Cellular and Molecular Mechanisms of VSMC Phenotypic Switching in Type 2 Diabetes
Abstract
1. Introduction
2. Obesity, Adipokines, and Atherosclerosis
2.1. Proatherogenic Adipokines and VSMC Dysfunction
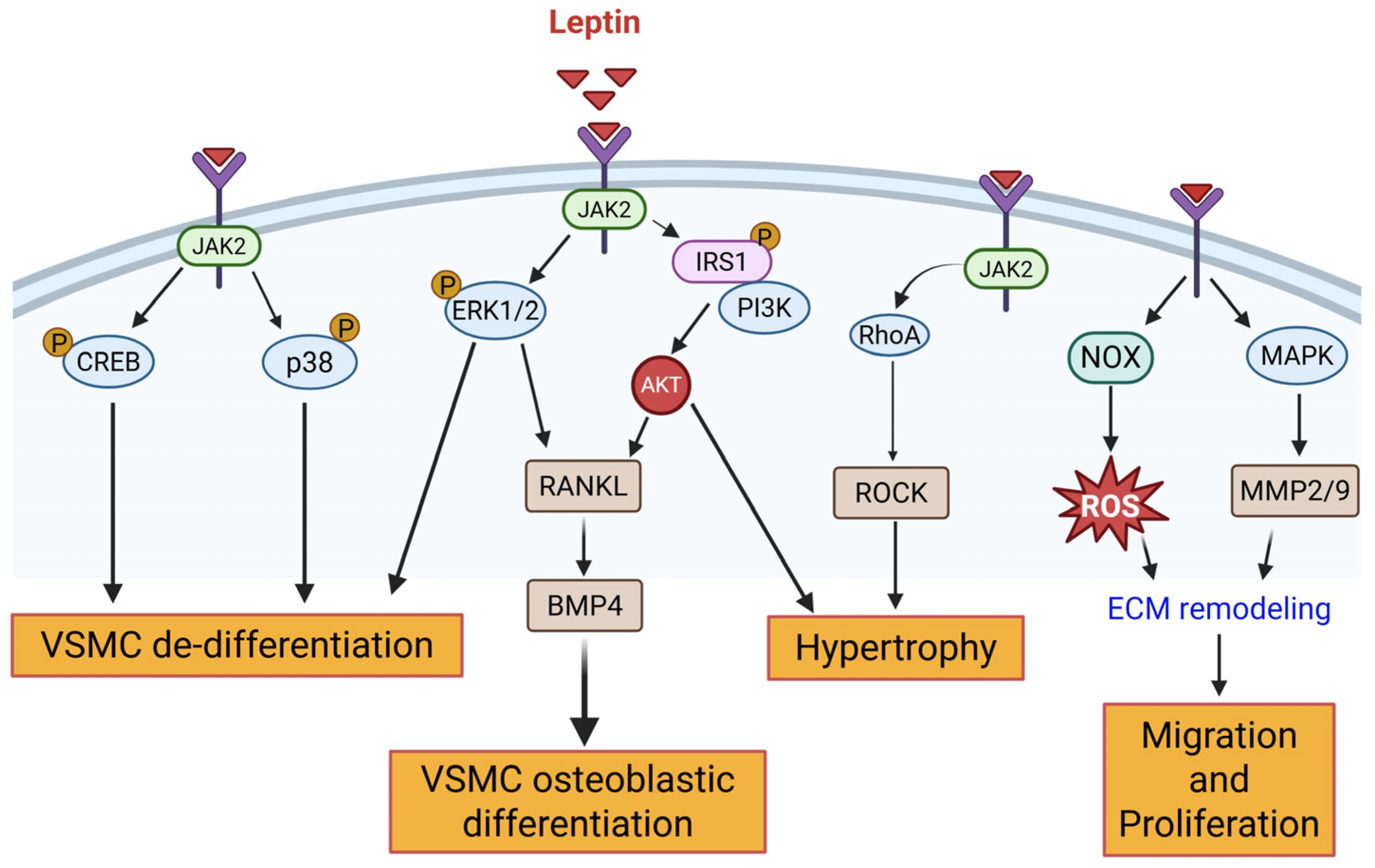
2.1.1. Leptin
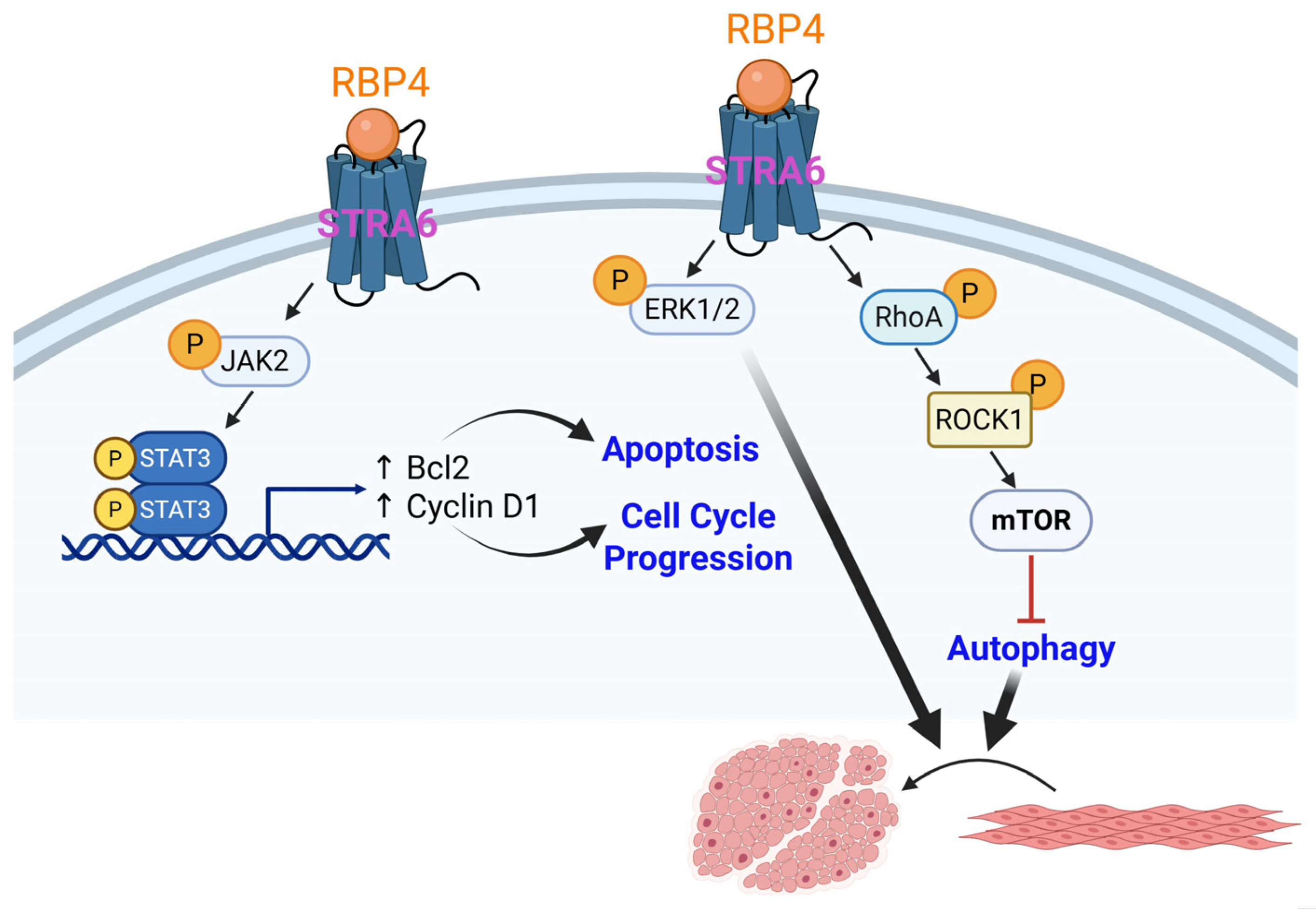
2.1.2. Retinol Binding Protein 4 (RBP4)
2.2. Anti-Atherogenic Adipokines and VSMC Dysfunction
2.2.1. Adiponectin
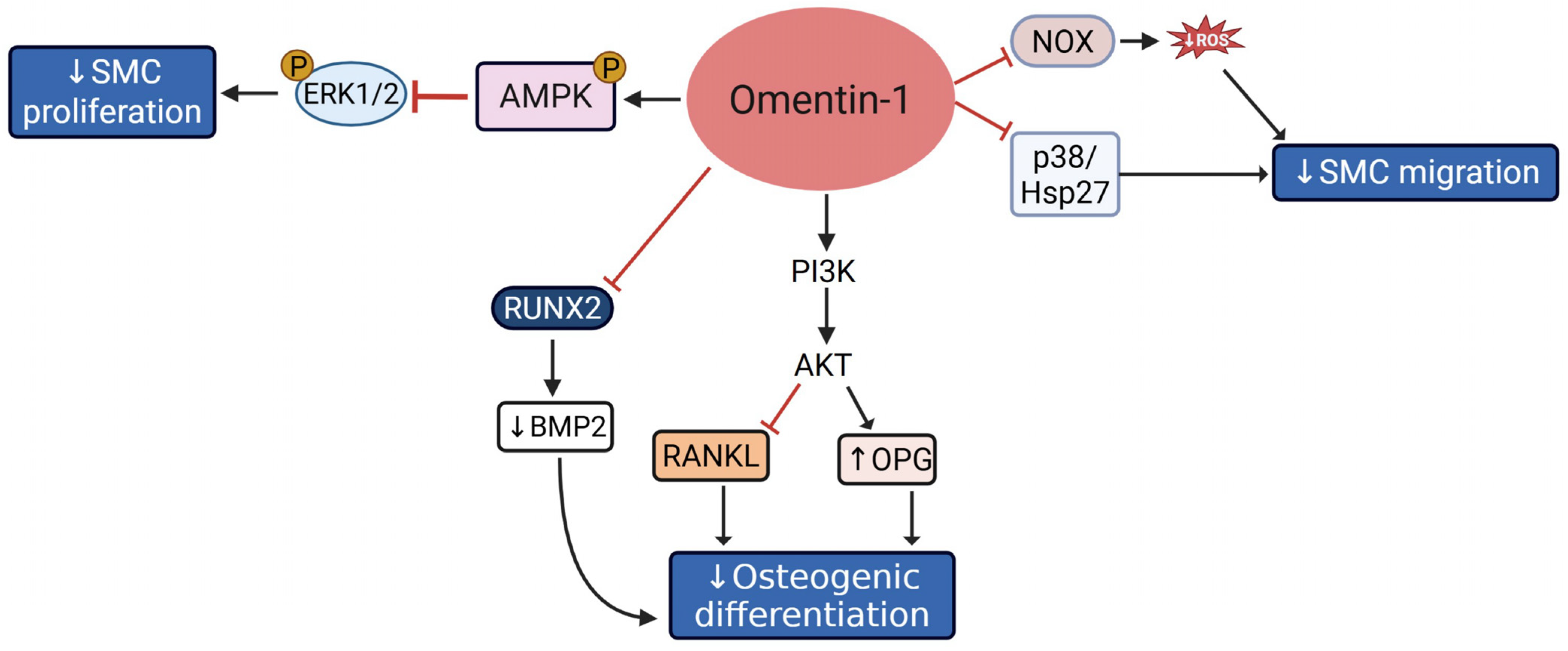
2.2.2. Omentin-1
3. Hyperglycemia, Atherosclerosis, and VSMC Dysfunction
3.1. High Glucose and VSMC Migration, Proliferation, and Inflammation
3.2. High Glucose and Calcifying VSMC
4. Hyperinsulinemia, Atherosclerosis, and VSMC Dysfunction
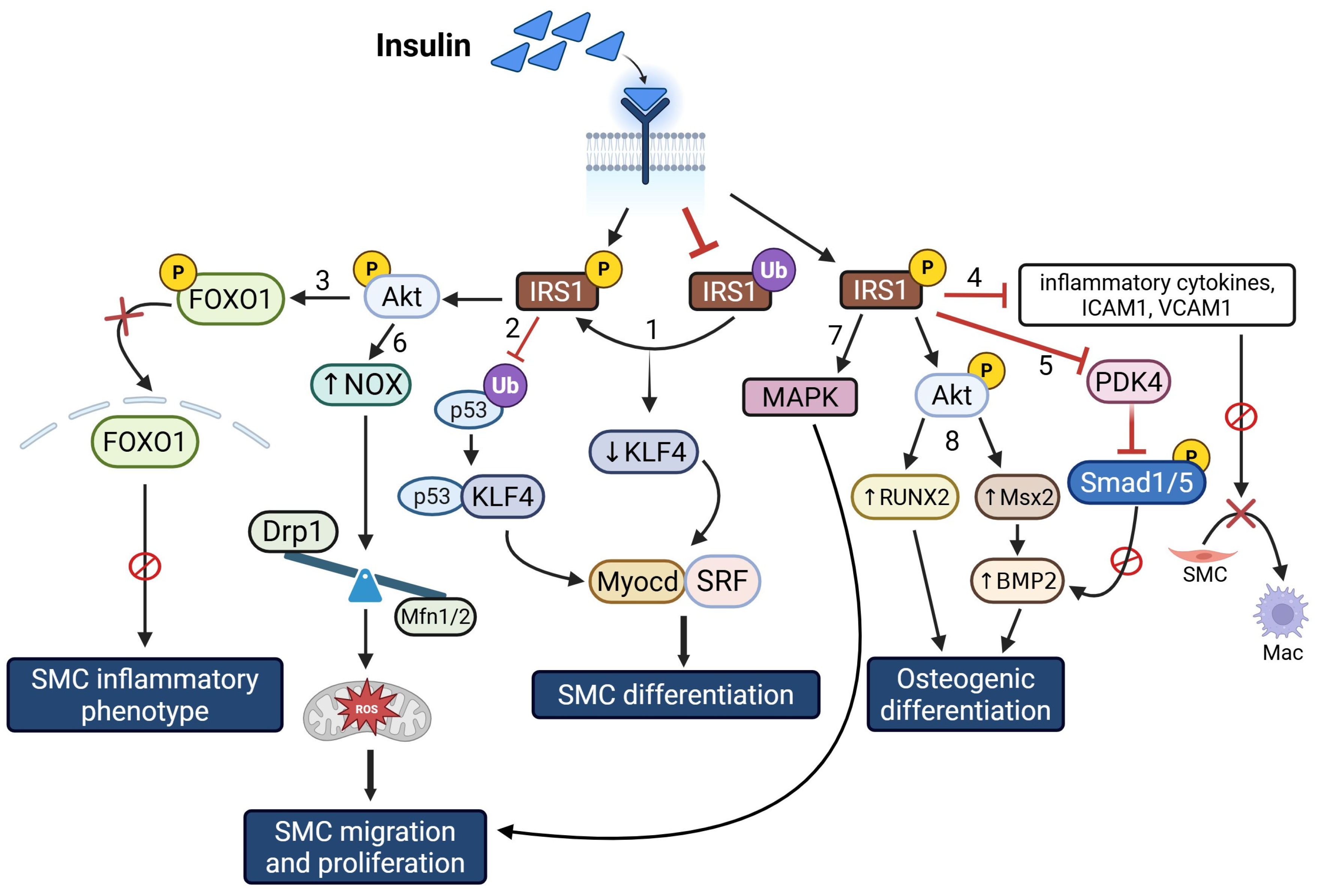
Insulin and VSMC Proliferation, Inflammation, and Calcification
5. Converging Mechanisms of VSMC Plasticity in T2D
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, D.; Kundu, R.; Basu, R.; Chakrabarti, P. Pathophysiological hallmarks in type 2 diabetes heterogeneity (review). Diabetol. Int. 2025, 16, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Avdic, T.; Carlsen, H.K.; Rawshani, A.; Gudbjörnsdottir, S.; Mandalenakis, Z.; Eliasson, B. Risk factors for and risk of all-cause and atherosclerotic cardiovascular disease mortality in people with type 2 diabetes and peripheral artery disease: An observational, register-based cohort study. Cardiovasc. Diabetol. 2024, 23, 127. [Google Scholar] [CrossRef] [PubMed]
- Pantea Stoian, A.; Roman, G. Cardiovascular Risk/Disease in Type 2 Diabetes Mellitus. In Type 2 Diabetes—From Pathophysiology to Cyber Systems; Pantea Stoian, A., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Fernando, S.; Bursill, C.A.; Nicholls, S.J.; Psaltis, P.J. Pathophysiology of Atherosclerosis. In Mechanisms of Vascular Disease: A Textbook for Vascular Specialists; Fitridge, R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–45. [Google Scholar]
- Ragino, Y.I.; Stakhneva, E.M.; Polonskaya, Y.V.; Kashtanova, E.V. The role of secretory activity molecules of visceral adipocytes in abdominal obesity in the development of cardiovascular disease: A review. Biomolecules 2020, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, K.M. Newly Discovered Adipokines: Pathophysiological Link Between Obesity and Cardiometabolic Disorders. Front. Physiol. 2020, 11, 568800. [Google Scholar] [CrossRef]
- Trovati, M.; Doronzo, G.; Barale, C.; Vaccheris, C.; Russo, I.; Cavalot, F. Leptin and vascular smooth muscle cells. Curr. Pharm. Des. 2014, 20, 625–634. [Google Scholar] [CrossRef]
- Juan, C.C.; Chuang, T.Y.; Lien, C.C.; Lin, Y.J.; Huang, S.W.; Kwok, C.F.; Ho, L.T. Leptin increases endothelin type A receptor levels in vascular smooth muscle cells. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E481–E487. [Google Scholar] [CrossRef]
- Zeidan, A.; Purdham, D.M.; Rajapurohitam, V.; Javadov, S.; Chakrabarti, S.; Karmazyn, M. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II- and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J. Pharmacol. Exp. Ther. 2005, 315, 1075–1084. [Google Scholar] [CrossRef]
- Benkhoff, S.; Loot, A.E.; Pierson, I.; Sturza, A.; Kohlstedt, K.; Fleming, I.; Shimokawa, H.; Grisk, O.; Brandes, R.P.; Schröder, K. Leptin potentiates endothelium-dependent relaxation by inducing endothelial expression of neuronal NO synthase. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1605–1612. [Google Scholar] [CrossRef]
- Ryan, M.J.; Coleman, T.T.; Sasser, J.M.; Pittman, K.M.; Hankins, M.W.; Stec, D.E. Vascular smooth muscle-specific deletion of the leptin receptor attenuates leptin-induced alterations in vascular relaxation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R960–R967. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Raman, P.; Khanal, S. Leptin in Atherosclerosis: Focus on Macrophages, Endothelial and Smooth Muscle Cells. Int. J. Mol. Sci. 2021, 22, 5446. [Google Scholar] [CrossRef]
- Zeidan, A.; Paylor, B.; Steinhoff, K.J.; Javadov, S.; Rajapurohitam, V.; Chakrabarti, S.; Karmazyn, M. Actin Cytoskeleton Dynamics Promotes Leptin-Induced Vascular Smooth Muscle Hypertrophy via RhoA/ROCK- and Phosphatidylinositol 3-Kinase/Protein Kinase B-Dependent Pathways. J. Pharmacol. Exp. Ther. 2007, 322, 1110–1116. [Google Scholar] [CrossRef]
- Liu, R.; Chen, B.; Chen, J.; Lan, J. Leptin upregulates smooth muscle cell expression of MMP-9 to promote plaque destabilization by activating AP-1 via the leptin receptor/MAPK/ERK signaling pathways. Exp. Ther. Med. 2018, 16, 5327–5333. [Google Scholar] [CrossRef]
- Li, L.; Mamputu, J.-C.; Wiernsperger, N.; Renier, G.V. Signaling Pathways Involved in Human Vascular Smooth Muscle Cell Proliferation and Matrix Metalloproteinase-2 Expression Induced by Leptin: Inhibitory Effect of Metformin. Diabetes 2005, 54, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.R.; Leifheit-Nestler, M.; Hubert, A.; Schumann, B.; Glückermann, R.; Eschholz, N.; Krüger, N.; Lutz, S.; Hasenfuss, G.; Konstantinides, S.; et al. Leptin promotes neointima formation and smooth muscle cell proliferation via NADPH oxidase activation and signalling in caveolin-rich microdomains. Cardiovasc. Res. 2013, 99, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.-P.; Zhang, L.-N.; Tian, G. Perivascular adipose tissue-derived leptin promotes vascular smooth muscle cell phenotypic switching via p38 mitogen-activated protein kinase in metabolic syndrome rats. Exp. Biol. Med. 2014, 239, 954–965. [Google Scholar] [CrossRef]
- Ganguly, R.; Khanal, S.; Mathias, A.; Gupta, S.; Lallo, J.; Sahu, S.; Ohanyan, V.; Patel, A.; Storm, K.; Datta, S.; et al. TSP-1 (Thrombospondin-1) Deficiency Protects ApoE(−/−) Mice Against Leptin-Induced Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e112–e127. [Google Scholar] [CrossRef]
- Liu, G.-Y.; Liang, Q.-H.; Cui, R.-R.; Liu, Y.; Wu, S.-S.; Shan, P.-F.; Yuan, L.-Q.; Liao, E.-Y. Leptin Promotes the Osteoblastic Differentiation of Vascular Smooth Muscle Cells From Female Mice by Increasing RANKL Expression. Endocrinology 2014, 155, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; Yang, Q.; Blüher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.-A.; Smith, U.; et al. Retinol-Binding Protein 4 and Insulin Resistance in Lean, Obese, and Diabetic Subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Flores-Cortez, Y.A.; Barragán-Bonilla, M.I.; Mendoza-Bello, J.M.; González-Calixto, C.; Flores-Alfaro, E.; Espinoza-Rojo, M. Interplay of retinol binding protein 4 with obesity and associated chronic alterations (Review). Mol. Med. Rep. 2022, 26, 244. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Lai, J.; Zhou, Y.; Lin, X.; Deng, G.; Zhang, Z.; Li, L. Circulating retinol binding protein 4 levels in coronary artery disease: A systematic review and meta-analysis. Lipids Health Dis. 2021, 20, 89. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Y.; Chen, H.; Wang, D.; Wang, M.; Ou, J.S.; Xia, M. Retinol-Binding Protein-Dependent Cholesterol Uptake Regulates Macrophage Foam Cell Formation and Promotes Atherosclerosis. Circulation 2017, 135, 1339–1354. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, S.D.; Wang, W. Elevated retinol binding protein 4 levels are associated with atherosclerosis in diabetic rats via JAK2/STAT3 signaling pathway. World J. Diabetes 2021, 12, 466–479. [Google Scholar] [CrossRef]
- Li, F.; Xia, K.; Sheikh, M.S.; Cheng, J.; Li, C.; Yang, T. Retinol binding protein 4 promotes hyperinsulinism-induced proliferation of rat aortic smooth muscle cells. Mol. Med. Rep. 2014, 9, 1634–1640. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, X.; Li, J.; Wang, W.; Ye, S. Retinol binding protein 4 promotes the phenotypic transformation of vascular smooth muscle cells under high glucose condition via modulating RhoA/ROCK1 pathway. Transl. Res. 2023, 259, 13–27. [Google Scholar] [CrossRef]
- Katsiki, N.; Mantzoros, C.; Mikhailidis, D.P. Adiponectin, lipids and atherosclerosis. Curr. Opin. Lipidol. 2017, 28, 347–354. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kihara, S.; Ouchi, N.; Nishida, M.; Arita, Y.; Kumada, M.; Ohashi, K.; Sakai, N.; Shimomura, I.; Kobayashi, H.; et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 2002, 106, 2767–2770. [Google Scholar] [CrossRef]
- Nawrocki, A.R.; Hofmann, S.M.; Teupser, D.; Basford, J.E.; Durand, J.L.; Jelicks, L.A.; Woo, C.W.; Kuriakose, G.; Factor, S.M.; Tanowitz, H.B.; et al. Lack of association between adiponectin levels and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Cullen, A.E.; Centner, A.M.; Deitado, R.; Ismaeel, A.; Koutakis, P.; Muller-Delp, J.; Salazar, G. AKT Mediates Adiponectin-Dependent Regulation of VSMC Phenotype. Cells 2023, 12, 2493. [Google Scholar] [CrossRef]
- Ding, M.; Carrão, A.C.; Wagner, R.J.; Xie, Y.; Jin, Y.; Rzucidlo, E.M.; Yu, J.; Li, W.; Tellides, G.; Hwa, J.; et al. Vascular smooth muscle cell-derived adiponectin: A paracrine regulator of contractile phenotype. J. Mol. Cell. Cardiol. 2012, 52, 474–484. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Li, Q.; Li, M.; Li, X. Adiponectin affects vascular smooth muscle cell proliferation and apoptosis through modulation of the mitofusin-2-mediated Ras-Raf-Erk1/2 signaling pathway. Mol. Med. Rep. 2015, 12, 4703–4707. [Google Scholar] [CrossRef]
- Ding, M.; Xie, Y.; Wagner, R.J.; Jin, Y.; Carrao, A.C.; Liu, L.S.; Guzman, A.K.; Powell, R.J.; Hwa, J.; Rzucidlo, E.M.; et al. Adiponectin induces vascular smooth muscle cell differentiation via repression of mammalian target of rapamycin complex 1 and FoxO4. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1403–1410. [Google Scholar] [CrossRef]
- Maahs, D.M.; Ogden, L.G.; Kinney, G.L.; Wadwa, P.; Snell-Bergeon, J.K.; Dabelea, D.; Hokanson, J.E.; Ehrlich, J.; Eckel, R.H.; Rewers, M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation 2005, 111, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.H.; Zhao, L.L.; Yuan, L.Q.; Wang, M.; Xie, H.; Liao, E.Y. Development of arterial calcification in adiponectin-deficient mice: Adiponectin regulates arterial calcification. J. Bone Miner. Res. 2009, 24, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Rattazzi, M.; Bennett, B.J.; Bea, F.; Kirk, E.A.; Ricks, J.L.; Speer, M.; Schwartz, S.M.; Giachelli, C.M.; Rosenfeld, M.E. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: Potential role of chondrocyte-like cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1420–1425. [Google Scholar] [CrossRef]
- Moore, Z.W.; Hui, D.Y. Apolipoprotein E inhibition of vascular hyperplasia and neointima formation requires inducible nitric oxide synthase. J. Lipid Res. 2005, 46, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.-K.; Wang, Y.-J.; Wang, Y.; Tang, Z.-Y.; Tan, P.; Huang, W.; Liu, Y.-S. Adiponectin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the AMPK/mTOR pathway. Exp. Cell Res. 2014, 323, 352–358. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.; Wang, R.; Sun, J.; Guo, B.; Wei, R.; Jia, Y. Adiponectin inhibits vascular smooth muscle cell calcification induced by beta-glycerophosphate through JAK2/STAT3 signaling pathway. J. Biosci. 2019, 44, 86. [Google Scholar] [CrossRef]
- As Habi, A.; Sadeghi, M.; Arab, A.; Hajianfar, H. The association between omentin and diabetes: A systematic review and meta-analysis of observational studies. Diabetes Metab. Syndr. Obes. 2019, 12, 1277–1286. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Bonadia, N.; Angelini, F.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Association between plasma omentin-1 levels in type 2 diabetic patients and peripheral artery disease. Cardiovasc. Diabetol. 2019, 18, 74. [Google Scholar] [CrossRef]
- Elsaid, N.H.; Sadik, N.A.; Ahmed, N.R.; Fayez, S.E.; Mohammed, N.A.E. Serum omentin-1 levels in type 2 diabetic obese women in relation to glycemic control, insulin resistance and metabolic parameters. J. Clin. Transl. Endocrinol. 2018, 13, 14–19. [Google Scholar] [CrossRef]
- Zengi, S.; Zengi, O.; Kirankaya, A.; Kucuk, S.H.; Kutanis, E.E.; Yigit, O. Serum omentin-1 levels in obese children. J. Pediatr. Endocrinol. Metab. 2019, 32, 247–251. [Google Scholar] [CrossRef]
- Lin, S.; Li, X.; Zhang, J.; Zhang, Y. Omentin-1: Protective impact on ischemic stroke via ameliorating atherosclerosis. Clin. Chim. Acta 2021, 517, 31–40. [Google Scholar] [CrossRef]
- Wu, D.M.; Wang, S.; Wen, X.; Han, X.R.; Wang, Y.J.; Shen, M.; Fan, S.H.; Zhang, Z.F.; Shan, Q.; Li, M.Q.; et al. Impact of serum omentin-1 levels on functional prognosis in nondiabetic patients with ischemic stroke. Am. J. Transl. Res. 2019, 11, 1854–1863. [Google Scholar] [PubMed]
- Wang, Y.; Sun, M.; Wang, Z.; Li, X.; Zhu, Y.; Li, Y. Omentin-1 ameliorates the attachment of the leukocyte THP-1 cells to HUVECs by targeting the transcriptional factor KLF2. Biochem. Biophys. Res. Commun. 2018, 498, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors αvβ3 and αvβ5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu-Ito, M.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kanemura, N.; Kambara, T.; Enomoto, T.; Yuasa, D.; Matsuo, K.; Ito, M.; et al. Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice. Cardiovasc. Res. 2015, 110, 107–117. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hwang, S.Y.; Hong, H.C.; Choi, H.Y.; Yang, S.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 103. [Google Scholar] [CrossRef]
- Xu, F.; Li, F.X.; Lin, X.; Zhong, J.Y.; Wu, F.; Shan, S.K.; Tan, C.M.; Yuan, L.Q.; Liao, X.B. Adipose tissue-derived omentin-1 attenuates arterial calcification via AMPK/Akt signaling pathway. Aging 2019, 11, 8760–8776. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Shibata, R.; Kanemura, N.; Ohashi, K.; Kambara, T.; Hiramatsu-Ito, M.; Enomoto, T.; Yuasa, D.; Joki, Y.; Matsuo, K.; et al. Adipose-derived protein omentin prevents neointimal formation after arterial injury. FASEB J. 2015, 29, 141–151. [Google Scholar] [CrossRef]
- Xie, H.; Xie, P.-L.; Wu, X.-P.; Chen, S.-M.; Zhou, H.-D.; Yuan, L.-Q.; Sheng, Z.-F.; Tang, S.-Y.; Luo, X.-H.; Liao, E.-Y. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc. Res. 2011, 92, 296–306. [Google Scholar] [CrossRef]
- Cosentino-Gomes, D.; Rocco-Machado, N.; Meyer-Fernandes, J.R. Cell Signaling through Protein Kinase C Oxidation and Activation. Int. J. Mol. Sci. 2012, 13, 10697–10721. [Google Scholar] [CrossRef]
- Srivastava, S.; Ramana, K.V.; Tammali, R.; Srivastava, S.K.; Bhatnagar, A. Contribution of Aldose Reductase to Diabetic Hyperproliferation of Vascular Smooth Muscle Cells. Diabetes 2006, 55, 901–910. [Google Scholar] [CrossRef]
- Nakamura, J.; Kasuya, Y.; Hamada, Y.; Nakashima, E.; Naruse, K.; Yasuda, Y.; Kato, K.; Hotta, N. Glucose-induced hyperproliferation of cultured rat aortic smooth muscle cells through polyol pathway hyperactivity. Diabetologia 2001, 44, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Trang, N.M.; Kim, E.N.; Jeong, H.G.; Jeong, G.S. Citropten Inhibits Vascular Smooth Muscle Cell Proliferation and Migration via the TRPV1 Receptor. ACS Omega 2024, 9, 29829–29839. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.; Venema, V.J.; Wang, X.; Ju, H.; Venema, R.C.; Marrero, M.B. Hyperglycemia enhances angiotensin II-induced janus-activated kinase/STAT signaling in vascular smooth muscle cells. J. Biol. Chem. 1999, 274, 32382–32386. [Google Scholar] [CrossRef]
- Shaw, S.; Wang, X.; Redd, H.; Alexander, G.D.; Isales, C.M.; Marrero, M.B. High Glucose Augments the Angiotensin II-induced Activation of JAK2 in Vascular Smooth Muscle Cells via the Polyol Pathway. J. Biol. Chem. 2003, 278, 30634–30641. [Google Scholar] [CrossRef]
- Liao, X.H.; Wang, N.; Zhao, D.W.; Zheng, D.L.; Zheng, L.; Xing, W.J.; Ma, W.J.; Bao, L.Y.; Dong, J.; Zhang, T.C. STAT3 Protein Regulates Vascular Smooth Muscle Cell Phenotypic Switch by Interaction with Myocardin. J. Biol. Chem. 2015, 290, 19641–19652. [Google Scholar] [CrossRef]
- Gao, D.; Hao, G.; Meng, Z.; Ning, N.; Yang, G.; Liu, Z.; Dong, X.; Niu, X. Rosiglitzone suppresses angiotensin II-induced production of KLF5 and cell proliferation in rat vascular smooth muscle cells. PLoS ONE 2015, 10, e0123724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shindo, T.; Manabe, I.; Fukushima, Y.; Tobe, K.; Aizawa, K.; Miyamoto, S.; Kawai-Kowase, K.; Moriyama, N.; Imai, Y.; Kawakami, H.; et al. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat. Med. 2002, 8, 856–863. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, J.-K.; Dong, L.-H.; Zheng, B.; Han, M. Krüppel-like factor (KLF) 5 mediates cyclin D1 expression and cell proliferation via interaction with c-Jun in Ang II-induced VSMCs. Acta Pharmacol. Sin. 2010, 31, 10–18. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zheng, B.; Tong, F.; Yang, Z.; Wang, Z.B.; Yang, B.M.; Sun, Y.; Zhang, X.H.; Zhao, Y.L.; Wen, J.K. iNOS-derived peroxynitrite mediates high glucose-induced inflammatory gene expression in vascular smooth muscle cells through promoting KLF5 expression and nitration. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2821–2834. [Google Scholar] [CrossRef]
- Su, G.; Chen, B.; Song, Y.; Yin, Q.; Wang, W.; Zhao, X.; Fan, S.; Lian, J.; Li, D.; Bi, J.; et al. Klf5-adjacent super-enhancer functions as a 3D genome structure-dependent transcriptional driver to safeguard ESC identity. Nat. Commun. 2025, 16, 5540. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Q.; Zhou, Z.; Huang, J.; Chaudhury, L.; Dong, J.T.; Chen, C. Krüppel-like factor 5 promotes breast cell proliferation partially through upregulating the transcription of fibroblast growth factor binding protein 1. Oncogene 2009, 28, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, S.T.; Zhou, B.; Lı, T. MiR-9 promotes the phenotypic switch of vascular smooth muscle cells by targeting KLF5. Turk. J. Med. Sci. 2019, 49, 928–938. [Google Scholar] [CrossRef]
- Fang, G.; Tian, Y.; Huang, S.; Zhang, X.; Liu, Y.; Li, Y.; Du, J.; Gao, S. KLF15 maintains contractile phenotype of vascular smooth muscle cells and prevents thoracic aortic dissection by interacting with MRTFB. J. Biol. Chem. 2024, 300, 107260. [Google Scholar] [CrossRef]
- Lu, Y.; Haldar, S.; Croce, K.; Wang, Y.; Sakuma, M.; Morooka, T.; Wang, B.; Jeyaraj, D.; Gray, S.J.; Simon, D.I.; et al. Kruppel-like Factor 15 Regulates Smooth Muscle Response to Vascular Injury—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1550–1552. [Google Scholar] [CrossRef]
- Yang, B.; Gao, X.; Sun, Y.; Zhao, J.; Chen, J.; Gao, L.; Zhao, L.; Li, Y. Dihydroartemisinin alleviates high glucose-induced vascular smooth muscle cells proliferation and inflammation by depressing the miR-376b-3p/KLF15 pathway. Biochem. Biophys. Res. Commun. 2020, 530, 574–580. [Google Scholar] [CrossRef]
- Kitamura, T. The role of FOXO1 in β-cell failure and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Colman, M.J.; Dansen, T.B.; Burgering, B.M.T. FOXO transcription factors as mediators of stress adaptation. Nat. Rev. Mol. Cell Biol. 2024, 25, 46–64. [Google Scholar] [CrossRef]
- Eijkelenboom, A.; Burgering, B.M. FOXOs: Signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 2013, 14, 83–97. [Google Scholar] [CrossRef]
- Su, D.; Coudriet, G.M.; Hyun Kim, D.; Lu, Y.; Perdomo, G.; Qu, S.; Slusher, S.; Tse, H.M.; Piganelli, J.; Giannoukakis, N.; et al. FoxO1 Links Insulin Resistance to Proinflammatory Cytokine IL-1β Production in Macrophages. Diabetes 2009, 58, 2624–2633. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zhang, M.N.; Chen, H.; Wang, X.; Zhao, K.; Li, X.; Song, X.; Tong, F. Salvianolic Acid B Alleviates High Glucose-Induced Vascular Smooth Muscle Cell Inflammation by Upregulating the miR-486a-5p Expression. Mediat. Inflamm. 2024, 2024, 4121166. [Google Scholar] [CrossRef] [PubMed]
- Roy, B. Pathophysiological Mechanisms of Diabetes-Induced Macrovascular and Microvascular Complications: The Role of Oxidative Stress. Med. Sci. 2025, 13, 87. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Carrillo-Sepulveda, M.A.; Spitler, K.; Pandey, D.; Berkowitz, D.E.; Matsumoto, T. Inhibition of TLR4 attenuates vascular dysfunction and oxidative stress in diabetic rats. J. Mol. Med. 2015, 93, 1341–1354. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.H.; Jiang, H.; Tang, Q.Z.; Yan, L.; Wang, D.; Liu, C.; Bian, Z.Y.; Li, H. Grape seed proanthocyanidins attenuate vascular smooth muscle cell proliferation via blocking phosphatidylinositol 3-kinase-dependent signaling pathways. J. Cell. Physiol. 2010, 223, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Fang, J.; Liu, Q.; Wang, Y.; Zhang, Z. Role of ROS-TRPM7-ERK1/2 axis in high concentration glucose-mediated proliferation and phenotype switching of rat aortic vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2017, 494, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, T.; Lin, J.; Ju, T.; Zhang, L. Inhibition of TRPM7 attenuates rat aortic smooth muscle cell proliferation induced by angiotensin II: Role of genistein. J. Cardiovasc. Pharmacol. 2015, 66, 16–24. [Google Scholar] [CrossRef]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, M.; Fan, X.-H.; Chen, J.-H.; Guan, Y.-Y.; Tang, Y.-B. Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ. Res. 2012, 111, 1137–1146. [Google Scholar] [CrossRef]
- Yogi, A.; Callera, G.E.; Antunes, T.T.; Tostes, R.C.; Touyz, R.M. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ. J. 2011, 75, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Morgan, J.I.; Spector, S. Benzodiazepines that bind at peripheral sites inhibit cell proliferation. Proc. Natl. Acad. Sci. USA 1984, 81, 753–756. [Google Scholar] [CrossRef]
- Hirsch, T.; Decaudin, D.; Susin, S.A.; Marchetti, P.; Larochette, N.; Resche-Rigon, M.; Kroemer, G. PK11195, a ligand of the mitochondrial benzodiazepine receptor, facilitates the induction of apoptosis and reverses Bcl-2-mediated cytoprotection. Exp. Cell Res. 1998, 241, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.; Veenman, L.; Singh, S.; Azrad, M.; Bode, J.; Vainshtein, A.; Caballero, B.; Marek, I.; Gavish, M. Classical and novel TSPO ligands for the mitochondrial TSPO can modulate nuclear gene expression: Implications for mitochondrial retrograde signaling. Int. J. Mol. Sci. 2017, 18, 786. [Google Scholar] [CrossRef]
- Hirsch, J.D.; Beyer, C.F.; Malkowitz, L.; Beer, B.; Blume, A.J. Mitochondrial benzodiazepine receptors mediate inhibition of mitochondrial respiratory control. Mol. Pharmacol. 1989, 35, 157–163. [Google Scholar] [CrossRef]
- Gatliff, J.; East, D.A.; Singh, A.; Alvarez, M.S.; Frison, M.; Matic, I.; Ferraina, C.; Sampson, N.; Turkheimer, F.; Campanella, M. A role for TSPO in mitochondrial Ca2+ homeostasis and redox stress signaling. Cell Death Dis. 2017, 8, e2896. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Han, Y.; Wu, L.; Xia, T.; Ren, H.; Yang, D.; Gu, D.; Wang, H.; Hu, C.; He, D.; et al. Translocator protein 18 kDa ligand alleviates neointimal hyperplasia in the diabetic rat artery injury model via activating PKG. Life Sci. 2019, 221, 72–82. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Li, Z.X.; Zhang, L.L.; Wang, D.; Liu, Y.P. Phenotypic plasticity of vascular smooth muscle cells in vascular calcification: Role of mitochondria. Front. Cardiovasc. Med. 2022, 9, 972836. [Google Scholar] [CrossRef]
- Campos, J.C.; Bozi, L.H.M.; Ferreira, J.C.B. Mitochondrial Biogenesis and Dynamics in Health and Disease. In Essential Aspects of Immunometabolism in Health and Disease; Camara, N.O.S., Alves-Filho, J.C., Moraes-Vieira, P.M.M.D., Andrade-Oliveira, V., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 31–51. [Google Scholar]
- Salabei, J.K.; Hill, B.G. Mitochondrial fission induced by platelet-derived growth factor regulates vascular smooth muscle cell bioenergetics and cell proliferation. Redox. Biol. 2013, 1, 542–551. [Google Scholar] [CrossRef]
- Zhuang, X.; Maimaitijiang, A.; Li, Y.; Shi, H.; Jiang, X. Salidroside inhibits high-glucose induced proliferation of vascular smooth muscle cells via inhibiting mitochondrial fission and oxidative stress. Exp. Ther. Med. 2017, 14, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Chiong, M.; Cartes-Saavedra, B.; Norambuena-Soto, I.; Mondaca-Ruff, D.; Morales, P.E.; García-Miguel, M.; Mellado, R. Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front. Cell Dev. Biol. 2014, 2, 72. [Google Scholar] [CrossRef]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Tai, S.; Hu, X.-Q.; Peng, D.-Q.; Zhou, S.-H.; Zheng, X.-L. The roles of autophagy in vascular smooth muscle cells. Int. J. Cardiol. 2016, 211, 1–6. [Google Scholar] [CrossRef]
- Xu, Z.J.; Xu, J.; Lei, W.J.; Wang, X.; Zou, Q.L.; Lv, L.C.; Liu, C.; Hu, W.M.; Xiang, Y.J.; Shen, J.Y.; et al. RANBP1 Regulates NOTCH3-Mediated Autophagy in High Glucose-Induced Vascular Smooth Muscle Cells. Front. Biosci. 2025, 30, 26850. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Sahu, S.; Chavez, R.J.; Raman, P. Trivalent chromium inhibits TSP-1 expression, proliferation, and O-GlcNAc signaling in vascular smooth muscle cells in response to high glucose in vitro. Am. J. Physiol. Cell Physiol. 2015, 308, C111–C122. [Google Scholar] [CrossRef]
- Raman, P.; Krukovets, I.; Marinic, T.E.; Bornstein, P.; Stenina, O.I. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J. Biol. Chem. 2007, 282, 5704–5714. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, X.; Wang, S.; Wang, B.; Chu, F.; Tian, Z.; Zhang, L.; Zhou, F. The role of O-GlcNAcylation in innate immunity and inflammation. J. Mol. Cell Biol. 2022, 14, mjac065. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Z.; Wen, H. Protein O-GlcNAcylation Regulates Innate Immune Cell Function. Front. Immunol. 2022, 13, 805018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, J.; Hou, C.; Luo, X.; Zhu, S.; Peng, Y.; Peng, C.; Li, P.; Meng, H.; Xia, Y.; et al. A ROS-mediated oxidation-O-GlcNAcylation cascade governs ferroptosis. Nat. Cell Biol. 2025, 27, 1288–1300. [Google Scholar] [CrossRef]
- Zhang, C.-C.; Li, Y.; Jiang, C.-Y.; Le, Q.-M.; Liu, X.; Ma, L.; Wang, F.-F. O-GlcNAcylation mediates H2O2-induced apoptosis through regulation of STAT3 and FOXO1. Acta Pharmacol. Sin. 2024, 45, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Chatham, J.C.; Patel, R.P. Protein glycosylation in cardiovascular health and disease. Nat. Rev. Cardiol. 2024, 21, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, S.; Lou, W.; Qian, H.; Xu, Z. Crosstalk between O-GlcNAcylation and phosphorylation in metabolism: Regulation and mechanism. Cell Death Differ. 2025, 32, 1181–1199. [Google Scholar] [CrossRef]
- Khanal, S.; Bhavnani, N.; Mathias, A.; Lallo, J.; Gupta, S.; Ohanyan, V.; Ferrell, J.M.; Raman, P. Deletion of Smooth Muscle O-GlcNAc Transferase Prevents Development of Atherosclerosis in Western Diet-Fed Hyperglycemic ApoE(−/−) Mice In Vivo. Int. J. Mol. Sci. 2023, 24, 7899. [Google Scholar] [CrossRef]
- Onuh, J.O.; Qiu, H. Serum response factor-cofactor interactions and their implications in disease. FEBS J. 2021, 288, 3120–3134. [Google Scholar] [CrossRef]
- Miano, J.M. Serum response factor: Toggling between disparate programs of gene expression. J. Mol. Cell. Cardiol. 2003, 35, 577–593. [Google Scholar] [CrossRef]
- Gualdrini, F.; Esnault, C.; Horswell, S.; Stewart, A.; Matthews, N.; Treisman, R. SRF Co-factors Control the Balance between Cell Proliferation and Contractility. Mol. Cell 2016, 64, 1048–1061. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.Z.; Hockemeyer, D.; McAnally, J.; Nordheim, A.; Olson, E.N. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 2004, 428, 185–189. [Google Scholar] [CrossRef]
- Tammali, R.; Saxena, A.; Srivastava, S.K.; Ramana, K.V. Aldose Reductase Regulates Vascular Smooth Muscle Cell Proliferation by Modulating G1/S Phase Transition of Cell Cycle. Endocrinology 2010, 151, 2140–2150. [Google Scholar] [CrossRef]
- Flores-Roco, A.; Lago, B.M.; Villa-Bellosta, R. Elevated glucose levels increase vascular calcification risk by disrupting extracellular pyrophosphate metabolism. Cardiovasc. Diabetol. 2024, 23, 405. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, P.; Chen, W.; Peng, D. Combined effects of hyperphosphatemia and hyperglycemia on the calcification of cultured human aortic smooth muscle cells. Exp. Ther. Med. 2019, 17, 863–868. [Google Scholar] [CrossRef]
- Lu, X.; Luo, Q.; Yu, D.; He, Y.; Chen, J. The Emerging Role of Lactate and Lactylation Modifications in the Pathophysiology of Atherosclerotic Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2025. [Google Scholar] [CrossRef]
- Chang, X.; Zheng, W.; Zhao, Y.; Niku, W.; Deng, B.; Liu, P.; Wang, Y. Association of Lactate with Risk of Cardiovascular Diseases: A Two-Sample Mendelian Randomization Study. Vasc. Health Risk Manag. 2024, 20, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.-L.; Yan, X.-J.; Ji, Y.; Wang, F.-F. Exploring a new mechanism between lactate and VSMC calcification: PARP1/POLG/UCP2 signaling pathway and imbalance of mitochondrial homeostasis. Cell Death Dis. 2023, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, J.-J.; Yang, R.; Han, X.-Q.; Sun, X.-J.; Ma, W.-Q.; Liu, N.-F. Lactate accelerates calcification in VSMCs through suppression of BNIP3-mediated mitophagy. Cell. Signal. 2019, 58, 53–64. [Google Scholar] [CrossRef]
- Hu, Y.; He, Z.; Li, Z.; Wang, Y.; Wu, N.; Sun, H.; Zhou, Z.; Hu, Q.; Cong, X. Lactylation: The novel histone modification influence on gene expression, protein function, and disease. Clin. Epigenetics 2024, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, J.C.; Zhang, J.L.; Wang, F.F.; Liu, R.P. A new mechanism of arterial calcification in diabetes: Interaction between H3K18 lactylation and CHI3L1. Clin. Sci. 2025, 139, 115–130. [Google Scholar] [CrossRef]
- Shi, L.L.; Hao, M.; Jin, Z.Y.; Peng, G.F.; Tang, Y.Y.; Kuang, H.Y. Liraglutide Alleviates Diabetic Atherosclerosis through Regulating Calcification of Vascular Smooth Muscle Cells. Dis. Markers 2022, 2022, 5013622. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, J.; Yang, W.; Zheng, H.; Xue, S. High mobility group box 1 (HMGB1) mediates high-glucose-induced calcification in vascular smooth muscle cells of saphenous veins. Inflammation 2013, 36, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Jin, J.S.; Son, S.M. Long Term Effect of High Glucose and Phosphate Levels on the OPG/RANK/RANKL/TRAIL System in the Progression of Vascular Calcification in rat Aortic Smooth Muscle Cells. Korean J. Physiol. Pharmacol. 2015, 19, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, A.; Golledge, J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 2009, 204, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, T.; Tu, Z.; Zhang, Y.; Wang, X.; Zang, D.; Xu, D.; Feng, Y.; He, F.; Ni, M.; et al. Both high glucose and phosphate overload promote senescence-associated calcification of vascular muscle cells. Int. Urol. Nephrol. 2022, 54, 2719–2731. [Google Scholar] [CrossRef]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef]
- Li, G.; Barrett, E.J.; Ko, S.H.; Cao, W.; Liu, Z. Insulin and insulin-like growth factor-I receptors differentially mediate insulin-stimulated adhesion molecule production by endothelial cells. Endocrinology 2009, 150, 3475–3482. [Google Scholar] [CrossRef]
- Vicent, D.; Ilany, J.; Kondo, T.; Naruse, K.; Fisher, S.J.; Kisanuki, Y.Y.; Bursell, S.; Yanagisawa, M.; King, G.L.; Kahn, C.R. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J. Clin. Investig. 2003, 111, 1373–1380. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Fulton, D.J. Mechanisms of vascular insulin resistance: A substitute Akt? Circ. Res. 2009, 104, 1035–1037. [Google Scholar] [CrossRef]
- Breen, D.M.; Giacca, A. Effects of insulin on the vasculature. Curr. Vasc. Pharmacol. 2011, 9, 321–332. [Google Scholar] [CrossRef]
- Wang, C.C.L.; Gurevich, I.; Draznin, B. Insulin Affects Vascular Smooth Muscle Cell Phenotype and Migration Via Distinct Signaling Pathways. Diabetes 2003, 52, 2562–2569. [Google Scholar] [CrossRef]
- Fu, J.; Yu, M.G.; Li, Q.; Park, K.; King, G.L. Insulin’s actions on vascular tissues: Physiological effects and pathophysiological contributions to vascular complications of diabetes. Mol. Metab. 2021, 52, 101236. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, J.; Xia, Y.; Qi, W.; Ishikado, A.; Park, K.; Yokomizo, H.; Huang, Q.; Cai, W.; Rask-Madsen, C.; et al. Homozygous receptors for insulin and not IGF-1 accelerate intimal hyperplasia in insulin resistance and diabetes. Nat. Commun. 2019, 10, 4427. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, J.; Park, K.; Shah, H.; Li, Q.; Wu, I.H.; King, G.L. Insulin receptors in vascular smooth muscle cells regulate plaque stability of atherosclerosis. Cardiovasc. Res. 2024, 120, 2017–2030. [Google Scholar] [CrossRef]
- Maier, K.G.; Han, X.; Sadowitz, B.; Gentile, K.L.; Middleton, F.A.; Gahtan, V. Thrombospondin-1: A proatherosclerotic protein augmented by hyperglycemia. J. Vasc. Surg. 2010, 51, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Sahu, S.; Ohanyan, V.; Haney, R.; Chavez, R.J.; Shah, S.; Yalamanchili, S.; Raman, P. Oral chromium picolinate impedes hyperglycemia-induced atherosclerosis and inhibits proatherogenic protein TSP-1 expression in STZ-induced type 1 diabetic ApoE−/− mice. Sci. Rep. 2017, 7, 45279. [Google Scholar] [CrossRef]
- Gupta, S.; Khanal, S.; Bhavnani, N.; Mathias, A.; Lallo, J.; Kiriakou, A.; Ferrell, J.; Raman, P. Sex-specific differences in atherosclerosis, thrombospondin-1, and smooth muscle cell differentiation in metabolic syndrome versus non-metabolic syndrome mice. Front. Cardiovasc. Med. 2022, 9, 1020006. [Google Scholar] [CrossRef]
- Xi, G.; Shen, X.; Wai, C.; White, M.F.; Clemmons, D.R. Hyperglycemia induces vascular smooth muscle cell dedifferentiation by suppressing insulin receptor substrate-1-mediated p53/KLF4 complex stabilization. J. Biol. Chem. 2019, 294, 2407–2421. [Google Scholar] [CrossRef]
- Abhijit, S.; Bhaskaran, R.; Narayanasamy, A.; Chakroborty, A.; Manickam, N.; Dixit, M.; Mohan, V.; Balasubramanyam, M. Hyperinsulinemia-induced vascular smooth muscle cell (VSMC) migration and proliferation is mediated by converging mechanisms of mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2013, 373, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-L.; Shao, J.-S.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 Promotes Osteogenesis and Suppresses Adipogenic Differentiation of Multipotent Mesenchymal Progenitors. J. Biol. Chem. 2003, 278, 45969–45977. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.S.; Cheng, S.L.; Pingsterhaus, J.M.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J. Clin. Investig. 2005, 115, 1210–1220. [Google Scholar] [CrossRef]
- Shao, J.-S.; Aly, Z.A.; Lai, C.-F.; Cheng, S.-L.; Cai, J.; Huang, E.; Behrmann, A.; Towler, D.A. Vascular Bmp–Msx2–Wnt Signaling and Oxidative Stress in Arterial Calcification. Ann. N. Y. Acad. Sci. 2007, 1117, 40–50. [Google Scholar] [CrossRef]
- Andrade, M.C.; Carmo, L.S.; Farias-Silva, E.; Liberman, M. Msx2 is required for vascular smooth muscle cells osteoblastic differentiation but not calcification in insulin-resistant ob/ob mice. Atherosclerosis 2017, 265, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; McVey, D.G.; Shen, D.; Huang, X.; Ye, S. Phenotypic Switching of Vascular Smooth Muscle Cells in Atherosclerosis. J. Am. Heart Assoc. 2023, 12, e031121. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Hernandez, G.; Raman, P. Cellular and Molecular Mechanisms of VSMC Phenotypic Switching in Type 2 Diabetes. Cells 2025, 14, 1365. https://doi.org/10.3390/cells14171365
Gupta S, Hernandez G, Raman P. Cellular and Molecular Mechanisms of VSMC Phenotypic Switching in Type 2 Diabetes. Cells. 2025; 14(17):1365. https://doi.org/10.3390/cells14171365
Chicago/Turabian StyleGupta, Shreya, Gilbert Hernandez, and Priya Raman. 2025. "Cellular and Molecular Mechanisms of VSMC Phenotypic Switching in Type 2 Diabetes" Cells 14, no. 17: 1365. https://doi.org/10.3390/cells14171365
APA StyleGupta, S., Hernandez, G., & Raman, P. (2025). Cellular and Molecular Mechanisms of VSMC Phenotypic Switching in Type 2 Diabetes. Cells, 14(17), 1365. https://doi.org/10.3390/cells14171365






