Assessment of Feasibility of the M2 Macrophage-Based Adoptive Gene Transfer Strategy for Osteoarthritis with a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Polarization and Expansion of Marrow-Derived M2 MΦs
2.3. Lentiviral Vector Preparations
2.4. Lentiviral Vector Transduction of Primary Murine M2 MΦs
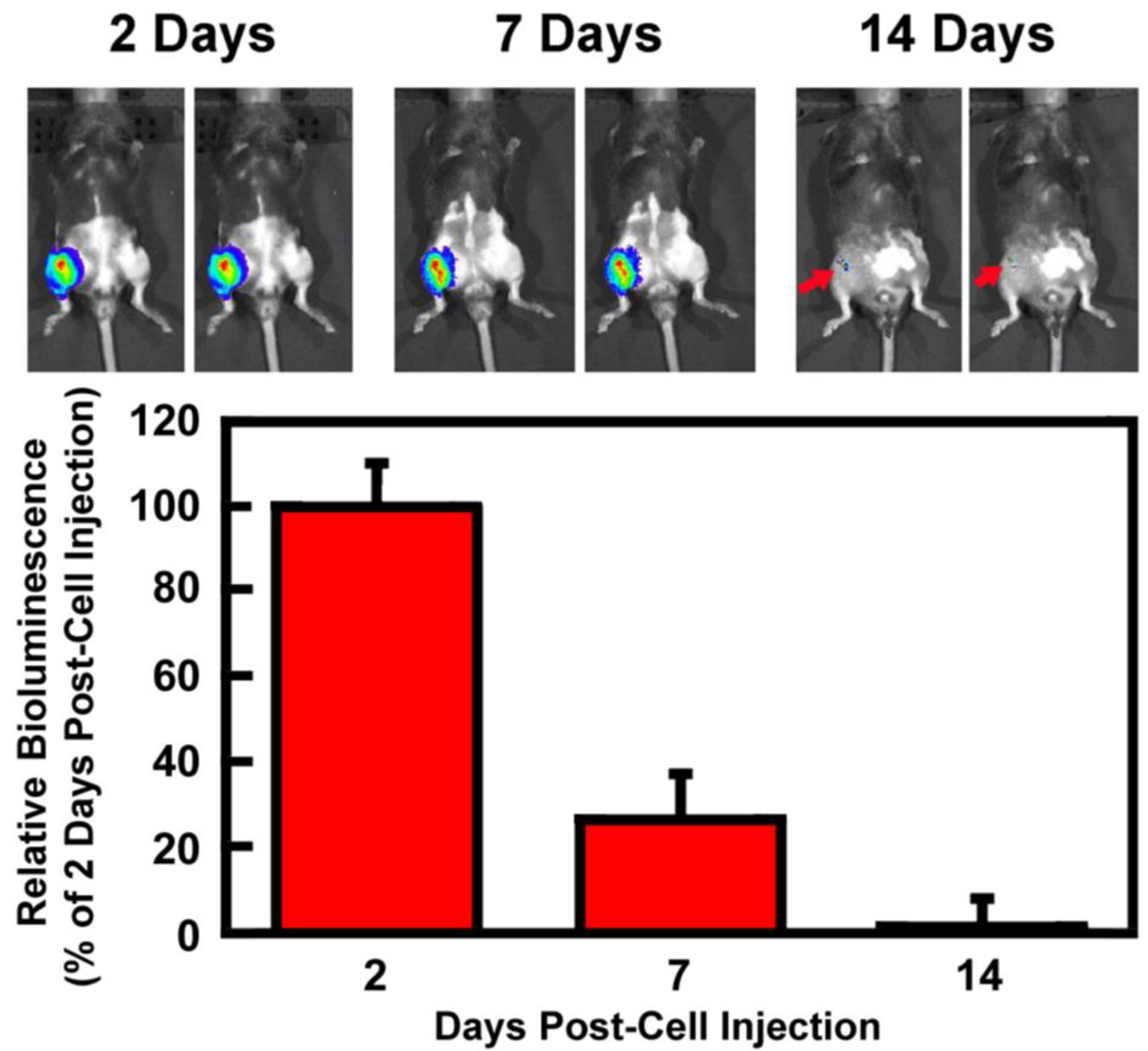
2.5. Bioluminescence-Based In Vivo Tracking of the Fate of Intraarticularly Injected M2 MΦs in Injured Knee Joints
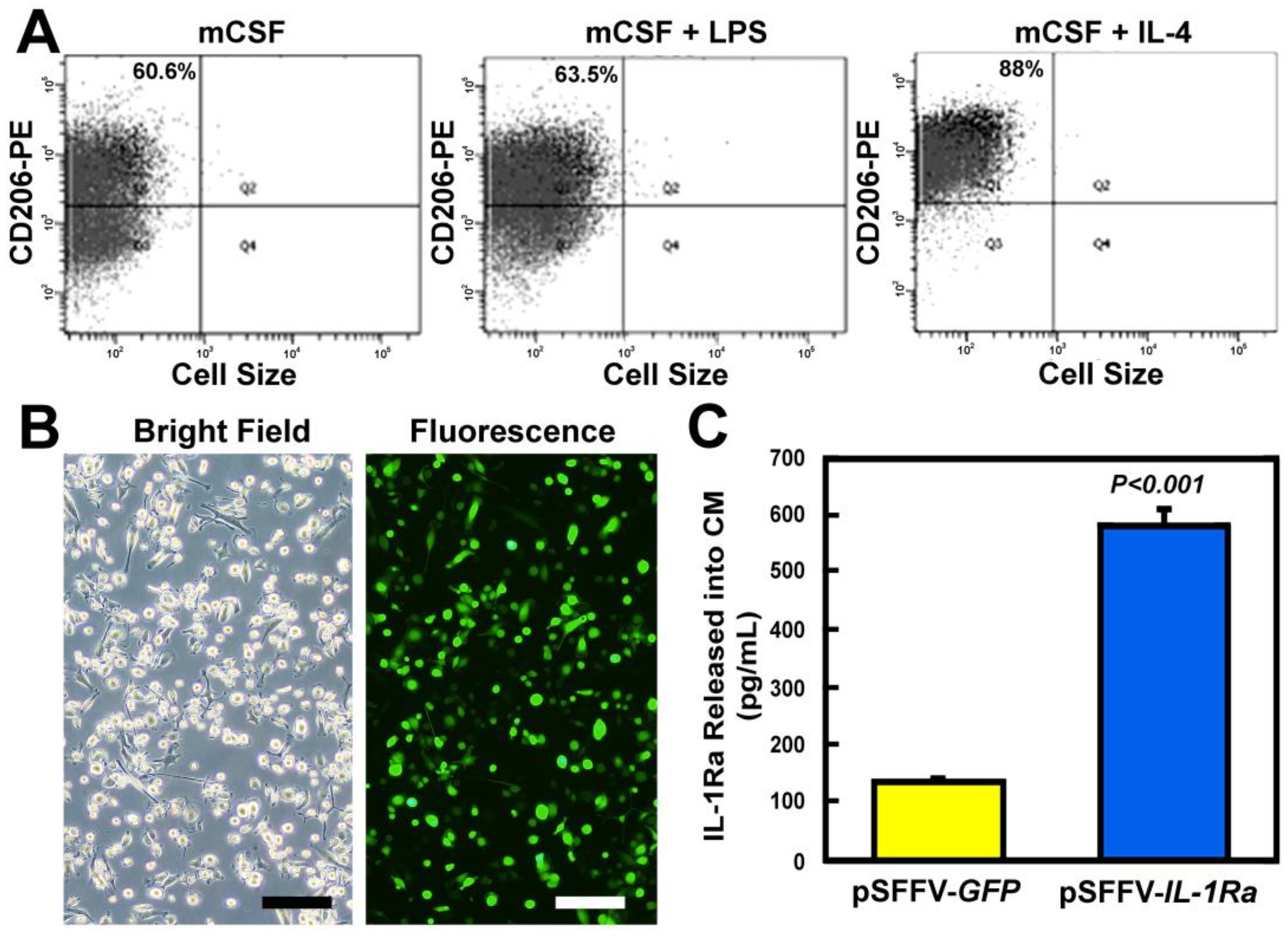
2.6. Flow Cytometry
2.7. A Mouse Tibial Plateau Injury Model
2.8. The IL-1Ra-Expressing M2 MΦ-Based Treatment Strategy for OA/PTOA
2.9. Plasma Cartilage Oligomeric Matrix Protein (COMP)
2.10. Histology
2.11. Histology-Based OA Severity Scoring Systems
2.12. Statistical Analyses
3. Results
3.1. Lentiviral-Transduced M2 MΦs Secreted Large Amounts of IL-1Ra Protein
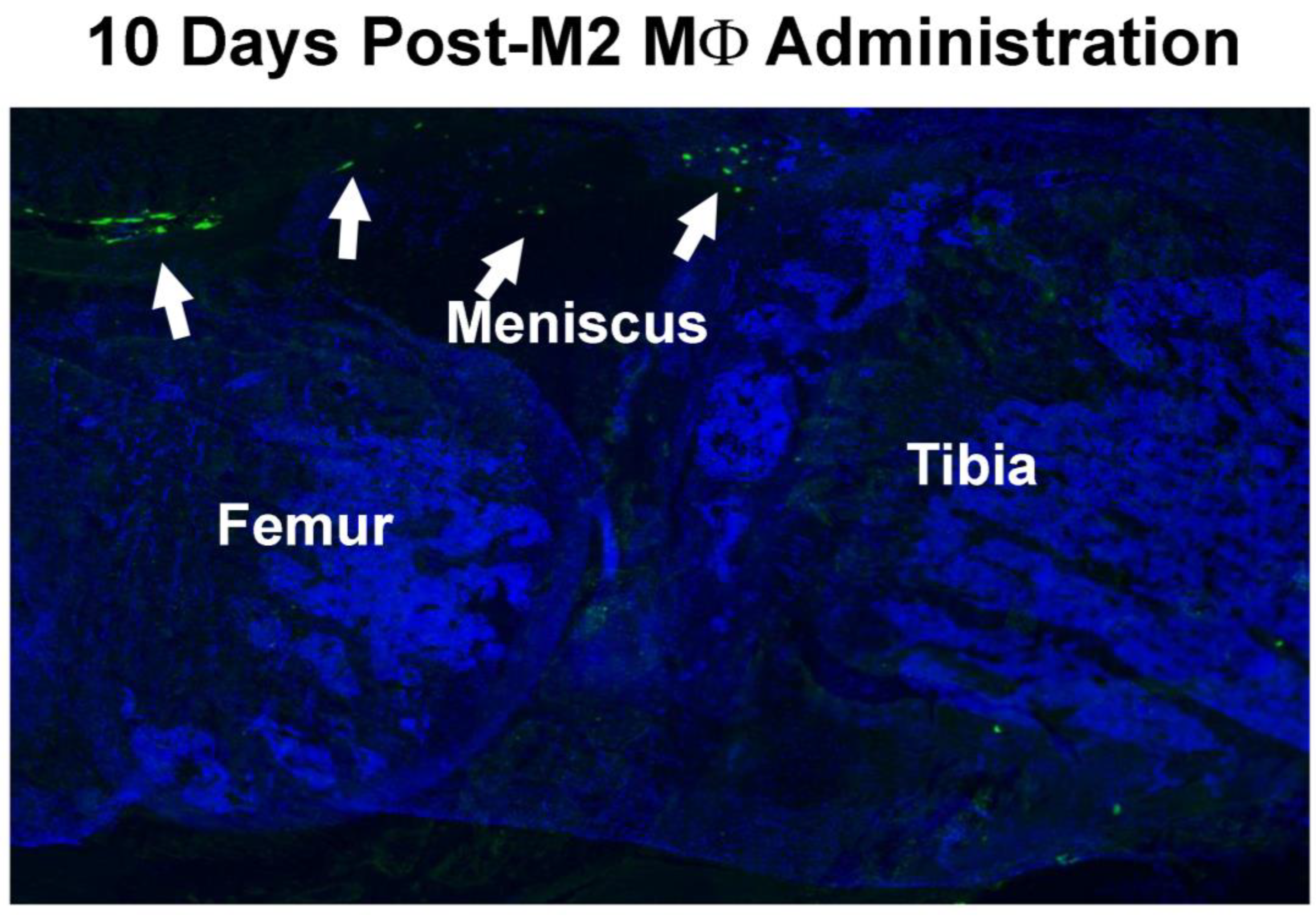
3.2. Retention of Intraarticularly Injected M2 MΦs in the Injured Joint
3.3. M2 MΦs-Based Adoptive IL-1Ra Gene Transfer Strategy Was Effective in Reducing Plasma Levels of COMP
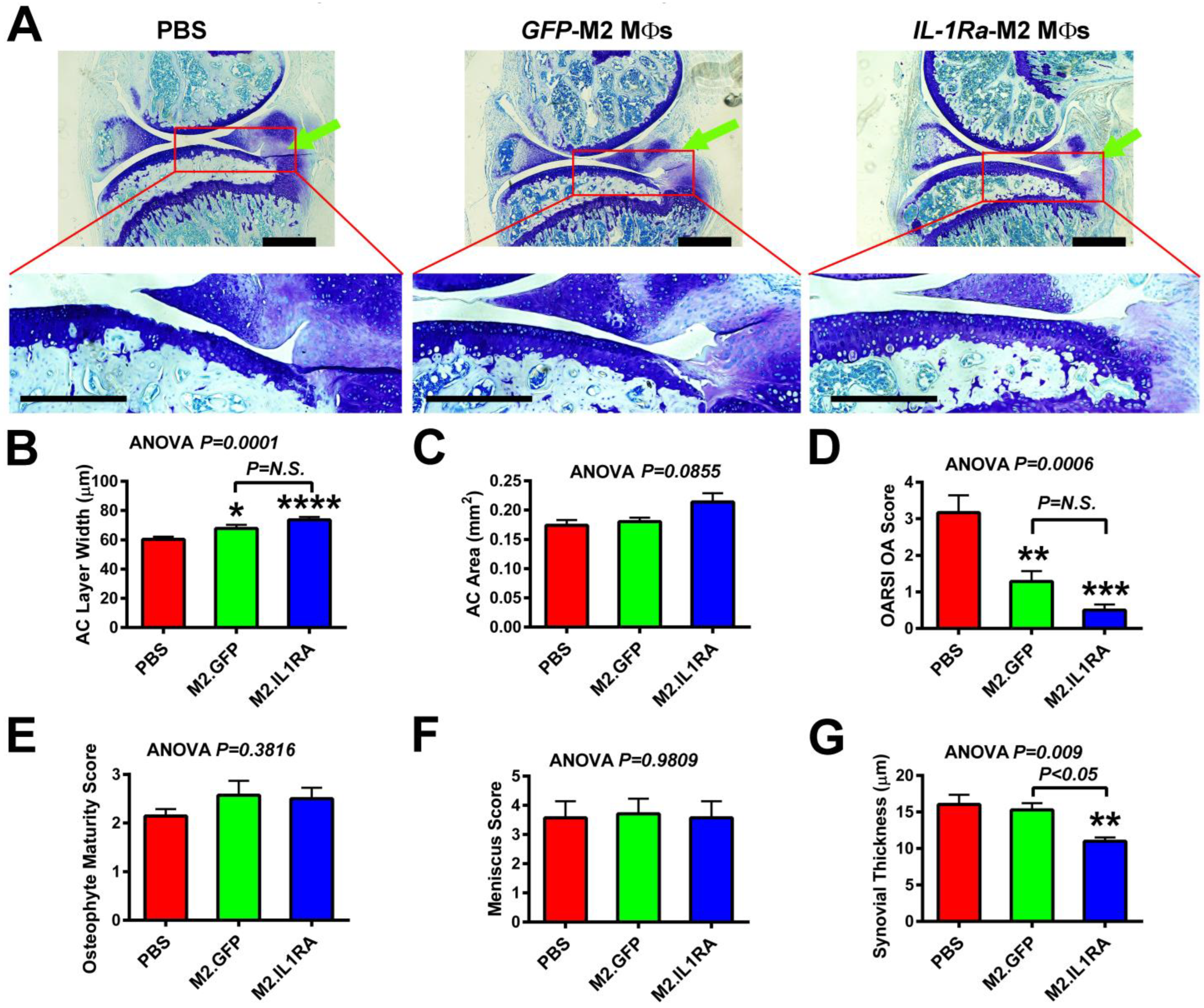
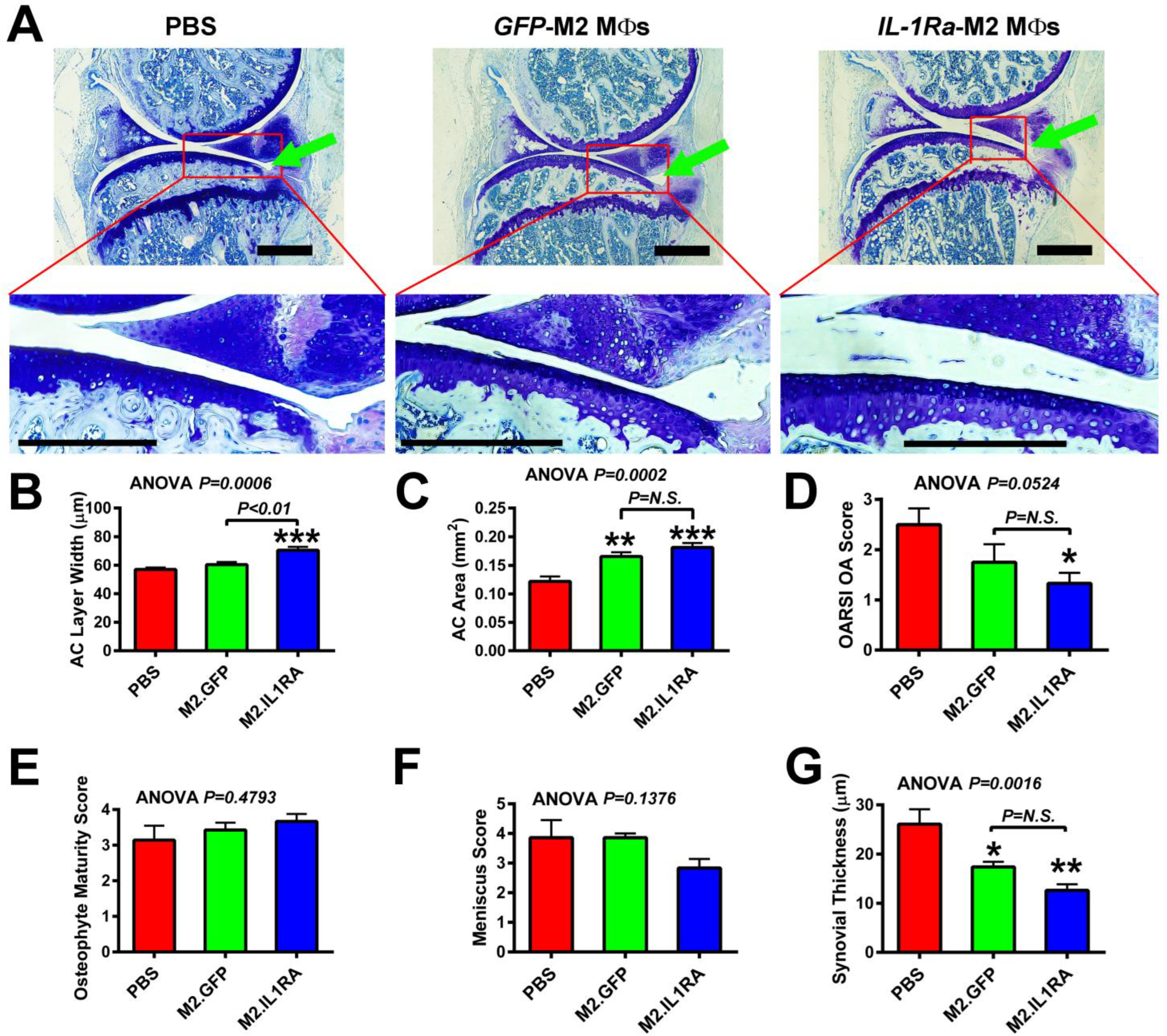
3.4. M2 MΦs-Based Adoptive IL-1Ra Gene Transfer Strategy, When Administered at 1 Week Post-Injury, Was Effective in Reducing Severity of OA
3.5. M2 MΦs-Based Adoptive IL-1Ra Gene Transfer Strategy, When Administered After Development of OA, Was Effective in Halting or Delaying OA Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, J.A.; Buckwalter, J.A. Post-traumatic osteoarthritis: The role of stress induced chondrocyte damage. Biorheology 2006, 43, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 2006, 20, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Eng. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpinski, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- Leifer, V.P.; Katz, J.N.; Losina, E. The burden of OA-health services and economics. Osteoarthr. Cartil. 2022, 30, 10–16. [Google Scholar] [CrossRef]
- Strobel, S.; Loparic, M.; Wendt, D.; Schenk, A.D.; Candrian, C.; Lindberg, R.L.; Moldovan, F.; Barbero, A.; Martin, I. Anabolic and catabolic responses of human articular chondrocytes to varying oxygen percentages. Arthritis Res. Ther. 2010, 12, R34. [Google Scholar] [CrossRef]
- Lotz, M.K.; Kraus, V.B. New developments in osteoarthritis. Posttraumatic osteoarthritis: Pathogenesis and pharmacological treatment options. Arthritis Res. Ther. 2010, 12, 211. [Google Scholar] [CrossRef]
- Blom, A.B.; van Lent, P.L.; Holthuysen, A.E.; van der Kraan, P.M.; Roth, J.; van Rooijen, N.; van den Berg, W.B. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2004, 12, 627–635. [Google Scholar] [CrossRef]
- Thomson, A.; Hilkens, C.M.U. Synovial Macrophages in Osteoarthritis: The Key to Understanding Pathogenesis? Front. Immunol. 2021, 12, 678757. [Google Scholar] [CrossRef]
- Lubis, A.M.; Lubis, V.K. Adult bone marrow stem cells in cartilage therapy. Acta Med. Indones. 2012, 44, 62–68. [Google Scholar]
- Matthews, G.L.; Hunter, D.J. Emerging drugs for osteoarthritis. Expert Opin. Emerg. Drugs 2011, 16, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Nuki, G.; Moskowitz, R.W.; Abramson, S.; Altman, R.D.; Arden, N.K.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef] [PubMed]
- Schindler, O.S. Current concepts of articular cartilage repair. Acta Orthop. Belg. 2011, 77, 709–726. [Google Scholar] [PubMed]
- Daheshia, M.; Yao, J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef]
- Elsaid, K.A.; Machan, J.T.; Waller, K.; Fleming, B.C.; Jay, G.D. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009, 60, 2997–3006. [Google Scholar] [CrossRef]
- Jotanovic, Z.; Mihelic, R.; Sestan, B.; Dembic, Z. Role of interleukin-1 inhibitors in osteoarthritis: An evidence-based review. Drugs Aging 2012, 29, 343–358. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Caron, J.P.; Evans, C.; Robbins, P.D.; Georgescu, H.I.; Jovanovic, D.; Fernandes, J.C.; Martel-Pelletier, J. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997, 40, 1012–1019. [Google Scholar] [CrossRef]
- Kraus, V.B.; Birmingham, J.; Stabler, T.V.; Feng, S.; Taylor, D.C.; Moorman, C.T., 3rd; Garrett, W.E.; Toth, A.P. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: A randomized controlled pilot trial (NCT00332254). Osteoarthr. Cartil. 2012, 20, 271–278. [Google Scholar] [CrossRef]
- D’Lima, D.; Hermida, J.; Hashimoto, S.; Colwell, C.; Lotz, M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006, 54, 1814–1821. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, Z.; Yu, C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J. Orthop. Res. 2004, 22, 742–750. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.Q.; Zhong, J.; Wu, X.L.; Chen, Q.; Peng, H.; Liu, S.Q. IL-17RA aptamer-mediated repression of IL-6 inhibits synovium inflammation in a murine model of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; O’Conor, C.J.; Kugler, L.E.; Cook, J.L.; Ateshian, G.A.; Hung, C.T. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann. Biomed. Eng. 2011, 39, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Noth, U.; Tuan, R.S. Concepts in gene therapy for cartilage repair. Injury 2008, 39 (Suppl. 1), S97–S113. [Google Scholar] [CrossRef] [PubMed]
- Punzi, L.; Galozzi, P.; Luisetto, R.; Favero, M.; Ramonda, R.; Oliviero, F.; Scanu, A. Post-traumatic arthritis: Overview on pathogenic mechanisms and role of inflammation. RMD Open 2016, 2, e000279. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Bondeson, J.; Blom, A.B.; Wainwright, S.; Hughes, C.; Caterson, B.; van den Berg, W.B. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010, 62, 647–657. [Google Scholar] [CrossRef]
- Haywood, L.; McWilliams, D.F.; Pearson, C.I.; Gill, S.E.; Ganesan, A.; Wilson, D.; Walsh, D.A. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003, 48, 2173–2177. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 2018, 180, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Fahy, N.; de Vries-van Melle, M.L.; Lehmann, J.; Wei, W.; Grotenhuis, N.; Farrell, E.; van der Kraan, P.M.; Murphy, J.M.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthr. Cartil. 2014, 22, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Fichadiya, A.; Bertram, K.L.; Ren, G.; Yates, R.M.; Krawetz, R.J. Characterizing heterogeneity in the response of synovial mesenchymal progenitor cells to synovial macrophages in normal individuals and patients with osteoarthritis. J. Inflamm. 2016, 13, 12. [Google Scholar] [CrossRef]
- Kraus, V.B.; McDaniel, G.; Huebner, J.L.; Stabler, T.V.; Pieper, C.F.; Shipes, S.W.; Petry, N.A.; Low, P.S.; Shen, J.; McNearney, T.A.; et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1613–1621. [Google Scholar] [CrossRef]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am. J. Trans. Res. 2020, 12, 261–268. [Google Scholar]
- Stiffel, V.; Rundle, C.H.; Sheng, M.H.; Das, S.; Lau, K.W. A Mouse Noninvasive Intraarticular Tibial Plateau Compression Loading-Induced Injury Model of Posttraumatic Osteoarthritis. Calcif. Tissue Int. 2020, 106, 158–171. [Google Scholar] [CrossRef]
- Dirschl, D.R.; Marsh, J.L.; Buckwalter, J.A.; Gelberman, R.; Olson, S.A.; Brown, T.D.; Llinias, A. Articular fractures. J. Am. Acad. Orthop. Surg. 2004, 12, 416–423. [Google Scholar] [CrossRef]
- Honkonen, S.E. Degenerative arthritis after tibial plateau fractures. J. Orthop. Trauma 1995, 9, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.O.; Labuz, D.; Keye, J.; Glauben, R.; Machelska, H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight. 2020, 5, e133093. [Google Scholar] [CrossRef] [PubMed]
- Jarrosson-Wuilleme, L.; Goujon, C.; Bernaud, J.; Rigal, D.; Darlix, J.L.; Cimarelli, A. Transduction of nondividing human macrophages with gammaretrovirus-derived vectors. J. Virol. 2006, 80, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Baylink, D.J.; Walter, M.H.; Lau, K.H.; Meng, X.; Wang, J.; Cherkas, A.; Tang, X.; Qin, X. Targeted 25-hydroxyvitamin D3 1alpha-hydroxylase adoptive gene therapy ameliorates dss-induced colitis without causing hypercalcemia in mice. Mol. Ther. 2015, 23, 339–351. [Google Scholar] [CrossRef]
- Stiffel, V.M.; Rundle, C.H.; Sheng, M.H.; Das, S.; Lau, K.W. A Novel EphA4 Signaling-Based Therapeutic Strategy for Osteoarthritis in Mice. J. Bone Miner. Res. 2022, 37, 660–674. [Google Scholar] [CrossRef]
- Sheng, M.H.; Rundle, C.H.; Lau, K.W. Microvesicles Released by Osteoclastic Cells Exhibited Chondrogenic, Osteogenic, and Anti-Inflammatory Activities: An Evaluation of the Feasibility of Their Use for Treatment of Osteoarthritis in a Mouse Model. Cells 2025, 14, 193. [Google Scholar] [CrossRef]
- Schwartz, M.; Yoles, E. Immune-based therapy for spinal cord repair: Autologous macrophages and beyond. J. Neurotrauma 2006, 23, 360–370. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S17–S23. [Google Scholar] [CrossRef]
- Kwok, J.; Onuma, H.; Olmer, M.; Lotz, M.K.; Grogan, S.P.; D’Lima, D.D. Histopathological analyses of murine menisci: Implications for joint aging and osteoarthritis. Osteoarthr. Cartil. 2016, 24, 709–718. [Google Scholar] [CrossRef]
- Jono, A.; Yanagihara, Y.; Kinoshita, T.; Takao, M.; Imai, Y. Establishment of a uniform histological evaluation method for early stage osteophytes in the destabilization of the medial meniscus mouse model. Osteoarthr. Cartil. Open 2023, 5, 100409. [Google Scholar] [CrossRef]
- Little, C.B.; Barai, A.; Burkhardt, D.; Smith, S.M.; Fosang, A.J.; Werb, Z.; Shah, M.; Thompson, E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009, 60, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yoshida, S.; Kubo, Y.; Yoshimura, T.; Kobayashi, Y.; Nakama, T.; Yamaguchi, M.; Ishikawa, K.; Oshima, Y.; Ishibashi, T. Different distributions of M1 and M2 macrophages in a mouse model of laser-induced choroidal neovascularization. Mol. Med. Rep. 2017, 15, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Recklies, A.D.; Baillargeon, L.; White, C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998, 41, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Saxne, T.; Heinegard, D.K. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann. Rheum. Dis. 1994, 53, 8–13. [Google Scholar] [CrossRef]
- Verma, P.; Dalal, K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: A novel diagnostic and prognostic biomarker. J. Orthop. Res. 2013, 31, 999–1006. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef]
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef]
- Stiffel, V.; Amoui, M.; Sheng, M.H.; Mohan, S.; Lau, K.H. EphA4 receptor is a novel negative regulator of osteoclast activity. J. Bone Miner. Res. 2014, 29, 804–819. [Google Scholar] [CrossRef]
- Yang, B.; Guo, H.; Zhang, Y.; Chen, L.; Ying, D.; Dong, S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE 2011, 6, e21679. [Google Scholar] [CrossRef]
- Ghivizzani, S.C.; Lechman, E.R.; Kang, R.; Tio, C.; Kolls, J.; Evans, C.H.; Robbins, P.D. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc. Natl. Acad. Sci. USA 1998, 95, 4613–4618. [Google Scholar] [CrossRef] [PubMed]
- Dharmavaram, R.M.; Liu, G.; Tuan, R.S.; Stokes, D.G.; Jimenez, S.A. Stable transfection of human fetal chondrocytes with a type II procollagen minigene: Expression of the mutant protein and alterations in the structure of the extracellular matrix in vitro. Arthritis Rheum. 1999, 42, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; von der Mark, K.; Aigner, T.; Park, J.; Schneider, H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Schneider, H. Ex vivo gene therapy approaches to cartilage repair. Adv. Drug Deliv. Rev. 2006, 58, 259–284. [Google Scholar] [CrossRef]
- Samuel, R.E.; Lee, C.R.; Ghivizzani, S.C.; Evans, C.H.; Yannas, I.V.; Olsen, B.R.; Spector, M. Delivery of plasmid DNA to articular chondrocytes via novel collagen-glycosaminoglycan matrices. Hum. Gene Ther. 2002, 13, 791–802. [Google Scholar] [CrossRef]
- Gelse, K.; Muhle, C.; Franke, O.; Park, J.; Jehle, M.; Durst, K.; Goken, M.; Hennig, F.; von der Mark, K.; Schneider, H. Cell-based resurfacing of large cartilage defects: Long-term evaluation of grafts from autologous transgene-activated periosteal cells in a porcine model of osteoarthritis. Arthritis Rheum. 2008, 58, 475–488. [Google Scholar] [CrossRef]
- Amin, S.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J., 3rd. Trends in fracture incidence: A population-based study over 20 years. J. Bone Miner. Res. 2014, 29, 581–589. [Google Scholar] [CrossRef]
- Caron, J.P.; Fernandes, J.C.; Martel-Pelletier, J.; Tardif, G.; Mineau, F.; Geng, C.; Pelletier, J.P. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996, 39, 1535–1544. [Google Scholar] [CrossRef]
- Fernandes, J.; Tardif, G.; Martel-Pelletier, J.; Lascau-Coman, V.; Dupuis, M.; Moldovan, F.; Sheppard, M.; Krishnan, B.R.; Pelletier, J.P. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: Prevention of osteoarthritis progression. Am. J. Pathol. 1999, 154, 1159–1169. [Google Scholar] [CrossRef]
- Patwari, P.; Lin, S.N.; Kurz, B.; Cole, A.A.; Kumar, S.; Grodzinsky, A.J. Potent inhibition of cartilage biosynthesis by coincubation with joint capsule through an IL-1-independent pathway. Scand. J. Med. Sci. Sports 2009, 19, 528–535. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Bellingan, G.J.; Caldwell, H.; Howie, S.E.; Dransfield, I.; Haslett, C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: Inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J. Immunol. 1996, 157, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Gautier, E.L.; Ivanov, S.; Lesnik, P.; Randolph, G.J. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood 2013, 122, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, M.; Pinno, K.; Ehnold, L.I.; Martens, N.; Martson, A.; Pap, T.; Starke, C.; Lohmann, C.H.; Bertrand, J. MMP-9 mediated Syndecan-4 shedding correlates with osteoarthritis severity. Osteoarthr. Cartil. 2021, 29, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Grabiec, A.M.; Travis, M.A. Culture of Human Monocyte-Derived Macrophages. Methods Mol. Biol. 2018, 1784, 1–11. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, M.H.-C.; Baylink, D.J.; Rundle, C.H.; Lau, K.-H.W. Assessment of Feasibility of the M2 Macrophage-Based Adoptive Gene Transfer Strategy for Osteoarthritis with a Mouse Model. Cells 2025, 14, 1067. https://doi.org/10.3390/cells14141067
Sheng MH-C, Baylink DJ, Rundle CH, Lau K-HW. Assessment of Feasibility of the M2 Macrophage-Based Adoptive Gene Transfer Strategy for Osteoarthritis with a Mouse Model. Cells. 2025; 14(14):1067. https://doi.org/10.3390/cells14141067
Chicago/Turabian StyleSheng, Matilda H.-C., David J. Baylink, Charles H. Rundle, and Kin-Hing William Lau. 2025. "Assessment of Feasibility of the M2 Macrophage-Based Adoptive Gene Transfer Strategy for Osteoarthritis with a Mouse Model" Cells 14, no. 14: 1067. https://doi.org/10.3390/cells14141067
APA StyleSheng, M. H.-C., Baylink, D. J., Rundle, C. H., & Lau, K.-H. W. (2025). Assessment of Feasibility of the M2 Macrophage-Based Adoptive Gene Transfer Strategy for Osteoarthritis with a Mouse Model. Cells, 14(14), 1067. https://doi.org/10.3390/cells14141067







