Drosophila as a Model for Studying the Roles of Lamins in Normal Tissues and Laminopathies
Abstract
1. Introduction
2. Lamins—General Introduction
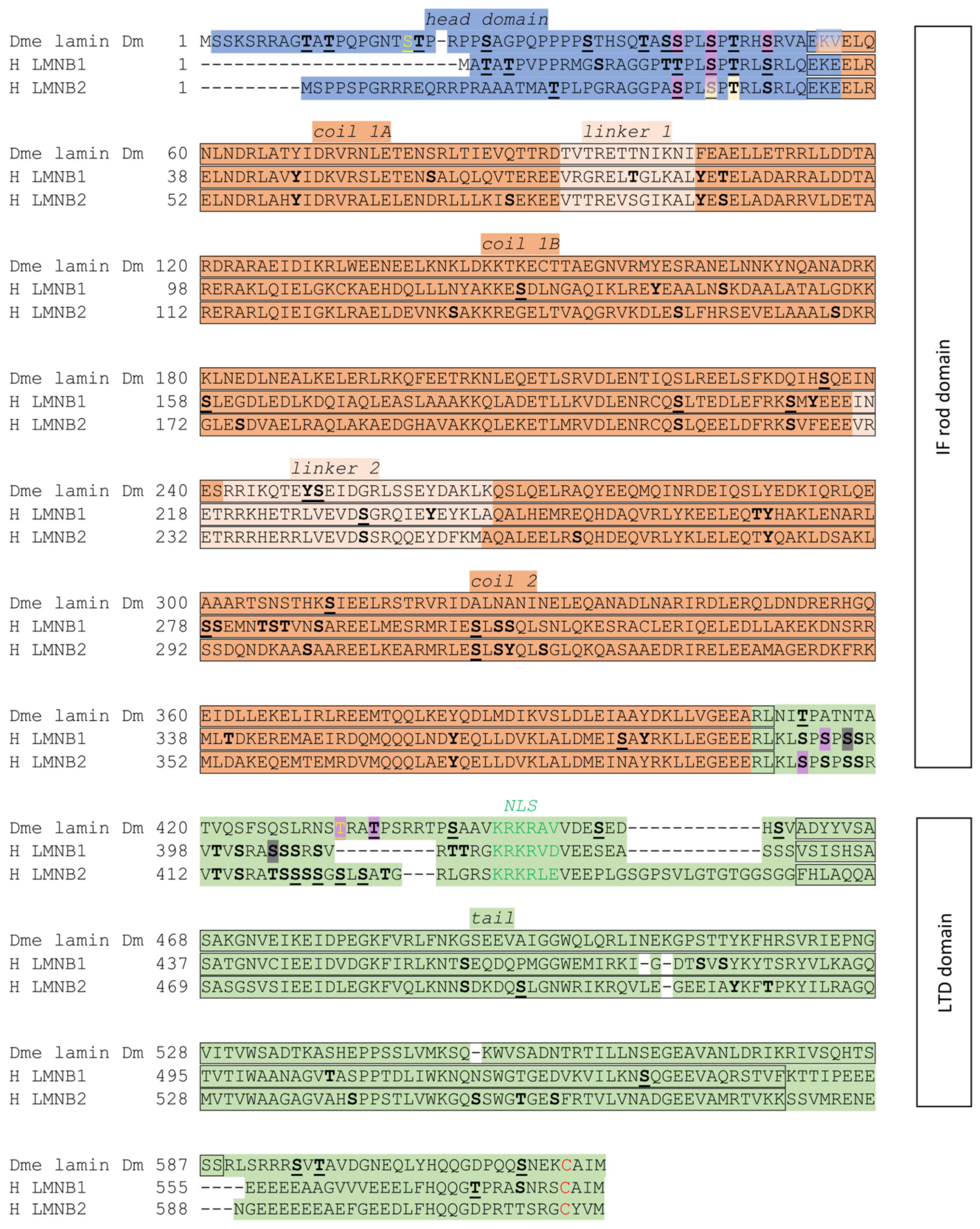
3. Fly Lamin Dm Structure and Function
4. Role of Lamin Dm in the Development and Tissue Functions of Drosophila
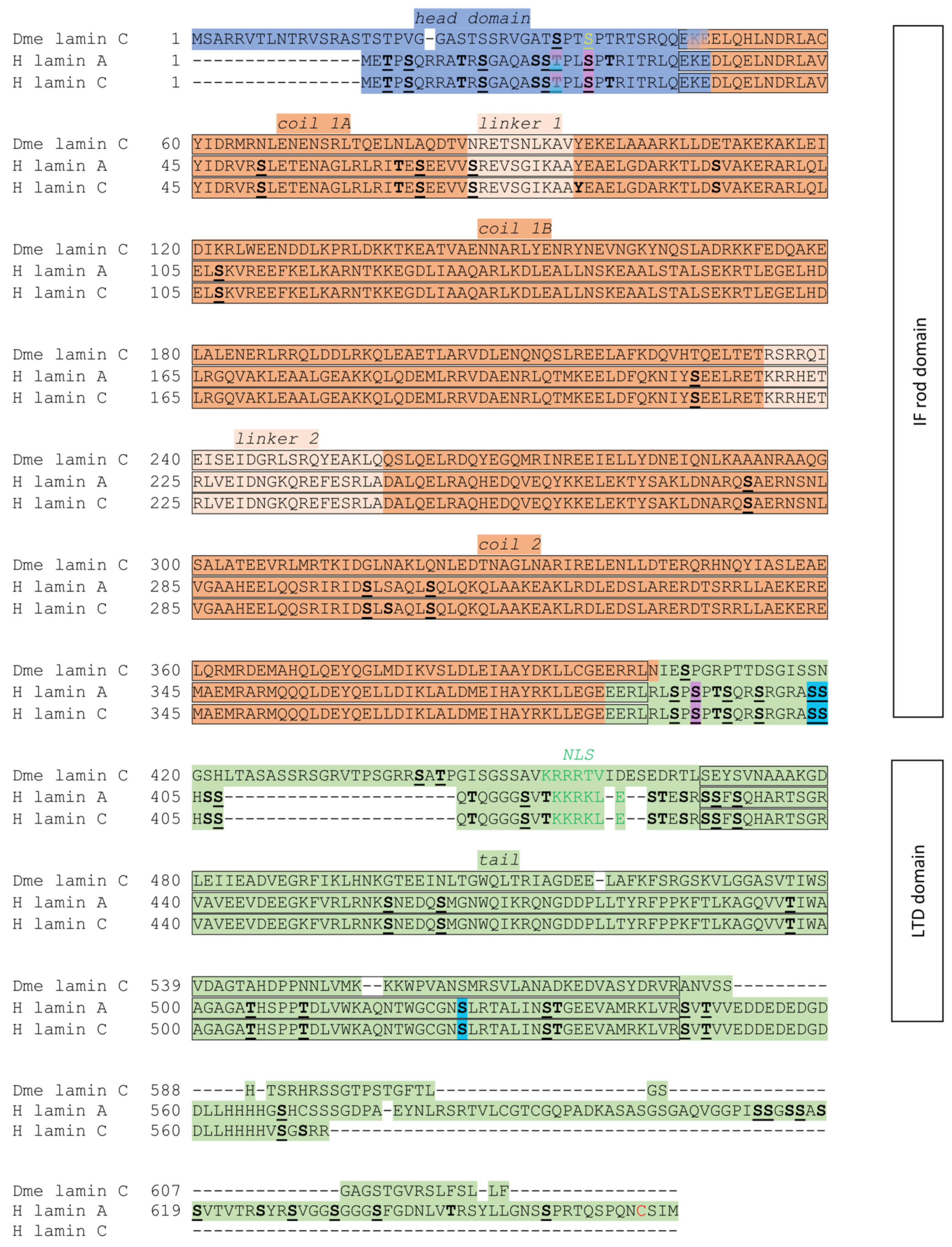
5. Drosophila Lamin C Structure and Function
| Reference | Fly Mutation | Tissue/Organ/Stage | Phenotype |
|---|---|---|---|
| [133] | Schulze et al., 2005: 1. wt; 2. G00158; 3. R401K; and 4. ΔN | Salivary glands Epithelial cells | 1. Normal nuclear rim staining; 2. O-ring aggregates; 3. strong O-ring phenotype lethality; 4. lamin C/lamin Dm aggregates, prepupal lethality. |
| [147] | Schulze et al., 2009: 1. N210K; 2. R401K; 3. K493W; 4. W557S; 5. L567P; and 6. ΔN and ΔC | Larval salivary gland nuclei | 1 and 2. O-ring lamin aggregates, chromatin defects, viable; 3. normal, viable; 4. chromatin defects, fully lethal for Mef2; 5. granules at NE, viable; 6. diffused internal lamins and chromatin, lethal with some configurations. |
| [145] | Dialynas et al., 2010: 1. wt; 2. LamC; and 3. ΔN mutant; | Larval muscles | 3. ΔN: muscle nuclei defects, lethality, disrupted NE components, cytoskeleton abnormalities, endocrine disruption. |
| [51] | Dialynas et al., 2012: 1. wt; 2. G489V; 3. N496I; 4. V528P; and 5. M553R | Larval body wall muscles | 2. Larval movement defects, extranuclear lamin C granules, NPC, Klaroid, and gp210 mislocalization, disrupted lamin Dm; 3. mild larval phenotype; 4. larval movement defect, lamin C granules, strong cytoplasmic Klaroid and NPC mislocalization, altered lamin Dm pattern; 5. larval movement defect, lamin C granules, NPC and Klaroid mislocalization, viability depends on used promoter. |
| [44] | Dialynas, 2015: 1. wt; 2. G489V; 3. N496I; 4. V528P; and 5. M553R | Larval body wall muscles | 1. Normal distribution of lamin C; similar nuclear strain as in mutants (except ΔN); no accumulation of redox markers; baseline expression of stress-related genes. 2. Upregulation of redox stress markers; nuclear accumulation; activation of Nrf2/Keap1 pathway; 21 genes affected similarly as in ΔN mutant. 4. Activation of redox stress pathway: increased CncC, p62 and Keap1 signal; moderate overlap in transcriptional profile with ΔN and G489V. 5. elevated redox stress markers; transcriptional profile overlaps partially with G489V/ΔN; 21 common genes affected both in ΔN and G489V mutants. |
| [156] | Chandran et al., 2019: 1. A177P; 2. R205W; 3. G489V; and 4. V528P | Adult indirect flight muscles | Fln-GAL4 driver: held-up wings in 2, 3, and 4; shorter sarcomeres in 2 and 3; disrupted Z-disks and M-lines in 2 and 3. DJ694-Gal4: mild phenotype; lamin/NPC aggregates; flightless phenotype. Rescue via AMPKα, dPGC-1, Thor, or S6K knockout. |
| [157] | Bhide et al., 2018: R205W, G489V | Semi intact hearts | Myofibril disorganization, lobulated nuclei, cytoplasmic lamin C and Otefin aggregates, elevated lamin C and Ref(2)P levels, more cytoplasmic foci, G489V milder than R205W, age-dependent phenotype worsening, reduced lifespan, enlarged lipid droplets, higher triglycerides, nuclear CncC accumulation. Atg1 overexpression reduces aggregates and improves heart and lifespan; CncC knockdown improves heart but not fat or lifespan; best rescue with combined Atg1 overexpression and CncC knockdown; Atg1 inhibition worsens phenotypes. |
| [142] | Shaw et al., 2022: 1. S37L; 2. ΔK47; 3. L74R; 4. R205W; 5. R237P; 6. G489V; 7. K521Q; and 8. R564P | Larval body wall muscles | ΔN lamin C: fully viable; 1, 2, 4, 5, and 7: lethal; 3: ~15% viable; 6 and 8: reduced survival; 1, 6, 7, and 8: cytoplasmic granules; 4: lamin C blebs and chromatin/actin defects; 8: granular lamin C at chromatin edge, NPCs at rim and in cytoplasm; NPCs: nuclear in wt and control, reduced in 6 and 7; increased nuclear strain in 2, 3, and 4; increased displacement and microtubule defects in 2 and 7; RNAi against Koi and MSP300 has no effect on nuclear strain. |
| [158] | Hinz et al., 2021: 1. R264Q; 2. R264W; and 3. R564P | Larval and adult IF muscles | 1 and 2: sterile in larval muscle, viable in IFM with age-related wing defects, stronger in females; 3: reduces larval motility; wt: nuclear lamin C with granules, normal NPCs and lamin Dm, thickened microtubules; 1: intranuclear lamin C ovoids, irregular lamin Dm, NPC aggregates, disorganized microtubules; 2: fragmented NE lamin C, lamin Dm in granular NE. protrusions, NPC granules on DNA+/– areas, disrupted microtubules. |
| [146] | Walker et al., 2023: K521W and R564P | Larval muscles, fat body | 1 and 2: sterile; 2: enlarged larval muscles (esp. segment 8), reduced motility, ~50% shorter lifespan in cardiac muscle; wt: lamin C at NE with granules, Otefin in nuclear granules, TMEM43 at NE; 1: lamin C in large NE protrusions, DAPI excluded, Otefin at NE, TMEM43 in protrusions and NE; 2: lamin C c:ytoplasmic near nuclei, Otefin at NE and cytoplasm (no colocalization), TMEM43 mostly cytoplasmic; 2 in fat body: cytoplasmic lamin C, weak NE signal. |
6. Muscle-Related Laminopathies Based on Fly Lamin C
7. Progeria HGPS and Other Rare Disorder Models Based on Lamin C
8. Summary of Discussion of Laminopathies and Other Rare Disorders Model Based on Lamins
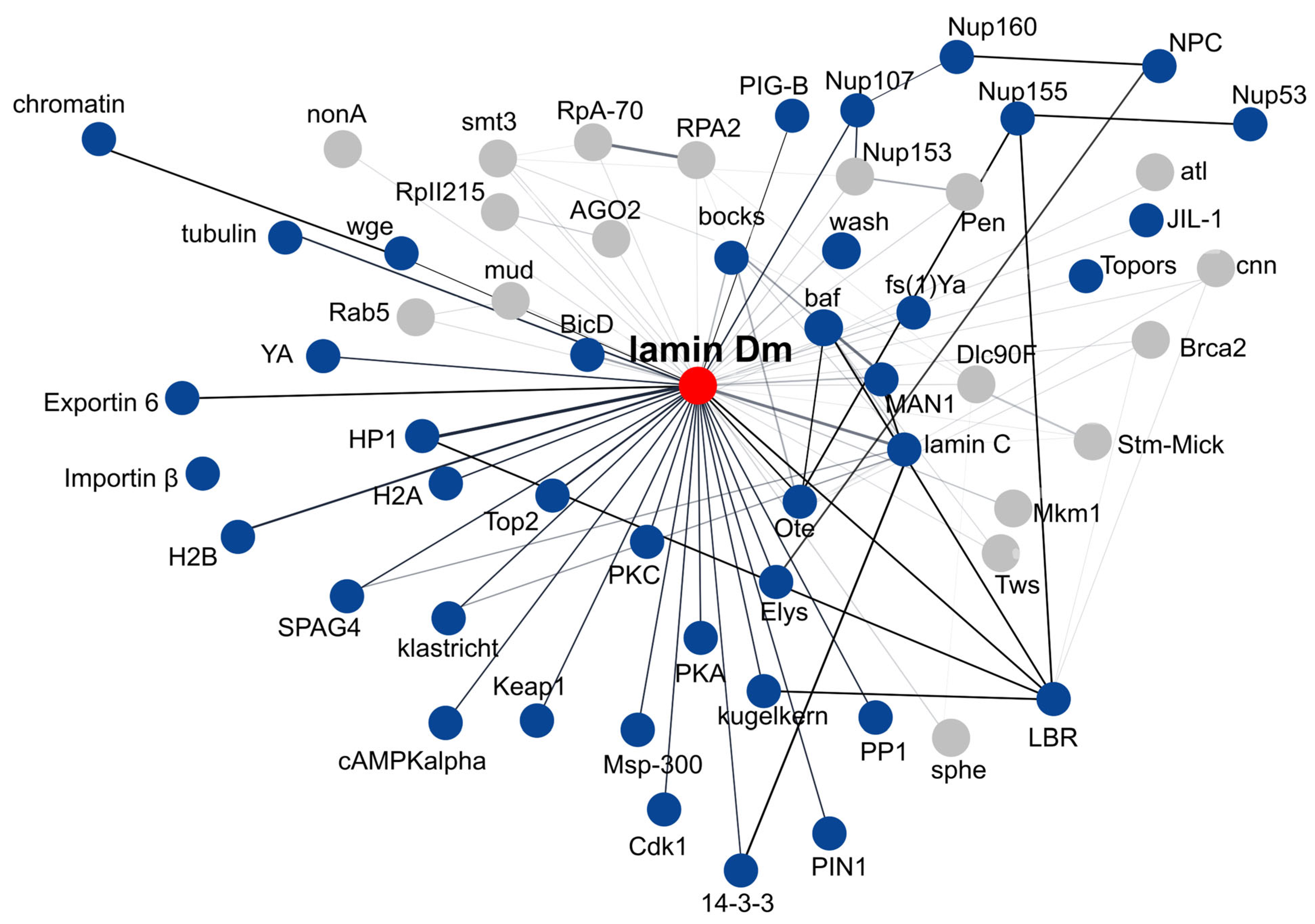
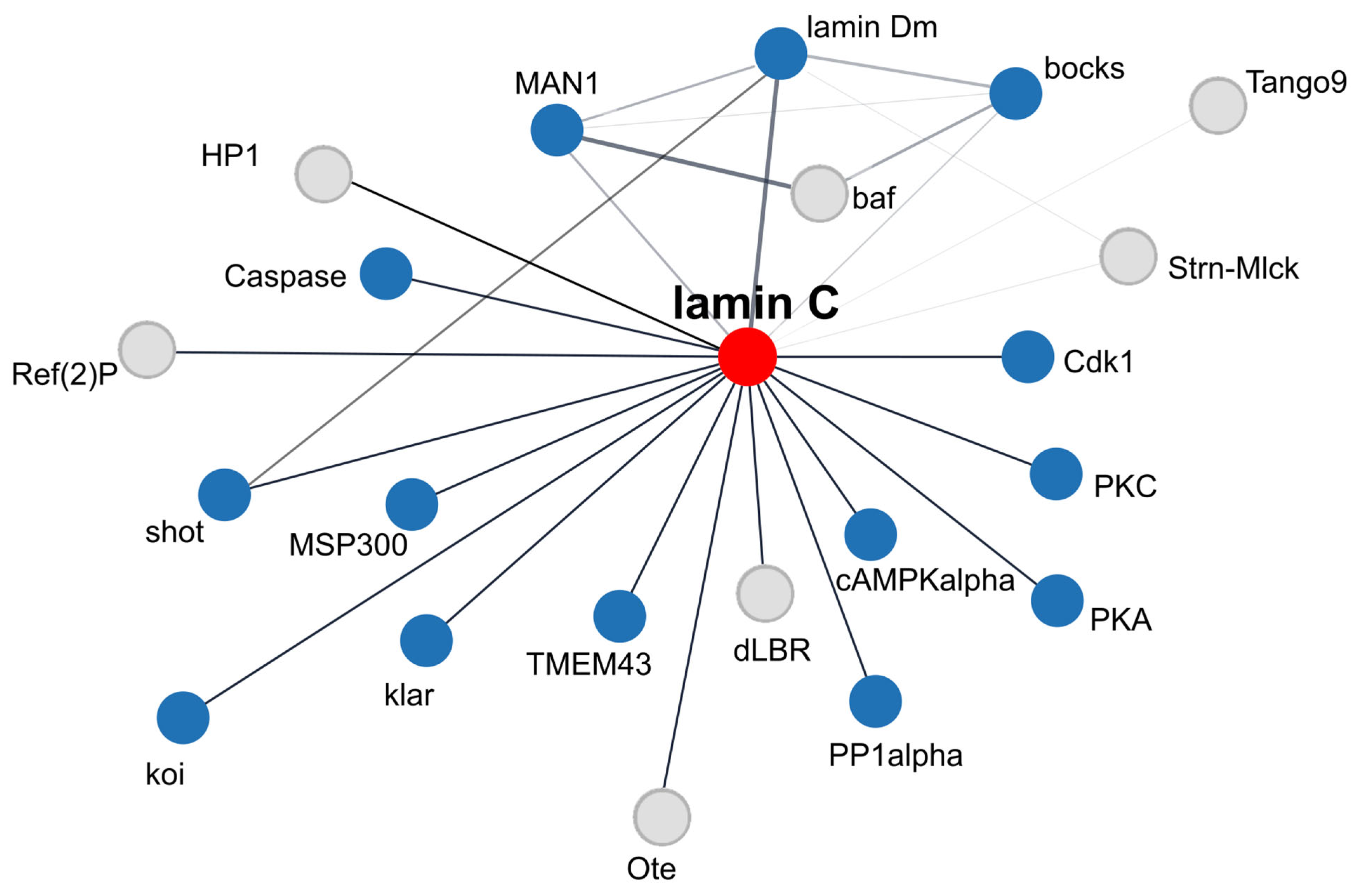
9. Lamin Dm and Lamin C Interaction Networks
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NE | Nuclear envelope |
| LINC | Linker of nucleoskeleton and cytoskeleton |
| EDMD | Emery–Dreifuss muscular dystrophy |
| ADLD | Autosomal dominant leukodystrophy |
| CMT | Charcot–Marie–Tooth |
| HGPS | Hutchinson–Gilford progeria syndrome |
| APLD | Acquired partial lipodystrophy (Barraquer–Simons syndrome) |
| LGMD | Limb–girdle muscular dystrophy |
| PHA | Pelger–Hue’t anomaly |
| HEM/GRBGD | Greenberg hydrops-ectopic calcification-moth-eaten skeletal dysplasia |
| BOS | Buschke–Ollendorff syndrome |
| ECM | Extracellular matrix |
| INM | Inner nuclear membrane |
| NL | Nuclear lamina |
| IF | Intermediate filaments |
| NLS | Nuclear localization signal/sequence |
| NPC | Nuclear pore complex |
| Cdk | Cyclin-Dependent Kinase |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| Gsk | Glycogen Synthase Kinase |
| ERK | Extracellular Signal-Regulated Kinase |
| MAPK/APK | Mitogen-Activated Protein Kinase/Activated Protein Kinase |
| S/MAR | Scaffold/matrix attachment region |
| cAMP | Cyclic Adenosine Monophosphate |
| GFP | Green Fluorescent Protein |
| CNS | Central Nervous System |
| IMD | Immune deficiency |
| Top2 | Topoisomerase II |
| SRPK | Serine/Arginine Protein Kinase |
| LEM | LAP2-Emerin-MAN1 Domain Protein |
| MEF | Myocyte Enhancer Factor 2 |
| MYF | Myogenic Factor |
| DMD | Duchenne Muscular Dystrophy |
| TOR | Target of Rapamycin |
| Nrf | Nuclear Factor Erythroid |
| NOX | NADPH Oxidase |
| IFM | Indirect flight muscle |
| AMPK | Activated protein kinase |
| FOXO | Forkhead box O |
| NPS | Neuropeptide S |
| HSF | Heat Shock Factor |
| CMD | Congenital muscular dystrophy |
| TM | Transmembrane |
| TMEM | Transmembrane Protein |
| BAF | Barrier-to-Autointegration Factor |
| LAP | Lamina-associated polypeptide |
| MAN | Inner Nuclear Membrane Protein |
| LEMD1 | LEM Domain-Containing 1 |
| EMD | Emerin |
| ANKL | Ankyrin Repeat and LEM Domain-Containing |
| BOKS | Barrier-to-Autointegration Factor (BAF)-binding protein of KASH domain |
| OTE | Otefin |
| NES | Nuclear Export Signal |
| MSC | MAN1/Src1p/C-terminal motif |
| ANK | Ankyrin repeats |
| RM | RNA Recognition Motif |
| GIY-YIG | Endonuclease Domain (a conserved DNA-binding motif) |
| LBR | Lamin B Receptor |
| HEM | Hydrops-Ectopic calcification-Moth-eaten skeletal dysplasia |
| LBS | Lamin binding site |
| CHD | Chromodomain-Helicase-DNA-binding protein |
| ABS | Acting binding side |
| AMC | Arthrogryposis multiplex congenital |
| MCPHRb | MicrocephalyRetinoblastoma |
| GSSG | Glutathione Disulfide |
| GSH | Reduced glutathione |
| wt | Wild type |
References
- Bank, E.M.; Gruenbaum, Y. Caenorhabditis Elegans as a Model System for Studying the Nuclear Lamina and Laminopathic Diseases. Nucleus 2011, 2, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Rzepecki, R.; Gruenbaum, Y. Invertebrate Models of Lamin Diseases. Nucleus 2018, 9, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Plantié, E.; Migocka-Patrzałek, M.; Daczewska, M.; Jagla, K. Model Organisms in the Fight against Muscular Dystrophy: Lessons from Drosophila and Zebrafish. Molecules 2015, 20, 6237–6253. [Google Scholar] [CrossRef] [PubMed]
- Döring, V.; Stick, R. Gene Structure of Nuclear Lamin LIII of Xenopus Laevis; a Model for the Evolution of IF Proteins from a Lamin-like Ancestor. EMBO J. 1990, 9, 4073–4081. [Google Scholar] [CrossRef]
- Arbach, H.E.; Harland-Dunaway, M.; Chang, J.K.; Wills, A.E. Extreme Nuclear Branching in Healthy Epidermal Cells of the Xenopus Tail Fin. J. Cell Sci. 2018, 131, jcs217513. [Google Scholar] [CrossRef]
- Lehner, C.F.; Stick, R.; Eppenberger, H.M.; Migg, E.A. Differential Expression of Nuclear Lamin Proteins during Chicken Development. J. Cell Biol. 1987, 105, 577–587. [Google Scholar] [CrossRef]
- Stewart, C.L.; Kozlov, S.; Fong, L.G.; Young, S.G. Mouse Models of the Laminopathies. Exp. Cell Res. 2007, 313, 2144–2156. [Google Scholar] [CrossRef]
- Taniura, H.; Glass, C.; Gerace, L. A Chromatin Binding Site in the Tail Domain of Nuclear Lamins That Interacts with Core Histones. J. Cell Biol. 1995, 131, 33–44. [Google Scholar] [CrossRef]
- Habtemichael, N.; Wünsch, D.; Bier, C.; Tillmann, S.; Unruhe, B.; Frauenknecht, K.; Heinrich, U.R.; Mann, W.J.; Stauber, R.H.; Knauer, S.K. Cloning and Functional Characterization of the Guinea Pig Apoptosis Inhibitor Protein Survivin. Gene 2010, 469, 9–17. [Google Scholar] [CrossRef]
- Miyagoe-Suzuki, Y.; Takeda, S. Gene Therapy for Muscle Disease. Exp. Cell Res. 2010, 316, 3087–3092. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 24, eabd5758. [Google Scholar] [CrossRef]
- Lisowska, M.; Rowińska, M.; Suszyńska, A.; Bearzi, C.; Izabela, Ł.; Hanusek, J.; Kunik, A.; Dzianisava, V.; Rzepecki, R.; Machowska, M.; et al. Human IPSC-Derived Muscle Cells as a New Model for Investigation of EDMD1 Pathogenesis. Int. J. Mol. Sci. 2025, 26, 1539. [Google Scholar] [CrossRef]
- Rabl, C. Über Zelltheilung. Morphol. Jahrb. 1885, 10, 214–330. [Google Scholar]
- Zhimulev, I.F.; Koryakov, D.E. Polytene Chromosomes. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Balbiani, E.G. Sur La Structure Du Noyau Des Cellules Salivaires Chez Les Larves de Chironomus. Zool. Anz. 1881, 4, 637–641. [Google Scholar]
- Kostoff, D. Discoid Structure of the Spireme. J. Hered. 1930, 21, 259–265. [Google Scholar] [CrossRef]
- Ashburner, M. Drosophila: A Laboratory Handbook, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2005; ISBN 0879693215. [Google Scholar]
- Jennings, B.H. Drosophila—A Versatile Model in Biology & Medicine. Mater. Today 2011, 14, 190–195. [Google Scholar] [CrossRef]
- Deal, S.L.; Yamamoto, S. Neuronal Lamin Regulates Motor Circuit Integrity and Controls Motor Function and Lifespan. Cell Stress 2018, 2, 225–232. [Google Scholar] [CrossRef] [PubMed]
- McGurk, L.; Berson, A.; Bonini, N.M. Drosophila as an in Vivo Model for Human Neurodegenerative Disease. Genetics 2015, 201, 377–402. [Google Scholar] [CrossRef] [PubMed]
- Mirzoyan, Z.; Sollazzo, M.; Allocca, M.; Valenza, A.M.; Grifoni, D.; Bellosta, P. Drosophila Melanogaster: A Model Organism to Study Cancer. Front. Genet. 2019, 10, 51. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Partridge, L. Drosophila as a Model for Ageing. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 2707–2717. [Google Scholar] [CrossRef]
- Farkas, G.; Leibovitch, B.A.; Elgin, S.C.R. Chromatin Organization and Transcriptional Control of Gene Expression in Drosophila. Gene 2000, 253, 117–136. [Google Scholar] [CrossRef]
- Filion, G.J.; van Bemmel, J.G.; Braunschweig, U.; Talhout, W.; Kind, J.; Ward, L.D.; Brugman, W.; de Castro, I.J.; Kerkhoven, R.M.; Bussemaker, H.J.; et al. Systematic Protein Location Mapping Reveals Five Principal Chromatin Types in Drosophila Cells. Cell 2010, 143, 212–224. [Google Scholar] [CrossRef]
- Briand, N.; Collas, P. Lamina-Associated Domains: Peripheral Matters and Internal Affairs. Genome Biol. 2020, 21, 85. [Google Scholar] [CrossRef]
- Ing-Simmons, E.; Vaid, R.; Bing, X.Y.; Levine, M.; Mannervik, M.; Vaquerizas, J.M. Independence of Chromatin Conformation and Gene Regulation during Drosophila Dorsoventral Patterning. Nat. Genet. 2021, 53, 487–499. [Google Scholar] [CrossRef]
- Kahn, T.G.; Savitsky, M.; Kuong, C.; Jacquier, C.; Cavalli, G.; Chang, J.M.; Schwartz, Y.B. Topological Screen Identifies Hundreds of Cp190- and CTCF-Dependent Drosophila Chromatin Insulator Elements. Sci. Adv. 2023, 9, eade0090. [Google Scholar] [CrossRef] [PubMed]
- Pindyurin, A.V.; Ilyin, A.A.; Ivankin, A.V.; Tselebrovsky, M.V.; Nenasheva, V.V.; Mikhaleva, E.A.; Pagie, L.; Van Steensel, B.; Shevelyov, Y.Y. The Large Fraction of Heterochromatin in Drosophila Neurons Is Bound by Both B-Type Lamin and HP1a. Epigenet. Chromatin 2018, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.R.; Laemmli, U.K. The Structure of Histone-Depleted Metaphase Chromosomes. Cell 1977, 12, 817–828. [Google Scholar] [CrossRef]
- Belmont, A.S.; Braunfeld, M.B.; Sedat, J.W.; Agard, D.A. Large-Scale Chromatin Structural Domains within Mitotic and Interphase Chromosomes in Vivo and in Vitro. Chromosoma 1989, 98, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.A.; Berrios, M.; Blobel, G. Isolation and Characterization of a Proteinaceous Subnuclear Fraction Composed of Nuclear Matrix, Peripheral Lamina, and Nuclear Pore Complexes from Embryos of Drosophila Melanogaster. J. Cell Biol. 1982, 92, 674–686. [Google Scholar] [CrossRef]
- Fisher, P.A.; Blobel, G. Preparation of a Nuclear Matrix-Pore Complex-Lamina Fraction from Embryos of Drosophila Melanogaster. Methods Enzymol. 1983, 96, 589–596. [Google Scholar] [CrossRef]
- Hochstrasser, M.; Mathog, D.; Gruenbaum, Y.; Saumweber, H.; Sedat, J.W. Spatial Organization of Chromosomes in the Salivary Gland Nuclei of Drosophila Melanogaster. J. Cell Biol. 1986, 102, 112–123. [Google Scholar] [CrossRef]
- Vergnes, L.; Péterfy, M.; Bergo, M.O.; Young, S.G.; Reue, K. Lamin B1 Is Required for Mouse Development and Nuclear Integrity. Proc. Natl. Acad. Sci. USA 2004, 101, 10428–10433. [Google Scholar] [CrossRef]
- Aaronson, R.P.; Blobel, G. Isolation of Nuclear Pore Complexes in Association with a Lamina. Proc. Natl. Acad. Sci. USA 1975, 72, 1007–1011. [Google Scholar] [CrossRef]
- Richards, S.A.; Muter, J.; Ritchie, P.; Lattanzi, G.; Hutchison, C.J. The Accumulation of Un-Repairable DNA Damage in Laminopathy Progeria Fibroblasts Is Caused by ROS Generation and Is Prevented by Treatment with N-Acetyl Cysteine. Hum. Mol. Genet. 2011, 20, 3997–4004. [Google Scholar] [CrossRef]
- Melcer, S.; Gruenbaum, Y.; Krohne, G. Invertebrate Lamins. Exp. Cell Res. 2007, 313, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Pałka, M.; Tomczak, A.; Grabowska, K.; Machowska, M.; Piekarowicz, K. Laminopathies: What Can Humans Learn from Fruit Flies. Cell. Mol. Biol. Lett. 2018, 23, 32. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.; Stick, R. Evolution of the Lamin Protein Family: What Introns Can Tell. Nucleus 2012, 3, 44–59. [Google Scholar] [CrossRef]
- Gruenbaum, Y.; Foisner, R. Lamins: Nuclear Intermediate Filament Proteins with Fundamental Functions in Nuclear Mechanics and Genome Regulation. Annu. Rev. Biochem. 2015, 84, 131–164. [Google Scholar] [CrossRef]
- Gruenbaum, Y.; Goldman, R.D.; Meyuhas, R.; Mills, E.; Margalit, A.; Fridkin, A.; Dayani, Y.; Prokocimer, M.; Enosh, A. The Nuclear Lamina and Its Functions in the Nucleus. Int. Rev. Cytol. 2003, 226, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Prokocimer, M.; Davidovich, M.; Nissim-Rafinia, M.; Wiesel-Motiuk, N.; Bar, D.Z.; Barkan, R.; Meshorer, E.; Gruenbaum, Y. Nuclear Lamins: Key Regulators of Nuclear Structure and Activities. J. Cell. Mol. Med. 2009, 13, 1059–1085. [Google Scholar] [CrossRef]
- Osouda, S.; Nakamura, Y.; De Saint Phalle, B.; McConnell, M.; Horigome, T.; Sugiyama, S.; Fisher, P.A.; Furukawa, K. Null Mutants of Drosophila B-Type Lamin Dm0 Show Aberrant Tissue Differentiation Rather than Obvious Nuclear Shape Distortion or Specific Defects during Cell Proliferation. Dev. Biol. 2005, 284, 219–232. [Google Scholar] [CrossRef]
- Dialynas, G.; Shrestha, O.K.; Ponce, J.M.; Zwerger, M.; Thiemann, D.A.; Young, G.H.; Moore, S.A.; Yu, L.; Lammerding, J.; Wallrath, L.L. Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway. PLoS Genet. 2015, 11, e1005231. [Google Scholar] [CrossRef]
- Turgay, Y.; Eibauer, M.; Goldman, A.E.; Shimi, T.; Khayat, M.; Ben-Harush, K.; Dubrovsky-Gaupp, A.; Sapra, K.T.; Goldman, R.D.; Medalia, O. The Molecular Architecture of Lamins in Somatic Cells. Nature 2017, 543, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, Y.; Aebi, U. Intermediate Filaments: A Dynamic Network That Controls Cell Mechanics. F1000Prime Rep. 2014, 6, 54. [Google Scholar] [CrossRef]
- Xie, W.; Burke, B. Quick Guide Lamins. Curr. Biol. 2016, 26, 348–350. [Google Scholar] [CrossRef]
- Gruenbaum, Y.; Medalia, O. Lamins: The Structure and Protein Complexes. Curr. Opin. Cell Biol. 2015, 32, 7–12. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, R.; Gruenbaum, Y.; Medalia, O. Nuclear Lamins: Thin Filaments with Major Functions. Trends Cell Biol. 2018, 28, 34–45. [Google Scholar] [CrossRef]
- Naetar, N.; Ferraioli, S.; Foisner, R. Lamins in the Nuclear Interior-Life Outside the Lamina. J. Cell Sci. 2017, 130, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Dialynas, G.; Flannery, K.M.; Zirbel, L.N.; Nagy, P.L.; Mathews, K.D.; Moore, S.A.; Wallrath, L.L. LMNA Variants Cause Cytoplasmic Distribution of Nuclear Pore Proteins in Drosophila and Human Muscle. Hum. Mol. Genet. 2012, 21, 1544–1556. [Google Scholar] [CrossRef]
- Zaremba-Czogalla, M.; Piekarowicz, K.; Wachowicz, K.; Kozioł, K.; Dubińska-Magiera, M.; Rzepecki, R. The Different Function of Single Phosphorylation Sites of Drosophila Melanogaster Lamin Dm and Lamin C. PLoS ONE 2012, 7, e32649. [Google Scholar] [CrossRef]
- Bank, E.M.; Gruenbaum, Y. The Nuclear Lamina and Heterochromatin: A Complex Relationship. Biochem. Soc. Trans. 2011, 39, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ben-Shahar, T.R.; Riemer, D.; Treinin, M.; Spann, P.; Weber, K.; Fire, A.; Gruenbaum, Y. Essential Roles for Caenorhabditis Elegans Lamin Gene in Nuclear Organization, Cell Cycle Progression, and Spatial Organization of Nuclear Pore Complexes. Mol. Biol. Cell 2000, 11, 3937–3947. [Google Scholar] [CrossRef]
- Harel, A.; Goldberg, M.; Ulitzur, N.; Gruenbaum, Y. Structural Organization and Biological Roles of the Nuclear Lamina. Gene Ther. Mol. Biol. 1998, 1, 529–542. [Google Scholar]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bursteinas, B.; et al. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Vaughan, O.A.; Whitfieid, W.G.E.; Hutchison, C.J. Functions of the Nuclear Lamins. Protoplasma 2000, 211, 1–7. [Google Scholar] [CrossRef]
- Goldberg, M.W.; Huttenlauch, I.; Hutchison, C.J.; Stick, R. Filaments Made from A- and B-Type Lamins Differ in Structure and Organization. J. Cell Sci. 2008, 121, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Dechat, T.; Adam, S.A.; Taimen, P.; Shimi, T.; Goldman, R.D. Nuclear Lamins. Cold Spring Harb. Perspect. Biol. 2010, 2, a000547. [Google Scholar] [CrossRef]
- Machowska, M.; Piekarowicz, K.; Rzepecki, R. Regulation of Lamin Properties and Functions: Does Phosphorylation Do It All? Open Biol. 2015; 5, 150094. [Google Scholar] [CrossRef]
- Torvaldson, E.; Kochin, V.; Eriksson, J.E. Phosphorylation of Lamins Determine Their Structural Properties and Signaling Functions. Nucleus 2015, 6, 166–171. [Google Scholar] [CrossRef]
- Simon, D.N.; Wilson, K.L. Partners and Post-Translational Modifications of Nuclear Lamins. Chromosoma 2013, 122, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Stuurman, N.; Heins, S.; Aebi, U. Nuclear Lamins: Their Structure, Assembly, and Interactions. J. Struct. Biol. 1998, 122, 42–66. [Google Scholar] [CrossRef]
- Wilson, K.L.; Foisner, R. Lamin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2010, 2, a000554. [Google Scholar] [CrossRef] [PubMed]
- Sobo, J.M.; Alagna, N.S.; Sun, S.X.; Wilson, K.L.; Reddy, K.L. Lamins: The Backbone of the Nucleocytoskeleton Interface. Curr. Opin. Cell Biol. 2024, 86, 102313. [Google Scholar] [CrossRef] [PubMed]
- Melcon, G.; Koslov, S.; Cutler, D.A.; Sullivan, T.; Hernandez, L.; Zhao, P.; Mitchell, S.; Nader, G.; Bakay, M.; Rottman, J.N.; et al. Loss of Emerin at the Nuclear Envelope Disrupts the Rb1/E2F and MyoD Pathways during Muscle Regeneration. Hum. Mol. Genet. 2006, 15, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Brachner, A.; Foisner, R. Evolvement of LEM-Proteins as Chromatin Tethers at the Nuclear Periphery. Biochem. Soc. Trans. 2011, 39, 1735–1741. [Google Scholar] [CrossRef]
- Barton, L.J.; Soshnev, A.A.; Geyer, P.K. Networking in the Nucleus: A Spotlight on LEM-Domain Proteins. Curr. Opin. Cell Biol. 2015, 34, 1–8. [Google Scholar] [CrossRef]
- Brachner, A.; Foisner, R. Lamina-Associated Polypeptide (LAP)2α and Other LEM Proteins in Cancer Biology. Adv. Exp. Med. Biol. 2014, 773, 143–163. [Google Scholar] [CrossRef]
- Kim, D.i.; Birendra, K.C.; Kyle, J. Roux Making the LINC: SUN and KASH protein interactions. Biol. Chem. 2015, 396, 295–310. [Google Scholar] [CrossRef]
- Gruenbaum, Y.; Landesman, Y.; Drees, B.; Bare, J.W.; Saumweber, H.; Paddy, M.R.; Sedat, J.W.; Smith, D.E.; Benton, B.M.; Fisher, P.A. Drosophila Nuclear Lamin Precursor Dm0 Is Translated from Either of Two Developmentally Regulated MRNA Species Apparently Encoded by a Single Gene. J. Cell Biol. 1988, 106, 585–596. [Google Scholar] [CrossRef]
- Smith, D.E.; Fisher, P.A. Identification, Developmental Regulation, and Response to Heat Shock of Two Antigenically Related Forms of a Major Nuclear Envelope Protein in Drosophila Embryos: Application of an Improved Method for Affinity Purification of Antibodies Using Polypeptides. J. Cell Biol. 1984, 99, 20–28. [Google Scholar] [CrossRef]
- Smith, D.E.; Gruenbaum, Y.; Berrios, M.; Fisher, P.A. Biosynthesis and Interconversion of Drosophila Nuclear Lamin Isoforms during Normal Growth and in Response to Heat Shock. J. Cell Biol. 1987, 105, 771–790. [Google Scholar] [CrossRef]
- McConnell, M.; Whalen, A.M.; Smith, D.E.; Fisher, P.A. Heat Shock-Induced Changes in the Structural Stability of Proteinaceous Karyoskeletal Elements in Vitro and Morphological Effects in Situ. J. Cell Biol. 1987, 105, 1087–1098. [Google Scholar] [CrossRef]
- Smith, D.E.; Fisher, P.A. Interconversion of Drosophila Nuclear Lamin Isoforms during Oogenesis, Early Embryogenesis, and upon Entry of Cultured Cells into Mitosis. J. Cell Biol. 1989, 108, 255–265. [Google Scholar] [CrossRef]
- Stuurman, N.; Maus, N.; Fisher, P.A. Interphase Phosphorylation of the Drosophila Nuclear Lamin: Site-Mapping Using a Monoclonal Antibody. J. Cell Sci. 1995, 108, 3137–3144. [Google Scholar] [CrossRef]
- Zaremba-Czogalla, M.; Gagat, P.; Kozioł, K.; Dubińska-Magiera, M.; Sikora, J.; Dadlez, M.; Rzepecki, R. Identification of New in Vivo Phosphosites on Lamin Dm-the Evidence of Heterogeneity of Phosphorylation Sites in Different Drosophila Tissues. Nucleus 2011, 2, 478–488. [Google Scholar] [CrossRef]
- Rzepecki, R.; Fisher, P.A. In Vivo Phosphorylation of Drosophila Melanogaster Nuclear Lamins during Both Interphase and Mitosis. Cell. Mol. Biol. Lett. 2002, 7, 859–876. [Google Scholar]
- Susan, J.M.; Kabakoff, B.; Fisher, P.A.; Lennarz, W.J. Characterization of Polyprenylation of Drosophila Nuclear Lamins. Dyn. Membr. Assem. 1992, H63, 189–196. [Google Scholar] [CrossRef]
- Schneider, U.; Mini, T.; Jenö, P.; Fisher, P.A.; Stuurman, N. Phosphorylation of the Major Drosophila Lamin In Vivo: Site Identification during Both M-Phase (Meiosis) and Interphase by Electrospray Ionization Tandem Mass Spectrometry. Biochemistry 1999, 38, 4620–4632. [Google Scholar] [CrossRef]
- Lin, L.; Fisher, P.A. Immunoaffinity Purification and Functional Characterization of Interphase and Meiotic Drosophila Nuclear Lamin Isoforms. J. Biol. Chem. 1990, 265, 12596–12601. [Google Scholar] [CrossRef]
- Dessev, G.; Goldman, R. Meiotic Breakdown of Nuclear Envelope in Oocytes of Spisula Solidissima Involves Phosphorylation and Release of Nuclear Lamin. Dev. Biol. 1988, 130, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Rowińska, M.; Aleksandra, T.; Jabłońska, J.; Piekarowicz, K.; Machowska, M.; Rzepecki, R. Heat Shock Alters the Distribution and in Vivo Interaction of Major Nuclear Structural Proteins, Lamin B and DNA Topoisomerase II, with Nucleic Acids. bioRxiv 2024, 1–46. [Google Scholar] [CrossRef]
- Lenz-Böhme, B.; Wismar, J.; Fuchs, S.; Reifegerste, R.; Buchner, E.; Betz, H.; Schmitt, B. Insertional Mutation of the Drosophila Nuclear Lamin Dm0 Gene Results in Defective Nuclear Envelopes, Clustering of Nuclear Pore Complexes, and Accumulation of Annulate Lamellae. J. Cell Biol. 1997, 137, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Di Palma, S.; Preisinger, C.; Peng, M.; Polat, A.N.; Heck, A.J.R.; Mohammed, S. Toward a Comprehensive Characterization of a Human Cancer Cell Phosphoproteome. J. Proteome Res. 2013, 12, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Malik, R.; Nigg, E.A.; Körner, R. Evaluation of the Low-Specificity Protease Elastase for Large-Scale Phosphoproteome Analysis. Anal. Chem. 2008, 80, 9526–9533. [Google Scholar] [CrossRef]
- Olsen, J.V.; Vermeulen, M.; Santamaria, A.; Kumar, C.; Miller, M.L.; Jensen, L.J.; Gnad, F.; Cox, J.; Jensen, T.S.; Nigg, E.A.; et al. Quantitative Phosphoproteomics Revealswidespread Full Phosphorylation Site Occupancy during Mitosis. Sci. Signal. 2010, 3, ra3. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Villén, J.; Beausoleil, S.A.; Mintseris, J.; Gygi, S.P. Phosphoproteome Analysis of Drosophila Melanogaster Embryos. J. Proteome Res. 2008, 7, 1675–1682. [Google Scholar] [CrossRef]
- Gardino, A.K.; Yaffe, M.B. 14-3-3 Proteins as Signaling Integration Points for Cell Cycle Control and Apoptosis. Semin. Cell Dev. Biol. 2011, 22, 688–695. [Google Scholar] [CrossRef]
- Sasse, B.; Lustig, A.; Aebi, U.; Stuurman, N. In Vitro Assembly of Drosophila Lamin Dm0 Lamin Polymerization Properties Are Conserved. Eur. J. Biochem. 1997, 250, 30–38. [Google Scholar] [CrossRef]
- Heitlinger, E.; Peter, M. The Role of the Head and Tail Domain in Lamin Structure Assembly: Analysis of Bacterially Expressed Chicken Lamin A and Truncated B, Lamins. J. Struct. Biol. 1992, 108, 74–91. [Google Scholar] [CrossRef]
- Stuurman, N.; Sasse, B.; Fisher, P.A. Intermediate Filament Protein Polymerization: Molecular Analysis of Drosophila Nuclear Lamin Head-to-Tail Binding. J. Struct. Biol. 1996, 15, 1–15. [Google Scholar] [CrossRef]
- Rzepecki, R.; Bogachev, S.S.; Kokoza, E.; Stuurman, N.; Fisher, P.A. In Vivo Association of Lamins with Nucleic Acids in Drosophila Melanogaster. J. Cell Sci. 1998, 111, 121–129. [Google Scholar] [CrossRef]
- Ludérus, M.E.; den Blaauwen, J.L.; de Smit, O.J.; Compton, D.A.; van Driel, R. Binding of Matrix Attachment Regions to Lamin Polymers Involves Single-Stranded Regions and the Minor Groove. Mol. Cell. Biol. 1994, 14, 6297–6305. [Google Scholar] [CrossRef] [PubMed]
- Uchino, R.; Sugiyama, S.; Katagiri, M.; Chuman, Y.; Furukawa, K. Non-Farnesylated B-Type Lamin Can Tether Chromatin inside the Nucleus and Its Chromatin Interaction Requires the Ig-Fold Region. Chromosoma 2017, 126, 125–144. [Google Scholar] [CrossRef]
- Bondarenko, S.M.; Sharakhov, I.V. Reorganization of the Nuclear Architecture in the Drosophila Melanogaster Lamin B Mutant Lacking the CaaX Box. Nucleus 2020, 11, 283–298. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Wang, S.; Heidinger, J.M.; Shumaker, D.K.; Adam, S.A.; Goldman, R.D.; Zheng, Y. A Mitotic Lamin B Matrix Induced by RanGTP Required for Spindle Assembly. Science 2006, 311, 1887–1893. [Google Scholar] [CrossRef]
- Yao, C.; Wang, C.; Li, Y.; Zavortink, M.; Archambault, V.; Girton, J.; Johansen, K.M.; Johansen, J. Evidence for a Role of Spindle Matrix Formation in Cell Cycle Progression by Antibody Perturbation. PLoS ONE 2018, 13, e0208022. [Google Scholar] [CrossRef]
- Ma, L.; Tsai, M.Y.; Wang, S.; Lu, B.; Chen, R.; Yates, J.R.; Zhu, X.; Zheng, Y. Requirement for Nudel and Dynein for Assembly of the Lamin B Spindle Matrix. Nat. Cell Biol. 2009, 11, 247–256. [Google Scholar] [CrossRef]
- Furukawa, K.; Ishida, K.; Tsunoyama, T.-a.; Toda, S.; Osoda, S.; Horigome, T.; Fisher, P.A.; Sugiyama, S. A-Type and B-Type Lamins Initiate Layer Assembly at Distinct Areas of the Nuclear Envelope in Living Cells. Exp. Cell Res. 2009, 315, 1181–1189. [Google Scholar] [CrossRef]
- Gaillard, C.; Delbarre, E.; Tramier, M.; Courvalin, J.; Buendia, B. The Truncated Prelamin A in Hutchinson–Gilford Progeria Syndrome Alters Segregation of A-Type and B-Type Lamin Homopolymers. Hum. Mol. Genet. 2006, 15, 1113–1122. [Google Scholar] [CrossRef]
- Schirmer, E.C.; Guan, T.; Gerace, L. Involvement of the Lamin Rod Domain in Heterotypic Lamin Interactions Important for Nuclear Organization. J. Cell Biol. 2001, 153, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Tanabe, K.; Katsube, H.; Inoue, Y.H. B-Type Nuclear Lamin and the Nuclear Pore Complex Nup107-160 Influences Maintenance of the Spindle Envelope Required for Cytokinesis in Drosophila Male Meiosis. Biol. Open 2016, 5, 1011–1021. [Google Scholar] [CrossRef]
- Prüfert, K.; Vogel, A.; Krohne, G. The Lamin CxxM Motif Promotes Nuclear Membrane Growth. J. Cell Sci. 2004, 117, 6105–6116. [Google Scholar] [CrossRef]
- Ralle, T.; Grund, C.; Franke, W.W.; Stick, R. Intranuclear Membrane Structure Formations by CaaX-Containing Nuclear Proteins. J. Cell Sci. 2004, 117, 6095–6104. [Google Scholar] [CrossRef]
- Guillemin, K.; Williams, T.; Krasnow, M.A. A Nuclear Lamin Is Required for Cytoplasmic Organization and Egg Polarity in Drosophila. Nat. Cell Biol. 2001, 3, 848–851. [Google Scholar] [CrossRef]
- Munoz-Alarcon, A.; Pavlovic, M.; Wismar, J.; Schmitt, B.; Eriksson, M.; Kylsten, P.; Dushay, M.S. Characterization of Lamin Mutation Phenotypes in Drosophila and Comparison to Human Laminopathies. PLoS ONE 2007, 2, e532. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Zheng, Y. Age-Associated Loss of Lamin-B Leads to Systemic Inflammation and Gut Hyperplasia. Cell 2014, 159, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Dopie, J.; Rajakylä, E.K.; Joensuu, M.S.; Huet, G. Genome-Wide RNAi Screen for Nuclear Actin Reveals a Network of Cofilin Regulators. J. Cell Sci. 2015, 128, 2388–2400. [Google Scholar] [CrossRef] [PubMed]
- Penfield, L.; Montell, D.J. Nuclear Lamin Facilitates Collective Border Cell Invasion into Confined Spaces in Vivo. J. Cell Biol. 2023, 222, e202212101. [Google Scholar] [CrossRef]
- Petrovsky, R.; Großhans, J. Expression of Lamina Proteins Lamin and Kugelkern Suppresses Stem Cell Proliferation. Nucleus 2018, 9, 104–118. [Google Scholar] [CrossRef]
- Brandt, A.; Papagiannouli, F.; Wagner, N.; Wilsch-Bräuninger, M.; Braun, M.; Furlong, E.E.; Loserth, S.; Wenzl, C.; Pilot, F.; Vogt, N.; et al. Developmental Control of Nuclear Size and Shape by Kugelkern and Kurzkern. Curr. Biol. 2006, 16, 543–552. [Google Scholar] [CrossRef]
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear Lamins: Major Factors in the Structural Organization and Function of the Nucleus and Chromatin. Genes Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef]
- van Steensel, B.; Belmont, A.S. Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell 2017, 169, 780–791. [Google Scholar] [CrossRef]
- Ho, C.Y.; Jaalouk, D.E.; Vartiainen, M.K.; Lammerding, J. Lamin A/C and Emerin Regulate MKL1-SRF Activity by Modulating Actin Dynamics. Nature 2013, 497, 507–511. [Google Scholar] [CrossRef]
- Patterson, K.; Molofsky, A.B.; Acosta, S.; Cater, C.; Fischer, J.A. The Functions of Klarsicht and Nuclear Lamin in Developmentally Regulated Nuclear Migrations of Photoreceptor Cells in the Drosophila Eye. Mol. Biol. Cell 2004, 15, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.A.; Berrios, M. Cell-Free Nuclear Assembly and Disassembly in Drosophila. In Methods in Cell Biology; Berrios, M., Ed.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 397–416. ISBN 9780125641555. [Google Scholar]
- Klapper, M.; Exner, K.; Kempf, A.; Gehrig, C.; Stuurman, N.; Fisher, P.A.; Krohne, G. Assembly of A- and B-Type Lamins Studied in Vivo with the Baculovirus System. J. Cell Sci. 1997, 110, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Harel, A.; Brandeis, M.; Rechsteiner, T.; Richmond, T.J.; Weiss, A.M.; Gruenbaum, Y. The Tail Domain of Lamin Dm0 Binds Histones H2A and H2B. Proc. Natl. Acad. Sci. USA 1999, 96, 2852–2857. [Google Scholar] [CrossRef]
- Meller, V.H.; Fisher, P.A. Nuclear Distribution of Drosophila DNA Topoisomerase II Is Sensitive to Both RNase and DNase. J. Cell Sci. 1995, 108, 1651–1657. [Google Scholar] [CrossRef]
- Rzepecki, R.; Fisher, P.A. During Both Interphase and Mitosis, DNA Topoisomerase II Interacts with DNA as Well as RNA through the Protein’s C-Terminal Domain. J. Cell Sci. 2000, 113, 1635–1647. [Google Scholar] [CrossRef]
- Fisher, P.A.; Lin, L.; McConnell, M.; Greenleaf, A.; Lee, J.M.; Smith, D.E. Heat Shock-Induced Appearance of RNA Polymerase II in Karyoskeletal Protein-Enriched (Nuclear “Matrix”) Fractions Correlates with Transcriptional Shutdown in Drosophila Melanogaster. J. Biol. Chem. 1989, 264, 3464–3469. [Google Scholar] [CrossRef] [PubMed]
- Rzepecki, R. The Properties of Plant (Cucurbita Pepo) Nuclear Matrix Preparations Are Strongly Affected by the Preparation Method. Cell. Mol. Biol. Lett. 2000, 5, 151–169. [Google Scholar]
- Belmont, A.S.; Zhai, Y.; Thilenius, A. Lamin B Distribution and Association with Peripheral Chromatin Revealed by Optical Sectioning and Electron Microscopy Tomography. J. Cell Biol. 1993, 123, 1671–1685. [Google Scholar] [CrossRef]
- Höger, T.H.; Krohne, G.; Kleinschmidt, J.A. Interaction of Xenopus Lamins A and LII with Chromatin in Vitro Mediated by a Sequence Element in the Carboxyterminal Domain. Exp. Cell Res. 1991, 197, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Simos, G.; Blobel, G.; Georgatos, S.D. Binding of Lamin A to Polynucleosomes. J. Biol. Chem. 1991, 266, 9211–9215. [Google Scholar] [CrossRef]
- Glass, J.R.; Gerace, L. Lamins A and C Bind and Assemble at the Surface of Mitotic Chromosomes. J. Cell Biol. 1990, 111, 1047–1057. [Google Scholar] [CrossRef]
- Glass, C.A.; Glass, J.R.; Taniura, H.; Hasel, K.W.; Blevitt, J.M.; Gerace, L. The Alpha-Helical Rod Domain of Human Lamins A and C Contains a Chromatin Binding Site. EMBO J. 1993, 12, 4413–4424. [Google Scholar] [CrossRef]
- Ulitzur, N.; Harel, A.; Feinstein, N.; Gruenbaum, Y. Lamin Activity Is Essential for Nuclear Envelope Assembly in a Drosophila Embryo Cell-Free Extract. J. Cell Biol. 1992, 119, 17–25. [Google Scholar] [CrossRef]
- Shimi, T.; Pfleghaar, K.; Kojima, S.I.; Pack, C.G.; Solovei, I.; Goldman, A.E.; Adam, S.A.; Shumaker, D.K.; Kinjo, M.; Cremer, T.; et al. The A- and B-Type Nuclear Lamin Networks: Microdomains Involved in Chromatin Organization and Transcription. Genes Dev. 2008, 22, 3409–3421. [Google Scholar] [CrossRef]
- Bossie, C.A.; Sanders, M.M. A CDNA from Drosophila Melanogaster Encodes a Lamin C-like Intermediate Filament Protein. J. Cell Sci. 1993, 104, 1263–1272. [Google Scholar] [CrossRef]
- Riemer, D.; Stuurman, N.; Berrios, M.; Hunter, C.; Fisher, P.A.; Weber, K. Expression of Drosophila Lamin C Is Developmentally Regulated: Analogies with Vertebrate A-Type Lamins. J. Cell Sci. 1995, 108, 3189–3198. [Google Scholar] [CrossRef]
- Schulze, S.R.; Curio-Penny, B.; Li, Y.; Imani, R.A.; Rydberg, L.; Geyer, P.K.; Wallrath, L.L. Molecular Genetic Analysis of the Nested Drosophila Melanogaster Lamin C Gene. Genetics 2005, 171, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Stuurman, N.; Delbecque, J.-P.; Callaerts, P.; Aebi, U. Ectopic Overexpression of Drosophila Lamin C Is Stage-Specific Lethal. Exp. Cell Res. 1999, 248, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Molecular Structure, Assembly Mechanism, and Integration Into Functionally Distinct Intracellular Scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789. [Google Scholar] [CrossRef] [PubMed]
- Riemer, D.; Weber, K. The Organization of the Gene for Drosophila Lamin C: Limited Homology with Vertebrate Lamin Genes and Lack of Homology versus the Drosophila Lamin Dmo Gene. Eur. J. Cell Biol. 1994, 63, 299–306. [Google Scholar] [PubMed]
- Maus, N.; Stuurman, N.; Fisher, P.A. Disassembly of the Drosophila Nuclear Lamina in a Homologous Cell-Free System. J. Cell Sci. 1995, 108, 2027–2035. [Google Scholar] [CrossRef]
- Stuurman, N. Identification of a Conserved Phosphorylation Site Modulating Nuclear Lamin Polymerization. FEBS Lett. 1997, 401, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Nakagawa, J.; Dorée, M.; Labbé, J.C.; Nigg, E.A. In Vitro Disassembly of the Nuclear Lamina and M Phase-Specific Phosphorylation of Lamins by Cdc2 Kinase. Cell 1990, 61, 591–602. [Google Scholar] [CrossRef]
- Heald, R.; McKeon, F. Mutations of Phosphorylation Sites in Lamin A That Prevent Nuclear Lamina Disassembly in Mitosis. Cell 1990, 61, 579–589. [Google Scholar] [CrossRef]
- Haas, M.; Jost, E. Functional Analysis of Phosphorylation Sites in Human Lamin A Controlling Lamin Disassembly, Nuclear Transport and Assembly. Eur. J. Cell Biol. 1993, 62, 237–247. [Google Scholar]
- Shaw, N.M.; Rios-Monterrosa, J.L.; Fedorchak, G.R.; Ketterer, M.R.; Coombs, G.S.; Lammerding, J.; Wallrath, L.L. Effects of Mutant Lamins on Nucleo-Cytoskeletal Coupling in Drosophila Models of LMNA Muscular Dystrophy. Front. Cell Dev. Biol. 2022, 10, 934586. [Google Scholar] [CrossRef]
- Harborth, J.; Elbashir, S.M.; Bechert, K.; Tuschl, T.; Weber, K. Identification of Essential Genes in Cultured Mammalian Cells Using Small Interfering RNAs. J. Cell Sci. 2001, 114, 4557–4565. [Google Scholar] [CrossRef]
- Uchino, R.; Nonaka, Y.; Horigome, T.; Sugiyama, S.; Furukawa, K. Loss of Drosophila A-Type Lamin C Initially Causes Tendon Abnormality Including Disintegration of Cytoskeleton and Nuclear Lamina in Muscular Defects. Dev. Biol. 2013, 373, 216–227. [Google Scholar] [CrossRef]
- Dialynas, G.; Speese, S.; Budnik, V.; Geyer, P.K.; Wallrath, L.L. The Role of Drosophila Lamin C in Muscle Function and Gene Expression. Development 2010, 137, 3067–3077. [Google Scholar] [CrossRef]
- Walker, S.G.; Langland, C.J.; Viles, J.; Hecker, L.A.; Wallrath, L.L. Drosophila Models Reveal Properties of Mutant Lamins That Give Rise to Distinct Diseases. Cells 2023, 12, 1142. [Google Scholar] [CrossRef]
- Schulze, S.R.; Curio-Penny, B.; Speese, S.; Dialynas, G.; Cryderman, D.E.; McDonough, C.W.; Nalbant, D.; Petersen, M.; Budnik, V.; Geyer, P.K.; et al. A Comparative Study of Drosophila and Human A-Type Lamins. PLoS ONE 2009, 4, e7564. [Google Scholar] [CrossRef]
- Zwerger, M.; Jaalouk, D.E.; Lombardi, M.L.; Isermann, P.; Mauermann, M.; Dialynas, G.; Herrmann, H.; Wallrath, L.; Lammerding, J. Myopathic Lamin Mutations Impair Nuclear Stability in Cells and Tissue and Disrupt Nucleo-Cytoskeletal Coupling. Hum. Mol. Genet. 2013, 22, 2335–2349. [Google Scholar] [CrossRef]
- Gurudatta, B.V.; Shashidhara, L.S.; Parnaik, V.K. Lamin C and Chromatin Organization in Drosophila. J. Genet. 2010, 89, 37–49. [Google Scholar] [CrossRef]
- Akter, R.; Rivas, D.; Geneau, G.; Drissi, H.; Duque, G. Effect of Lamin A/C Knockdown on Osteoblast Differentiation and Function. J. Bone Miner. Res. 2009, 24, 283–293. [Google Scholar] [CrossRef]
- Maresca, G.; Natoli, M.; Nardella, M.; Arisi, I.; Trisciuoglio, D.; Desideri, M.; Brandi, R.; D’Aguanno, S.; Nicotra, M.R.; D’Onofrio, M.; et al. LMNA Knock-Down Affects Differentiation and Progression of Human Neuroblastoma Cells. PLoS ONE 2012, 7, e45513. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Perez, D.; White, E. Lamin Proteolysis Facilitates Nuclear Events During Apoptosis. J. Cell Biol. 1996, 135, 1441–1455. [Google Scholar] [CrossRef]
- Sullivan, T.; Escalante-Alcalde, D.; Bhatt, H.; Anver, M.; Bhat, N.; Nagashima, K.; Stewart, C.L.; Burke, B. Loss of A-Type Lamin Expression Compromises Nuclear Envelope Integrity Leading to Muscular Dystrophy. J. Cell Biol. 1999, 147, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Chojnowski, A.; Boudier, T.; Lim, J.S.Y.; Ahmed, S.; Ser, Z.; Stewart, C.; Burke, B. A-Type Lamins Form Distinct Filamentous Networks with Differential Nuclear Pore Complex Associations. Curr. Biol. 2016, 26, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.; Hoskins, V.E.; Melendez-Perez, A.J.; Harr, J.C.; Gordon, M.; Reddy, K.L. Lamin C Is Required to Establish Genome Organization after Mitosis. Genome Biol. 2021, 22, 305. [Google Scholar] [CrossRef]
- Chandran, S.; Suggs, J.A.; Wang, B.J.; Han, A.; Bhide, S.; Cryderman, D.E.; Moore, S.A.; Bernstein, S.I.; Wallrath, L.L.; Melkani, G.C. Suppression of Myopathic Lamin Mutations by Muscle-Specific Activation of AMPK and Modulation of Downstream Signaling. Hum. Mol. Genet. 2019, 28, 351–371. [Google Scholar] [CrossRef]
- Bhide, S.; Trujillo, A.S.; O’Connor, M.T.; Young, G.H.; Cryderman, D.E.; Chandran, S.; Nikravesh, M.; Wallrath, L.L.; Melkani, G.C. Increasing Autophagy and Blocking Nrf2 Suppress Laminopathy-Induced Age-Dependent Cardiac Dysfunction and Shortened Lifespan. Aging Cell 2018, 17, e12747. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.E.; Walker, S.G.; Xiong, A.; Gogal, R.A.; Schnieders, M.J.; Wallrath, L.L. In Silico and in Vivo Analysis of Amino Acid Substitutions That Cause Laminopathies. Int. J. Mol. Sci. 2021, 22, 11226. [Google Scholar] [CrossRef] [PubMed]
- Masuko, K.; Furuhashi, H.; Komaba, K.; Numao, E.; Nakajima, R.; Fuse, N.; Kurata, S. Nuclear Lamin Is Required for Winged Eye-Mediated Transdetermination of Drosophila Imaginal Disc. Genes Cells 2018, 23, 724–731. [Google Scholar] [CrossRef]
- Estrella, N.L.; Desjardins, C.A.; Nocco, S.E.; Clark, A.L.; Maksimenko, Y.; Naya, F.J. MEF2 Transcription Factors Regulate Distinct Gene Programs in Mammalian Skeletal Muscle Differentiation. J. Biol. Chem. 2015, 290, 1256–1268. [Google Scholar] [CrossRef]
- Duffy, J.B. GAL4 System in Drosophila: A Fly Geneticist’s Swiss Army Knife. Genesis 2002, 34, 1–15. [Google Scholar] [CrossRef]
- Östlund, C.; Bonne, G.; Schwartz, K.; Worman, H.J. Properties of Lamin A Mutants Found in Emery-Dreifuss Muscular Dystrophy, Cardiomyopathy and Dunnigan-Type Partial Lipodystrophy. J. Cell Sci. 2001, 114, 4435–4445. [Google Scholar] [CrossRef]
- Raharjo, W.H.; Enarson, P.; Sullivan, T.; Stewart, C.L.; Burke, B. Nuclear Envelope Defects Associated with LMNA Mutations Cause Dilated Cardiomyopathy and Emery-Dreifuss Muscular Dystrophy. J. Cell Sci. 2001, 114, 4447–4457. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear Heme Oxygenase-1 (HO-1) Modulates Subcellular Distribution and Activation of Nrf2, Impacting Metabolic and Anti-Oxidant Defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef]
- Andrysiak, K.; Machaj, G.; Priesmann, D.; Woźnicka, O.; Martyniak, A.; Ylla, G.; Krüger, M.; Pyza, E.; Potulska-Chromik, A.; Kostera-Pruszczyk, A.; et al. Dysregulated Iron Homeostasis in Dystrophin-Deficient Cardiomyocytes: Correction by Gene Editing and Pharmacological Treatment. Cardiovasc. Res. 2024, 120, 69–81. [Google Scholar] [CrossRef]
- Rajasekaran, N.S.; Shelar, S.B.; Jones, D.P.; Hoidal, J.R. Reductive Stress Impairs Myogenic Differentiation. Redox Biol. 2020, 34, 101492. [Google Scholar] [CrossRef]
- Bronisz-Budzyńska, I.; Kozakowska, M.; Pietraszek-Gremplewicz, K.; Madej, M.; Józkowicz, A.; Łoboda, A.; Dulak, J. NRF2 Regulates Viability, Proliferation, Resistance to Oxidative Stress, and Differentiation of Murine Myoblasts and Muscle Satellite Cells. Cells 2022, 11, 3321. [Google Scholar] [CrossRef]
- Petrillo, S.; Pelosi, L.; Piemonte, F.; Travaglini, L.; Forcina, L.; Catteruccia, M.; Petrini, S.; Verardo, M.; D’Amico, A.; Musaró, A.; et al. Oxidative Stress in Duchenne Muscular Dystrophy: Focus on the NRF2 Redox Pathway. Hum. Mol. Genet. 2017, 26, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Kourakis, S.; Timpani, C.A.; Campelj, D.G.; Hafner, P.; Gueven, N.; Fischer, D.; Rybalka, E. Standard of Care versus New-Wave Corticosteroids in the Treatment of Duchenne Muscular Dystrophy: Can We Do Better? Orphanet J. Rare Dis. 2021, 16, 117. [Google Scholar] [CrossRef]
- Tsakiri, E.N.; Sykiotis, G.P.; Papassideri, I.S.; Terpos, E.; Dimopoulos, M.A.; Gorgoulis, V.G.; Bohmann, D.; Ioannis, P.T. Proteasome Dysfunction in Drosophila Signals to an Nrf2- Dependent Regulatory Circuit Aiming to Restore Proteostasis and Prevent Premature Aging. Aging Cell 2013, 12, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Pitoniak, A.; Bohmann, D. Mechanisms and Functions of Nrf2 Signaling in Drosophila. Free Radic. Biol. Med. 2015, 88, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darley-Usmar, V.; Zhang, J. KEAP1-NRF2 Signaling and Autophagy in Protection against Oxidative and Reductive Proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar] [CrossRef]
- Carlson, J.; Neidviecky, E.; Cook, I.; Cross, B.; Deng, H. Interaction with B-Type Lamin Reveals the Function of Drosophila Keap1 Xenobiotic Response Factor in Nuclear Architecture. Mol. Biol. Rep. 2024, 51, 556. [Google Scholar] [CrossRef]
- Carlson, J.; Price, L.; Cook, I.; Deng, H. Drosophila Keap1 Xenobiotic Response Factor Regulates Developmental Transcription through Binding to Chromatin. Dev. Biol. 2022, 481, 139–147. [Google Scholar] [CrossRef]
- Xiao, C.; Hull, D.; Qiu, S.; Yeung, J.; Zheng, J.; Barwell, T.; Meldrum Robertson, R.; Seroude, L. Expression of Heat Shock Protein 70 Is Insufficient to Extend Drosophila Melanogaster Longevity. G3 Genes Genomes Genet. 2019, 9, 4197–4207. [Google Scholar] [CrossRef]
- Alagar Boopathy, L.R.; Jacob-Tomas, S.; Alecki, C.; Vera, M. Mechanisms Tailoring the Expression of Heat Shock Proteins to Proteostasis Challenges. J. Biol. Chem. 2022, 298, 101796. [Google Scholar] [CrossRef]
- Ashburner, M.; Bonner, J.J. The Induction of Gene Activity in Drosophila by Heat Shock. Cell 1979, 17, 241–254. [Google Scholar] [CrossRef]
- Hasper, J.; Welle, K.; Swovick, K.; Hryhorenko, J.; Ghaemmaghami, S.; Buchwalter, A. Long Lifetime and Tissue-Specific Accumulation of Lamin A/C in Hutchinson–Gilford Progeria Syndrome. J. Cell Biol. 2024, 223, e202307049. [Google Scholar] [CrossRef]
- Salpingidou, G.; Smertenko, A.; Hausmanowa-Petrucewicz, I.; Hussey, P.J.; Hutchison, C.J. A Novel Role for the Nuclear Membrane Protein Emerin in Association of the Centrosome to the Outer Nuclear Membrane. J. Cell Biol. 2007, 178, 897–904. [Google Scholar] [CrossRef]
- Markiewicz, E.; Venables, R.; Alvarez-Reyes, M.; Quinlan, R.; Dorobek, M.; Hausmanowa-Petrucewicz, I.; Hutchison, C. Increased Solubility of Lamins and Redistribution of Lamin C in X-Linked Emery-Dreifuss Muscular Dystrophy Fibroblasts. J. Struct. Biol. 2002, 140, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.J.; Alvarez-Reyes, M.; Vaughan, O.A. Lamins in Disease: Why Do Ubiquitously Expressed Nuclear Envelope Proteins Give Rise to Tissue-Specific Disease Phenotypes? J. Cell Sci. 2001, 114, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Y.; Walker, D.L.; Dong, H.; Conley, C.; Johansen, J.; Johansen, K.M. JIL-1: A Novel Chromosomal Tandem Kinase Implicated in Transcriptional Regulation in Drosophila. Mol. Cell 1999, 4, 129–135. [Google Scholar] [CrossRef]
- Goldberg, M.; Lu, H.; Stuurman, N.; Ashery-Padan, R.; Weiss, A.M.; Yu, J.; Bhattacharyya, D.; Fisher, P.A.; Gruenbaum, Y.; Wolfner, M.F. Interactions among Drosophila Nuclear Envelope Proteins Lamin, Otefin, and YA. Mol. Cell. Biol. 1998, 18, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Mukai, T.; Mori-Yoshimura, M.; Nishikawa, A.; Hokkoku, K.; Sonoo, M.; Nishino, I.; Takahashi, Y. Emery-Dreifuss Muscular Dystrophy-Related Myopathy with TMEM43 Mutations. Muscle Nerve 2019, 59, E5–E7. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Shumaker, D.K.; Erdos, M.R.; Eriksson, M.; Goldman, A.E.; Gordon, L.B.; Gruenbaum, Y.; Khuon, S.; Mendez, M.; Varga, R.; et al. Accumulation of Mutant Lamin A Causes Progressive Changes in Nuclear Architecture in Hutchinson-Gilford Progeria Syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 8963–8968. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Weyhersmüller, A.; Blauth, A.; Schuhmann, T.; Heckmann, M.; Krohne, G.; Samakovlis, C. The Drosophila LEM-Domain Protein MAN1 Antagonizes BMP Signaling at the Neuromuscular Junction and the Wing Crossveins. Dev. Biol. 2010, 339, 1–13. [Google Scholar] [CrossRef]
- Brandt, A.; Petrovsky, R.; Kriebel, M.; Großhans, J. Use of Farnesyl Transferase Inhibitors in an Ageing Model in Drosophila. J. Dev. Biol. 2023, 11, 40. [Google Scholar] [CrossRef]
- Brandt, A.; Krohne, G.; Großhans, J. The Farnesylated Nuclear Proteins KUGELKERN and LAMIN B Promote Aging-like Phenotypes in Drosophila Flies. Aging Cell 2008, 7, 541–551. [Google Scholar] [CrossRef]
- Polychronidou, M.; Hellwig, A.; Grosshans, J. Farnesylated Nuclear Proteins Kugelkern and Lamin Dm0 Affect Nuclear Morphology by Directly Interacting with the Nuclear Membrane. Mol. Biol. Cell 2010, 21, 3409–3420. [Google Scholar] [CrossRef] [PubMed]
- Beard, G.S.; Bridger, J.M.; Kill, I.R.; Tree, D.R.P. Towards a Drosophila Model of Hutchinson-Gilford Progeria Syndrome. Biochem. Soc. Trans. 2008, 36, 1389–1392. [Google Scholar] [CrossRef]
- Verstraeten, V.L.R.M.; Ji, J.Y.; Cummings, K.S.; Lee, R.T.; Lammerding, J. Increased Mechanosensitivity and Nuclear Stiffness in Hutchinson-Gilford Progeria Cells: Effects of Farnesyltransferase Inhibitors. Aging Cell 2008, 7, 383–393. [Google Scholar] [CrossRef]
- Glynn, M.W.; Glover, T.W. Incomplete Processing of Mutant Lamin A in Hutchinson-Gilford Progeria Leads to Nuclear Abnormalities, Which Are Reversed by Farnesyltransferase Inhibition. Hum. Mol. Genet. 2005, 14, 2959–2969. [Google Scholar] [CrossRef]
- Mallampalli, M.P.; Huyer, G.; Bendale, P.; Gelb, M.H.; Michaelis, S. Inhibiting Farnesylation Reverses the Nuclear Morphology Defect in a HeLa Cell Model for Hutchinson-Gilford Progeria Syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 14416–14421. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Q.; Medalia, O. Lamins and Chromatin Join Forces. Adv. Biol. Regul. 2024, 95, 101059. [Google Scholar] [CrossRef]
- Dubińska-Magiera, M.; Kozioł, K.; Machowska, M.; Piekarowicz, K.; Filipczak, D.; Rzepecki, R. Emerin Is Required for Proper Nucleus Reassembly after Mitosis: Implications for New Pathogenetic Mechanisms for Laminopathies Detected in EDMD1 Patients. Cells 2019, 8, 240. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.-W.; Tewari, M.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef]
- Benedicto, I.; Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Carmona, R.M.; Espinós-Estévez, C.; Gonzalo, P.; de la Fuente-Pérez, M.; Andrés-Manzano, M.J.; González-Gómez, C.; et al. Endothelial Cell-Specific Progerin Expression Does Not Cause Cardiovascular Alterations and Premature Death. Aging Cell 2024, 24, e14389. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, L.; Sánchez-López, A.; González-Gómez, C.; Andrés-Manzano, M.J.; Dorado, B.; Andrés, V. Vascular Smooth Muscle Cell-Specific Progerin Expression Provokes Contractile Impairment in a Mouse Model of Hutchinson-Gilford Progeria Syndrome That Is Ameliorated by Nitrite Treatment. Cells 2020, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Toyama, B.H.; Savas, J.N.; Park, S.K.; Harris, M.S.; Ingolia, N.T.; Yates, J.R.; Hetzer, M.W. Identification of Long-Lived Proteins Reveals Exceptional Stability of Essential Cellular Structures. Cell 2013, 154, 971–982. [Google Scholar] [CrossRef]
- Razafsky, D.; Ward, C.; Potter, C.; Zhu, W.; Xue, Y.; Kefalov, V.J.; Fong, L.G.; Young, S.G.; Hodzic, D. Lamin B1 and Lamin B2 Are Long-Lived Proteins with Distinct Functions in Retinal Development. Mol. Biol. Cell 2016, 27, 1928–1937. [Google Scholar] [CrossRef]
- Coombs, G.S.; Rios-Monterrosa, J.L.; Lai, S.; Dai, Q.; Goll, A.C.; Ketterer, M.R.; Valdes, M.F.; Uche, N.; Benjamin, I.J.; Wallrath, L.L. Modulation of Muscle Redox and Protein Aggregation Rescues Lethality Caused by Mutant Lamins. Redox Biol. 2021, 48, 102196. [Google Scholar] [CrossRef]
- Puttaraju, M.; Jackson, M.; Klein, S.; Shilo, A.; Bennett, C.F.; Gordon, L.; Rigo, F.; Misteli, T. Systematic Screening Identifies Therapeutic Antisense Oligonucleotides for Hutchinson–Gilford Progeria Syndrome. Nat. Med. 2021, 27, 526–535. [Google Scholar] [CrossRef]
- Gunage, R.D.; Dhanyasi, N.; Reichert, H.; VijayRaghavan, K. Drosophila Adult Muscle Development and Regeneration. Semin. Cell Dev. Biol. 2017, 72, 56–66. [Google Scholar] [CrossRef]
- Baylies, M.K.; Bate, M.; Gomez, M.R. Myogenesis: A View from Drosophila. Cell 1998, 93, 921–927. [Google Scholar] [CrossRef]
- Dos Santos, M.; Shah, A.M.; Zhang, Y.; Bezprozvannaya, S.; Chen, K.; Xu, L.; Lin, W.; McAnally, J.R.; Bassel-Duby, R.; Liu, N.; et al. Opposing Gene Regulatory Programs Governing Myofiber Development and Maturation Revealed at Single Nucleus Resolution. Nat. Commun. 2023, 14, 4333. [Google Scholar] [CrossRef] [PubMed]
- Keshishian, H.; Broadie, K.; Chiba, A.; Bate, M. The Drosophila Neuromuscular Junction: A Model System for Studying Synaptic Development and and Function. Annu. Rev. Neurosci. 1996, 19, 545–575. [Google Scholar] [CrossRef]
- Frost, B.; Bardai, F.H.; Feany, M.B. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr. Biol. 2016, 26, 129–136. [Google Scholar] [CrossRef]
- Yamamoto, S.; Jaiswal, M.; Charng, W.L.; Gambin, T.; Karaca, E.; Mirzaa, G.; Wiszniewski, W.; Sandoval, H.; Haelterman, N.A.; Xiong, B.; et al. A Drosophila Genetic Resource of Mutants to Study Mechanisms Underlying Human Genetic Diseases. Cell 2014, 159, 200–214. [Google Scholar] [CrossRef]
- Link, N.; Chung, H.; Jolly, A.; Withers, M.; Tepe, B.; Arenkiel, B.R.; Shah, P.S.; Krogan, N.J.; Aydin, H.; Geckinli, B.B.; et al. Mutations in ANKLE2, a ZIKA Virus Target, Disrupt an Asymmetric Cell Division Pathway in Drosophila Neuroblasts to Cause Microcephaly. Dev. Cell 2019, 51, 713–729.e6. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Krohne, G. LEM-Domain Proteins: New Insights into Lamin-Interacting Proteins. Int. Rev. Cytol. 2007, 261, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Mandigo, T.R.; Turcich, B.D.; Anderson, A.J.; Hussey, M.R.; Folker, E.S. Drosophila Emerins Control LINC Complex Localization and Transcription to Regulate Myonuclear Position. J. Cell Sci. 2019, 132, jcs235580. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.A.; Coon, L.A.; Thomas, R.; Mandigo, T.R.; Wynn, E.; Folker, E.S. Ensconsin-Dependent Changes in Microtubule Organization and LINC Complex-Dependent Changes in Nucleus-Nucleus Interactions Result in Quantitatively Distinct Myonuclear Positioning Defects. Mol. Biol. Cell 2021, 32, ar27. [Google Scholar] [CrossRef]
- Wagner, N.; Schmitt, J.; Krohne, G. Two Novel LEM-Domain Proteins Are Splice Products of the Annotated Drosophila Melanogaster Gene CG9424 (Bocksbeutel). Eur. J. Cell Biol. 2004, 82, 605–616. [Google Scholar] [CrossRef]
- Duan, T.; Kitzman, S.C.; Geyer, P.K. Survival of Drosophila Germline Stem Cells Requires the Chromatin-Binding Protein Barrier-to-Autointegration Factor. Development 2020, 147, dev186171. [Google Scholar] [CrossRef]
- Wagner, N.; Kagermeier, B.; Loserth, S.; Krohne, G. The Drosophila Melanogaster LEM-Domain Protein MAN1. Eur. J. Cell Biol. 2006, 85, 91–105. [Google Scholar] [CrossRef]
- Pinto, B.S.; Wilmington, S.R.; Hornick, E.E.L.; Wallrath, L.L.; Geyer, P.K. Tissue-Specific Defects Are Caused by Loss of the Drosophila MAN1 LEM Domain Protein. Genetics 2008, 180, 133–145. [Google Scholar] [CrossRef]
- Barton, L.J.; Wilmington, S.R.; Martin, M.J.; Skopec, H.M.; Lovander, K.E.; Pinto, B.S.; Geyer, P.K. Unique and Shared Functions of Nuclear Lamina LEM Domain Proteins in Drosophila. Genetics 2014, 197, 653–665. [Google Scholar] [CrossRef]
- Gozalo, A.; Duke, A.; Lan, Y.; Pascual-Garcia, P.; Talamas, J.A.; Nguyen, S.C.; Shah, P.P.; Jain, R.; Joyce, E.F.; Capelson, M. Core Components of the Nuclear Pore Bind Distinct States of Chromatin and Contribute to Polycomb Repression. Mol. Cell 2020, 77, 67–81. [Google Scholar] [CrossRef]
- Katsani, K.R.; Karess, R.E.; Dostatni, N.; Doye, V. In Vivo Dynamics of Drosophila Nuclear Envelope Components. Mol. Biol. Cell 2008, 19, 3652–3666. [Google Scholar] [CrossRef]
- Mehsen, H.; Boudreau, V.; Garrido, D.; Bourouh, M.; Larouche, M.; Maddox, P.S.; Swan, A.; Archambault, V. PP2A-B55 Promotes Nuclear Envelope Reformation after Mitosis in Drosophila. J. Cell Biol. 2018, 217, 4106–4123. [Google Scholar] [CrossRef]
- Doronin, S.A.; Ilyin, A.A.; Kononkova, A.D.; Solovyev, M.A.; Olenkina, O.M.; Nenasheva, V.V.; Mikhaleva, E.A.; Lavrov, S.A.; Ivannikova, A.Y.; Simonov, R.A.; et al. Nucleoporin Elys Attaches Peripheral Chromatin to the Nuclear Pores in Interphase Nuclei. Commun. Biol. 2024, 7, 783. [Google Scholar] [CrossRef]
- Shevelyov, Y.Y. Interactions of Chromatin with the Nuclear Lamina and Nuclear Pore Complexes. Int. J. Mol. Sci. 2023, 24, 15771. [Google Scholar] [CrossRef]
- Shevelyov, Y.Y.; Ulianov, S.V. The Nuclear Lamina as an Organizer of Chromosome Architecture. Cells 2019, 8, 136. [Google Scholar] [CrossRef]
- Capelson, M.; Corces, V.G. The Ubiquitin Ligase DTopors Directs the Nuclear Organization of a Chromatin Insulator. Mol. Cell 2005, 20, 105–116. [Google Scholar] [CrossRef]
- Mattout, A.; Goldberg, M.; Tzur, Y.; Margalit, A.; Gruenbaum, Y. Specific and Conserved Sequences in D. Melanogaster and C. Elegans Lamins and Histone H2A Mediate the Attachment of Lamins to Chromosomes. J. Cell Sci. 2007, 120, 77–85. [Google Scholar] [CrossRef]
- Yamamoto-Hino, M.; Kawaguchi, K.; Ono, M.; Furukawa, K.; Goto, S. Lamin Is Essential for Nuclear Localization of the GPI Synthesis Enzyme PIG-B and GPI-Anchored Protein Production in Drosophila. J. Cell Sci. 2020, 133, jcs238527. [Google Scholar] [CrossRef]
- Yamamoto-Hino, M.; Katsumata, E.; Suzuki, E.; Maeda, Y.; Kinoshita, T.; Goto, S. Nuclear Envelope Localization of PIG-B Is Essential for GPI-Anchor Synthesis in Drosophila. J. Cell Sci. 2018, 131, jcs218024. [Google Scholar] [CrossRef]
- Napoletano, F.; Ferrari Bravo, G.; Voto, I.A.P.; Santin, A.; Celora, L.; Campaner, E.; Dezi, C.; Bertossi, A.; Valentino, E.; Santorsola, M.; et al. The Prolyl-Isomerase PIN1 Is Essential for Nuclear Lamin-B Structure and Function and Protects Heterochromatin under Mechanical Stress. Cell Rep. 2021, 36, 109694. [Google Scholar] [CrossRef]
- Milbradt, J.; Hutterer, C.; Bahsi, H.; Wagner, S.; Sonntag, E.; Horn, A.H.C.; Kaufer, B.B.; Mori, Y.; Sticht, H.; Fossen, T.; et al. The Prolyl Isomerase Pin1 Promotes the Herpesvirus-Induced Phosphorylation-Dependent Disassembly of the Nuclear Lamina Required for Nucleocytoplasmic Egress. PLoS Pathog. 2016, 12, e1005825. [Google Scholar] [CrossRef]
- Li, Z.; Tang, J.; Guo, F. Identification of 14-3-3 Proteins Phosphopeptide-Binding Specificity Using an Affinity-Based Computational Approach. PLoS ONE 2016, 11, e0147467. [Google Scholar] [CrossRef]
- Busayavalasa, K.; Chen, X.; Farrants, A.K.Ö.; Wagner, N.; Sabri, N. The Nup155-Mediated Organisation of Inner Nuclear Membrane Proteins Is Independent of Nup155 Anchoring to the Metazoan Nuclear Pore Complex. J. Cell Sci. 2012, 125, 4214–4218. [Google Scholar] [CrossRef]
- Jiang, X.; Xia, L.; Chen, D.; Yang, Y.; Huang, H.; Yang, L.; Zhao, Q.; Shen, L.; Wang, J.; Chen, D. Otefin, a Nuclear Membrane Protein, Determines the Fate of Germline Stem Cells in Drosophila via Interaction with Smad Complexes. Dev. Cell 2008, 14, 494–506. [Google Scholar] [CrossRef]
- Duan, T.; Cupp, R.; Geyer, P.K. Drosophila Female Germline Stem Cells Undergo Mitosis without Nuclear Breakdown. Curr. Biol. 2021, 31, 1450–1462.e3. [Google Scholar] [CrossRef]
- Jones, S.D.; Miller, J.E.B.; Amos, M.M.; Hernández, J.M.; Piaszynski, K.M.; Geyer, P.K. Emerin Preserves Stem Cell Survival through Maintenance of Centrosome and Nuclear Lamina Structure. Development 2024, 151, dev204219. [Google Scholar] [CrossRef]
- Deng, H.; Kerppola, T.K. Regulation of Drosophila Metamorphosis by Xenobiotic Response Regulators. PLoS Genet. 2013, 9, e1003263. [Google Scholar] [CrossRef]
- Hamed, M.; Antonin, W. Dunking into the Lipid Bilayer: How Direct Membrane Binding of Nucleoporins Can Contribute to Nuclear Pore Complex Structure and Assembly. Cells 2021, 10, 3601. [Google Scholar] [CrossRef]
- Ptak, C.; Wozniak, R.W. Nucleoporins and Chromatin Metabolism. Curr. Opin. Cell Biol. 2016, 40, 153–160. [Google Scholar] [CrossRef]
- Capelson, M. You Are Who Your Friends Are—Nuclear Pore Proteins as Components of Chromatin-Binding Complexes. FEBS Lett. 2023, 597, 2769–2781. [Google Scholar] [CrossRef]
- Kotb, N.M.; Ulukaya, G.; Chavan, A.; Nguyen, S.C.; Proskauer, L.; Joyce, E.F.; Hasson, D.; Jagannathan, M.; Rangan, P. Genome Organization Regulates Nuclear Pore Complex Formation and Promotes Differentiation during Drosophila Oogenesis. Genes Dev. 2024, 38, 436–454. [Google Scholar] [CrossRef]
- Nobari, P.; Doye, V.; Boumendil, C. Metazoan Nuclear Pore Complexes in Gene Regulation and Genome Stability. DNA Repair 2023, 130, 103565. [Google Scholar] [CrossRef]
- Takenawa, T.; Suetsugu, S. The WASP–WAVE Protein Network: Connecting the Membrane to the Cytoskeleton. Nat. Rev. Mol. Cell Bology 2007, 8, 37–48. [Google Scholar] [CrossRef]
- Miyamoto, K.; Teperek, M.; Yusa, K.; Allen, G.E.; Bradshaw, C.R.; Gurdon, J.B. Nuclear Wave1 Is Required for Reprogramming Transcription in Oocytes and for Normal Development. Science 2013, 341, 1002–1005. [Google Scholar] [CrossRef]
- Cao, L.; Ghasemi, F.; Way, M.; Jégou, A.; Romet-Lemonne, G. Regulation of Branched versus Linear Arp2/3-generated Actin Filaments. EMBO J. 2023, 42, e113008. [Google Scholar] [CrossRef]
- Verboon, J.M.; Rincon-Arano, H.; Werwie, T.R.; Delrow, J.J.; Scalzo, D.; Nandakumar, V.; Groudine, M.; Parkhurst, S.M. Wash Interacts with Lamin and Affects Global Nuclear Organization. Curr. Biol. 2015, 25, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, M.; Dubińska-Magiera, M.; Sopel, M.; Rzepecka, D.; Hutchison, C.J.; Goldberg, M.W.; Rzepecki, R. Embryonic and Adult Isoforms of XLAP2 Form Microdomains Associated with Chromatin and the Nuclear Envelope. Cell Tissue Res. 2011, 344, 97–110. [Google Scholar] [CrossRef] [PubMed]
| Reference | Type of Manipulation or Strain | Tissue, Organ, Stage | Phenotype |
|---|---|---|---|
| [84] | Lenz-Böhme et al., 1997 LamP (P-element insertion mutant in lamin Dm) | Larvae, pupae, adults | Developmental delay; impaired locomotor responses; minority (5–10%) reached adulthood; adults displayed sterility; abnormal gonadal development; defective oogenesis; malformed nuclei; nuclear pore complexes (NPCs) clustered and mislocalized; lamin Dm protein remained detectable in tissues despite phenotypic abnormalities. |
| [106] | Guillemin et al., 2001 misgsz18 (loss-of-function allele of lamin Dm) | Germline, embryos, larval stages | Disturbed dorsal–ventral egg polarity in oocyte (gurken transcripts and Gurken protein mislocalization); reduced levels of lamin Dm in oocyte and nurse cells; normal level in epidermal cells/ |
| [43] | Osouda et al., 2005 1. Lam14/Df; 2. misgsz18/Df; 3. Lam14/CyO; 4. LamP; 5. Lam14/LamP; 6. Lam9; 7. Lam14 | CNS, imaginal disks, gonads, digestive tract, pupae, third-instar larvae | 1. Lamin Dm absent in the CNS at L3. Overall, ~76% survival to the late pupal stage; ~1% reached adulthood. 2. No lamin Dm expression in CNS at L3; late pupal survival 77%; adult emergence ~3%. 3. Normal lamin Dm expression, fertility, and 100% adult emergence. 4. Lamin Dm expressed in larval brain and imaginal disks. In total, 89% survived to late pupal stage; 65% reached adulthood. All adults were sterile. 5. Lamin Dm absent in CNS and imaginal disks at L3; 72% survived to pupation; 45% became adults; and 45% of the adults were sterile. 6. Complete absence of lamin Dm in L3 CNS and imaginal disks. Overall, 59% pupal survival; no adults emerged. 7. Lamin Dm detectable in the CNS at L2 but undetectable at L3. Defective gonad formation, delayed CNS development, proliferative abnormalities in the larval midgut. EcRB1 levels reduced. Approximately 63% reached the pupal stage; no adult flies emerged. |
| [108] | Chen et al., 2014 Gal4-UAS knockdown Cg-Gal4/+; tub-Gal80ts/Lam RNAi | Fat body, midgut | Age-related lamin B depletion in the fat body: transcriptional dysregulation, upregulation of over 100 immune-related genes, systemic inflammation observed, IMD pathway hyperactivation, progressive midgut hyperplasia, indicating a loss of heterochromatin-mediated gene silencing, and impaired tissue homeostasis. |
| [109] | Dopie, 2015 RNAi-mediated knockdown of lamin Dm, Nup98, and exportin 6 | Cultured S2 cells, male meiotic cells | Lamin Dm depletion in cultured cells: disrupted nuclear actin organization lead to altered localization of actin and dysregulation of cofilin regulators. In the male germline, concurrent knockdown of lamin Dm and Nup107 impaired cytokinesis and mislocalization of nuclear lamins,. |
| [103] | Hayashi et al., 2016 Gal4-UAS; lamin Dm and Nup107 knockdown | Neural stem cells, larval neuroblasts | Simultaneous depletion of lamin Dm and components of the Nup107–160 complex compromised the integrity of the spindle envelope during mitosis. This disruption led to misalignment of chromosomes, spindle pole defects, and impaired mitotic progression, suggesting a cooperative role of lamin and NPCs in mitotic architecture. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, A.; Rowińska, M.; Tomczak, A.; Rzepecki, R. Drosophila as a Model for Studying the Roles of Lamins in Normal Tissues and Laminopathies. Cells 2025, 14, 1303. https://doi.org/10.3390/cells14171303
Zielińska A, Rowińska M, Tomczak A, Rzepecki R. Drosophila as a Model for Studying the Roles of Lamins in Normal Tissues and Laminopathies. Cells. 2025; 14(17):1303. https://doi.org/10.3390/cells14171303
Chicago/Turabian StyleZielińska, Aleksandra, Marta Rowińska, Aleksandra Tomczak, and Ryszard Rzepecki. 2025. "Drosophila as a Model for Studying the Roles of Lamins in Normal Tissues and Laminopathies" Cells 14, no. 17: 1303. https://doi.org/10.3390/cells14171303
APA StyleZielińska, A., Rowińska, M., Tomczak, A., & Rzepecki, R. (2025). Drosophila as a Model for Studying the Roles of Lamins in Normal Tissues and Laminopathies. Cells, 14(17), 1303. https://doi.org/10.3390/cells14171303





