Next-Generation mRNA Vaccines in Melanoma: Advances in Delivery and Combination Strategies
Abstract
1. Introduction
1.1. Overview mRNA Vaccines (Oncology Focus)
1.2. Why Melanoma Is a Leading Target (Mutational Burden, Immunogenicity)
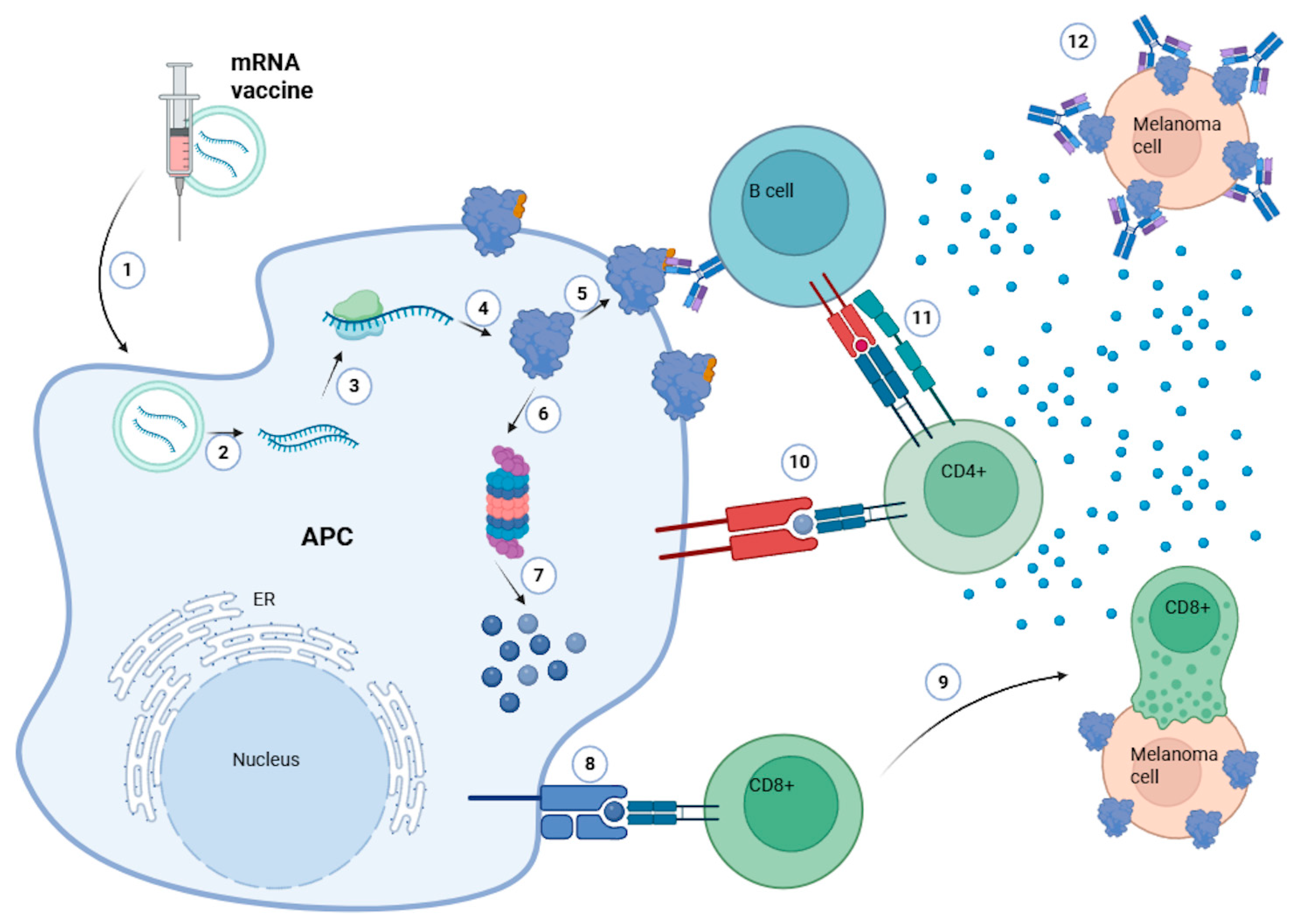
2. mRNA Vaccine Design and Targets in Melanoma
2.1. Tumor-Associated vs. Tumor-Specific Neoantigens
2.2. Personalized vs. Off-the-Shelf
2.3. Tumor Antigens, Neoantigens, Costimulatory Molecules
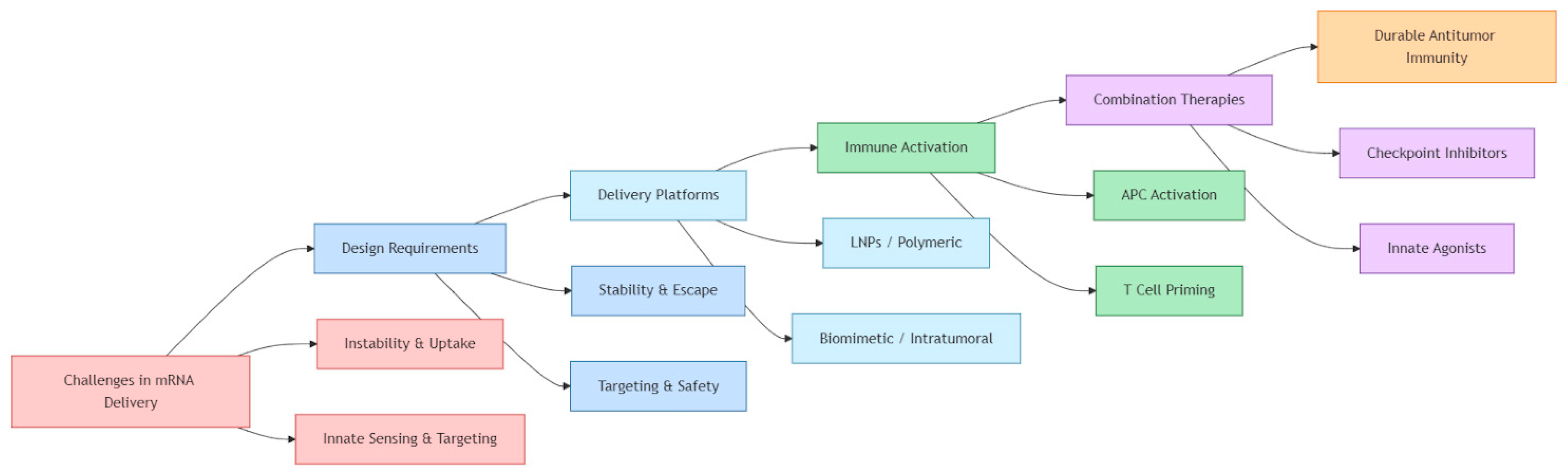
3. Delivery Challenges and Requirements
3.1. Stability, Uptake, Endosomal Escape, Targeting Lymph Nodes or Tumor
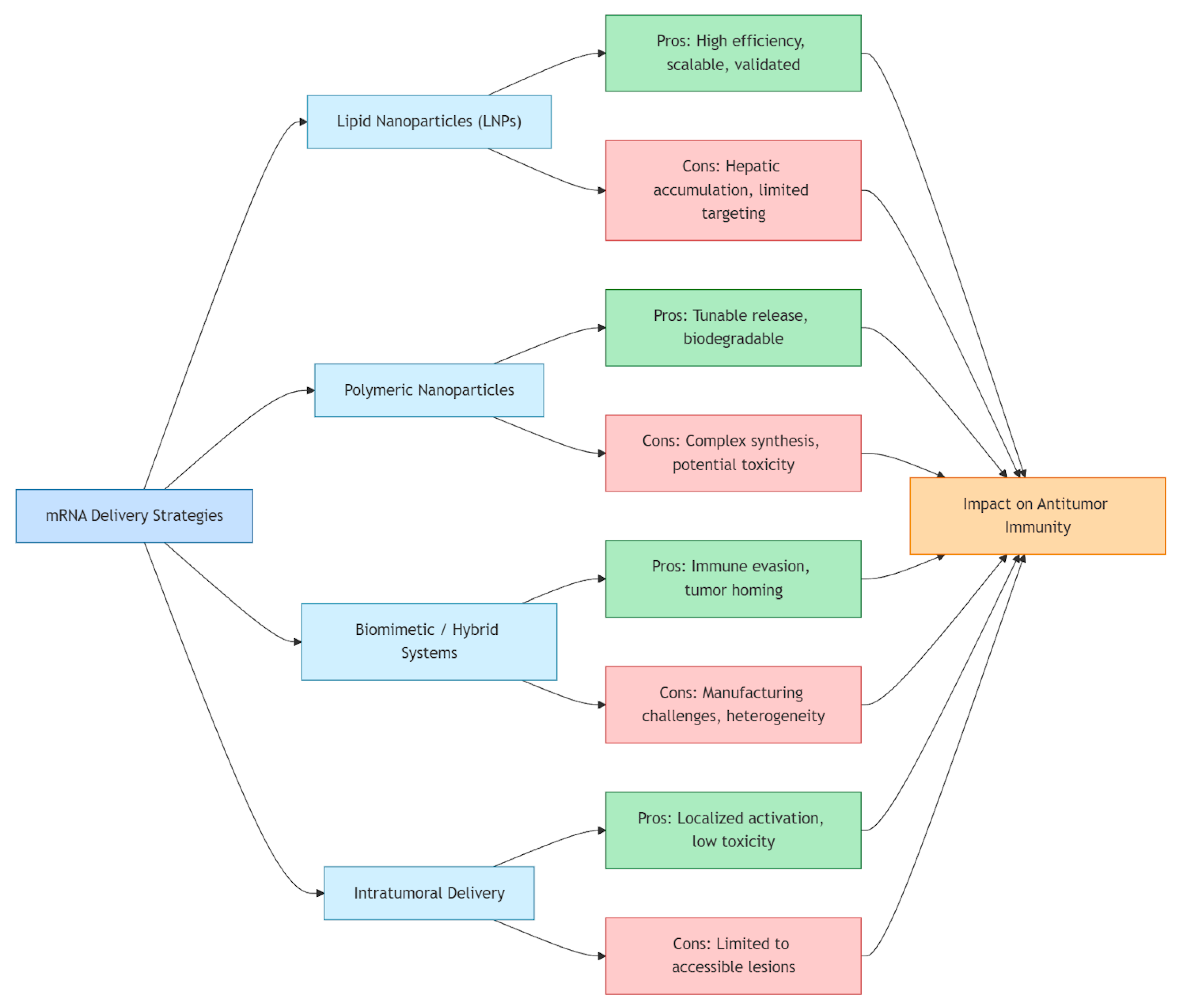
3.2. Lipid Nanoparticles (LNPs)
3.3. Polymeric Nanoparticles
3.4. Biomimetic and Hybrid Systems
3.5. Intratumoral Delivery Strategies
4. Clinical and Preclinical Evidence
4.1. Key Trials (e.g., Moderna mRNA-4157/V940, BioNTech FixVac)
4.2. Intratumoral vs. Systemic Delivery Outcomes
5. Integration with Combination Therapies
6. Future Directions
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Shi, M.; Wang, Y.; You, J. Progress and Prospects of MRNA-Based Drugs in Pre-Clinical and Clinical Applications. Signal Transduct. Target. Ther. 2024, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Laila, U.E.; An, W.; Xu, Z.X. Emerging Prospects of MRNA Cancer Vaccines: Mechanisms, Formulations, and Challenges in Cancer Immunotherapy. Front. Immunol. 2024, 15, 1448489. [Google Scholar] [CrossRef]
- Shariati, A.; Khani, P.; Nasri, F.; Afkhami, H.; Khezrpour, A.; Kamrani, S.; Shariati, F.; Alavimanesh, S.; Modarressi, M.H. MRNA Cancer Vaccines from Bench to Bedside: A New Era in Cancer Immunotherapy. Biomark. Res. 2024, 12, 157. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges and Prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Yaremenko, A.V.; Khan, M.M.; Zhen, X.; Tang, Y.; Tao, W. Clinical Advances of MRNA Vaccines for Cancer Immunotherapy. Med 2025, 6, 100562. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.M.; Vu, T.T.; Nguyen, M.N.; Tran-Nguyen, T.S.; Huynh, C.T.; Ha, Q.T.; Nguyen, H.N.; Tran, L.S. Neoantigen-Based MRNA Vaccine Exhibits Superior Anti-Tumor Activity Compared to Synthetic Long Peptides in an In Vivo Lung Carcinoma Model. Cancer Immunol. Immunother. 2025, 74, 145. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.J.; Xue, Y.; Chen, T.T.; Sun, X.K.; Shi, H.Y. Neoantigen-Based Immunotherapy in Lung Cancer: Advances, Challenges and Prospects. Cancers 2025, 17, 1953. [Google Scholar] [CrossRef] [PubMed]
- Sade-Feldman, M.; Yizhak, K.; Bjorgaard, S.L.; Ray, J.P.; de Boer, C.G.; Jenkins, R.W.; Lieb, D.J.; Chen, J.H.; Frederick, D.T.; Barzily-Rokni, M.; et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 2018, 175, 998–1013.e20. [Google Scholar] [CrossRef]
- Georgopoulos, A.P.; James, L.M.; Sanders, M. MRNA Multipeptide-HLA Class II Immunotherapy for Melanoma. Cells 2025, 14, 1430. [Google Scholar] [CrossRef]
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified MRNA in LNPs Constitutes a Competitive Technology for Prophylactic Vaccines. NPJ Vaccines 2017, 2, 29. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified MRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef]
- Feng, H.; Jin, Y.; Wu, B. Strategies for Neoantigen Screening and Immunogenicity Validation in Cancer Immunotherapy (Review). Int. J. Oncol. 2025, 66, 43. [Google Scholar] [CrossRef]
- Georgoulias, G.; Zaravinos, A. Genomic Landscape of the Immunogenicity Regulation in Skin Melanomas with Diverse Tumor Mutation Burden. Front. Immunol. 2022, 13, 1006665. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Li, Y.; Zeng, Y.; Zhang, Y.; Jiang, Q.; Zhang, Q.; Zhu, J.; Gong, J. Advancements in Melanoma Immunotherapy: The Emergence of Extracellular Vesicle Vaccines. Cell Death Discov. 2024, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Motavaf, M.; Nebo, I.; Luyten, S.; Osei-Opare, K.D.; Gru, A.A. Advancements in Melanoma Treatment: A Review of PD-1 Inhibitors, T-VEC, MRNA Vaccines, and Tumor-Infiltrating Lymphocyte Therapy in an Evolving Landscape of Immunotherapy. J. Clin. Med. 2025, 14, 1200. [Google Scholar] [CrossRef]
- Gurjao, C.; Tsukrov, D.; Imakaev, M.; Luquette, L.J.; Mirny, L.A. Is Tumor Mutational Burden Predictive of Response to Immunotherapy? Elife 2023, 12. [Google Scholar] [CrossRef]
- Andrews, M.C.; Li, G.; Graf, R.P.; Fisher, V.A.; Mitchell, J.; Aboosaiedi, A.; O’Rourke, H.; Shackleton, M.; Iddawela, M.; Oxnard, G.R.; et al. Predictive Impact of Tumor Mutational Burden on Real-World Outcomes of First-Line Immune Checkpoint Inhibition in Metastatic Melanoma. JCO Precis. Oncol. 2024, 8, e2300640. [Google Scholar] [CrossRef]
- Ning, B.; Liu, Y.; Wang, M.; Li, Y.; Xu, T.; Wei, Y. The Predictive Value of Tumor Mutation Burden on Clinical Efficacy of Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 748674. [Google Scholar] [CrossRef]
- Sias, F.; Zoroddu, S.; Migheli, R.; Bagella, L. Untangling the Role of MYC in Sarcomas and Its Potential as a Promising Therapeutic Target. Int. J. Mol. Sci. 2025, 26, 1973. [Google Scholar] [CrossRef]
- Lauss, M.; Phung, B.; Borch, T.H.; Harbst, K.; Kaminska, K.; Ebbesson, A.; Hedenfalk, I.; Yuan, J.; Nielsen, K.; Ingvar, C.; et al. Molecular Patterns of Resistance to Immune Checkpoint Blockade in Melanoma. Nat. Commun. 2024, 15, 3075. [Google Scholar] [CrossRef]
- Nsengimana, J.; Laye, J.; Filia, A.; O’Shea, S.; Muralidhar, S.; Poźniak, J.; Droop, A.; Chan, M.; Walker, C.; Parkinson, L.; et al. β-Catenin–Mediated Immune Evasion Pathway Frequently Operates in Primary Cutaneous Melanomas. J. Clin. Investig. 2018, 128, 2048–2063. [Google Scholar] [CrossRef]
- Zoroddu, S.; Lucariello, A.; De Luca, A.; Bagella, L. Dysregulation of MiRNAs in Soft Tissue Sarcomas. Cells 2024, 13, 1853. [Google Scholar] [CrossRef]
- Zoroddu, S.; Sias, F.; Bagella, L. The Double Life of MicroRNAs in Bone Sarcomas: Oncogenic Drivers and Tumor Suppressors. Int. J. Mol. Sci. 2025, 26, 4814. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised Neoantigen Therapy MRNA-4157 (V940) plus Pembrolizumab versus Pembrolizumab Monotherapy in Resected Melanoma (KEYNOTE-942): A Randomised, Phase 2b Study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Danelli, L. Personalized Neoantigen Therapy for Melanoma Immunotherapy: Cancer Immunotherapy. Nat. Cancer 2024, 5, 1783. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Hamzat, Y.; Alqudah, A.; Alzoubi, L. Neoantigen Vaccines: Advancing Personalized Cancer Immunotherapy. Open Explor. 2025, 5, 1003190. [Google Scholar] [CrossRef]
- Singh, P.; Khatib, M.N.; Roopashree, R.; Kaur, M.; Srivastava, M.; Barwal, A.; Rajput, G.V.S.; Rajput, P.; Syed, R.; Sharma, G.; et al. Advancements and Challenges in Personalized Neoantigen-Based Cancer Vaccines. Oncol. Rev. 2025, 19, 1541326. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Talukder, A.H.; Lim, S.A.; Kim, K.; Pan, K.; Melendez, B.; Bradley, S.D.; Jackson, K.R.; Khalili, J.S.; Wang, J.; et al. SLC45A2: A Melanoma Antigen with High Tumor Selectivity and Reduced Potential for Autoimmune Toxicity. Cancer Immunol. Res. 2017, 5, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, S.; Pop, O.T.; Hahn, M.; Aebischer, V.; Seitz, C.M.; Schroeder, C.; Liebmann, A.; Abele, M.; Wild, H.; Bien, E.; et al. Expression of the Tumor Antigens NY-ESO-1, Tyrosinase, MAGE-A3, and TPTE in Pediatric and Adult Melanoma: A Retrospective Case Control Study. Virchows Arch. 2024, 485, 335. [Google Scholar] [CrossRef] [PubMed]
- Rohaan, M.W.; Gomez-Eerland, R.; van den Berg, J.H.; Geukes Foppen, M.H.; van Zon, M.; Raud, B.; Jedema, I.; Scheij, S.; de Boer, R.; Bakker, N.A.M.; et al. MART-1 TCR Gene-Modified Peripheral Blood T Cells for the Treatment of Metastatic Melanoma: A Phase I/IIa Clinical Trial. Immuno-Oncol. Technol. 2022, 15, 100089. [Google Scholar] [CrossRef]
- Apavaloaei, A.; Zhao, Q.; Hesnard, L.; Cahuzac, M.; Durette, C.; Larouche, J.D.; Hardy, M.P.; Vincent, K.; Brochu, S.; Laverdure, J.P.; et al. Tumor Antigens Preferentially Derive from Unmutated Genomic Sequences in Melanoma and Non-Small Cell Lung Cancer. Nat. Cancer 2025, 6, 1419–1437. [Google Scholar] [CrossRef]
- Martini, D.J.; Wu, C.J. The Future of Personalized Cancer Vaccines. Cancer Discov. 2025, 15, OF1–OF10. [Google Scholar] [CrossRef]
- Fu, J.; Wu, S.; Bao, N.; Wu, L.; Qu, H.; Wang, Z.; Dong, H.; Wu, J.; Jin, Y. A Universal Strategy of Anti-Tumor MRNA Vaccine by Harnessing “Off-the-Shelf” Immunity. Adv. Sci. 2025, 12, 2401287. [Google Scholar] [CrossRef]
- Carri, I.; Schwab, E.; Trivino, J.C.; von Euw, E.M.; Nielsen, M.; Mordoh, J.; Barrio, M.M. VACCIMEL, an Allogeneic Melanoma Vaccine, Efficiently Triggers T Cell Immune Responses against Neoantigens and Alloantigens, as Well as against Tumor-Associated Antigens. Front. Immunol. 2024, 15, 1496204. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhao, X.; Hu, J.; Jiao, Y.; Yan, Y.; Pan, X.; Wang, X.; Jiao, F. MRNA Vaccines in the Context of Cancer Treatment: From Concept to Application. J. Transl. Med. 2025, 23, 12. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Lin, A.; Xu, C.; Li, Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H.; et al. Algorithm for Optimized MRNA Design Improves Stability and Immunogenicity. Nature 2023, 621, 396–403. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, X.; Yang, J.; Li, H.; Hao, Y.; Feng, X. Optimization of the 5′ Untranslated Region of MRNA Vaccines. Sci. Rep. 2024, 14, 19845. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Xu, Z.; Miao, L.; Huang, L. MRNA Vaccine with Antigen-Specific Checkpoint Blockade Induces an Enhanced Immune Response against Established Melanoma. Mol. Ther. 2018, 26, 420–434. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, M.; Zhong, Y.; Gao, Y.; Fan, D.; Lu, X. Diverse Nanoparticles Deliver MRNA to Enhance Tumor Immunotherapy. BMB Rep. 2025, 58, 124. [Google Scholar] [CrossRef]
- Lu, R.M.; Hsu, H.E.; Perez, S.J.L.P.; Kumari, M.; Chen, G.H.; Hong, M.H.; Lin, Y.S.; Liu, C.H.; Ko, S.H.; Concio, C.A.P.; et al. Current Landscape of MRNA Technologies and Delivery Systems for New Modality Therapeutics. J. Biomed. Sci. 2024, 31, 89. [Google Scholar] [CrossRef]
- Zwolsman, R.; Darwish, Y.B.; Kluza, E.; van der Meel, R. Engineering Lipid Nanoparticles for MRNA Immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2025, 17, e70007. [Google Scholar] [CrossRef]
- Cao, Z.; Ren, L.; Niu, L.; Zhao, R.; Liu, N.; Zhuang, Q.; Pan, F.; Liu, Z.; Cheng, Y.; Yang, Y.; et al. A Coordinative Dendrimer-Based Nanovaccine for Cancer Treatment. Matter 2023, 6, 3574–3597. [Google Scholar] [CrossRef]
- Smith, R.; Wafa, E.I.; Geary, S.M.; Ebeid, K.; Alhaj-Suliman, S.O.; Salem, A.K. Cationic Nanoparticles Enhance T Cell Tumor Infiltration and Antitumor Immune Responses to a Melanoma Vaccine. Sci. Adv. 2022, 8, eabk3150. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.E.I.; Filtjens, J.; Brabants, E.; Kancheva, D.; Debraekeleer, A.; Brughmans, J.; Jacobs, L.; Bardet, P.M.R.; Knetemann, E.; Lefesvre, P.; et al. Intratumoral Delivery of Lipid Nanoparticle-Formulated MRNA Encoding IL-21, IL-7, and 4-1BBL Induces Systemic Anti-Tumor Immunity. Nat. Commun. 2024, 15, 10635. [Google Scholar] [CrossRef]
- Boehm, D.T.; Landreth, K.M.; Kilic, E.S.; Lee, K.S.; Misra, B.; Bobbala, S.; Damron, F.H.; Liu, T.W. Intratumoral Administration of MRNA COVID-19 Vaccine Delays Melanoma Growth in Mice. Sci. Rep. 2025, 15, 5337. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Jacob, E.M.; Huang, J.; Chen, M. Lipid Nanoparticle-Based MRNA Vaccines: A New Frontier in Precision Oncology. Precis. Clin. Med. 2024, 7, pbae017. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, X.; Wang, K.; Liang, L.; Zhang, H. An Overview of Nanoparticle-Based Delivery Platforms for MRNA Vaccines for Treating Cancer. Vaccines 2024, 12, 727. [Google Scholar] [CrossRef]
- Ramadan, E.; Ahmed, A.; Naguib, Y.W. Advances in MRNA LNP-Based Cancer Vaccines: Mechanisms, Formulation Aspects, Challenges, and Future Directions. J. Pers. Med. 2024, 14, 1092. [Google Scholar] [CrossRef]
- Kon, E.; Ad-El, N.; Hazan-Halevy, I.; Stotsky-Oterin, L.; Peer, D. Targeting Cancer with MRNA–Lipid Nanoparticles: Key Considerations and Future Prospects. Nat. Rev. Clin. Oncol. 2023, 20, 739–754. [Google Scholar] [CrossRef]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. Recent Advances in MRNA Cancer Vaccines: Meeting Challenges and Embracing Opportunities. Front. Immunol. 2023, 14, 1246682. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Z.-C.; Li, S.; Ma, J.; Wei, C.; Yu, D.; Stelzel, J.L.; Ni, B.Y.X.; Miao, Y.; Van Batavia, K.; et al. An MRNA Lipid Nanoparticle-Incorporated Nanofiber-Hydrogel Composite for Cancer Immunotherapy. Nat. Commun. 2025, 16, 5707. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, J.; Zhou, J.; Jiang, W.; Chen, Z.; Wu, X.; Guo, B.; Wu, Y.; Yang, F. Engineered Lipid Nanoparticles with Synergistic Dendritic Cell Targeting and Enhanced Endosomal Escape for Boosted MRNA Cancer Vaccines. Mater. Today Bio 2025, 34, 102107. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, S.; Zeng, J.; Zhang, S.; Li, M.; Yue, F.; Chen, Z.; Dong, Y.; Zeng, Y.; Luo, J. A Biodegradable Lipid Nanoparticle Delivers a Cas9 Ribonucleoprotein for Efficient and Safe in Situ Genome Editing in Melanoma. Acta Biomater. 2024, 190, 531–547. [Google Scholar] [CrossRef]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables MRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, 2303261. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, F.; Zhang, Y. Present and Future of Cancer Nano-Immunotherapy: Opportunities, Obstacles and Challenges. Mol. Cancer 2025, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Xu, J.; Zhang, T.; Chen, Q.; Sun, T.; Jiang, C. Advanced Nano-Based Strategies for MRNA Tumor Vaccine. Acta Pharm. Sin. B 2024, 14, 170–189. [Google Scholar] [CrossRef]
- Hofstraat, S.R.J.; Anbergen, T.; Zwolsman, R.; Deckers, J.; van Elsas, Y.; Trines, M.M.; Versteeg, I.; Hoorn, D.; Ros, G.W.B.; Bartelet, B.M.; et al. Nature-Inspired Platform Nanotechnology for RNA Delivery to Myeloid Cells and Their Bone Marrow Progenitors. Nat. Nanotechnol. 2025, 20, 532–542. [Google Scholar] [CrossRef]
- Imani, S.; Tagit, O.; Pichon, C. Neoantigen Vaccine Nanoformulations Based on Chemically Synthesized Minimal MRNA (CmRNA): Small Molecules, Big Impact. NPJ Vaccines 2024, 9, 14. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Karlsson, J.; Hemmati, S.; Yu, H.; Tzeng, S.Y.; Pardoll, D.M.; Green, J.J. Biodegradable Lipophilic Polymeric MRNA Nanoparticles for Ligand-Free Targeting of Splenic Dendritic Cells for Cancer Vaccination. Proc. Natl. Acad. Sci. USA 2023, 120, e2301606120. [Google Scholar] [CrossRef]
- Wusiman, D.; Wang, Y.; Wang, M.; Wang, J.; Wu, R.; Tuo, Z.; Wang, Z.; Yu, Q.; An, Z.; Cho, W.C.; et al. Biomimetic Nanovaccines in Cancer Therapy: Mechanisms, Efficacy, and Clinical Translation. Mater Today Bio 2025, 34, 102116. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Li, T.; Mei, J.; Chen, Y. Recent Advances in Biomimetic Nanodelivery Systems for Cancer Immunotherapy. Mater. Today Bio 2025, 32, 101726. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Nie, G.; Li, N. Unlocking the Power of Nanomedicine: Cell Membrane-Derived Biomimetic Cancer Nanovaccines for Cancer Treatment. Med 2024, 5, 660–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Wu, Q.; Gong, C. Biomimetic Nanosystems Based on Cell Membranes (BNCMs) for Cancer Immunotherapy. MedComm-Biomater. Appl. 2024, 3, e106. [Google Scholar] [CrossRef]
- Poudel, K.; Ji, Z.; Njauw, C.N.; Rajadurai, A.; Bhayana, B.; Sullivan, R.J.; Kim, J.O.; Tsao, H. Fabrication and Functional Validation of a Hybrid Biomimetic Nanovaccine (HBNV) against KitK641E-Mutant Melanoma. Bioact. Mater. 2024, 46, 347. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Xie, C.; Xia, X. Recent Progress in MRNA Cancer Vaccines. Hum. Vaccines Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, J.C.; Rong, L.; He, Y.; Wang, X.; Lin, X.; Li, W.; Wu, Y.; Kuwentrai, C.; Su, C.; et al. The Guided Fire from within: Intratumoral Administration of MRNA-Based Vaccines to Mobilize Memory Immunity and Direct Immune Responses against Pathogen to Target Solid Tumors. Cell Discov. 2024, 10, 127. [Google Scholar] [CrossRef]
- Kong, B.; Kim, Y.; Kim, E.H.; Suk, J.S.; Yang, Y. MRNA: A Promising Platform for Cancer Immunotherapy. Adv. Drug Deliv. Rev. 2023, 199, 114993. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Y.; He, J.; Sun, X. Path towards MRNA Delivery for Cancer Immunotherapy from Bench to Bedside. Theranostics 2024, 14, 96. [Google Scholar] [CrossRef]
- Khazaei Monfared, Y.; Mahmoudian, M.; Zakeri-Milani, P.; Cecone, C.; Hayashi, T.; Ishii, K.J.; Conde, J.; Matencio, A.; Trotta, F. Intratumoural Delivery of MRNA Loaded on a Cationic Hyper-Branched Cyclodextrin-Based Polymer Induced an Anti-Tumour Immunological Response in Melanoma. Cancers 2023, 15, 3748. [Google Scholar] [CrossRef]
- Xu, Z.; Fisher, D.E. MRNA Melanoma Vaccine Revolution Spurred by the COVID-19 Pandemic. Front. Immunol. 2023, 14, 1155728. [Google Scholar] [CrossRef]
- Di Trani, C.A.; Cirella, A.; Arrizabalaga, L.; Alvarez, M.; Bella, Á.; Fernandez-Sendin, M.; Russo-Cabrera, J.S.; Gomar, C.; Ardaiz, N.; Teijeira, A.; et al. Intratumoral Injection of IL-12-Encoding MRNA Targeted to CSFR1 and PD-L1 Exerts Potent Anti-Tumor Effects without Substantial Systemic Exposure. Mol. Ther. Nucleic Acids 2023, 33, 599. [Google Scholar] [CrossRef]
- Hieken, T.J.; Ariyan, C. Personalized MRNA Vaccines and Contemporary Melanoma Practice. Ann. Surg. Oncol. 2025, 32, 1–2. [Google Scholar] [CrossRef]
- Global Phase 3 Trial Investigates MRNA Vaccine for Melanoma Treatment. Available online: https://www.targetedonc.com/view/global-phase-3-trial-investigates-mrna-vaccine-for-melanoma-treatment? (accessed on 30 July 2025).
- Featured Trial. Available online: https://trials.modernatx.com/study/?id=mRNA-4157-P201 (accessed on 30 July 2025).
- Moderna and Merck Announce MRNA-4157/V940, an Investigational Personalized MRNA Cancer Vaccine, in Combination with KEYTRUDA® (Pembrolizumab), Met Primary Efficacy Endpoint in Phase 2b KEYNOTE-942 Trial—Merck.Com. Available online: https://www.merck.com/news/moderna-and-merck-announce-mrna-4157-v940-an-investigational-personalized-mrna-cancer-vaccine-in-combination-with-keytruda-pembrolizumab-met-primary-efficacy-endpoint-in-phase-2b-keynote-94/ (accessed on 30 July 2025).
- Moderna and Merck Announce MRNA-4157/V940, an Investigational Personalized MRNA Cancer Vaccine, in Combination with KEYTRUDA® (Pembrolizumab), Was Granted Breakthrough Therapy Designation by the FDA for Adjuvant Treatment of Patients with High-Risk Melanoma Following Complete Resection—Merck.Com. Available online: https://www.merck.com/news/moderna-and-merck-announce-mrna-4157-v940-an-investigational-personalized-mrna-cancer-vaccine-in-combination-with-keytruda-pembrolizumab-was-granted-breakthrough-therapy-designation-by-the/ (accessed on 30 July 2025).
- Khattak, M.A.; Carlino, M.S.; Meniawy, T.; Taylor, M.H.; Ansstas, G.; Kim, K.B.; McKean, M.; Sullivan, R.J.; Faries, M.B.; Tran, T.; et al. Individualized Neoantigen Therapy MRNA-4157 (V940) plus Pembrolizumab in Resected Melanoma: 3-Year Update from the MRNA-4157-P201 (KEYNOTE-942) Trial. J. Clin. Oncol. 2024, 42, LBA9512. [Google Scholar] [CrossRef]
- BNT111 Melanoma Vaccine—Vax-Before-Travel. Available online: https://www.vax-before-travel.com/vaccines/bnt111-melanoma-vaccine? (accessed on 30 July 2025).
- BioNTech’s MRNA Immunotherapy BNT111 Yields Positive Topline Results in Advanced Melanoma. Available online: https://www.dermatologytimes.com/view/biontech-s-mrna-immunotherapy-bnt111-yields-positive-topline-results-in-advanced-melanoma? (accessed on 30 July 2025).
- BNT111/Cemiplimab Shows Significant ORR Improvement in Stage III/IV Melanoma. Available online: https://www.targetedonc.com/view/bnt111-cemiplimab-shows-significant-orr-improvement-in-stage-iii-iv-melanoma? (accessed on 30 July 2025).
- BioNTech Announces Positive Topline Phase 2 Results for MRNA Immunotherapy Candidate BNT111 in Patients with Advanced Melanoma | BioNTech. Available online: https://investors.biontech.de/news-releases/news-release-details/biontech-announces-positive-topline-phase-2-results-mrna? (accessed on 30 July 2025).
- BioNTech, Regeneron’s MRNA Cancer Vaccine Combo Clears Phase II in Melanoma—BioSpace. Available online: https://www.biospace.com/drug-development/biontech-regenerons-mrna-cancer-vaccine-combo-clears-phase-ii-in-melanoma? (accessed on 30 July 2025).
- Luke, J.J.; Davar, D.; Andtbacka, R.H.; Bhardwaj, N.; Brody, J.D.; Chesney, J.; Coffin, R.; de Baere, T.; de Gruijl, T.D.; Fury, M.; et al. Society for Immunotherapy of Cancer (SITC) Recommendations on Intratumoral Immunotherapy Clinical Trials (IICT): From Premalignant to Metastatic Disease. J. Immunother. Cancer 2024, 12, 8378. [Google Scholar] [CrossRef] [PubMed]
- Lakshmipathi, J.; Santha, S.; Li, M.; Qian, Y.; Roy, S.F.; Luheshi, N.; Politi, K.; Bosenberg, M.; Eyles, J.; Muthusamy, V. Intratumoral IL12 MRNA Administration Activates Innate and Adaptive Pathways in Checkpoint Inhibitor-Resistant Tumors Resulting in Complete Responses. Cancer Immunol. Immunother. 2025, 74, 250. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Srilatha, M.; Farran, B.; Nagaraju, G.P. MRNA Vaccines and Their Delivery Strategies: A Journey from Infectious Diseases to Cancer. Mol. Ther. 2023, 32, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, L.; Wang, Y.; Liu, Q.; Liu, J.; Long, H.; Li, Q.; Luo, L.; Peng, Y. Local Administration of MRNA Encoding Cytokine Cocktail Confers Potent Anti-Tumor Immunity. Front. Immunol. 2024, 15, 1455019. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhang, C.; Zhang, X.; Yan, J.; Zeng, C.; Talebian, F.; Lynch, K.; Zhao, W.; Hou, X.; Du, S.; et al. Intratumoral Delivery of IL-12 and IL-27 MRNA Using Lipid Nanoparticles for Cancer Immunotherapy. J. Control. Release 2022, 345, 306–313. [Google Scholar] [CrossRef]
- Sauer, K.; Rakhra, K.; Wu, K.; Mehta, N.K.; Michaelson, J.S.; Baeuerle, P.A. Intratumoral Injection and Retention Hold Promise to Improve Cytokine Therapies for Cancer. Front. Oncol. 2024, 14, 1456658. [Google Scholar] [CrossRef]
- Aunins, E.A.; Phan, A.T.; Alameh, M.G.; Dwivedi, G.; Cruz-Morales, E.; Christian, D.A.; Tam, Y.; Bunkofske, M.E.; Peñafiel, A.Z.; O’Dea, K.M.; et al. An Il12 MRNA-LNP Adjuvant Enhances MRNA Vaccine-Induced CD8 T Cell Responses. Sci. Immunol. 2025, 10, eads1328. [Google Scholar] [CrossRef]
- Xie, C.; Yao, R.; Xia, X. The Advances of Adjuvants in MRNA Vaccines. NPJ Vaccines 2023, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Riu, F.; Ibba, R.; Zoroddu, S.; Sestito, S.; Lai, M.; Piras, S.; Sanna, L.; Bordoni, V.; Bagella, L.; Carta, A. Design, Synthesis, and Biological Screening of a Series of 4′-Fluoro-Benzotriazole-Acrylonitrile Derivatives as Microtubule-Destabilising Agents (MDAs). J. Enzym. Inhib. Med. Chem. 2022, 37, 2223–2240. [Google Scholar] [CrossRef]
- Zoroddu, S.; Sanna, L.; Bordoni, V.; Lyu, W.; Murineddu, G.; Pinna, G.A.; Forcales, S.V.; Sala, A.; Kelvin, D.J.; Bagella, L. RNAseq Analysis of Novel 1,3,4-Oxadiazole Chalcogen Analogues Reveals Anti-Tubulin Properties on Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 11263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yang, N.; Jin, J.; Zhang, Z.; Lu, H.; Xu, L.; Chen, Y.; Jin, L.; Zhou, L.; Yang, H.; et al. Preclinical and Clinical Evaluation of Intratumoral Injection of an IL-12 Expressing SKV-012 Oncolytic Virus for Advanced Solid Tumors. J. Immunother. Cancer 2025, 13, e011642. [Google Scholar] [CrossRef]
- Imani, S.; Li, X.; Chen, K.; Maghsoudloo, M.; Jabbarzadeh Kaboli, P.; Hashemi, M.; Khoushab, S.; Li, X. Computational Biology and Artificial Intelligence in MRNA Vaccine Design for Cancer Immunotherapy. Front. Cell Infect. Microbiol. 2024, 14, 1501010. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Kong, H. Advances in Personalized Cancer Vaccine Development: AI Applications from Neoantigen Discovery to MRNA Formulation. BioChem 2025, 5, 5. [Google Scholar] [CrossRef]
- Vasudevan, K.; Dhanushkumar, T.; Hebbar, S.R.; Selvam, P.K.; Rambabu, M.; Anbarasu, K.; Rohini, K. Multi-Omics and AI-Driven Immune Subtyping to Optimize Neoantigen-Based Vaccines for Colorectal Cancer. Sci. Rep. 2025, 15, 19333. [Google Scholar] [CrossRef]
- Bravi, B. Development and Use of Machine Learning Algorithms in Vaccine Target Selection. npj Vaccines 2024, 9, 15. [Google Scholar] [CrossRef]
- Skerritt, J.H. Considerations for MRNA Product Development, Regulation and Deployment Across the Lifecycle. Vaccines 2025, 13, 473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoroddu, S.; Bagella, L. Next-Generation mRNA Vaccines in Melanoma: Advances in Delivery and Combination Strategies. Cells 2025, 14, 1476. https://doi.org/10.3390/cells14181476
Zoroddu S, Bagella L. Next-Generation mRNA Vaccines in Melanoma: Advances in Delivery and Combination Strategies. Cells. 2025; 14(18):1476. https://doi.org/10.3390/cells14181476
Chicago/Turabian StyleZoroddu, Stefano, and Luigi Bagella. 2025. "Next-Generation mRNA Vaccines in Melanoma: Advances in Delivery and Combination Strategies" Cells 14, no. 18: 1476. https://doi.org/10.3390/cells14181476
APA StyleZoroddu, S., & Bagella, L. (2025). Next-Generation mRNA Vaccines in Melanoma: Advances in Delivery and Combination Strategies. Cells, 14(18), 1476. https://doi.org/10.3390/cells14181476






