Retinoic Acid Modulates Immune Differentiation in a Human Small Intestinal In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cord Blood Collection and Cell Isolation
2.3. CD4+ T Cell Isolation
2.4. Preliminary Experiments with Retinoic Acid (RA)
2.5. PKH Staining
2.6. Setup of the Intestinal Cell Culture Models
2.7. Cell Harvesting and Flow Cytometry
2.8. Data Acquisition and Analysis
2.9. Statistical Analysis
2.10. Ethics
3. Results
3.1. Creation of a 3D Intestinal Model Permissive for the Generation of Inflammatory Monocytes and Activated Memory T Cells
3.2. Epithelial Cells and T Cells Fail to Promote M/DC Precursor Cell Differentiation
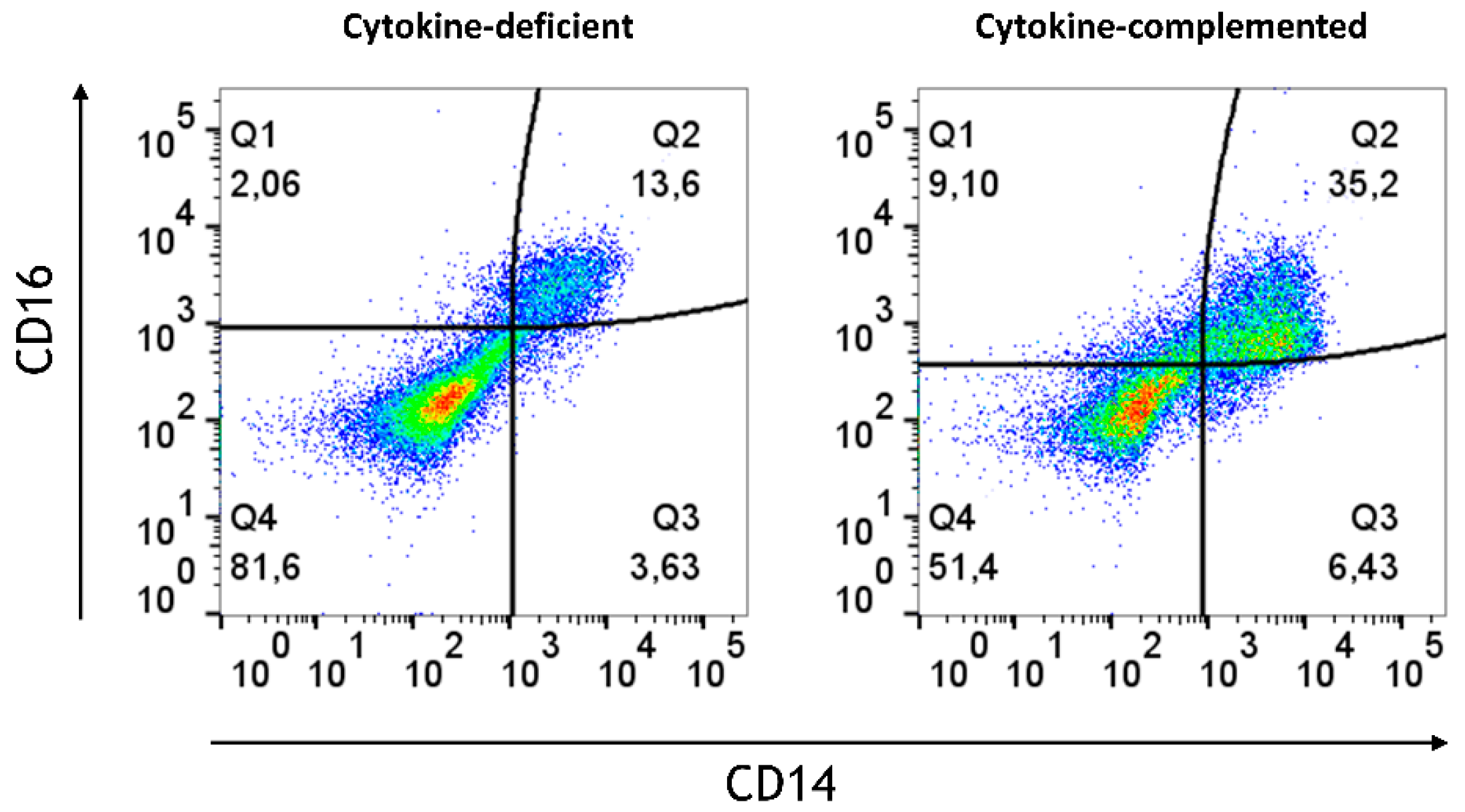
3.3. Pro-Inflammatory Cytokines Fail to Rescue CD103+ DC Differentiation but Promote Inflammatory Monocyte Differentiation in the 3D Model
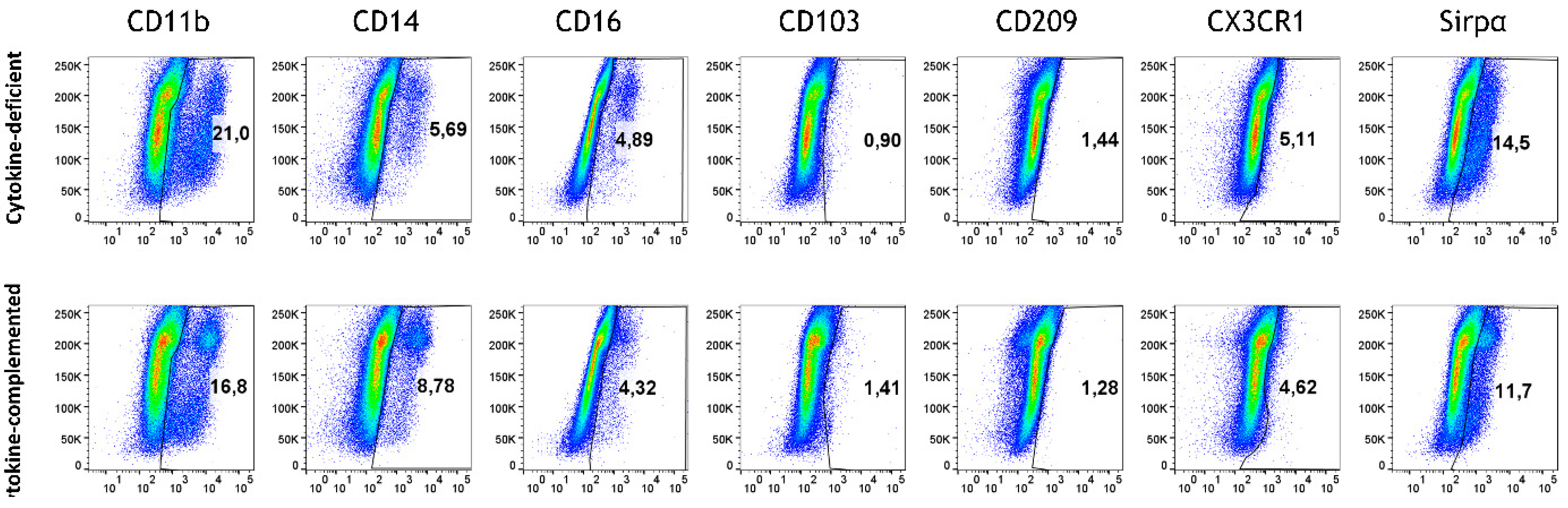
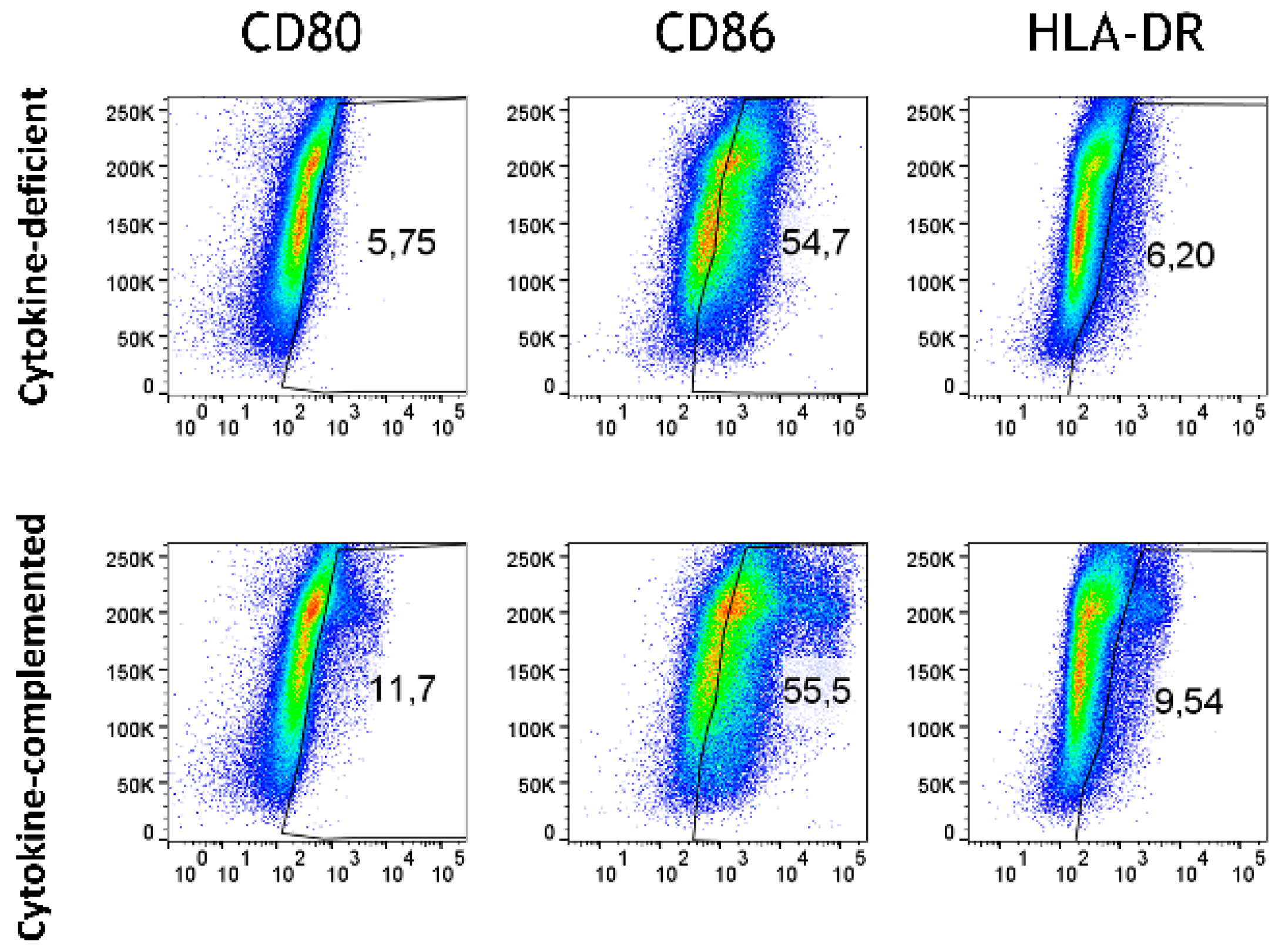
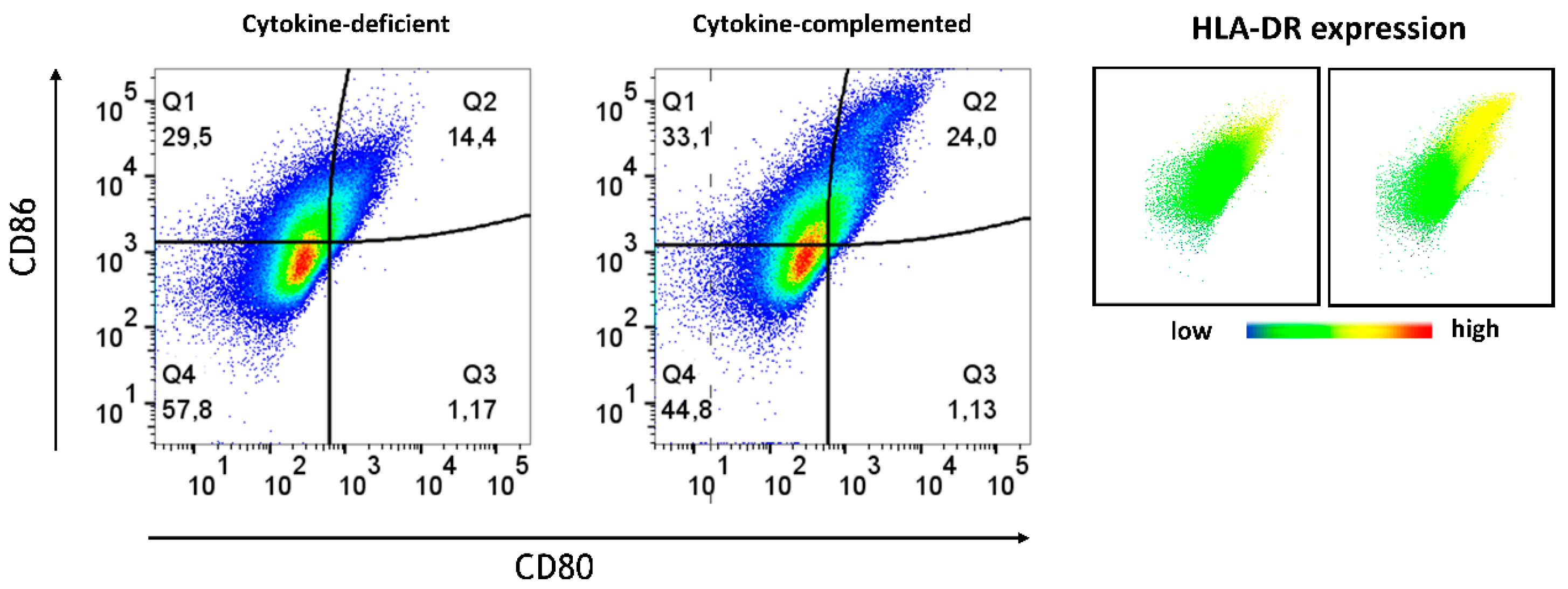
3.4. Increased Expression of Antigen-Presenting Cell (APC) Activation Markers and T Cell Activation in Response to Pro-Inflammatory Cytokines
3.5. Retinoic Acid (RA) Promotes the Generation of CD103+ DCs at the Expense of Sirpα+ DCs from M/DC Precursor Cells in Suspension Cultures
3.6. RA-Generated CD103+ DCs and CX3CR1+ Macrophages Remain Stable After Integration into the Cytokine-Deficient 3D Intestinal Model
3.7. Limited Induction of Regulatory Cells and Persistent Inflammatory Monocytes in the RA-Primed Cytokine-Complemented Model
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of disease: Inflammatory bowel diseases. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–165. [Google Scholar]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 2014, 27, 1–7. [Google Scholar] [CrossRef]
- Scott, C.L.; Aumeunier, A.M.; Mowat, A.M. Intestinal CD103+ dendritic cells: Master regulators of tolerance? Trends Immunol. 2011, 32, 412–419. [Google Scholar] [CrossRef]
- Jung, C.; Hugot, J.-P.; Barreau, F. Peyer′ s Patches: The Immune Sensors of the Intestine. Int. J. Inflamm. 2010, 2010, 823710. [Google Scholar]
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. An overview of in vitro, ex vivo and in vivo models for studying the transport of drugs across intestinal barriers. Adv. Drug Deliv. Rev. 2021, 175, 113795. [Google Scholar] [CrossRef]
- Sato, T.; Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef]
- Costa, J.; Ahluwalia, A. Advances and current challenges in intestinal in vitro model engineering: A digest. Front. Bioeng. Biotechnol. 2019, 7, 144. [Google Scholar] [CrossRef]
- Schimpel, C.; Passegger, C.; Egger, S.; Tam-Amersdorfer, C.; Strobl, H. A novel 3D cell culture model to study the human small intestinal immune landscape. Eur. J. Immunol. 2023, 53, 2250131. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Wang, B.-Y.; Wang, T.-T.; Wang, F.-F.; Guo, Y.-X.; Hua, R.-X.; Shang, H.-W.; Lu, X.; Xu, J.-D. Functions of dendritic cells and its association with intestinal diseases. Cells 2021, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Locatelli, F. Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. WJG 2011, 17, 3761. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Kayama, H.; Nishimura, J.; Sekido, Y.; Osawa, H.; Barman, S.; Ogino, T.; Takahashi, H.; Haraguchi, N.; Hata, T. CD103+ dendritic cell function is altered in the colons of patients with ulcerative colitis. Inflamm. Bowel Dis. 2017, 23, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.K.; Brynjólfsson, S.F.; Dige, A.; Uronen-Hansson, H.; Börjesson, L.G.; Bengtsson, J.L.; Gudjonsson, S.; Öhman, L.; Agnholt, J.; Sjövall, H. Macrophage and dendritic cell subsets in IBD: ALDH+ cells are reduced in colon tissue of patients with ulcerative colitis regardless of inflammation. Mucosal Immunol. 2016, 9, 171–182. [Google Scholar] [CrossRef]
- Bekiaris, V.; Persson, E.K.; Agace, W.W. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol. Rev. 2014, 260, 86–101. [Google Scholar] [CrossRef]
- Kämpfer, A.A.; Urbán, P.; Gioria, S.; Kanase, N.; Stone, V.; Kinsner-Ovaskainen, A. Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state. Toxicol. In Vitro 2017, 45, 31–43. [Google Scholar] [CrossRef]
- Noel, G.; Baetz, N.W.; Staab, J.F.; Donowitz, M.; Kovbasnjuk, O.; Pasetti, M.F.; Zachos, N.C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 2017, 7, 45270. [Google Scholar]
- Song, A.T.; Sindeaux, R.H.; Li, Y.; Affia, H.; Agnihotri, T.; Leclerc, S.; van Vliet, P.P.; Colas, M.; Guimond, J.-V.; Patey, N. Developmental role of macrophages modeled in human pluripotent stem cell-derived intestinal tissue. Cell Rep. 2024, 43, 113616. [Google Scholar] [CrossRef]
- des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.-J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Porchet, N.; Aubert, J.-P.; Swallow, D.; Gum, J.R.; Kim, Y.S.; Real, F.X.; Zweibaum, A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 1993, 106, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Schimpel, C.; Teubl, B.; Absenger, M.; Meindl, C.; Fröhlich, E.; Leitinger, G.; Zimmer, A.; Roblegg, E. Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles. Mol. Pharm. 2014, 11, 808–818. [Google Scholar] [CrossRef] [PubMed]
- McKenna, H.J. Role of hematopoietic growth factors/flt3 ligand in expansion and regulation of dendritic cells. Curr. Opin. Hematol. 2001, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.-J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. In Vitro 2010, 24, 1441–1449. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Yamada, M.; Kikuyama, M.; Maruyama, Y.; Iwaoka, Y.; Hirayama, K.; Nagata, S. Adsorptive depletion of elevated proinflammatory CD14+ CD16+ DR++ monocytes in patients with inflammatory bowel disease. Off. J. Am. Coll. Gastroenterol. ACG 2008, 103, 1210–1216. [Google Scholar] [CrossRef]
- Koch, S.; Kucharzik, T.; Heidemann, J.; Nusrat, A.; Luegering, A. Investigating the role of proinflammatory CD16+ monocytes in the pathogenesis of inflammatory bowel disease. Clin. Exp. Immunol. 2010, 161, 332–341. [Google Scholar] [CrossRef]
- Tindemans, I.; Joosse, M.E.; Samsom, J.N. Dissecting the heterogeneity in T-cell mediated inflammation in IBD. Cells 2020, 9, 110. [Google Scholar] [CrossRef]
- Bakdash, G.; Vogelpoel, L.T.; Van Capel, T.M.; Kapsenberg, M.L.; de Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef]
- Mann, E.R.; Bernardo, D.; English, N.R.; Landy, J.; Al-Hassi, H.O.; Peake, S.T.; Man, R.; Elliott, T.R.; Spranger, H.; Lee, G.H. Compartment-specific immunity in the human gut: Properties and functions of dendritic cells in the colon versus the ileum. Gut 2016, 65, 256–270. [Google Scholar] [CrossRef]
- Snyder, L.M.; Arora, J.; Kennett, M.J.; Weaver, V.; Cantorna, M.T. Retinoid signaling in intestinal epithelial cells is essential for early survival from gastrointestinal infection. Front. Immunol. 2020, 11, 559635. [Google Scholar] [CrossRef]
- Denning, T.L.; Norris, B.A.; Medina-Contreras, O.; Manicassamy, S.; Geem, D.; Madan, R.; Karp, C.L.; Pulendran, B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 2011, 187, 733–747. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.; Gao, T.; Wang, Y.; Ren, X.; Liu, S.; Zhang, S.; Zhou, P.; Lyu, J.; Bai, H. Causal role of immune cells in inflammatory bowel disease: A Mendelian randomization study. Medicine 2024, 103, e37537. [Google Scholar] [CrossRef] [PubMed]
- Eri, R.; Kodumudi, K.N.; Summerlin, D.J.; Srinivasan, M. Suppression of colon inflammation by CD80 blockade: Evaluation in two murine models of inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, C.; Duftner, C.; Grubeck-Loebenstein, B.; Schirmer, M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology 2006, 117, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Leal, R.F.; Planell, N.; Kajekar, R.; Lozano, J.J.; Ordás, I.; Dotti, I.; Esteller, M.; Masamunt, M.C.; Parmar, H.; Ricart, E. Identification of inflammatory mediators in patients with Crohn’s disease unresponsive to anti-TNFα therapy. Gut 2015, 64, 233–242. [Google Scholar] [CrossRef]
- Hasegawa, A.; Iwamura, C.; Kitajima, M.; Hashimoto, K.; Otsuyama, K.-i.; Ogino, H.; Nakayama, T.; Shirai, M. Crucial role for CD69 in the pathogenesis of dextran sulphate sodium-induced colitis. PLoS ONE 2013, 8, e65494. [Google Scholar] [CrossRef]
- Radulovic, K.; Niess, J.H. CD69 is the crucial regulator of intestinal inflammation: A new target molecule for IBD treatment? J. Immunol. Res. 2015, 2015, 497056. [Google Scholar] [CrossRef]
- Willerford, D.M.; Chen, J.; Ferry, J.A.; Davidson, L.; Ma, A.; Alt, F.W. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 1995, 3, 521–530. [Google Scholar] [CrossRef]
- Camelo, A.; Barlow, J.L.; Drynan, L.F.; Neill, D.R.; Ballantyne, S.J.; Wong, S.H.; Pannell, R.; Gao, W.; Wrigley, K.; Sprenkle, J. Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J. Gastroenterol. 2012, 47, 1198–1211. [Google Scholar] [CrossRef]
- Bain, C.; Montgomery, J.; Scott, C.; Kel, J.; Girard-Madoux, M.; Martens, L.; Zangerle-Murray, T.; Ober-Blöbaum, J.; Lindenbergh-Kortleve, D.; Samsom, J. TGFβR signalling controls CD103+ CD11b+ dendritic cell development in the intestine. Nat. Commun. 2017, 8, 620. [Google Scholar] [CrossRef]
- Liu, Q.; Wen, W.; Tang, L.; Qin, C.-J.; Lin, Y.; Zhang, H.-L.; Wu, H.; Ashton, C.; Wu, H.-P.; Ding, J. Inhibition of SIRPα in dendritic cells potentiates potent antitumor immunity. Oncoimmunology 2016, 5, e1183850. [Google Scholar] [CrossRef]
- Ness, S.; Lin, S.; Gordon, J.R. Regulatory dendritic cells, T cell tolerance, and dendritic cell therapy for immunologic disease. Front. Immunol. 2021, 12, 633436. [Google Scholar] [CrossRef]
- Zeng, R.; Bscheider, M.; Lahl, K.; Lee, M.; Butcher, E.C. Generation and transcriptional programming of intestinal dendritic cells: Essential role of retinoic acid. Mucosal Immunol. 2016, 9, 183–193. [Google Scholar] [CrossRef]
- Den Hartog, G.; Van Altena, C.; Savelkoul, H.F.; Van Neerven, R. The mucosal factors retinoic acid and TGF-β1 induce phenotypically and functionally distinct dendritic cell types. Int. Arch. Allergy Immunol. 2013, 162, 225–236. [Google Scholar] [CrossRef]
- Watchmaker, P.B.; Lahl, K.; Lee, M.; Baumjohann, D.; Morton, J.; Kim, S.J.; Zeng, R.; Dent, A.; Ansel, K.M.; Diamond, B. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat. Immunol. 2014, 15, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Cassani, B.; Villablanca, E.J.; De Calisto, J.; Wang, S.; Mora, J.R. Vitamin A and immune regulation: Role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Asp. Med. 2012, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.-M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β–and retinoic acid–dependent mechanism. J. Exp. Med. 2007, 204, 1757. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, H.; Fu, X.; Zhang, M.; Sun, F.; Fan, H. Dynamic role of macrophage CX3CR1 expression in inflammatory bowel disease. Immunol. Lett. 2021, 232, 39–44. [Google Scholar] [CrossRef]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.-Y. Tissue-specific role of CX3CR1 expressing immune cells and their relationships with human disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of inflammatory bowel disease: A comprehensive review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Targan, S.R. Biologic therapy of inflammatory bowel disease. Gastroenterology 2002, 122, 1592–1608. [Google Scholar] [CrossRef]
- Joshi, A.; Soni, A.; Acharya, S. In vitro models and ex vivo systems used in inflammatory bowel disease. In Vitro Models 2022, 1, 213–227. [Google Scholar] [CrossRef]
- Katsandegwaza, B.; Horsnell, W.; Smith, K. Inflammatory bowel disease: A review of pre-clinical murine models of human disease. Int. J. Mol. Sci. 2022, 23, 9344. [Google Scholar] [CrossRef]
- Walrath, T.; Dyamenahalli, K.U.; Hulsebus, H.J.; McCullough, R.L.; Idrovo, J.-P.; Boe, D.M.; McMahan, R.H.; Kovacs, E.J. Age-related changes in intestinal immunity and the microbiome. J. Leucoc. Biol. 2021, 109, 1045–1061. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, C.; Wang, Q.; Feng, S.; Fang, Y.; Zhang, S. The impact of aging on intestinal mucosal immune function and clinical applications. Front. Immunol. 2022, 13, 1029948. [Google Scholar] [CrossRef] [PubMed]

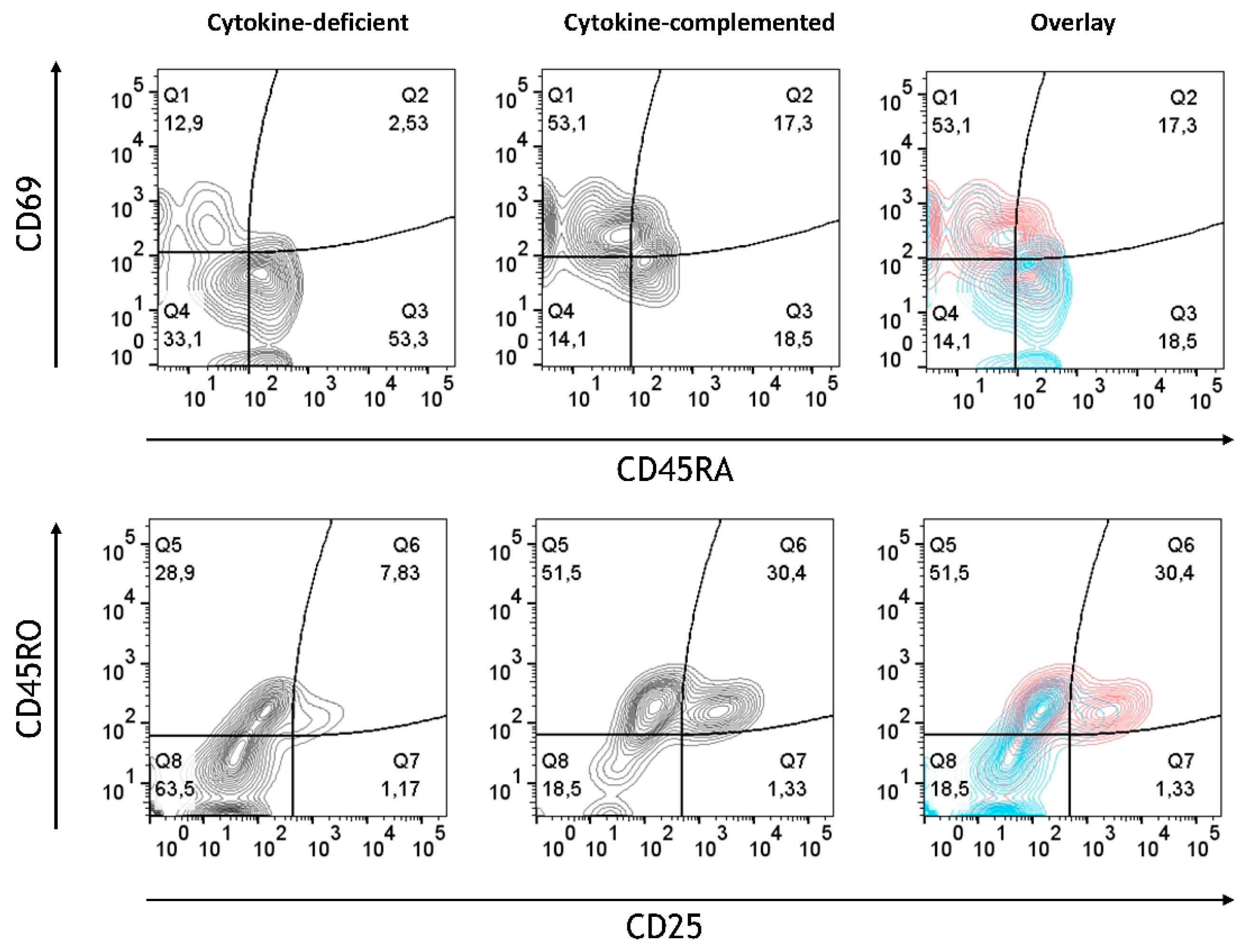
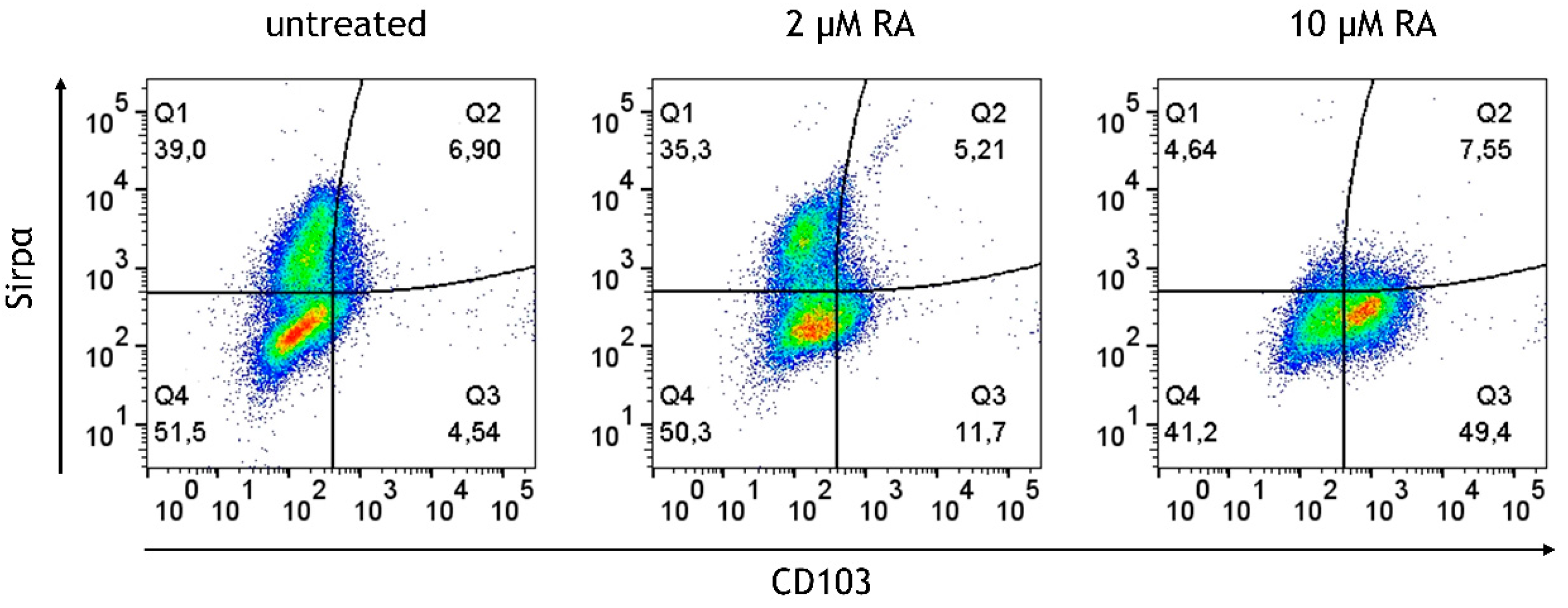
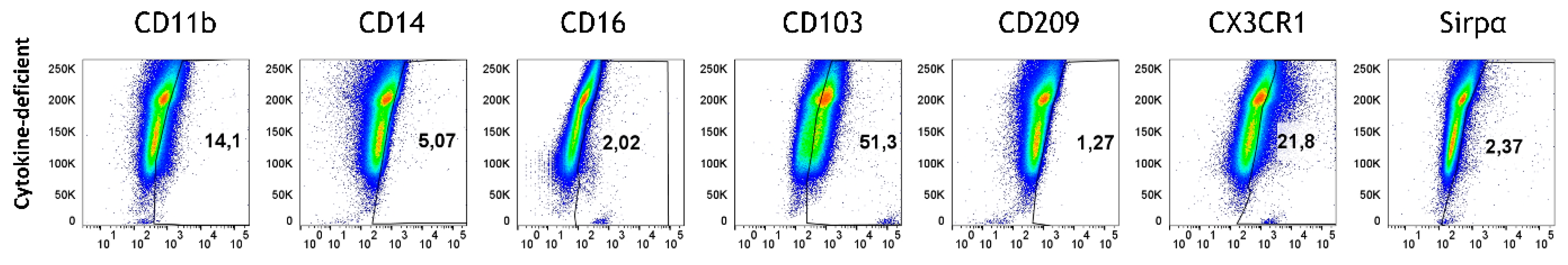

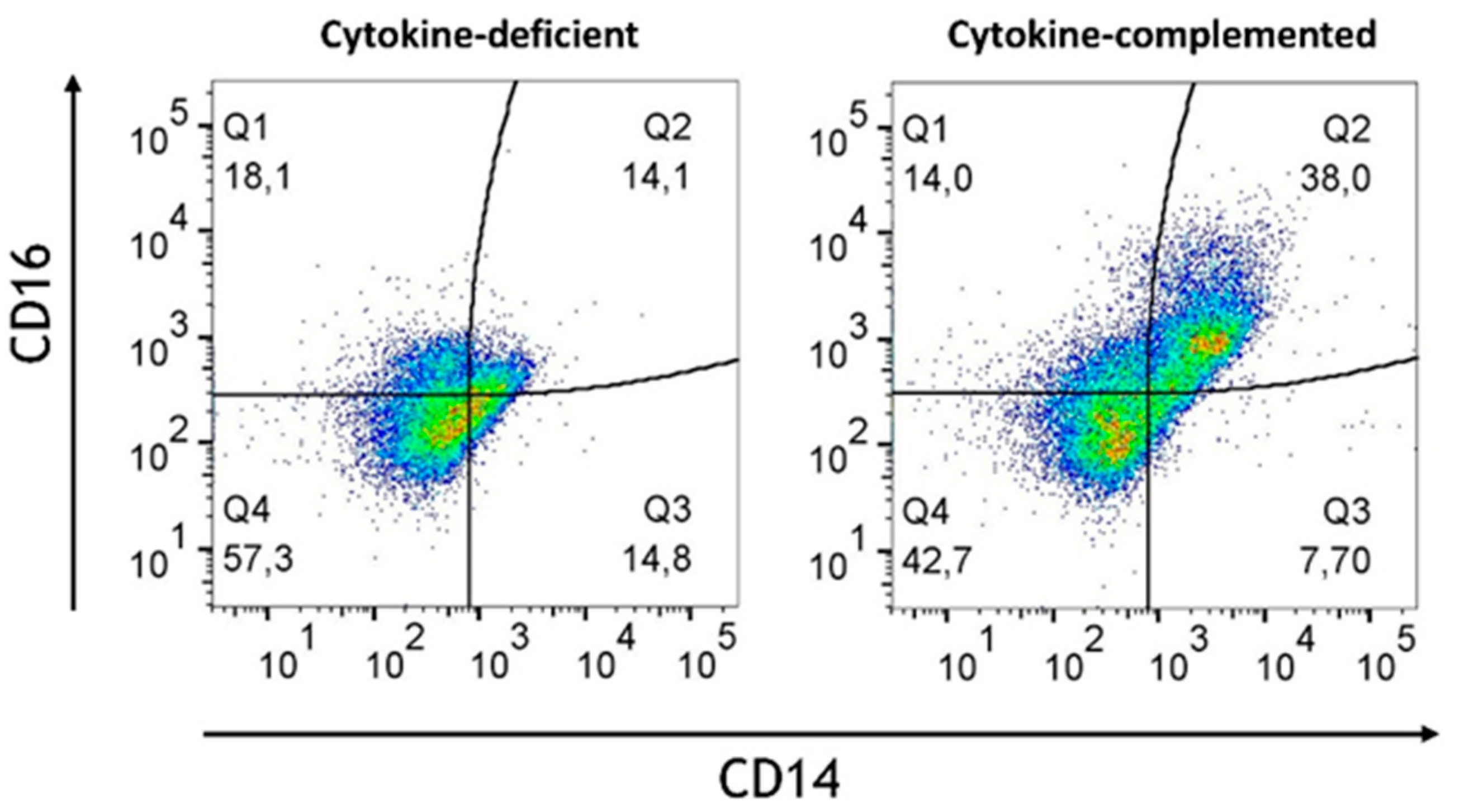

| Modell | Immune Phenotype | T Cell Phenotype | Immune State | Main Finding | Inflammatory Context |
|---|---|---|---|---|---|
| Cytokine-complemented with RA-primed M/DC precursors | CD14++, CD16++, CD103+, CX3CR1- | Memory T cells (CD45RO+) with signs of activation (CD25+, CD69+) | Moderate inflammation or “physiological inflammation” | Increased pro-inflammatory monocytes (CD14+CD16+), partially regulatory DCs (CD103+), but does not fully suppress. T cells are inflammation, activated (CD25+, CD69+). | RA modulates inflammation but does not fully counterbalance pro-inflammatory signals. |
| Cytokine-complemented with untreated M/DC precursors | CD14++, CD16++, CD103- | Strong T cell activation with high expression of CD45RO+ (memory) and CD25+, CD69+ (active T cells) | High, IBD-like inflammation | High pro-inflammatory monocyte activity (CD14+, CD16+), no CD103+ regulatory cells, strong T cell activation (CD25+, CD69+), leading to uncontrolled inflammation. | Uncontrolled inflammation, no regulatory modulation; model represents active, uncontrolled inflammation as seen in IBD, with a severe lack of immune regulation (CD103+ DCs are absent) |
| Cytokine-deficient with RA-primed M/DC precursors | CD103++, CX3CR1++ | Memory T cells (CD45RO+), with no signs of activation (CD25- CD69-) | Low inflammatory state | RA-priming fosters immune tolerance, with abundant regulatory cells (CD103+, CX3CR1+). T cells show memory phenotype (CD45RO+) without activation. | RA promotes immune homeostasis, inducing regulatory cells and maintaining a balanced immune environment. |
| Cytokine-deficient with untreated M/DC precursors | Few monocytes/macrophages, CD103- | Naive T cells (CD45RO+) with minimal activation (CD25- CD69-) | Immune quiescent or “suppressive state” | Epithelial cells maintain quiescence, minimal immune activation, few monocytes, no CD103+ cells, only naive T cells | Epithelial cells create an inhibitory milieu, preventing immune activation and maintaining quiescence. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schimpel, C.; Passegger, C.; Tam-Amersdorfer, C.; Strobl, H. Retinoic Acid Modulates Immune Differentiation in a Human Small Intestinal In Vitro Model. Cells 2025, 14, 1300. https://doi.org/10.3390/cells14171300
Schimpel C, Passegger C, Tam-Amersdorfer C, Strobl H. Retinoic Acid Modulates Immune Differentiation in a Human Small Intestinal In Vitro Model. Cells. 2025; 14(17):1300. https://doi.org/10.3390/cells14171300
Chicago/Turabian StyleSchimpel, Christa, Christina Passegger, Carmen Tam-Amersdorfer, and Herbert Strobl. 2025. "Retinoic Acid Modulates Immune Differentiation in a Human Small Intestinal In Vitro Model" Cells 14, no. 17: 1300. https://doi.org/10.3390/cells14171300
APA StyleSchimpel, C., Passegger, C., Tam-Amersdorfer, C., & Strobl, H. (2025). Retinoic Acid Modulates Immune Differentiation in a Human Small Intestinal In Vitro Model. Cells, 14(17), 1300. https://doi.org/10.3390/cells14171300






