Overcome Drug Resistance in Triple-Negative Breast Cancer. Cells. 2025; 14(18):1482. https://doi.org/10.3390/cells14181482
Chicago/Turabian StyleSingh, Desh Deepak, Dharmendra Kumar Yadav, and Dongyun Shin. 2025. "Targeting the CXCR4/CXCL12 Axis to Overcome Drug Resistance in Triple-Negative Breast Cancer" Cells 14, no. 18: 1482. https://doi.org/10.3390/cells14181482
APA StyleSingh, D. D., Yadav, D. K., & Shin, D. (2025). Targeting the CXCR4/CXCL12 Axis to Overcome Drug Resistance in Triple-Negative Breast Cancer. Cells, 14(18), 1482. https://doi.org/10.3390/cells14181482








