Microvascular, Biochemical, and Clinical Impact of Hyperbaric Oxygen Therapy in Recalcitrant Diabetic Foot Ulcers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants Selection
2.2. Participants and Diabetic Foot Ulcer Characteristics
2.3. Laboratory and Molecular Analysis
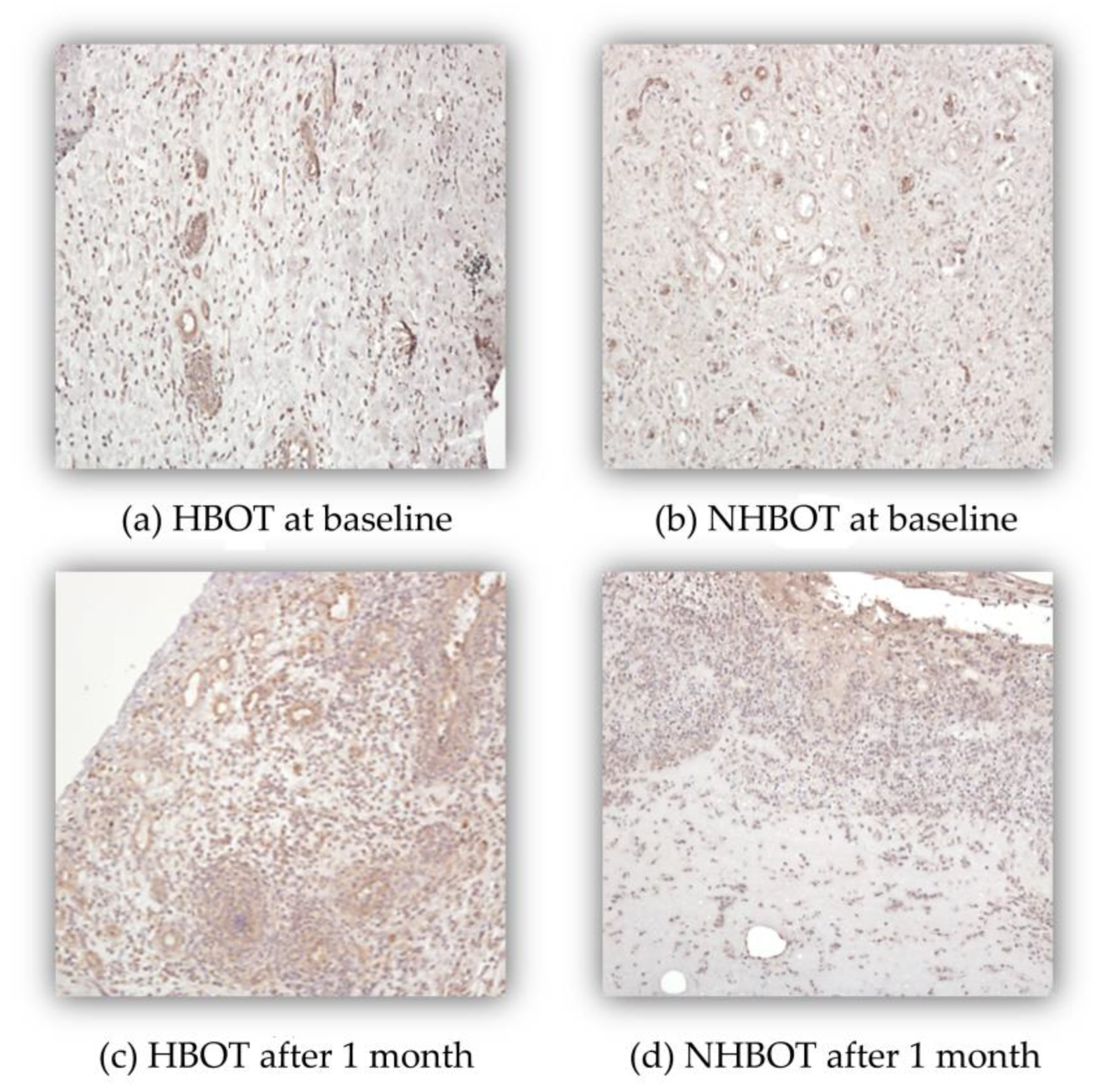
2.4. Immunohistochemistry Assays
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. DFU Evolution and Clinical Outcome in the Short Term
3.3. Metabolic Parameters
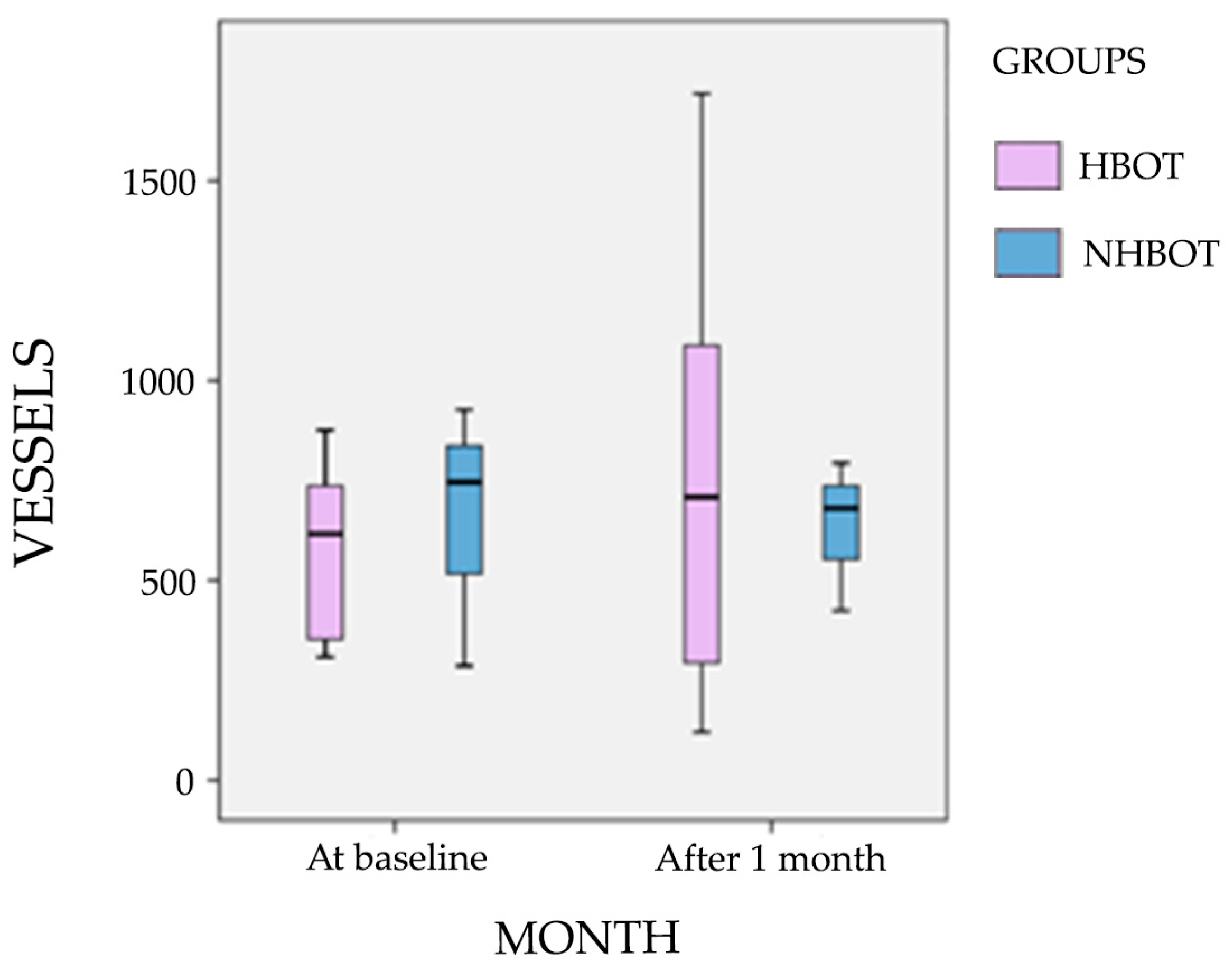
3.4. Microvascular Markers and Vessel Density
3.5. Long-Term Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Avidin Biotin Complex |
| ABI | Ankle Brachial Index |
| ATA | Atmospheres Absolute |
| BSI | Bovine Serum Albumin |
| CD | Cluster of Differentiation |
| CRP | C-reactive Protein |
| DAB | Diaminobenzidine |
| DFU | Diabetic Foot Ulcer |
| DM | Diabetes Mellitus |
| DPN | Diabetic Peripheral Neuropathy |
| EDTA | Ethylenediamine Tetraacetic Acid |
| ELISA | Enzyme-linked Immunosorbent Assay |
| EPC | Endothelial Progenitor Cells |
| ESR | Erythrocyte Sedimentation Rate |
| HbA1C | Glycated Hemoglobin A |
| HBOT | Hyperbaric Oxygen Therapy |
| HDL | High-density lipoprotein |
| H&E | Hematoxylin and Eosin |
| kPa | Kilopascal |
| LDL | Low-density Lipoprotein |
| LEA | Lower Extremity Amputation |
| mg/dL | Milligrams per deciliter |
| NHBOT | Non- Hyperbaric Oxygen Therapy |
| NRT | Non-randomized Trial |
| PlGF | Placental Growth Factor |
| PAD | Peripheral Arterial Disease |
| PBS | Phosphate Buffered Saline |
| pO2 | Tissue Oxygen Pressure |
| RCT | Randomized Controlled Trial |
| SD | Standard Deviation |
| SDF1-α | Stromal-derived Factor-Alpha |
| SWM | Semmes–Weinstein Monofilament |
| TcPO2 | Transcutaneous Partial Pressure of Oxygen |
| TUC | Texas University Classification |
| UK | United Kingdom |
| VEGF | Vascular Endothelial Growth Factor |
References
- Wild, S.; Roglic, G.; Green, A.; Sicreee, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://diabetesatlas.org (accessed on 5 May 2025).
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, 1.e1–1.e18; quiz 19–20. [Google Scholar] [CrossRef]
- Laing, T.; Hanson, R.; Chan, F.; Bouchier-Hayes, D. The role of endothelial dysfunction in the pathogenesis of impaired diabetic wound healing: A novel therapeutic target? Med. Hypotheses 2007, 69, 1029–1031. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, C.X.; Xu, B.; Yu, Z. Diabetic foot ulcers: Classification, risk factors and management. World J. Diabetes 2022, 13, 1049–1065. [Google Scholar] [CrossRef]
- Oyebode, O.A.; Jere, S.W.; Houreld, N.N. Current Therapeutic Modalities for the Management of Chronic Diabetic Wounds of the Foot. J. Diabetes Res. 2023, 2023, 1359537. [Google Scholar] [CrossRef]
- Costa, C.; Incio, J.; Soares, R. Angiogenesis and chronic inflammation: Cause or consequence? Angiogenesis 2007, 10, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, T.; Wang, J.; Huang, X.; Deng, X.; Cao, Y.; Zhou, M.; Zhao, C. An update on potential biomarkers for diagnosing diabetic foot ulcer at early stage. Biomed. Pharmacother. 2021, 133, 110991. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Brachvogel, B.; Odorisio, T.; Koch, M. Regulation of angiogenesis: Wound healing as a model. Prog. Histochem. Cytochem. 2007, 42, 115–170. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
- Gupta, S.; Mujawdiya, P.; Maheshwari, G.; Sagar, S. Dynamic Role of Oxygen in Wound Healing: A Microbial, Immunological, and Biochemical Perspective. Arch. Razi Inst. 2022, 77, 513–523. [Google Scholar] [CrossRef]
- Stavrou, D. Neovascularisation in wound healing. J. Wound Care 2008, 17, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, O.C. Angiogenesis and vasculogenesis: Inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J. Vasc. Surg. 2007, 45 (Suppl. A), A39–A47. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4344. [Google Scholar] [CrossRef] [PubMed]
- DA Cruz, D.L.M.; Oliveira-Pinto, J.; Mansilha, A. The role of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers: A systematic review with meta-analysis of randomized controlled trials on limb amputation and ulcer healing. Int. Angiol. A J. Int. Union Angiol. 2022, 41, 63–73. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef]
- Laitonjam, M.; Khan, M.M.; Krishna, D.; Cheruvu, V.P.R.; Minz, R. Reconstruction of Foot and Ankle Defects: A Prospective Analysis of Functional and Aesthetic Outcomes. Cureus 2023, 15, e40946. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Young, B.A.; Lin, E.; Von Korff, M.; Simon, G.; Ciechanowski, P.; Ludman, E.J.; Everson-Stewart, S.; Kinder, L.; Oliver, M.; Boyko, E.J.; et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am. J. Manag. Care 2008, 14, 15–23. [Google Scholar]
- Monteiro-Soares, M.; Dinis-Ribeiro, M. External validation and optimisation of a model for predicting foot ulcers in patients with diabetes. Diabetologia 2010, 53, 1525–1533. [Google Scholar] [CrossRef]
- Smieja, M.; Hunt, D.L.; Edelman, D.; Etchells, E.; Cornuz, J.; Simel, D.L. Clinical examination for the detection of protective sensation in the feet of diabetic patients. International Cooperative Group for Clinical Examination Research. J. Gen. Intern. Med. 1999, 14, 418–424. [Google Scholar] [CrossRef]
- Leenstra, B.; de Kleijn, R.; Kuppens, G.; Verhoeven, B.A.N.; Hinnen, J.W.; de Borst, G.J. Photo-Optical Transcutaneous Oxygen Tension Measurement Is of Added Value to Predict Diabetic Foot Ulcer Healing: An Observational Study. J. Clin. Med. 2020, 9, 3291. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef]
- Younes, N.A.; Albsoul, A.M. The DEPA scoring system and its correlation with the healing rate of diabetic foot ulcers. J. Foot Ankle Surg. 2004, 43, 209–213. [Google Scholar] [CrossRef]
- Beckert, S.; Witte, M.; Wicke, C.; Königsrainer, A.; Coerper, S. A new wound-based severity score for diabetic foot ulcers: A prospective analysis of 1,000 patients. Diabetes Care 2006, 29, 988–992. [Google Scholar] [CrossRef]
- Soares, R.; Balogh, G.; Guo, S.; Gärtner, F.; Russo, J.; Schmitt, F. Evidence for the notch signaling pathway on the role of estrogen in angiogenesis. Mol. Endocrinol. 2004, 18, 2333–2343. [Google Scholar] [CrossRef]
- Löndahl, M.; Katzman, P.; Nilsson, A.; Hammarlund, C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care 2010, 33, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Duzgun, A.P.; Satir, H.Z.; Ozozan, O.; Saylam, B.; Kulah, B.; Coskun, F. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J. Foot Ankle Surg. 2008, 47, 515–519. [Google Scholar] [CrossRef]
- Abidia, A.; Laden, G.; Kuhan, G.; Johnson, B.F.; Wilkinson, A.R.; Renwick, P.M.; Masson, E.A.; McCollum, P.T. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: A double-blind randomised-controlled trial. Eur. J. Vasc. Endovasc. Surg. 2003, 25, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Kuo, Y.R.; Wu, R.W.; Liu, R.T.; Hsu, C.S.; Wang, F.S.; Yang, K.D. Extracorporeal shockwave treatment for chronic diabetic foot ulcers. J. Surg. Res. 2009, 152, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Faglia, E.; Favales, F.; Aldeghi, A.; Calia, P.; Quarantiello, A.; Oriani, G.; Michael, M.; Campagnoli, P.; Morabito, A. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care 1996, 19, 1338–1343. [Google Scholar] [CrossRef]
- Kessler, L.; Bilbault, P.; Ortéga, F.; Grasso, C.; Passemard, R.; Stephan, D.; Pinget, M.; Schneider, F. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: A prospective randomized study. Diabetes Care 2003, 26, 2378–2382. [Google Scholar] [CrossRef]
- Kalani, M.; Jörneskog, G.; Naderi, N.; Lind, F.; Brismar, K. Hyperbaric oxygen (HBO) therapy in treatment of diabetic foot ulcers. Long-term follow-up. J. Diabetes Complicat. 2002, 16, 153–158. [Google Scholar] [CrossRef]
- Doctor, N.; Pandya, S.; Supe, A. Hyperbaric oxygen therapy in diabetic foot. J. Postgrad. Med. 1992, 38, 112–114. [Google Scholar]
- Löndahl, M. Hyperbaric oxygen therapy as treatment of diabetic foot ulcers. Diabetes Metab. Res. Rev. 2012, 28 (Suppl. 1), 78–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wu, R.W.; Hsu, M.C.; Hsieh, C.J.; Chou, M.C. Adjunctive Hyperbaric Oxygen Therapy for Healing of Chronic Diabetic Foot Ulcers: A Randomized Controlled Trial. J Wound Ostomy Continence Nurs. 2017, 44, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.; Sousa, J. Long-term evaluation of chronic diabetic foot ulcers, non-healed after hyperbaric oxygen therapy. Rev. Port Cir. Cardiotorac. Vasc. 2005, 12, 227–237. [Google Scholar]
- Zamboni, W.A.; Wong, H.P.; Stephenson, L.L.; Pfeifer, M.A. Evaluation of hyperbaric oxygen for diabetic wounds: A prospective study. Undersea Hyperb. Med. 1997, 24, 175–179. [Google Scholar]
- de Meijer, V.E.; Van’t Sant, H.P.; Spronk, S.; Kusters, F.J.; den Hoed, P.T. Reference value of transcutaneous oxygen measurement in diabetic patients compared with nondiabetic patients. J. Vasc. Surg. 2008, 48, 382–388. [Google Scholar] [CrossRef]
- Chen, C.E.; Ko, J.Y.; Fong, C.Y.; Juhn, R.J. Treatment of diabetic foot infection with hyperbaric oxygen therapy. Foot Ankle Surg. 2010, 16, 91–95. [Google Scholar] [CrossRef]
- Albuquerque e Sousa, J. Oxigenoterapia hiperbárica (OTHB). Perspectiva histórica, efeitos fisiológicos e aplicações clínicas. Med. Interna 2007, 14, 219–227. [Google Scholar]
- Thom, S.R. Hyperbaric oxygen: Its mechanisms and efficacy. Plast Reconstr. Surg. 2011, 127 (Suppl. 1), 131S–141S. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, F.; Yang, J.; Li, Z.; Hou, X.; Wang, Y.; Gao, C. Effects of stromal cell derived factor-1 and CXCR4 on the promotion of neovascularization by hyperbaric oxygen treatment in skin flaps. Mol. Med. Rep. 2013, 8, 1118–1124. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Dhamodharan, U.; Karan, A.N.; Anandharaj, A.; Rajesh, K.; Ramkumar, K.M. Role of Nrf2 in MALAT1/HIF-1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic. Biol. Med. 2020, 156, 168–175. [Google Scholar] [CrossRef]
- Costa, P.Z.; Soares, R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013, 92, 1037–1045. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. The Hyperoxic-Hypoxic Paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
- Oropallo, A.R.; Serena, T.E.; Armstrong, D.G.; Niederauer, M.Q. Molecular Biomarkers of Oxygen Therapy in Patients with Diabetic Foot Ulcers. Biomolecules 2021, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Soares, M.; Martins-Mendes, D.; Vaz-Carneiro, A.; Sampaio, S.; Dinis-Ribeiro, M. Classification systems for lower extremity amputation prediction in subjects with active diabetic foot ulcer: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2014, 30, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. The pathophysiology of diabetic foot: A narrative review. J. Yeungnam Med. Sci. 2023, 40, 328–334. [Google Scholar] [CrossRef]
- Du, W.; Ren, L.; Hamblin, M.H.; Fan, Y. Endothelial Cell Glucose Metabolism and Angiogenesis. Biomedicines 2021, 9, 147. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Lindenmann, J.; Kamolz, L.; Graier, W.; Smolle, J.; Smolle-Juettner, F.M. Hyperbaric Oxygen Therapy and Tissue Regeneration: A Literature Survey. Biomedicines 2022, 10, 3145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, W.; Xu, Y.; Liu, D. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcers: An updated systematic review and meta-analysis. Asian J. Surg. 2022, 45, 68–78. [Google Scholar] [CrossRef] [PubMed]
| Timepoint | Baseline (M0) | 1 Month (M1) | 3 Months (M3) | 6 Months (M6) | 12 Months (M12) | 36 Months (M36) |
|---|---|---|---|---|---|---|
| Clinical Evaluation (e.g., ulcer healing, LEA, recurrence, death) | Yes | Yes | Yes | Yes | Yes | Yes |
| Digital Wound Measurement | Yes | Yes | Yes | Yes | Yes | No |
| Blood Biomarkers (e.g., CRP, VEGF, PlGF, SDF1-α) | Yes | No | Yes | Yes | No | No |
| Histological Analysis (CD31) | Yes (subset only) | Yes (subset only) | No | No | No | No |
| Variables | Global (n = 20) | NHBOT DFU (n = 6) | HBOT DFU (n = 14) | p Value |
|---|---|---|---|---|
| Subject Characterization | ||||
| Age [mean (SD)] | 62 (12) | 63 (11) | 61 (13) | 0.8 * |
| Male gender [n (%)] | 17 (85) | 6 (100) | 11 (79) | 0.5 γ |
| Visual impairment [n (%)] | 16 (80) | 5 (83) | 11 (79) | 1.0 γ |
| Physical impairment [n (%)] | 13 (65) | 4 (67) | 9 (64) | 1.0 γ |
| Past or present smoker [n (%)] | 12 (60) | 4 (67) | 8 (57) | 0.4 γ |
| DM and Its Complications | ||||
| Type 2 [n (%)] | 19 (95) | 6 (100) | 13 (93) | 1.0 γ |
| Duration (in years) [mean (SD)] | 18 (9) | 15 (7) | 19 (10) | 0.3 * |
| Insulin use [n (%)] | 15 (70) | 5 (83) | 9 (64) | 0.7 γ |
| Cardiovascular complication history [n (%)] | 6 (30) | 1 (17) | 5 (36) | 0.6 γ |
| Retinopathy complication history [n (%)] | 18 (90) | 6 (100) | 12 (86) | 1.0 γ |
| Nephropathy complication history [n (%)] | 11 (55) | 5 (83) | 6 (43) | 0.2 γ |
| Cerebrovascular complication history [n (%)] | 3 (15) | 0 (0) | 3 (21) | 0.5 γ |
| PAD complication history [n (%)] | 17 (85) | 6 (100) | 11 (79) | 0.5 γ |
| Neuropathy complication history [n (%)] | 17 (85) | 4 (67) | 13 (93) | 0.2 γ |
| Metabolic complication history [n (%)] | 9 (45) | 2 (33) | 7 (50) | 0.6 γ |
| Complications count [median (range)] | 4 (5) | 4 (2) | 4 (2) | 1.0 α |
| DFU Foot Characterization | ||||
| Foot deformity [n (%)] | 11 (55) | 2 (33) | 9 (64) | 0.3 γ |
| Total foot pulses ≤ 1 [n (%)] | 19 (95) | 6 (100) | 13 (93) | 1.0 γ |
| ABI < 0.8 [n (%)] | 5 (25) | 2 (33) | 3 (21) | 0.6 γ |
| TcPO2 [median (range)] | 19 (67) | 24 (34) | 16 (67) | 0.6 α |
| Intermittent claudication [n (%)] | 12 (60) | 5 (83) | 7 (50) | 0.3 γ |
| DPN symptoms [n (%)] | 18 (90) | 5 (83) | 13 (93) | 0.5 γ |
| Altered SWM sensation [n (%)] a | 14 (88) | 3 (60) | 11 (100) | 0.08 γ |
| Previous DFU [n (%)] | 14 (70) | 6 (100) | 8 (57) | 0.1 γ |
| Previous LEA [n (%)] | 7 (35) | 3 (50) | 4 (29) | 0.6 γ |
| DFU Characterization | ||||
| Texas grade | ||||
| III (Bone or joint) [n (%)] | 20 (100) | 6 (100) | 14 (100) | 1.0 γ |
| Texas stage | ||||
| B (Infection) [n (%)] | 1 (5) | 0 (0) | 1 (7) | 1.0 γ |
| D (Infection plus ischemia) [n (%)] | 19 (95) | 6 (100) | 13 (93) | |
| Located at toes [n (%)] | 5 (25) | 3 (50) | 2 (14) | 0.1 γ |
| Variables | Global (n = 20) | NHBOT DFU Patients (n = 6) | Paired Samples Tests p Value | HBOT DFU Patients (n = 14) | Paired Samples Tests p Value | Independent Samples Tests p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| M0 | M3 | M0 | M3 | M0 | M3 | M0 | M3 | |||
| Baseline ulcer area (in cm2) [median (range)] | 11.0 (31.1) a | 2.7 (24.8) b | 12.1 (16.1) | 13.1 (23.5) b | NP | 7.3 (31.1) a | 2.7 (16.3) | 0.001 * | 0.4 α | NP |
| Mean depth (in mm) [median (range)] | 2.3 (11.6) c | 1.7 (6.5) d | 1.8 (4.7) | 3.5 (3.6) b | NP | 2.3 (11.6) c | 1.4 (6.5) a | 0.1 * | 0.6 α | NP |
| Maximum depth (in mm) [median (range)] | 4.6 (18.3) c | 3.3 (10.7) d | 3.6 (8.2) | 7.9 (5.2) b | NP | 4.6 (18.1) c | 3.2 (10.7) a | 0.03 * | 0.3 α | NP |
| Volume (in cm3) [median (range)] | 1.2 (24.8) c | 0.4 (13.8) d | 1.5 (10.4) | 7.0 (13.6) b | NP | 1.2 (24.5) c | 0.4 (10.7) a | 0.006 * | 0.8 α | NP |
| Variables | Global (n = 20) | NHBOT DFU Patients (n = 6) | HBOT DFU Patients (n = 14) | Comparison Between Groups Fisher’s Exact Test p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M3 | M6 | M12 | M3 | M6 | M12 | M3 | M6 | M12 | M3 | M6 | M12 | |
| Complete healing/improvement [n (%)] | 15 (75) | 15 (75) | 12 (60) | 1 (17) | 1 (17) | 1 (17) | 14 (100) | 14 (100) | 11 (79) | <0.001 | <0.001 | 0.02 |
| Major LEA or death [n (%)] | 5 (25) | 5 (25) | 8 (40) | 5 (83) | 5 (83) | 5 (83) | 0 (0) | 0 (0) | 3 (21) | |||
| Variables | Global | NHBOT (n = 6) | p Value | HBOT (n = 14) | p Value | NHBOT vs. HBOT p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M0 | M3 | M6 | M0 | M3 | M0 | M3 | M0 | M3 | |||
| Glucose (mg/dL) [mean (SD)] | 174 (78) | 212 (82) | 211 (75) | 194 (110) | 236 (109) | 0.5 ε | 165 (64) | 200 (68) | 0.04 ε | 0.5 ж | 0.4 ж |
| HbA1C (in %) [mean (SD)] | 8.0 (1.8) | 8.3 (1.8) | 8.2 (1.6) | 8.3 (2.1) | 9.5 (2.9) | 0.2 ε | 7.9 (1.7) | 7.8 (1.1) | 1.0 ε | 0.6 ж | 0.09 ж |
| Hemoglobin (g/dL) [mean (SD)] | 12 (1) | 12 (2) | 12 (2) | 12 (1) | 11 (2) | 0.3 ε | 12 (1) | 12 (2) | 0.9 ε | 0.8 ж | 0.7 ж |
| Leukocytes (×103/dL) [mean (SD)] | 10.0 (3.4) | 10.0 (3.7) | 7.9 (1.8) | 11.5 (4.1) | 12.5 (4.4) | 0.4 ε | 9.4 (3.0) | 8.7 (2.7) | 0.4 ε | 0.2 ж | 0.09 ж |
| Platelets (×103/dL) [mean (SD)] | 271 (74) | 265 (79) | 229 (76) | 256 (57) | 306 (90) | 0.2 ε | 291 (76) | 244 (67) | 0.04 ε | 0.6 ж | 0.2 ж |
| Total cholesterol (mg/dL) [mean (SD)] | 168 (52) | 153 (37) | 151 (24) | 163 (68) | 168 (57) | 0.5 ε | 170 (46) | 147 (26) | 0.1 ε | 0.8 ж | 0.5 ж |
| LDL cholesterol (mg/dL) [mean (SD)] | 102 (46) | 84 (32) | 83 (24) | 97 (57) | 97 (53) | 0.5 ε | 104 (43) | 79 (20) | 0.07 ε | 0.8 ж | 0.5 ж |
| HDL cholesterol (mg/dL) [mean (SD)] | 42 (9) | 41 (12) | 42 (17) | 43 (10) | 36 (13) | 0.1 ε | 41 (9) | 43 (11) | 0.6 ε | 0.7 ж | 0.3 ж |
| Triglycerides (mg/dL) [mean (SD)] | 131 (56) | 143 (85) | 131 (70) | 112 (44) | 177 (82) | 0.1 ε | 133 (60) | 130 (86) | 0.9 ε | 0.5 ж | 0.3 ж |
| Uric acid (mg/dL) [mean (SD)] | 5 (2) | 6 (2) | 6 (2) | 5 (5) | 7 (7) | 0.04 ε | 5 (6) | 5 (6) | 0.3 ε | 0.9 ж | 0.2 ж |
| Urea (mg/dL) [mean (SD)] | 58 (25) | 69 (47) | 63 (34) | 66 (33) | 92 (65) | 0.1 ε | 54 (21) | 57 (32) | 0.5 ε | 0.4 ж | 0.1ж |
| Creatinine (mg/dL) [median (range)] | 1.4 (7.3) | 1.5 (8.2) | 1.1 (0.6) | 1.3 (7.3) | 1.6 (8.2) | 0.2 Ϫ | 1.0 (1.8) | 0.9 (2.9) | 0.6 Ϫ | 0.6 α | 1.0 α |
| Microalbuminuria [n (%)] | 11 (55) | 12 (60) | 9 (45) | 4 (67) | 4 (67) | 1.0 γ | 7 (50) | 8 (57) | 0.9 γ | 0.3 γ | 0.6 γ |
| Erythrocyte sedimentation rate (mm) [median (range)] | 65 (97) | 37 (111) | 42 (107) | 104 (32) | 33 (16) | 0.02 | 58 (72) | 30 (111) | 0.04 Ϫ | 0.1 α | 0.6 α |
| C-reactive protein (mg/dL) [median (range)] | 0.9 (18) | 0.3 (51) | 0.3 (9) | 2.1 (11) | 11.8 (22.4) | 0.1 Ϫ | 0.7 (15.7) | 0.3 (5.4) | 0.08 Ϫ | 0.2 α | 0.03 α |
| VEGF (pg/mL) [median (range)] | 90 (236) | 63 (344) | 47 (29) | 189 (236) | 99 (344) | 0.9 Ϫ | 72 (268) | 56 (129) | 0.06 Ϫ | 0.6 α | 0.06 α |
| PlGF (pg/mL) [median (range)] | 13.4 (66.0) | 8.5 (65.8) | 10.5 (51.2) | 3.1 (14.7) | 7.4 (12.1) | 0.7 Ϫ | 14.8 (65.7) | 9.6 (64.0) | 0.08 Ϫ | 0.2 α | 0.4 α |
| SDF1-α (pg/mL) [mean (SD)] | 1890 (687) | 2132 (563) | 1738 (709) | 2297 (514) | 2455 (476) | 0.4 ε | 1716 (691) | 2017 (562) | 0.1 ε | 0.08 ж | 0.1 ж |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins-Mendes, D.; Costa, R.; Rodrigues, I.; Camacho, Ó.; Coelho, P.B.; Paixão-Dias, V.; Luís, C.; Pereira, A.C.; Fernandes, R.; Lima, J.; et al. Microvascular, Biochemical, and Clinical Impact of Hyperbaric Oxygen Therapy in Recalcitrant Diabetic Foot Ulcers. Cells 2025, 14, 1196. https://doi.org/10.3390/cells14151196
Martins-Mendes D, Costa R, Rodrigues I, Camacho Ó, Coelho PB, Paixão-Dias V, Luís C, Pereira AC, Fernandes R, Lima J, et al. Microvascular, Biochemical, and Clinical Impact of Hyperbaric Oxygen Therapy in Recalcitrant Diabetic Foot Ulcers. Cells. 2025; 14(15):1196. https://doi.org/10.3390/cells14151196
Chicago/Turabian StyleMartins-Mendes, Daniela, Raquel Costa, Ilda Rodrigues, Óscar Camacho, Pedro Barata Coelho, Vítor Paixão-Dias, Carla Luís, Ana Cláudia Pereira, Rúben Fernandes, Jorge Lima, and et al. 2025. "Microvascular, Biochemical, and Clinical Impact of Hyperbaric Oxygen Therapy in Recalcitrant Diabetic Foot Ulcers" Cells 14, no. 15: 1196. https://doi.org/10.3390/cells14151196
APA StyleMartins-Mendes, D., Costa, R., Rodrigues, I., Camacho, Ó., Coelho, P. B., Paixão-Dias, V., Luís, C., Pereira, A. C., Fernandes, R., Lima, J., & Soares, R. (2025). Microvascular, Biochemical, and Clinical Impact of Hyperbaric Oxygen Therapy in Recalcitrant Diabetic Foot Ulcers. Cells, 14(15), 1196. https://doi.org/10.3390/cells14151196









