Calcium Unified: Understanding How Calcium’s Atomic Properties Impact Human Health
Abstract
1. Overview
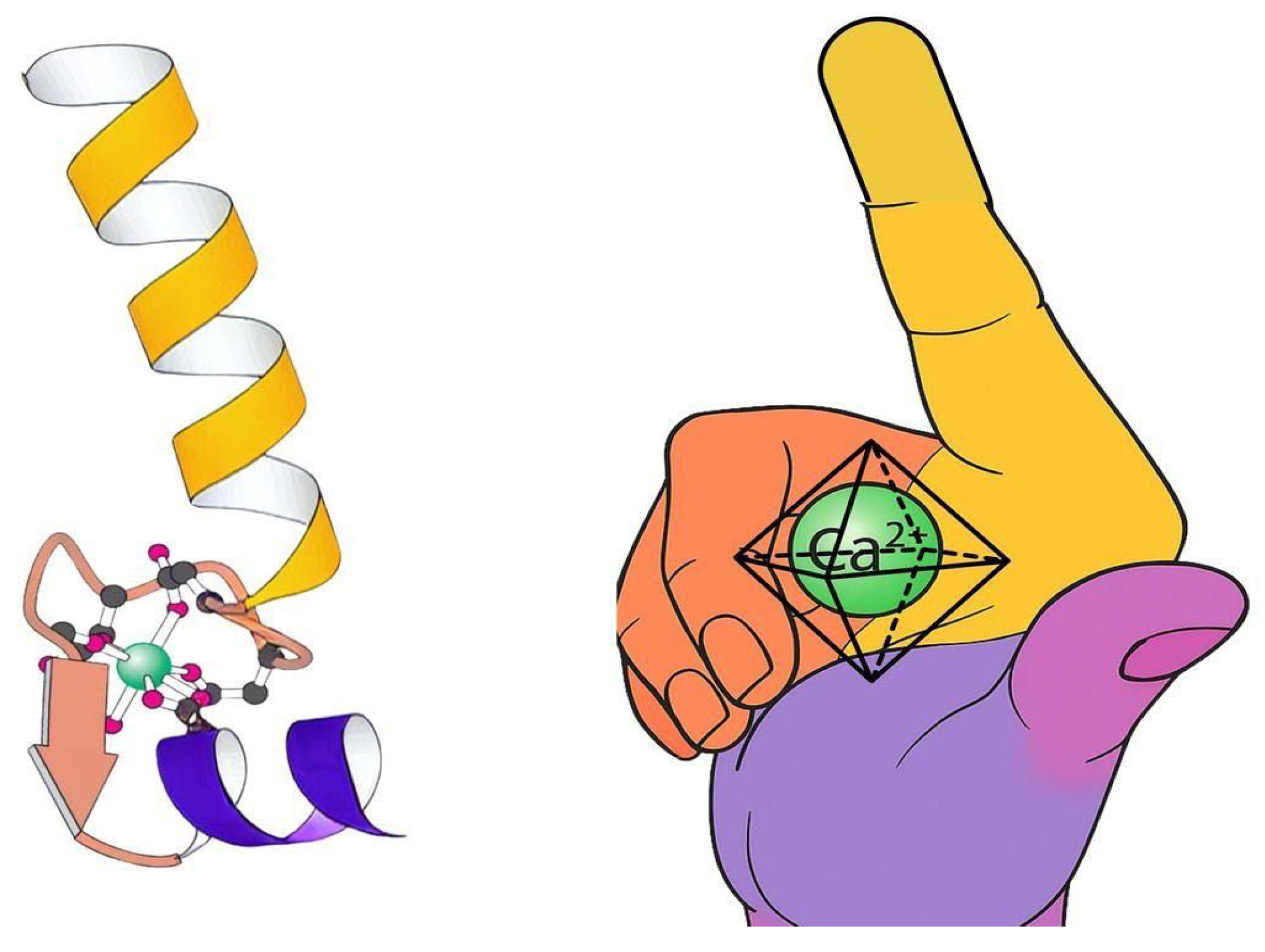
2. Calcium’s Coordination Geometry: From Platonic Solids to Protein Recognition
2.1. Understanding Calcium Geometry
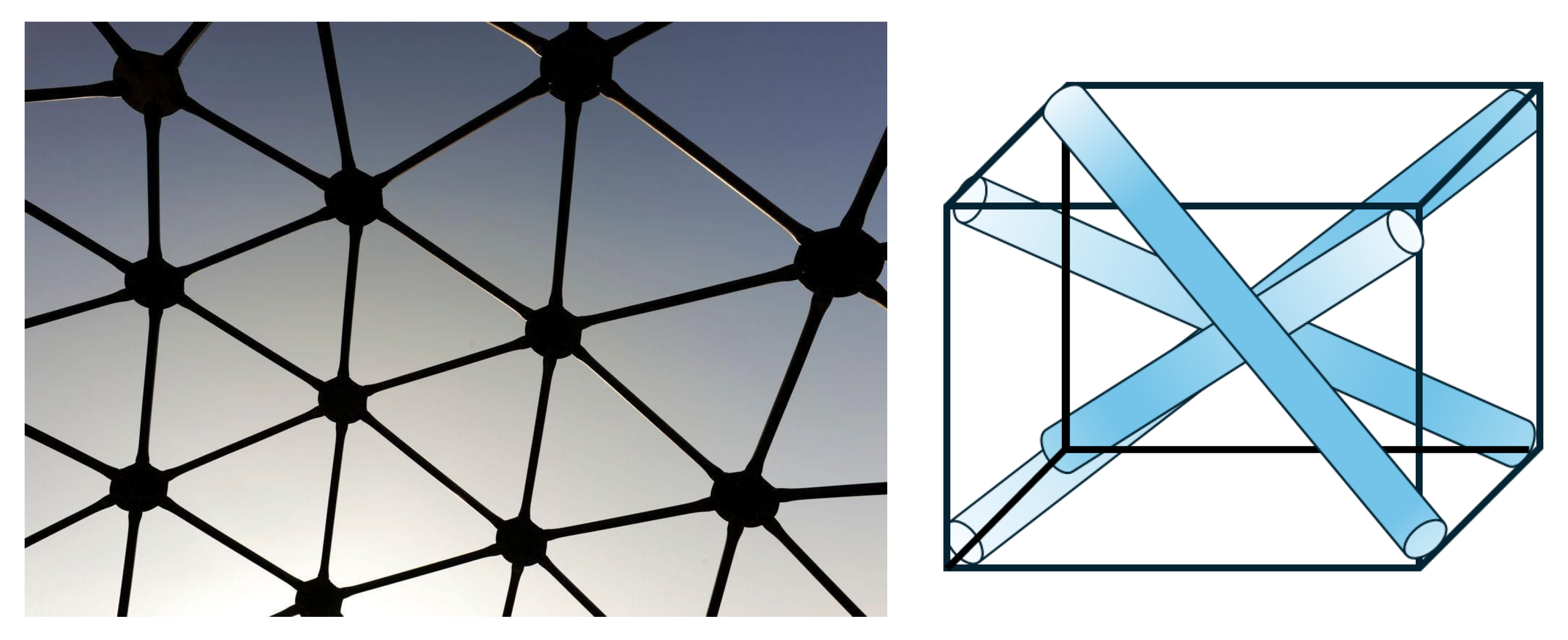
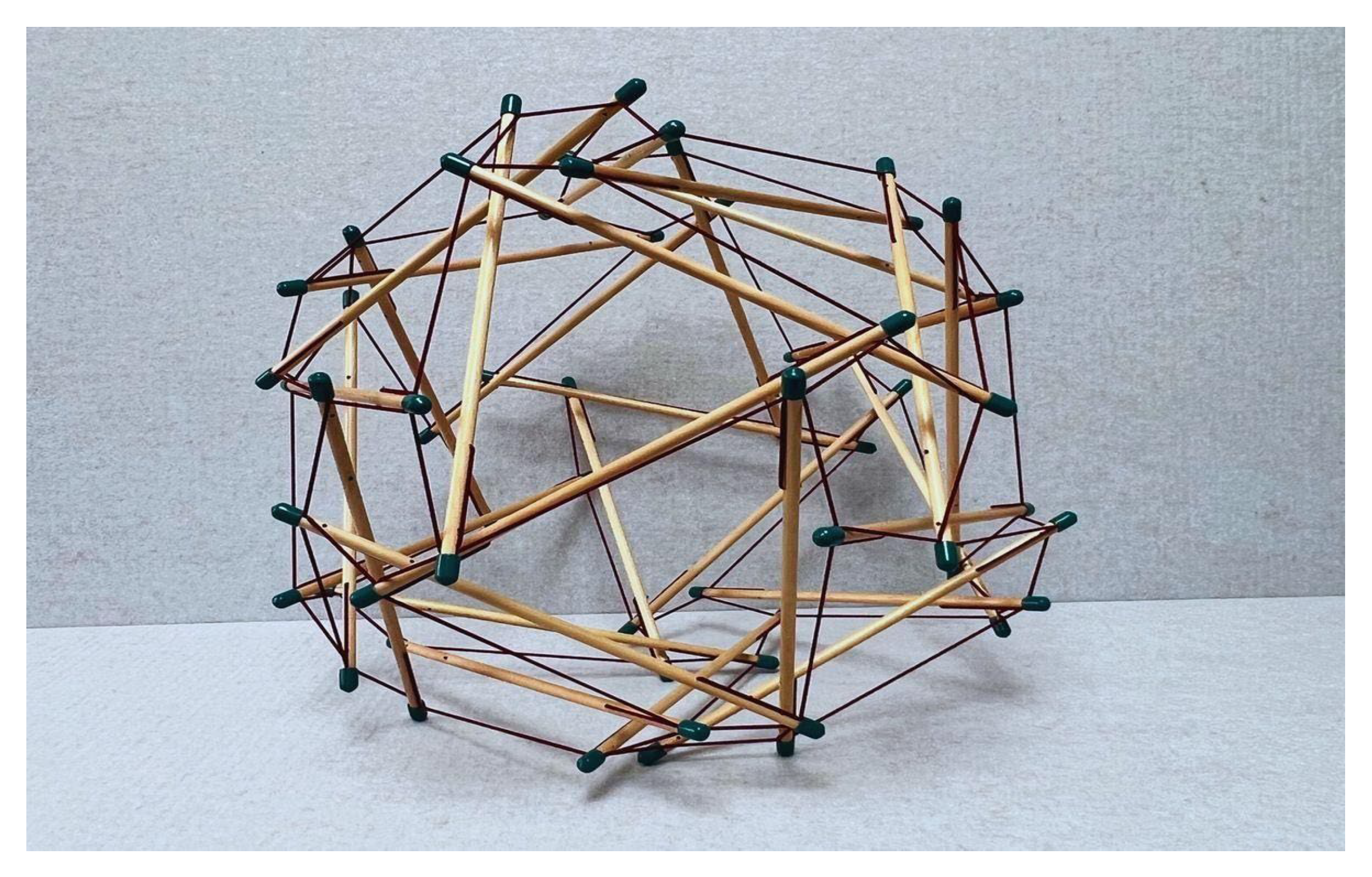
- a. Continuous tension network: A network of elements under constant tension that forms the primary force-bearing system, noting that tensile elements require less energy for production and maintenance.
- b. Discontinuous compression: Isolated rigid elements that resist compression but do not directly touch one another, allowing for porous arrangements rather than bulk solids that offer greater structural efficiency and versatility.
- c. Self-stabilizing equilibrium: The structure maintains its integrity through a balance of opposing forces, creating prestressed mechanical stability.
- d. Triangulation and prestress: Employs triangulated arrangements that efficiently distribute forces throughout the structure, providing both flexibility and strength, and offering complementary means to stabilize discrete networks.
- e. Energy-minimizing efficiency: Achieves structural integrity with minimal material by optimizing force distribution.
- f. Hierarchical organization: Tensegrity systems manifest across multiple scales, with smaller units serving as building blocks for larger structures. This nested hierarchy directly aligns with Ingber’s principle [7] that “structural efficiency is maximized and evolution accelerated through the use of hierarchical networks,” enabling emergent properties to arise at each level.
2.2. The Metal That Moves Us
2.3. Calcium as a Second Messenger in Cellular Signaling
2.3.1. Calcium’s Physical Chemistry in Biological Systems
2.3.2. Signal Initiation and Propagation
2.3.3. Changes in Protein Conformation and Functional Outcomes
2.3.4. Ca2+ Is Equally Attuned to Mechanical First Messengers
2.3.5. ECM ↔ Ca2+: A Dynamic Reciprocity
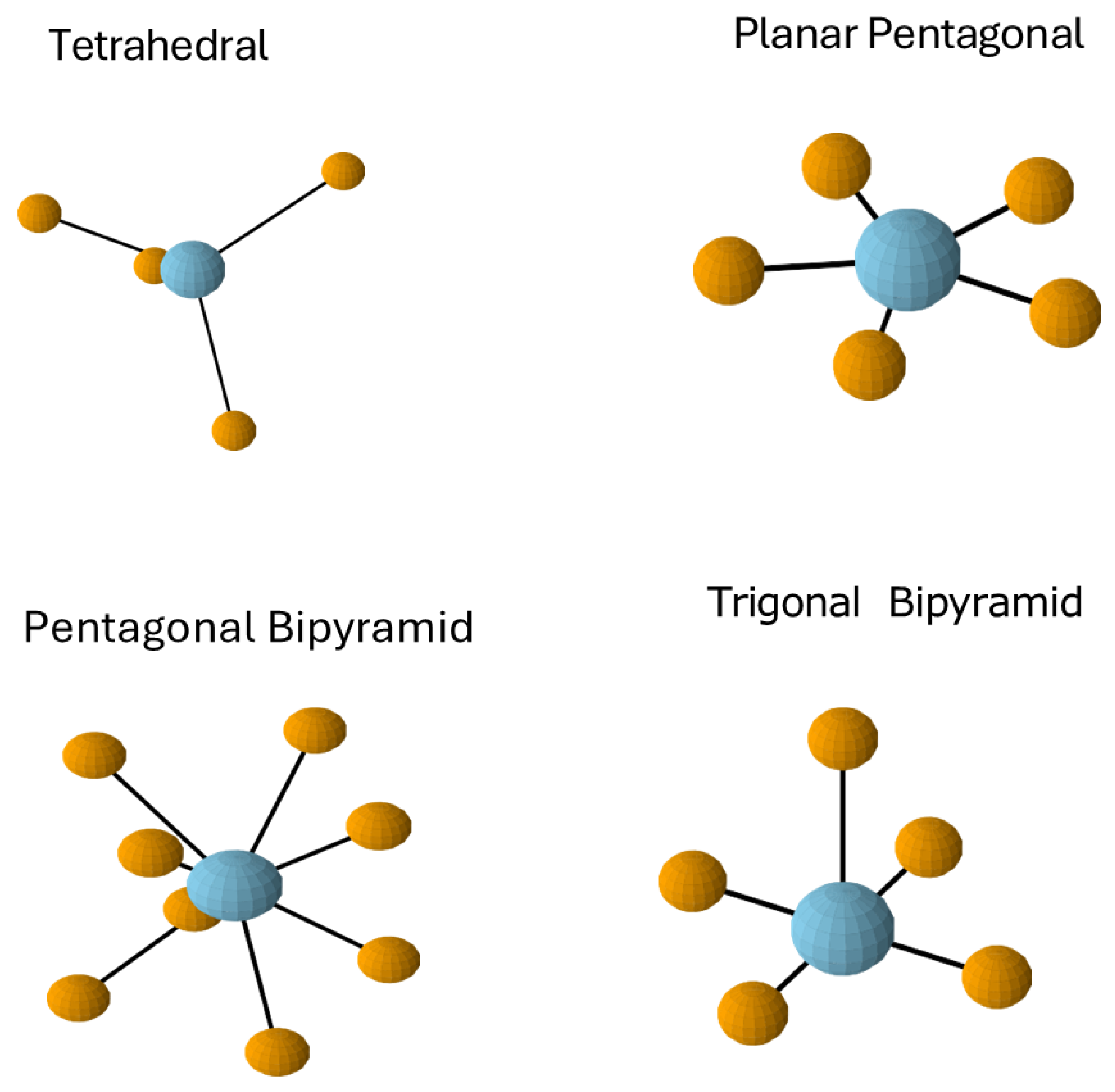
2.4. Coordination Preferences and Geometric Complementarity
2.5. The EF-Hand Motif
3. The EF-Hand: Geometric Specialization in Calcium Signaling
3.1. The EF-Hand: Nature’s Calcium-Specific Sensor
3.2. Functional Consequences of Coordination Geometry
4. Calcium as a Helical Mediator
4.1. Calcium’s Biological Role Through the Lens of Tensegrity
4.2. The EF-Hand as a Helical, Molecular Tension System
5. Discussion: Bridging Geometry and Biology
5.1. Calcium’s Impact Across Multiple Scales
5.2. Beyond Hydrogen Bonds
5.3. Calcium Keeps Things Moving: The Dynein-Amic Role of Ca2+ in Molecular Motors
5.4. Calmodulin: Nature’s Tension-Mediator
- Muscle contraction
- Neurotransmitter release
- Gene transcription
- Metabolism
- Cell proliferation
- Cytoskeletal dynamics
- Ion channel function
- Memory formation
- Brain tissue (especially in neurons);
- Cardiac muscle;
- Skeletal muscle;
- Smooth muscle;
- Pancreatic cells;
- Immune cells.
- Cytoplasm (primary location);
- Nucleus (where it regulates transcription factors);
- Associated with plasma membrane (regulating ion channels);
- Bound to the cytoskeleton;
- Present at synaptic junctions in neurons;
- Prevalence and Abundance.
5.5. Integration of Frameworks
5.6. Further Clinical Significance and Biomedical Applications
- a. S100 proteins in disease processes: The S100 family demonstrates how variations in calcium-binding domains produce wide-ranging pathophysiological effects in cancer, metabolic disorders, and neurological diseases [149,151]. S100A8/S100A9 as inflammation markers connect molecular geometry to diagnostic medicine [152].
- b. Neuroprotective mechanisms: Calcium-binding proteins like calbindin, calretinin, and parvalbumin influence neuronal vulnerability to neurodegenerative processes, bridging molecular biophysics to clinical neurology [153].
- c. Microbial virulence regulation: EF-hand proteins regulate virulence factors in pathogens like Pseudomonas aeruginosa, connecting molecular geometry to infectious disease mechanisms [154].
6. Future Directions
6.1. Bridging Calcium Coordination Geometry and Clinical Practice
6.2. Mechanistic Studies of Ca2+ Channels in Fascial Networks
6.3. ECM Remodeling Through Calcium-Mediated Tensegrity
6.4. Coordination Chemistry Effects on Biomechanical Property Modulation via Mesenchyme Healing
- Modulation of Mesenchymal Cell Biomechanics: Tripeptides and coordination chemistry can significantly enhance the mechanical properties of human mesenchymal stem cells (hMSCs), as demonstrated by a ~2-fold increase in Young’s modulus, which correlates with improved proliferation and wound healing capacity. This suggests that specific chemical cues can direct mesenchymal cell mechanics without inducing unwanted differentiation, supporting tissue regeneration [161].
- Molecular Mechanisms and Biomechanical Restoration: Mesenchymal stem cells (MSCs) can restore impaired biomechanical properties in damaged tissues by regulating collagen content and gene expression. In diabetic skin, MSCs correct biomechanical deficits by modulating miR-29a and increasing collagen, directly impacting tissue strength and healing [162]. Additionally, disruption of mechanotransduction pathways, such as through focal adhesion kinase (FAK) inhibition, can reduce fibrosis and contracture, restore collagen architecture, and improve biomechanical properties in healing tissues, highlighting the interplay between chemical signaling and mechanical outcomes [163,164].
- Bone and Connective Tissue Regeneration: Coordination chemistry strategies, such as gallic acid-calcium grafts, create multifunctional biomaterials that regulate the microenvironment for bone regeneration, influencing inflammation, vascularization, and osteogenic differentiation through pathways like integrin/PI3K/Akt [165]. MSCs also enhance bone healing and biomechanical strength by promoting the release of growth factors (b-FGF, VEGF, OPG) and improving bone mineral density and mechanical parameters, including maximum load and stiffness in fracture models [166].
6.5. Translational Implications: Drug Development and Biomarker Innovation
6.5.1. Biomarker Development
- Calcium-binding protein conformational markers utilizing conformation-sensitive antibodies that specifically recognize EF-hand domain geometry changes upon Ca2+ binding, enabling sensitive detection of disease-associated states such as early-stage malignant melanoma through exosome analysis [175,176].
6.5.2. Therapeutic Development:
- Calcium-based biomaterials for fascial repair and regeneration utilizing geometric coordination principles to create nanorods, nanowires, nanofilms, and 3D nanoframes that accelerate wound healing by modulating the local microenvironment and promoting orderly tissue repair through calcium’s coordination geometry [168,178].
- Targeted interventions based on EF-hand protein modulation and coordination chemistry to precisely control calcium signaling pathways critical for cell migration, proliferation, and matrix remodeling during fascial healing, enabling therapeutic control over calcium-binding protein conformational states [168].
6.6. Cross-Scale Research Framework
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Yang, W.; Lee, H.W.; Hellinga, H.; Yang, J.J. Structural analysis, identification, and design of calcium-binding sites in proteins. Proteins Struct. Funct. Bioinform. 2002, 47, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Borbolis, F.; Ploumi, C.; Palikaras, K. Calcium-mediated regulation of mitophagy: Implications in neurodegenerative diseases. npj Metab. Health. Dis. 2025, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Vitto, V.; Lanza, G.; Danese, A.; Giorgi, C.; Gafà, R.; Ramaccini, D.; Aguiari, G.; Modesti, L.; Pinton, P. Mitochondrial Ca2+ Signaling in Health, Disease and Therapy. Cells 2021, 10, 1317. [Google Scholar] [CrossRef]
- Fuller, R.B. Synergetics: Explorations in the Geometry of Thinking; Macmillan: New York, NY, USA, 1975. [Google Scholar]
- Ingber, D.E. The origin of cellular life. BioEssays 2000, 22, 1160–1170. [Google Scholar] [CrossRef]
- Bisht, D.; Pandey, G.; Bheri, M.; Ghosh, S. Calcium signaling and transport machinery: Potential for development of stress tolerance in plants. Curr. Plant Biol. 2022, 29, 100235. [Google Scholar] [CrossRef]
- Bootman, M.; Bultynck, G. Fundamentals of cellular calcium signaling: A primer. Cold Spring Harb. Perspect. Biol. 2019, 12, a038802. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, P.; Da Conceicao, V.; Sun, Y.; Ahamad, N.; Saraiva, L.; Selvaraj, S.; Singh, B. Calcium signaling regulates autophagy and apoptosis. Cells 2021, 10, 2125. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Trebak, M.; Kinet, J. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Pihán, P.; Hetz, C. Calcium signaling at the endoplasmic reticulum: Fine-tuning stress responses. Cell Calcium 2017, 70, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, P.; Cheung, M.; Cieplak, P. Determining the atomic charge of calcium ion requires the information of its coordination geometry in an EF-hand motif. J. Chem. Phys. 2020, 154, 124104. [Google Scholar] [CrossRef]
- Cheung, M.; Nde, J.; Cieplak, P.; Zhang, P.; Jennings, N.; Eliaz, Y. CaXML: Chemistry-informed machine learning explains mutual changes between protein conformations and calcium ions in calcium-binding proteins using structural and topological features. Protein Sci. A Publ. Protein Soc. 2025, 34, e70023. [Google Scholar] [CrossRef]
- Kim, Y.; Song, C.; Kim, H.; Park, Y.; An, M.; Jung, H. Structural insights into calcium-induced conformational changes in human gelsolin. Biochem. Biophys. Res. Commun. 2024, 735, 150826. [Google Scholar] [CrossRef] [PubMed]
- Meirovitch, E.; Mendelman, N. SRLS Analysis of 15N-1H NMR Relaxation from the Protein S100A1: Dynamic Structure, Calcium Binding, and Related Changes in Conformational Entropy. J. Phys. Chem. B 2021, 125, 805–816. [Google Scholar] [CrossRef]
- Jiang, J.; Kirberger, M.; Tang, S.; Deng, X.; Yang, J. Design of Calcium-Binding Proteins to Sense Calcium. Molecules 2020, 25, 2148. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Nagata, Y.; Schneider, P.; Hunger, J.; Gunkel, L.; Sutter, J.; Krevert, C. Deciphering Spectroscopic Signatures of Competing Ca2+—Peptide Interactions. J. Phys. Chem. B 2024, 128, 10688–10698. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Chen, Z.; Wang, L.; Luo, X.; Li, Y.; Wang, R.; Ding, Y.; Zhang, X. Isolation of a novel calcium-binding peptide from wheat germ protein hydrolysates and the prediction for its mechanism of combination. Food Chem. 2018, 239, 416–426. [Google Scholar] [CrossRef]
- Bao, X.; Yang, B.; Lv, Y.; Ren, C.; Guo, S. A study of the soluble complexes formed during calcium binding by soybean protein hydrolysates. J. Food Sci. 2008, 73, C117–C121. [Google Scholar] [CrossRef]
- Ren, P.; Jing, Z.; Liu, C.; Qi, R. Many-body effect determines the selectivity for Ca2+ and Mg2+ in proteins. Proc. Natl. Acad. Sci. USA 2018, 115, E7495–E7501. [Google Scholar] [CrossRef]
- Berridge, M. Elementary and global aspects of calcium signalling. J. Physiol. 1997, 499, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.; Brownlee, C. The generation of Ca2+ signals in plants. Annu. Rev. Plant Biol. 2004, 55, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Kretsinger, R. Structure and evolution of calcium-modulated proteins. CRC Crit. Rev. Biochem. 1980, 8, 119–174. [Google Scholar] [CrossRef]
- Woll, K.; Van Petegem, F. Calcium Release Channels: Structure and Function of IP3 Receptors and Ryanodine Receptors. Physiol. Rev. 2021, 102, 209–268. [Google Scholar] [CrossRef]
- Nakashima, R.; Marks, A.; Santulli, G.; Yuan, Q. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef]
- Finholm, I.; Farrugia, G.; Bellampalli, S.; Serlin, H.; Whiteman, S.; Alcaino, C.; Mercado-Perez, A.; Knutson, K.; Beyder, A.; Linden, D. Intestinal enteroendocrine cells rely on ryanodine and IP3 calcium store receptors for mechanotransduction. J. Physiol. 2022, 601, 287–305. [Google Scholar] [CrossRef]
- Carrasco, M.; Hidalgo, J.; Silva, M.; Puentes, N.; Galaz, J.; Valdés, J.; Jaimovich, E. NF-κB activation by depolarization of skeletal muscle cells depends on ryanodine and IP3 receptor-mediated calcium signals. Am. J. Physiol. Cell Physiol. 2007, 292, C1960–C1970. [Google Scholar] [CrossRef]
- Yule, D.I.; Betzenhauser, M.J.; Joseph, S.K. Linking structure to function: Recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis. Cell Calcium 2010, 47, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, V.; Taylor, C.W. Challenges for coordination of Ca2+ signaling by IP3. Curr. Opin. Cell Biol. 2016, 40, 52–58. [Google Scholar]
- Ramírez, O.; Lobos, P.; Härtel, S.; Hidalgo, C.; Cerda, M.; Couve, A.; Córdova, A. Ryanodine receptor-mediated Ca2+ release and atlastin-2 GTPase activity contribute to IP3-induced dendritic Ca2+ signals in primary hippocampal neurons. Cell Calcium 2021, 96, 102399. [Google Scholar] [CrossRef] [PubMed]
- Soeller, C.; Crossman, D.; Gilbert, R.; Cannell, M.B. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 14958–14963. [Google Scholar] [CrossRef] [PubMed]
- Meissner, G.; Elferdink, M.; Yamaguchi, N.; Kral, M.; Chirasani, V.; Heitmann, S.; Carter, J. Structural and functional interactions between the EF hand domain and S2–S3 loop in the type-1 ryanodine receptor ion channel. J. Biol. Chem. 2023, 300, 105606. [Google Scholar] [CrossRef]
- Clarke, O.; Grassucci, R.; Georges, A.; Marks, A.; Mancia, F.; Reiken, S.; Hendrickson, W.; Frank, J.; Zalk, R. Structure of a mammalian ryanodine receptor. Nature 2014, 517, 44–49. [Google Scholar] [CrossRef]
- Raunser, S.; Efremov, R.; Aebersold, R.; Leitner, A. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 2014, 517, 39–43. [Google Scholar] [CrossRef]
- Nayak, A.; Samsó, M. Ca2+ inactivation of the mammalian ryanodine receptor type 1 in a lipidic environment revealed by cryo-EM. eLife 2022, 11, e75568. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Xu, L.; Pasek, D.; Meissner, G.; Gomez, A. Two EF-hand motifs in ryanodine receptor calcium release channels contribute to isoform-specific regulation by calmodulin. Cell Calcium 2017, 66, 62–70. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Xiao, Z.; Sun, B.; Zhang, L.; Wang, R.; Liu, Y.; Chen, S. The EF-hand Ca2+ Binding Domain Is Not Required for Cytosolic Ca2+ Activation of the Cardiac Ryanodine Receptor. J. Biol. Chem. 2015, 291, 2150–2160. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Lai, R.; Li, G.; Cui, Q. Flexibility of Binding Site is Essential to the Ca2+ Selectivity in EF-Hand Calcium-Binding Proteins. J. Am. Chem. Soc. 2024, 146, 7628–7639. [Google Scholar] [CrossRef] [PubMed]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2017, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B 2017, 7, 3–17. [Google Scholar] [CrossRef]
- Hashimura, H.; Morimoto, Y.; Hirayama, Y.; Ueda, M. Calcium responses to external mechanical stimuli in the multicellular stage of Dictyostelium discoideum. Sci. Rep. 2022, 12, 12428. [Google Scholar] [CrossRef]
- Dey, K.; Roca, E.; Ramorino, G.; Sartore, L. Progress in the mechanical modulation of cell functions in tissue engineering. Biomater. Sci. 2020, 8, 7033–7081. [Google Scholar] [CrossRef]
- Shuaib, A.; Motan, D.; Bhattacharya, P.; McNabb, A.; Skerry, T.M.; Lacroix, D. Heterogeneity in The Mechanical Properties of Integrins Determines Mechanotransduction Dynamics in Bone Osteoblasts. Sci. Rep. 2019, 9, 13113. [Google Scholar] [CrossRef] [PubMed]
- DuFort, C.; Paszek, M.; Weaver, V. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Wosik, J. Mechanical Forces, Nucleus, Chromosomes, and Chromatin. Biomolecules 2025, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Moeder, W.; Yoshioka, K. Opening the gates: Insights into guard cell signaling in plant immunity. Trends Plant Sci. 2016, 21, 295–308. [Google Scholar] [CrossRef]
- Vivo, M.; Rosti, V.; Cervone, S.; Lanzuolo, C. Chromatin plasticity in mechanotransduction. Curr. Opin. Cell Biol. 2024, 88, 102376. [Google Scholar] [CrossRef]
- Morival, J.; Hazelwood, A.; Lammerding, J. Feeling the force from within-new tools and insights into nuclear mechanotransduction. J. Cell Sci. 2025, 138, JCS263615. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Richert, A.; Medjkane, S.; Hénon, S.; Weitzman, J. Cell geometry and the cytoskeleton impact the nucleo-cytoplasmic localisation of the SMYD3 methyltransferase. Sci. Rep. 2020, 10, 20598. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.; Liu, Y.; Yang, C.; Lee, O. Mechanotransduction of mesenchymal stem cells and hemodynamic implications. Chin. J. Physiol. 2023, 66, 55–64. [Google Scholar] [CrossRef]
- Verma, S.; Negi, N.; Narwal, P.; Kumari, P.; Kisku, A.; Gahlot, P.; Mittal, N.; Kumar, D. Calcium Signaling in Coordinating Plant Development, Circadian Oscillations and Environmental Stress Responses in Plants. Environ. Exp. Bot. 2022, 201, 104935. [Google Scholar] [CrossRef]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.; Thuleau, P.; Mazars, C. Calcium Sensors as Key Hubs in Plant Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef]
- Kölling, M.; Kumari, P.; Bürstenbinder, K. Calcium- and calmodulin-regulated microtubule-associated proteins as signal-integration hubs at the plasma membrane–cytoskeleton nexus. J. Exp. Bot. 2018, 70, 387–396. [Google Scholar] [CrossRef]
- Paupe, V.; Prudent, J. New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem. Biophys. Res. Commun. 2017, 500, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Xiang, Y. Actin cytoskeleton as actor in upstream and downstream of calcium signaling in plant cells. Int. J. Mol. Sci. 2019, 20, 1403. [Google Scholar] [CrossRef]
- Black, C.B.; Huang, H.W.; Cowan, J.A. Biological coordination chemistry of magnesium, sodium, and potassium ions, Protein and nucleotide binding sites. Coord. Chem. Rev. 1994, 135, 165–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Wen, X.; Ma, H. Current status on COVID-19 diagnosis. MedOne 2020, 4, 54–58. [Google Scholar]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nat. Mater. 2011, 10, 452–458. [Google Scholar] [CrossRef]
- Kirberger, M.; Wang, X.; Deng, H.; Yang, W.; Chen, G.; Yang, J.J. Statistical analysis of structural characteristics of protein Ca2+-binding sites. J. Biol. Inorg. Chem. 2008, 13, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Walsh, M.P.; Vogel, H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix–loop–helix EF-hand motifs. Biochem. J. 2007, 405, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Babu, Y.S.; Sack, J.S.; Greenhough, T.J.; Bugg, C.E.; Means, A.R.; Cook, W.J. Three-dimensional structure of calmodulin. Nature 1988, 315, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ikura, M.; Clore, G.M.; Gronenborn, A.M.; Zhu, G.; Klee, C.B.; Bax, A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science 1992, 256, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Marsden, B.J.; Shaw, G.S.; Sykes, B.D. Calcium binding proteins. Biochem. Cell Biol. 1990, 68, 587–601. [Google Scholar] [CrossRef]
- Strynadka, N.C.; James, M.N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu. Rev. Biochem. 1989, 58, 951–999. [Google Scholar] [CrossRef]
- Schwierz, N. Kinetic pathways of water exchange in the first hydration shell of magnesium. J. Chem. Phys. 2020, 152, 224106. [Google Scholar] [CrossRef]
- Grotz, K.; Schwierz, N.; Cruz-León, S. Optimized Magnesium Force Field Parameters for Biomolecular Simulations with Accurate Solvation, Ion-Binding, and Water-Exchange Properties. J. Chem. Theory Comput. 2021, 17, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Terakura, K.; Ikeda, T.; Boero, M. Hydration properties of magnesium and calcium ions from constrained first principles molecular dynamics. J. Chem. Phys. 2007, 127, 074503. [Google Scholar] [CrossRef]
- Politi, Y.; Metzler, R.A.; Abrecht, M.; Gilbert, B.; Wilt, F.H.; Sagi, I.; Addadi, L.; Weiner, S.; Gilbert, P.U.P.A. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proc. Natl. Acad. Sci. USA 2010, 102, 20515–20520. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, N.; Li, M.; Zhang, F. Comparison of the ionic effects of Ca2+ and Mg2+ on nucleic acids in liquids. J. Mol. Liq. 2021, 344, 117781. [Google Scholar] [CrossRef]
- Allnér, O.; Nilsson, L.; Villa, A. Magnesium ion–water coordination and exchange in biomolecular simulations. J. Chem. Theory Comput. 2012, 8, 1493–1502. [Google Scholar] [CrossRef]
- Okumus, D.; Arslan, S.; Uğur, Ş. Effects of different ionic radii on the optical, electronic, elastic and vibrational properties of calcium chalcogenides: An ab initio study. J. Phys. Chem. Solids 2009, 70, 674–678. [Google Scholar]
- Krebs, J.; Heizmann, C.W. Calcium-binding proteins and the EF-hand principle. In Calcium: A Matter of Life or Death; Elsevier: Amsterdam, The Netherlands, 2007; pp. 51–93. [Google Scholar]
- Grabarek, Z. Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Błoński, S.; Aureille, J.; Badawi, S.; Zaremba, D.; Pernet, L.; Grichine, A.; Fraboulet, S.; Korczyk, P.; Recho, P.; Guilluy, C.; et al. Direction of epithelial folding defines impact of mechanical forces on epithelial state. Dev. Cell 2021, 56, 3222–3234. [Google Scholar] [CrossRef] [PubMed]
- Luettgau, H.C. The action of calcium ions on potassium contractures of single muscle fibres. J. Physiol. 1963, 168, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Langer, G.; Serena, S.; Nudd, L. Cation exchange in heart cell culture: Correlation with effects on contractile force. J. Mol. Cell. Cardiol. 1974, 6, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef]
- He, L.; Tao, J.; Maity, D.; Si, F.; Wu, Y.; Wu, T.; Prasath, V.; Wirtz, D.; Sun, S. Role of membrane-tension gated Ca2+ flux in cell mechanosensation. J. Cell Sci. 2018, 131, jcs208470. [Google Scholar] [CrossRef]
- Lin, Y.; Annamdevula, N.S.; Kashef, J.; Nguyen, T.A. Calcium signature triggers recruitment of cadherin 11 and modulates membrane tension during Xenopus cranial neural crest cell migration. Dev. Cell 2024, 33, 119–126. [Google Scholar]
- Ingber, D.E.; Jamieson, J.D. Cells as tensegrity structures: Architectural regulation of histodifferentiation by physical forces transduced over basement membrane. In Gene Expression During Normal and Malignant Differentiation; Andersson, L.C., Gahmberg, C.G., Ekblom, P., Eds.; Academic Press: Cambridge, MA, USA, 1985; pp. 13–32. [Google Scholar]
- Ingber, D.E. Cellular tensegrity: Defining new rules of biological design that govern the cytoskeleton. J. Cell. Sci. 1993, 104, 613–627. [Google Scholar] [CrossRef]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Homsher, E.; Gordon, A.; Regnier, M. Regulation of contraction in striated muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef]
- Regnier, M.; Tesi, C.; Piroddi, N.; McMichael, J.; Poggesi, C.; Kreutziger, K. Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J. Mol. Cell. Cardiol. 2011, 50, 165–174. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Brownlee, C.; Wheeler, G.L. Ca2+ signalling in plants and algae. Curr. Opin. Plant Biol. 2025, 11, 379–387. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Halloran, B. The response of bone to unloading. J. Bone Miner. Metab. 1999, 17, 233–244. [Google Scholar] [CrossRef]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin Mediates Bone Response to Mechanical Unloading Through Antagonizing Wnt/β-Catenin Signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef]
- Kawao, N.; Moritake, A.; Tatsumi, K.; Kaji, H. Roles of Irisin in the Linkage from Muscle to Bone During Mechanical Unloading in Mice. Calcif. Tissue Int. 2018, 103, 24–34. [Google Scholar] [CrossRef]
- Sun, X.; McLamore, E.; Kishore, V.; Fites, K.; Slipchenko, M.; Porterfield, D.; Akkus, O. Mechanical stretch induced calcium efflux from bone matrix stimulates osteoblasts. Bone 2012, 50, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; McLamore, E.; Porterfield, D.; Akkus, O. Calcium Efflux from Bone Matrix in Response to Mechanical Loading. In Proceedings of the ASME 2011 Summer Bioengineering Conference, Proceedings of the ASME 2011 Summer Bioengineering Conference, Farmington, PA, USA, 22–25 June 2011; ASME: New York, NY, USA, 2011; pp. 505–506. [Google Scholar] [CrossRef]
- Zhovner, M.; Kalinkevich, A.; Danilchenko, S.; Kuznetsov, V.; Wang, J.; Li, H.; He, J.; Feng, X. A Mechanical Device to Evaluate the Effects of Dynamic Loading in Weak-acid Medium on the Bioapatite of Devitalized Cortical Bone. Exp. Tech. 2020, 44, 591–596. [Google Scholar] [CrossRef]
- Erickson, H.P. Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc. Natl. Acad. Sci. USA 1994, 91, 10114–10118. [Google Scholar] [CrossRef]
- Rief, M.; Gautel, M.; Oesterhelt, F.; Fernandez, J.M.; Gaub, H.E. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science 1997, 276, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Gautel, M.; Djinović-Carugo, K. The sarcomeric cytoskeleton: From molecules to motion. J. Exp. Biol. 2016, 219, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, B.W.; Fritschy, J.M.; Murmann, P.; Troxler, H.; Durussel, I.; Heizmann, C.W.; Cox, J.A. S100A5 is a novel calcium-, zinc-, and copper ion-binding protein of the EF-hand superfamily. J. Biol. Chem. 2000, 275, 30623–30630. [Google Scholar] [CrossRef]
- Cox, J.A.; Durussel, I.; Comte, M.; Nef, S.; Nef, P.; Lenz, S.E.; Gundelfinger, E.D. Cation binding and conformational changes in VILIP and NCS-1, two neuron-specific calcium-binding proteins. J. Biol. Chem. 1994, 269, 32807–32813. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.P.; Spartz, E.J.; Hong, W.; Luo, L.; Kopito, R.R. Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat. Commun. 2019, 6, 6768. [Google Scholar] [CrossRef] [PubMed]
- Morikis, V.; Jo, M.; Ha, T.; Simon, S. Bond tension on neutrophil LFA-1 regulates membrane calcium flux from the outside-in. J. Immunol. 2019, 202, 64.13. [Google Scholar] [CrossRef]
- Ingber, D.E.; Wang, N.; Stamenović, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhang, W.; Zhang, J.; Zhou, J.; Wang, J.; Chen, L.; Wang, L.; Hodgkins, A.; Iyer, V.; Huang, X.; et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat. Methods 2021, 11, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Kapustin, A.; Davies, J.; Reynolds, J.; McNair, R.; Jones, G.; Sidibé, A.; Schurgers, L.; Skepper, J.; Proudfoot, D.; Mayr, M.; et al. Calcium regulates key components of vascular smooth muscle cell–derived matrix vesicles to enhance mineralization. Circ. Res. 2011, 109, e1–e12. [Google Scholar] [CrossRef]
- Bäck, M.; Michel, J. From organic and inorganic phosphates to valvular and vascular calcifications. Cardiovasc. Res. 2021, 117, 2016–2029. [Google Scholar] [CrossRef] [PubMed]
- Kalya, S.; Rosenthal, A. Extracellular matrix changes regulate calcium crystal formation in articular cartilage. Curr. Opin. Rheumatol. 2005, 17, 325–329. [Google Scholar] [CrossRef]
- Maurer, P.; Hohenester, E. Structural and functional aspects of calcium binding in extracellular matrix proteins. Matrix Biol. 1997, 15, 569–580. [Google Scholar] [CrossRef]
- Murthy, S.; Karkossa, I.; Schmidt, C.; Hoffmann, A.; Hagemann, T.; Rothe, K.; Seifert, O.; Anderegg, U.; Von Bergen, M.; Schubert, K.; et al. Danger signal extracellular calcium initiates differentiation of monocytes into SPP1/osteopontin-producing macrophages. Cell Death Dis. 2022, 13, 53. [Google Scholar] [CrossRef]
- Brown, E. Physiology and pathophysiology of the extracellular calcium-sensing receptor. Am. J. Med. 1999, 106, 238–253. [Google Scholar] [CrossRef]
- Hrabetova, S.; Masri, D.; Tao, L.; Xiao, F.; Nicholson, C. Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J. Physiol. 2009, 587, 4029–4049. [Google Scholar] [CrossRef]
- Egelman, D.M.; Montague, P.R. Calcium dynamics in the extracellular space of mammalian neural tissue. Biophys. J. 1999, 76, 1856–1867. [Google Scholar] [CrossRef]
- Smith, M.L.; Gourdon, D.; Little, W.C.; Kubow, K.E.; Eguiluz, R.A.; Luna-Morris, S.; Vogel, V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007, 5, e268. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1917. [Google Scholar]
- Atiyah, M.; Sutcliffe, P. Polyhedra in physics, chemistry and geometry. Milan J. Math. 2003, 71, 33–58. [Google Scholar] [CrossRef]
- Kepler, J. The Harmony of the World; American Philosophical Society: Philadelphia, PA, USA, 1997. [Google Scholar]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Ingber, D.E. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2014, 35, 564–577. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity I: Cell structure and hierarchical systems biology. J. Cell. Sci. 2003, 116, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.B.; Ingber, D.E. Multi-scale modeling reveals use of hierarchical tensegrity principles at the molecular, multi-molecular, and cellular levels. Extrem. Mech. Lett. 2018, 20, 21–28. [Google Scholar] [CrossRef]
- Ingber, D.E. Integrins as mechanochemical transducers. Curr. Opin. Cell Biol. 1991, 3, 841–848. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity II: How structural networks influence cellular information processing networks. J. Cell. Sci. 2003, 116, 1397–1408. [Google Scholar] [CrossRef]
- Ingber, D.E. The architecture of life. Sci. Am. 1998, 278, 48–57. [Google Scholar] [CrossRef]
- Xu, F.; Wang, H.; Teat, S.; Liu, W.; Xia, Q.; Li, Z.; Li, J. Synthesis, structure and enhanced photoluminescence properties of two robust, water stable calcium and magnesium coordination networks. Dalton Trans. 2015, 44, 20459–20463. [Google Scholar] [CrossRef]
- Kesseli, F.; Lauer, C.; Baker, I.; Mirica, K.; Van Citters, D. Identification of a calcium phosphoserine coordination network in an adhesive organo-apatitic bone cement system. Acta Biomater. 2020, 105, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Wang, K.; Wang, Y.; Cheng, Y.; Gan, J. The negative electrostatic potential of the coordination between calcium and carboxyl/oxygen group in calcium-peptide complexes contributes to calcium utilization. Food Chem. 2024, 464, 141909. [Google Scholar] [CrossRef]
- Gaur, R.; Susmitha, A.; Chary, K.; Mishra, L. A water soluble calcium–sodium based coordination polymer: Selective release of calcium at specific binding sites on proteins. RSC Adv. 2014, 4, 24038–24041. [Google Scholar] [CrossRef]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [PubMed]
- Sakato, M.; Sakakibara, H.; King, S. Chlamydomonas outer arm dynein alters conformation in response to Ca2+. Mol. Biol. Cell 2007, 18, 3620–3634. [Google Scholar] [CrossRef] [PubMed]
- Smith, E. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol. Biol. Cell 2002, 13, 3303–3313. [Google Scholar] [CrossRef]
- Ikura, M.; Ames, J.B. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: Two ways to promote multifunctionality. Proc. Natl. Acad. Sci. USA 2006, 103, 1159–1164. [Google Scholar] [CrossRef]
- Iqbal, Z.; Shariq Iqbal, M.; Singh, S.P.; Buaboocha, T. Ca2+/calmodulin complex triggers CAMTA transcriptional machinery under stress in plants: Signaling cascade and molecular regulation. Front. Plant Sci. 2020, 11, 598327. [Google Scholar] [CrossRef]
- Means, A.; Dedman, J. Calmodulin—An intracellular calcium receptor. Nature 1980, 285, 73–77. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Friedberg, F.; Rhoads, A. Evolutionary aspects of calmodulin. IUBMB Life 2001, 51, 215–221. [Google Scholar] [CrossRef]
- Solà, C.; Tusell, J.; Serratosa, J. Comparative study of the pattern of expression of calmodulin messenger RNAs in the mouse brain. Neuroscience 1996, 75, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Toutenhoofd, S.L.; Foletti, D.; Wicki, R.; Rhyner, J.A.; Garcia, F.; Tolon, R.; Strehler, E.E. Characterization of the human CALM2 calmodulin gene and comparison of the transcriptional activity of CALM1, CALM2 and CALM3. Cell Calcium 1998, 23, 323–338. [Google Scholar] [CrossRef]
- Munk, M.; Alcalde, J.; Lorentzen, L.; Villalobo, A.; Berchtold, M.; Panina, S. The impact of calmodulin on the cell cycle analyzed in a novel human cellular genetic system. Cell Calcium 2020, 88, 102207. [Google Scholar] [CrossRef] [PubMed]
- Colomer, J.; Agell, N.; Engel, P.; Bachs, O. Expression of calmodulin and calmodulin binding proteins in lymphoblastoid cells. J. Cell. Physiol. 1994, 159, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.H. Studying complex adaptive systems. J. Syst. Sci. Complex. 2006, 19, 1–8. [Google Scholar] [CrossRef]
- Guimberteau, J.C.; Sawaya, E.T.; Armstrong, C. New Perspectives on the Organization of Living Tissue and the Ongoing Connective Tissue/Fascia Nomenclature Debate, as Revealed by Intra-Tissue Endoscopy That Provides Real-Time Images During Surgical Procedures. Life 2025, 15, 791. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, J. Fascia and Tensegrity the Quintessence of a Unified Systems Conception. Int. J. Anat. Appl. Physiol. 2021, 7, 174–178. [Google Scholar] [CrossRef]
- Scarr, G.; Blyum, L.; Levin, S.M.; de Solórzano, S.L. Moving beyond Vesalius: Why anatomy needs a mapping update. Med. Hypotheses 2024, 183, 111257. [Google Scholar] [CrossRef]
- Wal, J. The Fascia as the Organ of Innerness—An Holistic Approach Based Upon a Phenomenological Embryology and Morphology. Int. J. Ther. Massage Bodyw. 2015, 8, 39–52. [Google Scholar]
- Van Der Wal, J. The Architecture of the Connective Tissue in the Musculoskeletal System—An Often Overlooked Functional Parameter as to Proprioception in the Locomotor Apparatus. Int. J. Ther. Massage Bodyw. 2009, 2, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.R.; Chagot, B.; Chazin, W.J. EF-hand calcium-binding proteins. Encycl. Life Sci. 2010. [Google Scholar] [CrossRef]
- Schäfer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef]
- Chazin, W.J. Relating form and function of EF-hand calcium binding proteins. Acc. Chem. Res. 2011, 44, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, T.; Miao, H.; Liang, B. The calcium binding protein S100A11 and its roles in diseases. Front. Cell Dev. Biol. 2021, 9, 693262. [Google Scholar] [CrossRef]
- Foell, D.; Frosch, M.; Sorg, C.; Roth, J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin. Chim. Acta 2004, 344, 37–51. [Google Scholar] [CrossRef]
- Fairless, R.; Williams, S.K.; Diem, R. Calcium-binding proteins as determinants of central nervous system neuronal vulnerability to disease. Int. J. Mol. Sci. 2019, 20, 2146. [Google Scholar] [CrossRef]
- Kayastha, B.B.; Kubo, A.; Burch-Konda, J.; Dohmen, R.; McCoy, J.; Rogers, R.; Mares, S.; Bevere, J.; Huckaby, A.; Witt, W.; et al. EF-hand protein, EfhP, specifically binds Ca2+ and mediates Ca2+ regulation of virulence in a human pathogen Pseudomonas aeruginosa. Sci. Rep. 2022, 12, 8635. [Google Scholar] [CrossRef]
- Min, J.; Yu, Y.J.; Lee, E. Biomimetic calcium-based nanomaterials: Design principles for biomedical applications. Biomater. Res. 2024, 28, 3. [Google Scholar]
- Xian, S.; Huo, F.; Shah, B. Calcium-based metal-organic frameworks for carbon dioxide capture: Synthesis, modification, and applications. ChemSusChem 2020, 13, 6449–6458. [Google Scholar]
- Loiselle, A.; Cao, C.; Mendias, C.; Ciufo, D.; Korcari, A.; Li, H.; Pitt, G.; Buckley, M.; Rodeo, S. Increased Ca2+ signaling through CaV1.2 induces tendon hypertrophy with increased collagen fibrillogenesis and biomechanical properties. FASEB J. 2023, 37, e23007. [Google Scholar] [CrossRef]
- Li, J.; Wickström, S.; Albigès-Rizo, C.; Manet, S.; Faurobert, E.; Miroshnikova, Y. Calcium signaling mediates a biphasic mechanoadaptive response of endothelial cells to cyclic mechanical stretch. Mol. Biol. Cell 2023, 32, 1724–1736. [Google Scholar] [CrossRef]
- Meyer, G.; Smith, L.; Lieber, R. Systems analysis of biological networks in skeletal muscle function. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 55–71. [Google Scholar] [CrossRef]
- Sun, R.; Sheng, W.; Janssen, L.; Mukherjee, S. Ca2+/calmodulin-dependent protein kinase IIβ and IIδ mediate TGFβ-induced transduction of fibronectin and collagen in human pulmonary fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L510–L519. [Google Scholar] [CrossRef]
- Sharma, S.; Tewari, D.; Ali, R.; Kulkarni, M.; Kulkarni, C.; Chattopadhyay, N.; Porwal, K.; Verma, S. Tripeptide-induced modulation of mesenchymal stem cell biomechanics stimulates proliferation and wound healing. Chem. Commun. 2020, 56, 3043–3046. [Google Scholar] [CrossRef]
- Xu, J.; Liechty, K.; Beason, D.; Hodges, M.; Soslowsky, L.; Zgheib, C.; Hu, J. Mechanisms of mesenchymal stem cell correction of the impaired biomechanical properties of diabetic skin: The role of miR-29a. Wound Repair Regen. 2016, 24, 237–246. [Google Scholar] [CrossRef]
- Fischer, K.; Padmanabhan, J.; Tevlin, R.; Mermin-Bunnell, A.; Carlomagno, T.; Trotsyuk, A.; Bonham, C.; Leeolou, M.; Januszyk, M.; Kussie, H.; et al. Disrupting mechanotransduction decreases fibrosis and contracture in split-thickness skin grafting. Sci. Transl. Med. 2022, 14, eabj9152. [Google Scholar] [CrossRef]
- Tager, A.; Tschumperlin, D.; Liu, F. Biomechanical regulation of mesenchymal cell function. Curr. Opin. Rheumatol. 2013, 25, 92–100. [Google Scholar] [CrossRef]
- Zhou, Z.; Musskaya, O.; Zhao, X.; Glazov, I.; Chen, Y.; Zhang, L.; Chen, F.; Luo, Y.; Qu, X.; Zhao, J.; et al. Photothermal switch by gallic acid-calcium grafts synthesized by coordination chemistry for sequential treatment of bone tumor and regeneration. Biomaterials 2024, 312, 122724. [Google Scholar] [CrossRef]
- Dang, J.; Ling, W. Effect of mesenchymal stem cells on biomechanics of stress fracture rabbit model after healing. Pak. J. Pharm. Sci. 2021, 34, 1673–1678. [Google Scholar] [PubMed]
- Wang, Y.; Zhang, Y.; Ossipov, D.; Hilborn, J.; Ding, P.; Shi, L. Self-Healing Polymeric Hydrogel Formed by Metal-Ligand Coordination Assembly: Design, Fabrication, and Biomedical Applications. Macromol. Rapid Commun. 2019, 40, e1800837. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.; Wang, H.; Wu, J. Application of metal-based biomaterials in wound repair. Eng. Regen. 2021, 2, 137–153. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, Y.; Wang, L.; Li, W.; Han, L. Calcium signaling of primary chondrocytes and ATDC5 chondrogenic cells under osmotic stress and mechanical stimulation. J. Biomech. 2022, 145, 111388. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Vassilieva, I.O.; Pugovkina, N.A.; Nikolsky, N.N.; Negulyaev, Y.A.; Morachevskaya, E.A.; Chubinskiy-Nadezhdin, V.I. Local calcium signalling is mediated by mechanosensitive ion channels in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 563–568. [Google Scholar] [CrossRef]
- Polte, T.; Eichler, G.; Wang, N.; Ingber, D. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol.-Cell Physiol. 2001, 286, C518–C528. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Tensegrity: The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997, 59, 575–599. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.H. Osteocyte Calcium Signaling—A Potential Translator of Mechanical Load to Mechanobiology. Bone 2021, 153, 116136. [Google Scholar] [CrossRef]
- Barrera, J.; Bonham, C.; Greco, A.; Sivaraj, D.; Maan, Z.; Keller, A.; Trotsyuk, A.; Mermin-Bunnell, A.; Dohi, T.; Kwon, S.; et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat. Commun. 2021, 12, 5256. [Google Scholar] [CrossRef]
- Choi, S.; Paek, S.; Choi, D.; Paek, S.; Park, J. Detecting early-stage malignant melanoma using a calcium switch-enriched exosome subpopulation containing tumor markers as a sample. Biosens. Bioelectron. 2021, 198, 113828. [Google Scholar] [CrossRef]
- Thangaraj, K.; Sharma, Y.; Phanindranath, R.; Sudhakar, D. Conformational scanning of individual EF-hand motifs of calcium sensor protein centrin-1. Biochem. Biophys. Res. Commun. 2021, 570, 67–73. [Google Scholar] [CrossRef]
- Lim, G.; Paek, S.; Seo, S.; Kang, J.; Paek, S.; Paek, S.; Cho, I.; Park, J.; Kim, D. Conformation-sensitive antibody-based point-of-care immunosensor for serum Ca2+ using two-dimensional sequential binding reactions. Biosens. Bioelectron. 2016, 85, 611–617. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Ye, H. Fascia Layer—A Novel Target for the Application of Biomaterials in Skin Wound Healing. Int. J. Mol. Sci. 2023, 24, 2936. [Google Scholar] [CrossRef]

| Level of Organization | Approximate Scale | Examples | Calcium-Related Structures, Functions, and Tensegrity Balance |
|---|---|---|---|
| Subatomic | 10−15 m | Electrons, protons, neutrons | Electron orbital interactions that enable calcium’s bonding properties; electron density distributions create tensional balance in coordination sphere |
| Atomic | 10−10 m | Individual calcium atoms, oxygen atoms | Atomic radius of calcium (114 pm) creates optimal tensional geometry for coordinating 6–8 ligands in balanced triangulated arrangements |
| Ionic | 10−10 m | Ca2+ ions, Mg2+ ions, phosphate ions | Electrostatic forces between calcium ions and oxygen ligands establish precise tensional equilibrium in coordination complexes |
| Moleular | 10−9 m | ATP, glucose, amino acids, water | Calcium-binding loops create tensegrity-based pocket structures; water molecules form dynamic tensional networks around calcium ions |
| Macromolecular | 10−8 m | Proteins (calmodulin, troponin C), DNA, integrins | EF-hand domains function as tensional springs; calcium binding induces balanced conformational shifts for signal transduction |
| Cytoskeletal | 10−8 to 10−7 m | Actin filaments, microtubules, intermediate filaments | Calcium regulates tensional states of cytoskeletal networks; modulates compression-tension balance between microtubules and actin filaments |
| Supramolecular | 10−7 m | Protein complexes, focal adhesions, desmosomes | Calcium-dependent tensional integrity of adhesion complexes; maintains balanced tension across gap junctions and intercellular connections |
| Subcellular | 10−7 to 10−6 m | Mitochondria, endoplasmic reticulum, nucleus | Calcium gradients establish tensional homeostasis between organelles; ER serves as tensional reservoir for calcium-mediated structural stability |
| Cellular | 10−6 to 10−4 m | Neurons, muscle cells, osteoblasts | Calcium waves regulate cellular prestress; modulates tensegrity-based mechanotransduction through cytoskeletal-membrane-nucleus continuum |
| Extracellular matrix | 10−6–10−4 m | Collagen networks, elastin fibers, proteoglycans | Calcium-dependent ECM tensional integrity; balances compression-tension elements in fibronectin networks; regulates matrix prestress |
| Tissue | 10−4–10−2 m | Muscle tissue, bone tissue, epithelium | Calcium mediates tissue-level tensional states; maintains tensegrity balance in bone mineralization; coordinates contractile forces across tissues |
| Organ | 10−2–10−1 m | Heart, bones, brain | Coordinated calcium signaling balances tensional forces in cardiac contraction; maintains tensegrity-based structural integrity of organs |
| System | 10−1–1 m | Skeletal system, nervous system, cardiovascular system | Calcium regulation establishes tensional equilibrium across body systems; coordinates balanced force distribution throughout musculoskeletal network |
| Organism | 1 to 2 m | Whole human body | Integrated calcium homeostasis maintains whole-body tensegrity balance; orchestrates tensional harmony across all biological scales |
| First Messengers | Second Messengers |
|---|---|
| External ligands (hormones, neurotransmitters) | Internal signaling molecules (Ca2+, cAMP, IP3) |
| Bind to cell surface receptors | Released/activated inside the cell |
| Initiate signaling cascade | Amplify and transmit signals |
| Cannot cross membrane barriers | Operate within cellular compartments |
| Property | Effect on Protein Binding | Comparison to Mg2+ |
|---|---|---|
| Large ionic radius (114 pm) | Enables flexible, high coordination (6–8 sites) | vs. Mg2+ (86 pm): rigid, strict 6-coordination |
| Intermediate charge density (12.6 C/mm3) | Matches protein site electrostatics optimally | vs. Mg2+ (23.3 C/mm3): overly tight binding |
| Flexible geometry | Supports rapid, reversible interactions | vs. Mg2+: static octahedral geometry only |
| Selectivity over Mg2+ | Many-body effects and geometric fit favor Ca2+ | Geometric constraints exclude smaller Mg2+ |
| Process | Signal Initiation | Protein Conformation Change | Functional Outcome | Key References |
|---|---|---|---|---|
| Nerve conduction | Neurotransmitter/hormone triggers Ca2+ influx | Ca2+ binds to synaptic proteins | Neurotransmission | [10,23] |
| Muscle contraction | Ca2+ release via channels | Ca2+ binds to contractile proteins | Muscle contraction | [23,24] |
| Cell survival/death | SOCE, Ca2+ channel activation | Ca2+ modulates autophagy/apoptosis proteins | Cell fate decisions | [40] |
| Protein Family | Key Members | Primary Function | Associated Diseases/Disorders | Calcium Binding Domain |
|---|---|---|---|---|
| EF-Hand Proteins | ||||
| Calmodulin (CaM) | CaM1, CaM2, CaM3 | Universal calcium signal transducer; regulates > 100 target proteins | Cardiac arrhythmias, CPVT, Long QT syndrome, neurodevelopmental disorders, certain cancers | 4 EF-hands |
| Troponin C | cTnC, sTnC | Muscle contraction regulation | Hypertrophic cardiomyopathy, dilated cardiomyopathy, heart failure | 4 EF-hands |
| Calcineurin | CnA, CnB | Phosphatase activity, immune response, cardiac development | Cardiac hypertrophy, immunodeficiency, transplant rejection, Down syndrome | 4 EF-hands (in CnB) |
| S100 proteins | S100B, S100A1-A16 | Tissue-specific regulation, inflammation | Alzheimer’s disease, melanoma, psoriasis, rheumatoid arthritis, cancer progression | 2 EF-hands |
| Calbindin | Calbindin-D28k | Calcium buffering in neurons | Parkinson’s disease, epilepsy, Alzheimer’s disease | 6 EF-hands |
| Parvalbumin | α, β isoforms | Calcium buffering in fast-twitch muscles and neurons | ALS, epilepsy, autism spectrum disorders | 3 EF-hands |
| Calretinin | CR | Neuronal calcium buffering | Mesothelioma, colon cancer, Huntington’s disease | 6 EF-hands |
| Annexins | Annexins A1-A13 | Membrane organization, vesicle trafficking, calcium homeostasis | Cancer, inflammation, autoimmune disorders, thrombosis | Type II calcium binding sites |
| C2-Domain Proteins | ||||
| Protein Kinase C | PKC-α, β, γ | Signal transduction, cell proliferation | Cancer, diabetes, cardiovascular disease, Alzheimer’s disease | C2 domain |
| Synaptotagmins | Syt1-17 | Neurotransmitter release, membrane fusion | Epilepsy, neurodevelopmental disorders, psychiatric disorders | C2 domains |
| Calcium Channels | ||||
| Voltage-gated Ca2+ channels | CaV1.1-1.4, CaV2.1-2.3, CaV3.1-3.3 | Calcium influx, excitation-contraction coupling | Migraine, epilepsy, ataxia, hypokalemic periodic paralysis, Timothy syndrome | EF-hand-like domains |
| Ryanodine receptors | RyR1, RyR2, RyR3 | Calcium release from SR/ER | Malignant hyperthermia, central core disease, CPVT, heart failure | EF-hand-like domains |
| IP3 receptors | IP3R1, IP3R2, IP3R3 | Calcium release from ER | Spinocerebellar ataxia, Alzheimer’s disease, Huntington’s disease | EF-hand-like domains |
| STIM/Orai | STIM1, STIM2, Orai1-3 | Store-operated calcium entry | SCID, Stormorken syndrome, tubular aggregate myopathy, York platelet syndrome | EF-hand (in STIM) |
| TRP channels | TRPV, TRPC, TRPM, TRPA, TRPP, TRPML | Sensory transduction, calcium homeostasis | Polycystic kidney disease, mucolipidosis type IV, pain syndromes, cancer | Various |
| ECM Proteins | ||||
| Fibrillin | Fibrillin-1, -2, -3 | ECM structural organization, growth factor regulation | Marfan syndrome, congenital contractural arachnodactyly | cbEGF domains |
| Matrix Gla Protein (MGP) | MGP | Inhibits tissue calcification | Vascular calcification, Keutel syndrome | Gla domains |
| BM-40/SPARC/Osteonectin | SPARC | Cell–matrix interactions, tissue remodeling | Osteogenesis imperfecta, cataracts, cancer progression | EF-hand pair |
| Calcium Sensing Proteins | ||||
| Calcium-sensing receptor | CaSR | Extracellular calcium sensing | Familial hypocalciuric hypercalcemia, autosomal dominant hypocalcemia, hyperparathyroidism | Venus flytrap domain |
| Neuronal calcium sensors | NCS-1, VILIPs, KChIPs, GCAPs | Neuronal calcium signaling | Schizophrenia, bipolar disorder, retinal degeneration | EF-hands |
| Calcium Buffers/Transporters | ||||
| Calsequestrin | CASQ1, CASQ2 | SR calcium storage | Catecholaminergic polymorphic ventricular tachycardia (CPVT), malignant hyperthermia | Acidic domains |
| PMCA pumps | PMCA1-4 | Calcium extrusion from cells | Hearing loss, neurological disorders, cardiovascular disease | Acidic regions |
| SERCA pumps | SERCA1-3 | Calcium sequestration into SR/ER | Brody disease, Darier disease, heart failure | Transmembrane domains |
| NCX exchangers | NCX1-3 | Sodium-calcium exchange | Cardiac arrhythmias, heart failure, hypertension | α-repeats |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkness, K.B.; Sharkey, J.; Scarlata, S. Calcium Unified: Understanding How Calcium’s Atomic Properties Impact Human Health. Cells 2025, 14, 1066. https://doi.org/10.3390/cells14141066
Kirkness KB, Sharkey J, Scarlata S. Calcium Unified: Understanding How Calcium’s Atomic Properties Impact Human Health. Cells. 2025; 14(14):1066. https://doi.org/10.3390/cells14141066
Chicago/Turabian StyleKirkness, Karen B., John Sharkey, and Suzanne Scarlata. 2025. "Calcium Unified: Understanding How Calcium’s Atomic Properties Impact Human Health" Cells 14, no. 14: 1066. https://doi.org/10.3390/cells14141066
APA StyleKirkness, K. B., Sharkey, J., & Scarlata, S. (2025). Calcium Unified: Understanding How Calcium’s Atomic Properties Impact Human Health. Cells, 14(14), 1066. https://doi.org/10.3390/cells14141066








