Targeting Diabetic Retinopathy with Human iPSC-Derived Vascular Reparative Cells in a Type 2 Diabetes Model
Abstract
1. Introduction
2. Materials and Methods
2.1. hiPSC Generation and Culture
2.2. Animal Study
2.3. Electroretinogram (ERG)
2.4. Immunohistochemistry
2.5. Optical Coherence Tomography (OCT)
2.6. Proteomic Analysis
2.7. Statistical Analysis
3. Results
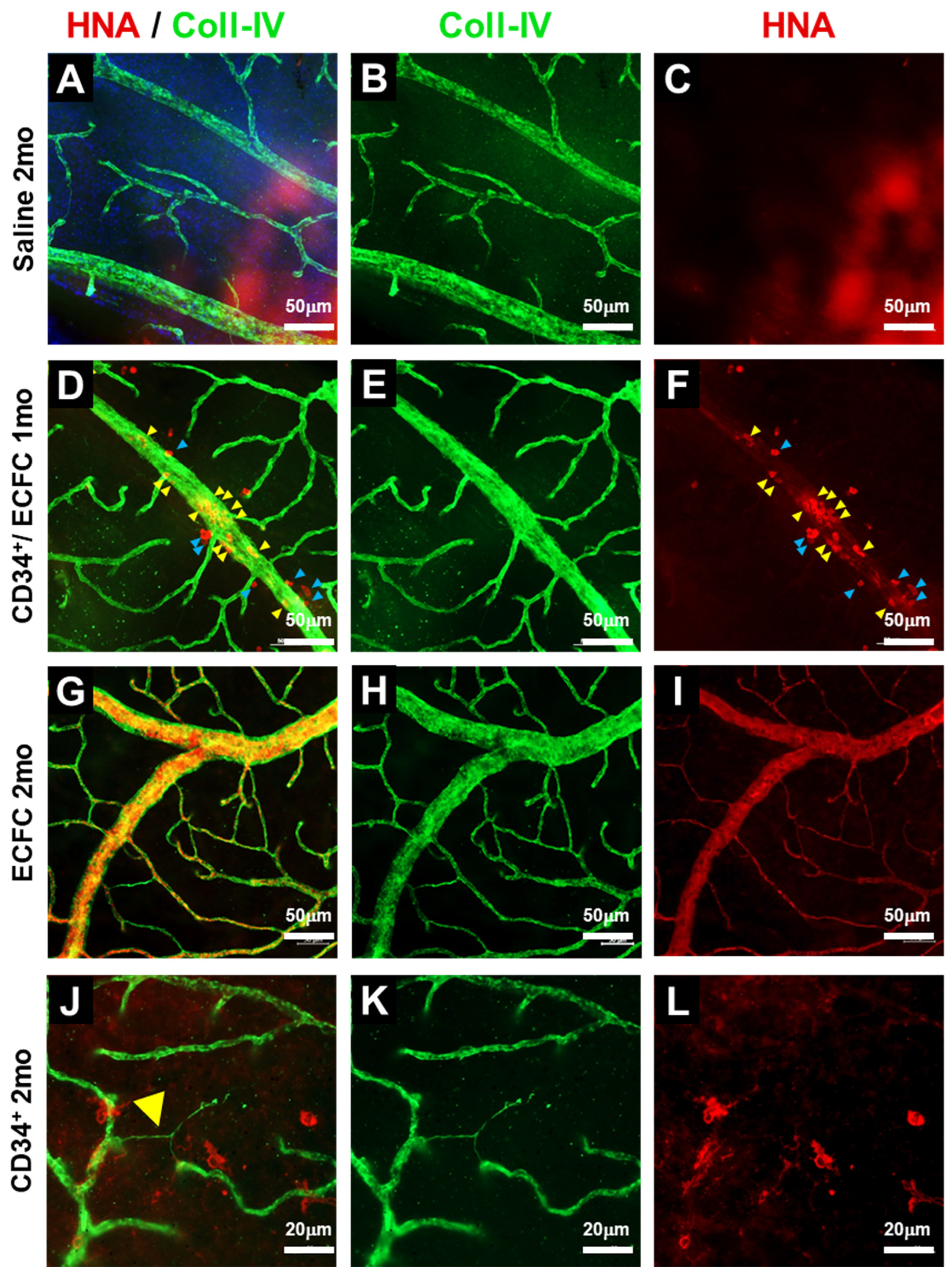
3.1. KNA+ Cells Differentiate into Pericytes in db/db Mice with DR
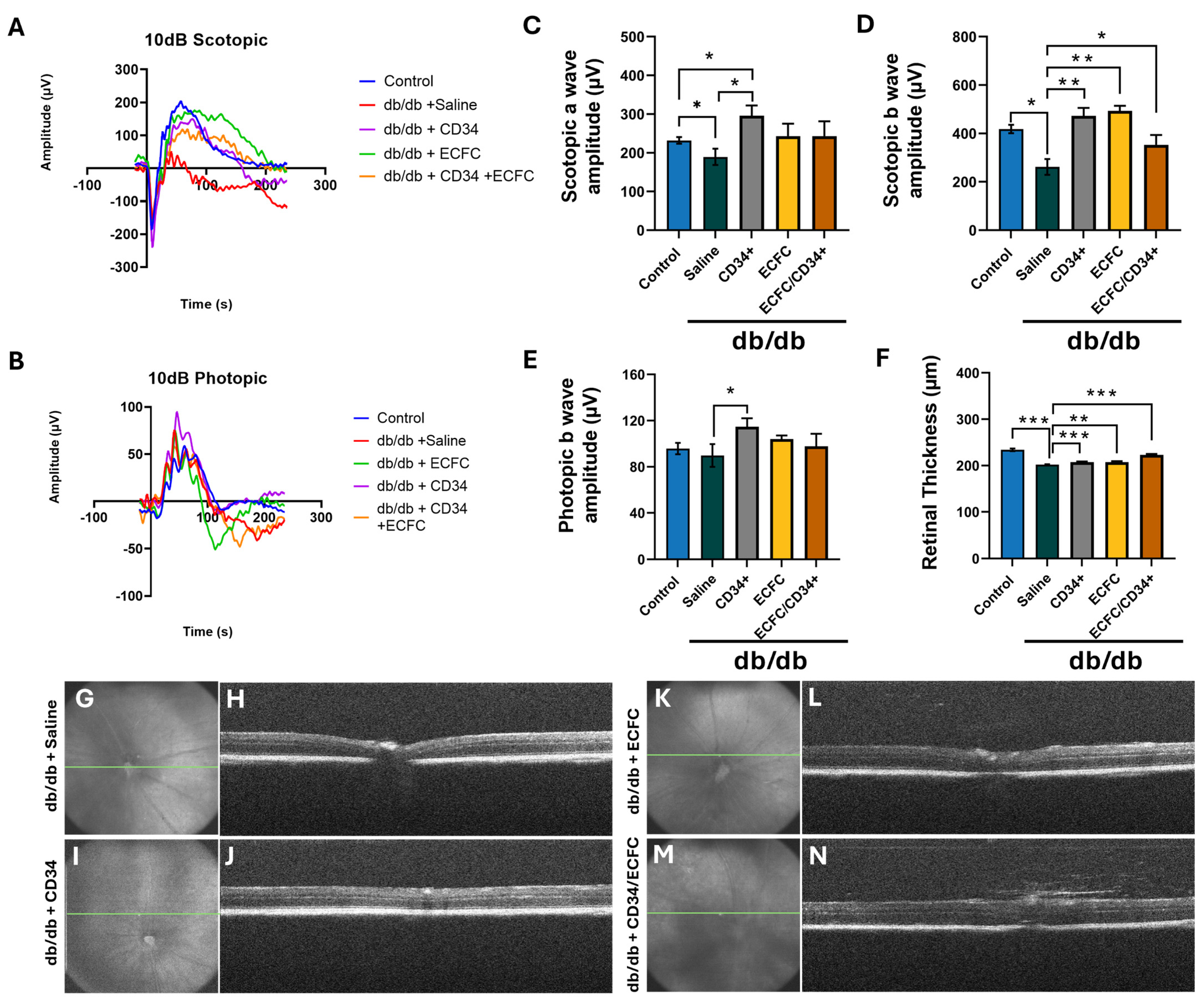
3.2. hiPSC-CD34+ and hiPS-ECFCsAlone and in Combination Resulted in Correction of Diabetes Induced Structural and Functional Retinal Pathology
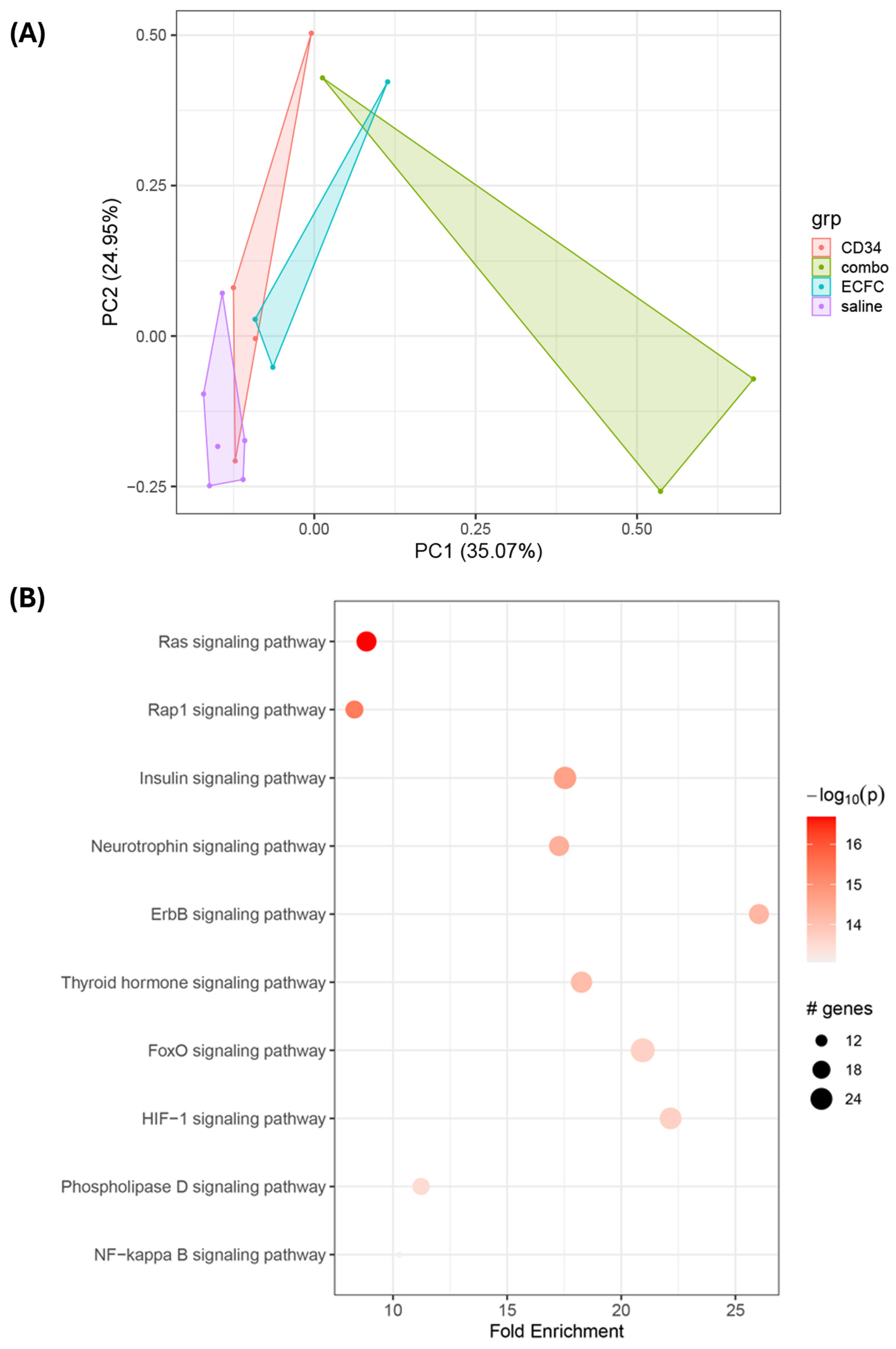
3.3. Proteomic Analysis of Signaling Mechanisms in iPSC-Injected db/db Retinas
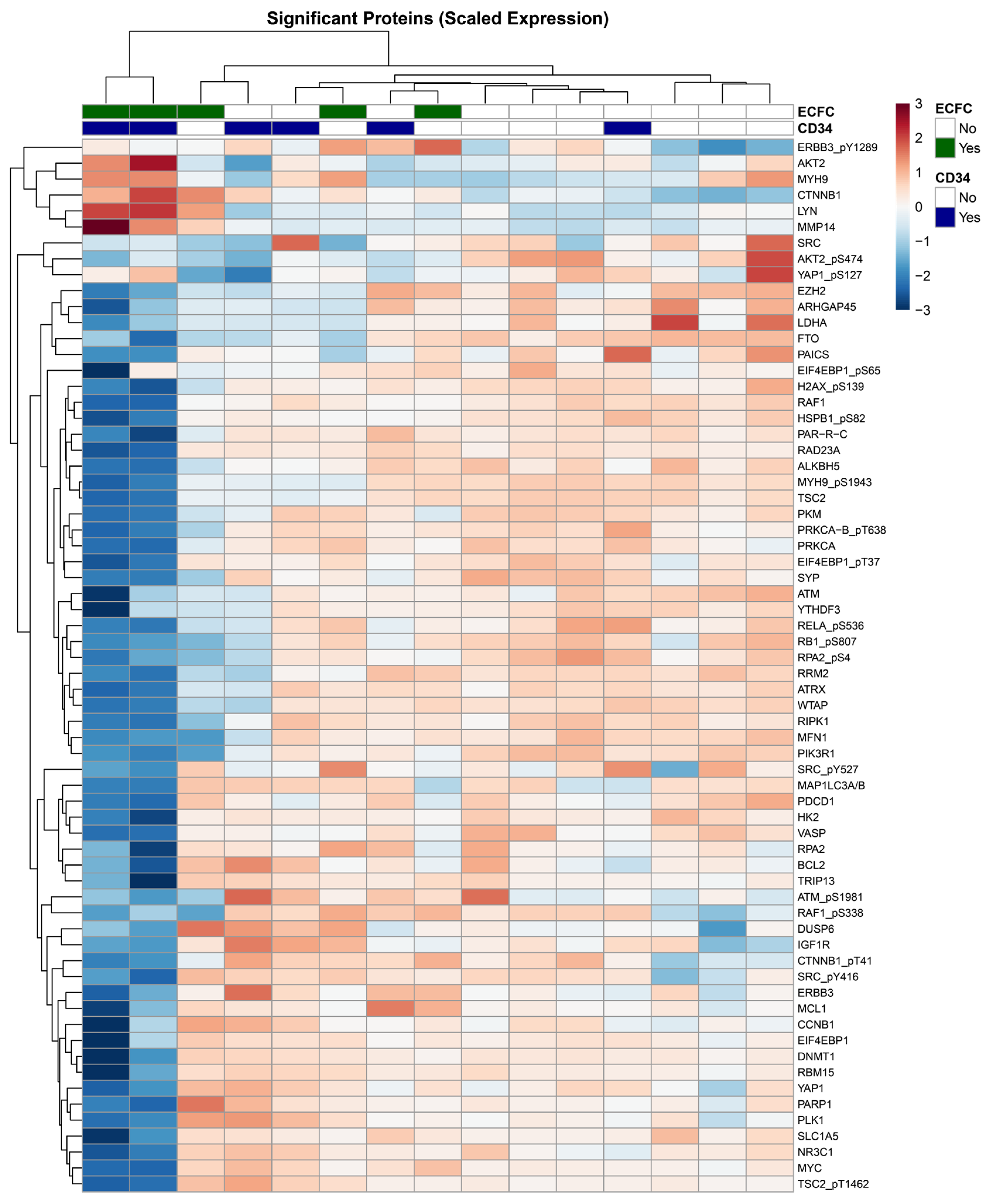
3.4. Protein Expression Profiling Reveals Interactional Effects of Combination CD34+ and ECFC Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moss, S.E.; Klein, R.; Klein, B.E. The 14-year incidence of visual loss in a diabetic population. Ophthalmology 1998, 105, 998–1003. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef]
- Klein, B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007, 14, 179–183. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Tooke, J.E. Microcirculation and diabetes. Br. Med. Bull. 1989, 45, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: Preliminary phase 1 clinical trial findings. Investig. Ophthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779. [Google Scholar] [CrossRef] [PubMed]
- Delewi, R.; Andriessen, A.; Tijssen, J.G.; Zijlstra, F.; Piek, J.J.; Hirsch, A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A meta-analysis of randomised controlled clinical trials. Heart 2013, 99, 225–232. [Google Scholar] [CrossRef]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.; et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef]
- Jarajapu, Y.P.; Hazra, S.; Segal, M.; Li Calzi, S.; Jadhao, C.; Qian, K.; Mitter, S.K.; Raizada, M.K.; Boulton, M.E.; Grant, M.B. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS ONE 2014, 9, e93965. [Google Scholar] [CrossRef]
- Lin, Y.; Banno, K.; Gil, C.H.; Myslinski, J.; Hato, T.; Shelley, W.C.; Gao, H.; Xuei, X.; Liu, Y.; Basile, D.P.; et al. Origin, prospective identification, and function of circulating endothelial colony-forming cells in mice and humans. JCI Insight 2023, 8, e164781. [Google Scholar] [CrossRef]
- Lin, Y.; Gil, C.H.; Banno, K.; Yokoyama, M.; Wingo, M.; Go, E.; Prasain, N.; Liu, Y.; Hato, T.; Naito, H.; et al. ABCG2-Expressing Clonal Repopulating Endothelial Cells Serve to Form and Maintain Blood Vessels. Circulation 2024, 150, 451–465. [Google Scholar] [CrossRef]
- Jarajapu, Y.P.; Bhatwadekar, A.D.; Caballero, S.; Hazra, S.; Shenoy, V.; Medina, R.; Kent, D.; Stitt, A.W.; Thut, C.; Finney, E.M.; et al. Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes 2013, 62, 1258–1269. [Google Scholar] [CrossRef]
- Prasain, N.; Lee, M.R.; Vemula, S.; Meador, J.L.; Yoshimoto, M.; Ferkowicz, M.J.; Fett, A.; Gupta, M.; Rapp, B.M.; Saadatzadeh, M.R.; et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat. Biotechnol. 2014, 32, 1151–1157. [Google Scholar] [CrossRef]
- Gil, C.H.; Chakraborty, D.; Vieira, C.P.; Prasain, N.; Li Calzi, S.; Fortmann, S.D.; Hu, P.; Banno, K.; Jamal, M.; Huang, C.; et al. Specific mesoderm subset derived from human pluripotent stem cells ameliorates microvascular pathology in type 2 diabetic mice. Sci. Adv. 2022, 8, eabm5559. [Google Scholar] [CrossRef] [PubMed]
- Li Calzi, S.; Shaw, L.C.; Moldovan, L.; Shelley, W.C.; Qi, X.; Racette, L.; Quigley, J.L.; Fortmann, S.D.; Boulton, M.E.; Yoder, M.C.; et al. Progenitor cell combination normalizes retinal vascular development in the oxygen-induced retinopathy (OIR) model. JCI Insight 2019, 4, e129224. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Zimmerlin, L.; Evans-Moses, R.; Thomas, J.; Huo, J.S.; Kanherkar, R.; He, A.; Ruzgar, N.; Grebe, R.; Bhutto, I.; et al. Vascular progenitors generated from tankyrase inhibitor-regulated naive diabetic human iPSC potentiate efficient revascularization of ischemic retina. Nat. Commun. 2020, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; McCarrel, T.M.; Grant, M.B. Novel Methods to Mobilize, Isolate, and Expand Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5728. [Google Scholar] [CrossRef]
- Piau, O.; Brunet-Manquat, M.; L’Homme, B.; Petit, L.; Birebent, B.; Linard, C.; Moeckes, L.; Zuliani, T.; Lapillonne, H.; Benderitter, M.; et al. Generation of transgene-free hematopoietic stem cells from human induced pluripotent stem cells. Cell Stem Cell 2023, 30, 1610–1623.e7. [Google Scholar] [CrossRef]
- Fortmann, S.D.; Frey, B.F.; Rosencrans, R.F.; Adu-Rutledge, Y.; Ready, V.E.; Kilchrist, K.V.; Welner, R.S.; Boulton, M.E.; Saban, D.R.; Grant, M.B. Prenatally derived macrophages support choroidal health and decline in age-related macular degeneration. J. Exp. Med. 2025, 222, e20242007. [Google Scholar] [CrossRef]
- Sheskey, S.R.; Antonetti, D.A.; Renteria, R.C.; Lin, C.M. Correlation of Retinal Structure and Visual Function Assessments in Mouse Diabetes Models. Investig. Ophthalmol. Vis. Sci. 2021, 62, 20. [Google Scholar] [CrossRef]
- Danielsson, S.B.; Garcia-Llorca, A.; Reynisson, H.; Eysteinsson, T. Mouse microphthalmia-associated transcription factor (Mitf) mutations affect the structure of the retinal vasculature. Acta Ophthalmol. 2022, 100, 911–918. [Google Scholar] [CrossRef]
- Garcia-Llorca, A.; Eysteinsson, T. The Microphthalmia-Associated Transcription Factor (MITF) and Its Role in the Structure and Function of the Eye. Genes 2024, 15, 1258. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, M.; Zhao, P.; Li, S.; Zhang, L.; Huang, L.; Huang, Y.; Fei, P.; Yang, Y.; Zhang, S.; et al. Catenin alpha 1 mutations cause familial exudative vitreoretinopathy by overactivating Norrin/beta-catenin signaling. J. Clin. Investig. 2021, 131, e139869. [Google Scholar] [CrossRef] [PubMed]
- Weh, E.; Lutrzykowska, Z.; Smith, A.; Hager, H.; Pawar, M.; Wubben, T.J.; Besirli, C.G. Hexokinase 2 is dispensable for photoreceptor development but is required for survival during aging and outer retinal stress. Cell Death Dis. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Rajala, A.; Soni, K.; Rajala, R.V.S. Metabolic and Non-metabolic Roles of Pyruvate Kinase M2 Isoform in Diabetic Retinopathy. Sci. Rep. 2020, 10, 7456. [Google Scholar] [CrossRef] [PubMed]
- Rajala, A.; Bhat, M.A.; Teel, K.; Gopinadhan Nair, G.K.; Purcell, L.; Rajala, R.V.S. The function of lactate dehydrogenase A in retinal neurons: Implications to retinal degenerative diseases. PNAS Nexus 2023, 2, pgad038. [Google Scholar] [CrossRef]
- Ruzycki, P.A.; Zhang, X.; Chen, S. CRX directs photoreceptor differentiation by accelerating chromatin remodeling at specific target sites. Epigenetics Chromatin 2018, 11, 42. [Google Scholar] [CrossRef]
- Martowicz, A.; Trusohamn, M.; Jensen, N.; Wisniewska-Kruk, J.; Corada, M.; Ning, F.C.; Kele, J.; Dejana, E.; Nyqvist, D. Endothelial beta-Catenin Signaling Supports Postnatal Brain and Retinal Angiogenesis by Promoting Sprouting, Tip Cell Formation, and VEGFR (Vascular Endothelial Growth Factor Receptor) 2 Expression. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2273–2288. [Google Scholar] [CrossRef]
- Amado-Azevedo, J.; Reinhard, N.R.; van Bezu, J.; van Nieuw Amerongen, G.P.; van Hinsbergh, V.W.M.; Hordijk, P.L. The minor histocompatibility antigen 1 (HMHA1)/ArhGAP45 is a RacGAP and a novel regulator of endothelial integrity. Vasc. Pharmacol. 2018, 101, 38–47. [Google Scholar] [CrossRef]
- Nag, T.C.; Wadhwa, S. Differential expression of syntaxin-1 and synaptophysin in the developing and adult human retina. J. Biosci. 2001, 26, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Widden, H.; Placzek, W.J. The multiple mechanisms of MCL1 in the regulation of cell fate. Commun. Biol. 2021, 4, 1029. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.; Sengupta, N.; Afzal, A.; Chang, K.H.; Li Calzi, S.; Guberski, D.L.; Kern, T.S.; Grant, M.B. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007, 56, 960–967. [Google Scholar] [CrossRef]
- Hazra, S.; Jarajapu, Y.P.; Stepps, V.; Caballero, S.; Thinschmidt, J.S.; Sautina, L.; Bengtsson, N.; Licalzi, S.; Dominguez, J.; Kern, T.S.; et al. Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia 2013, 56, 644–653. [Google Scholar] [CrossRef]
- Jarajapu, Y.P.; Caballero, S.; Verma, A.; Nakagawa, T.; Lo, M.C.; Li, Q.; Grant, M.B. Blockade of NADPH oxidase restores vasoreparative function in diabetic CD34+ cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5093–5104. [Google Scholar] [CrossRef]
- Yoder, M.C.; Ingram, D.A. The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: Is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochim. Biophys. Acta 2009, 1796, 50–54. [Google Scholar] [CrossRef]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef]
- Ingram, D.A.; Mead, L.E.; Moore, D.B.; Woodard, W.; Fenoglio, A.; Yoder, M.C. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 2005, 105, 2783–2786. [Google Scholar] [CrossRef]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef]
- Medina, R.J.; O’Neill, C.L.; Sweeney, M.; Guduric-Fuchs, J.; Gardiner, T.A.; Simpson, D.A.; Stitt, A.W. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med. Genom. 2010, 3, 18. [Google Scholar] [CrossRef]
- Medina, R.J.; O’Neill, C.L.; O’Doherty, T.M.; Chambers, S.E.; Guduric-Fuchs, J.; Neisen, J.; Waugh, D.J.; Simpson, D.A.; Stitt, A.W. Ex vivo expansion of human outgrowth endothelial cells leads to IL-8-mediated replicative senescence and impaired vasoreparative function. Stem Cells 2013, 31, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Milbauer, L.C.; Enenstein, J.A.; Roney, M.; Solovey, A.; Bodempudi, V.; Nichols, T.C.; Hebbel, R.P. Blood outgrowth endothelial cell migration and trapping in vivo: A window into gene therapy. Transl. Res. 2009, 153, 179–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoon, C.H.; Hur, J.; Park, K.W.; Kim, J.H.; Lee, C.S.; Oh, I.Y.; Kim, T.Y.; Cho, H.J.; Kang, H.J.; Chae, I.H.; et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005, 112, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Beltramo, E.; Porta, M. Pericyte loss in diabetic retinopathy: Mechanisms and consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef]
- Zafar, S.; Sachdeva, M.; Frankfort, B.J.; Channa, R. Retinal Neurodegeneration as an Early Manifestation of Diabetic Eye Disease and Potential Neuroprotective Therapies. Curr. Diab Rep. 2019, 19, 17. [Google Scholar] [CrossRef]
- Bhatt, Y.; Hunt, D.M.; Carvalho, L.S. The origins of the full-field flash electroretinogram b-wave. Front. Mol. Neurosci. 2023, 16, 1153934. [Google Scholar] [CrossRef]
- Sabapathy, V.; Kumar, S. hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J. Cell. Mol. Med. 2016, 20, 1571–1588. [Google Scholar] [CrossRef]
- Khateb, S.; Jha, S.; Bharti, K.; Banin, E. Cell-Based Therapies for Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2021, 1256, 265–293. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Esswein, P.; Ramirez, L.; Warren, E.; Gerecht, S. Derivation of functional retinal endothelial cells from human pluripotent stem cells for therapeutics and modeling. bioRxiv 2025. [Google Scholar] [CrossRef]
- Lei, Q.; Zhang, R.; Yuan, F.; Xiang, M. Integration and Differentiation of Transplanted Human iPSC-Derived Retinal Ganglion Cell Precursors in Murine Retinas. Int. J. Mol. Sci. 2024, 25, 12947. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, X.; Duan, C.; Tang, S.; Chen, J. Application of patient-derived induced pluripotent stem cells and organoids in inherited retinal diseases. Stem Cell Res. Ther. 2023, 14, 340. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzi, S.L.; Chakraborty, D.; Hu, P.; Prasad, R.; Adu-Rutledge, Y.; Vieira, C.; Sheini, F.; Boulton, M.E.; Yoder, M.C.; Cheng, C.; et al. Targeting Diabetic Retinopathy with Human iPSC-Derived Vascular Reparative Cells in a Type 2 Diabetes Model. Cells 2025, 14, 1352. https://doi.org/10.3390/cells14171352
Calzi SL, Chakraborty D, Hu P, Prasad R, Adu-Rutledge Y, Vieira C, Sheini F, Boulton ME, Yoder MC, Cheng C, et al. Targeting Diabetic Retinopathy with Human iPSC-Derived Vascular Reparative Cells in a Type 2 Diabetes Model. Cells. 2025; 14(17):1352. https://doi.org/10.3390/cells14171352
Chicago/Turabian StyleCalzi, Sergio Li, Dibyendu Chakraborty, Ping Hu, Ram Prasad, Yvonne Adu-Rutledge, Cristiano Vieira, Fadeela Sheini, Michael E. Boulton, Mervin C. Yoder, Changde Cheng, and et al. 2025. "Targeting Diabetic Retinopathy with Human iPSC-Derived Vascular Reparative Cells in a Type 2 Diabetes Model" Cells 14, no. 17: 1352. https://doi.org/10.3390/cells14171352
APA StyleCalzi, S. L., Chakraborty, D., Hu, P., Prasad, R., Adu-Rutledge, Y., Vieira, C., Sheini, F., Boulton, M. E., Yoder, M. C., Cheng, C., & Grant, M. B. (2025). Targeting Diabetic Retinopathy with Human iPSC-Derived Vascular Reparative Cells in a Type 2 Diabetes Model. Cells, 14(17), 1352. https://doi.org/10.3390/cells14171352








