Abstract
Although the calcium-dependent proteases, calpains, were discovered more than 60 years ago, we still know very little regarding their functions, mostly because very few studies are addressing questions related to specific members of this relatively large family of cysteine proteases. The “classical calpains”, calpain-1 and calpain-2, are ubiquitous and have received more attention because of the special roles they play in the brain. The authors have been studying the properties and functions of these two calpain isoforms in the brain for over 45 years, and this review will focus on what has been learned over this period of time. In particular, we will discuss the numerous studies that have led to the notion that calpain-1 and calpain-2 play opposite functions in the brain on processes ranging from neuronal survival or death, synaptic plasticity, and learning and memory to neurogenesis. Mechanisms underlying these opposite functions are starting to be understood and the findings support the notion that such opposite functions might be a general feature of these two isoforms in any type of cell. This review concludes with a discussion of the potential benefits of selective calpain-2 inhibitors for the treatment of a variety of neurological disorders.
1. Introduction
Much has been learned over the last 45 years regarding the properties and functions of various members of the family of the calcium-dependent proteases, calpains. These enzymes are found in all living organisms, reflecting their ancient evolutionary origins and essential roles in nearly all aspects of cellular biology. Since the discovery of calpains in 1964, these calcium-dependent, soluble cysteine proteases have been recognized as critical regulators of many cellular functions. Among them, calpain-1 and calpain-2—which are present in the brain—have emerged as key regulators of synaptic plasticity, neuronal survival and neurodegeneration, and neurogenesis. Initial biochemical characterizations in the late 1970s and early 1980s established that calpain-1 (also known as μ-calpain) and calpain-2 (also known as m-calpain) differ in their calcium requirements for activation, with calpain-1 responding to micromolar and calpain-2 to millimolar calcium concentrations. By the late 1980s, several studies demonstrated that calpains play a crucial role in cytoskeletal remodeling, particularly through the proteolysis of spectrin and other structural proteins, implicating them in activity-dependent synaptic reorganization.
The 1990s marked a turning point in our understanding of the functions of calpains in neurodegeneration as it was shown that prolonged calpain activation followed either seizure activity or traumatic brain injury (TBI), resulting in axonal and neuronal damage. The early 2000s brought significant advances in identifying specific substrates of calpain-1 and calpain-2, including two major types of ionotropic glutamate receptors—AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA (N-methyl-D-aspartate)—as well as several associated proteins that regulate their trafficking and insertion into postsynaptic membranes, directly linking calpain activity to learning and memory processes. Over the last 15 years, a number of studies have shown that calpain-1 activation is essential for long-term potentiation (LTP) of synaptic transmission by facilitating synaptic strengthening through proteolytic modification of key signaling proteins, while calpain-2 activation has an opposite function, reducing LTP magnitude and contributing to long-term depression (LTD) and synaptic weakening. Moreover, calpain-1 activation has been shown to be neuroprotective, while calpain-2 activation is directly associated with axonal degeneration and neuronal death. More recent studies have revealed that dysregulation of these proteases is a major contributor to neurodegenerative diseases, including cerebellar ataxia, Alzheimer’s, Huntington’s, and Parkinson’s diseases, as well as TBIs.
This review will explore the biochemical properties, activation mechanisms, and physiological roles of calpain-1 and calpain-2, focusing on key discoveries spanning five decades of research in the field. By examining the contributions of these two calpain isoforms to synaptic plasticity, learning and memory, and neurodegeneration, we aim to provide a comprehensive understanding of their distinct and opposite functions as well as their potential as therapeutic targets for neurological disorders.
2. The Early Period, 1978–1985: Focus on Synaptic Plasticity
The first report of the existence of a neutral, calcium-activated protease in rat brain was published in 1964 [1]. Surprisingly, it took another 16 years before two studies were published demonstrating the presence of calpain in muscles and identifying its endogenous inhibitor, calpastatin [2,3]. The following years saw an explosion of reports on the purification and properties of two forms of calpain with different requirements for activation by calcium. These were found in different organs, such as brain, muscle, liver, lens, and retina, as well as in different species, including rat, pig, and rabbit. Based on their calcium requirements, the two isoforms were originally named μ-calpain (now referred to as calpain-1), which is activated by micromolar concentrations of calcium, and m-calpain (now calpain-2), which requires millimolar calcium concentrations for activation. These two isoforms are heteromeric proteins composed of large homologous catalytic subunits of about 80 kDa and a shared small regulatory subunit (now known as calpain-4) of about 30 kDa. A high-molecular-weight endogenous inhibitor was also identified and named calpastatin—from calpain and statin, a suffix commonly used to indicate an inhibitor—with a molecular weight of 120 kDa. It was also proposed that during evolution, calpain originated from the fusion of genes coding for a thiol protease and a calcium-binding protein [4].
A number of substrates were also identified, including glial fibrillary acidic protein (GFAP), myelin, neurofilament protein, and, in the brain, a cytoskeletal protein named fodrin [5]. It was then realized that brain fodrin was identical to erythrocyte α-spectrin [6]. Interestingly, calpain activation results in partial truncation rather than complete degradation of all the substrate proteins, which strongly suggests that the function of this type of proteases is not simply degradative but is in fact a post-translational regulatory mechanism [7,8,9].
Our laboratory started to work on calpain in the late 1970s following the finding that glutamate receptors could be regulated by calpain. These studies showed that incubating synaptic membranes in the presence of calcium resulted in an increase in 3H-glutamate binding to its receptors [10]. Interestingly, the effect of calcium could be observed when membranes were first incubated with calcium, followed by calcium wash-out prior to the binding assay, indicating that the calcium treatment had resulted in an irreversible change in membrane properties. While several calcium-dependent processes could have been involved, the report by the Murachi laboratory of the presence of calpain in the brain led to the investigation of the possible role of calpain in this process. In a series of studies, the effect of calcium on 3H-glutamate binding in rat hippocampal membranes was found to be the result of calpain activation [11,12,13]. It was also determined that calpain-1 was present in hippocampal membranes [14] and brain fodrin (spectrin) was identified as a calpain substrate [5], confirming the results previously found in erythrocyte membranes [15].
Parallel studies in the laboratory of Gary Lynch indicated that the induction of LTP of synaptic transmission elicited by brief trains of high-frequency stimulation at hippocampal synapses was critically dependent on an influx of calcium [16]. All these results led to the hypothesis that calpain activation plays a critical role in synaptic plasticity and in learning and memory [17]. This hypothesis was further supported by the finding that a thiol protease inhibitor, leupeptin, could prevent olfactory discrimination learning in rats [18].
Interestingly, during this period, a report indicated that calpain-2 could be co-purified with the cAMP-dependent protein kinase (PKA), which can phosphorylate calpain-2 as well as other substrates [19]. The significance of this finding would not be realized until almost 30 years later (see below). Similarly, the calcium dependency for calpain-2 activation was found to be dramatically reduced in the presence of certain types of phospholipids, in particular phosphatidylinositol [20,21]. Furthermore, this effect was specific for the autolysis of calpain-2, resulting in a prolonged calpain-2 activation. The findings from this period were extensively reviewed in Zimmerman and Schlaepfer [22], which stressed the signature of this class of proteases, that is, the partial proteolytic processing of their substrates, thus establishing calpains as a unique post-translational regulatory mechanism.
3. Calpain and Neurodegeneration: 1985–1994
In the mid-1980s, the focus of calpain research in the brain turned to neurodegeneration. This was the result of a number of key findings, which raised the possibility that calpain activation was not limited to the regulation of physiological processes but could play a critical role in neurodegeneration. First, calpain-1 expression was found to be prominent in several structures in which degeneration occurs spontaneously, e.g., in spinal motoneurons and olfactory neurons. In addition, calpain was found in regions susceptible to age-related pathologies, including cerebellar Purkinje cells, substantia nigra, and subiculum [23]. This distribution suggested that calpain-1 could be involved in both normal and pathological neuronal death. Second, calpain activation was found to be related to excitotoxicity-induced neuronal damage [24,25], or following lesion-induced neurodegeneration in the dentate gyrus [26,27]. Third, calpain inhibitors were found to be neuroprotective following ischemic episodes in both in vitro and in vivo experiments [28,29,30,31,32]. Fourth, several evidence linked calpain and Alzheimer’s disease (AD). Calpain was found to be localized in senile plaques and in neurons exhibiting neurofibrillary degeneration in AD [33,34]. Calpain was also found to be involved in the proteolysis of the ß-amyloid precursor protein (APP) [35]. The widespread activation of calpain in the brain of patients with AD led to the proposal that calpain represented a molecular mechanism for neurodegeneration in the disease [36]. All these findings led Gary Lynch, Carol Cotman, and Ralph Bradshaw from UC Irvine to create a biopharmaceutical company, Cortex Pharmaceuticals (Cortex), to develop calpain inhibitors for the treatment of neurodegeneration. Cortex did develop several novel calpain inhibitors [37], which were found to protect neurons following focal ischemia [38] and attenuate motor and cognitive deficits in a rat model of TBI [39]. These findings supported the notion that calpain was a novel target to treat acute neurodegenerative disorders [40]. However, Cortex did not further pursue the clinical development of calpain inhibitors for the treatment of neurological disorders for reasons that only became clear about 15 years later (see below).
Nevertheless, parallel findings continued to support a critical role for calpain activation in synaptic plasticity and learning and memory. Thus, hippocampal LTP was reduced by calpain inhibitors both in vitro and in vivo [41,42,43,44]. Olfactory discrimination learning was blocked by a calpain inhibitor [18].
These multiple functions of calpain provided a confusing picture regarding the underlying mechanisms and, in particular, the processes involved in calpain activation under physiological or pathological conditions. While it was tempting to use the different calcium sensitivities of calpain-1 and calpain-2 to account for the selective activation of calpain-1 and calpain-2 in these conditions [17], there was no convincing evidence to support this idea. Increasing the confusion regarding these different functions were the findings related to changes in calpain activity with aging. Thus, calpain activity was found to increase with age [45], and a comparative study of brain calpain activity suggested that it could be an important element regulating longevity in mammalian species, when it was observed that brain calpain activity was highly negatively correlated with maximal lifespan across various species [46,47].
4. Calpain and Glutamate Receptors: 1995–2012
The initial identification of calpain substrates focused on cytoskeletal proteins, including spectrin [5], neurofilament proteins [15], tubulin and microtubule-associated proteins (MAPs) [48], as well as desmin and vimentin [49]. Additional substrates include various protein kinases, such as calcium/calmodulin-dependent protein kinase IV (CaM KIV) [50] and CaMKII [51], protein kinase C [52], glycogen synthase kinase 3 (GSK-3) [53], as well as various phosphatases [54,55]. AMPA receptors were then found to be cleaved by calpain in adult rat brain in their C-terminal domains [56,57] and, in particular, the GluA1 subunits of AMPA receptors [58]. Furthermore, NMDA receptors were regulated by calpain-mediated proteolysis, a mechanism that was extensively studied by the group of David Lynch [59,60,61,62,63,64]. In addition to partially proteolyzing glutamate receptors, calpain was found to degrade several postsynaptic proteins involved in anchoring and stabilizing glutamate receptors in postsynaptic densities. These included PSD-95 (postsynaptic density protein 95) [65], glutamate-receptor-interacting protein (GRIP) [66], and stargazin [67]. Interestingly, the state of phosphorylation of glutamate receptors was found to be an important regulator of calpain-mediated truncation of the receptors. Thus, phosphorylation of AMPA and NMDA receptors either reduced [60] or enhanced their susceptibility to calpain cleavage [68], while fyn-mediated phosphorylation protected AMPA receptors from calpain cleavage but enhanced calpain-mediated cleavage of NMDA receptors [69]. In contrast, src-mediated phosphorylation reduced NMDA receptor cleavage and did not affect AMPA receptor cleavage [69]. Such complex interactions between the phosphorylation states of calpain targets have now been reported for tau [70], the activator of the cyclin-dependent kinase 5 (cdk5), p35 [71], spectrin [72], the transcription factor C/EBP [73], and GSK3ß [74]. This should not be too surprising since calpain interactions with its substrates rely on their 3D structures, which are clearly sensitive to their state of phosphorylation.
In addition to cleaving ionotropic glutamate receptors, calpain was found to cleave the metabotropic glutamate receptor, mGluR1α, in its C-terminal domain [75]. In this particular case, the resulting effect of the cleavage was quite dramatic as it switched the function of mGluR1α from being neuroprotective to neurodegenerative. This step was found to be a key step in excitotoxicity [76]. These findings led to the development of a small cell-permeable decoy peptide that could prevent calpain-mediated truncation of the receptors and be neuroprotective under ischemic or excitotoxic conditions [76].
These complex interactions between phosphorylation mechanisms and calpain-mediated proteolysis are proposed to play important roles in a number of physiological processes, such as synaptic plasticity [77], cell migration [78], and the remodeling of cytoskeletal anchorage complexes [79]. They are also proposed to participate in several pathological processes, including neurodegeneration [80]—especially AD [81]—cerebral vasospasm [82], and muscle dysfunction [83].
During this period, molecular cloning spearheaded by the group of Hiroyuki Sorumachi led to the identification of the calpain family, which consists of 16 members encoded by 16 different genes, CAPN1-16, although CAPN4 is now designated as CAPNS1 [84,85,86,87]. Based on the organization of different domains of the family members, calpains have been classified as classical and non-classical calpains. While some of the members are tissue-specific—such as calpain-3, which is only expressed in skeletal muscles—many are ubiquitously expressed [88]. Structural studies have helped in understanding the mechanism of activation by calcium, as well as the fact that these enzymes prefer to proteolyze unstructured regions of their target proteins due to the steric constraints of their catalytic sites. In particular, the group of Peter Davis reported the mechanism of calpain activation following the binding of 10 calcium ions to calpain, resulting in a conformational change and the opening of the catalytic site between the PC1 and PC2 domains of calpain (Figure 1) ([89], see also https://www.igakuken.or.jp/calpain/MainPages/ResearchTheme.html (accessed on 14 August 2025)). The catalytic site consists of a ubiquitous triad of amino acids (Cys105/His262/Asn286), responsible for the cysteine protease designation of calpains.
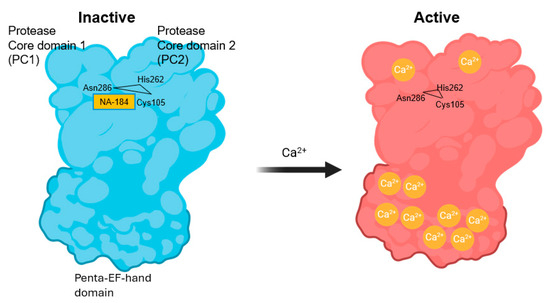
Figure 1.
Schematic representation of the activation of calpain by calcium. In the absence of calcium, calpain-1/2 is in an inactive state and the catalytic triad located between Protease Core domain 1 (PC1) and Protease Core domain 2 (PC2), Cys105/His262/Asn286, is not functional (left); this panel also shows the binding sites of NA184. Upon binding of 10 calcium ions to various sites of the Penta–EF–hand domains and PC1 and PC2 domains, the catalytic triad becomes functional (right).
It is also interesting to note that other members of the calpain family were linked to specific diseases; thus, loss-of-function mutations in calpain-3 were shown to be responsible for limb-girdle muscular dystrophy 2A [90,91]. Down-regulation of calpain-9 has been linked to gastric cancer [92].
Increasing evidence has linked calpain-1 and calpain-2 to stroke, TBI, and AD [36,93,94,95,96,97], spinal cord injury [98], Parkinson’s disease (PD) [99], and global neurodegeneration [100].
5. Opposite Functions of Calpain-1 and Calpain-2 in the Brain: 2012–2025
The high calcium requirement for activation of both calpain-1 and calpain-2 has long been a puzzle for scientists working in this field since it exceeds by far the resting levels of intracellular calcium, and even those likely to be present following cellular activation. It has been widely accepted that this requirement ensures that calpain activation could take place only near sources of calcium influx from extracellular or intracellular stores, resulting in a temporally and spatially limited stimulation of this potentially damaging proteolytic system. Importantly, phosphorylation of various residues in calpain-1 and calpain-2 was found to regulate their activity. Mitogen-activated protein kinase (MAP Kinase)-mediated phosphorylation of serine 50 was found to activate both calpains [101,102], while PKA-mediated phosphorylation of serine 369—which, as mentioned earlier, had been reported in 1985—resulted in calpain-2 inhibition [103,104]. While the mechanisms underlying these effects are not clearly understood, it is assumed that these phosphorylation events modify the interactions of calpain-1 and calpain-2 with cell membranes or other proteins.
In cultured hippocampal neurons, the brain-derived nerve growth factor (BDNF) stimulated calpain-2 but not calpain-1, an effect that was blocked by MAPK inhibitors. BDNF-induced calpain-2 activation was preferentially localized in dendrites and dendritic spines of hippocampal neurons and was associated with actin polymerization, which was prevented by calpain inhibition [102]. BDNF has been shown to stimulate local dendritic protein synthesis [105], and calpain-2-mediated PTEN (phosphatase and tensin homolog) truncation was found to be involved in BDNF-mediated stimulation of this local protein synthesis [106]. Conditional deletion of CAPNS1 in the brain, which disrupts both calpain-1 and calpain-2 activity, resulted in altered dendritic morphology and LTP impairment in hippocampus, thus confirming a critical role for calpain in LTP [107]. Activation of synaptic NMDA receptors has been shown to be neuroprotective by stimulating the Akt survival pathway, while activation of extrasynaptic NMDA receptors is linked to a neurodegenerative pathway [108,109]. In cultured hippocampal neurons, activation of synaptic NMDA receptors resulted in the stimulation of calpain-1 and the cleavage of two splice variants of PH domain and Leucine-rich repeat Protein Phosphatase 1 (PHLPP1)—PHLPP1α and PHLPP1β—which inhibit the Akt pathway [110]. In contrast, extrasynaptic NMDAR activation had no effect on PHLPP1 and the Akt pathways but resulted in calpain-2-mediated degradation of striatal-enriched protein tyrosine phosphatase (STEP) and neuronal death [110]. This finding provided the first evidence that calpain-1 and calpain-2 play opposite functions in the brain. The suprachiasmatic nucleus circadian oscillatory protein (SCOP)—also known as PHLPP1β, a negative regulator of the extracellular signal-regulated kinase (ERK)—has been shown to play an important role in learning and memory as its overexpression blocks long-term memory encoding [111]. Calpain-1-mediated SCOP degradation was found to be involved in LTP induction, while calpain-2-mediated stimulation of mTOR (mechanistic target of rapamycin)-dependent SCOP synthesis restricted the magnitude of potentiation during early consolidation [112]. Thus, opposite effects of calpain-1 and calpain-2 control the formation and extent of LTP in hippocampus. These results were confirmed by finding that LTP induction was absent in calpain-1 knock-out (ko) mice [113], while LTP magnitude was enhanced by a selective calpain-2 inhibitor [112]. The neuroprotective properties of calpain-1 were also further supported by studies in mice, dogs, and humans with null mutations or deletions of calpain-1 [114,115], which result in cerebellar ataxia. Detailed studies in mice revealed that a lack of calpain-1 was associated with increased apoptosis throughout the brain during the early postnatal period [115]. As there are no selective calpain-1 inhibitors, our understanding of the functions of calpain-1 is limited to the findings obtained by studies with the calpain-1 ko mice, which could be biased due to the long-term changes in the expression of a number of genes affected by calpain-1 deletion [116]. Conversely, the neurodegenerative properties of calpain-2 were confirmed by a wide range of studies, demonstrating the critical role of calpain-2 in several animal models of neurodegeneration, including acute glaucoma [117], TBI [118], repeated mild concussion [119], and seizure-induced neuropathology [120].
All the data are, therefore, consistent with the notion that, in the brain at least, calpain-1 and calpain-2 play opposite functions in synaptic plasticity and learning and memory as well as in neuroprotection/neurodegeneration [121,122]. Obviously, a critical question concerns the potential mechanism(s) that could account for these opposing functions. One of the mechanisms we identified is related to the different targets for calpain-1 and calpain-2. Thus, calpain-1 activation results in the cleavage and inactivation of SCOP, a negative regulator of ERK, and PI3-Akt, a pro-survival kinase [112,123]. On the other hand, calpain-2 degrades and inactivates STEP, resulting in the activation of p38 and downstream cell death signaling pathways [110,124,125]. Another mechanism we identified was provided by the existence of different (PSD-95/Dlg/ZO-1) (PDZ) binding domains in the C-terminal domains of calpain-1 and calpain-2 [121]. We postulated that these domains result in the association of calpain-1 and calpain-2 with different signaling pathways—involved in neuroprotection and synaptic plasticity for calpain-1, and in opposing synaptic plasticity and stimulating neuronal damage for calpain-2 [88,122,126] (Figure 2). Calpain-1 is likely to be associated with PSD-95, which also binds the NMDA receptors, as well as neuronal nitric oxide synthase (nNOS) and PHLPP1 [127] (Figure 2A). Interestingly, all the members of the calpain family exhibit a PDZ binding domain in their C-terminal domains, which suggests that they are all associated with distinct signaling pathways [88]. In searching for a PDZ binding partner for calpain-2, we identified tyrosine phosphatase, PTPN13—also known as Fas-Associated Protein 1 (FAP1)—a negative regulator of apoptosis [128]. Importantly, PTPN13 is selectively cleaved and inactivated following calpain-2 activation, resulting in the activation of downstream signaling pathways, including apoptosis and various protein kinases, such as c-Abl (Figure 2B). This pathway could account for the abnormal tau phosphorylation observed following TBI [128]. Moreover, a fragment of PTPN13 with a molecular weight of about 130 kD leaks into the blood and its levels were found to be correlated with the degree of injury following TBIs in both mice and humans [129]. Interestingly, PTPN13 has five PDZ binding domains and also binds PIP2 and PTEN in addition to calpain-2 [130,131,132] (Figure 2B). This could account for the activation of calpain-2 following stimulation of extrasynaptic NMDA receptors—resulting in the truncation of PTEN and PTPN13—as well as in the autolysis of calpain-2—resulting in the prolonged activation of calpain-2—which has been shown following TBI or status epilepticus [118,120].
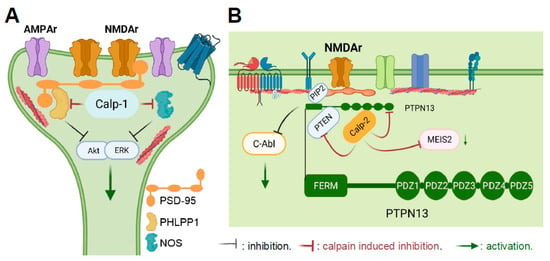
Figure 2.
Different subcellular localizations and key actions of calpain-1 and calpain-2 in neurons. (A) Subcellular localization of calpain-1. Calpain-1 is downstream of synaptic NMDA receptors and is in a complex of proteins linked to PSD-95, which includes PHLPP1 and NOS. By cleaving PHLPP1, calpain-1 activation results in ERK and Akt activation, which are both involved in synaptic plasticity and in neuronal survival. (B) Subcellular localization and major actions of calpain-2. Calpain-2 is downstream of extrasynaptic NMDA receptors and is in a complex of proteins linked to PTPN13, which includes PTEN. Calpain-2 activation results in the cleavage of PTEN and of PTPN13, leading to stimulation of dendritic protein synthesis and c-Abl activation. This pathway is involved in both synaptic plasticity and in neurodegeneration. Calpain-2 also cleaves MEIS2, leading to reduced neurogenesis.
Many studies have shown that calpains participate in neurogenesis in adult mammalian brain [133,134,135,136,137]. As mentioned above, calpains play an important role in cell proliferation, survival, migration, and differentiation [135,137,138,139,140,141,142,143,144,145,146]. Participation of calpains in these processes is the result of their interactions with and cleavage of proteins involved in cell cycle regulation [142,144,147]—in particular cdk5 [148], which requires the binding of its regulatory subunit, p35. Calpain cleaves p35 into p25, which increases cdk5 activity and changes its substrate selectivity and subcellular localization. For a long time, the contributions of calpain-1 and calpain-2 in neurogenesis have been difficult to evaluate due to the lack of selective inhibitor or of mutant mice with selective down-regulation of calpain-1 or calpain-2. Calpain-1 is highly expressed in neural stem cells (NSCs) and immature neural progenitor cells (NPCs), while calpain-2 expression increases during neuronal differentiation [133,136,149], suggesting that calpain-1 and calpain-2 play different roles in neurogenesis. Using calpain-1 ko mice, we found that the proliferation of newborn cells was decreased in the dentate gyrus [150]. This decrease was comparable to that found in the dentate gyrus of the CAPNS1-ko mice [107,133]. These results suggest that calpain-1 plays a positive role in neurogenesis. In a very recent study, the transcription factor Myeloid Ecotropic Viral Integration Site 2 (MEIS2) was found to be a target of calpain-2 but not of calpain-1. Acute treatment of mice with a selective calpain-2 inhibitor, NA-184, resulted in increased MEIS2 levels in various brain regions, including the subventricular zone and the dentate gyrus, leading to an increase in neurogenesis [151]. Therefore, calpain-2 appears to be a negative regulator of neurogenesis. These findings further underscore the opposite functions of calpain-1 and calpain-2 in the brain.
6. Development of Selective Calpain-2 Inhibitors for the Treatment of Neurodegenerative Disorders
Calpains have been implicated in many disorders since their initial discovery. This topic has been covered by many interesting reviews throughout the years, and only a few are cited here due to space limitations [152,153,154,155,156,157]. In particular, the classical calpains have long been implicated in neurodegeneration broadly [158,159] and, more specifically, in stroke [160,161] and TBI [162,163]. Accordingly, numerous studies have tested calpain inhibitors to attenuate neurodegeneration in both stroke and TBI [163,164]. Some first-generation inhibitors showed promising effects in TBI models [165], though others failed to demonstrate efficacy [166,167]. As discussed previously, some of the inhibitors generated by Cortex Pharmaceuticals were also tested in animal models of neurodegeneration [38]. For example, administration of the keto-amide inhibitor, AK295, 15 min post-lateral fluid percussion injury reduced cognitive and motor impairments [39] but did not affect neuronal damage or calpain-mediated spectrin degradation [168]. Similarly, pre-injury administration of the non-selective calpain inhibitor, MDL-28710, mitigated short-term diffuse axonal injury in a rat impact acceleration model [169] and provided neuroprotection in the corpus callosum following fluid percussion injury. Notably, neuroprotection was limited to a 4-h window, though repeated dosing extended efficacy [170]. However, MDL-28710 did not prevent axonal transport deficits after stretch injury [171]. Even more advanced, BBB-permeable inhibitors, such as SNJ-1945 and MDL-28170, failed to provide sufficient efficacy or a clinically relevant therapeutic window in controlled cortical impact (CCI) models [165,166].
These outcomes may reflect limitations in specificity, potency, and isoform selectivity [172], as well as an incomplete understanding of the differential roles of calpain isoforms in the brain. As discussed above, calpain-1 and calpain-2 have now been found to exhibit opposing functions in synaptic plasticity and neurodegeneration [121]. Specifically, calpain-1 is required for theta-burst-induced LTP and is neuroprotective, while calpain-2 restricts LTP and promotes neurodegeneration [110,111,112]. The non-selectivity of earlier inhibitors likely masked these divergent effects and hindered their therapeutic development. Therefore, it is the balance between calpain-1 and calpain-2 activity that is responsible for many critical processes regulating cellular homeostasis in neurons and other cell types (Figure 3).
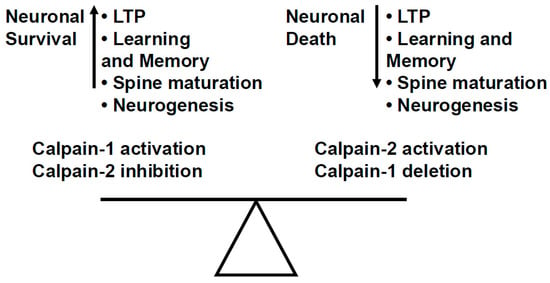
Figure 3.
The balance between calpain-1 and calpain-2 activity plays a critical role in neuronal homeostasis. Calpain-1 activation and calpain-2 inhibition result in enhanced LTP and learning and memory, enhanced dendritic spine maturation, neuronal survival, and enhanced neurogenesis. On the other hand, calpain-2 activation and calpain-1 deletion/inhibition result in opposite functions, i.e., neuronal death, decreased LTP and learning and memory, inhibited spine maturation, and inhibited neurogenesis.
These findings imply that selective calpain-2 inhibitors could be extremely beneficial in a number of both acute and chronic neurological disorders, including TBI and ischemic damage, as well as AD and PD [173,174].
Although the first selective calpain-2 inhibitor we identified, NA-101 (Figure 4), was found to selectively bind to the catalytic triad [175] and exhibited favorable pharmacological characteristics, it was not suitable for clinical advancement. OSIRIS Data Warrior analysis yielded a drug-likeness score of -13.6, well below the threshold for typical drug candidates. Furthermore, NA-101 displayed a non-monotonic (inverted U-shaped) dose–response curve in CCI models, where low doses were neuroprotective and high doses worsened injury [118].
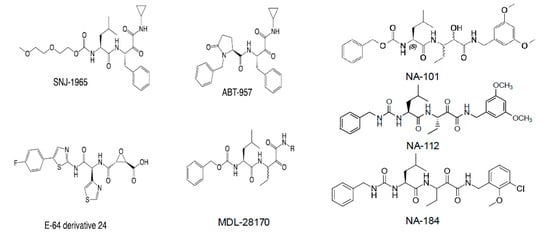
Figure 4.
Structures of different calpain inhibitors. While SNJ-1965, ABT-957, E-64 derivative 24, and MDL-28170 are not selective for calpain-1 or calpain-2, NA-101, NA-112, and NA-184 are selective for calpain-2 over calpain-1. Note that ABT-957 was tested in a Phase I clinical trial for Alzheimer’s disease. While no significant side-effects were observed, AbbVie decided not to pursue the clinical development due to the lack of observable pharmacological effects at the doses tested [176].
Consequently, we initiated a medicinal chemistry campaign to develop improved analogs. NA-101 shares a scaffold with other calpain inhibitors, including those in clinical development (e.g., ABT-957 by AbbVie; SNJ-1945 by Senju), as well as with clinically tested ketoamide serine protease inhibitors such as telaprevir and boceprevir (Figure 4). NA-101 is a peptidyl α-ketoamide that forms a reversible covalent bond with the calpain catalytic triad shown in Figure 1. Among reversible covalent calpain inhibitors, α-ketoamides offer particularly stable electrophilic warheads [177,178], making them promising leads.
Molecular dynamics simulations revealed that NA-101 adopts different binding poses in calpain-1 and calpain-2 due to isoform-specific differences in domain 3. Notably, calpain-1 lacks the hydrogen bond interaction between catalytic histidine and the P1′ methoxy group seen in calpain-2 [175]. This suggested that isoform selectivity could be improved through targeted substitutions at the P3 and P1′ positions. To guide structure-based optimization, we employed SILCS (Site Identification by Ligand Competitive Saturation) simulations [179], which generate fragment probability maps (FragMaps) of protein surfaces. At the P1′ site, calpain-1 favored substitutions at the para-position of the phenyl ring, while calpain-2 favored meta-position substitutions and accommodated additional H-bond donors/acceptors at the ortho-position.
Using this information, we synthesized ~130 analogs and screened them for selectivity toward human calpain-1 and calpain-2. Two compounds, NA-112 and NA-184, emerged as lead candidates based on their low Ki for calpain-2 and excellent isoform selectivity (Figure 4). Substitutions at the P3 site did not improve selectivity, likely due to its conformational flexibility. However, replacing the carbamate linker with a urea linker at this site enhanced both binding stability and metabolic resistance.
Importantly, both NA-112 and NA-184 cross the blood–brain barrier (BBB) and achieve >20-fold selectivity for calpain-2 over calpain-1 in vivo. In CCI models of TBI, both compounds provided robust neuroprotection and reduced circulating levels of the calpain-2 biomarker P13BP at 24 h post-injury [180].
NA-184 is a potent in vivo inhibitor of mouse calpain-2 with an IC50 of 130 nM and an ED50 of 0.13 mg/kg for neuroprotection in CCI models, with no calpain-1 inhibition up to 10 mg/kg. Against human calpain-2, NA-184 has an IC50 of 1.3 nM and does not inhibit calpain-1 at concentrations up to 10 µM.
As mentioned above, NA-184 has an additional property that makes it an interesting drug to address age-related neuronal disorders as it stimulates neurogenesis through increased levels of the transcription factor MEIS2 [151]. Stimulating neurogenesis—particularly in the adult hippocampus and subventricular zone—can offer several potential benefits for age-related neuronal disorders such as AD, PD, and age-associated cognitive decline. Hippocampal neurogenesis is closely linked to learning, memory, and pattern separation [181]. Aging and neurodegenerative diseases are associated with reduced neurogenesis, which correlates with cognitive deficits [182]. Stimulating neurogenesis could enhance neuronal survival, replenish neuronal populations, and improve synaptic plasticity, potentially restoring memory and learning abilities. New neurons can integrate into existing neural circuits and may help rebuild damaged networks [183]. This type of plasticity is particularly relevant for diseases like AD, where synaptic loss and network disruption are hallmarks. Moreover, neurogenesis is often accompanied by increased levels of neurotrophic factors such as BDNF and IGF-1 (insulin-like growth factor 1) [184]. These factors promote neuronal survival, reduce inflammation, and support the health of existing neurons—providing a neuroprotective environment. Finally, depression is commonly comorbid with neurodegenerative diseases, and hippocampal neurogenesis is implicated in the antidepressant response [185]. Enhancing neurogenesis might alleviate mood disturbances, improving quality of life and cognitive performance indirectly. Chronic inflammation impairs neurogenesis and contributes to cognitive decline. Stimulating neurogenesis may counteract neuroinflammation, either directly or indirectly by modulating microglial and astrocytic responses [186]. Interestingly, a recent study appears to confirm that neurogenesis is present in the dentate gyrus of adult humans [187], suggesting that stimulating neurogenesis in humans could have broad beneficial effects.
Altogether, these findings strongly support the development of specific calpain-2 inhibitors for the treatment of a variety of neurological disorders. The coming years should provide clinical validation to this hypothesis as clinical trials of NA-184 for TBI treatment are expected to be initiated in 2026.
7. Conclusions and Future Directions
Looking back at the state of knowledge on calpains in the early 1980s, it is clear that much has been learned regarding all the different features of calpain-1 and calpain-2. We know how these enzymes are activated by brief changes in calcium concentrations and/or phosphorylation status, a large number of substrates have been identified, and their participation in many important neuronal functions has been elucidated. A critical feature of these two isoforms is the existence of different PDZ binding domains, resulting in their association with different signaling pathways, which are mostly responsible for their opposite functions. While calpain-1 is required for hippocampal LTP and learning and memory, calpain-2 limits the magnitude of LTP and the extent of learning and memory. Similarly, calpain-1 is neuroprotective and stimulates neurogenesis, while calpain-2 is neurodegenerative and inhibits neurogenesis. These findings have led to the identification of selective calpain-2 inhibitors, which have been found to facilitate learning and memory and limit the extent of neuronal damage in several animal models of acute injury, including glaucoma and mild and severe concussions. They have also been found to stimulate neurogenesis in adult mouse brain. Several of these inhibitors are in the final stage of pre-IND studies and are scheduled to enter clinical trials for the treatment of various neurological disorders. The next few years should provide definitive answers regarding the successful translation of the pre-clinical studies to human therapeutic indications.
8. Limitations
We realize that we might have missed important studies in our literature search, and we apologize to the authors of these studies. It is also the case that we focused on the roles of calpain-1 and calpain-2 in the brain since there is still little information regarding the specific roles of these two calpain isoforms in other tissues and organs. It is our hope that this review will encourage scientists to expand on our studies in order to provide more information on this topic, as well as on the roles of the other members of the calpain family, which are still understudied.
Funding
This research was funded by NIH, grant number NS130825, DoD, grant number W81XWH-19-1-0329. Xiaoning Bi is also supported by an endowed Chair from the Sarkaria family.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors want to acknowledge the enormous contributions of Gary Lynch, in whose lab both of us started to work on the roles of calpains in the brain. They also want to thank Athar Chishti, who generated the calpain-1 knock-out mice, which have been immensely helpful to understand the functions of calpain-1 in the brain, and generously provided them to us. They also want to acknowledge the contributions of all the postdocs, graduate and undergraduate students, and lab assistants who have worked on many aspects of the work presented in this review.
Conflicts of Interest
Michel Baudry and Xiaoning Bi are co-founders of NeurAegis, Inc, a biotech company developing selective calpain-2 inhibitors for the treatment of neurological disorders. The authors declare no conflict of interest.
Abbreviations
| TBI | Traumatic brain injury |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| NMDA | N-methyl-D-aspartate |
| LTP | Long-term potentiation |
| LTD | Long-term depression |
| GFAP | Glial fibrillary acidic protein |
| PKA | cAMP-dependent protein kinase |
| AD | Alzheimer’s disease |
| APP | ß-amyloid precursor protein |
| MAPs | Microtubule-associated proteins |
| CaM KIV | Calcium/calmodulin-dependent protein kinase IV |
| GSK-3ß | Glycogen synthase kinase 3 |
| PSD-95 | Postsynaptic density protein 95 |
| GRIP | Glutamate-receptor-interacting protein |
| Cdk5 | Cyclin-dependent kinase 5 |
| PD | Parkinson’s disease |
| BDNF | Brain-derived nerve growth factor |
| MAP Kinase | Mitogen-activated protein kinase |
| PTEN | Phosphatase and tensin homolog |
| PHLPP1 | PH domain and Leucine-rich repeat Protein Phosphatase 1 |
References
- Guroff, G. A Neutral, Calcium-Activated Proteinase from the Soluble Fraction of Rat Brain. J. Biol. Chem. 1964, 239, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sugita, H.; Ishiura, S.; Suzuki, K.; Imahori, K. Ca-activated neutral protease and its inhibitors: In vitro effect on intact myofibrils. Muscle Nerve 1980, 3, 335–339. [Google Scholar] [CrossRef]
- Murachi, T.; Tanaka, K.; Hatanaka, M.; Murakami, T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv. Enzyme Regul. 1980, 19, 407–424. [Google Scholar] [CrossRef]
- Ohno, S.; Emori, Y.; Imajoh, S.; Kawasaki, H.; Kisaragi, M.; Suzuki, K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature 1984, 312, 566–570. [Google Scholar] [CrossRef]
- Siman, R.; Baudry, M.; Lynch, G. Brain fodrin: Substrate for calpain I, an endogenous calcium-activated protease. Proc. Natl. Acad. Sci. USA 1984, 81, 3572–3576. [Google Scholar] [CrossRef]
- Glenney, J.R., Jr.; Glenney, P. Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell 1983, 34, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Suzuki, K.; Hata, S.; Kawabata, Y.; Sorimachi, H. Structure, activation, and biology of calpain. Diabetes 2004, 53 (Suppl. S1), S12–S18. [Google Scholar] [CrossRef]
- Croall, D.E.; Ersfeld, K. The calpains: Modular designs and functional diversity. Genome Biol. 2007, 8, 218. [Google Scholar] [CrossRef]
- Baudry, M.; Lynch, G. Regulation of glutamate receptors by cations. Nature 1979, 282, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Lynch, G. Regulation of hippocampal glutamate receptors: Evidence for the involvement of a calcium-activated protease. Proc. Natl. Acad. Sci. USA 1980, 77, 2298–2302. [Google Scholar] [CrossRef]
- Baudry, M.; Bundman, M.C.; Smith, E.K.; Lynch, G.S. Micromolar calcium stimulates proteolysis and glutamate binding in rat brain synaptic membranes. Science 1981, 212, 937–938. [Google Scholar] [CrossRef]
- Baudry, M.; Smith, E.; Lynch, G. Influences of temperature, detergents, and enzymes on glutamate receptor binding and its regulation by calcium in rat hippocampal membranes. Mol. Pharmacol. 1981, 20, 280–286. [Google Scholar] [CrossRef]
- Siman, R.; Baudry, M.; Lynch, G. Purification from synaptosomal plasma membranes of calpain I, a thiol protease activated by micromolar calcium concentrations. J. Neurochem. 1983, 41, 950–956. [Google Scholar] [CrossRef]
- Pant, H.C.; Virmani, M.; Gallant, P.E. Calcium-induced proteolysis of spectrin and band 3 protein in rat erythrocyte membranes. Biochem. Biophys. Res. Commun. 1983, 117, 372–377. [Google Scholar] [CrossRef]
- Lynch, G.; Larson, J.; Kelso, S.; Barrionuevo, G.; Schottler, F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 1983, 305, 719–721. [Google Scholar] [CrossRef]
- Lynch, G.; Baudry, M. The biochemistry of memory: A new and specific hypothesis. Science 1984, 224, 1057–1063. [Google Scholar] [CrossRef]
- Staubli, U.; Baudry, M.; Lynch, G. Olfactory discrimination learning is blocked by leupeptin, a thiol protease inhibitor. Brain Res. 1985, 337, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, U.J.; Schlaepfer, W.W. Characterization of calcium-activated neutral protease (CANP)-associated protein kinase from bovine brain and its phosphorylation of neurofilaments. Biochem. Biophys. Res. Commun. 1985, 129, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Coolican, S.A.; Hathaway, D.R. Effect of L-alpha-phosphatidylinositol on a vascular smooth muscle Ca2+-dependent protease. Reduction of the Ca2+ requirement for autolysis. J. Biol. Chem. 1984, 259, 11627–11630. [Google Scholar] [CrossRef] [PubMed]
- Pontremoli, S.; Melloni, E.; Sparatore, B.; Salamino, F.; Michetti, M.; Sacco, O.; Horecker, B.L. Role of phospholipids in the activation of the Ca2+-dependent neutral proteinase of human erythrocytes. Biochem. Biophys. Res. Commun. 1985, 129, 389–395. [Google Scholar] [CrossRef]
- Zimmerman, U.J.; Schlaepfer, W.W. Calcium-activated neutral protease (CANP) in brain and other tissues. Prog. Neurobiol. 1984, 23, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Siman, R.; Gall, C.; Perlmutter, L.S.; Christian, C.; Baudry, M.; Lynch, G. Distribution of calpain I, an enzyme associated with degenerative activity, in rat brain. Brain Res. 1985, 347, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Siman, R.; Noszek, J.C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron 1988, 1, 279–287. [Google Scholar] [CrossRef]
- Siman, R.; Noszek, J.C.; Kegerise, C. Calpain I activation is specifically related to excitatory amino acid induction of hippocampal damage. J. Neurosci. 1989, 9, 1579–1590. [Google Scholar] [CrossRef]
- Seubert, P.; Ivy, G.; Larson, J.; Lee, J.; Shahi, K.; Baudry, M.; Lynch, G. Lesions of entorhinal cortex produce a calpain-mediated degradation of brain spectrin in dentate gyrus. I. Biochemical studies. Brain Res. 1988, 459, 226–232. [Google Scholar] [CrossRef]
- Ivy, G.; Seubert, P.; Lynch, G.; Baudry, M. Lesions of entorhinal cortex produce a calpain-mediated degradation of brain spectrin in dentate gyrus. II. Anatomical studies. Brain Res. 1988, 459, 233–240. [Google Scholar] [CrossRef]
- Inuzuka, T.; Tamura, A.; Sato, S.; Kirino, T.; Toyoshima, I.; Miyatake, T. Suppressive effect of E-64c on ischemic degradation of cerebral proteins following occlusion of the middle cerebral artery in rats. Brain Res. 1990, 526, 177–179. [Google Scholar] [CrossRef]
- Arai, A.; Kessler, M.; Lee, K.; Lynch, G. Calpain inhibitors improve the recovery of synaptic transmission from hypoxia in hippocampal slices. Brain Res. 1990, 532, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, L.; Mehdi, S.; Lee, K.S. Improved posthypoxic recovery with a membrane-permeable calpain inhibitor. Eur. J. Pharmacol. 1991, 209, 123–125. [Google Scholar] [CrossRef]
- Roberts-Lewis, J.M.; Savage, M.J.; Marcy, V.R.; Pinsker, L.R.; Siman, R. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J. Neurosci. 1994, 14, 3934–3944. [Google Scholar] [CrossRef]
- Hong, S.C.; Goto, Y.; Lanzino, G.; Soleau, S.; Kassell, N.F.; Lee, K.S. Neuroprotection with a calpain inhibitor in a model of focal cerebral ischemia. Stroke 1994, 25, 663–669. [Google Scholar] [CrossRef]
- Nilsson, E.; Alafuzoff, I.; Blennow, K.; Blomgren, K.; Hall, C.M.; Janson, I.; Karlsson, I.; Wallin, A.; Gottfries, C.G.; Karlsson, J.O. Calpain and calpastatin in normal and Alzheimer-degenerated human brain tissue. Neurobiol. Aging 1990, 11, 425–431. [Google Scholar] [CrossRef]
- Nixon, R.A.; Saito, K.I.; Grynspan, F.; Griffin, W.R.; Katayama, S.; Honda, T.; Mohan, P.S.; Shea, T.B.; Beermann, M. Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer’s disease. Ann. N. York Acad. Sci. 1994, 747, 77–91. [Google Scholar] [CrossRef]
- Siman, R.; Card, J.P.; Davis, L.G. Proteolytic processing of beta-amyloid precursor by calpain I. J. Neurosci. 1990, 10, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Elce, J.S.; Hamos, J.E.; Nixon, R.A. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: A potential molecular basis for neuronal degeneration. Proc. Natl. Acad. Sci. USA 1993, 90, 2628–2632. [Google Scholar] [CrossRef]
- Li, Z.; Patil, G.S.; Golubski, Z.E.; Hori, H.; Tehrani, K.; Foreman, J.E.; Eveleth, D.D.; Bartus, R.T.; Powers, J.C. Peptide alpha-keto ester, alpha-keto amide, and alpha-keto acid inhibitors of calpains and other cysteine proteases. J. Med. Chem. 1993, 36, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Hayward, N.J.; Elliott, P.J.; Sawyer, S.D.; Baker, K.L.; Dean, R.L.; Akiyama, A.; Straub, J.A.; Harbeson, S.L.; Li, Z.; et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke 1994, 25, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Saatman, K.E.; Murai, H.; Bartus, R.T.; Smith, D.H.; Hayward, N.J.; Perri, B.R.; McIntosh, T.K. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc. Natl. Acad. Sci. USA 1996, 93, 3428–3433. [Google Scholar] [CrossRef]
- Bartus, R.T.; Elliott, P.J.; Hayward, N.J.; Dean, R.L.; Harbeson, S.; Straub, J.A.; Li, Z.; Powers, J.C. Calpain as a novel target for treating acute neurodegenerative disorders. Neurol. Res. 1995, 17, 249–258. [Google Scholar] [CrossRef]
- Oliver, M.W.; Baudry, M.; Lynch, G. The protease inhibitor leupeptin interferes with the development of LTP in hippocampal slices. Brain Res. 1989, 505, 233–238. [Google Scholar] [CrossRef] [PubMed]
- del Cerro, S.; Larson, J.; Oliver, M.W.; Lynch, G. Development of hippocampal long-term potentiation is reduced by recently introduced calpain inhibitors. Brain Res. 1990, 530, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Staubli, U.; Larson, J.; Thibault, O.; Baudry, M.; Lynch, G. Chronic administration of a thiol-proteinase inhibitor blocks long-term potentiation of synaptic responses. Brain Res. 1988, 444, 153–158. [Google Scholar] [CrossRef]
- Denny, J.B.; Polan-Curtain, J.; Ghuman, A.; Wayner, M.J.; Armstrong, D.L. Calpain inhibitors block long-term potentiation. Brain Res. 1990, 534, 317–320. [Google Scholar] [CrossRef]
- Bahr, B.A.; Vanderklish, P.W.; Ha, L.T.; Tin, M.T.; Lynch, G. Spectrin breakdown products increase with age in telencephalon of mouse brain. Neurosci. Lett. 1991, 131, 237–240. [Google Scholar] [CrossRef]
- Baudry, M.; DuBrin, R.; Beasley, L.; Leon, M.; Lynch, G. Low levels of calpain activity in Chiroptera brain: Implications for mechanisms of aging. Neurobiol. Aging 1986, 7, 255–258. [Google Scholar] [CrossRef]
- Baudry, M.; Simonson, L.; Dubrin, R.; Lynch, G. A comparative study of soluble calcium-dependent proteolytic activity in brain. J. Neurobiol. 1986, 17, 15–28. [Google Scholar] [CrossRef]
- Billger, M.; Wallin, M.; Karlsson, J.O. Proteolysis of tubulin and microtubule-associated proteins 1 and 2 by calpain I and II. Difference in sensitivity of assembled and disassembled microtubules. Cell Calcium 1988, 9, 33–44. [Google Scholar] [CrossRef]
- Nelson, W.J.; Traub, P. Proteolysis of vimentin and desmin by the Ca2+-activated proteinase specific for these intermediate filament proteins. Mol. Cell Biol. 1983, 3, 1146–1156. [Google Scholar] [CrossRef]
- McGinnis, K.M.; Whitton, M.M.; Gnegy, M.E.; Wang, K.K. Calcium/calmodulin-dependent protein kinase IV is cleaved by caspase-3 and calpain in SH-SY5Y human neuroblastoma cells undergoing apoptosis. J. Biol. Chem. 1998, 273, 19993–20000. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.S.; de Mattos-Dutra, A.; Wannmacher, C.M.; Pessoa-Pureur, R. Ca(2+)-mediated phosphorylation and proteolysis activity associated with the cytoskeletal fraction from cerebral cortex of rats. Neurochem. Res. 1996, 21, 1489–1495. [Google Scholar] [CrossRef]
- Shea, T.B.; Spencer, M.J.; Beermann, M.L.; Cressman, C.M.; Nixon, R.A. Calcium influx into human neuroblastoma cells induces ALZ-50 immunoreactivity: Involvement of calpain-mediated hydrolysis of protein kinase C. J. Neurochem. 1996, 66, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Goni-Oliver, P.; Lucas, J.J.; Avila, J.; Hernandez, F. N-terminal cleavage of GSK-3 by calpain: A new form of GSK-3 regulation. J. Biol. Chem. 2007, 282, 22406–22413. [Google Scholar] [CrossRef]
- Pain, S.; Monstero-Lastres, A.; Falet, H.; Brohard-Bohn, B.; Fraiz, N.; Bachelot-Loza, C.; Cano, E.; Rendu, F. Calpain controls the balance between protein tyrosine kinase and tyrosine phosphatase activities during platelet activation. FEBS Lett. 1999, 453, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Gil-Henn, H.; Volohonsky, G.; Elson, A. Regulation of protein-tyrosine phosphatases alpha and epsilon by calpain-mediated proteolytic cleavage. J. Biol. Chem. 2001, 276, 31772–31779. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Tocco, G.; Baudry, M. Calpain-mediated regulation of AMPA receptors in adult rat brain. Neuroreport 1994, 6, 61–64. [Google Scholar] [CrossRef]
- Bi, X.; Chang, V.; Molnar, E.; McIlhinney, R.A.; Baudry, M. The C-terminal domain of glutamate receptor subunit 1 is a target for calpain-mediated proteolysis. Neuroscience 1996, 73, 903–906. [Google Scholar] [CrossRef]
- Bi, X.; Chen, J.; Dang, S.; Wenthold, R.J.; Tocco, G.; Baudry, M. Characterization of calpain-mediated proteolysis of GluR1 subunits of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors in rat brain. J. Neurochem. 1997, 68, 1484–1494. [Google Scholar] [CrossRef]
- Bi, X.; Rong, Y.; Chen, J.; Dang, S.; Wang, Z.; Baudry, M. Calpain-mediated regulation of NMDA receptor structure and function. Brain Res. 1998, 790, 245–253. [Google Scholar] [CrossRef]
- Bi, R.; Rong, Y.; Bernard, A.; Khrestchatisky, M.; Baudry, M. Src-mediated tyrosine phosphorylation of NR2 subunits of N-methyl-D-aspartate receptors protects from calpain-mediated truncation of their C-terminal domains. J. Biol. Chem. 2000, 275, 26477–26483. [Google Scholar] [CrossRef]
- Guttmann, R.P.; Baker, D.L.; Seifert, K.M.; Cohen, A.S.; Coulter, D.A.; Lynch, D.R. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J. Neurochem. 2001, 78, 1083–1093. [Google Scholar] [CrossRef]
- Guttmann, R.P.; Sokol, S.; Baker, D.L.; Simpkins, K.L.; Dong, Y.; Lynch, D.R. Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ. J. Pharmacol. Exp. Ther. 2002, 302, 1023–1030. [Google Scholar] [CrossRef]
- Simpkins, K.L.; Guttmann, R.P.; Dong, Y.; Chen, Z.; Sokol, S.; Neumar, R.W.; Lynch, D.R. Selective activation induced cleavage of the NR2B subunit by calpain. J. Neurosci. 2003, 23, 11322–11331. [Google Scholar] [CrossRef]
- Wu, H.Y.; Lynch, D.R. Calpain and synaptic function. Mol. Neurobiol. 2006, 33, 215–236. [Google Scholar] [CrossRef]
- Lu, X.; Rong, Y.; Baudry, M. Calpain-mediated degradation of PSD-95 in developing and adult rat brain. Neurosci. Lett. 2000, 286, 149–153. [Google Scholar] [CrossRef]
- Lu, X.; Wyszynski, M.; Sheng, M.; Baudry, M. Proteolysis of glutamate receptor-interacting protein by calpain in rat brain: Implications for synaptic plasticity. J. Neurochem. 2001, 77, 1553–1560. [Google Scholar] [CrossRef]
- Yu, L.; Rostamiani, K.; Hsu, Y.T.; Wang, Y.; Bi, X.; Baudry, M. Calpain-mediated regulation of stargazin in adult rat brain. Neuroscience 2011, 178, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yuen, E.Y.; Liu, W.; Yan, Z. The phosphorylation state of GluR1 subunits determines the susceptibility of AMPA receptors to calpain cleavage. J. Biol. Chem. 2007, 282, 16434–16440. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Lu, X.; Bernard, A.; Khrestchatisky, M.; Baudry, M. Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J. Neurochem. 2001, 79, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Litersky, J.M.; Scott, C.W.; Johnson, G.V. Phosphorylation, calpain proteolysis and tubulin binding of recombinant human tau isoforms. Brain Res. 1993, 604, 32–40. [Google Scholar] [CrossRef]
- Kamei, H.; Saito, T.; Ozawa, M.; Fujita, Y.; Asada, A.; Bibb, J.A.; Saido, T.C.; Sorimachi, H.; Hisanaga, S. Suppression of calpain-dependent cleavage of the CDK5 activator p35 to p25 by site-specific phosphorylation. J. Biol. Chem. 2007, 282, 1687–1694. [Google Scholar] [CrossRef]
- Nedrelow, J.H.; Cianci, C.D.; Morrow, J.S. c-Src binds alpha II spectrin’s Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176. J. Biol. Chem. 2003, 278, 7735–7741. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, S.F.; Qian, S.W.; Zhang, Y.Y.; Liu, Y.; Tang, Q.Q.; Li, X. Phosphorylation prevents C/EBPbeta from the calpain-dependent degradation. Biochem. Biophys. Res. Commun. 2012, 419, 550–555. [Google Scholar] [CrossRef]
- Ma, S.; Liu, S.; Huang, Q.; Xie, B.; Lai, B.; Wang, C.; Song, B.; Li, M. Site-specific phosphorylation protects glycogen synthase kinase-3beta from calpain-mediated truncation of its N and C termini. J. Biol. Chem. 2012, 287, 22521–22532. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wong, T.P.; Chery, N.; Gaertner, T.; Wang, Y.T.; Baudry, M. Calpain-mediated mGluR1alpha truncation: A key step in excitotoxicity. Neuron 2007, 53, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, M.; Baudry, M. Neuroprotection by cell permeable TA-mGluR1 peptide in ischemia: Synergy between carrier and cargo sequences. Neuroscientist 2008, 14, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Standley, S.; Baudry, M. Posttranslational regulation of ionotropic glutamate receptors and synaptic plasticity. Int. Rev. Neurobiol. 1998, 42, 227–284. [Google Scholar] [CrossRef]
- Franco, S.J.; Huttenlocher, A. Regulating cell migration: Calpains make the cut. J. Cell Sci. 2005, 118, 3829–3838. [Google Scholar] [CrossRef]
- Lebart, M.C.; Benyamin, Y. Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J. 2006, 273, 3415–3426. [Google Scholar] [CrossRef]
- Camins, A.; Verdaguer, E.; Folch, J.; Pallas, M. Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev. 2006, 12, 135–148. [Google Scholar] [CrossRef]
- Mi, K.; Johnson, G.V. The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2006, 3, 449–463. [Google Scholar] [CrossRef]
- Tani, E. Molecular mechanisms involved in development of cerebral vasospasm. Neurosurg. Focus 2002, 12, ECP1. [Google Scholar] [CrossRef] [PubMed]
- Dargelos, E.; Poussard, S.; Brule, C.; Daury, L.; Cottin, P. Calcium-dependent proteolytic system and muscle dysfunctions: A possible role of calpains in sarcopenia. Biochimie 2008, 90, 359–368. [Google Scholar] [CrossRef]
- Sorimachi, H.; Imajoh-Ohmi, S.; Emori, Y.; Kawasaki, H.; Ohno, S.; Minami, Y.; Suzuki, K. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and mu-types. Specific expression of the mRNA in skeletal muscle. J. Biol. Chem. 1989, 264, 20106–20111. [Google Scholar] [CrossRef]
- Sorimachi, H.; Toyama-Sorimachi, N.; Saido, T.C.; Kawasaki, H.; Sugita, H.; Miyasaka, M.; Arahata, K.; Ishiura, S.; Suzuki, K. Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J. Biol. Chem. 1993, 268, 10593–10605. [Google Scholar] [CrossRef]
- Sorimachi, H.; Hata, S.; Ono, Y. Calpain chronicle—An enzyme family under multidisciplinary characterization. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 287–327. [Google Scholar] [CrossRef]
- Ono, Y.; Sorimachi, H. Calpains: An elaborate proteolytic system. Biochim. Biophys. Acta 2012, 1824, 224–236. [Google Scholar] [CrossRef]
- Baudry, M.; Su, W.; Bi, X. The calpain proteolytic system. Encycl. Cell Biol. 2023, 1, 852–864. [Google Scholar]
- Campbell, R.L.; Davies, P.L. Structure-function relationships in calpains. Biochem. J. 2012, 447, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Richard, I.; Broux, O.; Allamand, V.; Fougerousse, F.; Chiannilkulchai, N.; Bourg, N.; Brenguier, L.; Devaud, C.; Pasturaud, P.; Roudaut, C.; et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 1995, 81, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Herasse, M.; Ono, Y.; Fougerousse, F.; Kimura, E.; Stockholm, D.; Beley, C.; Montarras, D.; Pinset, C.; Sorimachi, H.; Suzuki, K.; et al. Expression and functional characteristics of calpain 3 isoforms generated through tissue-specific transcriptional and posttranscriptional events. Mol. Cell Biol. 1999, 19, 4047–4055. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Mukai, H.; Hino, F.; Asada, K.; Kato, I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn. J. Cancer Res. 2000, 91, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Kampfl, A.; Posmantur, R.M.; Zhao, X.; Schmutzhard, E.; Clifton, G.L.; Hayes, R.L. Mechanisms of calpain proteolysis following traumatic brain injury: Implications for pathology and therapy: Implications for pathology and therapy: A review and update. J. Neurotrauma 1997, 14, 121–134. [Google Scholar] [CrossRef]
- Grynspan, F.; Griffin, W.R.; Cataldo, A.; Katayama, S.; Nixon, R.A. Active site-directed antibodies identify calpain II as an early-appearing and pervasive component of neurofibrillary pathology in Alzheimer’s disease. Brain Res. 1997, 763, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Markgraf, C.G.; Velayo, N.L.; Johnson, M.P.; McCarty, D.R.; Medhi, S.; Koehl, J.R.; Chmielewski, P.A.; Linnik, M.D. Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke 1998, 29, 152–158. [Google Scholar] [CrossRef]
- Wang, K.K. Calpain and caspase: Can you tell the difference?, by kevin K.W. Wang. Trends Neurosci. 2000, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A. A “protease activation cascade” in the pathogenesis of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000, 924, 117–131. [Google Scholar] [CrossRef]
- Ray, S.K.; Matzelle, D.D.; Sribnick, E.A.; Guyton, M.K.; Wingrave, J.M.; Banik, N.L. Calpain inhibitor prevented apoptosis and maintained transcription of proteolipid protein and myelin basic protein genes in rat spinal cord injury. J. Chem. Neuroanat. 2003, 26, 119–124. [Google Scholar] [CrossRef]
- Samantaray, S.; Ray, S.K.; Banik, N.L. Calpain as a potential therapeutic target in Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2008, 7, 305–312. [Google Scholar] [CrossRef]
- Ray, S.K.; Banik, N.L. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: Therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr. Drug Targets CNS Neurol. Disord. 2003, 2, 173–189. [Google Scholar] [CrossRef]
- Glading, A.; Bodnar, R.J.; Reynolds, I.J.; Shiraha, H.; Satish, L.; Potter, D.A.; Blair, H.C.; Wells, A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol. Cell Biol. 2004, 24, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Zadran, S.; Jourdi, H.; Rostamiani, K.; Qin, Q.; Bi, X.; Baudry, M. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J. Neurosci. 2010, 30, 1086–1095. [Google Scholar] [CrossRef]
- Shiraha, H.; Glading, A.; Chou, J.; Jia, Z.; Wells, A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol. Cell Biol. 2002, 22, 2716–2727. [Google Scholar] [CrossRef]
- Smith, S.D.; Jia, Z.; Huynh, K.K.; Wells, A.; Elce, J.S. Glutamate substitutions at a PKA consensus site are consistent with inactivation of calpain by phosphorylation. FEBS Lett. 2003, 542, 115–118. [Google Scholar] [CrossRef]
- Tanaka, J.; Horiike, Y.; Matsuzaki, M.; Miyazaki, T.; Ellis-Davies, G.C.; Kasai, H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 2008, 319, 1683–1687. [Google Scholar] [CrossRef]
- Briz, V.; Hsu, Y.T.; Li, Y.; Lee, E.; Bi, X.; Baudry, M. Calpain-2-mediated PTEN degradation contributes to BDNF-induced stimulation of dendritic protein synthesis. J. Neurosci. 2013, 33, 4317–4328. [Google Scholar] [CrossRef]
- Amini, M.; Ma, C.L.; Farazifard, R.; Zhu, G.; Zhang, Y.; Vanderluit, J.; Zoltewicz, J.S.; Hage, F.; Savitt, J.M.; Lagace, D.C.; et al. Conditional disruption of calpain in the CNS alters dendrite morphology, impairs LTP, and promotes neuronal survival following injury. J. Neurosci. 2013, 33, 5773–5784. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Arnold, F.J.; Bading, H. A calcium microdomain near NMDA receptors: On switch for ERK-dependent synapse-to-nucleus communication. Nat. Neurosci. 2001, 4, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Wang, Y.; Briz, V.; Chishti, A.; Bi, X.; Baudry, M. Distinct roles for mu-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J. Neurosci. 2013, 33, 18880–18892. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Phan, T.; Mansuy, I.M.; Storm, D.R. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell 2007, 128, 1219–1229. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Briz, V.; Hsu, Y.T.; Bi, X.; Baudry, M. A molecular brake controls the magnitude of long-term potentiation. Nat. Commun. 2014, 5, 3051. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Wang, Y.; Bi, X.; Baudry, M. Different patterns of electrical activity lead to long-term potentiation by activating different intracellular pathways. J. Neurosci. 2015, 35, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Forman, O.P.; De Risio, L.; Mellersh, C.S. Missense mutation in CAPN1 is associated with spinocerebellar ataxia in the Parson Russell Terrier dog breed. PLoS ONE 2013, 8, e64627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hersheson, J.; Lopez, D.; Hammer, M.; Liu, Y.; Lee, K.H.; Pinto, V.; Seinfeld, J.; Wiethoff, S.; Sun, J.; et al. Defects in the CAPN1 Gene Result in Alterations in Cerebellar Development and Cerebellar Ataxia in Mice and Humans. Cell Rep. 2016, 16, 79–91. [Google Scholar] [CrossRef]
- Su, W.; Zhou, Q.; Wang, Y.; Chishti, A.; Li, Q.Q.; Dayal, S.; Shiehzadegan, S.; Cheng, A.; Moore, C.; Bi, X.; et al. Deletion of the Capn1 Gene Results in Alterations in Signaling Pathways Related to Alzheimer’s Disease, Protein Quality Control and Synaptic Plasticity in Mouse Brain. Front. Genet. 2020, 11, 334. [Google Scholar] [CrossRef]
- Wang, Y.; Lopez, D.; Davey, P.G.; Cameron, D.J.; Nguyen, K.; Tran, J.; Marquez, E.; Liu, Y.; Bi, X.; Baudry, M. Calpain-1 and calpain-2 play opposite roles in retinal ganglion cell degeneration induced by retinal ischemia/reperfusion injury. Neurobiol. Dis. 2016, 93, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Lopez, D.; Lee, M.; Dayal, S.; Hurtado, A.; Bi, X.; Baudry, M. Protection against TBI-Induced Neuronal Death with Post-Treatment with a Selective Calpain-2 Inhibitor in Mice. J. Neurotrauma 2018, 35, 105–117. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Nham, A.; Sherbaf, A.; Quach, D.; Yahya, E.; Ranburger, D.; Bi, X.; Baudry, M. Calpain-2 as a therapeutic target in repeated concussion-induced neuropathy and behavioral impairment. Sci. Adv. 2020, 6, eaba5547. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yahya, E.; Quach, D.; Bi, X.; Baudry, M. Calpain-2 activation in mouse hippocampus plays a critical role in seizure-induced neuropathology. Neurobiol. Dis. 2021, 147, 105149. [Google Scholar] [CrossRef]
- Baudry, M.; Bi, X. Calpain-1 and Calpain-2: The Yin and Yang of Synaptic Plasticity and Neurodegeneration. Trends Neurosci. 2016, 39, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Bi, X.; Baudry, M. Calpain-1 and Calpain-2 in the Brain: New Evidence for a Critical Role of Calpain-2 in Neuronal Death. Cells 2020, 9, 2698. [Google Scholar] [CrossRef]
- Brunet, A.; Park, J.; Tran, H.; Hu, L.S.; Hemmings, B.A.; Greenberg, M.E. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell Biol. 2001, 21, 952–965. [Google Scholar] [CrossRef]
- Deacon, K.; Mistry, P.; Chernoff, J.; Blank, J.L.; Patel, R. p38 Mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol. Biol. Cell 2003, 14, 2071–2087. [Google Scholar] [CrossRef]
- Xu, J.; Kurup, P.; Zhang, Y.; Goebel-Goody, S.M.; Wu, P.H.; Hawasli, A.H.; Baum, M.L.; Bibb, J.A.; Lombroso, P.J. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J. Neurosci. 2009, 29, 9330–9343. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, X.; Baudry, M. Calpain-2 as a therapeutic target for acute neuronal injury. Expert Opin. Ther. Targets 2018, 22, 19–29. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Hillier, B.J.; Lim, W.A.; Bredt, D.S. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J. Biol. Chem. 1999, 274, 27467–27473. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hall, R.A.; Lee, M.; Kamgar-Parsi, A.; Bi, X.; Baudry, M. The tyrosine phosphatase PTPN13/FAP-1 links calpain-2, TBI and tau tyrosine phosphorylation. Sci. Rep. 2017, 7, 11771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brazdzionis, J.; Dong, F.; Patchana, T.; Ghanchi, H.; Podkovik, S.; Wiginton, J.G.T.; Marino, M.; Duong, J.; Wacker, M.; et al. P13BP, a Calpain-2-Mediated Breakdown Product of PTPN13, Is a Novel Blood Biomarker for Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Freiss, G.; Chalbos, D. PTPN13/PTPL1: An important regulator of tumor aggressiveness. Anticancer Agents Med. Chem. 2011, 11, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, N.S.; Schepens, J.T.; Valiente, M.; Hendriks, W.J.; Pulido, R. PTEN-PDZ domain interactions: Binding of PTEN to PDZ domains of PTPN13. Methods 2015, 77–78, 147–156. [Google Scholar] [CrossRef]
- Kock, G.; Dicks, M.; Yip, K.T.; Kohl, B.; Putz, S.; Heumann, R.; Erdmann, K.S.; Stoll, R. Molecular Basis of Class III Ligand Recognition by PDZ3 in Murine Protein Tyrosine Phosphatase PTPN13. J. Mol. Biol. 2018, 430, 4275–4292. [Google Scholar] [CrossRef]
- Santos, D.M.; Xavier, J.M.; Morgado, A.L.; Sola, S.; Rodrigues, C.M. Distinct regulatory functions of calpain 1 and 2 during neural stem cell self-renewal and differentiation. PLoS ONE 2012, 7, e33468. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.; Lindberg, O.R.; Kuhn, H.G. Radixin inhibition decreases adult neural progenitor cell migration and proliferation in vitro and in vivo. Front. Cell Neurosci. 2013, 7, 161. [Google Scholar] [CrossRef]
- Chung, K.M.; Park, H.; Jung, S.; Ha, S.; Yoo, S.J.; Woo, H.; Lee, H.J.; Kim, S.W.; Kim, E.K.; Moon, C.; et al. Calpain Determines the Propensity of Adult Hippocampal Neural Stem Cells to Autophagic Cell Death Following Insulin Withdrawal. Stem Cells 2015, 33, 3052–3064. [Google Scholar] [CrossRef]
- Machado, V.M.; Morte, M.I.; Carreira, B.P.; Azevedo, M.M.; Takano, J.; Iwata, N.; Saido, T.C.; Asmussen, H.; Horwitz, A.R.; Carvalho, C.M.; et al. Involvement of calpains in adult neurogenesis: Implications for stroke. Front. Cell Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y. Regulation of TET protein stability by calpains. Cell Rep. 2014, 6, 278–284. [Google Scholar] [CrossRef]
- Huttenlocher, A.; Palecek, S.P.; Lu, Q.; Zhang, W.; Mellgren, R.L.; Lauffenburger, D.A.; Ginsberg, M.H.; Horwitz, A.F. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 1997, 272, 32719–32722. [Google Scholar] [CrossRef]
- Yajima, Y.; Kawashima, S. Calpain function in the differentiation of mesenchymal stem cells. Biol. Chem. 2002, 383, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Moyen, C.; Goudenege, S.; Poussard, S.; Sassi, A.H.; Brustis, J.J.; Cottin, P. Involvement of micro-calpain (CAPN 1) in muscle cell differentiation. Int. J. Biochem. Cell Biol. 2004, 36, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, F.; Marcilhac, A.; Chebli, K.; Benyamin, Y.; Rossel, M. Calpain 2 expression pattern and sub-cellular localization during mouse embryogenesis. Int. J. Dev. Biol. 2008, 52, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Gage, F.H.; Eisch, A.J.; Lagace, D.C. Making a neuron: Cdk5 in embryonic and adult neurogenesis. Trends Neurosci. 2009, 32, 575–582. [Google Scholar] [CrossRef]
- Moudilou, E.N.; Mouterfi, N.; Exbrayat, J.M.; Brun, C. Calpains expression during Xenopus laevis development. Tissue Cell 2010, 42, 275–281. [Google Scholar] [CrossRef]
- Storr, S.J.; Carragher, N.O.; Frame, M.C.; Parr, T.; Martin, S.G. The calpain system and cancer. Nat. Rev. Cancer 2011, 11, 364–374. [Google Scholar] [CrossRef]
- Lade, A.; Ranganathan, S.; Luo, J.; Monga, S.P. Calpain induces N-terminal truncation of beta-catenin in normal murine liver development: Diagnostic implications in hepatoblastomas. J. Biol. Chem. 2012, 287, 22789–22798. [Google Scholar] [CrossRef]
- Liang, Z.; Brown, R.C.; Fletcher, J.C.; Opsahl-Sorteberg, H.G. Calpain-Mediated Positional Information Directs Cell Wall Orientation to Sustain Plant Stem Cell Activity, Growth and Development. Plant Cell Physiol. 2015, 56, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Nalabothula, N.; Ghosh, M.; Popp, O.; Jochum, M.; Machleidt, W.; Gil-Parrado, S.; Holak, T.A. Identification of calpain cleavage sites in the G1 cyclin-dependent kinase inhibitor p19(INK4d). Biol. Chem. 2006, 387, 329–335. [Google Scholar] [CrossRef]
- Kawauchi, T. Cdk5 regulates multiple cellular events in neural development, function and disease. Dev. Growth Differ. 2014, 56, 335–348. [Google Scholar] [CrossRef]
- Wu, J.Q.; Habegger, L.; Noisa, P.; Szekely, A.; Qiu, C.; Hutchison, S.; Raha, D.; Egholm, M.; Lin, H.; Weissman, S.; et al. Dynamic transcriptomes during neural differentiation of human embryonic stem cells revealed by short, long, and paired-end sequencing. Proc. Natl. Acad. Sci. USA 2010, 107, 5254–5259. [Google Scholar] [CrossRef]
- Baudry, M.; Su, W.; Seinfeld, J.; Sun, J.; Bi, X. Role of Calpain-1 in Neurogenesis. Front. Mol. Biosci. 2021, 8, 685938. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Reece, T.; Shahi, R.; Ta, K.; Bi, X. Calpain-2 inhibition or deletion enhances levels of the transcription factor, MEIS2, and stimulates neurogenesis. BMS-CN-2025-281 2025.
- Saez, M.E.; Ramirez-Lorca, R.; Moron, F.J.; Ruiz, A. The therapeutic potential of the calpain family: New aspects. Drug Discov. Today 2006, 11, 917–923. [Google Scholar] [CrossRef]
- Potz, B.A.; Abid, M.R.; Selke, F.W. Role of calpain in pathogenesis of human disease processes. J. Nat.Sci. 2016, 2, e218. [Google Scholar] [PubMed]
- Miyazaki, T. Calpain and Cardiometabolic Diseases. Int. J. Mol. Sci. 2023, 24, 16782. [Google Scholar] [CrossRef]
- Vanderklish, P.W.; Bahr, B.A. The pathogenic activation of calpain: A marker and mediator of cellular toxicity and disease states. Int. J. Exp. Pathol. 2000, 81, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug Discov. 2016, 15, 854–876. [Google Scholar] [CrossRef]
- Spinozzi, S.; Albini, S.; Best, H.; Richard, I. Calpains for dummies: What you need to know about the calpain family. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140616. [Google Scholar] [CrossRef]
- Vosler, P.S.; Brennan, C.S.; Chen, J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 2008, 38, 78–100. [Google Scholar] [CrossRef] [PubMed]
- Yildiz-Unal, A.; Korulu, S.; Karabay, A. Neuroprotective strategies against calpain-mediated neurodegeneration. Neuropsychiatr. Dis. Treat. 2015, 11, 297–310. [Google Scholar] [CrossRef]
- Koumura, A.; Nonaka, Y.; Hyakkoku, K.; Oka, T.; Shimazawa, M.; Hozumi, I.; Inuzuka, T.; Hara, H. A novel calpain inhibitor, ((1S)-1((((1S)-1-benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-methylbutyl) carbamic acid 5-methoxy-3-oxapentyl ester, protects neuronal cells from cerebral ischemia-induced damage in mice. Neuroscience 2008, 157, 309–318. [Google Scholar] [CrossRef]
- Anagli, J.; Han, Y.; Stewart, L.; Yang, D.; Movsisyan, A.; Abounit, K.; Seyfried, D. A novel calpastatin-based inhibitor improves postischemic neurological recovery. Biochem. Biophys. Res. Commun. 2009, 385, 94–99. [Google Scholar] [CrossRef]
- Liu, S.; Yin, F.; Zhang, J.; Qian, Y. The role of calpains in traumatic brain injury. Brain Inj. 2014, 28, 133–137. [Google Scholar] [CrossRef]
- Cagmat, E.B.; Guingab-Cagmat, J.D.; Vakulenko, A.V.; Hayes, R.L.; Anagli, J. Potential Use of Calpain Inhibitors as Brain Injury Therapy. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; Frontiers in Neuroengineering: Boca Raton, FL, USA, 2015. [Google Scholar]
- Siklos, M.; BenAissa, M.; Thatcher, G.R. Cysteine proteases as therapeutic targets: Does selectivity matter? A systematic review of calpain and cathepsin inhibitors. Acta Pharm. Sin. B 2015, 5, 506–519. [Google Scholar] [CrossRef]
- Thompson, S.N.; Carrico, K.M.; Mustafa, A.G.; Bains, M.; Hall, E.D. A pharmacological analysis of the neuroprotective efficacy of the brain- and cell-permeable calpain inhibitor MDL-28170 in the mouse controlled cortical impact traumatic brain injury model. J. Neurotrauma 2010, 27, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Bains, M.; Cebak, J.E.; Gilmer, L.K.; Barnes, C.C.; Thompson, S.N.; Geddes, J.W.; Hall, E.D. Pharmacological analysis of the cortical neuronal cytoskeletal protective efficacy of the calpain inhibitor SNJ-1945 in a mouse traumatic brain injury model. J. Neurochem. 2013, 125, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Schoch, K.M.; von Reyn, C.R.; Bian, J.; Telling, G.C.; Meaney, D.F.; Saatman, K.E. Brain injury-induced proteolysis is reduced in a novel calpastatin-overexpressing transgenic mouse. J. Neurochem. 2013, 125, 909–920. [Google Scholar] [CrossRef]
- Saatman, K.E.; Zhang, C.; Bartus, R.T.; McIntosh, T.K. Behavioral efficacy of posttraumatic calpain inhibition is not accompanied by reduced spectrin proteolysis, cortical lesion, or apoptosis. J. Cereb. Blood Flow Metab. 2000, 20, 66–73. [Google Scholar] [CrossRef]
- Buki, A.; Farkas, O.; Doczi, T.; Povlishock, J.T. Preinjury administration of the calpain inhibitor MDL-28170 attenuates traumatically induced axonal injury. J. Neurotrauma 2003, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Liu, E.; Wang, J.; Chen, Y.; Yu, J.; Baker, A.J. Calpain inhibitor MDL-28170 reduces the functional and structural deterioration of corpus callosum following fluid percussion injury. J. Neurotrauma 2007, 24, 960–978. [Google Scholar] [CrossRef]
- Ma, M.; Li, L.; Wang, X.; Bull, D.L.; Shofer, F.S.; Meaney, D.F.; Neumar, R.W. Short-duration treatment with the calpain inhibitor MDL-28170 does not protect axonal transport in an in vivo model of traumatic axonal injury. J. Neurotrauma 2012, 29, 445–451. [Google Scholar] [CrossRef]
- Donkor, I.O. Calpain inhibitors: A survey of compounds reported in the patent and scientific literature. Expert. Opin. Ther. Pat. 2011, 21, 601–636. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Baudry, M. Targeting calpain-2 for Alzheimer’s disease treatment. Med. Res. Arch. 2023, 11. [Google Scholar] [CrossRef]
- Baudry, M.; Luo, Y.L.; Bi, X. Calpain-2 Inhibitors as Therapy for Traumatic Brain Injury. Neurotherapeutics 2023, 20, 1592–1602. [Google Scholar] [CrossRef]
- Chatterjee, P.; Botello-Smith, W.M.; Zhang, H.; Qian, L.; Alsamarah, A.; Kent, D.; Lacroix, J.J.; Baudry, M.; Luo, Y. Can Relative Binding Free Energy Predict Selectivity of Reversible Covalent Inhibitors? J. Am. Chem. Soc. 2017, 139, 17945–17952. [Google Scholar] [CrossRef]
- Lon, H.K.; Mendonca, N.; Goss, S.; Othman, A.A.; Locke, C.; Jin, Z.; Rendenbach-Mueller, B. Pharmacokinetics, Safety, Tolerability, and Pharmacodynamics of Alicapistat, a Selective Inhibitor of Human Calpains 1 and 2 for the Treatment of Alzheimer Disease: An Overview of Phase 1 Studies. Clin. Pharmacol. Drug Dev. 2019, 8, 290–303. [Google Scholar] [CrossRef]
- Li, Z.; Ortega-Vilain, A.C.; Patil, G.S.; Chu, D.L.; Foreman, J.E.; Eveleth, D.D.; Powers, J.C. Novel peptidyl alpha-keto amide inhibitors of calpains and other cysteine proteases. J. Med. Chem. 1996, 39, 4089–4098. [Google Scholar] [CrossRef]
- Ovat, A.; Li, Z.Z.; Hampton, C.Y.; Asress, S.A.; Fernandez, F.M.; Glass, J.D.; Powers, J.C. Peptidyl alpha-ketoamides with nucleobases, methylpiperazine, and dimethylaminoalkyl substituents as calpain inhibitors. J. Med. Chem. 2010, 53, 6326–6336. [Google Scholar] [CrossRef]
- Ustach, V.D.; Lakkaraju, S.K.; Jo, S.; Yu, W.; Jiang, W.; MacKerell, A.D., Jr. Optimization and Evaluation of Site-Identification by Ligand Competitive Saturation (SILCS) as a Tool for Target-Based Ligand Optimization. J. Chem. Inf. Model. 2019, 59, 3018–3035. [Google Scholar] [CrossRef]
- Baudry, M.; Wang, Y.; Bi, X.; Luo, Y.L.; Wang, Z.; Kamal, Z.; Shirokov, A.; Sullivan, E.; Lagasca, D.; Khalil, H.; et al. Identification and neuroprotective properties of NA-184, a calpain-2 inhibitor. Pharmacol. Res. Perspect. 2024, 12, e1181. [Google Scholar] [CrossRef]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O’Carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Babcock, K.R.; Page, J.S.; Fallon, J.R.; Webb, A.E. Adult Hippocampal Neurogenesis in Aging and Alzheimer’s Disease. Stem Cell Rep. 2021, 16, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Khalil, M.H. The BDNF-Interactive Model for Sustainable Hippocampal Neurogenesis in Humans: Synergistic Effects of Environmentally-Mediated Physical Activity, Cognitive Stimulation, and Mindfulness. Int. J. Mol. Sci. 2024, 25, 12924. [Google Scholar] [CrossRef]
- Eisch, A.J.; Petrik, D. Depression and hippocampal neurogenesis: A road to remission? Science 2012, 338, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, C.T. Microglial activation—Tuning and pruning adult neurogenesis. Front. Pharmacol. 2012, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, I.; Paterlini, M.; Zamboni, M.; Ziegenhain, C.; Giatrellis, S.; Saghaleyni, R.; Bjorklund, A.; Alkass, K.; Tata, M.; Druid, H.; et al. Identification of proliferating neural progenitors in the adult human hippocampus. Science 2025, 389, 58–63. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).