The Cytotoxic Potential of Humanized γδ T Cells Against Human Cancer Cell Lines in In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Humanized Gamma Delta (γδ) Humanized T Cell Receptor (HuTCR)-T1 Mice
2.2. Isolation of Mononuclear Cells (MNCs) and Gamma-Delta (γδ) T Cells
2.3. Cell Lines and Co-Culture
2.4. Lactate Dehydrogenase (LDH) Cytotoxicity and Apoptosis Assay
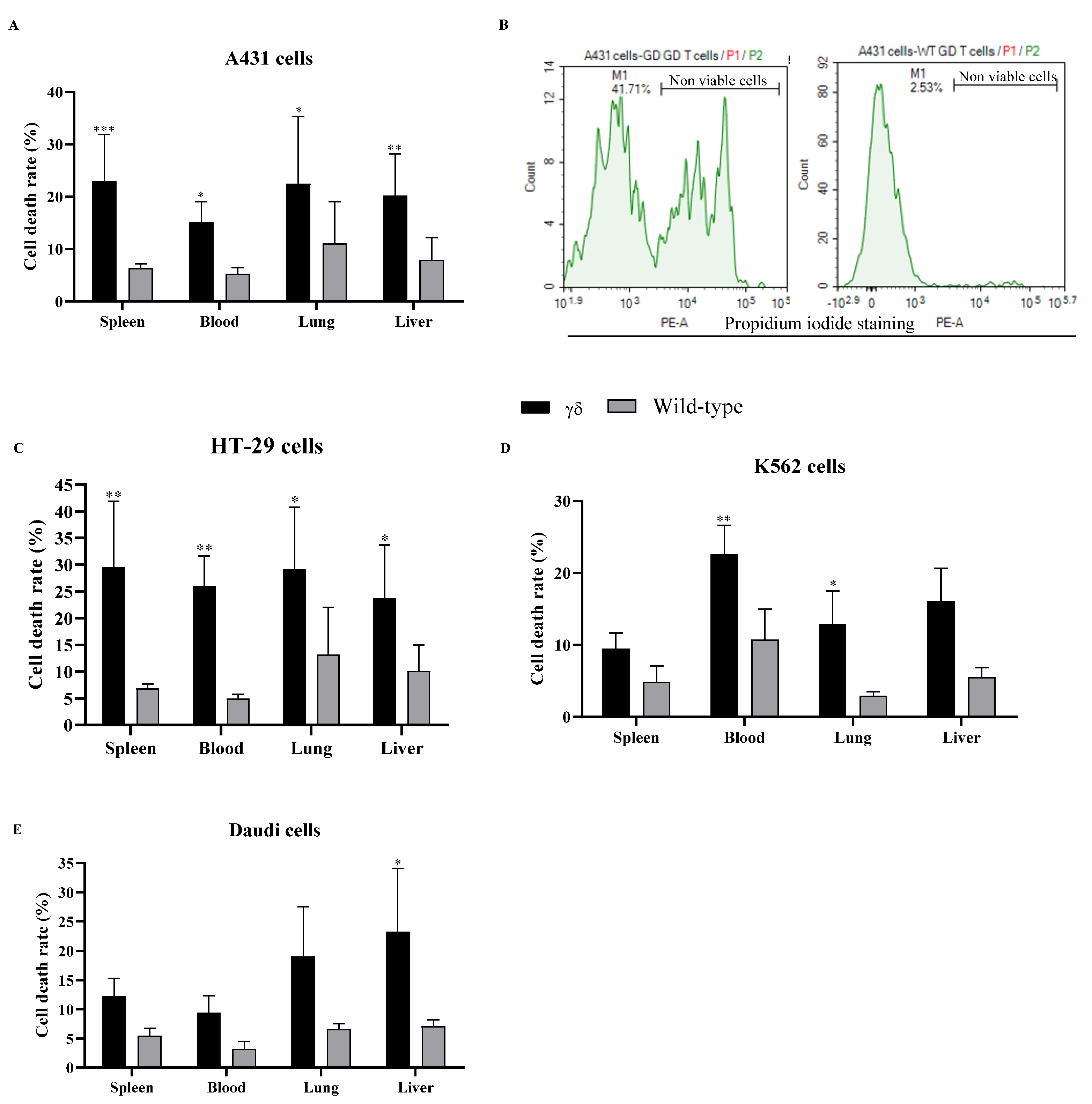
2.5. Propidium Iodide (PI) Staining for Cell Death
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) and Enzyme-Linked Immunosorbent Spot (ELISPOT) Assay
2.7. Statistical Analysis
3. Results
3.1. Humanized T Cell Receptor (HuTCR)-T1 Mice Gamma Delta (γδ) T Cells Demonstrated Greater Cytotoxicity Against Human Cancer Cells
3.2. HuTCR-T1 Mice γδ T Cells Enhanced the Production of Anticancer Cytokine-Specific ELISPOTs in Response to Stimulation
3.3. HuTCR-T1 Mice γδ T Cells Increased the Levels of Anticancer Cytokines and Granzyme B in Response to Stimulation
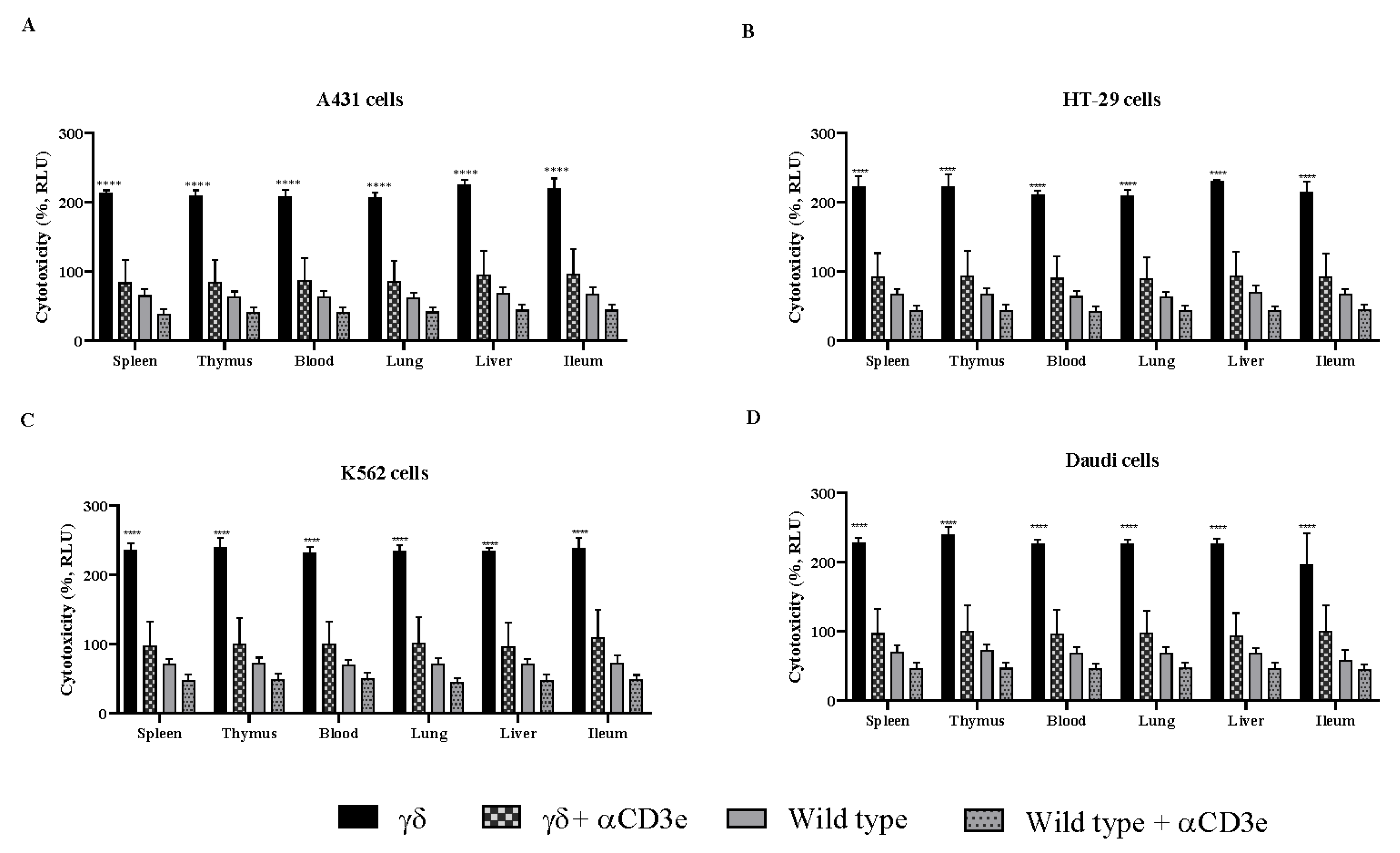
3.4. αCD3e Effectively Abrogated the Functional Effect of γδ T Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bank, I.; DePinho, R.A.; Brenner, M.B.; Cassimeris, J.; Alt, F.W.; Chess, L. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature 1986, 322, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.B.; McLean, J.; Dialynas, D.P.; Strominger, J.L.; Smith, J.A.; Owen, F.L.; Seidman, J.G.; Ip, S.; Rosen, F.; Krangel, M.S. Identification of a putative second T-cell receptor. Nature 1986, 322, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Iwashima, M.; Kaplan, K.B.; Elliott, J.F.; Davis, M.M. A new T-cell receptor gene located within the alpha locus and expressed early in T-cell differentiation. Nature 1987, 327, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A.C. Gammadelta T Cell Update: Adaptate Orchestrators of Immune Surveillance. J. Immunol. 2019, 203, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Holtmeier, W.; Kabelitz, D. Gammadelta T cells link innate and adaptive immune responses. Chem. Immunol. Allergy 2005, 86, 151–183. [Google Scholar] [PubMed]
- Papadopoulou, M.; Sanchez Sanchez, G.; Vermijlen, D. Innate and adaptive γδ T cells: How, when, and why. Immunol. Rev. 2020, 298, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, W.; Pan, M.; Scully, E.; Girardi, M.; Augenlicht, L.H.; Craft, J.; Yin, Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J. Exp. Med. 2003, 198, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Girardi, M.; Oppenheim, D.E.; Steele, C.R.; Lewis, J.M.; Glusac, E.; Filler, R.; Hobby, P.; Sutton, B.; Tigelaar, R.E.; Hayday, A.C. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001, 294, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Lanca, T.; Silva-Santos, B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology 2012, 1, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Chiplunkar, S.V. Lysis of aminobisphosphonate-sensitized MCF-7 breast tumor cells by Vγ9Vδ2 T cells. Cancer Immun. 2010, 10, 10. [Google Scholar] [PubMed]
- Yazdanifar, M.; Barbarito, G.; Bertaina, A.; Airoldi, I. Gammadelta T Cells: The Ideal Tool for Cancer Immunotherapy. Cells 2020, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, Q.; Li, Y.; Lu, L.; Xiang, Z.; Yin, Z.; Kabelitz, D.; Wu, Y. γδ T cells: Origin and fate, subsets, diseases and immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Kronenberg, M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J. Clin. Investig. 2004, 114, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Uldrich, A.P.; Le Nours, J.; Pellicci, D.G.; Gherardin, N.A.; McPherson, K.G.; Lim, R.T.; Patel, O.; Beddoe, T.; Gras, S.; Rossjohn, J.; et al. CD1d-lipid antigen recognition by the γδ TCR. Nat. Immunol. 2013, 14, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Le Nours, J.; Andrews, D.M.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Picard, D.; Anderson, B.; Chaudhary, V.; Luoma, A.; Jabri, B.; Adams, E.J.; Savage, P.B.; Bendelac, A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR. Eur. J. Immunol. 2012, 42, 2505–2510. [Google Scholar] [CrossRef] [PubMed]
- Paget, C.; Chow, M.T.; Duret, H.; Mattarollo, S.R.; Smyth, M.J. Role of γδ T cells in α-galactosylceramide-mediated immunity. J. Immunol. 2012, 188, 3928–3939. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Motoki, K.; Akimoto, K.; Natori, T.; Sakai, T.; Sawa, E.; Yamaji, K.; Koezuka, Y.; Kobayashi, E.; Fukushima, H. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J. Med. Chem. 1995, 38, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.; Weng, G.W.; Vallas, M.M.; Lovos, D.; Chen, E.; Sheiffele, P.; Weng, W. Metabolomics analysis reveals resembling metabolites between humanized γδ TCR mice and human plasma. Sci. Rep. 2024, 14, 29321. [Google Scholar] [CrossRef] [PubMed]
- Weng, W. Genetically Modified Non-Human Having Humanized Gamma and Delta TCR Variable Genes. Patent Application Publication US20240114883A1, 10 January 2023. [Google Scholar]
- Jungblut, M.; Oeltze, K.; Zehnter, I.; Hasselmann, D.; Bosio, A. Standardized preparation of single-cell suspensions from mouse lung tissue using the gentleMACS Dissociator. J. Vis. Exp. 2009, 1266. [Google Scholar] [PubMed]
- Lee, J.S.; Oka, K.; Obara, M.; Nishimukai, M.; Yoo, Y.C.; Yamada, K.; Tsukahara, T.; Nakayama, K.; Hara, H.; Ishizuka, S. Improved isolation methods for mucosal leukocytes from small and large intestines in rats. Biosci. Biotechnol. Biochem. 2009, 73, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jianyu, X.; Park, M.S.; DeMarte, L.; Hovhannisyan, Z.; Couri, R.; Stanners, C.P.; Yeretssian, G.; Mayer, L. Characterizing CEACAM5 interaction with CD8α and CD1d in intestinal homeostasis. Mucosal Immunol. 2014, 7, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.; Nieda, M.; Koezuka, Y.; Porcelli, S.; Suzuki, K.; Tadokoro, K.; Durrant, S.; Juji, T. Human invariant valpha24+ natural killer T cells activated by alpha-galactosylceramide (KRN7000) have cytotoxic anti-tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology 2000, 99, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Y.; Yue, L.; Wan, L.; Ma, X.; Yang, Q.; Tian, X.; Li, Y.; Wang, K.; Wei, S.; et al. Efficacy of natural killer T and gammadelta T cells in mesothelin-targeted immunotherapy of pancreatic cancer. Front. Immunol. 2025, 16, 1524899. [Google Scholar] [CrossRef] [PubMed]
- Fisch, P.; Oettel, K.; Fudim, N.; Surfus, J.E.; Malkovsky, M.; Sondel, P.M. MHC-unrestricted cytotoxic and proliferative responses of two distinct human γ/δ T cell subsets to Daudi cells. J. Immunol. 1992, 148, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, G.; Zhu, X. gammadelta T cells in hematological malignancies: Mechanisms and therapeutic strategies. Blood Sci. 2025, 7, e00213. [Google Scholar] [CrossRef] [PubMed]
- Schneiders, F.L.; Prodohl, J.; Ruben, J.M.; O’Toole, T.; Scheper, R.J.; Bonneville, M.; Scotet, E.; Verheul, H.M.; de Gruijl, T.D.; van der Vliet, H.J. CD1d-restricted antigen presentation by Vγ9Vδ2-T cells requires trogocytosis. Cancer Immunol. Res. 2014, 2, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Mackinnon, S.; Lowdell, M.W. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy 2012, 14, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Mensurado, S.; Blanco-Dominguez, R.; Silva-Santos, B. The emerging roles of γδ T cells in cancer immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D.; Wesch, D.; Pitters, E.; Zoller, M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J. Immunol. 2004, 173, 6767–6776. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.; Lai, N.; Wang, E.; Smith, T.; Yang, X.; Lin, B. A rapid detection method for apoptosis and necrosis measurement using the Cellometer imaging cytometry. Apoptosis 2011, 16, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.M.; List, J.; Heiduk, M.; Decker, R.; von Renesse, J.; Meinecke, A.C.; Aust, D.E.; Welsch, T.; Weitz, J.; Seifert, L. Gamma-delta T cells stimulate IL-6 production by pancreatic stellate cells in pancreatic ductal adenocarcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 3233–3240. [Google Scholar] [CrossRef] [PubMed]
- Couzi, L.; Pitard, V.; Sicard, X.; Garrigue, I.; Hawchar, O.; Merville, P.; Moreau, J.F.; Déchanet-Merville, J. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 2012, 119, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, N.; La Mendola, C.; Orlando, V.; Meraviglia, S.; Todaro, M.; Stassi, G.; Sireci, G.; Fournié, J.J.; Dieli, F. Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood 2011, 118, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Sakuta, K.; Noguchi, A.; Ariyoshi, N.; Sato, K.; Sato, S.; Sato, K.; Hosoi, A.; Nakajima, J.; Yoshida, Y.; et al. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 2008, 10, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Yuan, D.; Guo, Y.; Yan, R.; Li, K. Immune Effects of γδ T Cells in Colorectal Cancer: A Review. Front. Immunol. 2020, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, Z.; Ren, X.; Song, S.; Zhang, Y.; Wang, Y. γδ T cells, a key subset of T cell for cancer immunotherapy. Front. Immunol. 2025, 16, 1562188. [Google Scholar] [CrossRef] [PubMed]
- Ramstead, A.G.; Jutila, M.A. Complex role of γδ T-cell-derived cytokines and growth factors in cancer. J. Interferon Cytokine Res. 2012, 32, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-γ: Teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Hoeres, T.; Holzmann, E.; Smetak, M.; Birkmann, J.; Wilhelm, M. PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-Cells in response to leukemia. Oncoimmunology 2019, 8, 1550618. [Google Scholar] [CrossRef] [PubMed]
- Duhindan, N.; Farley, A.J.; Humphreys, S.; Parker, C.; Rossiter, B.; Brooks, C.G. Patterns of lymphokine secretion amongst mouse gamma delta T cell clones. Eur. J. Immunol. 1997, 27, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, M.F.; Muller, U.; Huang, S.; Zinkernagel, R.M.; Aguet, M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 1995, 148, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, F.J.; Lienard, D.; Matter, M.; Ruegg, C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006, 6, 6. [Google Scholar] [PubMed]
- Johansson, A.; Hamzah, J.; Payne, C.J.; Ganss, R. Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 7841–7846. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Aymeric, L.; Locher, C.; Mattarollo, S.R.; Delahaye, N.F.; Pereira, P.; Boucontet, L.; Apetoh, L.; Ghiringhelli, F.; Casares, N.; et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 2011, 208, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Duarte, J.D.G.; Ostrouska, S.; Behren, A. Gammadelta T Cells in the Tumor Microenvironment-Interactions With Other Immune Cells. Front. Immunol. 2022, 13, 894315. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, Y.; Teo, H.Y.; Liu, H. Targeting Cytokine Signals to Enhance γδ T Cell-Based Cancer Immunotherapy. Front. Immunol. 2022, 13, 914839. [Google Scholar] [CrossRef]
- Lechner, M.G.; Cheng, M.I.; Patel, A.Y.; Hoang, A.T.; Yakobian, N.; Astourian, M.; Pioso, M.S.; Rodriguez, E.D.; McCarthy, E.C.; Hugo, W.; et al. Inhibition of IL-17A Protects against Thyroid Immune-Related Adverse Events while Preserving Checkpoint Inhibitor Antitumor Efficacy. J. Immunol. 2022, 209, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, A.A.; Pawlowski, N.N.; Grollich, K.; Blessenohl, M.; Westermann, J.; Zeitz, M.; Loddenkemper, C.; Hoffmann, J.C. Human peripheral gammadelta T cells possess regulatory potential. Immunology 2009, 128, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.F. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Petrasca, A.; Doherty, D.G. Human Vδ2(+) γδ T Cells Differentially Induce Maturation, Cytokine Production, and Alloreactive T Cell Stimulation by Dendritic Cells and B Cells. Front. Immunol. 2014, 5, 650. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, J.; Olislagers, V.; Poupot, R.; Fournie, J.J.; Goldman, M. Human gamma delta T cells induce dendritic cell maturation. Clin. Immunol. 2002, 103 Pt 1, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Mace, T.A.; Shakya, R.; Pitarresi, J.R.; Swanson, B.; McQuinn, C.W.; Loftus, S.; Nordquist, E.; Cruz-Monserrate, Z.; Yu, L.; Young, G.; et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018, 67, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Sapochnik, M.; Haedo, M.R.; Fuertes, M.; Ajler, P.; Carrizo, G.; Cervio, A.; Sevlever, G.; Stalla, G.K.; Arzt, E. Autocrine IL-6 mediates pituitary tumor senescence. Oncotarget 2017, 8, 4690–4702. [Google Scholar] [CrossRef] [PubMed]
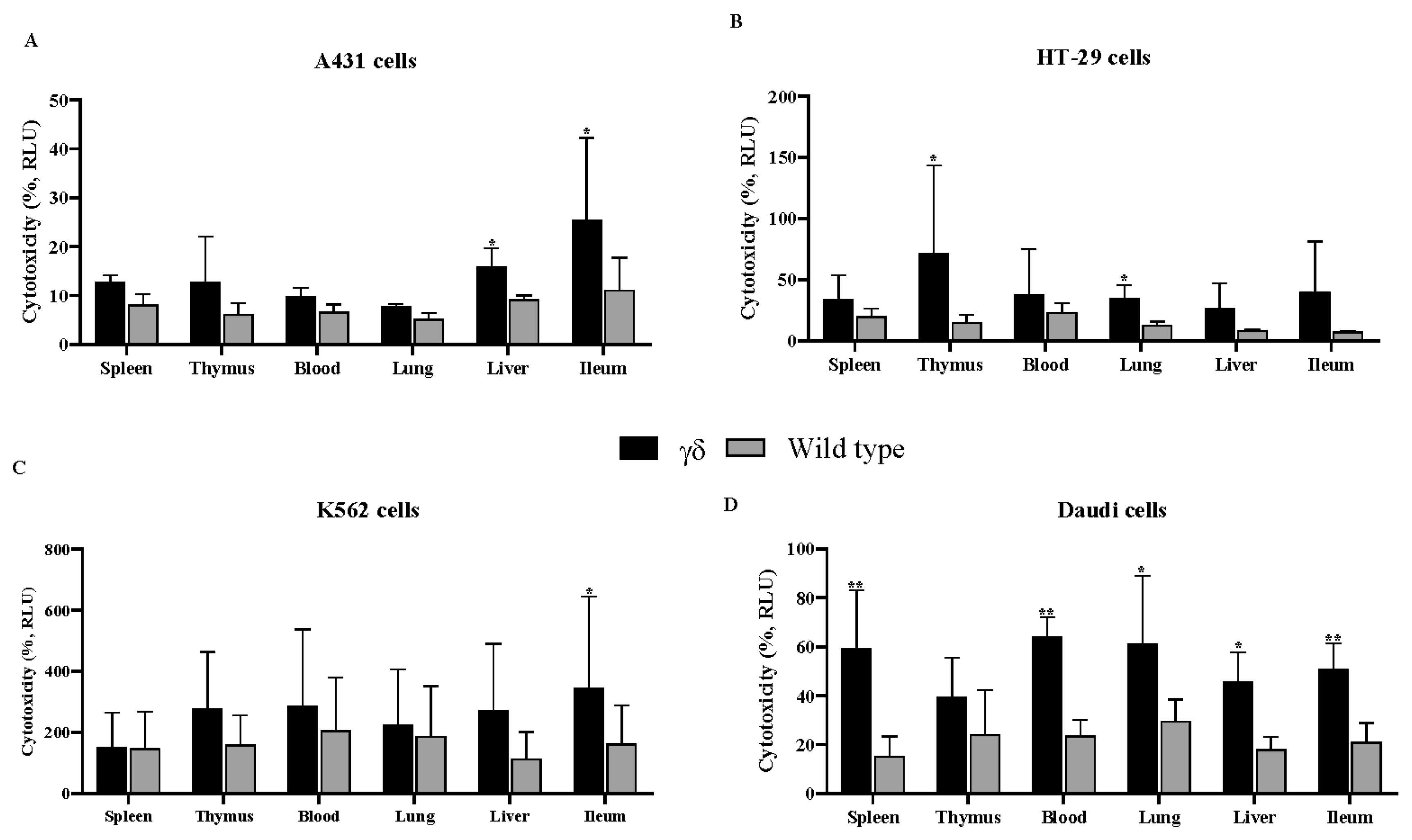
| Spleen | Thymus | Blood | Lung | Liver | Ileum | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-γ | TNF-α | IL-17 | IFN-γ | TNF-α | IL-17 | IFN-γ | TNF-α | IL-17 | IFN-γ | TNF-α | IL-17 | IFN-γ | TNF-α | IL-17 | IFN-γ | TNF-α | IL-17 | |||||||||||||||||||
| Cell line | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT | γδ | WT |
| A431 | *** 20 | 0 | ** 17 | 2.7 | * 4 | 0 | **** 24 | 1.6 | ** 14 | 2.2 | 3 | 0 | * 12 | 1.6 | * 12 | 0.5 | 2 | 0 | * 15 | 4.1 | ** 18 | 3.8 | 2 | 2.6 | 5 | 4.4 | 2 | 2.6 | 1 | 0.2 | ** 15 | 1.7 | 2 | 0 | ** 4 | 0 |
| HT-29 | *** 35 | 2 | ** 22 | 2 | 3 | 2 | * 21 | 6 | * 17 | 2 | 4 | 2 | * 20 | 2 | * 13 | 2 | 2 | 3 | * 24 | 4 | 9 | 5 | 4 | 1 | 7 | 1 | 9 | 10 | * 7 | 3 | 8 | 4 | 6 | 4 | 3 | 1 |
| K562 | **** 21 | 5 | *** 27 | 10 | 3 | 5 | * 8 | 1 | 4 | 1 | 4 | 1 | * 8 | 1 | 3 | 1 | 1 | 2 | * 10 | 1 | * 9 | 1 | 4 | 2 | **** 25 | 5 | ** 22 | 7 | 2 | * 8 | **** 20 | 4 | *** 21 | 5 | 2 | 5 |
| Daudi | ** 8 | 0 | * 8 | 0 | 3 | 0 | 4 | 0 | 1 | 0.6 | 1 | 0.6 | * 5 | 0.8 | * 4 | 0 | 3 | 0.4 | * 9 | 0 | ** 12 | 1.3 | 4 | 0.7 | * 16 | 6.8 | * 13 | 4.8 | * 7 | 2.1 | ** 13 | 2.5 | * 11 | 2.1 | 5 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michael, H.; Lenihan, A.T.; Vallas, M.M.; Weng, G.W.; Barber, J.; He, W.; Chen, E.; Sheiffele, P.; Weng, W. The Cytotoxic Potential of Humanized γδ T Cells Against Human Cancer Cell Lines in In Vitro. Cells 2025, 14, 1197. https://doi.org/10.3390/cells14151197
Michael H, Lenihan AT, Vallas MM, Weng GW, Barber J, He W, Chen E, Sheiffele P, Weng W. The Cytotoxic Potential of Humanized γδ T Cells Against Human Cancer Cell Lines in In Vitro. Cells. 2025; 14(15):1197. https://doi.org/10.3390/cells14151197
Chicago/Turabian StyleMichael, Husheem, Abigail T. Lenihan, Mikaela M. Vallas, Gene W. Weng, Jonathan Barber, Wei He, Ellen Chen, Paul Sheiffele, and Wei Weng. 2025. "The Cytotoxic Potential of Humanized γδ T Cells Against Human Cancer Cell Lines in In Vitro" Cells 14, no. 15: 1197. https://doi.org/10.3390/cells14151197
APA StyleMichael, H., Lenihan, A. T., Vallas, M. M., Weng, G. W., Barber, J., He, W., Chen, E., Sheiffele, P., & Weng, W. (2025). The Cytotoxic Potential of Humanized γδ T Cells Against Human Cancer Cell Lines in In Vitro. Cells, 14(15), 1197. https://doi.org/10.3390/cells14151197






