Impact of Donor and Host Age on Systemic Cell Therapy to Treat Age-Related Macular Degeneration
Abstract
1. Introduction
2. Methods and Materials
2.1. Animals
2.2. SOD2 KD Mouse Model
2.3. Preparation of RPE65-Programmed HSPC
2.4. Systemic Administration of RPE65-Programmed HSPC
2.5. Electroretinography (ERG)
2.6. Optical Coherence Tomography (OCT)
2.7. Histopathology
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
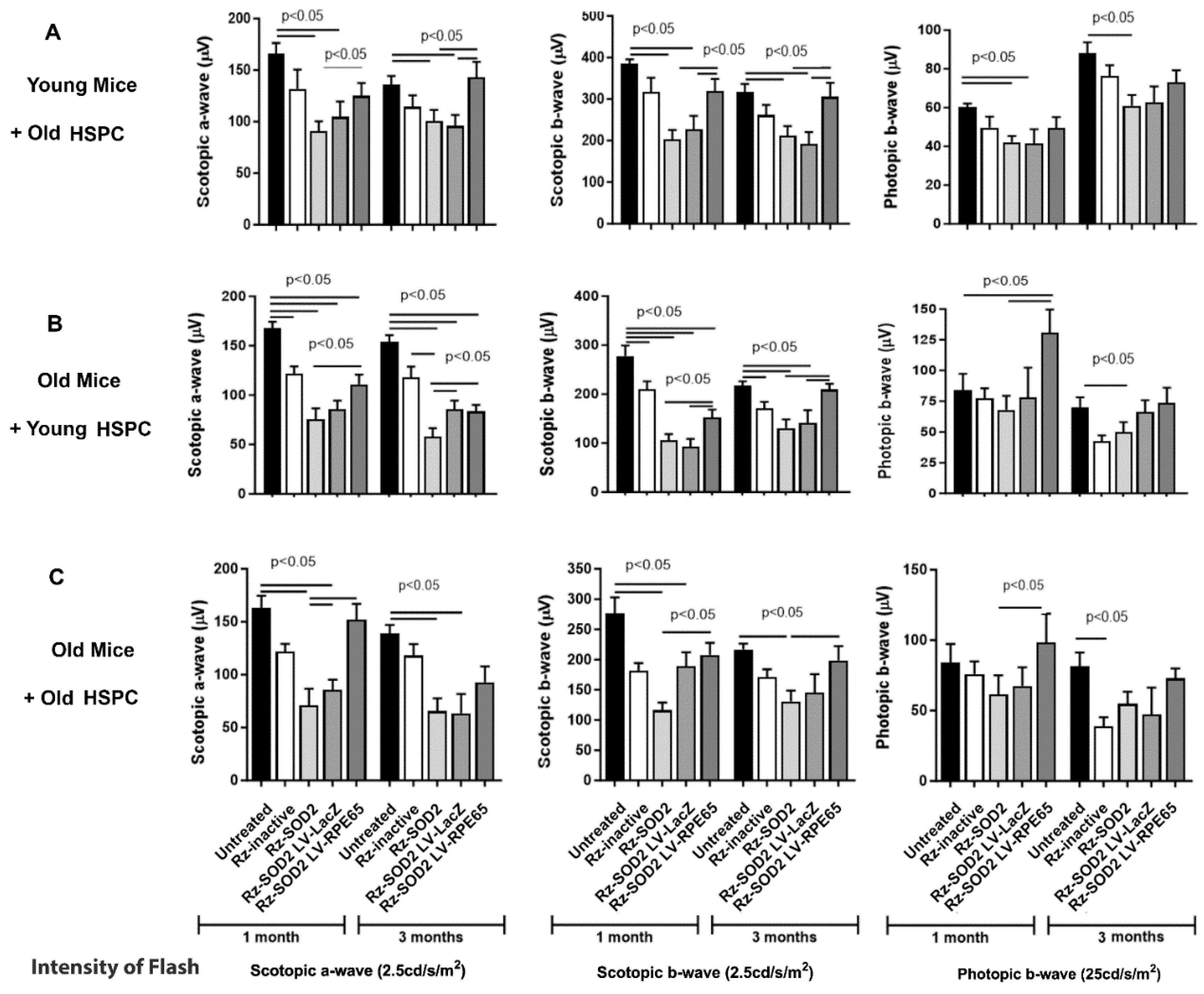
3.1. Improvement in Visual Function
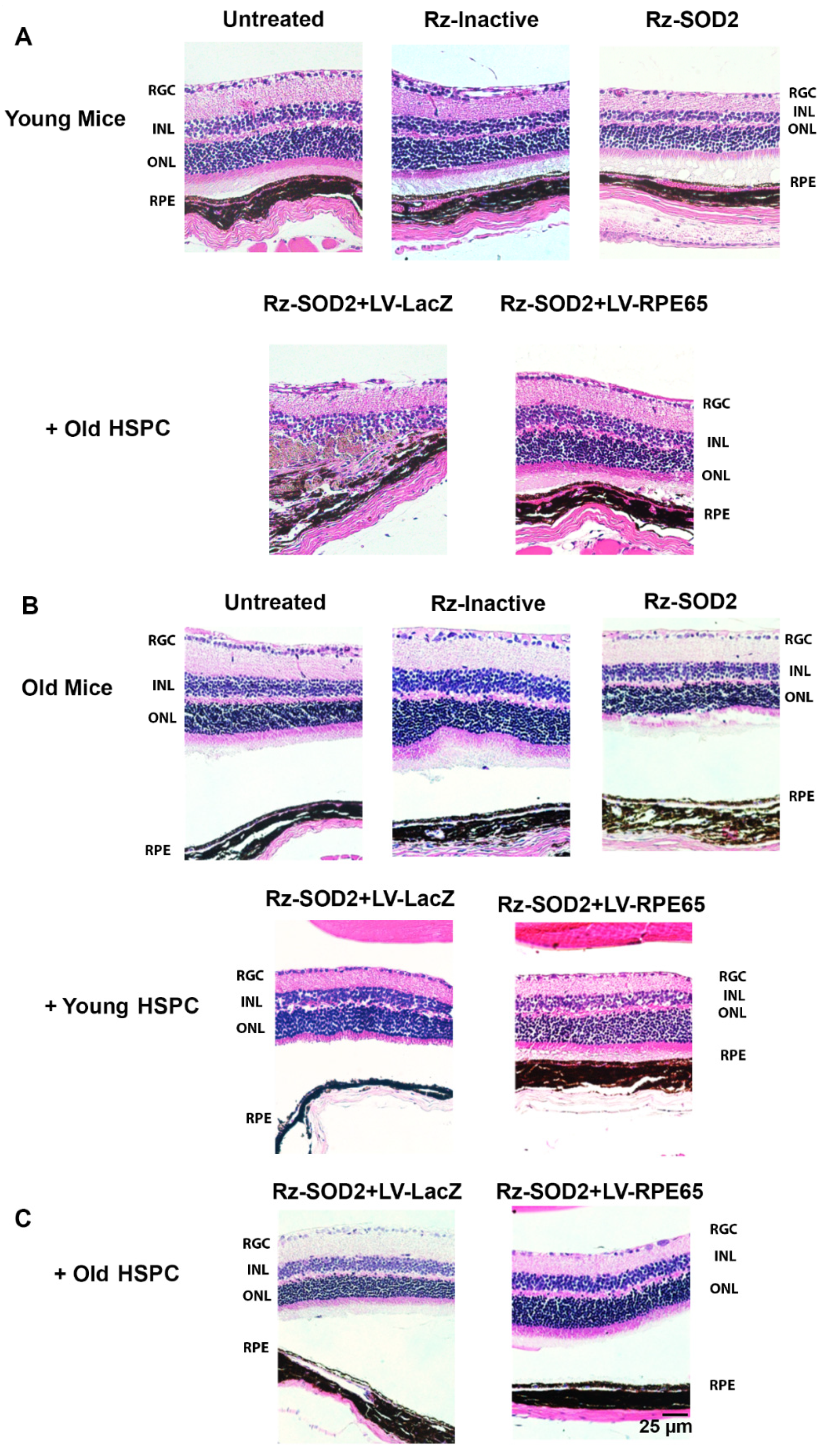
3.2. Improvement in Retinal Structure and Morphology
3.3. Localization of HSPCs to the RPE Layer in SOD2 KD Mice
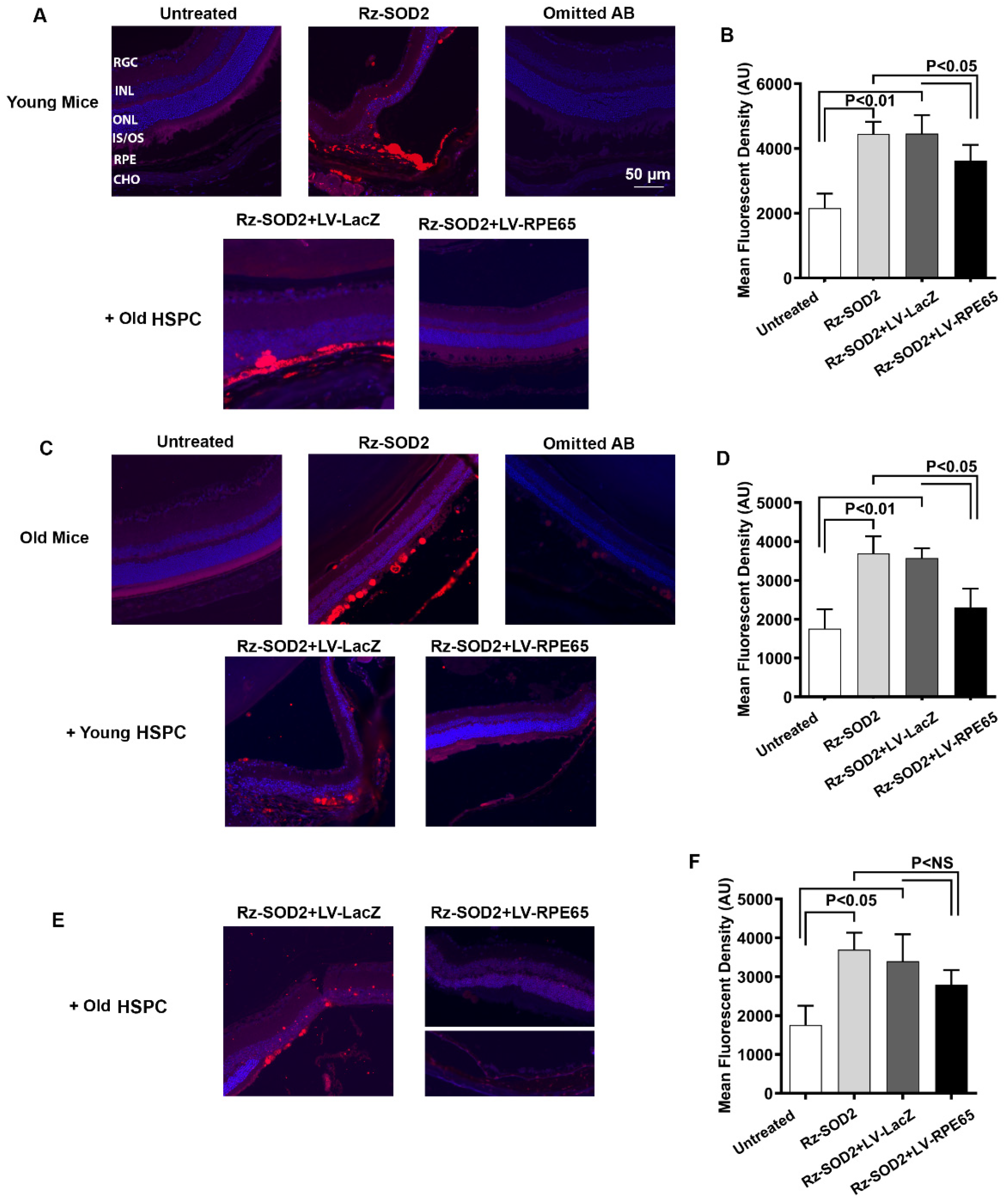
3.4. Reduction in Oxidative Damage and Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pay, S.L.; Qi, X.; Willard, J.F.; Godoy, J.; Sankhavaram, K.; Horton, R.; Mitter, S.K.; Quigley, J.L.; Chang, L.J.; Grant, M.B.; et al. Improving the Transduction of Bone Marrow-Derived Cells with an Integrase-Defective Lentiviral Vector. Hum. Gene Ther. Methods 2018, 29, 44–59. [Google Scholar] [CrossRef]
- Qi, X.; Pay, S.L.; Yan, Y.; Thomas, J., Jr.; Lewin, A.S.; Chang, L.J.; Grant, M.B.; Boulton, M.E. Systemic Injection of RPE65-Programmed Bone Marrow-Derived Cells Prevents Progression of Chronic Retinal Degeneration. Mol. Ther. 2017, 25, 917–927. [Google Scholar] [CrossRef]
- Sengupta, N.; Caballero, S.; Sullivan, S.M.; Chang, L.J.; Afzal, A.; Li Calzi, S.; Kielczewski, J.L.; Prabarakan, S.; Ellis, E.A.; Moldovan, L.; et al. Regulation of adult hematopoietic stem cells fate for enhanced tissue-specific repair. Mol. Ther. 2009, 17, 1594–1604. [Google Scholar] [CrossRef]
- Abid, M.B.; Estrada-Merly, N.; Zhang, M.J.; Chen, K.; Allan, D.; Bredeson, C.; Sabloff, M.; Guru Murthy, G.S.; Badar, T.; Hashmi, S.; et al. Impact of Donor Age on Allogeneic Hematopoietic Cell Transplantation Outcomes in Older Adults with Acute Myeloid Leukemia. Transplant. Cell Ther. 2023, 29, 578 e1–578 e9. [Google Scholar] [CrossRef]
- Colvin, M.M.; Smith, C.A.; Tullius, S.G.; Goldstein, D.R. Aging and the immune response to organ transplantation. J. Clin. Investig. 2017, 127, 2523–2529. [Google Scholar] [CrossRef]
- Dayoub, J.C.; Cortese, F.; Anzic, A.; Grum, T.; de Magalhaes, J.P. The effects of donor age on organ transplants: A review and implications for aging research. Exp. Gerontol. 2018, 110, 230–240. [Google Scholar] [CrossRef]
- Gao, X.; Lu, A.; Tang, Y.; Schneppendahl, J.; Liebowitz, A.B.; Scibetta, A.C.; Morris, E.R.; Cheng, H.; Huard, C.; Amra, S.; et al. Influences of donor and host age on human muscle-derived stem cell-mediated bone regeneration. Stem Cell Res. Ther. 2018, 9, 316. [Google Scholar] [CrossRef]
- Liang, Y.; Van Zant, G.; Szilvassy, S.J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 2005, 106, 1479–1487. [Google Scholar] [CrossRef]
- Pruitt, A.; Gao, F.; De Togni, E.; Cochran, H.; Godbole, S.; Slade, M.; Abboud, R. Impact of donor age and relationship on outcomes of peripheral blood haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2023, 58, 855–862. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Malek, G. Age-Related Macular Degeneration Revisited: From Pathology and Cellular Stress to Potential Therapies. Front. Cell Dev. Biol. 2020, 8, 612812. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef]
- Schultz, N.M.; Bhardwaj, S.; Barclay, C.; Gaspar, L.; Schwartz, J. Global Burden of Dry Age-Related Macular Degeneration: A Targeted Literature Review. Clin. Ther. 2021, 43, 1792–1818. [Google Scholar] [CrossRef]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68-80. [Google Scholar] [CrossRef]
- Flores, R.; Carneiro, A.; Vieira, M.; Tenreiro, S.; Seabra, M.C. Age-Related Macular Degeneration: Pathophysiology, Management, and Future Perspectives. Ophthalmologica 2021, 244, 495–511. [Google Scholar] [CrossRef]
- Geiger, H.; Van Zant, G. The aging of lympho-hematopoietic stem cells. Nat. Immunol. 2002, 3, 329–333. [Google Scholar] [CrossRef]
- Mantovani, C.; Raimondo, S.; Haneef, M.S.; Geuna, S.; Terenghi, G.; Shawcross, S.G.; Wiberg, M. Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Exp. Cell Res. 2012, 318, 2034–2048. [Google Scholar] [CrossRef]
- Van Zant, G.; Liang, Y. The role of stem cells in aging. Exp. Hematol. 2003, 31, 659–672. [Google Scholar] [CrossRef]
- Perales, M.A.; Tomlinson, B.; Zhang, M.J.; St Martin, A.; Beitinjaneh, A.; Gibson, J.; Hogan, W.; Kekre, N.; Lazarus, H.; Marks, D.; et al. Alternative donor transplantation for acute myeloid leukemia in patients aged >/=50 years: Young HLA-matched unrelated or haploidentical donor? Haematologica 2020, 105, 407–413. [Google Scholar] [CrossRef]
- Ogden, D.A.; Mickliem, H.S. The fate of serially transplanted bone marrow cell populations from young and old donors. Transplantation 1976, 22, 287–293. [Google Scholar] [CrossRef]
- Dinenno, F.A.; Seals, D.R.; DeSouza, C.A.; Tanaka, H. Age-related decreases in basal limb blood flow in humans: Time course, determinants and habitual exercise effects. J. Physiol. 2001, 531 Pt 2, 573–579. [Google Scholar] [CrossRef]
- Proctor, D.N.; Shen, P.H.; Dietz, N.M.; Eickhoff, T.J.; Lawler, L.A.; Ebersold, E.J.; Loeffler, D.L.; Joyner, M.J. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J. Appl. Physiol. 1998, 85, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Shewchuk, R. Comparison of calf muscle blood flow responses to rhythmic exercise between mean age 25- and 74-year-old men. Proc. Soc. Exp. Biol. Med. 1980, 164, 550–555. [Google Scholar] [CrossRef]
- Justilien, V.; Pang, J.J.; Renganathan, K.; Zhan, X.; Crabb, J.W.; Kim, S.R.; Sparrow, J.R.; Hauswirth, W.W.; Lewin, A.S. SOD2 knockdown mouse model of early AMD. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4407–4420. [Google Scholar] [CrossRef]
- Qi, X.; Francelin, C.; Mitter, S.; Boye, S.L.; Gu, H.; Quigley, J.; Grant, M.B.; Boulton, M.E. beta-secretase 1 overexpression by AAV-mediated gene delivery prevents retina degeneration in a mouse model of age-related macular degeneration. Mol. Ther. 2023, 31, 2042–2055. [Google Scholar] [CrossRef]
- Francelin, C.; Godoy, J.; Qi, X.; Silva, J.A.F.; Grant, M.B.; Boulton, M.E. Characterizing temporal and spatial recruitment of systemically administered RPE65-programmed bone marrow-derived cells to the retina in a mouse model of age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2987–2994. [Google Scholar] [CrossRef]
- El Assaad, N.; Chebly, A.; Salame, R.; Achkar, R.; Bou Atme, N.; Akouch, K.; Rafoul, P.; Hanna, C.; Abou Zeid, S.; Ghosn, M.; et al. Anti-aging based on stem cell therapy: A scoping review. World J. Exp. Med. 2024, 14, 97233. [Google Scholar] [CrossRef] [PubMed]
- DeZern, A.E.; Franklin, C.; Tsai, H.L.; Imus, P.H.; Cooke, K.R.; Varadhan, R.; Jones, R.J. Relationship of donor age and relationship to outcomes of haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv. 2021, 5, 1360–1368. [Google Scholar] [CrossRef]
- Goodell, M.A.; Rando, T.A. Stem cells and healthy aging. Science 2015, 350, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Tomany, S.C.; Meuer, S.M.; Huang, G.H. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology 2002, 109, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Zhang, X.; Honeycutt, A.A.; Lesesne, S.B.; Saaddine, J.; Vision Health Cost-Effectiveness Study, G. Forecasting age-related macular degeneration through the year 2050: The potential impact of new treatments. Arch. Ophthalmol. 2009, 127, 533–540. [Google Scholar] [CrossRef]
- Flurkey, K.; Currer, J.M.; Harrison, D.E. Chapter 20—Mouse Models in Aging Research. In The Mouse in Biomedical Research, 2nd ed.; Fox, J.G., Davisson, M.T., Quimby, F.W., Barthold, S.W., Newcomer, C.E., Smith, A.L., Eds.; Academic Press: Burlington, MA, USA, 2007; pp. 637–672. [Google Scholar]
- Caballero, S.; Sengupta, N.; Afzal, A.; Chang, K.H.; Li Calzi, S.; Guberski, D.L.; Kern, T.S.; Grant, M.B. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007, 56, 960–967. [Google Scholar] [CrossRef]
- Simpson, E.; Dazzi, F. Bone Marrow Transplantation 1957–2019. Front. Immunol. 2019, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y. Human CD34-negative hematopoietic stem cells: The current understanding of their biological nature. Exp. Hematol. 2021, 96, 13–26. [Google Scholar] [CrossRef]
- Vieira, C.P.; Fortmann, S.D.; Hossain, M.; Longhini, A.L.; Hammer, S.S.; Asare-Bediako, B.; Crossman, D.K.; Sielski, M.S.; Adu-Agyeiwaah, Y.; Dupont, M.; et al. Selective LXR agonist DMHCA corrects retinal and bone marrow dysfunction in type 2 diabetes. JCI Insight 2020, 5, e137230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, W.; Chen, Z.; Kang, H. In vitro differentiation of human CD45(+) CD34(+) induced hematopoietic progenitor cells from iPSCs using a monolayer system. Transfus. Apher. Sci. 2024, 63, 103866. [Google Scholar] [CrossRef]
- Thongsa-Ad, U.; Wongpan, A.; Wongkummool, W.; Chaiwijit, P.; Uppakara, K.; Chaiyakitpattana, G.; Singpant, P.; Tong-Ngam, P.; Chukhan, A.; Pabuprappap, W.; et al. Improving hematopoietic differentiation from human induced pluripotent stem cells by the modulation of Hippo signaling with a diarylheptanoid derivative. Stem Cell Res. Ther. 2024, 15, 60. [Google Scholar] [CrossRef]
- Cotrim, C.C.; Toscano, L.; Messias, A.; Jorge, R.; Siqueira, R.C. Intravitreal use of bone marrow mononuclear fraction containing CD34(+) stem cells in patients with atrophic age-related macular degeneration. Clin. Ophthalmol. 2017, 11, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, M.S.; Strano, A.; Guan, K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells 2022, 40, 546–555. [Google Scholar] [CrossRef]
- Simpson, D.J.; Olova, N.N.; Chandra, T. Cellular reprogramming and epigenetic rejuvenation. Clin. Epigenet. 2021, 13, 170. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Fu, X.; Xu, Y. The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Front. Immunol. 2017, 8, 645. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Sugita, S.; Horiuchi, M.; Masuda, T.; Fujii, S.; Makabe, K.; Kawasaki, A.; Hayashi, T.; Kuwahara, A.; Kishino, A.; et al. Low Immunogenicity and Immunosuppressive Properties of Human ESC- and iPSC-Derived Retinas. Stem Cell Rep. 2021, 16, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, Y.; Li, J.; Guan, J.; Yang, S.; Wang, Q. Generation of a gene corrected human isogenic iPSC line (CPGHi001-A-1) from a hearing loss patient with the TMC1 p.M418K mutation using CRISPR/Cas9. Stem Cell Res. 2022, 60, 102736. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francelin, C.; Qi, X.; Godoy, J.; Bicknell, B.T.; Prasad, R.; Grant, M.B.; Boulton, M.E. Impact of Donor and Host Age on Systemic Cell Therapy to Treat Age-Related Macular Degeneration. Cells 2025, 14, 1360. https://doi.org/10.3390/cells14171360
Francelin C, Qi X, Godoy J, Bicknell BT, Prasad R, Grant MB, Boulton ME. Impact of Donor and Host Age on Systemic Cell Therapy to Treat Age-Related Macular Degeneration. Cells. 2025; 14(17):1360. https://doi.org/10.3390/cells14171360
Chicago/Turabian StyleFrancelin, Carolina, Xiaoping Qi, Juliana Godoy, Brenton T. Bicknell, Ram Prasad, Maria B. Grant, and Michael E. Boulton. 2025. "Impact of Donor and Host Age on Systemic Cell Therapy to Treat Age-Related Macular Degeneration" Cells 14, no. 17: 1360. https://doi.org/10.3390/cells14171360
APA StyleFrancelin, C., Qi, X., Godoy, J., Bicknell, B. T., Prasad, R., Grant, M. B., & Boulton, M. E. (2025). Impact of Donor and Host Age on Systemic Cell Therapy to Treat Age-Related Macular Degeneration. Cells, 14(17), 1360. https://doi.org/10.3390/cells14171360







