Abstract
Endothelial dysfunction (ED) has been identified as a precursor to micro- and macroangiopathic complications and an independent risk factor for major adverse cardiac events (MACEs). Recent studies have identified a novel risk factor for ED: severe aortic stenosis (AS). Traditionally linked to other established risk factors for endothelial cell dysregulation, AS has emerged as a contributor to ED, which is supported by the improvement of endothelial function following transcatheter (TAVR) or surgical (SAVR) interventions. Furthermore, the observation of ED in patients with a dysfunctional bicuspid aortic valve (BAV) at a younger age suggests a distinct impact of AS on ED. A promising hypothesis is a hemodynamic theory suggesting that changes in the shear stress of the ascending aortic wall and peripheral vessels, along with subclinical hemolysis caused by turbulent blood flow, could lead to reduced nitric oxide (NO) bioavailability. Current hypotheses on ED have yet to consider the influence of concomitant aortic stenosis in BAV. Additionally, studies examining potential intravascular hemolysis in BAV patients or the impact of surgical treatment of this defect on endothelial function are scarce. The aim of this review is to summarize the current knowledge on the mechanisms underlying ED in patients with AS or BAV and to identify possible directions for future research.
1. Introduction
Endothelial dysfunction (ED) is a broadly defined heterogeneous spectrum of pathophysiological phenomena that leads to the loss of endothelial homeostatic functions. This process is a recognized precursor of micro- and macroangiopathic complications [1,2] and an independent risk factor for major adverse cardiac events (MACEs) [3]. Numerous established and emerging risk factors contribute to endothelial dysregulation through various mechanisms, including hypertension, diabetes, obesity, dyslipidaemia, smoking, and other chronic diseases [4,5,6,7,8,9,10,11,12]. There are various methods to assess endothelial function; some allow for the evaluation of regional endothelial cell function, while others are used for systemic assessment. Invasive techniques are employed for regional functional assessment, involving evaluations of large vessels, such as coronary artery vasomotor responses measured by quantitative coronary angiography or intravascular ultrasound [13,14] as well as microcirculation assessments typically performed through coronary blood flow (CBF) measurements, with coronary flow reserve (CFR) considered the gold standard [15,16]. Conversely, non-invasive methods are widely used to evaluate systemic endothelial function [1,17,18], as they avoid the risks associated with invasive procedures. These include techniques such as the most widely used method, flow-mediated dilation (FMD) of the brachial artery [1,18,19], the laser Doppler flowmetry, and the peripheral arterial tonometry of arterioles located in the distal parts of upper extremities.
2. Aortic Stenosis (AS) as an Independent Risk Factor for Systemic Endothelial Dysfunction
Aortic valve disease manifesting as clinically significant stenosis has been identified as a potential independent risk factor for systemic endothelial dysfunction; however, this effect appears to be highly heterogeneous, as evidenced by studies showing reduced FMD values that do not consistently correspond with the severity of aortic stenosis [20]. There are several hypothetical mechanisms that explain the impact of AS on endothelial cell function. The potential etiopathogenesis of endothelial dysfunction in patients with AS is based on the reduced area available for blood flow due to the narrowed aortic valve. According to Bernoulli’s principle, this forces blood to flow through a smaller opening at a higher velocity [21]. Beyond the stenosis, the bloodstream disperses, causing irregularities in flow direction. These alterations generate turbulent blood flow, marked by the presence of vortices and velocity fluctuations, which result in localized increases in wall shear stress (WSS) within the ascending aorta. The high flow velocity generates greater frictional forces between the blood and the endothelium [22,23,24]. Concurrently, in peripheral arteries, decreased blood flow leads to reduced WSS [25]. The hemodynamic consequences in both the ascending aorta and peripheral arteries appear to influence ED. WSS is a critical biomechanical signal that regulates endothelial function. Proper WSS values promote vascular homeostasis by activating endothelial mechanoreceptors (e.g., integrins and glycocalyx complexes) and initiating signaling pathways that enhance nitric oxide (NO) production, suppress inflammation, and limit vascular smooth muscle cell (VSMC) proliferation [26,27]. Altered WSS values have been found to be strongly associated with the atherogenic process [28]. Low WSS in peripheral arteries has been shown to lead to the activation of pro-inflammatory factors, such as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), resulting in an increased expression of inflammatory cytokines and adhesion molecules, like intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). This, in turn, promotes leukocyte infiltration and the development of inflammation in the endothelium [29,30]. Additionally, endothelial nitric oxide synthase (eNOS) activity is reduced under low WSS conditions, leading to a decrease in NO bioavailability. NO bioavailability is a key factor for vasodilation and protection against atherogenic processes, such as platelet aggregation and VSMC proliferation [29]. Furthermore, gene expression profiles in endothelial cells change, predisposing them to a pro-atherogenic phenotype. These changes include increased vascular permeability, endothelial cell proliferation, and oxidative stress [29,31]. The disruption of the balance between protective and damaging processes is attributed to the insufficient stimulation of endothelial mechanoreceptors under low WSS [32,33,34]. The change in the metabolic profile of endothelial cells also occurs in response to increased shear stress [26]. Zeller J et al. proposed a hypothesis that increased WSS induces the accelerated dissociation of the pentameric isoform of the C-reactive protein (pCRP) into its monomeric subunits (mCRP) [35]. This form of CRP (mCRP) stimulates the secretion of pro-inflammatory cytokines, enhances leukocyte chemotaxis and adhesion to endothelial cells through the induction of adhesion molecule expression, and activates platelets by promoting their aggregation—thereby amplifying local inflammation [36,37,38]. The Piezo-type mechanosensitive ion channel component 1 (Piezo-1) may also be responsible for the direct activation of monocytes in response to increased wall shear stress [39]. The same receptor, along with other mechanoreceptors and vascular endothelial growth factor receptor 2 (VEGFR2), increases the expression of pro-inflammatory cytokines and induces phenotypic changes in valvular interstitial cells (VICs) under elevated wall shear stress, promoting calcification and thereby amplifying the mechanical stimulus. It is also possible that this vicious cycle mechanism applies to vascular endothelial cells as well, especially since endothelial cells usually exhibit a higher expression of VEGFR2 [40,41]. In the ascending aorta, increased WSS has been shown to have a detrimental effect on regional endothelial cells by activating signaling pathways such as NF-κB [29]. The injury to these endothelial cells may be reflected in elevated circulating endothelial microparticles (EMPs) [42]. Increased WSS may also disrupt the integrity of red blood cells, leading to the accumulation of extracellular hemoglobin (eHb) and hemoglobin microvesicles [43,44,45,46]. An indirect indication of increased hemolysis is the elevated level of the NO inhibitor, asymmetric dimethylarginine (ADMA), in individuals with significant AS [20], as red blood cells are potential reservoirs of ADMA [47]. The release of eHb and hemoglobin microvesicles during hemolysis leads to the conversion of NO to nitrites (NO2−) or nitrates (NO3−) through the action of NO scavengers. Furthermore, the release of arginase-1 (ARG-1) during hemolysis results in a reduction in arginine, the substrate for NO synthesis, thereby further diminishing the bioavailability of endogenous NO [48,49]. NO, in addition to its well-known vasodilatory function, has been shown to have protective effects against atherogenesis, including mitigating oxidative stress, platelet activation, inflammation, and VSMC proliferation [50]. Furthermore, hemoglobin microvesicles and free hemoglobin have been observed to participate in oxidative processes, generating reactive oxygen species (ROS) that accelerate inflammatory processes in the endothelium and damage its structure [51,52]. Furthermore, the release of ARG-1 during hemolysis reduces the availability of arginine, which is the substrate for NO synthesis [48,49]. Consequently, the bioavailability of endogenous NO is diminished. Nitric oxide, in addition to its well-known vasodilatory function, exerts protective effects against atherogenesis, including mitigating oxidative stress, platelet activation, inflammation, and smooth-muscle cell proliferation [50]. Furthermore, the previously mentioned arginase-1, in addition to decreasing the substrate for NO synthesis, promotes oxidative stress and is considered an important factor in the etiopathogenesis of ED in the presence of more established risk factors such as diabetes, hypertension, or obesity [53]. As a result of these phenomena, the systemic dysregulation of endothelial homeostasis occurs, which is reflected, among other things, by a decrease in FMD [10]. It has been posited that the circulating EMPs previously discussed do not contribute to the etiopathogenesis of systemic endothelial dysfunction. However, they are recognized as a marker of impaired endothelial integrity [54,55]. Furthermore, their levels have been observed to increase in the early stages of atherosclerosis [56], and they are acknowledged as negative prognostic markers of cardiovascular diseases [57,58] (Figure 1 and Table 1).
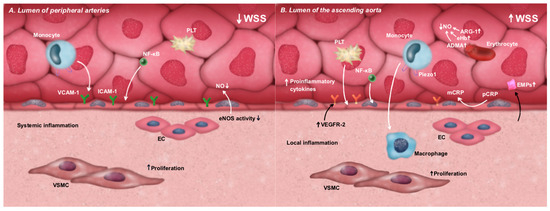
Figure 1.
The impact of WSS disturbances on the metabolic shift in endothelial cells toward a pro-inflammatory phenotype and the associated ED. (A). Low WSS in peripheral arteries leads to the activation of the NF-κB pathway and the increased expression of adhesion molecules, such as ICAM-1 and VCAM-1, promoting leukocyte adhesion and transmigration. Simultaneously, low WSS reduces eNOS activity, resulting in decreased NO bioavailability, enhanced platelet aggregation, and VSMC proliferation. (B). High WSS in the ascending aorta activates mechanosensitive pathways involving NF-κB, VEGFR2, and Piezo-1 receptors, as well as the dissociation of pCRP into mCRP. These events contribute to the increased secretion of pro-inflammatory cytokines, leukocyte adhesion, platelet aggregation, and enhanced VSMC proliferation. Additionally, subclinical hemolysis—frequently present under high shear stress conditions—leads to the release of NO scavengers, further reducing NO bioavailability and exacerbating local inflammatory responses. ADMA—asymmetric dimethylarginine; ARG-1—arginase-1; EC—endothelial cells; ED—endothelial dysfunction; eHb—extracellular hemoglobin; EMPs—endothelial microparticles; eNOS—endothelial nitric oxide synthase; ICAM-1—intracellular adhesion molecule 1; mCRP—monomeric isoform of C-reactive protein; NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells; NO—nitric oxide; pCRP—pentameric isoform of C-reactive protein; Piezo-1—Piezo-type mechanosensitive ion channel component 1; VCAM-1—vascular cell adhesion molecule 1; VEGFR2—vascular endothelial growth factor receptor 2; VSMC—vascular smooth muscle cell; WSS—wall shear stress.

Table 1.
Causes of reduced nitric oxide (NO) bioavailability induced by changes in wall shear stress (WSS) and hemolysis in patients with clinically significant aortic stenosis (AS).
Aortic stenosis is the most common acquired valvular heart disease in developed countries, with the highest prevalence in the elderly population [59,60]. Thus, AS is frequently associated with other comorbid conditions such as hypertension, diabetes, obesity, and smoking [61,62,63]. These diseases are well-established risk factors for the development of endothelial dysfunction [64] and serve as major drivers in the progression of aortic valve sclerosis [65]. The coexistence of these ED-related risk factors with AS may serve as a counterargument to the hypotheses presented above. Opponents of the notion that AS is an independent risk factor for ED might argue that endothelial cell dysfunction in these patients arises from their comorbidities rather than from AS itself. Below, we present the existing body of scientific evidence and the conclusions drawn from it, which we believe lay the foundation for resolving this debate and recognizing AS to be an independent contributor to the development of ED. At the same time, we emphasize the necessity of further research to deepen our understanding of this issue.
2.1. Arguments Supporting the Notion of Aortic Stenosis as an Independent Risk Factor for the Loss of Endothelial Function
A compelling body of evidence substantiates AS to be an independent risk factor for ED, as evidenced by the enhancement of FMD after the management of significant aortic stenosis in patients exhibiting risk factors for endothelial dysfunction. Comella et al. [66] observed an augmentation in FMD following transcatheter aortic valve replacement (TAVR), both in the early and late follow-up phases, encompassing patients with risk factors for endothelial dysfunction (hypertension, type 2 diabetes, and smoking). A notable limitation of the study was the inclusion of a relatively small sample size (n = 27). In contrast, the study by Vitez et al. [67] demonstrated a significant enhancement in FMD in a group of 43 patients following TAVR in both early and late follow-up assessments. Additionally, an improvement in cardiac autonomic function, as measured by high-resolution ECG, was observed. The study by Moscarelli et al. [68] demonstrated a significant improvement in endothelial function in the late period after TAVR, and a significant improvement in FMD was also demonstrated after surgical aortic valve replacement (SAVR), with the improvement being greater in the surgical group compared to patients undergoing the transcatheter procedure.
Furthermore, Irace et al. [25] observed an increase in wall shear stress in the common carotid artery in patients undergoing SAVR, which could support the hypothetical decrease in WSS in the peripheral arteries as a potential component of endothelial dysfunction development. However, the impact on WSS in the ascending aorta, as well as other markers assessing endothelial cell function, were not examined.
In contrast, an earlier observation by Chenevard et al. [69] found no improvement in endothelial function in patients undergoing SAVR using the same ED marker as the Moscarelli et al. [68] group (FMD assessment).
Noteworthy, Horn et al. [70] observed an enhancement in FMD in patients following transcatheter aortic valve implantation (TAVI), concurrently detecting peripheral increases in WSS and a concomitant decrease in endothelial-derived microparticles. As previously delineated, EMPs are a recognized indicator of impaired endothelial integrity and a negative prognostic factor for cardiovascular diseases.
The most extensive study to date that has confirmed the improvement of endothelial function in patients with clinically significant AS undergoing TAVR was conducted by Quast et al. [71]. Utilizing patient observations, an experimental animal model with patient biological sample transfer, and computational fluid dynamics simulations, this study not only confirmed the improvement of FMD in a significant number of patients undergoing transcatheter aortic valve implantation, further solidifying the belief that AS is an independent risk factor for ED but also brought us closer to answering questions about the potential etiopathogenesis of this phenomenon. Observations during this study, such as the reduction in peak velocity in ascending aortic MR and a decrease in extracellular hemoglobin, erythrocyte microparticles, and hemoglobin microvesicles, suggest the possibility of a valid hypothesis regarding the phenomenon of WSS in the ascending aorta, causing subclinical hemolysis with subsequent consequences for systemic endothelial function. It is also noteworthy that even relatively low levels of extracellular hemoglobin, in the absence of overt signs of hemolysis, have been shown to reduce the bioavailability of endogenous NO, which is a finding that has also been confirmed in studies on stored red blood cells [72] (Table 2).

Table 2.
Experimental, clinical, and translational studies supporting individual hypotheses on the impact of aortic stenosis on endothelial dysfunction (the description of individual hypotheses in the pathophysiology of endothelial dysfunction development in the text).
2.2. Bicuspid Aortic Valve (BAV) as Additional Potential Evidence of Independent Aortic Valve Dysfunction’s Impact on Impaired Endothelial Function
Another piece of evidence supporting the independent impact of significant AS on endothelial dysfunction may be its manifestation in the population of patients with a bicuspid aortic valve. These patients often develop significant aortic valve defects at a young age and are typically free from other recognized risk factors for ED [73,74]. While an established correlation exists between impaired endothelial function and BAV, as evidenced by various markers of endothelial dysregulation in patients with BAV, such as increased circulating EMPs [75] or decreased FMD levels [76,77,78], the number of theories about the development of ED in this population underscores the complexity of the underlying process, and the still unclear etiopathogenesis of this phenomenon, especially when associated with aortic dilation and/or aortic stenosis [75,77,79,80,81,82]. Genetic and hemodynamic theories have been identified as the predominant hypotheses. Recent studies have identified mutations in proteins such as Notch-1 [80], ROBO4 [83], or GATA4 [84] in patients with BAV. These proteins are considered key mediators in the signaling pathways involved in the dysregulation of the endothelial-to-mesenchymal transition (EndoMT) process. This process is regarded as crucial in heart development and wound healing [85]; thus, its dysregulation may be a direct cause of endothelial homeostasis loss [83]. Various microRNAs (miRNAs) also play a regulatory role in the proper progression of EndoMT. For instance, the microRNA-200 (miR-200) family appears to suppress this process [86]. While specific miRNA expression patterns have been associated with concurrent endothelial dysfunction in BAV, their precise role remains uncertain [87,88]. Another potential mutation is Knox20, which is responsible for the dysregulation of NO synthesis [89]. Hemodynamic hypotheses, however, largely focused on the local (in the ascending aorta) dysfunction of endothelial cells caused by increased WSS [90,91,92], also suggesting potential pathways leading to systemic dysfunction. A proper, laminar shear stress pattern leads to increased tension in platelet endothelial cell adhesion molecule 1 (PECAM-1), one of the most well-characterized mechanotransducers in endothelial cells (EC) [93,94], triggering its association with the vimentin cytoskeleton [95]. This interaction results in the activation of proto-oncogenic tyrosine kinase (Src), the vascular endothelial growth factor receptor (VEGFR), phosphatidylinositol 3-kinase (PI3K), and eNOS [96]. Furthermore, when the endothelium remains intact, other mechanosensitive pathways, such as transforming growth factor-beta (TGF-β), are activated. TGF-β plays a role in regulating the proliferation of vascular smooth muscle cells through endothelial heparan sulfate proteoglycans (HSPGs) [97,98] and leads to the activation of the Krüppel-like factor 2 (KLF2) signaling cascade [98,99]. KLF2 activation is responsible for inducing eNOS activity and production, thereby increasing NO synthesis, which is essential for maintaining vascular and endothelial tone [100]. In regions of turbulent blood flow, where shear stress is altered, the endothelial cell phenotype is modified, leading to increased endothelial permeability, the disruption of the proliferation-apoptosis balance, and enhanced monocyte adhesive properties [101]. What is more, the turbulent blood flow present in BAV leads to the impairment of the TGF-beta pathway, directly affecting phenotypic and structural changes in endothelial cells [98,99], reducing NO production [100], and dysregulating the previously mentioned EndoMT process [102,103]. Although reports indicate reduced eNOS expression in BAV patients [104], the observations made by Kotlarczyk et al. [105] provide an intriguing perspective. Their study identified regions of the ascending aorta with higher eNOS expression in BAV patients compared to those with TAV. However, despite this increased expression, they observed lower NO bioavailability. They hypothesized that this phenomenon results from oxidative stress induced by elevated WSS and an altered response to free radicals in smooth muscle cells (SMCs) of the medial layer of the ascending aorta in BAV patients [106,107]. Under oxidative stress conditions, including arginine deficiency and the depletion of the essential cofactor tetrahydrobiopterin (BH4) [108], eNOS can become “uncoupled” from NO production and instead generate the superoxide radical (O₂●−) [109]. Superoxide radicals oxidize tetrahydrobiopterin and react with NO, forming peroxynitrite (ONOO−), which further oxidizes tetrahydrobiopterin, perpetuating a vicious cycle of increasing oxidative stress, further eNOS uncoupling, reduced NO bioavailability, and generalized endothelial dysfunction [108,109]. It also suggests that turbulent blood flow may cause disruptions in the expression of proteins responsible for atherosclerosis processes, such as Calponin 1 (CNN1) [110] or Kruppel-like factor 4 (KLF4) [111] (Table 3).

Table 3.
The classification of studies on the etiopathogenesis of endothelial dysfunction (ED) in patients with a bicuspid aortic valve (BAV) according to the investigated hypothesis: genetic, hemodynamic, or focusing on endothelial nitric oxide synthase (eNOS) activity or ED markers, taking into account both experimental and clinical studies.
Despite the evident association between the presence of BAV-AS and endothelial dysfunction, there is still a lack of evidence-based medicine (EBM) studies that quantitatively assess the relationship between the severity of AS in the context of BAV and endothelial dysfunction, for example, by evaluating the degree of impairment in flow-mediated dilation (FMD) or the levels of inflammatory and prothrombotic markers. There is currently a paucity of studies evaluating the potential presence of subclinical hemolysis, indicated by increased extracellular hemoglobin and hemoglobin microvesicles, and their potential impact on the bioavailability of endogenous NO in BAV patients. A potential indicator of increased hemolysis in patients with BAV could be the presence of elevated levels of ADMA, a well-documented NO synthase inhibitor, in this patient population [112]. This phenomenon has also been observed in patients with severely stenotic tricuspid aortic valves [20].
Additionally, there is a paucity of studies that directly compare endothelial function following BAV treatment with significant aortic defects. Conducting such research could facilitate a more comprehensive understanding of the pathogenesis of ED in patients with BAV and provide a framework for the appropriate qualification of patients with bicuspid aortic valves for surgical treatment.
3. Future Perspectives
The hypothesis that clinically significant aortic stenosis is an independent risk factor for systemic endothelial dysfunction has emerged recently, and its validity must be established through further research. Despite the growing number of studies and large body of evidence supporting the validity of this theory, the potential etiopathogenesis remains unclear. A promising avenue for furthering our understanding of the isolated impact of clinically significant AS on endothelial cell function lies in the study of patients with a bicuspid aortic valve, who typically do not have other recognized risk factors for ED development. The independent impact of BAV on the development of ED should be considered [78]; however, future studies are needed in order to analyze the potential role of genetic factors in the coexistence of BAV and ED. The impact of concurrent aortic valve defects on red blood cell integrity and vascular endothelial function should be taken into consideration, which could clarify the etiopathogenesis of ED in these patients (Figure 2).
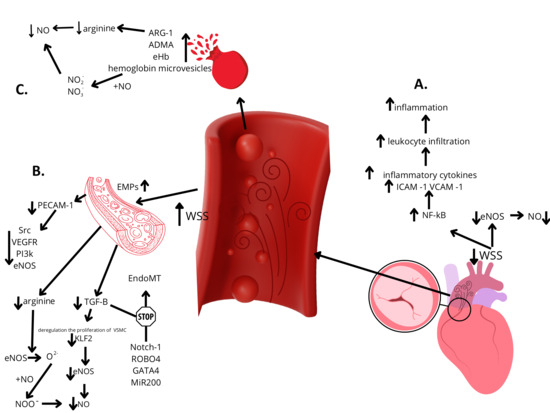
Figure 2.
Hypotheses on the development of endothelial dysfunction in patients with bicuspid aortic valve stenosis. Legends: A—Lower shear stress within peripheral arteries leads to the decreased activity of eNOS and increased activation of the pro-inflammatory transcription factor NF-κB, resulting in enhanced local inflammation and reduced NO bioavailability. B—Increased shear stress within the ascending aorta leads to a reduction in the substrates necessary for nitric oxide (NO) synthesis, such as arginine, and impairs the activation of mechanosensitive pathways, including PECAM-1 and TGF-β. This results in decreased eNOS activity and reduced NO bioavailability. Furthermore, diminished TGF-β signaling, together with genetic factors, contributes to the inhibition of the EndoMT process. C—Subclinical hemolysis induced by localized increases in shear stress leads to the release of nitric oxide scavengers and arginine inhibitors—the key substrate for NO synthesis. This results in reduced nitric oxide bioavailability. ADMA—asymmetric dimethylarginine, ARG-1—arginase-1, EMPs—circulating endothelial microparticles; EndoMT—endothelial-to-mesenchymal transition; eHb—extracellular hemoglobin; eNOS—endothelial nitric oxide synthase; ICAM-1—intracellular adhesion molecule 1; KLF2—Krüppel-like factor 2; MiR200—microRNA-200; NF-kB—nuclear factor kappa-light-chain-enhancer of activated B cells; NO—nitric oxide; NO2−—nitrites; NO3−—nitrates; PECAM-1—platelet endothelial cell adhesion molecule 1; PI3k—phosphatidylinositol 3-kinase; Src—proto-oncogenic tyrosine kinase; TGF-β—transforming growth factor-beta; VCAM-1—vascular cell adhesion molecule 1; VEGFR—vascular endothelial growth factor receptor; VSMC—vascular smooth muscle cell; WSS—wall shear stress.
Author Contributions
Conceptualization, M.M. and A.D.; methodology, M.M. and A.D.; formal analysis, M.M.; resources, M.M. and A.D.; writing—original draft preparation, M.M.; writing—review and editing, A.D.; supervision, A.D. and W.B.; project administration, A.D. and W.B.; funding acquisition, A.D. and W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| ADMA | asymmetric dimethylarginine |
| ARG-1 | arginase-1 |
| AS | aortic stenosis |
| BAV | bicuspid aortic valve |
| BH4 | tetrahydrobiopterin |
| CBF | coronary blood flow |
| CFR | coronary flow reserve |
| CNN1 | calponin 1 |
| EC | endothelial cells |
| ED | endothelial dysfunction |
| eHb | extracellular hemoglobin |
| EMPs | endothelial microparticles |
| EndoMT | endothelial-to-mesenchymal transition |
| eNOS | endothelial nitric oxide synthase |
| FMD | flow-mediated dilation |
| HSPG | heparan sulfate proteoglycans |
| ICAM-1 | intracellular adhesion molecule 1 |
| KLF2 | Krüppel-like factor 2 |
| KLF4 | Kruppel-like factor 4 |
| MACE | major adverse cardiac events |
| mCRP | monomeric isoform of C-reactive protein |
| mi-R200 | microRNA-200 |
| miRNAs | microRNAs |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| NO2− | nitrites |
| NO3− | nitrates |
| O₂●− | superoxide radical |
| ONOO− | peroxynitrite |
| pCRP | pentameric isoform of C-reactive protein |
| PECAM-1 | platelet endothelial cell adhesion molecule 1 |
| PI3K | phosphatidylinositol 3-kinase |
| Piezo-1 | Piezo-type mechanosensitive ion channel component 1 |
| ROS | reactive oxygen species |
| SAVR | surgical aortic valve replacement |
| SMCs | smooth muscle cells |
| Src | proto-oncogenic tyrosine kinase |
| TAVI | transcatheter aortic valve implantation |
| TAVR | transcatheter aortic valve replacement |
| TGF-β | transforming growth factor-beta |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGFR | vascular endothelial growth factor receptor |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| VICs | valvular interstitial cells |
| VSMC | vascular smooth muscle cell |
| WSS | wall shear stress |
References
- Anderson, T.J.; Uehata, A.; Gerhard, M.D.; Meredith, I.T.; Knab, S.; Delagrange, D.; Lieberman, E.H.; Ganz, P.; Creager, M.A.; Yeung, A.C.; et al. Close Relation of Endothelial Function in the Human Coronary and Peripheral Circulations. J. Am. Coll. Cardiol. 1995, 26, 1235–1241. [Google Scholar] [CrossRef]
- Kwak, B.R.; Bäck, M.; Bochaton-Piallat, M.-L.; Caligiuri, G.; Daemen, M.J.A.P.; Davies, P.F.; Hoefer, I.E.; Holvoet, P.; Jo, H.; Krams, R.; et al. Biomechanical Factors in Atherosclerosis: Mechanisms and Clinical Implications†. Eur. Heart J. 2014, 35, 3013–3020. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Kwon, T.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow—Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta—Analysis. J. Am. Heart Assoc. 2024, 4, e002270. [Google Scholar] [CrossRef]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E.; Epstein, S.E. Abnormal Endothelium-Dependent Vascular Relaxation in Patients with Essential Hypertension. N. Engl. J. Med. 1990, 323, 22–27. [Google Scholar] [CrossRef]
- JBrush, E.; Faxon, D.P.; Salmon, S.; Jacobs, A.K.; Ryan, T.J. Abnormal endothelium-dependent coronary vasomotion in hypertensive patients. J. Am. Coll. Cardiol. 1992, 19, 809–815. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Larson, M.G.; Keyes, M.J.; Mitchell, G.F.; Vasan, R.S.; Keaney, J.F.; Lehman, B.T.; Fan, S.; Osypiuk, E.; Vita, J.A. Clinical Correlates and Heritability of Flow-Mediated Dilation in the Community. Circulation 2004, 109, 613–619. [Google Scholar] [CrossRef]
- McVeigh, G.E.; Brennan, G.M.; Johnston, G.D.; McDermott, B.J.; McGrath, L.T.; Henry, W.R.; Andrews, J.W.; Hayes, J.R. Impaired Endothelium-Dependent and Independent Vasodilation in Patients with Type 2 (Non-Insulin-Dependent) Diabetes Mellitus. Diabetologia 1992, 35, 771–776. [Google Scholar] [CrossRef]
- Nitenberg, A.; Ledoux, S.; Valensi, P.; Sachs, R.; Attali, J.-R.; Antony, I. Impairment of Coronary Microvascular Dilation in Response to Cold Pressor–Induced Sympathetic Stimulation in Type 2 Diabetic Patients With Abnormal Stress Thallium Imaging. Diabetes 2001, 50, 1180–1185. [Google Scholar] [CrossRef][Green Version]
- Williams, S.B.; Cusco, J.A.; Roddy, M.A.; Johnstone, M.T.; Creager, M.A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1996, 27, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial Function in Cardiovascular Medicine: A Consensus Paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Itani, H.; Richmond, B.; Arslanbaeva, L.; Vergeade, A.; Rahman, S.M.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G.; et al. Tobacco Smoking Induces Cardiovascular Mitochondrial Oxidative Stress, Promotes Endothelial Dysfunction, and Enhances Hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Carmeliet, P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell Metab. 2019, 30, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Ludmer, P.L.; Selwyn, A.P.; Shook, T.L.; Wayne, R.R.; Mudge, G.H.; Alexander, R.W.; Ganz, P. Paradoxical Vasoconstriction Induced by Acetylcholine in Atherosclerotic Coronary Arteries. N. Engl. J. Med. 1986, 315, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Liew, G.Y.H.; Nicholls, S.J.; Nelson, A.J.; Leong, D.P.; Carbone, A.; Copus, B.; Wong, D.T.L.; Beltrame, J.F.; Worthley, S.G.; et al. Coronary Β2-Adrenoreceptors Mediate Endothelium-Dependent Vasoreactivity in Humans: Novel Insights from an in Vivo Intravascular Ultrasound Study. Eur. Heart J. 2012, 33, 495–504. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Camici, P. Advances in Coronary Microvascular Dysfunction. Heart Lung Circ. 2009, 18, 19–27. [Google Scholar] [CrossRef]
- Camici, P.G.; Filippo, C. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2024, 356, 830–840. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef]
- Takase, B.; Uehata, A.; Akima, T.; Nagai, T.; Nishioka, T.; Hamabe, A.; Satomura, K.; Ohsuzu, F.; Kurita, A. Endothelium-Dependent Flow-Mediated Vasodilation in Coronary and Brachial Arteries in Suspected Coronary Artery Disease. Am. J. Cardiol. 1998, 82, 1535–1539. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the Ultrasound Assessment of Endothelial-Dependent Flow-Mediated Vasodilation of the Brachial Artery: A Report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Schumm, J.; Luetzkendorf, S.; Rademacher, W.; Franz, M.; Schmidt-Winter, C.; Kiehntopf, M.; Figulla, H.R.; Brehm, B.R. In Patients with Aortic Stenosis Increased Flow-Mediated Dilation Is Independently Associated with Higher Peak Jet Velocity and Lower Asymmetric Dimethylarginine Levels. Am. Heart J. 2011, 161, 893–899. [Google Scholar] [CrossRef]
- Yoganathan, A.P. Fluid mechanics of aortic stenosis. Eur. Heart J. 1988, 9, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A.; Topper, J.N.; Nagel, T.; Anderson, K.R.; Garcia-Cardeña, G. Endothelial Dysfunction, Hemodynamic Forces, and Atherogenesis. Ann. N. Y. Acad. Sci. 2000, 902, 230–240. [Google Scholar] [CrossRef]
- Michail, M.; Hughes, A.D.; Comella, A.; Cameron, J.N.; Gooley, R.P.; McCormick, L.M.; Mathur, A.; Parker, K.H.; Brown, A.J.; Cameron, J.D. Acute Effects of Transcatheter Aortic Valve Replacement on Central Aortic Hemodynamics in Patients With Severe Aortic Stenosis. Hypertension 2020, 75, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- van Ooij, P.; Garcia, J.; Potters, W.V.; Malaisrie, S.C.; Collins, J.D.; Carr, J.C.; Markl, M.; Barker, A.J. Age-Related Changes in Aortic 3D Blood Flow Velocities and Wall Shear Stress: Implications for the Identification of Altered Hemodynamics in Patients with Aortic Valve Disease. J. Magn. Reson. Imaging 2016, 43, 1239–1249. [Google Scholar] [CrossRef]
- Irace, C.; Gnasso, A.; Cirillo, F.; Leonardo, G.; Ciamei, M.; Crivaro, A.; Renzulli, A.; Cotrufo, M. Arterial Remodeling of the Common Carotid Artery After Aortic Valve Replacement in Patients With Aortic Stenosis. Stroke 2002, 33, 2446–2450. [Google Scholar] [CrossRef] [PubMed]
- Baratchi, S.; Khoshmanesh, K.; Woodman, O.L.; Potocnik, S.; Peter, K.; McIntyre, P. Molecular Sensors of Blood Flow in Endothelial Cells. Trends Mol. Med. 2017, 23, 850–868. [Google Scholar] [CrossRef]
- Traub, O.; Berk, B.C. Laminar Shear Stress. Arter. Thromb. Vasc. Biol. 1998, 18, 677–685. [Google Scholar] [CrossRef]
- Moerman, A.M.; Korteland, S.; Dilba, K.; van Gaalen, K.; Poot, D.H.J.; van Der Lugt, A.; Verhagen, H.J.M.; Wentzel, J.J.; van Der Steen, A.F.W.; Gijsen, F.J.H.; et al. The Correlation Between Wall Shear Stress and Plaque Composition in Advanced Human Carotid Atherosclerosis. Front. Bioeng. Biotechnol. 2022, 9, 828577. [Google Scholar] [CrossRef]
- Katoh, K. Effects of Mechanical Stress on Endothelial Cells In Situ and In Vitro. Int. J. Mol. Sci. 2023, 24, 16518. [Google Scholar] [CrossRef]
- Baeriswyl, D.C.; Prionisti, I.; Peach, T.; Tsolkas, G.; Chooi, K.Y.; Vardakis, J.; Morel, S.; Diagbouga, M.R.; Bijlenga, P.; Cuhlmann, S.; et al. Disturbed Flow Induces a Sustained, Stochastic NF-ΚB Activation Which May Support Intracranial Aneurysm Growth in Vivo. Sci. Rep. 2019, 9, 4738. [Google Scholar] [CrossRef]
- Wentzel, J.J.; Chatzizisis, Y.S.; Gijsen, F.J.H.; Giannoglou, G.D.; Feldman, C.L.; Stone, P.H. Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: Current understanding and remaining questions. Cardiovasc. Res. 2012, 96, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Storch, A.S.; Rocha, H.N.M.; Garcia, V.P.; da S. Batista, G.M.; Mattos, J.D.; Campos, M.O.; Fuly, A.L.; da Nóbrega, A.C.L.; Fernandes, I.A.; Rocha, N.G. Oscillatory Shear Stress Induces Hemostatic Imbalance in Healthy Men. Thromb. Res. 2018, 170, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tresoldi, C.; Pellegata, A.F.; Mantero, S. Cells and Stimuli in Small-Caliber Blood Vessel Tissue Engineering. Regen. Med. 2015, 10, 505–527. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, D.; Birukov, K.G. Mechanosensing and Mechanoregulation of Endothelial Cell Functions. Compr. Physiol. 2019, 8, 873–904. [Google Scholar]
- Zeller, J.; Loseff-Silver, J.; Khoshmanesh, K.; Baratchi, S.; Lai, A.; Nero, T.L.; Roy, A.; Watson, A.; Dayawansa, N.; Sharma, P.; et al. Shear-Sensing by C-Reactive Protein: Linking Aortic Stenosis and Inflammation. Circ. Res. 2024, 135, 1033–1047. [Google Scholar] [CrossRef]
- Eisenhardt, S.U.; Habersberger, J.; Murphy, A.; Chen, Y.-C.; Woollard, K.J.; Bassler, N.; Qian, H.; von zur Muhlen, C.; Hagemeyer, C.E.; Ahrens, I.; et al. Dissociation of Pentameric to Monomeric C-Reactive Protein on Activated Platelets Localizes Inflammation to Atherosclerotic Plaques. Circ. Res. 2009, 105, 128–137. [Google Scholar] [CrossRef]
- Zeller, J.; Bogner, B.; McFadyen, J.D.; Kiefer, J.; Braig, D.; Pietersz, G.; Krippner, G.; Nero, T.L.; Morton, C.J.; Shing, K.S.C.T.; et al. Transitional Changes in the Structure of C-Reactive Protein Create Highly pro-Inflammatory Molecules: Therapeutic Implications for Cardiovascular Diseases. Pharmacol. Ther. 2022, 235, 108165. [Google Scholar] [CrossRef]
- Thiele, J.R.; Habersberger, J.; Braig, D.; Schmidt, Y.; Goerendt, K.; Maurer, V.; Bannasch, H.; Scheichl, A.; Woollard, K.J.; von Dobschütz, E.; et al. Dissociation of Pentameric to Monomeric C-Reactive Protein Localizes and Aggravates Inflammation. Circulation 2014, 130, 35–50. [Google Scholar] [CrossRef]
- Baratchi, S.; Zaldivia, M.T.K.; Wallert, M.; Loseff-Silver, J.; Al-Aryahi, S.; Zamani, J.; Thurgood, P.; Salim, A.; Htun, N.M.; Stub, D.; et al. Transcatheter Aortic Valve Implantation Represents an Anti-Inflammatory Therapy Via Reduction of Shear Stress–Induced, Piezo-1–Mediated Monocyte Activation. Circulation 2020, 142, 1092–1105. [Google Scholar] [CrossRef]
- Dayawansa, N.H.; Baratchi, S.; Peter, K. Uncoupling the Vicious Cycle of Mechanical Stress and Inflammation in Calcific Aortic Valve Disease. Front. Cardiovasc. Med. 2022, 9, 783543. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Z.; Daitoku, K.; Fukuda, I.; Motomura, S.; Matsumiya, T.; Imaizumi, T.; Furukawa, K.-I.; Seya, K. Human Aortic Valve Interstitial Cells Obtained from Patients with Aortic Valve Stenosis Are Vascular Endothelial Growth Factor Receptor 2 Positive and Contribute to Ectopic Calcification. J. Pharmacol. Sci. 2021, 145, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, P.N.D.; Verena, S.; Thomas, H.; Friedhelm, B.; Manfred, O.; Christoph, B.; Martin, M. Increased levels of circulating microparticles in patients with severe aortic valve stenosis. Thromb. Haemost. 2008, 99, 711–719. [Google Scholar] [CrossRef]
- Frimat, M.; Boudhabhay, I.; Roumenina, L.T. Hemolysis Derived Products Toxicity and Endothelium: Model of the Second Hit. Toxins 2019, 11, 660. [Google Scholar] [CrossRef]
- Herold, S.; Exner, M.; Nauser, T. Kinetic and Mechanistic Studies of the NO•-Mediated Oxidation of Oxymyoglobin and Oxyhemoglobin. Biochemistry 2001, 40, 3385–3395. [Google Scholar] [CrossRef] [PubMed]
- Eich, R.F.; Li, T.; Lemon, D.D.; Doherty, D.H.; Curry, S.R.; Aitken, J.F.; Mathews, A.J.; Johnson, K.A.; Smith, R.D.; Phillips George, N.; et al. Mechanism of NO-Induced Oxidation of Myoglobin and Hemoglobin. Biochemistry 1996, 35, 6976–6983. [Google Scholar] [CrossRef] [PubMed]
- Schechter, A.N.; Gladwin, M.T. Hemoglobin and the Paracrine and Endocrine Functions of Nitric Oxide. N. Engl. J. Med. 2024, 348, 1483–1485. [Google Scholar] [CrossRef]
- Davids, M.; van Hell, A.J.; Visser, M.; Nijveldt, R.J.; van Leeuwen, P.A.M.; Teerlink, T. Role of the human erythrocyte in generation and storage of asymmetric dimethylarginine. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H1762–H1770. [Google Scholar] [CrossRef] [PubMed]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The Clinical Sequelae of Intravascular Hemolysis and Extracellular Plasma HemoglobinA Novel Mechanism of Human Disease. JAMA 2005, 293, 1653–1662. [Google Scholar] [CrossRef]
- Morris, C.R.; Kato, G.J.; Poljakovic, M.; Wang, X.; Blackwelder, W.C.; Sachdev, V.; Hazen, S.L.; Vichinsky, E.P.; Morris, S.M.; Gladwin, M.T. Dysregulated Arginine Metabolism, Hemolysis-Associated Pulmonary Hypertension, and Mortality in Sickle Cell Disease. JAMA 2005, 294, 81–90. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Gbotosho, O.T.; Kapetanaki, M.G.; Kato, G.J. The Worst Things in Life are Free: The Role of Free Heme in Sickle Cell Disease. Front. Immunol. 2021, 11, 561917. [Google Scholar] [CrossRef] [PubMed]
- Vallelian, F.; Buehler, P.W.; Schaer, D.J. Hemolysis, free hemoglobin toxicity, and scavenger protein therapeutics. Blood 2022, 140, 1837–1844. [Google Scholar] [CrossRef]
- Mahdi, A.; Kövamees, O.; Pernow, J. Improvement in endothelial function in cardiovascular disease—Is arginase the target? Int. J. Cardiol. 2020, 301, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rautou, P.-E.; Vion, A.-C.; Amabile, N.; Chironi, G.; Simon, A.; Tedgui, A.; Boulanger, C.M.; Weber, C.; Mause, S. Microparticles, Vascular Function, and Atherothrombosis. Circ. Res. 2011, 109, 593–606. [Google Scholar] [CrossRef]
- Dignat-George, F.; Boulanger, C.M. The Many Faces of Endothelial Microparticles. Arter. Thromb. Vasc. Biol. 2011, 31, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Wassmann, S.; Ahlers, P.; Kosiol, S.; Nickenig, G. Circulating CD31+/Annexin V+ Apoptotic Microparticles Correlate with Coronary Endothelial Function in Patients With Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2006, 26, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sinning, J.-M.; Losch, J.; Walenta, K.; Böhm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef]
- Amabile, N.; Guérin, A.P.; Tedgui, A.; Boulanger, C.M.; London, G.M. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: A pilot study. Nephrol. Dial. Transplant. 2012, 27, 1873–1880. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease: Developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Llndroos, M.; Kupari, M.; Valvanne, J.; Strandberg, T.; Heikkilä, J.; Tllvis, R. Factors associated with calcific aortic valve degeneration in the elderly. Eur. Heart J. 1994, 15, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Lind, B.K.; Kitzman, D.W.; Gersh, B.J.; Siscovick, D.S. Association of Aortic-Valve Sclerosis with Cardiovascular Mortality and Morbidity in the Elderly. N. Engl. J. Med. 2024, 341, 142–147. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical Factors Associated With Calcific Aortic Valve Disease. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice: Developed by the Task Force for Cardiovascular Disease Prevention in Clinical Practice with Representatives of the European Society of Cardiology and 12 Medical Societies With the Special Contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Pohle, K.; Mäffert, R.; Ropers, D.; Moshage, W.; Stilianakis, N.; Daniel, W.G.; Achenbach, S. Progression of Aortic Valve Calcification. Circulation 2001, 104, 1927–1932. [Google Scholar] [CrossRef]
- Comella, A.; Michail, M.; Chan, J.; Cameron, J.D.; Gooley, R.; Mathur, A.; Hughes, A.D.; Brown, A.J. Patients with Aortic Stenosis Exhibit Early Improved Endothelial Function Following Transcatheter Aortic Valve Replacement: The EFAST Study. Int. J. Cardiol. 2021, 332, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Vitez, L.; Starc, V.; Jug, B.; Bunc, M. Improved Endothelial and Autonomic Function after Transcatheter Aortic Valve Implantation. Rev. Cardiovasc. Med. 2023, 24, 140. [Google Scholar] [CrossRef]
- Moscarelli, M.; Devito, F.; Fattouch, K.; Lancellotti, P.; Ciccone, M.M.; Rizzo, P.; Gaudino, M.; Marchese, A.; Angelini, G.; Speziale, G. The Effect of Surgical versus Transcatheter Aortic Valve Replacement on Endothelial Function. An Observational Study. Int. J. Surg. 2019, 63, 1–7. [Google Scholar] [CrossRef]
- Chenevard, R.; Bechir, M.; Hurlimann, D.; Ruschitzka, F.; Turina, J.; Luscher, T.F.; Noll, G. Persistent Endothelial Dysfunction in Calcified Aortic Stenosis beyond Valve Replacement Surgery. Heart 2006, 92, 1862. [Google Scholar] [CrossRef]
- Horn, P.; Stern, D.; Veulemans, V.; Heiss, C.; Zeus, T.; Merx, M.W.; Kelm, M.; Westenfeld, R. Improved Endothelial Function and Decreased Levels of Endothelium-Derived Microparticles after Transcatheter Aortic Valve Implantation. EuroIntervention 2015, 10, 1456–1463. [Google Scholar] [CrossRef]
- Quast, C.; Bönner, F.; Polzin, A.; Veulemans, V.; Chennupati, R.; Gyamfi Poku, I.; Pfeiler, S.; Kramser, N.; Nankinova, M.; Staub, N.; et al. Aortic Valve Stenosis Causes Accumulation of Extracellular Hemoglobin and Systemic Endothelial Dysfunction. Circulation 2024, 150, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Donadee, C.; Raat, N.J.H.; Kanias, T.; Tejero, J.; Lee, J.S.; Kelley, E.E.; Zhao, X.; Liu, C.; Reynolds, H.; Azarov, I.; et al. Nitric Oxide Scavenging by Red Blood Cell Microparticles and Cell-Free Hemoglobin as a Mechanism for the Red Cell Storage Lesion. Circulation 2011, 124, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Tzemos, N.; Therrien, J.; Yip, J.; Thanassoulis, G.; Tremblay, S.; Jamorski, M.T.; Webb, G.D.; Siu, S.C. Outcomes in Adults With Bicuspid Aortic Valves. JAMA 2008, 300, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Alegret, J.M.; Ligero, C.; Vernis, J.M.; Beltrán-Debón, R.; Aragonés, G.; Duran, I.; Palazón, O.; Hernández-Aparicio, A. Factors Related to the Need for Surgery after the Diagnosis of Bicuspid Aortic Valve: One Center´s Experience under a Conservative Approach. Int. J. Med. Sci. 2013, 10, 176–182. [Google Scholar] [CrossRef][Green Version]
- Alegret, J.M.; Martínez-Micaelo, N.; Aragonès, G.; Beltrán-Debón, R. Circulating endothelial microparticles are elevated in bicuspid aortic valve disease and related to aortic dilation. Int. J. Cardiol. 2016, 217, 35–41. [Google Scholar] [CrossRef]
- Tzemos, N.; Lyseggen, E.; Silversides, C.; Jamorski, M.; Tong, J.H.; Harvey, P.; Floras, J.; Siu, S. Endothelial Function, Carotid–Femoral Stiffness, and Plasma Matrix Metalloproteinase-2 in Men With Bicuspid Aortic Valve and Dilated Aorta. J. Am. Coll. Cardiol. 2010, 55, 660–668. [Google Scholar] [CrossRef]
- Ali, O.A.; Chapman, M.; Nguyen, T.H.; Chirkov, Y.Y.; Heresztyn, T.; Mundisugih, J.; Horowitz, J.D. Interactions between Inflammatory Activation and Endothelial Dysfunction Selectively Modulate Valve Disease Progression in Patients with Bicuspid Aortic Valve. Heart 2014, 100, 800. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Li, Y.; Deng, Y.-B.; Liu, Y.-N.; Zhang, J.; Sun, J.; Zhu, Y.; Li, L.; Tang, Q.-Y.; Zhou, W. Enlarged Size and Impaired Elastic Properties of the Ascending Aorta Are Associated with Endothelial Dysfunction and Elevated Plasma Matrix Metalloproteinase-2 Level in Patients with Bicuspid Aortic Valve. Ultrasound Med. Biol. 2018, 44, 955–962. [Google Scholar] [CrossRef]
- Vaturi, M.; Perl, L.; Leshem-Lev, D.; Dadush, O.; Bental, T.; Shapira, Y.; Yedidya, I.; Greenberg, G.; Kornowski, R.; Sagie, A.; et al. Circulating Endothelial Progenitor Cells in Patients With Dysfunctional Versus Normally Functioning Congenitally Bicuspid Aortic Valves. Am. J. Cardiol. 2011, 108, 272–276. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Crapanzano, F.; Schirone, L.; Allegra, A.; Pisano, C.; Ruvolo, G.; Forte, M.; Greco, E.; Cavarretta, E.; Marullo, A.G.M.; et al. Deregulation of Notch1 Pathway and Circulating Endothelial Progenitor Cell (EPC) Number in Patients with Bicuspid Aortic Valve with and without Ascending Aorta Aneurysm. Sci. Rep. 2018, 8, 13834. [Google Scholar] [CrossRef]
- Gavriliuk, N.D.; Tatiana, A.D.G.; Olga, B.I.; Alexandr, A.Z.; Tatiana, F.S.; Vladimir, E.U.; Nina, A.P.; Olga, M.M. Asymmetric Dimethylarginine in Patients with Ascending Aortic Aneurysms. AORTA 2016, 4, 219–225. [Google Scholar] [CrossRef] [PubMed]
- van de Pol, V.; Bons, L.R.; Lodder, K.; Kurakula, K.B.; Sanchez-Duffhues, G.; Siebelink, H.-M.J.; Roos-Hesselink, J.W.; DeRuiter, M.C.; Goumans, M.-J. Endothelial Colony Forming Cells as an Autologous Model to Study Endothelial Dysfunction in Patients with a Bicuspid Aortic Valve. Int. J. Mol. Sci. 2019, 20, 3251. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Poujade, F.-A.; Bergman, O.; Gådin, J.R.; Simon, N.; Lång, K.; Franco-Cereceda, A.; Body, S.C.; Björck, H.M.; Eriksson, P. Endothelial/Epithelial Mesenchymal Transition in Ascending Aortas of Patients With Bicuspid Aortic Valve. Front. Cardiovasc. Med. 2019, 6, 182. [Google Scholar] [CrossRef]
- Gehlen, J.; Stundl, A.; Debiec, R.; Fontana, F.; Krane, M.; Sharipova, D.; Nelson, C.P.; Al-Kassou, B.; Giel, A.-S.; Sinning, J.-M.; et al. Elucidation of the Genetic Causes of Bicuspid Aortic Valve Disease. Cardiovasc. Res. 2023, 119, 857–866. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Hill, L.; Browne, G.; Tulchinsky, E. ZEB/miR-200 feedback loop: At the crossroads of signal transduction in cancer. Int. J. Cancer 2013, 132, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Poggio, P.; Songia, P.; Moschetta, D.; Valerio, V.; Myasoedova, V.; Perrucci, G.L.; Pompilio, G. MiRNA Profiling Revealed Enhanced Susceptibility to Oxidative Stress of Endothelial Cells from Bicuspid Aortic Valve. J. Mol. Cell Cardiol. 2019, 131, 146–154. [Google Scholar] [CrossRef]
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Aragonés, G.; Faiges, M.; Alegret, J.M. MicroRNAs Clustered within the 14q32 Locus Are Associated with Endothelial Damage and Microparticle Secretion in Bicuspid Aortic Valve Disease. Front. Physiol. 2017, 8, 648. [Google Scholar] [CrossRef]
- Odelin, G.; Faure, E.; Maurel-Zaffran, C.; Zaffran, S. Krox20 Regulates Endothelial Nitric Oxide Signaling in Aortic Valve Development and Disease. J. Cardiovasc. Dev. Dis. 2019, 6, 39. [Google Scholar] [CrossRef]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 126, 821–828. [Google Scholar] [CrossRef]
- Meierhofer, C.; Schneider, E.P.; Lyko, C.; Hutter, A.; Martinoff, S.; Markl, M.; Hager, A.; Hess, J.; Stern, H.; Fratz, S. Wall Shear Stress and Flow Patterns in the Ascending Aorta in Patients with Bicuspid Aortic Valves Differ Significantly from Tricuspid Aortic Valves: A Prospective Study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol. 2017, 219, 382–408. [Google Scholar] [CrossRef]
- Brazil, M. Go with the flow. Nat. Rev. Drug Discov. 2005, 4, 883. [Google Scholar] [CrossRef]
- Osawa, M.; Masuda, M.; Harada, N.; Lopes, R.B.; Fujiwara, K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur. J. Cell Biol. 1997, 72, 229–237. [Google Scholar]
- Conway, D.E.; Breckenridge, M.T.; Hinde, E.; Gratton, E.; Chen, C.S.; Schwartz, M.A. Fluid Shear Stress on Endothelial Cells Modulates Mechanical Tension across VE-Cadherin and PECAM-1. Curr. Biol. 2013, 23, 1024–1030. [Google Scholar] [CrossRef]
- Fleming, I.; Fisslthaler, B.; Dixit, M.; Busse, R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 2005, 118, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Ettenson, D.S.; Koo, E.W.Y.; Januzzi, J.L.; Edelman, E.R. Endothelial heparan sulfate is necessary but not sufficient for control of vascular smooth muscle cell growth. J. Cell Physiol. 2000, 184, 93–100. [Google Scholar] [CrossRef]
- Baker, A.B.; Ettenson, D.S.; Jonas, M.; Nugent, M.A.; Iozzo, R.V.; Edelman, E.R. Endothelial Cells Provide Feedback Control for Vascular Remodeling Through a Mechanosensitive Autocrine TGF-β Signaling Pathway. Circ. Res. 2008, 103, 289–297. [Google Scholar] [CrossRef]
- Walshe, T.E.; Paz, N.G.D.; D’Amore, P.A. The Role of Shear-Induced Transforming Growth Factor-β Signaling in the Endothelium. Arter. Thromb. Vasc. Biol. 2013, 33, 2608–2617. [Google Scholar] [CrossRef]
- Fontijn, R.D.; Volger, O.L.; van der Pouw-Kraan, T.C.; Doddaballapur, A.; Leyen, T.; Baggen, J.M.; Boon, R.A.; Horrevoets, A.J.G. Expression of Nitric Oxide-Transporting Aquaporin-1 Is Controlled by KLF2 and Marks Non-Activated Endothelium In Vivo. PLoS ONE 2016, 10, e0145777. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.-S.; Chien, S. Shear Stress–Initiated Signaling and Its Regulation of Endothelial Function. Arter. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Dijke, P.T.; Egorova, A.D.; Goumans, M.-J.T.H.; Poelmann, R.E.; Hierck, B.P. TGF-β Signaling in Endothelial-to-Mesenchymal Transition: The Role of Shear Stress and Primary Cilia. Sci. Signal 2012, 5(pt2). [Google Scholar] [CrossRef]
- Muylaert, D.E.P.; de Jong, O.G.; Slaats, G.G.G.; Nieuweboer, F.E.; Fledderus, J.O.; Goumans, M.-J.; Hierck, B.P.; Verhaar, M.C. Environmental Influences on Endothelial to Mesenchymal Transition in Developing Implanted Cardiovascular Tissue-Engineered Grafts. Tissue Eng. Part. B Rev. 2015, 22, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Aicher, D.; Urbich, C.; Zeiher, A.; Dimmeler, S.; Schäfers, H.-J. Endothelial Nitric Oxide Synthase in Bicuspid Aortic Valve Disease. Ann. Thorac. Surg. 2007, 83, 1290–1294. [Google Scholar] [CrossRef]
- Kotlarczyk, M.P.; Billaud, M.; Green, B.R.; Hill, J.C.; Shiva, S.; Kelley, E.E.; Phillippi, J.A.; Gleason, T.G. Regional Disruptions in Endothelial Nitric Oxide Pathway Associated With Bicuspid Aortic Valve. Ann. Thorac. Surg. 2016, 102, 1274–1281. [Google Scholar] [CrossRef]
- Phillippi, J.A.; Eskay, M.A.; Kubala, A.A.; Pitt, B.R.; Gleason, T.G. Altered Oxidative Stress Responses and Increased Type I Collagen Expression in Bicuspid Aortic Valve Patients. Ann. Thorac. Surg. 2010, 90, 1893–1898. [Google Scholar] [CrossRef]
- Phillippi, J.A.; Klyachko, E.A.; Kenny, J.P.; Eskay, M.A.; Gorman, R.C.; Gleason, T.G. Basal and Oxidative Stress–Induced Expression of Metallothionein Is Decreased in Ascending Aortic Aneurysms of Bicuspid Aortic Valve Patients. Circulation 2009, 119, 2498–2506. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Hsu, P.-L.; Chen, J.-S.; Wang, C.-Y.; Wu, H.-L.; Mo, F.-E. Shear-Induced CCN1 Promotes Atheroprone Endothelial Phenotypes and Atherosclerosis. Circulation 2019, 139, 2877–2891. [Google Scholar] [CrossRef]
- Jiang, Y.-Z.; Jiménez, J.M.; Ou, K.; McCormick, M.E.; Zhang, L.-D.; Davies, P.F. Hemodynamic Disturbed Flow Induces Differential DNA Methylation of Endothelial Kruppel-Like Factor 4 Promoter In Vitro and In Vivo. Circ. Res. 2014, 115, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Drapisz, S.; Góralczyk, T.; Jamka-Miszalski, T.; Olszowska, M.; Undas, A. Nonstenotic bicuspid aortic valve is associated with elevated plasma asymmetric dimethylarginine. J. Cardiovasc. Med. 2013, 14, 446–452. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).