Potential of Lactoferrin Against the Radiation-Induced Brain Injury
Abstract
1. Introduction
2. Materials and Methods
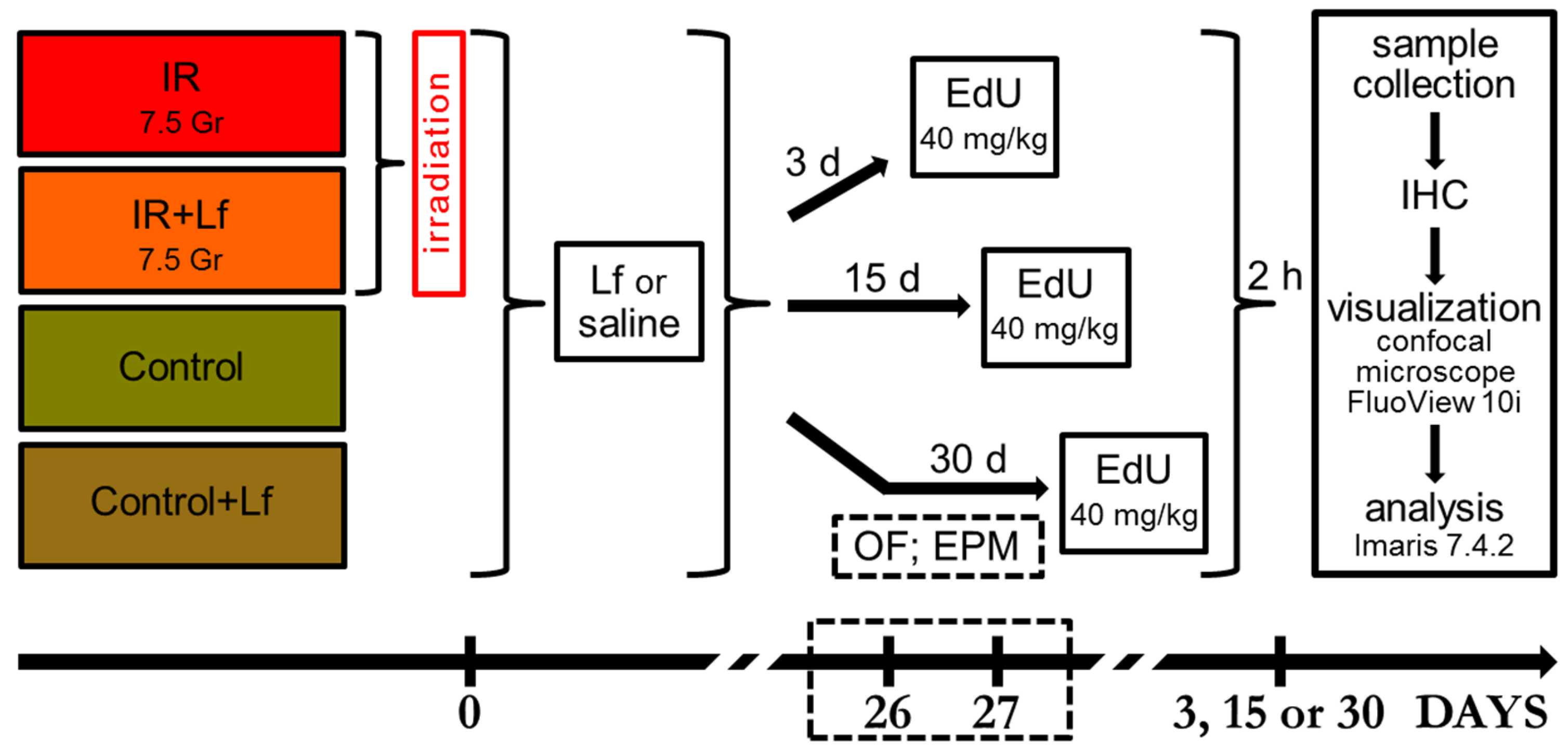
2.1. Experimental Groups
2.2. Weighing
2.3. Open Field Testing
2.4. Analysis of Elevated Plus-Maze Behavior
2.5. Sample Collection
2.6. Blood Tests
2.7. Immunocytochemical Analysis of Bone Marrow Cells
2.7.1. EdU Staining
2.7.2. Flow Cytometry
2.8. Immunohistochemical Analysis
2.8.1. Brain Sections
2.8.2. EdU Staining
2.8.3. DCX and NeuN Staining
2.8.4. Immunofluorescence Analysis
2.9. Statistical Analysis
3. Results
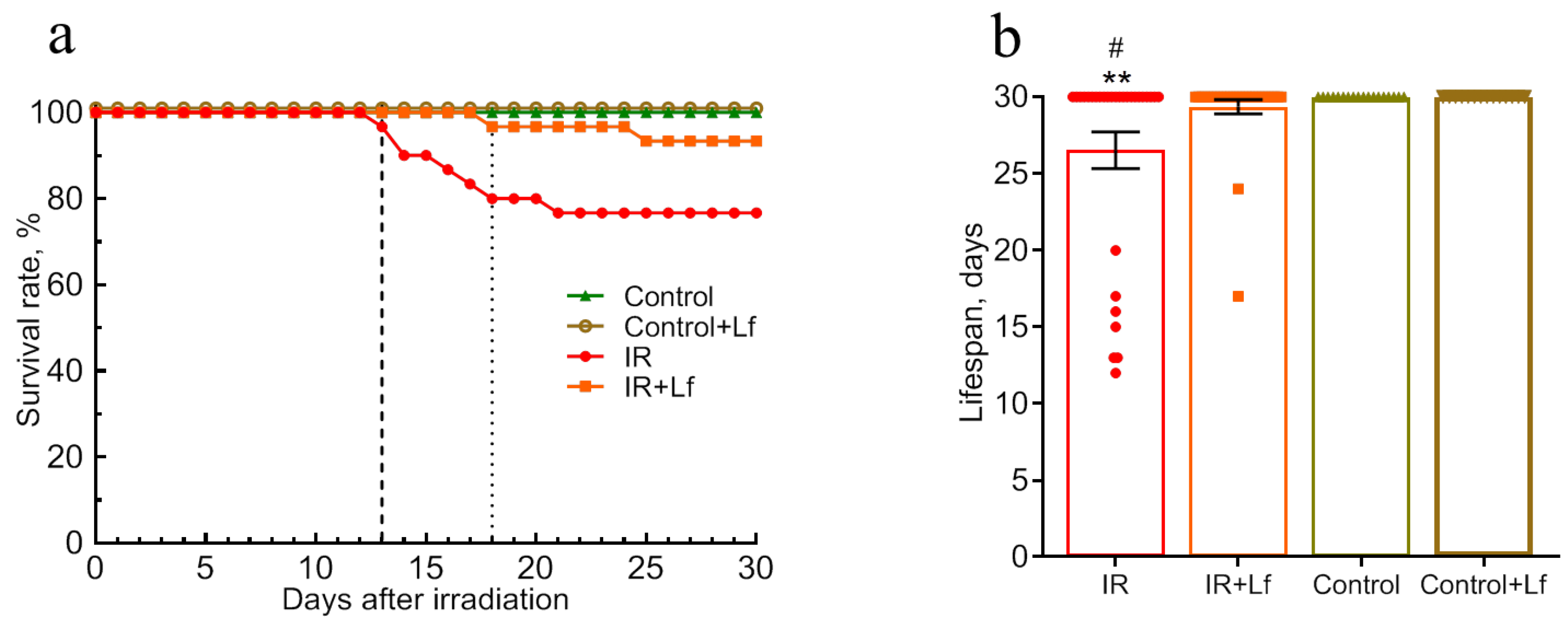
3.1. Treatment with Lf Increased the Survival Rate and the Mean Lifespan of Irradiated Mice During the Experiment (30 Days)
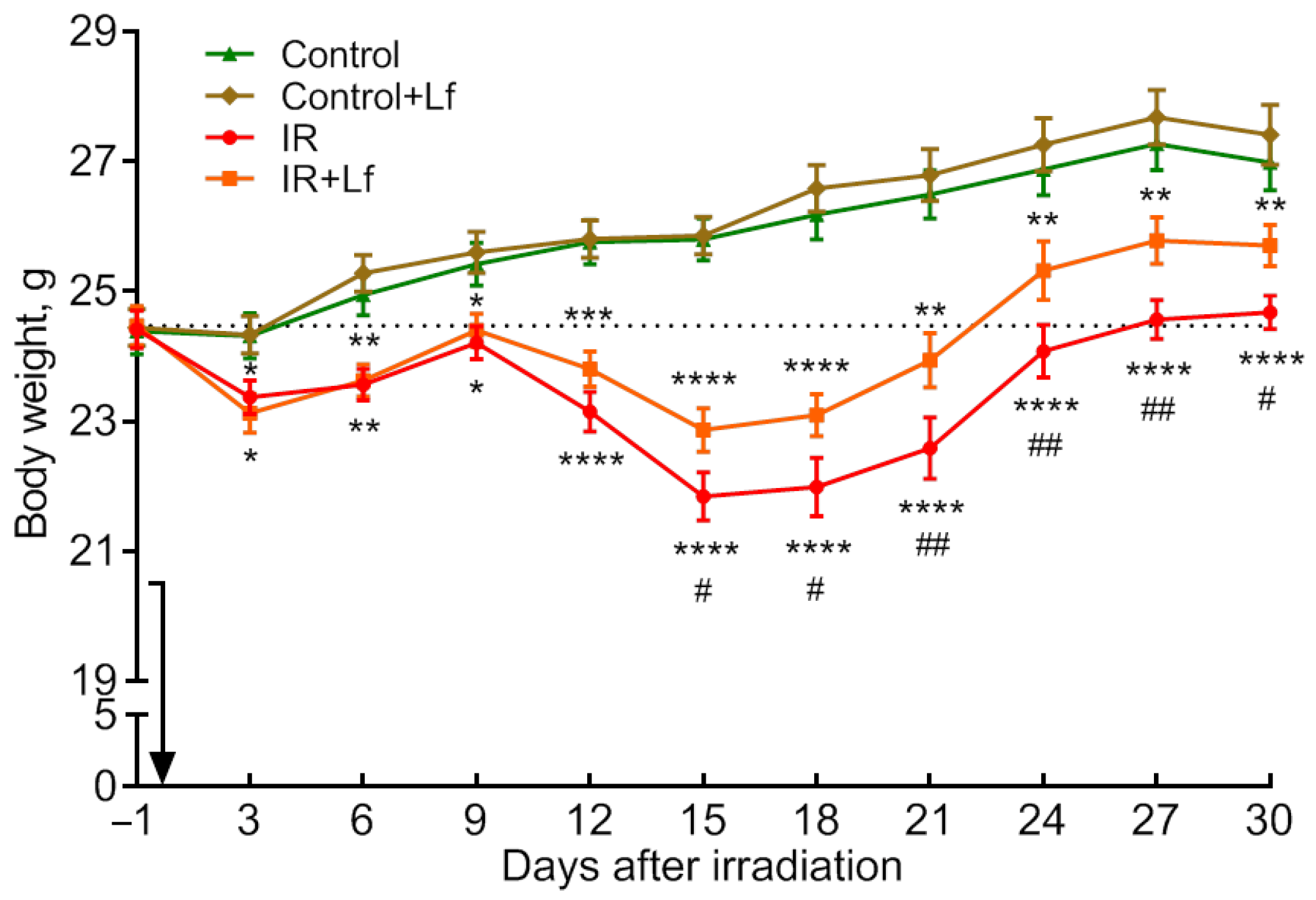
3.2. The Positive Effect of Lf on the Dynamics of Body Weight Recovery in Irradiated Mice
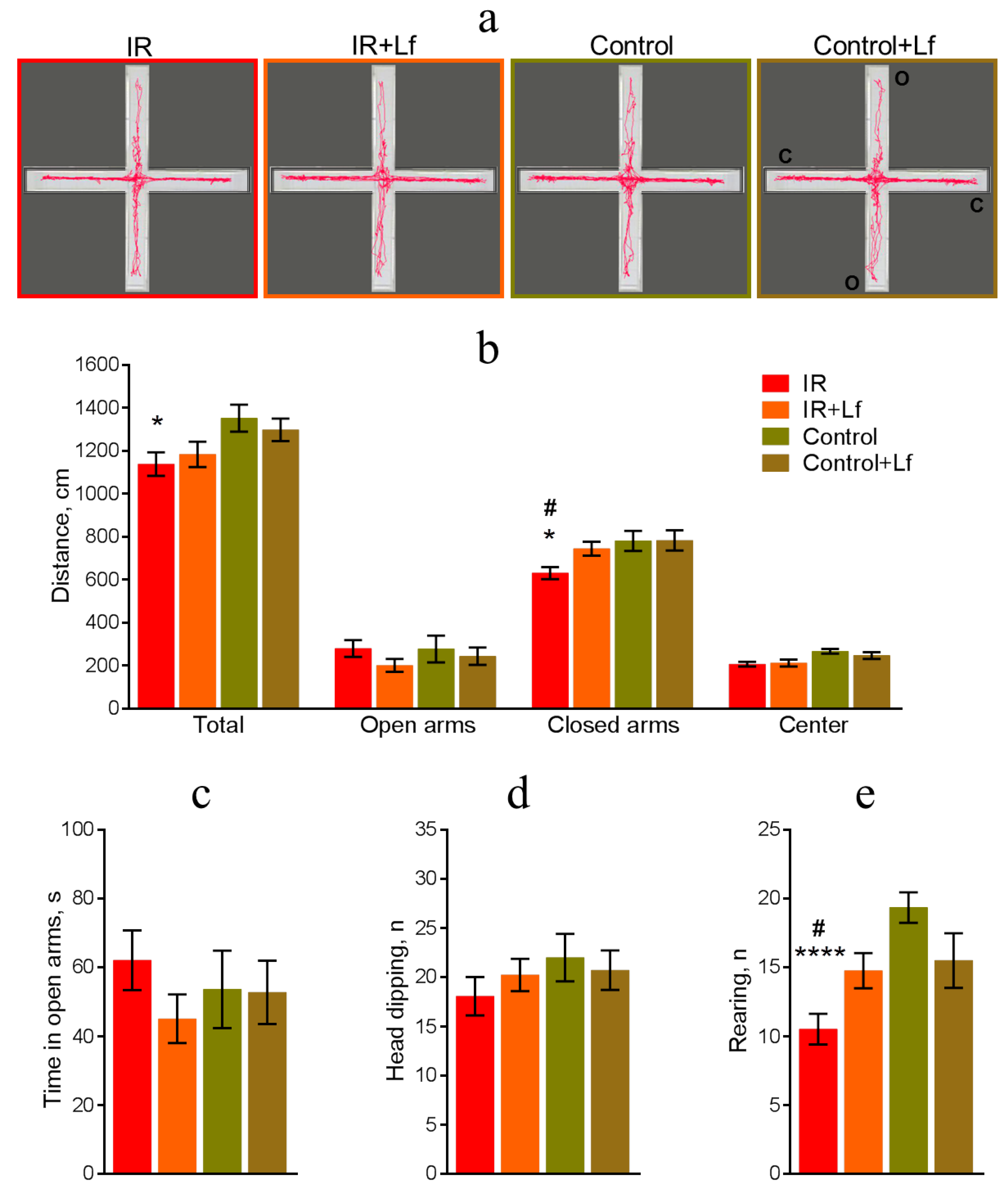
3.3. Lf Prevented Changes in the Behavior of Irradiated Mice
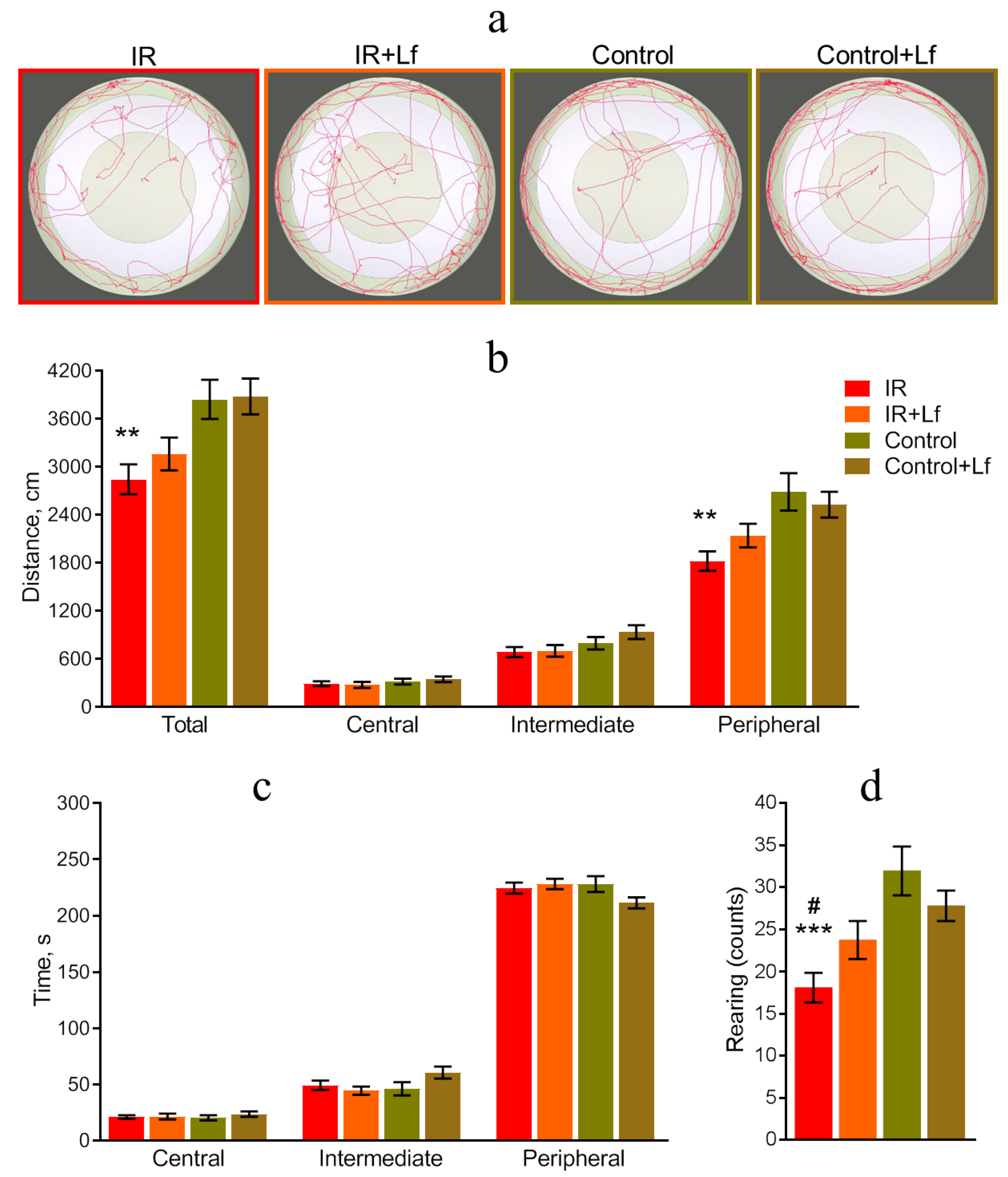
3.3.1. Open Field Test
3.3.2. Elevated Plus-Maze Testing
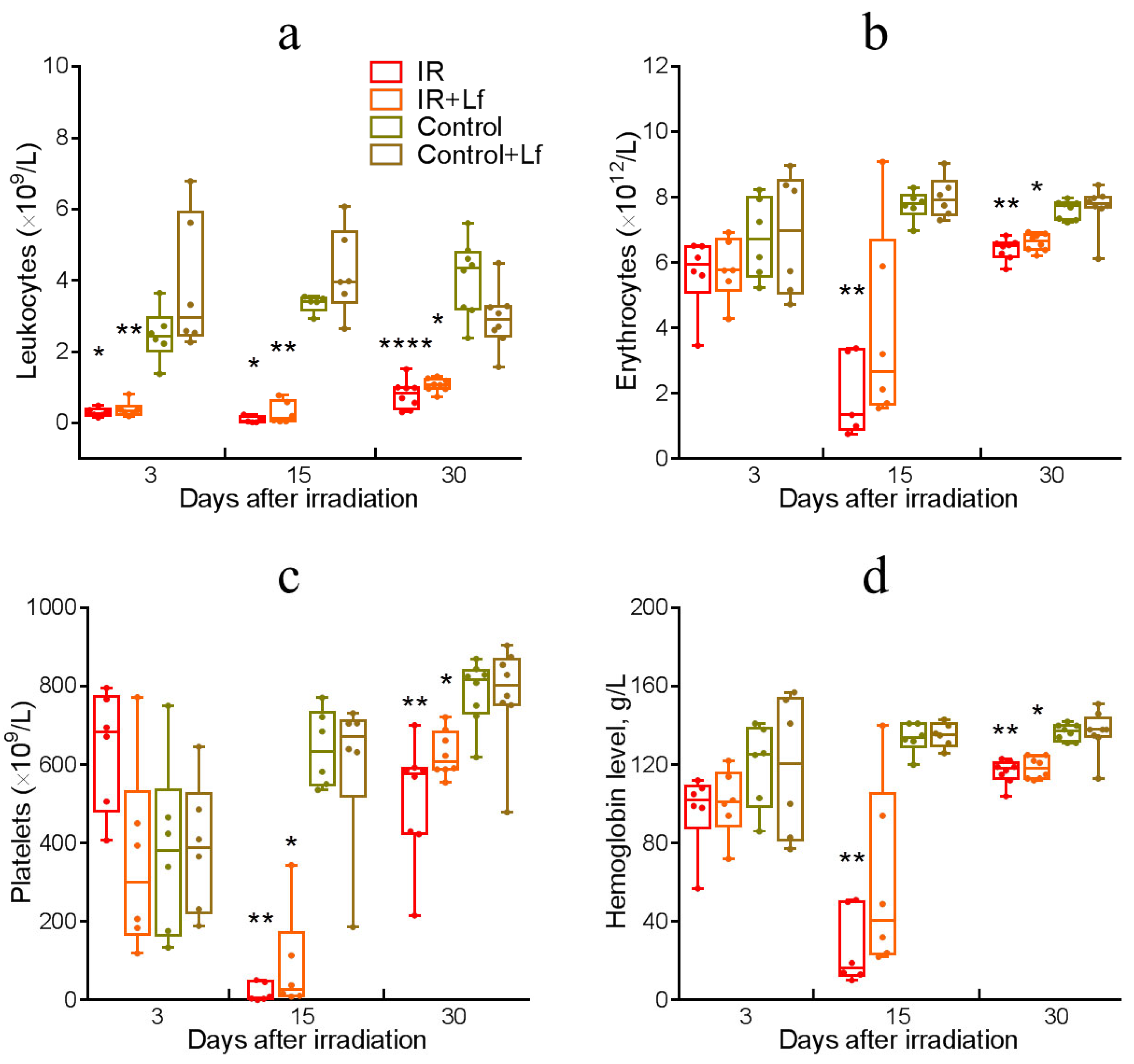
3.4. Dynamics of Blood Parameters in Irradiated Mice
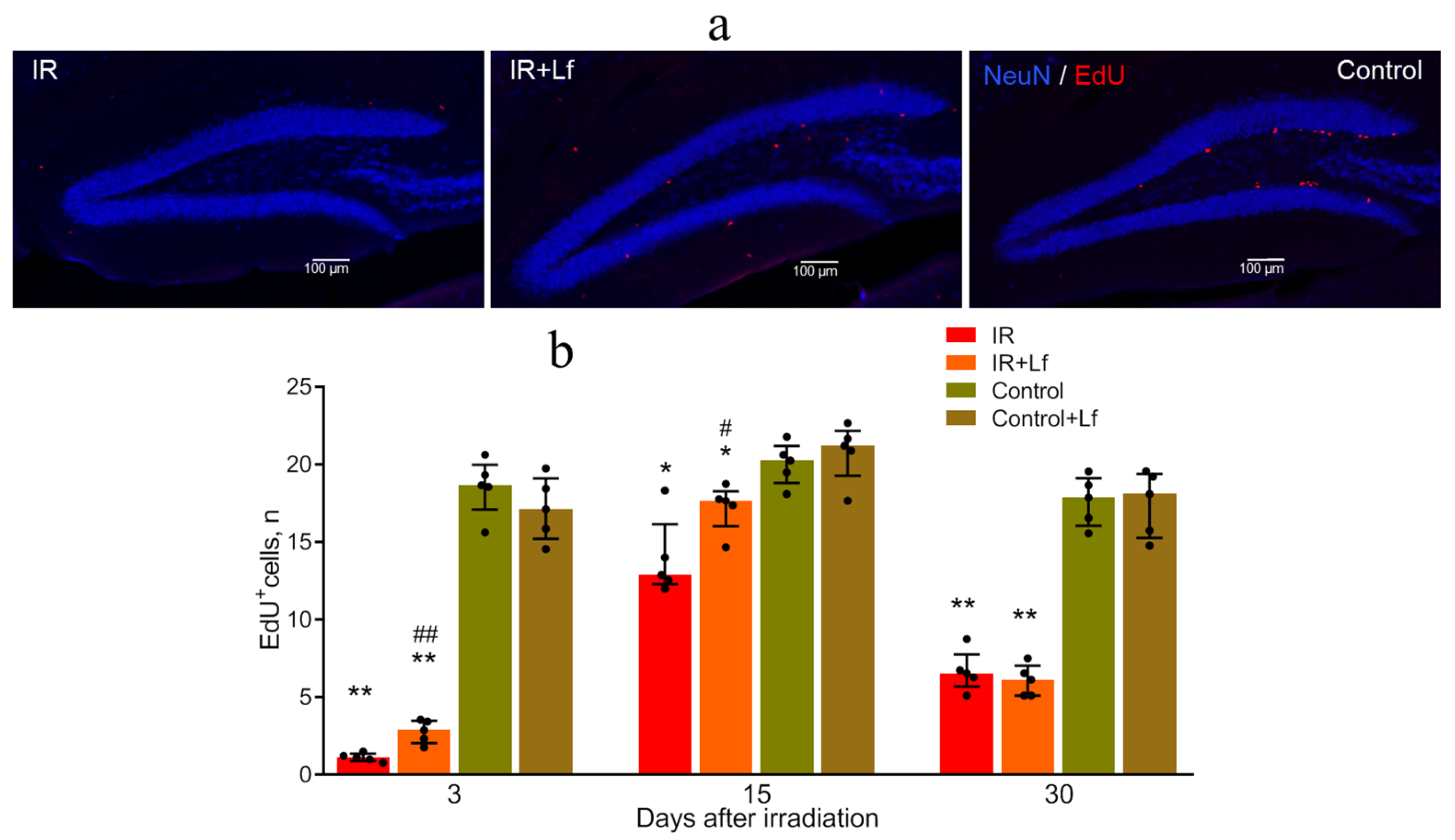
3.5. Dynamics of the Number of EdU+ Cells in the Bone Marrow of Irradiated Mice
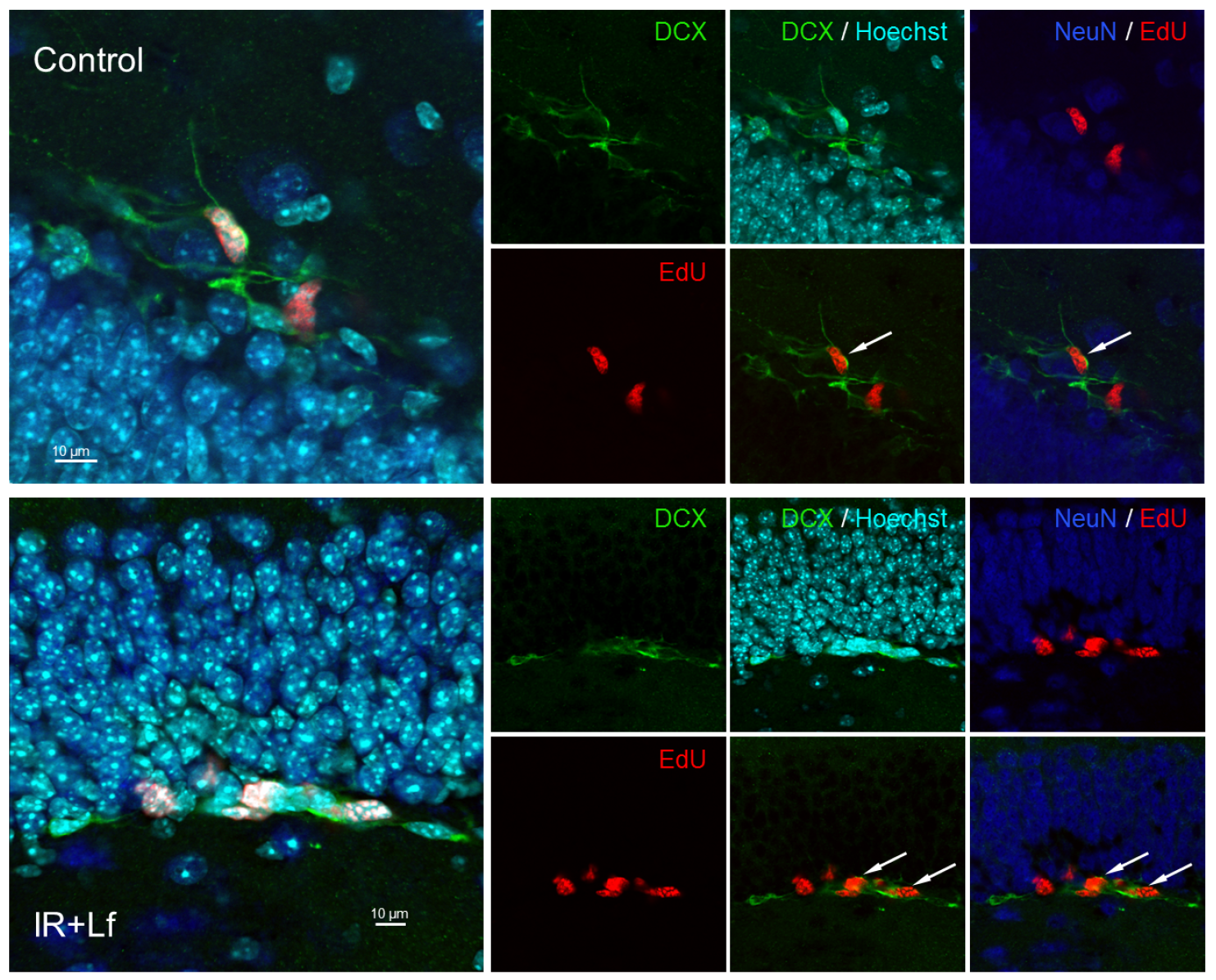
3.6. Radioprotective Effect of Lf on Mouse Brain Cells
3.6.1. Proliferating (EdU+) Cells
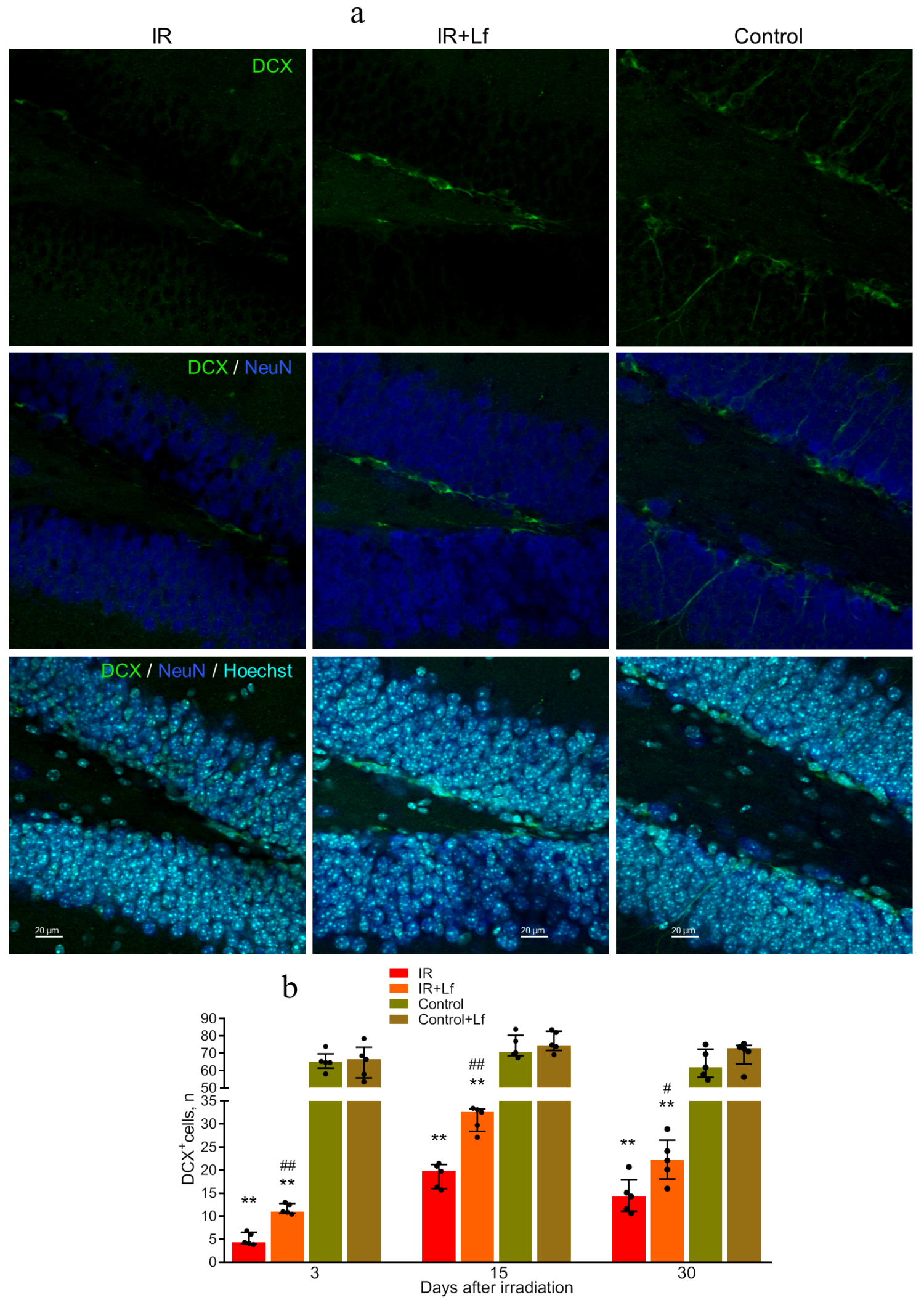
3.6.2. Immature Neurons (DCX+ Cells)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pazzaglia, S.; Briganti, G.; Mancuso, M.; Saran, A. Neurocognitive Decline Following Radiotherapy: Mechanisms and Therapeutic Implications. Cancers 2020, 12, 146. [Google Scholar] [CrossRef]
- Thomas, G.A.; Symonds, P. Radiation Exposure and Health Effects—Is It Time to Reassess the Real Consequences? Clin. Oncol. 2016, 28, 231–236. [Google Scholar] [CrossRef]
- Chang, D.S.; Lasley, F.D.; Das, I.J.; Mendonca, M.S.; Dynlacht, J.R. Acute Effects of Total Body Irradiation (TBI). In Basic Radiotherapy Physics and Biology; Springer: Cham, Switzerland, 2014; pp. 259–263. [Google Scholar]
- Wujanto, C.; Vellayappan, B.; Chang, E.L.; Chao, S.T.; Sahgal, A.; Lo, S.S. Radiotherapy to the Brain: What Are the Consequences of This Age-Old Treatment? Ann. Palliat. Med. 2021, 10, 936–952. [Google Scholar] [CrossRef]
- Roberts, S.A.; Spreadborough, A.R.; Bulman, B.; Barber, J.B.P.; Evans, D.G.R.; Scott, D. Heritability of Cellular Radiosensitivity: A Marker of Low-Penetrance Predisposition Genes in Breast Cancer? Am. J. Hum. Genet. 1999, 65, 784–794. [Google Scholar] [CrossRef]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-Induced Brain Injury: Current Concepts and Therapeutic Strategies Targeting Neuroinflammation. Neuro-Oncol. Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef]
- Balentova, S.; Adamkov, M. Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review. Int. J. Mol. Sci. 2015, 16, 27796–27815. [Google Scholar] [CrossRef]
- Tada, E.; Parent, J.M.; Lowenstein, D.H.; Fike, J.R. X-Irradiation Causes a Prolonged Reduction in Cell Proliferation in the Dentate Gyrus of Adult Rats. Neuroscience 2000, 99, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kopaeva, M.Y.; Cherepov, A.B.; Zaraiskaya, I.Y. Lactoferrin Has a Protective Effect on Mouse Brain Cells after Acute Gamma Irradiation of the Head. Bull. Exp. Biol. Med. 2023, 176, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Amelchenko, E.M.; Bezriadnov, D.V.; Chekhov, O.A.; Ivanova, A.A.; Kedrov, A.V.; Anokhin, K.V.; Lazutkin, A.A.; Enikolopov, G. Cognitive Flexibility Is Selectively Impaired by Radiation and Is Associated with Differential Recruitment of Adult-Born Neurons. J. Neurosci. 2023, 43, 6061–6083. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-Induced Brain Injury: A Review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef]
- Liu, R.; Page, M.; Solheim, K.; Fox, S.; Chang, S.M. Quality of Life in Adults with Brain Tumors: Current Knowledge and Future Directions. Neuro-Oncology 2009, 11, 330–339. [Google Scholar] [CrossRef]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme Sensitivity of Adult Neurogenesis to Low Doses of X-Irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar] [PubMed]
- Seed, T.M. Radiation protectants: Current status and future prospects. Health Phys. 2005, 89, 15. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Qin, L.; Huang, F.; Wang, X.; Yang, L.; Shi, H.; Wu, H.; Zhang, B.; Chen, Z.; Wu, X. Amentoflavone Protects Dopaminergic Neurons in MPTP-Induced Parkinson’s Disease Model Mice through PI3K/Akt and ERK Signaling Pathways. Toxicol. Appl. Pharmacol. 2017, 319, 80–90. [Google Scholar] [CrossRef]
- Hong, R.; Xie, A.; Jiang, C.; Guo, Y.; Zhang, Y.; Chen, J.; Shen, X.; Li, M.; Yue, X. A review of the biological activities of lactoferrin: Mechanisms and potential applications. Food Funct. 2024, 15, 8182–8199. [Google Scholar] [CrossRef]
- Nishimura, Y.; Homma-Takeda, S.; Kim, H.-S.; Kakuta, I. Radioprotection of Mice by Lactoferrin against Irradiation with Sublethal X-Rays. J. Radiat. Res. 2014, 55, 277–282. [Google Scholar] [CrossRef]
- Feng, L.; Li, J.; Qin, L.; Guo, D.; Ding, H.; Deng, D. Radioprotective Effect of Lactoferrin in Mice Exposed to Sublethal X-ray Irradiation. Exp. Ther. Med. 2018, 16, 3143–3148. [Google Scholar] [CrossRef]
- Kopaeva, M.Y.; Alchinova, I.B.; Cherepov, A.B.; Demorzhi, M.S.; Nesterenko, M.V.; Zarayskaya, I.Y.; Karganov, M.Y. New Properties of a Well-Known Antioxidant: Pleiotropic Effects of Human Lactoferrin in Mice Exposed to Gamma Irradiation in a Sublethal Dose. Antioxidants 2022, 11, 1833. [Google Scholar] [CrossRef]
- Kopaeva, M.Y.; Cherepov, A.B.; Nesterenko, M.V.; Zarayskaya, I.Y. Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure. Biology 2021, 10, 24. [Google Scholar] [CrossRef]
- Yong, S.J.; Veerakumarasivam, A.; Lim, W.L.; Chew, J. Neuroprotective Effects of Lactoferrin in Alzheimer’s and Parkinson’s Diseases: A Narrative Review. ACS Chem. Neurosci. 2023, 14, 1342–1355. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. GRN 000456 Cow’s Milk-Derived Lactoferrin; U.S. Food and Drug Administration’s Office of Food Additive Safety: Silver Spring, MD, USA, 2016. Available online: https://www.fda.gov/media/153787/download (accessed on 28 May 2025).
- Yamauchi, K.; Toida, T.; Nishimura, S.; Nagano, E.; Kusuoka, O.; Teraguchi, S.; Hayasawa, H.; Shimamura, S.; Tomita, M. 13-Week Oral Repeated Administration Toxicity Study of Bovine Lactoferrin in Rats. Food Chem. Toxicol. 2000, 38, 503–512. [Google Scholar] [CrossRef]
- Varadhachary, A.; Wolf, J.S.; Petrak, K.; O’Malley, B.W.; Spadaro, M.; Curcio, C.; Forni, G.; Pericle, F. Oral Lactoferrin Inhibits Growth of Established Tumors and Potentiates Conventional Chemotherapy. Int. J. Cancer 2004, 111, 398–403. [Google Scholar] [CrossRef]
- Troost, F.J.; Saris, W.H.M.; Brummer, R.-J.M. Recombinant Human Lactoferrin Ingestion Attenuates Indomethacin-Induced Enteropathy in Vivo in Healthy Volunteers. Eur. J. Clin. Nutr. 2003, 57, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.G.; Falchook, G.F.; Varadhachary, G.R.; Smith, D.P.; Davis, L.D.; Dhingra, H.M.; Hayes, B.P.; Varadhachary, A. Phase I Trial of Oral Talactoferrin Alfa in Refractory Solid Tumors. Invest. New Drugs 2006, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi Di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/eli/dir/2010/63/2019-06-26 (accessed on 28 May 2025).
- Liu, C.; Liu, J.; Hao, Y.; Gu, Y.; Yang, Z.; Li, H.; Li, R. 6,7,3′,4′-Tetrahydroxyisoflavone Improves the Survival of Whole-Body-Irradiated Mice via Restoration of Hematopoietic Function. Int. J. Radiat. Biol. 2017, 93, 793–802. [Google Scholar] [CrossRef]
- Alchinova, I.B.; Polyakova, M.V.; Yakovenko, E.N.; Medvedeva, Y.S.; Saburina, I.N.; Karganov, M.Y. Effect of Extracellular Vesicles Formed by Multipotent Mesenchymal Stromal Cells on Irradiated Animals. Bull. Exp. Biol. Med. 2019, 166, 574–579. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Gould, T.D., Ed.; Neuromethods; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 1–20. ISBN 978-1-60761-302-2. [Google Scholar]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Bekal, M.; Sun, L.; Ueno, S.; Moritake, T. Neurobehavioral Effects of Acute Low-Dose Whole-Body Irradiation. J. Radiat. Res. 2021, 62, 804–811. [Google Scholar] [CrossRef]
- Ivanova, A.; Gruzova, O.; Ermolaeva, E.; Astakhova, O.; Itaman, S.; Enikolopov, G.; Lazutkin, A. Synthetic Thymidine Analog Labeling without Misconceptions. Cells 2022, 11, 1888. [Google Scholar] [CrossRef]
- Amend, S.R.; Valkenburg, K.C.; Pienta, K.J. Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. J. Vis. Exp. 2016, 110, 53936. [Google Scholar] [CrossRef]
- Salic, A.; Mitchison, T.J. A Chemical Method for Fast and Sensitive Detection of DNA Synthesis in Vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Enikolopov, G. Identifying and Quantitating Neural Stem and Progenitor Cells in the Adult Brain. Methods Cell Biol. 2008, 85, 243–272. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Mandillo, S.; Tucci, V.; Hölter, S.M.; Meziane, H.; Al, M.; Kallnik, M.; Lad, H.V.; Nolan, P.M.; Ouagazzal, A.-M.; Coghill, E.L.; et al. Reliability, Robustness, and Reproducibility in Mouse Behavioral Phenotyping: A Cross-Laboratory Study. Physiol. Genom. 2008, 34, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the Elevated Plus-Maze and Open-Field Tests for the Assessment of Anxiety-Related Behaviour in Inbred Mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Buck, S.B.; Bradford, J.; Gee, K.R.; Agnew, B.J.; Clarke, S.T.; Salic, A. Detection of S-Phase Cell Cycle Progression Using 5-Ethynyl-2′-Deoxyuridine Incorporation with Click Chemistry, an Alternative to Using 5-Bromo-2′-Deoxyuridine Antibodies. BioTechniques 2008, 44, 927–929. [Google Scholar] [CrossRef]
- Flomerfelt, F.A.; Gress, R.E. Analysis of Cell Proliferation and Homeostasis Using EdU Labeling. Methods Mol. Biol. 2016, 1323, 211–220. [Google Scholar] [CrossRef]
- Nacher, J.; Crespo, C.; McEwen, B.S. Doublecortin Expression in the Adult Rat Telencephalon. Eur. J. Neurosci. 2001, 14, 629–644. [Google Scholar] [CrossRef]
- Raber, J.; Allen, A.R.; Rosi, S.; Sharma, S.; Dayger, C.; Davis, M.J.; Fike, J.R. Effects of Whole Body 56Fe Radiation on Contextual Freezing and Arc-Positive Cells in the Dentate Gyrus. Behav. Brain Res. 2013, 246, 162–167. [Google Scholar] [CrossRef]
- Vlkolinský, R.; Krucker, T.; Smith, A.L.; Lamp, T.C.; Nelson, G.A.; Obenaus, A. Effects of lipopolysaccharide on 56Fe-particle radiation-induced impairment of synaptic plasticity in the mouse hippocampus. Radiat. Res. 2007, 168, 462–470. [Google Scholar] [CrossRef]
- Seim, R.F.; Herring, L.E.; Mordant, A.L.; Willis, M.L.; Wallet, S.M.; Coleman, L.G.; Maile, R. Involvement of Extracellular Vesicles in the Progression, Diagnosis, Treatment, and Prevention of Whole-Body Ionizing Radiation-Induced Immune Dysfunction. Front. Immunol. 2023, 14, 1188830. [Google Scholar] [CrossRef]
- Kopaeva, M.Y.; Alchinova, I.B.; Nesterenko, M.V.; Cherepov, A.B.; Zarayskaya, I.Y.; Karganov, M.Y. Lactoferrin beneficially influences the recovery of physiological and behavioral indexes in mice exposed to acute gamma-irradiation. Patogenez. Pathog. 2020, 18, 29–33. (In Russian) [Google Scholar] [CrossRef]
- Van der Meeren, A.; Lebaron-Jacobs, L. Behavioural Consequences of an 8 Gy Total Body Irradiation in Mice: Regulation by Interleukin-4. Can. J. Physiol. Pharmacol. 2001, 79, 140–143. [Google Scholar] [CrossRef]
- Njamnshi, A.; Ahidjo, N.; Ngarka, L.; Nfor, L.; Mengnjo, M.; Njamnshi, W.; Basseguin Atchou, J.; Tatah, G.; Mbaku, L.; Dong, À.; et al. Characterization of the Cognitive and Motor Changes Revealed by the Elevated plus Maze in an Experimental Rat Model of Radiation-Induced Brain Injury. Adv. Biomed. Res. 2020, 9, 72. [Google Scholar] [CrossRef]
- Drayson, O.G.G.; Vozenin, M.-C.; Limoli, C.L. A Rigorous Behavioral Testing Platform for the Assessment of Radiation-Induced Neurological Outcomes. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 180, pp. 177–197. ISBN 978-0-323-99007-3. [Google Scholar]
- Li, Z.-T.; Wang, L.-M.; Yi, L.-R.; Jia, C.; Bai, F.; Peng, R.-J.; Yu, Z.-Y.; Xiong, G.-L.; Xing, S.; Shan, Y.-J.; et al. Succinate Ester Derivative of δ-Tocopherol Enhances the Protective Effects against 60Co γ-Ray-Induced Hematopoietic Injury through Granulocyte Colony-Stimulating Factor Induction in Mice. Sci. Rep. 2017, 7, 40380. [Google Scholar] [CrossRef] [PubMed]
- Solius, G.M.; Maltsev, D.I.; Belousov, V.V.; Podgorny, O.V. Recent Advances in Nucleotide Analogue-Based Techniques for Tracking Dividing Stem Cells: An Overview. J. Biol. Chem. 2021, 297, 101345. [Google Scholar] [CrossRef] [PubMed]
- Martí-Clúa, J. Methods for Inferring Cell Cycle Parameters Using Thymidine Analogues. Biology 2023, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-R.; Zhu, N.; Hao, Y.-T.; Yu, X.-C.; Li, Z.; Mao, R.-X.; Liu, R.; Kang, J.-W.; Hu, J.-N.; Li, Y. Radioprotective Effect of Whey Hydrolysate Peptides against γ-Radiation-Induced Oxidative Stress in BALB/c Mice. Nutrients 2021, 13, 816. [Google Scholar] [CrossRef]
- Abbott, L.C.; Nigussie, F. Adult Neurogenesis in the Mammalian Dentate Gyrus. Anat. Histol. Embryol. 2020, 49, 3–16. [Google Scholar] [CrossRef]
- Ribeiro, F.F.; Xapelli, S. An Overview of Adult Neurogenesis. In Recent Advances in NGF and Related Molecules. Advances in Experimental Medicine and Biology; Calzà, L., Aloe, L., Giardino, L., Eds.; Springer: Cham, Switzerland, 2021; Volume 1331, pp. 77–94. [Google Scholar] [CrossRef]
- Raber, J.; Rola, R.; LeFevour, A.; Morhardt, D.; Curley, J.; Mizumatsu, S.; VandenBerg, S.R.; Fike, J.R. Radiation-Induced Cognitive Impairments Are Associated with Changes in Indicators of Hippocampal Neurogenesis. Radiat. Res. 2004, 162, 39–47. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing Radiation-Induced Metabolic Oxidative Stress and Prolonged Cell Injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Tamminga, J.; Kovalchuk, O. Role of DNA Damage and Epigenetic DNA Methylation Changes in Radiation-Induced Genomic Instability and Bystander Effects in Germline in vivo. Curr. Mol. Pharmacol. 2011, 4, 115–125. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lönnerdal, B. Characterization of Mammalian Receptors for Lactoferrin. Biochem. Cell Biol. 2002, 80, 75–80. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Lactoferrin: Mammalian Lactoferrin Receptors: Structure and Function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Interactions of Lactoferrin with Cells Involved in Immune function. Biochem. Cell Biol. 2006, 84, 282–290. [Google Scholar] [CrossRef]
- Kopaeva, Y.; Cherepov, A.B.; Zarayskaya, I.Y.; Nesterenko, M.V. Transport of Human Lactoferrin into Mouse Brain: Administration Routes and Distribution. Bull. Exp. Biol. Med. 2019, 167, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lai, X.; Liang, X.; Chen, J.; Yang, Y.; Xu, W.; Qin, Q.; Qin, R.; Huang, X.; Xie, M.; et al. A Promise for Neuronal Repair: Reprogramming Astrocytes into Neurons in Vivo. Biosci. Rep. 2024, 44, BSR20231717. [Google Scholar] [CrossRef]
- Tan, Z.; Qin, S.; Yuan, Y.; Hu, X.; Huang, X.; Liu, H.; Pu, Y.; He, C.; Su, Z. NOTCH1 Signaling Regulates the Latent Neurogenic Program in Adult Reactive Astrocytes after Spinal Cord Injury. Theranostics 2022, 12, 4548–4563. [Google Scholar] [CrossRef]
- Ruggiero, M.; Cianciulli, A.; Calvello, R.; Lofrumento, D.D.; Saponaro, C.; Filannino, F.M.; Porro, C.; Panaro, M.A. Lactoferrin Attenuates Pro-Inflammatory Response and Promotes the Conversion into Neuronal Lineages in the Astrocytes. Int. J. Mol. Sci. 2025, 26, 405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, Z.; Zhu, X.; Shi, Y.; Tian, D.; Zhao, F.; Liu, N.; Hüppi, P.S.; Troy, F.A.; Wang, B. Lactoferrin Promotes Early Neurodevelopment and Cognition in Postnatal Piglets by Upregulating the BDNF Signaling Pathway and Polysialylation. Mol. Neurobiol. 2015, 52, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Guo, C. A Review on Lactoferrin and Central Nervous System Diseases. Cells 2021, 10, 1810. [Google Scholar] [CrossRef]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular Determinants of Milk Lactoferrin as a Bioactive Compound in Early Neurodevelopment and Cognition. J. Pediatr. 2016, 173, S29–S36. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopaeva, M.Y.; Cherepov, A.B.; Alchinova, I.B.; Shaposhnikova, D.A.; Rybakova, A.V.; Trashkov, A.P. Potential of Lactoferrin Against the Radiation-Induced Brain Injury. Cells 2025, 14, 1198. https://doi.org/10.3390/cells14151198
Kopaeva MY, Cherepov AB, Alchinova IB, Shaposhnikova DA, Rybakova AV, Trashkov AP. Potential of Lactoferrin Against the Radiation-Induced Brain Injury. Cells. 2025; 14(15):1198. https://doi.org/10.3390/cells14151198
Chicago/Turabian StyleKopaeva, Marina Yu., Anton B. Cherepov, Irina B. Alchinova, Daria A. Shaposhnikova, Anna V. Rybakova, and Alexandr P. Trashkov. 2025. "Potential of Lactoferrin Against the Radiation-Induced Brain Injury" Cells 14, no. 15: 1198. https://doi.org/10.3390/cells14151198
APA StyleKopaeva, M. Y., Cherepov, A. B., Alchinova, I. B., Shaposhnikova, D. A., Rybakova, A. V., & Trashkov, A. P. (2025). Potential of Lactoferrin Against the Radiation-Induced Brain Injury. Cells, 14(15), 1198. https://doi.org/10.3390/cells14151198







