Characterization of Disseminated Tumor Cells (DTCs) in Patients with Triple-Negative Breast Cancer (TNBC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Informed Consent
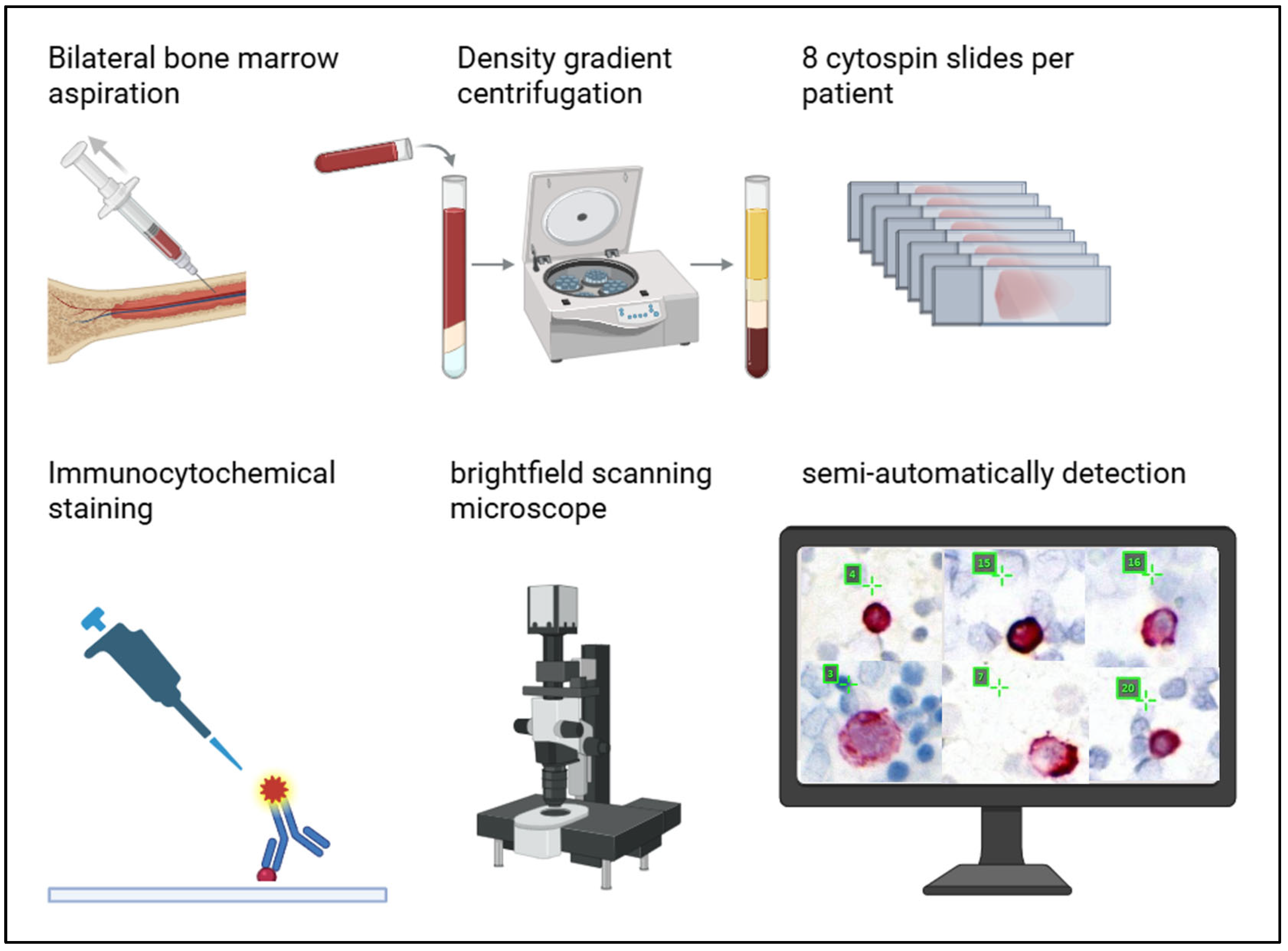
2.2. Bone Marrow Aspirates
2.3. Cell Culture for Reference Slides
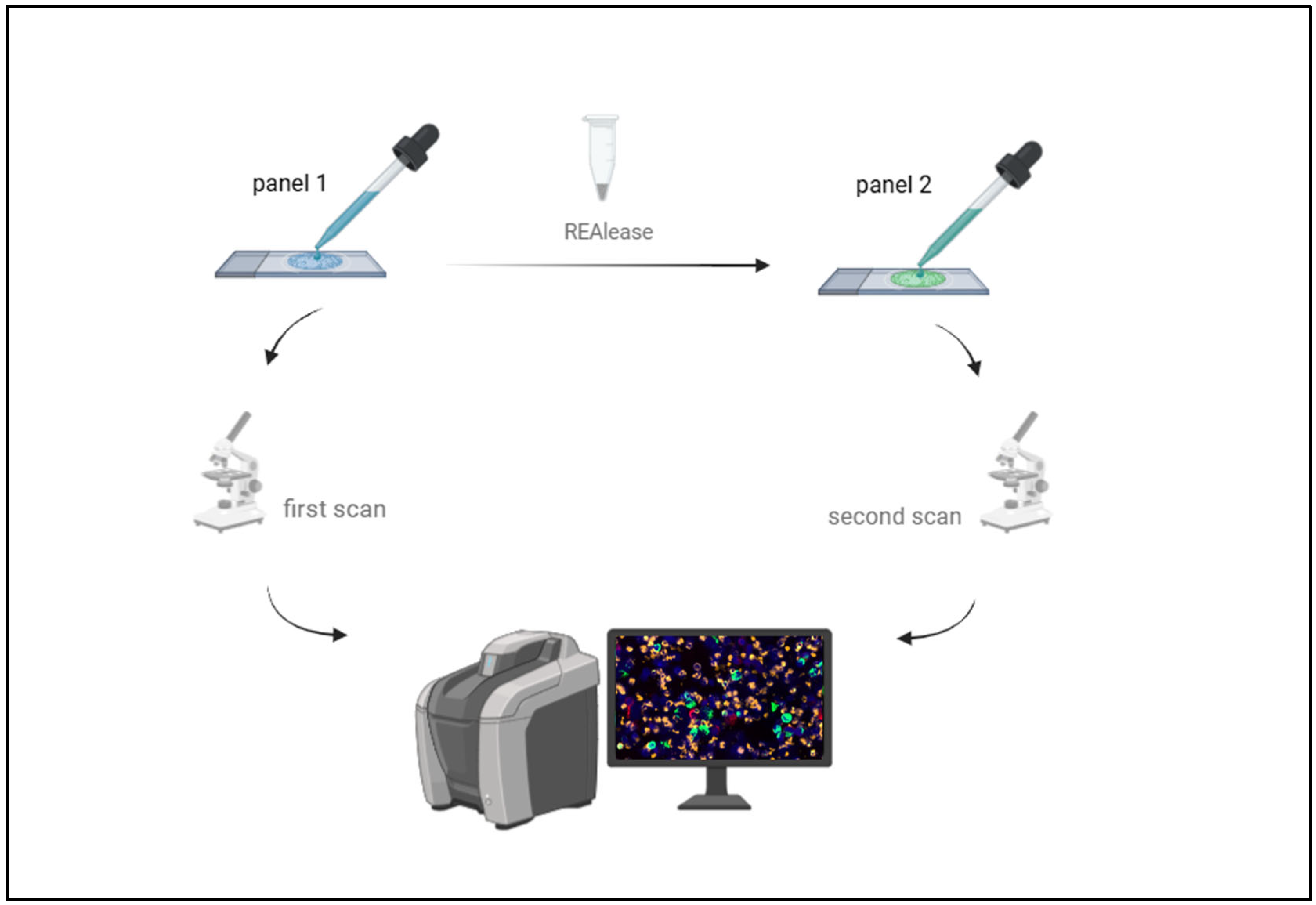
2.4. Sequential Immunofluorescence Staining and DTC Imaging
2.5. Release of Fluorochromes from APC- and FITC- Conjugated Antibodies for Further Staining
2.6. Immunohistochemical Staining of Matching Tumor Tissue
2.7. Statistical Analysis
3. Results
3.1. Staining of Reference Slides Using Sequential Immunofluorescence
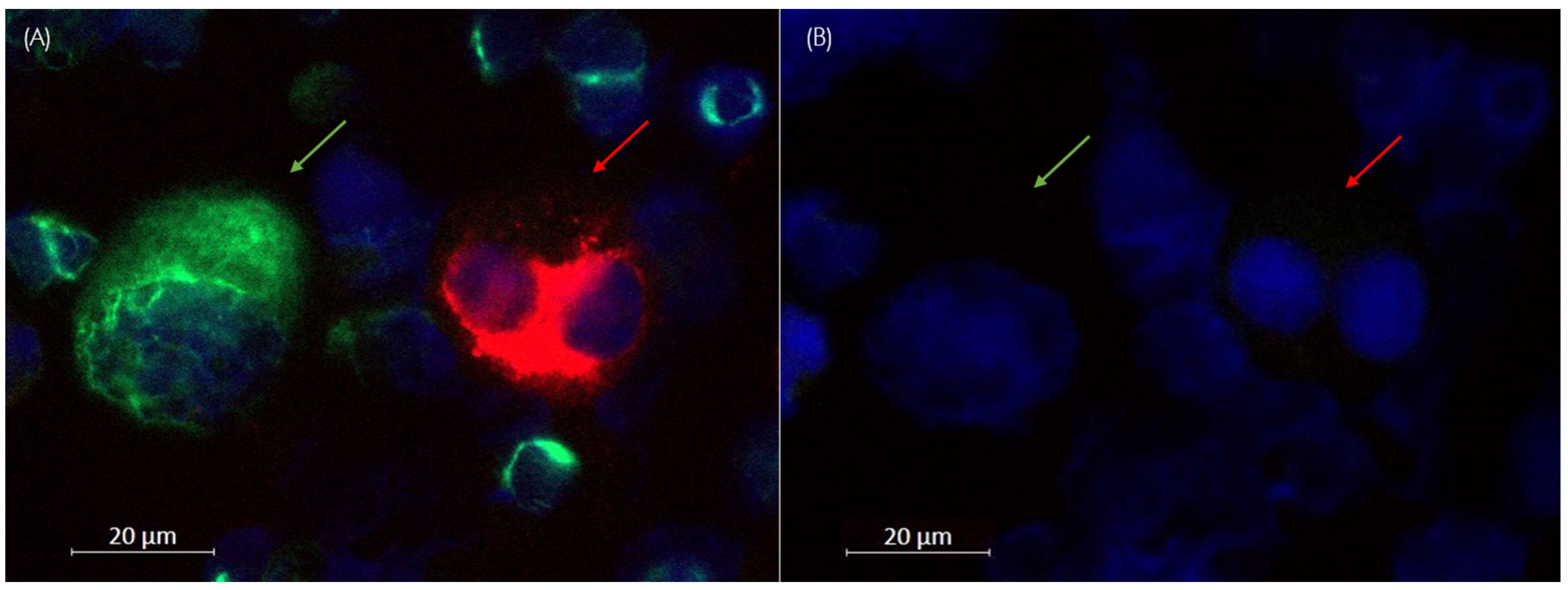
3.1.1. Control Cells for Marker Panels
3.1.2. Calculation of the Release Rate
3.1.3. Characterization of Control Cells
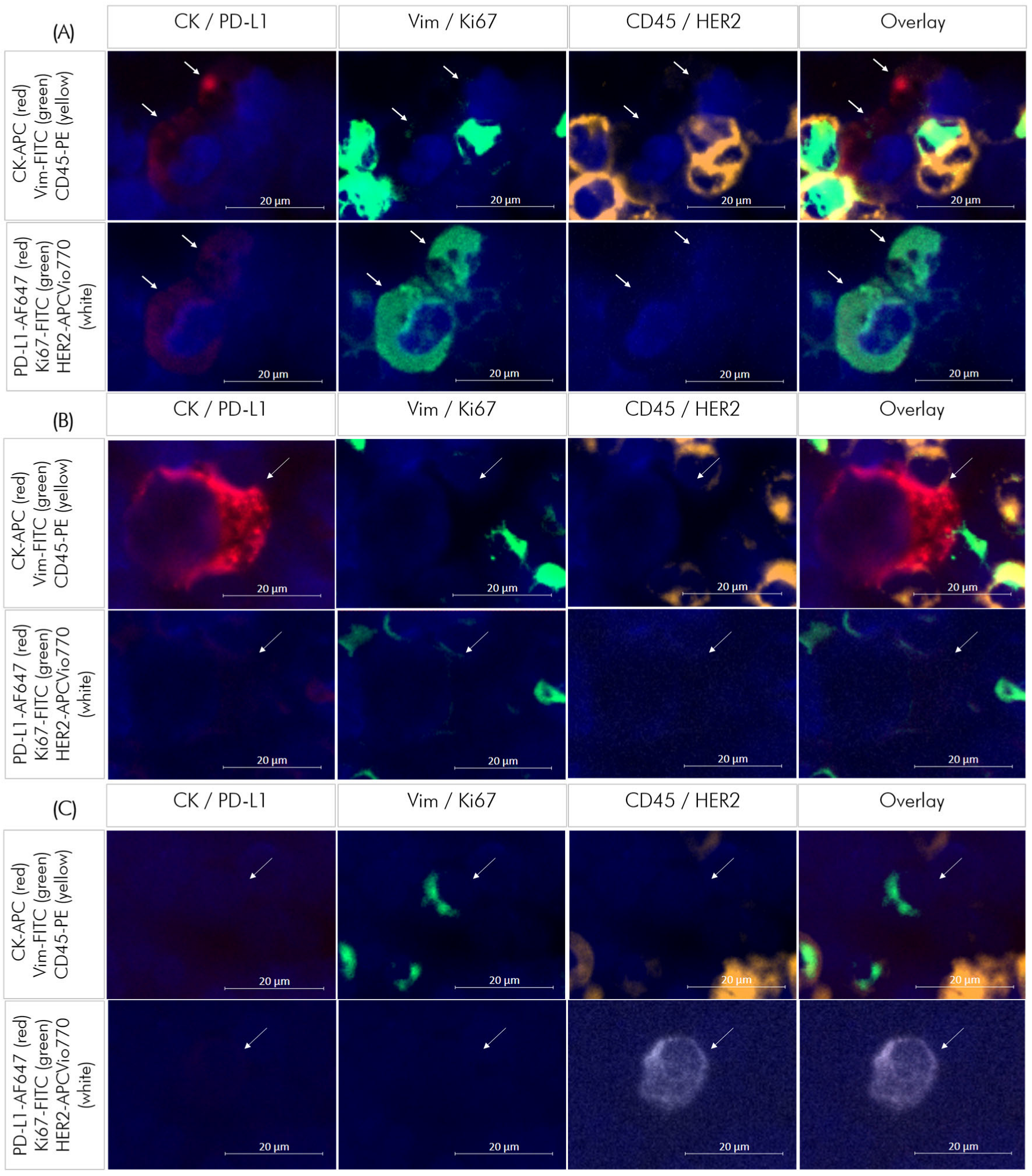
3.2. Identification and Quantification of DTC-Subtypes in Bone Marrow Samples
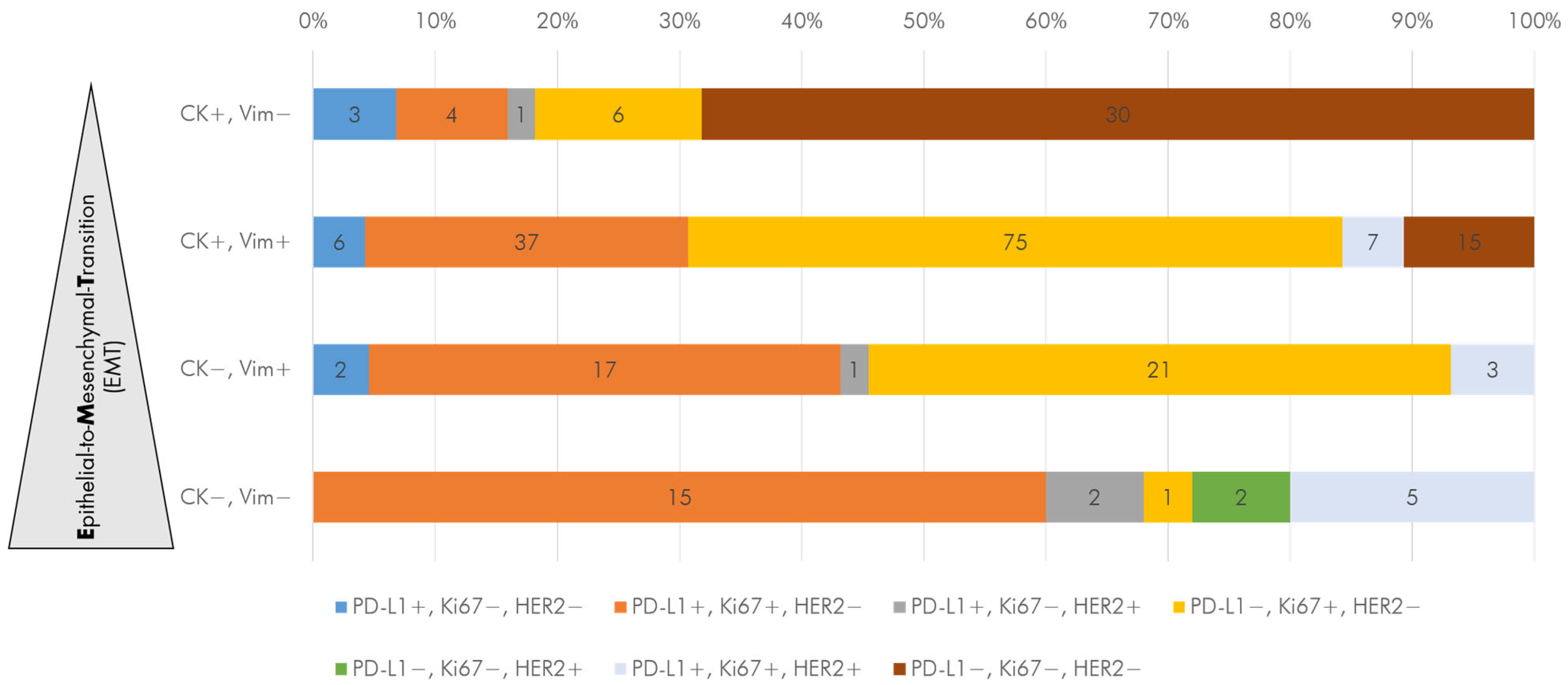
3.3. DTC Subtypes in Primary and Recurrent TNBC Patients
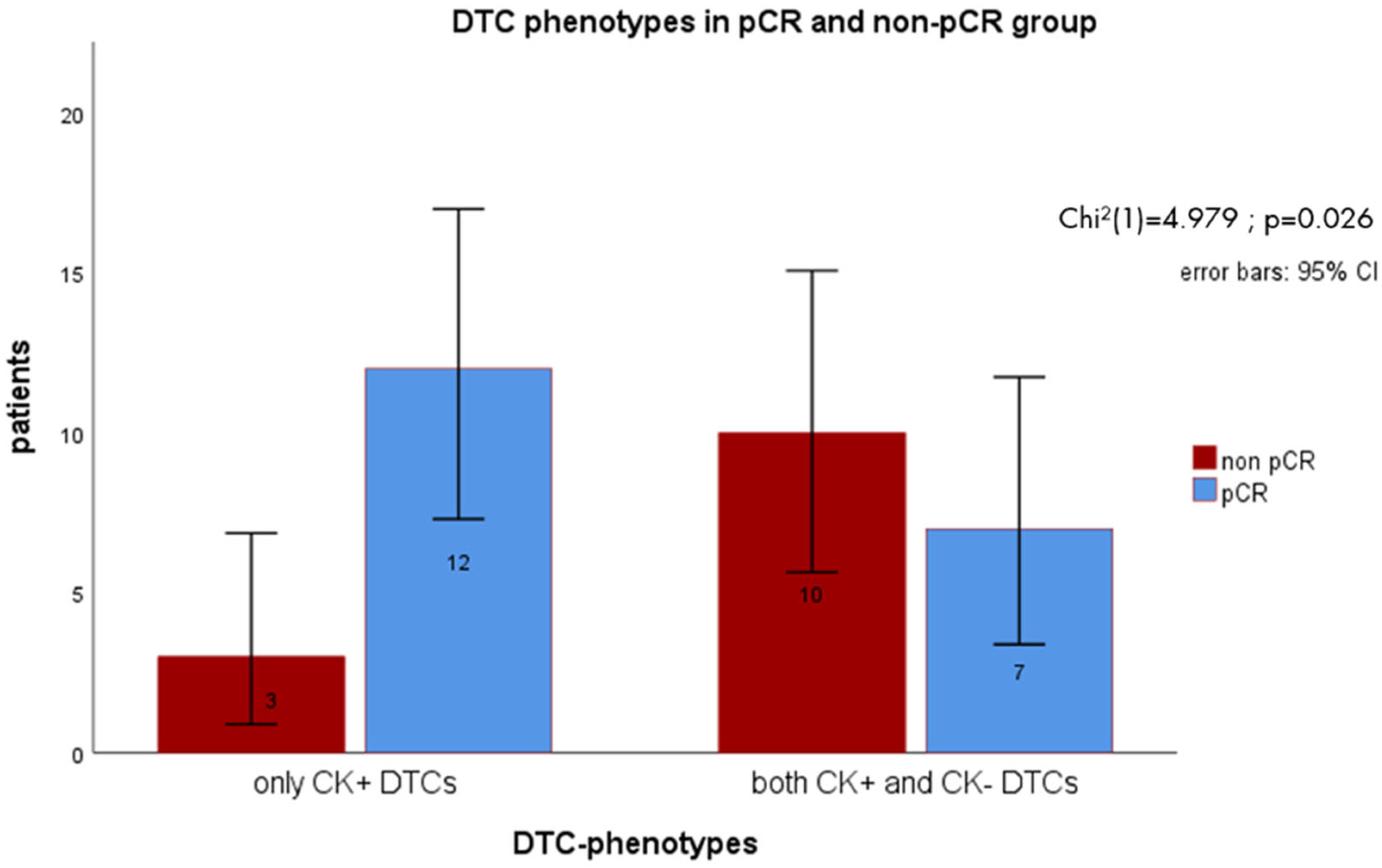
3.4. DTC Status and Subtypes After NACT in Patients with Primary TNBC Reaching pCR vs. Non-pCR
3.5. DTC Subtype Detection vs. Standard Detection of CK+ Cells
3.6. Correlation of DTC Analysis to Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNBC | Triple Negative Breast Cancer |
| DTCs | Disseminated Tumor Cells |
| NACT | Neoadjuvant Chemotherapy |
| pCR | Pathologic Complete Remission |
| CK | Cytokeratin |
| Vim | Vimentin |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| PD-L1 | Programmed Cell Death 1 Ligand 1 |
| BM | Bone Marrow |
| EMT | Epithelial–Mesenchymal Transition |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| IF | Immunofluorescence |
| IHC | Immunohistochemical |
| FISH | Fluorescence In Situ Hybridization |
| CISH | Chromogenic In Situ Hybridization |
| CNB | Core Needle Biopsy |
| DMEM | Dulbecco’s Modified Eagle Medium |
| RPMI | Roswell Park Memorial Institute |
| PBS | Phosphate-Buffered Saline |
| DAPI | 4′,6′-Diamidin-2-phenylindol |
| APC | Allophycocyanin |
| FITC | Fluoresceinisothiocyanat |
| PE | Phycoerythrin |
| TPS | Tumor Proportion Score |
| CPS | Combined Positive Score |
| CTCs | Circulating Tumor Cells |
Appendix A
| Pat.-ID | Total DTCs | Total CK+ DTCs | Total CK- DTCs | Total Vim+ DTCs | Total Ki67+ DTCs | Total PD-L1+ DTCs | Total HER2+ DTCs |
|---|---|---|---|---|---|---|---|
| 731 | 3 | 3 | 0 | 3 | 2 | 0 | 0 |
| 744 | 7 | 3 | 4 | 5 | 5 | 5 | 0 |
| 762 | 14 | 6 | 8 | 7 | 13 | 13 | 0 |
| 801 | 3 | 3 | 0 | 2 | 1 | 2 | 0 |
| 844 | 11 | 7 | 4 | 7 | 6 | 9 | 2 |
| 1085 | 10 | 1 | 9 | 2 | 7 | 9 | 6 |
| 1123 | 10 | 5 | 5 | 3 | 7 | 8 | 0 |
| 1139 | 15 | 11 | 4 | 15 | 14 | 15 | 4 |
| 1230 | 3 | 1 | 2 | 1 | 1 | 3 | 3 |
| 1320 | 2 | 2 | 0 | 1 | 1 | 1 | 0 |
| 1892 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| 1893 | 3 | 3 | 0 | 3 | 2 | 3 | 0 |
| 2017 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 2037 | 4 | 3 | 1 | 3 | 4 | 3 | 0 |
| 2045 | 8 | 5 | 3 | 7 | 7 | 2 | 0 |
| 2108 | 6 | 5 | 1 | 5 | 6 | 0 | 0 |
| 2284 | 12 | 9 | 3 | 11 | 11 | 2 | 0 |
| 2492 | 10 | 10 | 0 | 6 | 6 | 1 | 0 |
| 2539 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| 2607 | 7 | 7 | 0 | 7 | 5 | 1 | 0 |
| 2735 | 4 | 4 | 0 | 0 | 0 | 0 | 0 |
| 2769 | 3 | 2 | 1 | 3 | 3 | 1 | 0 |
| 2803 | 5 | 3 | 2 | 5 | 5 | 0 | 0 |
| 2833 | 7 | 6 | 1 | 5 | 6 | 0 | 0 |
| 2909 | 2 | 2 | 0 | 1 | 1 | 0 | 0 |
| 2917 | 6 | 5 | 1 | 3 | 3 | 0 | 0 |
| 2927 | 8 | 6 | 2 | 7 | 6 | 0 | 1 |
| 2953 | 16 | 12 | 4 | 11 | 10 | 4 | 0 |
| 2973 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| 3122 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| 3217 | 3 | 3 | 0 | 3 | 3 | 3 | 1 |
| 3306 | 3 | 3 | 0 | 3 | 3 | 2 | 2 |
| 3313 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 3339 | 8 | 5 | 3 | 5 | 6 | 2 | 0 |
| 3353 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3427 | 7 | 6 | 1 | 7 | 7 | 6 | 0 |
| 3457 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| 3558 | 11 | 10 | 1 | 11 | 9 | 0 | 0 |
| 3569 | 12 | 9 | 3 | 11 | 12 | 0 | 0 |
| 3573 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| 3605 | 5 | 4 | 1 | 4 | 3 | 2 | 0 |
| 3625 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 3648 | 7 | 4 | 3 | 6 | 6 | 2 | 0 |
| 3652 | 3 | 3 | 0 | 1 | 2 | 0 | 0 |
| 3684 | 3 | 3 | 0 | 3 | 1 | 1 | 1 |
| sum | 253 | 184 | 69 | 184 | 190 | 103 | 21 |
| mean | 5.62 | 4.09 | 1.53 | 4.09 | 4.22 | 2.29 | 0.467 |
References
- Morgan, E.; O’Neill, C.; Shah, R.; Langselius, O.; Su, Y.; Frick, C.; Fink, H.; Bardot, A.; Walsh, P.M.; Woods, R.R.; et al. Metastatic recurrence in women diagnosed with non-metastatic breast cancer: A systematic review and meta-analysis. Breast Cancer Res. BCR 2024, 26, 171. [Google Scholar] [CrossRef]
- Ramamoorthi, G.; Kodumudi, K.; Gallen, C.; Zachariah, N.N.; Basu, A.; Albert, G.; Beyer, A.; Snyder, C.; Wiener, D.; Costa, R.L.B.; et al. Disseminated cancer cells in breast cancer: Mechanism of dissemination and dormancy and emerging insights on therapeutic opportunities. Semin. Cancer Biol. 2022, 78, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Coombes, R.C.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G.; et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Hartkopf, A.D.; Brucker, S.Y.; Taran, F.-A.; Harbeck, N.; von Au, A.; Naume, B.; Pierga, J.-Y.; Hoffmann, O.; Beckmann, M.W.; Rydén, L.; et al. Disseminated tumour cells from the bone marrow of early breast cancer patients: Results from an international pooled analysis. Eur. J. Cancer 2021, 154, 128–137. [Google Scholar] [CrossRef]
- Janni, W.; Vogl, F.D.; Wiedswang, G.; Synnestvedt, M.; Fehm, T.; Jückstock, J.; Borgen, E.; Rack, B.; Braun, S.; Sommer, H.; et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse--a European pooled analysis. Clin. Cancer Res. 2011, 17, 2967–2976. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Taran, F.-A.; Wallwiener, M.; Hahn, M.; Becker, S.; Solomayer, E.-F.; Brucker, S.Y.; Fehm, T.N.; Wallwiener, D. Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients—Results from a large single-centre analysis. Eur. J. Cancer 2014, 50, 2550–2559. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Fritz, A.J.; Zaidi, S.K.; van Wijnen, A.J.; Nickerson, J.A.; Imbalzano, A.N.; Lian, J.B.; Stein, J.L.; Stein, G.S. Epithelial-to-mesenchymal transition and cancer stem cells contribute to breast cancer heterogeneity. J. Cell. Physiol. 2018, 233, 9136–9144. [Google Scholar] [CrossRef]
- Jørgensen, C.L.T.; Forsare, C.; Bendahl, P.-O.; Falck, A.-K.; Fernö, M.; Lövgren, K.; Aaltonen, K.; Rydén, L. Expression of epithelial-mesenchymal transition-related markers and phenotypes during breast cancer progression. Breast Cancer Res. Treat. 2020, 181, 369–381. [Google Scholar] [CrossRef]
- Zou, H.; Yang, Z.; Chan, Y.-S.; Yeung, S.-K.A.; Alam, M.K.; Si, T.; Xu, T.; Yang, M. Single cell analysis of mechanical properties and EMT-related gene expression profiles in cancer fingers. iScience 2022, 25, 103917. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Epithelial-Mesenchymal Transition (EMT) and Regulation of EMT Factors by Steroid Nuclear Receptors in Breast Cancer: A Review and in Silico Investigation. J. Clin. Med. 2016, 5, 11. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.-J. Strategies for subtypes--dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.; Carey, L.A. Understanding and treating triple-negative breast cancer. Oncology 2008, 22, 1233–1239. [Google Scholar]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; van Siewertsz Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef]
- Won, K.-A.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie. LL Mammakarzinom—Kurzversion; Version 4.3. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Mammakarzinom_4_0/Version_4.4/LL_Mammakarzinom_Kurzversion_4.3.pdf (accessed on 1 June 2025).
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. npj Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Tarantino, P.; Viale, G.; Press, M.F.; Hu, X.; Penault-Llorca, F.; Bardia, A.; Batistatou, A.; Burstein, H.J.; Carey, L.A.; Cortes, J.; et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann. Oncol. 2023, 34, 645–659. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabières, C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 2015, 9, 1773–1782. [Google Scholar] [CrossRef]
- Xiang, X.; Yu, P.-C.; Long, D.; Liao, X.-L.; Zhang, S.; You, X.-M.; Zhong, J.-H.; Li, L.-Q. Prognostic value of PD -L1 expression in patients with primary solid tumors. Oncotarget 2018, 9, 5058–5072. [Google Scholar] [CrossRef] [PubMed]
- Planes-Laine, G.; Rochigneux, P.; Bertucci, F.; Chrétien, A.-S.; Viens, P.; Sabatier, R.; Gonçalves, A. PD-1/PD-L1 Targeting in Breast Cancer: The First Clinical Evidences Are Emerging. A Literature Review. Cancers 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Lüönd, F.; Sugiyama, N.; Bill, R.; Bornes, L.; Hager, C.; Tang, F.; Santacroce, N.; Beisel, C.; Ivanek, R.; Bürglin, T.; et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev. Cell 2021, 56, 3203–3221.e11. [Google Scholar] [CrossRef]
- Felipe Lima, J.; Nofech-Mozes, S.; Bayani, J.; Bartlett, J.M.S. EMT in Breast Carcinoma-A Review. J. Clin. Med. 2016, 5, 65. [Google Scholar] [CrossRef]
- Thiery, J.P.; Lim, C.T. Tumor dissemination: An EMT affair. Cancer Cell 2013, 23, 272–273. [Google Scholar] [CrossRef]
- Fehm, T.; Braun, S.; Muller, V.; Janni, W.; Gebauer, G.; Marth, C.; Schindlbeck, C.; Wallwiener, D.; Borgen, E.; Naume, B.; et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 2006, 107, 885–892. [Google Scholar] [CrossRef]
- König, T.; Dogan, S.; Höhn, A.K.; Weydandt, L.; Aktas, B.; Nel, I. Multi-Parameter Analysis of Disseminated Tumor Cells (DTCs) in Early Breast Cancer Patients with Hormone-Receptor-Positive Tumors. Cancers 2023, 15, 568. [Google Scholar] [CrossRef]
- Aktas, B.; Tewes, M.; Fehm, T.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. BCR 2009, 11, R46. [Google Scholar] [CrossRef]
- Balic, M.; Lin, H.; Young, L.; Hawes, D.; Giuliano, A.; McNamara, G.; Datar, R.H.; Cote, R.J. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin. Cancer Res. 2006, 12, 5615–5621. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: Meta-analyses of individual patient data from randomised trials. Lancet 2015, 386, 1353–1361. [Google Scholar] [CrossRef]
- Nel, I.; Herzog, H.; Aktas, B. Combined Analysis of Disseminated Tumor Cells (DTCs) and Circulating Tumor DNA (ctDNA) in a Patient Suffering from Triple Negative Breast Cancer Revealed Elevated Risk. Front. Biosci. 2022, 27, 208. [Google Scholar] [CrossRef] [PubMed]
- Rudin, L.I.; Osher, S.; Fatemi, E. Nonlinear total variation based noise removal algorithms. Phys. D Nonlinear Phenom. 1992, 60, 259–268. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Aitken, S.J.; Thomas, J.S.; Langdon, S.P.; Harrison, D.J.; Faratian, D. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann. Oncol. 2010, 21, 1254–1261. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Wolff, A.C.; Mangu, P.B.; Temin, S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Oncol. Pract. 2010, 6, 195–197. [Google Scholar] [CrossRef]
- Volmer, L.L.; Dannehl, D.; Matovina, S.; Taran, F.-A.; Walter, C.B.; Wallwiener, M.; Brucker, S.Y.; Hartkopf, A.D.; Engler, T. Comparing the HER2 Status of the Primary Tumor to That of Disseminated Tumor Cells in Early Breast Cancer. Int. J. Mol. Sci. 2024, 25, 5910. [Google Scholar] [CrossRef]
- D’Alterio, C.; Scala, S.; Sozzi, G.; Roz, L.; Bertolini, G. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin. Cancer Biol. 2020, 60, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.-F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Wimberger, P.; Blohmer, J.-U.; Krabisch, P.; Link, T.; Just, M.; Sinn, B.V.; Simon, E.; Solbach, C.; Fehm, T.; Denkert, C.; et al. The effect of denosumab on disseminated tumor cells (DTCs) of breast cancer patients with neoadjuvant treatment: A GeparX translational substudy. Breast Cancer Res. BCR 2023, 25, 32. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, J.; Lei, L.; He, H.; Chen, E.; Dong, J.; Yang, J. High Vimentin Expression Predicts a Poor Prognosis and Progression in Colorectal Cancer: A Study with Meta-Analysis and TCGA Database. BioMed Res. Int. 2018, 2018, 6387810. [Google Scholar] [CrossRef] [PubMed]
- Dauphin, M.; Barbe, C.; Lemaire, S.; Nawrocki-Raby, B.; Lagonotte, E.; Delepine, G.; Birembaut, P.; Gilles, C.; Polette, M. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer 2013, 81, 117–122. [Google Scholar] [CrossRef]
- Yamashita, N.; Tokunaga, E.; Kitao, H.; Hisamatsu, Y.; Taketani, K.; Akiyoshi, S.; Okada, S.; Aishima, S.; Morita, M.; Maehara, Y. Vimentin as a poor prognostic factor for triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 739–746. [Google Scholar] [CrossRef]
- Grasset, E.M.; Dunworth, M.; Sharma, G.; Loth, M.; Tandurella, J.; Cimino-Mathews, A.; Gentz, M.; Bracht, S.; Haynes, M.; Fertig, E.J.; et al. Triple-negative breast cancer metastasis involves complex epithelial-mesenchymal transition dynamics and requires vimentin. Sci. Transl. Med. 2022, 14, eabn7571. [Google Scholar] [CrossRef]
- Javir, G.; Joshi, K.; Khedkar, V.; Rojatkar, S. 6 α-Hydroxy-414, 1015-guainadien-8β, 12-olide induced cell cycle arrest via modulation of EMT and Wnt/β-catenin pathway in HER-2 positive breast cancer cells. J. Steroid Biochem. Mol. Biol. 2020, 197, 105514. [Google Scholar] [CrossRef]
- Jäger, B.A.S.; Finkenzeller, C.; Bock, C.; Majunke, L.; Jueckstock, J.K.; Andergassen, U.; Neugebauer, J.K.; Pestka, A.; Friedl, T.W.P.; Jeschke, U.; et al. Estrogen Receptor and HER2 Status on Disseminated Tumor Cells and Primary Tumor in Patients with Early Breast Cancer. Transl. Oncol. 2015, 8, 509–516. [Google Scholar] [CrossRef][Green Version]
- Krishnamurthy, S.; Bischoff, F.; Ann Mayer, J.; Wong, K.; Pham, T.; Kuerer, H.; Lodhi, A.; Bhattacharyya, A.; Hall, C.; Lucci, A. Discordance in HER2 gene amplification in circulating and disseminated tumor cells in patients with operable breast cancer. Cancer Med. 2013, 2, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, N.; Banys, M.; Neubauer, H.; Solomayer, E.-F.; Gall, C.; Hahn, M.; Becker, S.; Bachmann, R.; Wallwiener, D.; Fehm, T. HER2 status on persistent disseminated tumor cells after adjuvant therapy may differ from initial HER2 status on primary tumor. Anticancer Res. 2009, 29, 4019–4024. [Google Scholar] [PubMed]
- Matsushita, D.; Uenosono, Y.; Arigami, T.; Yanagita, S.; Okubo, K.; Kijima, T.; Miyazono, F.; Hamanoue, M.; Hokita, S.; Nakashima, S.; et al. Clinical significance of circulating tumor cells in the response to trastuzumab for HER2-negative metastatic gastric cancer. Cancer Chemother. Pharmacol. 2021, 87, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Mueller, V.; Banys-Paluchowski, M.; Fasching, P.A.; Friedl, T.W.P.; Hartkopf, A.; Huober, J.; Loehberg, C.; Rack, B.; Riethdorf, S.; et al. Efficacy of Lapatinib in Patients with HER2-Negative Metastatic Breast Cancer and HER2-Positive Circulating Tumor Cells-The DETECT III Clinical Trial. Clin. Chem. 2024, 70, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Ottewell, P.; Wilson, C. Bone-Targeted Agents in Breast Cancer: Do We Now Have All the Answers? Breast Cancer Basic Clin. Res. 2019, 13, 1178223419843501. [Google Scholar] [CrossRef]
- O’Carrigan, B.; Wong, M.H.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017, 10, CD003474. [Google Scholar] [CrossRef]



| n = 80 | % | |
|---|---|---|
| Age | ||
| Median (years) | 50 | |
| Range | 26–86 | |
| Menopausal status | ||
| pre- | 34 | 42.5 |
| peri- | 7 | 8.8 |
| post- | 39 | 48.8 |
| Tumor stage | ||
| Primary | 67 | 83.8 |
| Recurrent | 13 | 16.3 |
| Ki67 (CNB) | ||
| average (%) | 52 | |
| range (%) | 2–90 | |
| Nodal status N0 N1 | 59 21 | 73.8 26.2 |
| HER2 score (CNB) | ||
| 0 | 64 | 80.0 |
| 1+ | 12 | 15.0 |
| 2+/CISH neg | 4 | 5.0 |
| Grade (CNB) | ||
| G1 | 3 | 3.8 |
| G2 | 28 | 35.0 |
| G3 | 47 | 58.8 |
| n.a. | 2 | 2.5 |
| NACT | ||
| yes | 64 | 80.0 |
| combined with pembrolizumab | 9 | 14.1 |
| no | 16 | 20.0 |
| pCR | 39 | 60.9 |
| Cells/Marker | ZR75-1 | T98G | CaSki | MCF7 | Hematopoietic Cells |
|---|---|---|---|---|---|
| Cytokeratin | + | - | + | + | - |
| Vimentin | - | + | + | - | + |
| CD45 | - | - | - | - | + |
| PD-L1 | - | - | + | - | both * |
| Ki67 | + | + | + | + | - |
| HER2 | + | - | - | - | - |
| DAPI | + | + | + | + | + |
| Marker | Patients with DTCs, Positive for Marker (n) | Tumor Tissue * Positive (n) | Tumor Tissue * Negative (n) | Missing Data (n) |
|---|---|---|---|---|
| HER2 | 9 | 4 | 3 | 2 |
| PD-L1 | 27 | 10 | 10 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckardt, A.; Nel, I.; Weydandt, L.; Brochwitz, E.; Höhn, A.K.; Winter, K.; Aktas, B. Characterization of Disseminated Tumor Cells (DTCs) in Patients with Triple-Negative Breast Cancer (TNBC). Cells 2025, 14, 857. https://doi.org/10.3390/cells14120857
Eckardt A, Nel I, Weydandt L, Brochwitz E, Höhn AK, Winter K, Aktas B. Characterization of Disseminated Tumor Cells (DTCs) in Patients with Triple-Negative Breast Cancer (TNBC). Cells. 2025; 14(12):857. https://doi.org/10.3390/cells14120857
Chicago/Turabian StyleEckardt, Anne, Ivonne Nel, Laura Weydandt, Elisa Brochwitz, Anne Kathrin Höhn, Karsten Winter, and Bahriye Aktas. 2025. "Characterization of Disseminated Tumor Cells (DTCs) in Patients with Triple-Negative Breast Cancer (TNBC)" Cells 14, no. 12: 857. https://doi.org/10.3390/cells14120857
APA StyleEckardt, A., Nel, I., Weydandt, L., Brochwitz, E., Höhn, A. K., Winter, K., & Aktas, B. (2025). Characterization of Disseminated Tumor Cells (DTCs) in Patients with Triple-Negative Breast Cancer (TNBC). Cells, 14(12), 857. https://doi.org/10.3390/cells14120857







