PCSK9 Regulation of Lipid Metabolism in the Nervous System: Implications for Schwann Cell Function and Peripheral Neuropathy
Abstract
1. Introduction
2. PCSK9 Biology: Structure, Regulation, and Canonical Functions
2.1. Domain Architecture and Activation of PCSK9
2.2. Transcriptional Control by SREBP2 and HNF1α, and Post-Transcriptional Regulation
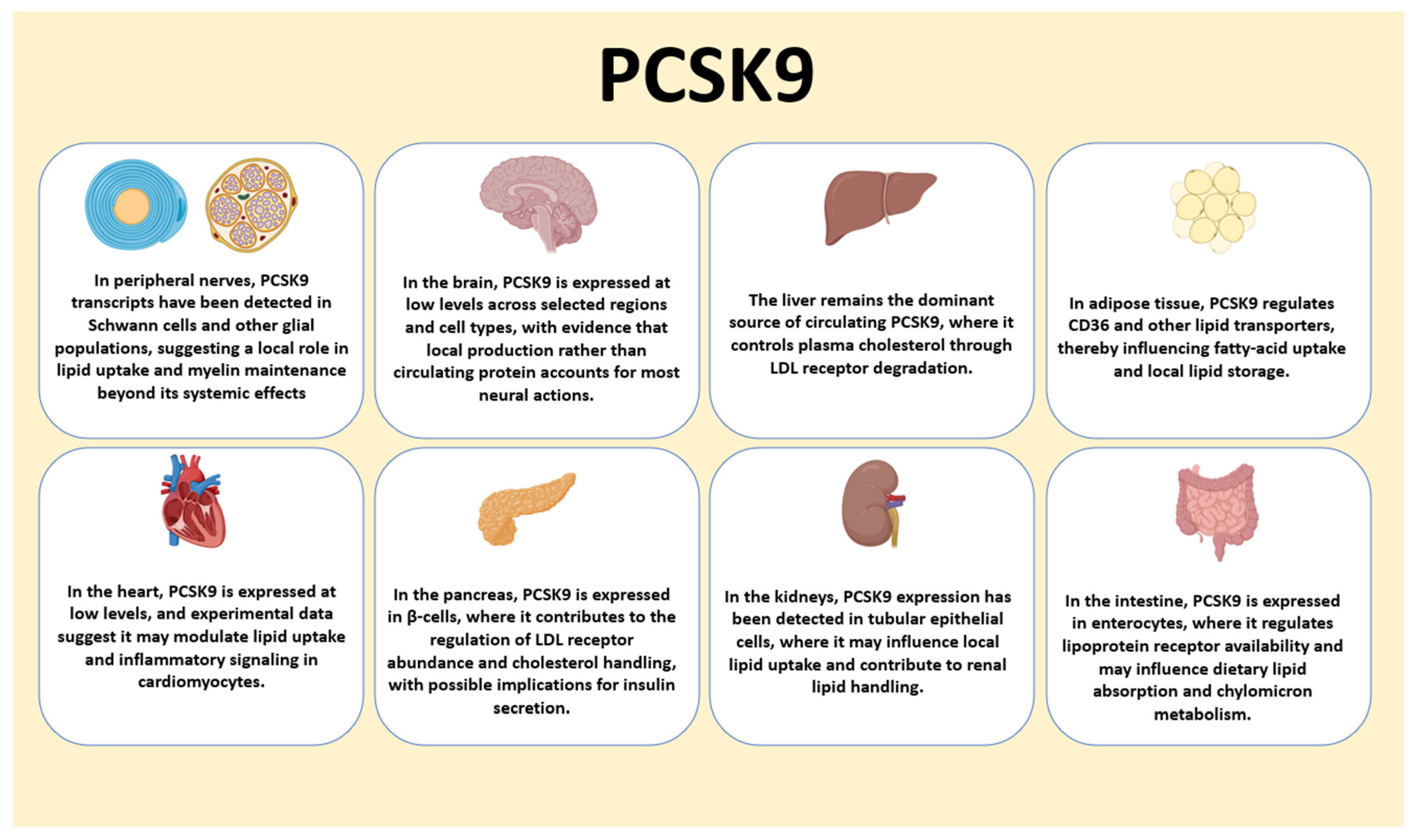
2.3. Secretion, Circulating Forms, and Tissue Distribution
2.4. Canonical Receptor Biology: LDLR Turnover and Systemic Cholesterol Control
2.5. Non-Hepatic Targets and Interactors: VLDLR, LRP1, ApoER2, CD36
3. Lipid Homeostasis in the Nervous System
3.1. Lipid Requirements of Neurons and Glia: Cholesterol, Sphingolipids, Plasmalogens
3.2. Uptake and Synthesis in the CNS and PNS Under Barrier Constraints
3.3. Key Transporters and Receptors in Neural Lipid Traffic
3.3.1. LDLR Family: LDLR, VLDLR, LRP1, ApoER2
3.3.2. ApoE-Containing Lipoproteins in the Brain
3.3.3. CD36 and Long-Chain Fatty Acid Uptake
3.3.4. ABCA1/ABCG1 and Cholesterol Efflux
3.4. Synthesis, β-Oxidation, and Lipid Turnover in Neural Cells
3.5. Mitochondrial Lipid Handling and Redox Balance in Myelinating Glia
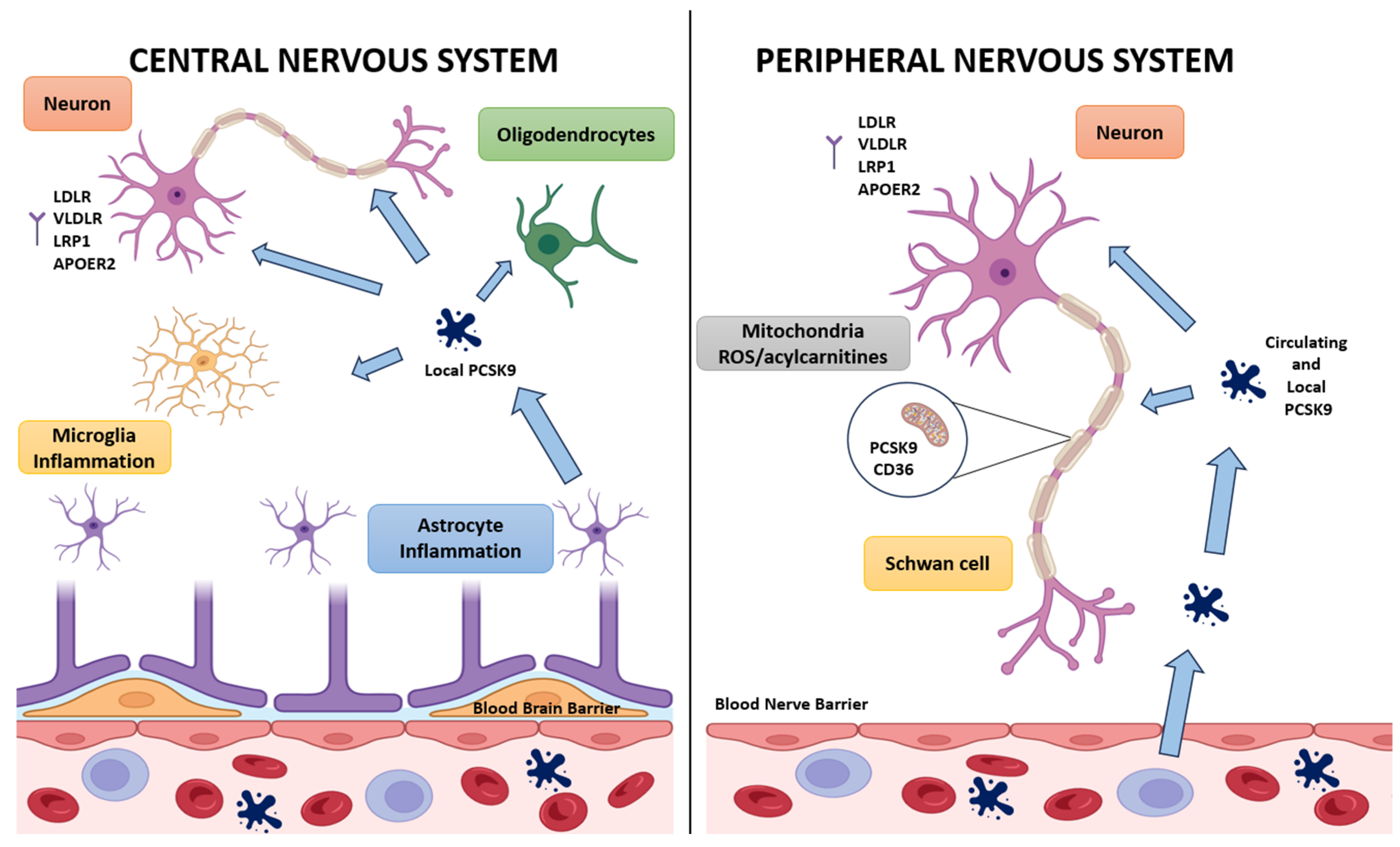
4. PCSK9 in the Central Nervous System
4.1. Expression Across Neural Cell Types and Developmental Regulation
4.2. Neurodevelopment and Synaptic Biology: Reelin Signaling Through ApoER2 and VLDLR
4.3. Apoptosis, Stress, and Inflammatory Signaling Linked to PCSK9
4.4. PCSK9 in Neurodegenerative and Demyelinating Contexts
4.5. Knowledge Gaps and Methodological Considerations: Local vs Circulating PCSK9 and Barrier Constraints
5. PCSK9 in the Peripheral Nervous System: Focus on Schwann Cells
5.1. Expression Across Schwann Cells, Satellite Glia, and Nociceptive Schwann Cells
5.2. Genetic PCSK9 Deficiency and Neuropathy Phenotypes
5.2.1. Behavioral and Electrophysiological Readouts
5.2.2. Hypomyelination and G-Ratio Changes
5.2.3. Axonal Pathology in Remak Bundles
5.3. Lipid Dysregulation in Peripheral Nerves: Droplets and Raft Composition
5.4. Macrophages and Nerve Regeneration: Debris Clearance and CD36
5.5. Pharmacological Inhibition of PCSK9 in Diabetic Peripheral Neuropathy
6. The PCSK9–CD36–Mitochondria Axis: A Working Model
6.1. Evidence That PCSK9 Regulates CD36 Abundance and Trafficking
6.2. Consequences for Fatty-Acid Influx in Schwann Cells
6.3. Mitochondrial Overload, Reactive Oxygen Species, and Bioenergetic Failure
6.4. Acylcarnitine Buildup as a Signature of Incomplete β-Oxidation
6.5. Integrated Model for Hypomyelination and Small-Fiber Injury
7. Clinical Implications: PCSK9 Inhibitors and Neural Safety
7.1. Pharmacologic Inhibition Versus Genetic Loss: What Differs and Why It Matters
7.2. Neural Outcomes in Large Cardiovascular Trials: Signals and Limits of Detection
7.3. Case Reports and Post-Marketing Pharmacovigilance: How to Interpret
7.4. Translational Angles: Targeting Lipid Flux Without Harming Myelin
7.5. Practical Guidance for Future RCTs in Lipid-Lowering and Neural Safety
7.6. PCSK9, Systemic Metabolic Disorders, and Peripheral Neuropathy
8. Future Directions and Open Questions
8.1. Cell Type Specific Roles Beyond Schwann Cells: Satellite and Enteric Glia
8.2. PCSK9 in Nerve Regeneration and Pain Biology
8.3. Human Tissue and iPSC-Derived Schwann Cell Models, Spatial and Transcriptomic Mapping
8.4. Biomarkers for Clinical Studies: Lipid Signatures, Mitochondrial Readouts, Imaging
8.5. Therapeutic Strategies: Schwann-Cell-Specific Modulation and CD36 Fine-Tuning
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tracey, T.J.; Steyn, F.J.; Wolvetang, E.J.; Ngo, S.T. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Grüter, T.; Mohamad, N.; Rilke, N.; Blusch, A.; Sgodzai, M.; Demir, S.; Pedreiturria, X.; Lemhoefer, K.; Gisevius, B.; Haghikia, A.; et al. Propionate Exerts Neuroprotective and Neuroregenerative Effects in the Peripheral Nervous System. Proc. Natl. Acad. Sci. USA 2023, 120, e2216941120. [Google Scholar] [CrossRef]
- Ball, A.; Wagner, J.; Rosoff, D.B.; Lohoff, F.W. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in the Central Nervous System. Neurosci. Biobehav. Rev. 2023, 149, 105155. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, A.K.; Techer, R.; Chemello, K.; Lambert, G.; Bourane, S. PCSK9 and the Nervous System: A No-Brainer? J. Lipid Res. 2023, 64, 100426. [Google Scholar] [CrossRef] [PubMed]
- Wuerch, E.; Yong, V.W. Cholesterol in the CNS: Functions, Recycling and Remyelination. J. Neuroinflamm. 2025, 22, 180. [Google Scholar] [CrossRef]
- Hornemann, T. Mini review: Lipids in Peripheral Nerve Disorders. Neurosci. Lett. 2021, 740, 135455. [Google Scholar] [CrossRef]
- Morant-Ferrando, B.; Jiménez-Blasco, D.; Alonso-Batan, P.; Agulla, J.; Lapresa, R.; García-Rodríguez, D.; Yunta-Sanchez, S.; López-Fabuel, I.; Fernández, E.; Carmeliet, P.; et al. Fatty Acid Oxidation Organizes Mitochondrial Supercomplexes to Sustain Astrocytic ROS and Cognition. Nat. Metab. 2023, 5, 1290–1320. [Google Scholar] [CrossRef]
- Jaafar, A.K.; Paulo-Ramos, A.; Rastoldo, G.; Veeren, B.; Planesse, C.; Bringart, M.; Rondeau, P.; Meilhac, O.; Lambert, G.; Bourane, S. PCSK9 Deficiency Promotes the Development of Peripheral Neuropathy. JCI Insight 2025, 10, e183786. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liao, M.; Mou, A.; Zheng, Q.; Yang, W.; Yu, Z.; Cui, Y.; Xia, X.; Qin, Y.; Chen, M.; et al. Rheb-Regulated Mitochondrial Pyruvate Metabolism of Schwann Cells Linked to Axon Stability. Dev. Cell 2021, 56, 2980–2994. e6. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Sasaki, Y.; Kim, S.; Strickland, A.; Workman, C.S.; Yang, K.; Gross, R.W.; Milbrandt, J. Aberrant Schwann Cell Lipid Metabolism Linked to Mitochondrial Deficits Leads to Axon Degeneration and Neuropathy. Neuron 2013, 77, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Kister, A.; Kister, I. Overview of Myelin, Major Myelin Lipids, and Myelin-Associated Proteins. Front. Chem. 2023, 10, 1041961. [Google Scholar] [CrossRef]
- Coppinger, C.; Movahed, M.R.; Azemawah, V.; Peyton, L.; Gregory, J.; Hashemzadeh, M. A Comprehensive Review of PCSK9 Inhibitors. J. Cardiovasc. Pharmacol. Ther. 2022, 27, 10742484221100107. [Google Scholar] [CrossRef] [PubMed]
- Stoekenbroek, R.M.; Lambert, G.; Cariou, B.; Hovingh, G.K. Inhibiting PCSK9—Biology beyond LDL Control. Nat. Rev. Endocrinol. 2018, 15, 52–62. [Google Scholar] [CrossRef]
- Poliakova, Т.; Wellington, C.L. Roles of Peripheral Lipoproteins and Cholesteryl Ester Transfer Protein in the Vascular Contributions to Cognitive Impairment and Dementia. Mol. Neurodegener. 2023, 18, 86. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, W.; Li, L.; Ma, H.; Zhu, M.; Li, X.; Feng, X. Targeting Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) to Tackle Central Nervous System Diseases: Role as a Promising Approach. Eur. J. Med. Res. 2025, 30, 690. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, S.; Dalt, L.D.; Cesare, G.D.; Stringhi, R.; D’Andrea, L.; Greca, F.L.; Cambria, C.; Vandermeulen, L.; Zianni, E.; Musardo, S.; et al. Neuronal PCSK9 Regulates Cognitive Performances via the Modulation of ApoER2 Synaptic Localization. Pharmacol. Res. 2025, 213, 107652. [Google Scholar] [CrossRef]
- Pärn, A.; Olsen, D.; Tuvikene, J.; Kaas, M.; Borisova, Е.; Bilgin, M.; Elhauge, M.; Vilstrup, J.; Madsen, P.; Ambrozkiewicz, M.C.; et al. PCSK9 Deficiency Alters Brain Lipid Composition without Affecting Brain Development and Function. Front. Mol. Neurosci. 2023, 15, 1084633. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.B.; Wang, H. The Shared Role of Cholesterol in Neuronal and Peripheral Inflammation. Pharmacol. Ther. 2023, 249, 108486. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-Function of CD36 and Importance of Fatty Acid Signal Transduction in Fat Metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef]
- Demers, A.; Samami, S.; Lauzier, B.; Des Rosiers, C.; Ngo Sock, E.T.; Ong, H.; Mayer, G. PCSK9 Induces CD36 Degradation and Affects Long-Chain Fatty Acid Uptake and Triglyceride Metabolism in Adipocytes and in Mouse Liver. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2517–2525. [Google Scholar] [CrossRef]
- Byun, J.H.; Lebeau, P.; Platko, K.; Carlisle, R.E.; Faiyaz, M.; Chen, J.; MacDonald, M.E.; Makda, Y.; Yousof, T.; Lynn, E.G.; et al. Inhibitory Antibodies against PCSK9 Reduce Surface CD36 and Mitigate Diet-Induced Renal Lipotoxicity. Kidney360 2022, 3, 1394–1410. [Google Scholar] [CrossRef]
- Follis, R.M.; Tep, C.; Genaro-Mattos, T.C.; Kim, M.L.; Ryu, J.C.; Morrison, V.E.; Chan, J.R.; Porter, N.A.; Carter, B.; Yoon, S.O. Metabolic Control of Sensory Neuron Survival by the P75 Neurotrophin Receptor in Schwann Cells. J. Neurosci. 2021, 41, 8710–8724. [Google Scholar] [CrossRef]
- Cui, N.; Feng, Y.; Wang, M.; Lu, X.; Huang, Y.; Chen, Y.; Shi, X. Protective Effect of Alirocumab, a PCSK9 Inhibitor, on the Sciatic Nerve of Rats with Diabetic Peripheral Neuropathy. Endocr. J. 2024, 71, 233–244. [Google Scholar] [CrossRef]
- Seidah, N.G. The PCSK9 Discovery, an Inactive Protease with Varied Functions in Hypercholesterolemia, Viral Infections, and Cancer. J. Lipid Res. 2021, 62, 100130. [Google Scholar] [CrossRef]
- Sundararaman, S.S.; Döring, Y.; van der Vorst, E.P.C. PCSK9: A Multi-Faceted Protein That Is Involved in Cardiovascular Biology. Biomedicines 2021, 9, 793. [Google Scholar] [CrossRef]
- Seidah, N.G.; Prat, A. The Multifaceted Biology of PCSK9. Endocr. Rev. 2021, 43, 558–582. [Google Scholar] [CrossRef]
- Willows, J.W.; Gunsch, G.; Paradie, E.; Blaszkiewicz, M.; Tonniges, J.R.; Pino, M.F.; Smith, S.R.; Sparks, L.M.; Townsend, K.L. Schwann Cells Contribute to Demyelinating Diabetic Neuropathy and Nerve Terminal Structures in White Adipose Tissue. iScience 2023, 26, 106189. [Google Scholar] [CrossRef]
- Hori, M.; Ishihara, M.; Yuasa, Y.; Makino, H.; Yanagi, K.; Tamanaha, T.; Kishimoto, I.; Kujiraoka, T.; Hattori, H.; Harada-Shiba, M. Removal of Plasma Mature and Furin-Cleaved Proprotein Convertase Subtilisin/Kexin 9 by Low-Density Lipoprotein-Apheresis in Familial Hypercholesterolemia: Development and Application of a New Assay for PCSK9. J. Clin. Endocrinol. Metab. 2014, 100, E41–E49. [Google Scholar] [CrossRef]
- Schroeder, K.; Beyer, T.P.; Hansen, R.J.; Han, B.; Pickard, R.T.; Wroblewski, V.J.; Kowala, M.C.; Eacho, P.I. Proteolytic Cleavage of Antigen Extends the Durability of an Anti-PCSK9 Monoclonal Antibody. J. Lipid Res. 2015, 56, 2124–2132. [Google Scholar] [CrossRef]
- Oleaga, C.; Hay, J.; Gurcan, E.; David, L.L.; Müeller, P.; Tavori, H.; Shapiro, M.D.; Pamir, N.; Fazio, S. Insights into the Kinetics and Dynamics of the Furin-Cleaved Form of PCSK9. J. Lipid Res. 2020, 62, 100003. [Google Scholar] [CrossRef]
- Kataoka, Y.; Harada-Shiba, M.; Hori, M.; Watanabe, M.; Kokubo, Y.; Noguchi, T.; Yasuda, S.; Miyamoto, Y. Circulating Furin-Cleaved Proprotein Convertase Subtilisin/Kexin Type 9 Concentration Predicts Future Coronary Events in Japanese Subjects. JACC Asia 2021, 1, 360–368. [Google Scholar] [CrossRef]
- Lipari, M.T.; Li, W.; Moran, P.; Kong-Beltran, M.; Sai, T.; Lai, J.; Lin, S.; Kolumam, G.; Zavala-Solorio, J.; Izrael-Tomasevic, A.; et al. Furin-Cleaved Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Is Active and Modulates Low Density Lipoprotein Receptor and Serum Cholesterol Levels. J. Biol. Chem. 2012, 287, 43482–43491. [Google Scholar] [CrossRef]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A Key Modulator of Cardiovascular Health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef]
- Schulz, R.; Schlüter, K.-D.; Laufs, U. Molecular and Cellular Function of the Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9). Basic Res. Cardiol. 2015, 110, 4. [Google Scholar] [CrossRef]
- Dong, B.; Wu, M.; Li, H.; Kraemer, F.B.; Adeli, K.; Seidah, N.G.; Park, S.W.; Liu, J. Strong Induction of PCSK9 Gene Expression through HNF1α and SREBP2: Mechanism for the Resistance to LDL-Cholesterol Lowering Effect of Statins in Dyslipidemic Hamsters. J. Lipid Res. 2010, 51, 1486–1495. [Google Scholar] [CrossRef]
- Luquero, A.; Badimon, L.; Badimon, L.; Borrell-Pages, M. PCSK9 Functions in Atherosclerosis Are Not Limited to Plasmatic LDL-Cholesterol Regulation. Front. Cardiovasc. Med. 2021, 8, 639727. [Google Scholar] [CrossRef]
- Xia, X.; Peng, Z.; Gu, H.; Wang, M.; Wang, G.; Zhang, D.-W. Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 764038. [Google Scholar] [CrossRef]
- Piper, D.E.; Jackson, S.; Liu, Q.; Romanow, W.G.; Shetterly, S.; Thibault, S.T.; Shan, B.; Walker, N.P.C. The Crystal Structure of PCSK9: A Regulator of Plasma LDL-Cholesterol. Structure 2007, 15, 545–552. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, H.; Kim, K.; Kim, Y.-K.; Yoon, D.; Park, S.W. Sterol-Dependent Regulation of Proprotein Convertase Subtilisin/Kexin Type 9 Expression by Sterol-Regulatory Element Binding Protein-2. J. Lipid Res. 2007, 49, 399–409. [Google Scholar] [CrossRef]
- Norata, G.D.; Tavori, H.; Pirillo, A.; Fazio, S.; Catapano, A.L. Biology of Proprotein Convertase Subtilisin Kexin 9: Beyond Low-Density Lipoprotein Cholesterol Lowering. Cardiovasc. Res. 2016, 112, 429–442. [Google Scholar] [CrossRef]
- Ferri, N. Proprotein Convertase Subtilisin/Kexin Type 9, Brain Cholesterol Homeostasis and Potential Implication for Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 120. [Google Scholar]
- van Solingen, C.; Oldebeken, S.R.; Salerno, A.G.; Wanschel, A.; Moore, K.J. High-Throughput Screening Identifies MicroRNAs Regulating Human PCSK9 and Hepatic Low-Density Lipoprotein Receptor Expression. Front. Cardiovasc. Med. 2021, 8, 667298. [Google Scholar] [CrossRef]
- Laudette, M.; Lindbom, M.; Arif, M.; Cinato, M.; Ruiz, M.; Doran, S.; Miljanovic, A.; Rutberg, M.; Andersson, L.; Klevstig, M.; et al. Cardiomyocyte-Specific PCSK9 Deficiency Compromises Mitochondrial Bioenergetics and Heart Function. Cardiovasc. Res. 2023, 119, 1537–1552. [Google Scholar] [CrossRef]
- Dlugosz, P.; Nimpf, J. The Reelin Receptors Apolipoprotein E Receptor 2 (ApoER2) and VLDL Receptor. Int. J. Mol. Sci. 2018, 19, 3090. [Google Scholar] [CrossRef]
- Pohlkamp, T.; Wasser, C.R.; Herz, J. Functional Roles of the Interaction of APP and Lipoprotein Receptors. Front. Mol. Neurosci. 2017, 10, 54. [Google Scholar] [CrossRef]
- Saitoski, K.; Ryaboshapkina, M.; Hamza, G.M.; Jarnuczak, A.F.; Berthault, C.; Carlotti, F.; Armanet, M.; Sengupta, K.; Underwood, C.R.; Andersson, S.; et al. Proprotein Convertase PCSK9 Affects Expression of Key Surface Proteins in Human Pancreatic Beta Cells via Intracellular and Extracellular Regulatory Circuits. J. Biol. Chem. 2022, 298, 102096. [Google Scholar] [CrossRef]
- Canclini, L.; Malvandi, A.M.; Uboldi, P.; Jabnati, N.; Grigore, L.; Zambon, A.; Baragetti, A.; Catapano, A.L. The Association of Proprotein Convertase Subtilisin/Kexin Type 9 to Plasma Low-Density Lipoproteins: An Evaluation of Different Methods. Metabolites 2021, 11, 861. [Google Scholar] [CrossRef]
- Sawaguchi, J.; Saeki, Y.; Oda, M.; Takamura, T.; Fujibayashi, K.; Wakasa, M.; Akao, H.; Kitayama, M.; Kawai, Y.; Kajinami, K. The Circulating Furin-Cleaved/Mature PCSK9 Ratio Has a Potential Prognostic Significance in Statin-Naïve Patients with Acute ST Elevation Myocardial Infarction. Atheroscler. Plus 2022, 50, 50–56. [Google Scholar] [CrossRef]
- Oleaga, C.; Shapiro, M.D.; Hay, J.; Müeller, P.; Miles, J.; Huang, C.; Friz, E.; Tavori, H.; Tóth, P.P.; Wójcik, C.; et al. Hepatic Sensing Loop Regulates PCSK9 Secretion in Response to Inhibitory Antibodies. J. Am. Coll. Cardiol. 2021, 78, 1437–1449. [Google Scholar] [CrossRef]
- Mikaeeli, S.; Ouadda, A.B.D.; Evagelidis, A.; Essalmani, R.; Ramos, O.H.P.; Fruchart-Gaillard, C.; Seidah, N.G. Insights into PCSK9-LDLR Regulation and Trafficking via the Differential Functions of MHC-I Proteins HFE and HLA-C. Cells 2024, 13, 857. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, X.; Yang, Z.; Zhu, X.; Liu, M.; Du, M.; Pan, X.; Wang, Y. PCSK9 Promotes LDLR Degradation by Preventing SNX17-Mediated LDLR Recycling. Circulation 2025, 151, 1512–1526. [Google Scholar] [CrossRef]
- Merrill, N.J.; Davidson, W.S.; He, Y.; Ludovico, I.D.; Sarkar, S.; Berger, M.; McDermott, J.; Eldik, L.J.V.; Wilcock, D.M.; Monroe, M.; et al. Human Cerebrospinal Fluid Contains Diverse Lipoprotein Subspecies Enriched in Proteins Implicated in Central Nervous System Health. Sci. Adv. 2023, 9, eadi5571. [Google Scholar] [CrossRef]
- Lagace, T.A. PCSK9 and LDLR Degradation: Regulatory Mechanisms in Circulation and in Cells. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef]
- Gu, H.; Adijiang, A.; Mah, M.C.-M.; Zhang, D. Characterization of the Role of EGF-A of Low Density Lipoprotein Receptor in PCSK9 Binding. J. Lipid Res. 2013, 54, 3345–3357. [Google Scholar] [CrossRef]
- Poirier, S.; Mayer, G.; Benjannet, S.; Bergeron, É.; Marcinkiewicz, J.; Nassoury, N.; Mayer, H.; Nimpf, J.; Prat, A.; Seidah, N.G. The Proprotein Convertase PCSK9 Induces the Degradation of Low Density Lipoprotein Receptor (LDLR) and Its Closest Family Members VLDLR and ApoER2. J. Biol. Chem. 2008, 283, 2363. [Google Scholar] [CrossRef]
- Canuel, M.; Sun, X.; Asselin, M.; Paramithiotis, E.; Prat, A.; Seidah, N.G. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Can Mediate Degradation of the Low Density Lipoprotein Receptor-Related Protein 1 (LRP-1). PLoS ONE 2013, 8, e64145. [Google Scholar] [CrossRef]
- Seidah, N.G.; Garçon, D. Expanding Biology of PCSK9: Roles in Atherosclerosis and Beyond. Curr. Atheroscler. Rep. 2022, 24, 821–830. [Google Scholar] [CrossRef]
- Zhang, L.; Song, K.; Zhu, M.; Shi, J.; Zhang, H.; Xu, L.; Chen, Y. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Lipid Metabolism, Atherosclerosis and Ischemic Stroke. Int. J. Neurosci. 2015, 126, 675–680. [Google Scholar] [CrossRef]
- Mayer, G.; Poirier, S.; Seidah, N.G. Annexin A2 Is a C-Terminal PCSK9-Binding Protein That Regulates Endogenous Low Density Lipoprotein Receptor Levels. J. Biol. Chem. 2008, 283, 31791–31801. [Google Scholar] [CrossRef]
- Seidah, N.G.; Poirier, S.; Denis, M.; Parker, R.A.; Miao, B.; Mapelli, C.; Prat, A.; Wassef, H.; Davignon, J.; Hajjar, K.A.; et al. Annexin A2 Is a Natural Extrahepatic Inhibitor of the PCSK9-Induced LDL Receptor Degradation. PLoS ONE 2012, 7, e41865. [Google Scholar] [CrossRef]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef]
- Barnes-Vélez, J.A.; Aksoy Yasar, F.B.; Hu, J. Myelin Lipid Metabolism and Its Role in Myelination and Myelin Maintenance. Innov. 2022, 4, 100360. [Google Scholar] [CrossRef]
- Sysoev, E.I.; Shenfeld, A.; Belashova, T.A.; Valina, A.A.; Zadorsky, S.P.; Ap, G. Amyloid Fibrils of the Myelin Basic Protein Are an Integral Component of Myelin in the Vertebrate Brain. Sci. Rep. 2025, 15, 29053. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q. Cholesterol Metabolism and Homeostasis in the Brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.Y.; Hartmann, H.; Ling, S. Central Nervous System Cholesterol Metabolism in Health and Disease. IUBMB Life 2022, 74, 826–841. [Google Scholar] [CrossRef]
- Silva, A.; Prior, R.; D’Antonio, M.; Swinnen, J.V.; Bosch, L.V.D. Lipid Metabolism Alterations in Peripheral Neuropathies. Neuron 2025, 113, 2556–2581. [Google Scholar] [CrossRef]
- Sharp, F.R.; DeCarli, C.; Jin, L.; Zhan, X. White Matter Injury, Cholesterol Dysmetabolism, and APP/Abeta Dysmetabolism Interact to Produce Alzheimer’s Disease (AD) Neuropathology: A Hypothesis and Review. Front. Aging Neurosci. 2023, 15, 1096206. [Google Scholar] [CrossRef]
- Berger, J.; Dorninger, F.; Forss-Petter, S.; Kunze, M. Peroxisomes in Brain Development and Function. Biochim. Biophys. Acta 2016, 1863, 934–955. [Google Scholar] [CrossRef]
- Roy, D.; Tedeschi, A. The Role of Lipids, Lipid Metabolism and Ectopic Lipid Accumulation in Axon Growth, Regeneration and Repair after CNS Injury and Disease. Cells 2021, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Kobayashi, M.; Morito, K.; Hasi, R.Y.; Aihara, M.; Hayashi, J.; Kawakami, R.; Tsuchiya, K.; Sango, K.; Tanaka, T. Peroxisomes Attenuate Cytotoxicity of Very Long-Chain Fatty Acids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159259. [Google Scholar] [CrossRef] [PubMed]
- Scarian, E.; Viola, C.; Dragoni, F.; Gerlando, R.D.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Bordoni, M.; Pansarasa, O. New Insights into Oxidative Stress and Inflammatory Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2698. [Google Scholar] [CrossRef]
- Sarkar, C.; Lipinski, M.M. Role and Function of Peroxisomes in Neuroinflammation. Cells 2024, 13, 1655. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty Acid Transporting Proteins: Roles in Brain Development, Aging, and Stroke. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 35. [Google Scholar] [CrossRef]
- Pan, Y.; Scanlon, M.J.; Owada, Y.; Yamamoto, Y.; Porter, C.J.H.; Nicolazzo, J.A. Fatty Acid-Binding Protein 5 Facilitates the Blood–Brain Barrier Transport of Docosahexaenoic Acid. Mol. Pharm. 2015, 12, 4375–4385. [Google Scholar] [CrossRef]
- Ubogu, E.E. Biology of the Human Blood-Nerve Barrier in Health and Disease. Exp. Neurol. 2020, 328, 113272. [Google Scholar] [CrossRef]
- Reinhold, A.; Rittner, H.L. Barrier Function in the Peripheral and Central Nervous System—A Review. Pflügers Arch. Eur. J. Physiol. 2017, 469, 123–134. [Google Scholar] [CrossRef]
- Reinhold, A.; Rittner, H.L. Characteristics of the Nerve Barrier and the Blood Dorsal Root Ganglion Barrier in Health and Disease. Exp. Neurol. 2020, 327, 113244. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Meehan, S.D. Axon Regeneration: Membrane Expansion and Lipidomics. Neural Regen. Res. 2021, 17, 989–990. [Google Scholar] [CrossRef]
- Cristobal, C.D.; Lee, H.-K. Development of Myelinating Glia: An Overview. Glia 2022, 70, 2237–2259. [Google Scholar] [CrossRef]
- Auderset, L.; Landowski, L.; Foa, L.; Young, K.M. Low Density Lipoprotein Receptor Related Proteins as Regulators of Neural Stem and Progenitor Cell Function. Stem Cells Int. 2016, 2016, 2108495. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Herz, J. The ApoE Receptors Vldlr and Apoer2 in Central Nervous System Function and Disease. J. Lipid Res. 2017, 58, 1036–1043. [Google Scholar] [CrossRef]
- Shinohara, M.; Tachibana, M.; Kanekiyo, T.; Bu, G. Role of LRP1 in the Pathogenesis of Alzheimer’s Disease: Evidence from Clinical and Preclinical Studies. J. Lipid Res. 2017, 58, 1267–1281. [Google Scholar] [CrossRef]
- He, Z.; Wang, G.; Wu, J.; Tang, Z.; Luo, M. The Molecular Mechanism of LRP1 in Physiological Vascular Homeostasis and Signal Transduction Pathways. Biomed. Pharmacother. 2021, 139, 111667. [Google Scholar] [CrossRef]
- Faissner, A. Low-Density Lipoprotein Receptor-Related Protein-1 (LRP1) in the Glial Lineage Modulates Neuronal Excitability. Front. Netw. Physiol. 2023, 3, 1190240. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Philips, G.T.; Herz, J. More than Cholesterol Transporters: Lipoprotein Receptors in CNS Function and Neurodegeneration. Neuron 2014, 83, 771–787. [Google Scholar] [CrossRef]
- Pifferi, F.; Laurent, B.; Plourde, M. Lipid Transport and Metabolism at the Blood-Brain Interface: Implications in Health and Disease. Front. Physiol. 2021, 12, 645646. [Google Scholar] [CrossRef]
- Koldamova, R.; Fitz, N.F.; Lefterov, I. ATP-Binding Cassette Transporter A1: From Metabolism to Neurodegeneration. Neurobiol. Dis. 2014, 72, 13–21. [Google Scholar] [CrossRef]
- Wahrle, S.E.; Jiang, H.; Parsadanian, M.; Kim, J.; Li, A.; Knoten, A.; Jain, S.; Hirsch-Reinshagen, V.; Wellington, C.L.; Bales, K.R.; et al. Overexpression of ABCA1 Reduces Amyloid Deposition in the PDAPP Mouse Model of Alzheimer Disease. J. Clin. Investig. 2008, 118, 671–682. [Google Scholar] [CrossRef]
- Blanchard, J.; Akay, L.A.; Dávila-Velderrain, J.; von Maydell, D.; Mathys, H.; Davidson, S.M.; Effenberger, A.H.; Chen, C.; Maner-Smith, K.; Hajjar, I.; et al. APOE4 Impairs Myelination via Cholesterol Dysregulation in Oligodendrocytes. Nature 2022, 611, 769–779. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Y.; Gui, Y. Neuronal ApoE4 in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Aging Neurosci. 2023, 15, 1199434. [Google Scholar] [CrossRef]
- Windham, I.A.; Powers, A.E.; Ragusa, J.V.; Wallace, E.D.; Zanellati, M.C.; Williams, V.H.; Wagner, C.H.; White, K.; Cohen, S. APOE Traffics to Astrocyte Lipid Droplets and Modulates Triglyceride Saturation and Droplet Size. J. Cell Biol. 2024, 223, e202305003. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Li, D.; He, C.; He, K.; Xue, T.; Wan, L.; Zhang, C.; Liu, Q. Astrocytic ApoE Reprograms Neuronal Cholesterol Metabolism and Histone-Acetylation-Mediated Memory. Neuron 2021, 109, 957–970.e8. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The Role of CD36 in Cardiovascular Disease. Cardiovasc. Res. 2020, 118, 115–129. [Google Scholar] [CrossRef]
- Dobri, A.-M.; Dudău, M.; Enciu, A.; Hinescu, M.E. CD36 in Alzheimer’s Disease: An Overview of Molecular Mechanisms and Therapeutic Targeting. Neuroscience 2021, 453, 301–311. [Google Scholar] [CrossRef]
- Ioghen, O.C.; Chiţoiu, L.; Gherghiceanu, M.; Ceafalan, L.C.; Hinescu, M.E. CD36–A Novel Molecular Target in the Neurovascular Unit. Eur. J. Neurosci. 2021, 53, 2500–2510. [Google Scholar] [CrossRef]
- Feng, M.; Zhou, Q.; Xie, H.; Liu, C.; Zheng, M.; Zhang, S.; Zhou, S.; Zhao, J. Role of CD36 in Central Nervous System Diseases. Neural Regen. Res. 2023, 19, 512–518. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Heather, L.C.; Luiken, J.J.F.P. CD36 as a Gatekeeper of Myocardial Lipid Metabolism and Therapeutic Target for Metabolic Disease. Physiol. Rev. 2024, 104, 727–764. [Google Scholar] [CrossRef]
- Rumora, A.E.; Kim, B.; Feldman, E.L. A Role for Fatty Acids in Peripheral Neuropathy Associated with Type 2 Diabetes and Prediabetes. Antioxid. Redox Signal. 2022, 37, 560–577. [Google Scholar] [CrossRef]
- Kim, W.S.; Rahmanto, A.S.; Kamili, A.; Rye, K.-A.; Guillemin, G.J.; Gelissen, I.C.; Jessup, W.; Hill, A.F.; Garner, B. Role of ABCG1 and ABCA1 in Regulation of Neuronal Cholesterol Efflux to Apolipoprotein E Discs and Suppression of Amyloid-β Peptide Generation. J. Biol. Chem. 2006, 282, 2851–2861. [Google Scholar] [CrossRef]
- Koldamova, R.; Staufenbiel, M.; Lefterov, I. Lack of ABCA1 Considerably Decreases Brain ApoE Level and Increases Amyloid Deposition in APP23 Mice. J. Biol. Chem. 2005, 280, 43224–43235. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Anderson, A.; Allen, L.B.; Korade, Ž.; Mirnics, K. Cholesterol Biosynthesis and Uptake in Developing Neurons. ACS Chem. Neurosci. 2019, 10, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Musolino, V.; Bosco, F.; Scicchitano, M.; Scarano, F.; Nucera, S.; Zito, M.C.; Ruga, S.; Carresi, C.; Macrì, R.; et al. Cholesterol Homeostasis: Researching a Dialogue between the Brain and Peripheral Tissues. Pharmacol. Res. 2020, 163, 105215. [Google Scholar] [CrossRef]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.; Newman, J.W.; Garvey, W.T. Plasma Acylcarnitine Profiles Suggest Incomplete Long-Chain Fatty Acid β-Oxidation and Altered Tricarboxylic Acid Cycle Activity in Type 2 Diabetic African-American Women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines—Old Actors Auditioning for New Roles in Metabolic Physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef]
- Chrast, R.; Saher, G.; Nave, K.-A.; Verheijen, M.H.G. Lipid Metabolism in Myelinating Glial Cells: Lessons from Human Inherited Disorders and Mouse Models. J. Lipid Res. 2011, 52, 419–434. [Google Scholar] [CrossRef]
- Yoon, H.; Shaw, J.L.; Haigis, M.C.; Greka, A. Lipid Metabolism in Sickness and in Health: Emerging Regulators of Lipotoxicity. Mol. Cell 2021, 81, 3708–3730. [Google Scholar] [CrossRef]
- Guerra, I.M.S.; Ferreira, H.B.; Melo, T.; Rocha, H.; Moreira, S.; Diogo, L.; Domingues, M.R.M.; Moreira, A.S.P. Mitochondrial Fatty Acid β-Oxidation Disorders: From Disease to Lipidomic Studies—A Critical Review. Int. J. Mol. Sci. 2022, 23, 13933. [Google Scholar] [CrossRef]
- Dustin, E.; Suárez-Pozos, E.; Stotesberry, C.; Qiu, S.; Palavicini, J.P.; Han, X.; Dupree, J.L. Compromised Myelin and Axonal Molecular Organization Following Adult-Onset Sulfatide Depletion. Biomedicines 2023, 11, 1431. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-deMiguel, P.; Martínez-Guardado, I.; Dalamitros, A.A.; Yañéz-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.R.; Saxena, S. Schwann Cells in Neuromuscular Disorders: A Spotlight on Amyotrophic Lateral Sclerosis. Cells 2025, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Wadan, A.S.; Shaaban, A.; El-Sadek, M.; Mostafa, S.A.S.; Moshref, A.S.; El-Hussein, A.; Ellakwa, D.E.; Mehanny, S.S. Mitochondrial-Based Therapies for Neurodegenerative Diseases: A Review of the Current Literature. Naunyn-Schmiedeberg Arch. Pharmacol. 2025, 398, 11357–11386. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, R.; Wang, X.; Li, T.; Dong, X.; Zhao, C.; Li, X. Integrating Lipidomics and Transcriptomics Reveals the Crosstalk Between Oxidative Stress and Neuroinflammation in Central Nervous System Demyelination. Front. Aging Neurosci. 2022, 14, 870957. [Google Scholar] [CrossRef]
- Escobar, E.L.N.; Mohotti, N.D.S.; Manolescu, M.; Radadiya, A.; Dhar, P.; Hartley, M.D. Reduced Cholesterol Alters the Biophysical Properties of Repaired Myelin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159637. [Google Scholar] [CrossRef]
- Seidah, N.G.; Benjannet, S.; Wickham, L.A.; Marcinkiewicz, J.; Jasmin, S.B.; Stifani, S.; Basak, A.; Prat, A.; Chrétien, M. The Secretory Proprotein Convertase Neural Apoptosis-Regulated Convertase 1 (NARC-1): Liver Regeneration and Neuronal Differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef]
- Lu, F.; Ferriero, D.M.; Jiang, X. Cholesterol in Brain Development and Perinatal Brain Injury: More than a Building Block. Curr. Neuropharmacol. 2021, 20, 1400–1412. [Google Scholar] [CrossRef]
- Qian, L.; Chai, A.B.; Gelissen, I.C.; Brown, A.J. Balancing Cholesterol in the Brain: From Synthesis to Disposal. Explor. Neuroprot. Ther. 2022, 2, 1–27. [Google Scholar] [CrossRef]
- Cesaro, A.; Bianconi, V.; Gragnano, F.; Moscarella, E.; Fimiani, F.; Monda, E.; Scudiero, O.; Limongelli, G.; Pirro, M.; Calabrò, P. Beyond Cholesterol Metabolism: The Pleiotropic Effects of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9). Genetics, Mutations, Expression, and Perspective for Long-term Inhibition. BioFactors 2020, 46, 367–380. [Google Scholar] [CrossRef]
- Lu, F.; Li, E.; Yang, X. The association between circulatory, local pancreatic PCSK9 and type 2 diabetes mellitus: The effects of antidiabetic drugs on PCSK9. Heliyon 2023, 9, e19371. [Google Scholar] [CrossRef]
- Rao, M.; Nelms, B.; Dong, L.; Salinas-Rios, V.; Rutlin, M.; Gershon, M.D.; Corfas, G. Enteric Glia Express Proteolipid Protein 1 and Are a Transcriptionally Unique Population of Glia in the Mammalian Nervous System. Glia 2015, 63, 2040–2057. [Google Scholar] [CrossRef]
- Pannese, E. Quantitative, Structural and Molecular Changes in Neuroglia of Aging Mammals: A Review. Eur. J. Histochem. 2021, 65, 3249. [Google Scholar] [CrossRef]
- Han, S.B.; Gim, Y.; Jang, E.H.; Hur, E.M. Functions and Dysfunctions of Oligodendrocytes in Neurodegenerative Diseases. Front. Cell. Neurosci. 2022, 16, 1083159. [Google Scholar] [CrossRef]
- Kent, S.A.; Miron, V.E. Microglia Regulation of Central Nervous System Myelin Health and Regeneration. Nat. Rev. Immunol. 2023, 24, 49–63. [Google Scholar] [CrossRef]
- Muzio, L.; Perego, J. CNS Resident Innate Immune Cells: Guardians of CNS Homeostasis. Int. J. Mol. Sci. 2024, 25, 4865. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Weeber, E.J. Reelin and apoE Actions on Signal Transduction, Synaptic Function and Memory Formation. Neuron Glia Biol. 2008, 4, 259–270. [Google Scholar] [CrossRef]
- Roubtsova, A.; Garçon, D.; Lacoste, S.; Chamberland, A.; Marcinkiewicz, J.; Métivier, R.; Sotin, T.; Paquette, M.; Bernard, S.; Cariou, B.; et al. PCSK9 Deficiency Results in a Specific Shedding of Excess LDLR in Female Mice Only: Role of Hepatic Cholesterol. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159217. [Google Scholar] [CrossRef]
- Kysenius, K.; Muggalla, P.; Mätlik, K.; Arumäe, U.; Huttunen, H.J. PCSK9 Regulates Neuronal Apoptosis by Adjusting ApoER2 Levels and Signaling. Cell. Mol. Life Sci. 2012, 69, 1903–1916. [Google Scholar] [CrossRef]
- Papotti, B.; Adorni, M.P.; Marchi, C.; Zimetti, F.; Ronda, N.; Panighel, G.; Lupo, M.G.; Vilella, A.; Giuliani, D.; Ferri, N.; et al. PCSK9 Affects Astrocyte Cholesterol Metabolism and Reduces Neuron Cholesterol Supplying In Vitro: Potential Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12192. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, T.; Luo, J.; Zhang, X.; Wang, T.; Wang, Y.; Ma, Y.; Yang, B.; Jia, J.; Dmytriw, A.A.; et al. PCSK9 Increases Vulnerability of Carotid Plaque by Promoting Mitochondrial Dysfunction and Apoptosis of Vascular Smooth Muscle Cells. CNS Neurosci. Ther. 2024, 30, e14640. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, T.; Li, G.; Xu, L.; Zhang, Y. PCSK9 Inhibitor Protects against Ischemic Cerebral Injury by Attenuating Inflammation via the GPNMB/CD44 Pathway. Int. Immunopharmacol. 2023, 126, 111195. [Google Scholar] [CrossRef]
- Shin, D.; Kim, S.; Lee, H.; Lee, H.-C.; Lee, J.; Park, H.E.; Fukai, M.; Choi, E.; Choi, S.; Koo, B.-J.; et al. PCSK9 Stimulates Syk, PKCδ, and NF-κB, Leading to Atherosclerosis Progression Independently of LDL Receptor. Nat. Commun. 2024, 15, 2789. [Google Scholar] [CrossRef]
- Papotti, B.; Bertolotti, M.; Marchi, C.; Adorni, M.P.; Chiari, A.; Bedin, R.; Lupo, M.G.; Elviri, L.; Remaggi, G.; Baldelli, E.; et al. Serum and Cerebrospinal Fluid Concentrations of PCSK9 and Hydroxysterols in Patients with Cognitive Impairment. Atherosclerosis 2022, 355, 26. [Google Scholar] [CrossRef]
- Zimetti, F.; Caffarra, P.; Ronda, N.; Favari, E.; Adorni, M.P.; Zanotti, I.; Bernini, F.; Barocco, F.; Spallazzi, M.; Galimberti, D.; et al. Increased PCSK9 Cerebrospinal Fluid Concentrations in Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 55, 315–320. [Google Scholar] [CrossRef]
- Husain, M.A.; Laurent, B.; Laurent, B.; Plourde, M.; Plourde, M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front. Neurosci. 2021, 15, 630502. [Google Scholar] [CrossRef]
- Mahley, R.W. Central Nervous System Lipoproteins ApoE and Regulation of Cholesterol Metabolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1305–1315. [Google Scholar] [CrossRef]
- Vigne, S.; Duc, D.; Peter, B.; Rebeaud, J.; Yersin, Y.; Ruiz, F.; Bressoud, V.; Collet, T.; Pot, C. Lowering Blood Cholesterol Does Not Affect Neuroinflammation in Experimental Autoimmune Encephalomyelitis. J. Neuroinflamm. 2022, 19, 42. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Qiao, H.; Yang, L.; Zhang, P.; Wang, J.; Zhang, C.; Jiang, W.; Xu, R.; Zhang, X. Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor Ameliorates Cerebral Ischemia in Mice by Inhibiting Inflammation. J. Stroke Cerebrovasc. Dis. 2023, 33, 107517. [Google Scholar] [CrossRef]
- Pu, S.; Jia, C.; Li, Z.; Zang, Y. Protective Mechanism of Proprotein Convertase Subtilisin-Like Kexin Type 9 Inhibitor on Rats with Middle Cerebral Artery Occlusion-Induced Cerebral Ischemic Infarction. Comput. Intell. Neurosci. 2022, 2022, 964262. [Google Scholar] [CrossRef] [PubMed]
- Apaijai, N.; Moisescu, D.M.; Palee, S.; McSweeney, C.M.; Saiyasit, N.; Maneechote, C.; Boonnag, C.; Chattipakorn, N.; Chattipakorn, S.C. Pretreatment With PCSK9 Inhibitor Protects the Brain Against Cardiac Ischemia/Reperfusion Injury Through a Reduction of Neuronal Inflammation and Amyloid Beta Aggregation. J. Am. Heart Assoc. 2019, 8, e010838. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, E.M.; Lohoff, F.W. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in the Brain and Relevance for Neuropsychiatric Disorders. Front. Neurosci. 2020, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, H.; Bigot, E.; Pichelin, M.; Guyomarch, B.; Boutoleau-Brétonnière, C.; May, C.L.; Derkinderen, P.; Cariou, B. PCSK9 Concentrations in Cerebrospinal Fluid Are Not Specifically Increased in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1519–1525. [Google Scholar] [CrossRef]
- Lee, J.S.; O’Connell, E.M.; Pacher, P.; Lohoff, F.W. PCSK9 and the Gut-Liver-Brain Axis: A Novel Therapeutic Target for Immune Regulation in Alcohol Use Disorder. J. Clin. Med. 2021, 10, 1758. [Google Scholar] [CrossRef]
- Mbikay, M.; Mayne, J.; Chrétien, M. Proprotein Convertases Subtilisin/Kexin Type 9, an Enzyme Turned Escort Protein: Hepatic and Extra Hepatic Functions. J. Diabetes 2013, 5, 391–405. [Google Scholar] [CrossRef]
- D’Arcangelo, G. Apoer2: A Reelin Receptor to Remember. Neuron 2005, 47, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Giannelli, S.; Staurenghi, E.; Cecci, R.; Floro, L.; Gamba, P.; Sottero, B.; Leonarduzzi, G. The Emerging Role of PCSK9 in the Pathogenesis of Alzheimer’s Disease: A Possible Target for the Disease Treatment. Int. J. Mol. Sci. 2024, 25, 13637. [Google Scholar] [CrossRef]
- Wagner, J.; Park, L.M.; Mukhopadhyay, P.; Mátyás, C.; Trojnár, E.; Damadzic, R.; Jung, J.; Bell, A.S.; Mavromatis, L.A.; Hamandi, A.; et al. PCSK9 Inhibition Attenuates Alcohol-Associated Neuronal Oxidative Stress and Cellular Injury. Brain Behav. Immun. 2024, 119, 494–506. [Google Scholar] [CrossRef]
- Papotti, B.; Palumbo, M.; Adorni, M.P.; Elviri, L.; Chiari, A.; Tondelli, M.; Bedin, R.; Baldelli, E.; Lancellotti, G.; Lupo, M.G.; et al. Influence of APOE4 Genotype on PCSK9-Lipids Association in Cerebrospinal Fluid and Serum of Patients in the Alzheimer’s Disease Continuum. J. Alzheimer’s Dis. 2024, 102, 162–172. [Google Scholar] [CrossRef]
- Benjannet, S.; Rhainds, D.; Hamelin, J.; Nassoury, N.; Seidah, N.G. The Proprotein Convertase (PC) PCSK9 Is Inactivated by Furin and/or PC5/6A. J. Biol. Chem. 2006, 281, 30561–30572. [Google Scholar] [CrossRef] [PubMed]
- Abdo, H.; Calvo-Enrique, L.; Martínez-López, J.A.; Song, J.; Zhang, M.-D.; Usoskin, D.; Manira, A.E.; Adameyko, I.; Hjerling-Leffler, J.; Ernfors, P. Specialized Cutaneous Schwann Cells Initiate Pain Sensation. Science 2019, 365, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Pannese, E. Biology and Pathology of Perineuronal Satellite Cells in Sensory Ganglia; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Milosavljević, A.; Jančić, J.; Mirčić, A.; Dožić, A.; Boljanović, J.; Milisavljević, M.; Ćetković, M. Morphological and Functional Characteristics of Satellite Glial Cells in the Peripheral Nervous System. Folia Morphol. 2020, 80, 745–755. [Google Scholar] [CrossRef]
- Hanani, M.; Verkhratsky, A. Satellite Glial Cells and Astrocytes, a Comparative Review. Neurochem. Res. 2021, 46, 2525–2537. [Google Scholar] [CrossRef]
- Andreeva, D.D.; Murashova, L.A.; Burzak, N.; Dyachuk, V. Satellite Glial Cells: Morphology, Functional Heterogeneity, and Role in Pain. Front. Cell. Neurosci. 2022, 16, 1019449. [Google Scholar] [CrossRef]
- Lu, J.; Wang, D.; Xu, J.; Zhang, H.; Yu, W. New Insights on the Role of Satellite Glial Cells. Stem Cell Rev. Rep. 2022, 19, 358–367. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, Y.; Da, X.; Wang, Y.; Chen, Z.; Xu, C. Satellite Glial Cells in Sensory Ganglia Play a Wider Role in Chronic Pain via Multiple Mechanisms. Neural Regen. Res. 2023, 19, 1056–1063. [Google Scholar] [CrossRef]
- Rastoldo, G.; Jaafar, A.K. Aurélie-Paulo-Ramos; Thouvenot, K.; Planesse, C.; Bringart, M.; Gonthier, M.; Lambert, G.; Bourane, S. Alteration of Nociceptive Schwann Cells in a Mouse Model of High-Fat Diet Induced Diabetic Peripheral Neuropathy. bioRxiv 2024. [Google Scholar] [CrossRef]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann Cell Functions in Peripheral Nerve Development and Repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Yuan, Y.; Huang, L.; Bian, M.; Yu, J.; Zou, J.; Jiang, L.; Meng, D.; Zhang, J. Inhibition of Schwann Cell Pyroptosis Promotes Nerve Regeneration in Peripheral Nerve Injury in Rats. Mediat. Inflamm. 2023, 2023, 721375. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, S.; Ming, L.; Yu, J.; Zuo, C.; Hu, D.; Luo, H.; Zhang, Q. Potential Role of Schwann Cells in Neuropathic Pain. Eur. J. Pharmacol. 2023, 956, 175955. [Google Scholar] [CrossRef]
- Donovan, L.J.; Brewer, C.L.; Bond, S.F.; Lopez, A.P.; Hansen, L.; Jordan, C.E.; González, O.C.; de Lecea, L.; Kauer, J.A.; Tawfik, V.L. Aging and Injury Drive Neuronal Senescence in the Dorsal Root Ganglia. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Perez-Matos, M.C.; Morales-Alvarez, M.C.; Mendivil, C.O. Lipids: A Suitable Therapeutic Target in Diabetic Neuropathy? Exp. Diabetes Res. 2017, 2017, 6943851. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, P.; Gylfadottir, S.S.; Kristensen, A.G.; Ramirez, J.D.; Cruz, P.F.; Le, N.; Shillo, P.; Tesfaye, S.; Rice, A.S.C.; Tankişi, H.; et al. Axonal Swellings Are Related to Type 2 Diabetes, but Not to Distal Diabetic Sensorimotor Polyneuropathy. Diabetologia 2021, 64, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Morbin, M.; Lombardi, R.; Borgna, M.; Mazzoleni, G.; Sghirlanzoni, A.; Pareyson, D. Axonal Swellings Predict the Degeneration of Epidermal Nerve Fibers in Painful Neuropathies. Neurology 2003, 61, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Yoshikawa, H.; Fujimura, H.; Naba, I.; Sumi-Akamaru, H.; Takayasu, S.; Itabe, H.; Sakoda, S. The Role of CD36 in Peripheral Nerve Remyelination after Crush Injury. Eur. J. Neurosci. 2003, 17, 2659–2666. [Google Scholar] [CrossRef]
- Viader, A.; Golden, J.P.; Baloh, R.H.; Schmidt, R.E.; Hunter, D.; Milbrandt, J. Schwann Cell Mitochondrial Metabolism Supports Long-Term Axonal Survival and Peripheral Nerve Function. J. Neurosci. 2011, 31, 10128–10140. [Google Scholar] [CrossRef]
- Beirowski, B.; Babetto, E.; Golden, J.P.; Chen, Y.-J.; Yang, K.; Gross, R.W.; Patti, G.J.; Milbrandt, J. Metabolic Regulator LKB1 Is Crucial for Schwann Cell-Mediated Axon Maintenance. Nat. Neurosci. 2014, 17, 1351–1361. [Google Scholar] [CrossRef]
- Schaeren-Wiemers, N.; Bonnet, A.; Erb, M.; Erne, B.; Bartsch, U.; Kern, F.; Mantei, N.; Sherman, D.L.; Suter, U. The Raft-Associated Protein MAL Is Required for Maintenance of Proper Axon–Glia Interactions in the Central Nervous System. J. Cell Biol. 2004, 166, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.B.; Chung, W.; Sloan, S.A.; Carson, G.A.; Zhou, L.; Lovelett, E.; Posada, S.; Zuchero, J.B.; Barres, B.A. Schwann Cells Use TAM Receptor-Mediated Phagocytosis in Addition to Autophagy to Clear Myelin in a Mouse Model of Nerve Injury. Proc. Natl. Acad. Sci. USA 2017, 114, E8072–E8080. [Google Scholar] [CrossRef]
- Grajchen, E.; Wouters, E.; van de Haterd, B.; Haidar, M.; Hardonnière, K.; Dierckx, T.; Broeckhoven, J.V.; Erens, C.; Hendrix, S.; Kerdine-Römer, S.; et al. CD36-Mediated Uptake of Myelin Debris by Macrophages and Microglia Reduces Neuroinflammation. J. Neuroinflamm. 2020, 17, 224. [Google Scholar] [CrossRef] [PubMed]
- Thumm, M.; Simons, M. Myelinophagy: Schwann Cells Dine In. J. Cell Biol. 2015, 210, 9–10. [Google Scholar] [CrossRef]
- Dimas, P.; Montani, L.; Pereira, J.A.; Moreno, D.; Trötzmüller, M.; Gerber, J.; Semenkovich, C.F.; Köfeler, H.; Suter, U. CNS Myelination and Remyelination Depend on Fatty Acid Synthesis by Oligodendrocytes. eLife 2019, 8, e44702. [Google Scholar] [CrossRef]
- Zheng, B.; He, Y.; Yin, S.; Zhu, X.; Zhao, Q.; Yang, H.; Wang, Z.; Zhu, R.; Cheng, Q. Unresolved Excess Accumulation of Myelin-Derived Cholesterol Contributes to Scar Formation after Spinal Cord Injury. Research 2023, 6, 0135. [Google Scholar] [CrossRef]
- Chung, H.; Ye, Q.; Park, Y.-J.; Zuo, Z.; Mok, J.-W.; Kanca, O.; Tattikota, S.G.; Lu, S.; Perrimon, N.; Lee, H.K.; et al. Very-Long-Chain Fatty Acids Induce Glial-Derived Sphingosine-1-Phosphate Synthesis, Secretion, and Neuroinflammation. Cell Metab. 2023, 35, 855–874.e5. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Wang, Y.; Du, W.; Yang, R.; Wu, J.; Li, Y. Lipid Droplet Accumulation in Microglia and Their Potential Roles. Lipids Health Dis. 2025, 24, 215. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Sumi, H.; Fujimura, H.; Yoshikawa, H.; Sakoda, S. Pioglitazone Promotes Peripheral Nerve Remyelination after Crush Injury through CD36 Upregulation. J. Peripher. Nerv. Syst. 2008, 13, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Wang, Y.; Bao, H.; Zhang, Z.; Yang, N.; Meng, J.; Zhang, Q.; Jian, A.; Ji, R.; Zhang, L.; et al. Lipid Synthesis, Triggered by PPARγ T166 Dephosphorylation, Sustains Reparative Function of Macrophages during Tissue Repair. Nat. Commun. 2024, 15, 7269. [Google Scholar] [CrossRef]
- Dalt, L.D.; Ruscica, M.; Bonacina, F.; Balzarotti, G.; Dhyani, A.; Cairano, E.D.; Baragetti, A.; Arnaboldi, L.; Metrio, S.D.; Pellegatta, F.; et al. PCSK9 Deficiency Reduces Insulin Secretion and Promotes Glucose Intolerance: The Role of the Low-Density Lipoprotein Receptor. Eur. Heart J. 2018, 40, 357–368. [Google Scholar] [CrossRef]
- Dalt, L.D.; Castiglioni, L.; Baragetti, A.; Audano, M.; Svecla, M.; Bonacina, F.; Pedretti, S.; Uboldi, P.; Benzoni, P.; Giannetti, F.; et al. PCSK9 Deficiency Rewires Heart Metabolism and Drives Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2021, 42, 3078–3090. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Fatty Acids and Evolving Roles of Their Proteins in Neurological, Cardiovascular Disorders and Cancers. Prog. Lipid Res. 2021, 83, 101116. [Google Scholar] [CrossRef]
- Rhea, E.M.; Rhea, E.M.; Banks, W.A.; Banks, W.A. Interactions of Lipids, Lipoproteins, and Apolipoproteins with the Blood-Brain Barrier. Pharm. Res. 2021, 38, 1469–1475. [Google Scholar] [CrossRef]
- Renne, M.F.; Hariri, H. Lipid Droplet-Organelle Contact Sites as Hubs for Fatty Acid Metabolism, Trafficking, and Metabolic Channeling. Front. Cell Dev. Biol. 2021, 9, 726261. [Google Scholar] [CrossRef]
- Lebeau, P.; Byun, J.H.; Platko, K.; Al-Hashimi, A.; Lhoták, Š.; MacDonald, M.E.; Mejía-Benítez, A.; Prat, A.; Igdoura, S.A.; Trigatti, B.L.; et al. Pcsk9 Knockout Exacerbates Diet-Induced Non-Alcoholic Steatohepatitis, Fibrosis and Liver Injury in Mice. JHEP Rep. 2019, 1, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-H.; Liu, T.; Wang, H.; Xue, L.; Wang, J. Fatty Acids Metabolism: The Bridge Between Ferroptosis and Ionizing Radiation. Front. Cell Dev. Biol. 2021, 9, 675617. [Google Scholar] [CrossRef]
- Obaseki, E.; Adebayo, D.; Bandyopadhyay, S.; Hariri, H. Lipid Droplets and Fatty Acid-induced Lipotoxicity: In a Nutshell. FEBS Lett. 2024, 598, 1207–1214. [Google Scholar] [CrossRef]
- Wang, Y.; Moura, A.K.; Zuo, R.; Wang, Z.; Roudbari, K.; Hu, J.Z.; Wang, M.; Li, P.; Zhang, Y.; Li, X.C. Defective Lipid Droplet Biogenesis Exacerbates Oleic Acid-Induced Cellular Homeostasis Disruption and Ferroptosis in Mouse Cardiac Endothelial Cells. Cell Death Discov. 2025, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Duong, Q.V.; Levitsky, Y.; Dessinger, M.J.; Bazil, J.N. Identifying Site-Specific Superoxide and Hydrogen Peroxide Production Rates from the Mitochondrial Electron Transport System Using a Computational Strategy. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Tyurina, Y.Y.; Tyurin, V.A.; Mohammadyani, D.; Angeli, J.P.F.; Баранoв, В.С.; Klein-Seetharaman, J.; Friedlander, R.M.; Mallampalli, R.K.; Conrad, M.; et al. Cardiolipin Signaling Mechanisms: Collapse of Asymmetry and Oxidation. Antioxid. Redox Signal. 2015, 22, 1667–1680. [Google Scholar] [CrossRef]
- Di Paolo, G.; Przedborski, S. When Schwann Cells Conspire with Mitochondria, Neighboring Axons Are under Attack by Glia-Derived Neurotoxic Lipids. Neuron 2013, 77, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macías-Cámara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann Cell Autophagy, Myelinophagy, Initiates Myelin Clearance from Injured Nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef]
- Cartelli, D.; Cavaletti, G.; Lauria, G.; Meregalli, C. Ubiquitin Proteasome System and Microtubules Are Master Regulators of Central and Peripheral Nervous System Axon Degeneration. Cells 2022, 11, 1358. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Summerhill, V.I.; Khotina, V.A.; Popov, M.А.; Uzokov, J.; Sukhorukov, V.N. Role of Mitochondria in the Chronification of Inflammation: Focus on Dysfunctional Mitophagy and Mitochondrial DNA Mutations. Gene Expr. 2023, 22, 329–344. [Google Scholar] [CrossRef]
- Espinoza, N.; Papadopoulos, V. Role of Mitochondrial Dysfunction in Neuropathy. Int. J. Mol. Sci. 2025, 26, 3195. [Google Scholar] [CrossRef]
- Goodman, S.G.; Steg, P.G.; Poulouin, Y.; Bhatt, D.L.; Bittner, V.; Díaz, R.; Garon, G.; Harrington, R.A.; Jukema, J.W.; Manvelian, G.; et al. Long-Term Efficacy, Safety, and Tolerability of Alirocumab in 8242 Patients Eligible for 3 to 5 Years of Placebo-Controlled Observation in the ODYSSEY OUTCOMES Trial. J. Am. Heart Assoc. 2023, 12, e029216. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Zhang, G.; Davidson, M.T.; Chaves, A.; Crown, S.B.; Johnson, J.M.; Slentz, D.H.; Grimsrud, P.A.; Muoio, D.M. Pyruvate-Supported Flux through Medium-Chain Ketothiolase Promotes Mitochondrial Lipid Tolerance in Cardiac and Skeletal Muscles. Cell Metab. 2023, 35, 1038–1056.e8. [Google Scholar] [CrossRef] [PubMed]
- Brandts, J.; Ray, K.K. Clinical Implications and Outcomes of the Orion Phase Iii Trials. Future Cardiol. 2020, 17, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H.; Hobbs, H.H. Sequence Variations inPCSK9,Low LDL, and Protection against Coronary Heart Disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Ubogu, E.; Dong, C.; Choudhary, A. Glial Derived Neurotrophic Factor: A Sufficient Essential Molecular Regulator of Mammalian Blood-Nerve Barrier Tight Junction Formation. Neural Regen. Res. 2020, 16, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Köenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lagace, T.A.; McNutt, M.; Horton, J.D.; Deisenhofer, J. Molecular Basis for LDL Receptor Recognition by PCSK9. Proc. Natl. Acad. Sci. USA 2008, 105, 1820–1825. [Google Scholar] [CrossRef]
- Rohrbach, S.; Li, L.; Novoyatleva, T.; Niemann, B.; Knapp, F.; Molenda, N.; Schulz, R. Impact of PCSK9 on CTRP9-Induced Metabolic Effects in Adult Rat Cardiomyocytes. Front. Physiol. 2021, 12, 593862. [Google Scholar] [CrossRef]
- Tang, V.T.; McCormick, J.; Xu, B.; Wang, Y.; Fang, H.; Wang, X.; Siemieniak, D.; Khoriaty, R.; Emmer, B.T.; Chen, X.; et al. Hepatic Inactivation of Murine Surf4 Results in Marked Reduction in Plasma Cholesterol. eLife 2022, 11, e82269. [Google Scholar] [CrossRef]
- Wright, R.S.; Ray, K.K.; Landmesser, U.; Köenig, W.; Raal, F.J.; Leiter, L.A.; Conde, L.G.; Han, J.; Schwartz, G.G. Effects of Inclisiran in Patients With Atherosclerotic Cardiovascular Disease. Mayo Clin. Proc. 2024, 99, 1222–1235. [Google Scholar] [CrossRef]
- Sinnaeve, P.; Schwartz, G.G.; Wojdyla, D.; Alings, M.; Bhatt, D.L.; Bittner, V.; Chiang, C.; Correa, R.; Díaz, R.; Dorobanţu, M.; et al. Effect of Alirocumab on Cardiovascular Outcomes after Acute Coronary Syndromes According to Age: An ODYSSEY OUTCOMES Trial Analysis. Eur. Heart J. 2019, 41, 2248–2258. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Estrella, A.M.; Skavdis, A.; Genao, E.P.; Martínez, I.F.; Guzman, E. Inclisiran for the Treatment of Cardiovascular Disease: A Short Review on the Emerging Data and Therapeutic Potential. Ther. Clin. Risk Manag. 2020, 16, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Wright, R.S.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-Term Efficacy and Safety of Inclisiran in Patients with High Cardiovascular Risk and Elevated LDL Cholesterol (ORION-3): Results from the 4-Year Open-Label Extension of the ORION-1 Trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef]
- White, H.D.; Schwartz, G.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.; Chiang, C.; Díaz, R.; Goodman, S.G.; Jukema, J.W.; Loy, M.; et al. Alirocumab after Acute Coronary Syndrome in Patients with a History of Heart Failure. Eur. Heart J. 2021, 43, 1554–1565. [Google Scholar] [CrossRef]
- Gencer, B.; Mach, F.; Guo, J.; Yu, C.; Ruzza, A.; Wang, H.; Kurtz, C.E.; Pedersen, T.R.; Keech, A.; Ott, B.R.; et al. Cognition After Lowering LDL-Cholesterol with Evolocumab. J. Am. Coll. Cardiol. 2020, 75, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Zimerman, A.; O’Donoghue, M.L.; Ran, X.; Im, K.; Ott, B.R.; Mach, F.; Zavitz, K.H.; Kurtz, C.E.; Monsalvo, M.L.; Wang, B.; et al. Long-Term Cognitive Safety of Achieving Very Low LDL Cholesterol with Evolocumab. NEJM Evid. 2024, 4, 2400112. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, J.; Gong, H.; Cai, X.; Han, X.; Gao, W. Psychiatric Disorders Associated with PCSK9 Inhibitors: A Real-world, Pharmacovigilance Study. CNS Neurosci. Ther. 2023, 30, e14522. [Google Scholar] [CrossRef]
- Zulehner, G.; Seidel, S.; Polanz, A.; Schörgenhofer, C.; Rommer, P.; Merrelaar, M.; von Roth, D.; Herkner, H.; Behrens, S.; Kienbacher, C.L. Lower Serum Cholesterol Levels as a Risk Factor for Critical Illness Polyneuropathy: A Matched Case–Control Study. Sci. Rep. 2023, 13, 20405. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Mach, F.; Zavitz, K.H.; Kurtz, C.E.; Schneider, J.; Wang, H.; Keech, A.; Pedersen, T.R.; Sabatine, M.S.; Sever, P.S.; et al. Design and Rationale of the EBBINGHAUS Trial: A Phase 3, Double-blind, Placebo-controlled, Multicenter Study to Assess the Effect of Evolocumab on Cognitive Function in Patients with Clinically Evident Cardiovascular Disease and Receiving Statin Background Lipid-lowering Therapy—A Cognitive Study of Patients Enrolled in the FOURIER Trial. Clin. Cardiol. 2017, 40, 59–65. [Google Scholar] [CrossRef]
- Gaudet, D.; López-Sendón, J.; Averna, M.; Bigot, G.; Banach, M.; Letierce, A.; Loy, M.; Samuel, R.; Manvelian, G.; Batsu, I.; et al. Safety and Efficacy of Alirocumab in a Real-Life Setting: The ODYSSEY APPRISE Study. Eur. J. Prev. Cardiol. 2020, 28, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Mazura, A.D.; Ohler, A.; Storck, S.E.; Kurtyka, M.; Scharfenberg, F.; Weggen, S.; Becker-Pauly, C.; Pietrzik, C.U. PCSK9 Acts as a Key Regulator of Aβ Clearance across the Blood–Brain Barrier. Cell. Mol. Life Sci. 2022, 79, 212. [Google Scholar] [CrossRef]
- Alannan, M.; Seidah, N.G.; Merched, A. PCSK9 in Liver Cancers at the Crossroads between Lipid Metabolism and Immunity. Cells 2022, 11, 4132. [Google Scholar] [CrossRef]
- Nishikido, T. Clinical Potential of Inclisiran for Patients with a High Risk of Atherosclerotic Cardiovascular Disease. Cardiovasc. Diabetol. 2023, 22, 20. [Google Scholar] [CrossRef]
- Goodman, S.G.; Steg, P.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Harrington, R.A.; Jukema, J.; White, H.D.; Zeiher, A.M.; et al. Safety of the Pcsk9 Inhibitor Alirocumab: Insights From 47,296 Patient-Years of Observation. Eur. Heart J.-Cardiovasc. Pharmacother. 2024, 10, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Bajaj, A.; Brousseau, M.E.; Taub, P.R. Harnessing RNA Interference for Cholesterol Lowering: The Bench-to-Bedside Story of Inclisiran. J. Am. Heart Assoc. 2024, 13, e032031. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Romano, M.; Vecchio, M. A Systematic Review of the Diagnostic Methods of Small Fiber Neuropathies in Rehabilitation. Diagnostics 2020, 10, 613. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Bitirgen, G.; Ferdousi, M.; Kalteniece, A.; Azmi, S.; D’Onofrio, L.; Lim, S.H.; Ponirakis, G.; Khan, A.; Gad, H.; et al. Corneal Confocal Microscopy to Image Small Nerve Fiber Degeneration: Ophthalmology Meets Neurology. Front. Pain Res. 2021, 2, 725363. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.M.; Wylęgała, A.; Gargiulo, L.; Inferrera, L.; Russo, M.; Mencucci, R.; Orzechowska-Wylęgała, B.; Aragona, E.; Mancini, M.; Quartarone, A. Corneal Sub-Basal Nerve Plexus in Non-Diabetic Small Fiber Polyneuropathies and the Diagnostic Role of In Vivo Corneal Confocal Microscopy. Stomatology 2023, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- Seijas-Amigo, J.; Mauriz-Montero, M.J.; Suárez-Artime, P.; Gayoso-Rey, M.; Estany-Gestal, A.; Casas-Martínez, A.; González-Freire, L.; Rodriguez-Vazquez, A.; Pérez-Rodríguez, N.; Villaverde-Piñeiro, L.; et al. Cognitive Function with PCSK9 Inhibitors: A 24-Month Follow-Up Observational Prospective Study in the Real World—MEMOGAL Study. Am. J. Cardiovasc. Drugs 2023, 23, 583–593. [Google Scholar] [CrossRef]
- Zhou, Z.; Ryan, J.; Tonkin, A.M.; Zoungas, S.; Lacaze, P.; Wolfe, R.; Orchard, S.G.; Murray, A.; Mcneil, J.J.; Yu, C.; et al. Association Between Triglycerides and Risk of Dementia in Community-Dwelling Older Adults: A Prospective Cohort Study. Neurology 2023, 101, E2288–E2299. [Google Scholar] [CrossRef]
- Quaytman, J.A.; David, N.L.; Venugopal, S.; Amorim, T.; Beatrice, B.; Toledo, F.G.S.; Miller, R.G.; Steinhauser, M.L.; Fazeli, P.K. Intermittent Fasting for Systemic Triglyceride Metabolic Reprogramming (IFAST): Design and Methods of a Prospective, Randomized, Controlled Trial. Contemp. Clin. Trials 2024, 146, 107698. [Google Scholar] [CrossRef]
- Cox, J.E.J.; Pham, K.D.; Keck, A.W.; Wright, Z.; Thomas, M.A.; Freeman, W.M.; Ocañas, S.R. Flow Cytometry Analysis of Microglial Phenotypes in the Murine Brain During Aging and Disease. Bio-Protocol 2024, 14, e5018. [Google Scholar] [CrossRef]
- Preka, E.; Romero, A.L.; Sun, Y.; Vilalta, Y.O.; Seitz, T.; Fragkopoulou, A.; Betsholtz, C.; Osman, A.M.; Blomgren, K. Rapid and Robust Isolation of Microglia and Vascular Cells from Brain Subregions for Integrative Single-Cell Analyses. Heliyon 2024, 10, e35838. [Google Scholar] [CrossRef]
- Chen, C.; Shu, Y.; Yan, C.; Li, H.; Huang, Z.; Shen, S.; Liu, C.; Jiang, Y.; Huang, S.; Wang, Z.; et al. Astrocyte-Derived Clusterin Disrupts Glial Physiology to Obstruct Remyelination in Mouse Models of Demyelinating Diseases. Nat. Commun. 2024, 15, 7791. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Alonso, J.; Calvo-Enrique, L.; Paricio-Montesinos, R.; Kumar, R.; Zhang, M.-D.; Poulet, J.F.A.; Ernfors, P.; Lewin, G.R. Sensory Schwann Cells Set Perceptual Thresholds for Touch and Selectively Regulate Mechanical Nociception. Nat. Commun. 2024, 15, 898. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, E.; Qu, C.; Ji, G.; Neugebauer, V.; Guerrero, M.; Rosen, H.; Roberts, E.; Porreca, F. Opposing Effects on Descending Control of Nociception by µ and κ Opioid Receptors in the Anterior Cingulate Cortex. Anesthesiology 2023, 140, 272–283. [Google Scholar] [CrossRef]
- An, Y.; Chen, S.; Deng, Y.; Wang, Z.V.; Funcke, J.; Shah, M.; Shan, B.; Gordillo, R.; Yoshino, J.; Klein, S.; et al. The Mitochondrial Dicarboxylate Carrier Prevents Hepatic Lipotoxicity by Inhibiting White Adipocyte Lipolysis. J. Hepatol. 2021, 75, 387–399. [Google Scholar] [CrossRef]
- Tong, L.; Yang, W.; Luo, W.; Zhang, L.; Mai, Y.; Li, Z.; Liu, S.; Lü, J.; Liu, P.; Li, Z. Disturbance of Fatty Acid Metabolism Promoted Vascular Endothelial Cell Senescence via Acetyl-CoA-Induced Protein Acetylation Modification. Oxidative Med. Cell. Longev. 2022, 2022, 1198607. [Google Scholar] [CrossRef]
- Wangler, M.F.; Lesko, B.; Dahal, R.; Jangam, S.; Bhadane, P.; Wilson, T.E.; McPheron, M.; Miller, M.J. Dicarboxylic Acylcarnitine Biomarkers in Peroxisome Biogenesis Disorders. Mol. Genet. Metab. 2023, 140, 107680. [Google Scholar] [CrossRef]
- Schwantje, M.; Mosegaard, S.; Knottnerus, S.J.G.; van Klinken, J.B.; Wanders, R.J.A.; van Lenthe, H.; Hermans, J.; IJlst, L.; Denis, S.; Jaspers, Y.R.J.; et al. Tracer-based Lipidomics Enables the Discovery of Disease-specific Candidate Biomarkers in Mitochondrial Β-oxidation Disorders. FASEB J. 2024, 38, e23478. [Google Scholar] [CrossRef]
- Toma, J.S.; Karamboulas, K.; Carr, M.; Kolaj, A.; Yuzwa, S.A.; Mahmud, N.; Storer, M.A.; Kaplan, D.R.; Miller, F.D. Peripheral Nerve Single-Cell Analysis Identifies Mesenchymal Ligands That Promote Axonal Growth. eNeuro 2020, 7, ENEURO.0066-20.2020. [Google Scholar] [CrossRef]
- Thomas, S.; Enders, J.; Kaiser, A.; Rovenstine, L.; Hauser, W.A.; Chadwick, A.; Wright, D.D. Abnormal Intraepidermal Nerve Fiber Density in Disease: A Scoping Review. Front. Neurol. 2023, 14, 1161077. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Aleksovska, K.; Attal, N.; Baron, R.; Bennett, D.L.H.; Bouhassira, D.; Cruccu, G.; Eisenberg, E.; Enax-Krumova, E.K.; Davis, K.D.; et al. Joint European Academy of Neurology–European Pain Federation–Neuropathic Pain Special Interest Group of the International Association for the Study of Pain Guidelines on Neuropathic Pain Assessment. Eur. J. Neurol. 2023, 30, 2177–2196. [Google Scholar] [CrossRef] [PubMed]
- Samtani, G.; Kim, S.-J.; Michaud, D.; Hillhouse, A.; Szule, J.A.; Konganti, K.; Li, J. Brain Region Dependent Molecular Signatures and Myelin Repair Following Chronic Demyelination. Front. Cell. Neurosci. 2023, 17, 1169786. [Google Scholar] [CrossRef] [PubMed]
- Kaplanis, S.I.; Kaffe, D.; Ktena, N.; Lygeraki, A.; Kolliniati, O.; Savvaki, M.; Karagogeos, D. Nicotinamide Enhances Myelin Production after Demyelination through Reduction of Astrogliosis and Microgliosis. Front. Cell. Neurosci. 2023, 17, 1201317. [Google Scholar] [CrossRef]
| Receptor/Transporter | Primary Functions | Tissue Distribution | PCSK9 Regulatory Effect | Mechanism of Action | Neural Consequences |
|---|---|---|---|---|---|
| LDLR Family | |||||
| LDLR | • LDL cholesterol uptake • Hepatic lipoprotein clearance • Cellular cholesterol homeostasis | Liver (high), CNS neurons, Peripheral tissues | Promotes degradation, Reduces surface availability | • Binds EGF-A repeat • Prevents receptor recycling • Diverts to lysosomal degradation • Both extracellular and intracellular pathways | • Reduced cholesterol uptake • Altered membrane composition |
| VLDLR | • VLDL and apoE lipoprotein uptake • Reelin signaling coreceptor • Neuronal migration guidance • Synaptic plasticity | CNS neurons, Astrocytes, Developing brain regions | Reduces surface abundance, Attenuates Reelin signaling | • Similar to LDLR routing • Lysosomal targeting after endocytosis | • Impaired synaptic plasticity • Reduced neurite complexity • Altered spine morphology |
| ApoER2 | • Lipoprotein uptake • Reelin signaling coreceptor • NMDA receptor trafficking • Long-term potentiation • Memory formation | CNS neurons, Hippocampus, Cortical regions | Lowers receptor levels, Reduces Reelin responses | • PCSK9 binding promotes degradation • Interference with recycling adaptors | • Decreased synaptic strength • Cognitive alterations • Reduced neuronal survival |
| LRP1 | • Multifunctional endocytic receptor • Lipid handling and signaling • Amyloid-β trafficking • Proteostasis regulation | CNS (widespread), Vascular cells, Neurons and glia | Promotes receptor degradation | • Biases toward lysosomal delivery • Reduces recycling efficiency | • Altered amyloid handling • Impaired proteostasis • Modified vascular function |
| Scavenger Receptors | |||||
| CD36 | • Long-chain fatty acid transporter • Lipid scavenging • Myelin debris clearance • Oxidized LDL uptake | Schwann cells (high), Macrophages, Endothelial cells, Glial cells | Promotes degradation, Reduces fatty acid influx, (Primary PNS target) | • Lysosomal and proteasomal pathways • Post-transcriptional regulation • Surface trafficking control | • Loss of PCSK9 → CD36 upregulation • Excessive fatty acid entry • Lipid droplet accumulation • Mitochondrial overload |
| Cholesterol Efflux | |||||
| ABCA1 | • Cholesterol efflux to apoA-I • ApoE lipidation in brain • Lipoprotein particle formation • Protection from sterol overload | Astrocytes, Microglia, Peripheral macrophages, Hepatocytes | Indirect regulation, (Not direct PCSK9 target) | • PCSK9 affects upstream cholesterol balance • Influences sterol-responsive transcription | • Enhanced brain ApoE levels • Improved neuronal lipid supply • Reduced amyloid deposition |
| ABCG1 | • Cholesterol efflux to HDL • Complements ABCA1 function • Cellular sterol homeostasis | Neural cells, Macrophages, Peripheral tissues | Indirect effects, (Secondary to receptor changes) | • Responds to altered intracellular sterol pools • Links to PCSK9-LDLR axis | • Modified cholesterol export • Altered membrane composition |
| Receptor Class | Normal PCSK9 Function | PCSK9 Deficiency Result | Clinical Relevance |
|---|---|---|---|
| LDLR Family | Increases receptor degradation → Reduces cholesterol uptake | Enhanced receptor availability → Improved cholesterol delivery | • Cardiovascular protection • Potential cognitive benefits |
| CD36 | Decreases surface availability → Limits fatty acid influx | Increased surface expression → Excessive lipid entry | • Peripheral neuropathy risk • Small-fiber dysfunction • Mitochondrial stress |
| Efflux Transporters | Indirectly modulates via cholesterol balance | Enhanced efflux capacity → Better lipid homeostasis | • Improved neural lipid circulation • Reduced lipotoxicity |
| Component | Normal Function | PCSK9 Deficiency Effect | Consequence |
|---|---|---|---|
| PCSK9 | Promotes CD36 degradation; limits fatty acid influx | Absent regulation | Loss of brake on lipid entry |
| CD36 | Fatty acid transporter; controlled surface expression | Increased abundance and activity | Enhanced fatty acid uptake |
| Fatty Acid Influx | Matched to oxidative capacity | Exceeds mitochondrial capacity | Substrate overload |
| Mitochondria | Efficient β-oxidation; stable cristae structure | Overloaded; structural disruption | Incomplete oxidation; ROS production |
| Acylcarnitines | Low levels; rapid turnover | Accumulation of multiple chain lengths | Metabolic stress marker |
| Myelin | Normal thickness; stable composition | Thinning; altered lipid ratios | Small-fiber hypomyelination |
| Axonal Function | Normal conduction; intact sensation | Slowed conduction; reduced sensation | Clinical neuropathy phenotype |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, A.; Śniegocki, M.; Ziółkowska, E.A. PCSK9 Regulation of Lipid Metabolism in the Nervous System: Implications for Schwann Cell Function and Peripheral Neuropathy. Cells 2025, 14, 1479. https://doi.org/10.3390/cells14181479
Nowacka A, Śniegocki M, Ziółkowska EA. PCSK9 Regulation of Lipid Metabolism in the Nervous System: Implications for Schwann Cell Function and Peripheral Neuropathy. Cells. 2025; 14(18):1479. https://doi.org/10.3390/cells14181479
Chicago/Turabian StyleNowacka, Agnieszka, Maciej Śniegocki, and Ewa A. Ziółkowska. 2025. "PCSK9 Regulation of Lipid Metabolism in the Nervous System: Implications for Schwann Cell Function and Peripheral Neuropathy" Cells 14, no. 18: 1479. https://doi.org/10.3390/cells14181479
APA StyleNowacka, A., Śniegocki, M., & Ziółkowska, E. A. (2025). PCSK9 Regulation of Lipid Metabolism in the Nervous System: Implications for Schwann Cell Function and Peripheral Neuropathy. Cells, 14(18), 1479. https://doi.org/10.3390/cells14181479









