The Activity of Human NK Cells Towards 3D Heterotypic Cellular Tumor Model of Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Isolation and Activation of Human NK Cells
2.3. Cell Viability Assay in Real-Time System
2.4. Spheroids Formation
2.5. Time-Lapse of Spheroid Formation Process
2.6. Fluorescence Microscopy
2.7. Live/Dead Staining
2.8. Calculation of the Spheroid Volumes
2.9. Flow Cytometry
2.10. Statistical Analysis
3. Results
3.1. Generation and Cultivation of 3D Heterotypic Breast Cancer Model
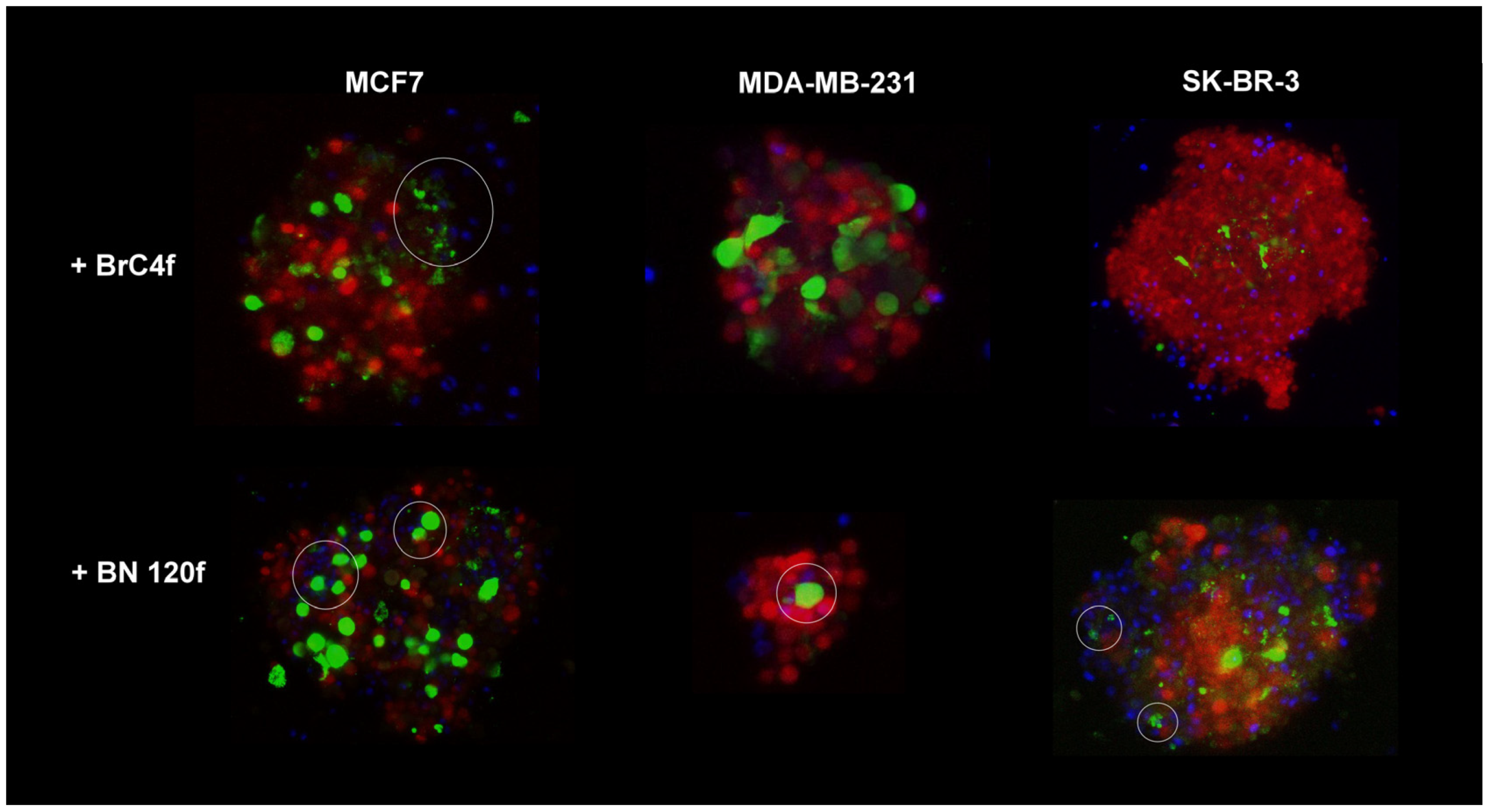
3.2. NK Cell Line Penetration in 3D Heterotypic Cellular BC Tumor Model
3.3. Cancer-Associated Fibroblasts Regulated MIC A/B, MUC1 and PD-L1 Expression on Tumor Cells in Spheroids
3.4. The Combination of Cytokines and NK Cells Increases Tumor Cell Killing
3.5. Evaluation of PB-NK Phenotype Changes Within a 3D Model upon Exposure to IL-15 or TGFβ1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D-models | two dimensions cellular culture |
| 3D-models | three dimensions cellular culture |
| 3D-2-models | three dimensions cellular culture from tumor and stromal cells |
| 3D-3-models | three dimensions cellular culture from tumor, stromal and immune cells |
| BC | breast cancer |
| BSA | bovine serum albumin |
| CAFs | cancer-associated fibroblasts |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| FBS | fetal bovine serum |
| FDA | fluorescein diacetate |
| FGF | fibroblast growth factor |
| GFP | Green fluorescent protein |
| IL-15 | interleukin 15 |
| MFI | mean fluorescent intensity |
| NK | natural killer |
| PB-NK | peripheral blood NK cells |
| PI | propidium iodide |
| TGFβ1 | transforming growth factor beta 1 |
| TME | tumor microenvironment |
References
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kong, D.; Liu, J.; Zhan, L.; Luo, L.; Zheng, W.; Zheng, Q.; Chen, C.; Sun, S. Breast Cancer Heterogeneity and Its Implication in Personalized Precision Therapy. Exp. Hematol. Oncol. 2023, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Tsydenova, I.A.; Dolgasheva, D.S.; Gaptulbarova, K.A.; Ibragimova, M.K.; Tsyganov, M.M.; Kravtsova, E.A.; Nushtaeva, A.A.; Litviakov, N.V. WNT-Conditioned Mechanism of Exit from Postchemotherapy Shock of Differentiated Tumour Cells. Cancers 2023, 15, 2765. [Google Scholar] [CrossRef] [PubMed]
- Razeghian, E.; Kameh, M.C.; Shafiee, S.; Khalafi, F.; Jafari, F.; Asghari, M.; Kazemi, K.; Ilkhani, S.; Shariatzadeh, S.; Haj-Mirzaian, A. The Role of the Natural Killer (NK) Cell Modulation in Breast Cancer Incidence and Progress. Mol. Biol. Rep. 2022, 49, 10935–10948. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Ben-Shmuel, A.; Gruper, Y.; Halperin, C.; Levi-Galibov, O.; Rosenberg-Fogler, H.; Barki, D.; Carradori, G.; Stein, Y.; Yagel, G.; Naumova, M.; et al. Cancer-Associated Fibroblasts Serve as Decoys to Suppress NK Cell Anti-Cancer Cytotoxicity in Breast Cancer. Cancer Discov. 2025, 15, 1247–1269. [Google Scholar] [CrossRef]
- Lobel, G.P.; Jiang, Y.; Simon, M.C. Tumor Microenvironmental Nutrients, Cellular Responses, and Cancer. Cell Chem. Biol. 2023, 30, 1015–1032. [Google Scholar] [CrossRef]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front. Immunol. 2017, 8, 760. [Google Scholar] [CrossRef]
- Levi, I.; Amsalem, H.; Nissan, A.; Darash-Yahana, M.; Peretz, T.; Mandelboim, O.; Rachmilewitz, J. Characterization of Tumor Infiltrating Natural Killer Cell Subset. Oncotarget 2015, 6, 13835–13843. [Google Scholar] [CrossRef] [PubMed]
- Mamessier, E.; Sylvain, A.; Thibult, M.-L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human Breast Cancer Cells Enhance Self Tolerance by Promoting Evasion from NK Cell Antitumor Immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Cavallo, F.; Defilippi, P.; Conti, L. The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front. Oncol. 2021, 11, 610303. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, K.; Redmond, W.L. Current Landscape and Future Prospects of Interleukin-2 Receptor (IL-2R) Agonists in Cancer Immunotherapy. Oncoimmunology 2025, 14, 2452654. [Google Scholar] [CrossRef] [PubMed]
- Berjis, A.; Muthumani, D.; Aguilar, O.A.; Pomp, O.; Johnson, O.; Finck, A.V.; Engel, N.W.; Chen, L.; Plachta, N.; Scholler, J.; et al. Pretreatment with IL-15 and IL-18 Rescues Natural Killer Cells from Granzyme B-Mediated Apoptosis after Cryopreservation. Nat. Commun. 2024, 15, 3937. [Google Scholar] [CrossRef]
- Ma, S.; Caligiuri, M.A.; Yu, J. Harnessing IL-15 Signaling to Potentiate NK Cell-Mediated Cancer Immunotherapy. Trends Immunol. 2022, 43, 833–847. [Google Scholar] [CrossRef]
- Gaggero, S.; Witt, K.; Carlsten, M.; Mitra, S. Cytokines Orchestrating the Natural Killer-Myeloid Cell Crosstalk in the Tumor Microenvironment: Implications for Natural Killer Cell-Based Cancer Immunotherapy. Front. Immunol. 2021, 11, 621225. [Google Scholar] [CrossRef]
- Chung, D.C.; Garcia-Batres, C.R.; Millar, D.G.; Wong, S.W.Y.; Elford, A.R.; Mathews, J.A.; Wang, B.X.; Nguyen, L.T.; Shaw, P.A.; Clarke, B.A.; et al. Generation of an Inhibitory NK Cell Subset by TGF-Β1/IL-15 Polarization. J. Immunol. 2024, 212, 1904–1912. [Google Scholar] [CrossRef]
- Witt, B.L.; Tollefsbol, T.O. Molecular, Cellular, and Technical Aspects of Breast Cancer Cell Lines as a Foundational Tool in Cancer Research. Life 2023, 13, 2311. [Google Scholar] [CrossRef]
- Petrosyan, V.; Dobrolecki, L.E.; LaPlante, E.L.; Srinivasan, R.R.; Bailey, M.H.; Welm, A.L.; Welm, B.E.; Lewis, M.T.; Milosavljevic, A. Immunologically “Cold” Triple Negative Breast Cancers Engraft at a Higher Rate in Patient Derived Xenografts. NPJ Breast Cancer 2022, 8, 104. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, S.; Yazdanparast, A.; Li, M.; Pawar, A.V.; Liu, Y.; Inavolu, S.M.; Cheng, L. Comprehensive Comparison of Molecular Portraits between Cell Lines and Tumors in Breast Cancer. BMC Genomics 2016, 17 (Suppl. 7), 525. [Google Scholar] [CrossRef]
- Rebelo, S.P.; Pinto, C.; Martins, T.R.; Harrer, N.; Estrada, M.F.; Loza-Alvarez, P.; Cabeçadas, J.; Alves, P.M.; Gualda, E.J.; Sommergruber, W.; et al. 3D-3-Culture: A Tool to Unveil Macrophage Plasticity in the Tumour Microenvironment. Biomaterials 2018, 163, 185–197. [Google Scholar] [CrossRef]
- Horvath, P.; Aulner, N.; Bickle, M.; Davies, A.M.; Nery, E.D.; Ebner, D.; Montoya, M.C.; Östling, P.; Pietiäinen, V.; Price, L.S.; et al. Screening out Irrelevant Cell-Based Models of Disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Cancer Drug Discovery: Recent Innovative Approaches to Tumor Modeling. Expert Opin. Drug Discov. 2016, 11, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Thongsin, N.; Wattanapanitch, M. A Three-Dimensional Immune-Oncology Model for Studying In Vitro Primary Human NK Cell Cytotoxic Activity. PLoS ONE 2022, 17, e0264366. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension: How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Troitskaya, O.; Novak, D.; Nushtaeva, A.; Savinkova, M.; Varlamov, M.; Ermakov, M.; Richter, V.; Koval, O. EGFR Transgene Stimulates Spontaneous Formation of MCF7 Breast Cancer Cells Spheroids with Partly Loss of HER3 Receptor. Int. J. Mol. Sci. 2021, 22, 12937. [Google Scholar] [CrossRef]
- Santo, V.E.; Estrada, M.F.; Rebelo, S.P.; Abreu, S.; Silva, I.; Pinto, C.; Veloso, S.C.; Serra, A.T.; Boghaert, E.; Alves, P.M.; et al. Adaptable Stirred-Tank Culture Strategies for Large Scale Production of Multicellular Spheroid-Based Tumor Cell Models. J. Biotechnol. 2016, 221, 118–129. [Google Scholar] [CrossRef]
- Nushtaeva, A.; Savinkova, M.; Ermakov, M.; Varlamov, M.; Novak, D.; Richter, V.; Koval, O. Breast Cancer Cells in 3D Model Alters Their Sensitivity to Hormonal and Growth Factors. Cell Tissue Biol. 2022, 16, 555–567. [Google Scholar] [CrossRef]
- Arez, F.; Preiss, L.; Gal, I.R.; Rebelo, S.P.; Badolo, L.; Brito, C.; Spangenberg, T.; Alves, P.M. Heterotypic Spheroids as a Strategy for 3D Culture of Cryopreserved Primary Human Hepatocytes in Stirred-Tank Systems. SLAS Discov. 2025, 31, 100210. [Google Scholar] [CrossRef]
- Herter, S.; Morra, L.; Schlenker, R.; Sulcova, J.; Fahrni, L.; Waldhauer, I.; Lehmann, S.; Reisländer, T.; Agarkova, I.; Kelm, J.M.; et al. A Novel Three-Dimensional Heterotypic Spheroid Model for the Assessment of the Activity of Cancer Immunotherapy Agents. Cancer Immunol. Immunother. 2017, 66, 129–140. [Google Scholar] [CrossRef]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-Cell Therapy in the Era of Solid Tumor Treatment: Current Challenges and Emerging Therapeutic Advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef]
- Franchi-Mendes, T.; Lopes, N.; Brito, C. Heterotypic Tumor Spheroids in Agitation-Based Cultures: A Scaffold-Free Cell Model That Sustains Long-Term Survival of Endothelial Cells. Front. Bioeng. Biotechnol. 2021, 9, 649949. [Google Scholar] [CrossRef] [PubMed]
- Nushtaeva, A.A.; Karpushina, A.A.; Ermakov, M.S.; Gulyaeva, L.F.; Gerasimov, A.V.; Sidorov, S.V.; Gayner, T.A.; Yunusova, A.Y.; Tkachenko, A.V.; Richter, V.A.; et al. Establishment of Primary Human Breast Cancer Cell Lines Using “Pulsed Hypoxia” Method and Development of Metastatic Tumor Model in Immunodeficient Mice. Cancer Cell Int. 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Nushtaeva, A.; Ermakov, M.; Abdurakhmanova, M.; Troitskaya, O.; Belovezhets, T.; Varlamov, M.; Gayner, T.; Richter, V.; Koval, O. “Pulsed Hypoxia” Gradually Reprograms Breast Cancer Fibroblasts into Pro-Tumorigenic Cells via Mesenchymal-Epithelial Transition. Int. J. Mol. Sci. 2023, 24, 2494. [Google Scholar] [CrossRef] [PubMed]
- Jiajia, L.; Shinghung, M.; Jiacheng, Z.; Jialing, W.; Dilin, X.; Shengquan, H.; Zaijun, Z.; Qinwen, W.; Yifan, H.; Wei, C. Assessment of Neuronal Viability Using Fluorescein Diacetate-Propidium Iodide Double Staining in Cerebellar Granule Neuron Culture. J. Vis. Exp. 2017, 55442. [Google Scholar] [CrossRef]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of In Vitro 3D Cell Model Developed from Human Hepatocellular Carcinoma (HepG2) Cell Line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef]
- Boyd, V.; Cholewa, O.M.; Papas, K.K. Limitations in the Use of Fluorescein Diacetate/Propidium Iodide (FDA/PI) and Cell Permeable Nucleic Acid Stains for Viability Measurements of Isolated Islets of Langerhans. Curr. Trends Biotechnol. Pharm. 2008, 2, 66–84. [Google Scholar]
- Nushtaeva, A.A.; Stepanov, G.A.; Semenov, D.V.; Juravlev, E.S.; Balahonova, E.A.; Gerasimov, A.V.; Sidorov, S.V.; Savelyev, E.I.; Kuligina, E.V.; Richter, V.A.; et al. Characterization of Primary Normal and Malignant Breast Cancer Cell and Their Response to Chemotherapy and Immunostimulatory Agents. BMC Cancer 2018, 18, 728. [Google Scholar] [CrossRef]
- Abdurakhmanova, M.; Ermakov, M.; Richter, V.; Koval, O.; Nushtaeva, A. The optimization of methods for the establishment of heterogeneous three-dimensional cellular models of breast cancer. Genes Cells 2022, 17, 91–103. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, W.; Tian, Z. NK Cell-Based Immunotherapy for Cancer. Semin. Immunol. 2017, 31, 37–54. [Google Scholar] [CrossRef]
- Zhu, S.; Yin, J.; Lu, X.; Jiang, D.; Chen, R.; Cui, K.; He, W.; Huang, N.; Xu, G. Influence of Experimental Variables on Spheroid Attributes. Sci. Rep. 2025, 15, 9751. [Google Scholar] [CrossRef]
- Tarazona, R.; Borrego, F.; Galiani, M.D.; Aguado, E.; Peña, J.; Coligan, J.E.; Solana, R. Inhibition of CD28-Mediated Natural Cytotoxicity by KIR2DL2 Does Not Require P56(Lck) in the NK Cell Line YT-Indy. Mol. Immunol. 2002, 38, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, A.; Weil, S.; Kloess, S.; Ansari, N.; Stelzer, E.H.K.; Cerwenka, A.; Steinle, A.; Koehl, U.; Koch, J. Cytotoxicity and Infiltration of Human NK Cells in In Vivo-like Tumor Spheroids. BMC Cancer 2015, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Yamashita, N.; Daimon, T.; Hirose, H.; Yamano, S.; Haratake, N.; Ishikawa, S.; Bhattacharya, A.; Fushimi, A.; Ahmad, R.; et al. MUC1-C Is a Master Regulator of MICA/B NKG2D Ligand and Exosome Secretion in Human Cancer Cells. J. Immunother. Cancer 2023, 11, e006238. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Rajabi, H.; Ahmad, R.; Jin, C.; Kufe, D. Targeting the MUC1-C Oncoprotein Inhibits Self-Renewal Capacity of Breast Cancer Cells. Oncotarget 2014, 5, 2622–2634. [Google Scholar] [CrossRef]
- Ziani, L.; Safta-Saadoun, T.B.; Gourbeix, J.; Cavalcanti, A.; Robert, C.; Favre, G.; Chouaib, S.; Thiery, J. Melanoma-Associated Fibroblasts Decrease Tumor Cell Susceptibility to NK Cell-Mediated Killing through Matrix-Metalloproteinases Secretion. Oncotarget 2017, 8, 19780–19794. [Google Scholar] [CrossRef]
- Oliviero, B.; Varchetta, S.; Mele, D.; Pessino, G.; Maiello, R.; Falleni, M.; Tosi, D.; Donadon, M.; Soldani, C.; Franceschini, B.; et al. MICA/B-Targeted Antibody Promotes NK Cell-Driven Tumor Immunity in Patients with Intrahepatic Cholangiocarcinoma. Oncoimmunology 2022, 11, 2035919. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef]
- Park, S.; Colville, M.J.; Paek, J.H.; Shurer, C.R.; Singh, A.; Secor, E.J.; Sailer, C.J.; Huang, L.-T.; Kuo, J.C.-H.; Goudge, M.C.; et al. Immunoengineering Can Overcome the Glycocalyx Armour of Cancer Cells. Nat. Mater. 2024, 23, 429–438. [Google Scholar] [CrossRef]
- McGuinness, C.; Britt, K.L. Estrogen Receptor Regulation of the Immune Microenvironment in Breast Cancer. J. Steroid Biochem. Mol. Biol. 2024, 240, 106517. [Google Scholar] [CrossRef]
- De Santis, P.; Perrone, M.; Guarini, C.; Santoro, A.N.; Laface, C.; Carrozzo, D.; Oliva, G.R.; Fedele, P. Early-Stage Triple Negative Breast Cancer: The Therapeutic Role of Immunotherapy and the Prognostic Value of Pathological Complete Response. Explor. Target. Anti-Tumor Ther. 2024, 5, 232–250. [Google Scholar] [CrossRef]
- Domingues, M.; Leite Pereira, C.; Sarmento, B.; Castro, F. Mimicking 3D Breast Tumor-Stromal Interactions to Screen Novel Cancer Therapeutics. Eur. J. Pharm. Sci. 2023, 190, 106560. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Francois, A.; Benoit, A.; Merlin, J.-L.; Bezdetnaya, L.; Vogin, G. Advanced Co-Culture 3D Breast Cancer Model for Investigation of Fibrosis Induced by External Stimuli: Optimization Study. Sci. Rep. 2020, 10, 21273. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-Z.; Chang, H.-Y. Recent Advances in Three-Dimensional Multicellular Spheroid Culture for Biomedical Research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Keely, P.J. Why the Stroma Matters in Breast Cancer: Insights into Breast Cancer Patient Outcomes through the Examination of Stromal Biomarkers. Cell Adh. Migr. 2012, 6, 249–260. [Google Scholar] [CrossRef]
- Nielsen, B.S.; Madsen, N.H.; Larsen, J.; Skandorff, I.; Gad, M.; Holmstrøm, K. Architectural Organization and Molecular Profiling of 3D Cancer Heterospheroids and Their Application in Drug Testing. Front. Oncol. 2024, 14, 1386097. [Google Scholar] [CrossRef]
- Rafaeva, M.; Jensen, A.R.D.; Horton, E.R.; Zornhagen, K.W.; Strøbech, J.E.; Fleischhauer, L.; Mayorca-Guiliani, A.E.; Nielsen, S.R.; Grønseth, D.S.; Kuś, F.; et al. Fibroblast-Derived Matrix Models Desmoplastic Properties and Forms a Prognostic Signature in Cancer Progression. Front. Immunol. 2023, 14, 1154528. [Google Scholar] [CrossRef]
- Baker, S.G.; Sauter, E.R. The Role of the Extracellular Matrix in Cancer Prevention. Cancers 2025, 17, 1491. [Google Scholar] [CrossRef]
- Maffini, M.V.; Soto, A.M.; Calabro, J.M.; Ucci, A.A.; Sonnenschein, C. The Stroma as a Crucial Target in Rat Mammary Gland Carcinogenesis. J. Cell Sci. 2004, 117, 1495–1502. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Liu, H.; Wang, Z.; Jia, W. Characterization of tumor-associated reactive astrocytes in gliomas by single-cell and bulk tumor sequencing. Front. Neurol. 2023, 14, 1193844. [Google Scholar] [CrossRef]
- Tong, L.; Jiménez-Cortegana, C.; Tay, A.H.M.; Wickström, S.; Galluzzi, L.; Lundqvist, A. NK Cells and Solid Tumors: Therapeutic Potential and Persisting Obstacles. Mol. Cancer 2022, 21, 206. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Truttschel, R.; Gong, M.M.; Humayun, M.; Virumbrales-Munoz, M.; Vitek, R.; Felder, M.; Gillies, S.D.; Sondel, P.; Wisinski, K.B.; et al. Evaluating Natural Killer Cell Cytotoxicity against Solid Tumors Using a Microfluidic Model. Oncoimmunology 2019, 8, 1553477. [Google Scholar] [CrossRef] [PubMed]
- Rozanov, D.V.; Rozanov, N.D.; Chiotti, K.E.; Reddy, A.; Wilmarth, P.A.; David, L.L.; Cha, S.W.; Woo, S.; Pevzner, P.; Bafna, V.; et al. MHC Class I Loaded Ligands from Breast Cancer Cell Lines: A Potential HLA-I-Typed Antigen Collection. J. Proteom. 2018, 176, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ferrari de Andrade, L.; Kumar, S.; Luoma, A.M.; Ito, Y.; Alves da Silva, P.H.; Pan, D.; Pyrdol, J.W.; Yoon, C.H.; Wucherpfennig, K.W. Inhibition of MICA and MICB Shedding Elicits NK-Cell-Mediated Immunity against Tumors Resistant to Cytotoxic T Cells. Cancer Immunol. Res. 2020, 8, 769–780. [Google Scholar] [CrossRef]
- Boneva, E.; Shivarov, V.; Ivanova, M. A Concise Review of the Role of the NKG2D Receptor and Its Ligands in Cancer. Immuno 2025, 5, 9. [Google Scholar] [CrossRef]
- Dhandapani, H.; Siddiqui, A.; Karadkar, S.; Tayalia, P. In Vitro 3D Spheroid Model Preserves Tumor Microenvironment of Hot and Cold Breast Cancer Subtypes. Adv. Healthc. Mater. 2023, 12, e2300164. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between Cancer-Associated Fibroblasts and Immune Cells in the Tumor Microenvironment: New Findings and Future Perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Chen, X.; Sandrine, I.K.; Yang, M.; Tu, J.; Yuan, X. MUC1 and MUC16: Critical for Immune Modulation in Cancer Therapeutics. Front. Immunol. 2024, 15, 1356913. [Google Scholar] [CrossRef]
- Chan, A.; Hong, D.-L.; Atzberger, A.; Kollnberger, S.; Filer, A.D.; Buckley, C.D.; McMichael, A.; Enver, T.; Bowness, P. CD56bright Human NK Cells Differentiate into CD56dim Cells: Role of Contact with Peripheral Fibroblasts. J. Immunol. 2007, 179, 89–94. [Google Scholar] [CrossRef]
- Guo, X.; Sunil, C.; Adeyanju, O.; Parker, A.; Huang, S.; Ikebe, M.; Tucker, T.A.; Idell, S.; Qian, G. PD-L1 Mediates Lung Fibroblast to Myofibroblast Transition through Smad3 and β-Catenin Signaling Pathways. Sci. Rep. 2022, 12, 3053. [Google Scholar] [CrossRef]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural Killer Cell Homing and Trafficking in Tissues and Tumors: From Biology to Application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef]
- Jia, H.; Yang, H.; Xiong, H.; Luo, K.Q. NK Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2023, 14, 1303605. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, M.; Steinle, A. Impairment of NKG2D-Mediated Tumor Immunity by TGF-β. Front. Immunol. 2019, 10, 2689. [Google Scholar] [CrossRef] [PubMed]
- Foltz, J.A.; Moseman, J.E.; Thakkar, A.; Chakravarti, N.; Lee, D.A. TGFβ Imprinting During Activation Promotes Natural Killer Cell Cytokine Hypersecretion. Cancers 2018, 10, 423. [Google Scholar] [CrossRef]
- Fionda, C.; Stabile, H.; Cerboni, C.; Soriani, A.; Gismondi, A.; Cippitelli, M.; Santoni, A. Hitting More Birds with a Stone: Impact of TGF-β on ILC Activity in Cancer. J. Clin. Med. 2020, 9, 143. [Google Scholar] [CrossRef]
- Hawke, L.G.; Mitchell, B.Z.; Ormiston, M.L. TGF-β and IL-15 Synergize through MAPK Pathways to Drive the Conversion of Human NK Cells to an Innate Lymphoid Cell 1-like Phenotype. J. Immunol. 2020, 204, 3171–3181. [Google Scholar] [CrossRef]
- Xu, J.; Gao, H.; Azhar, M.S.; Xu, H.; Chen, S.; Li, M.; Ni, X.; Yan, T.; Zhou, H.; Long, Q.; et al. Interleukin Signaling in the Regulation of Natural Killer Cells Biology in Breast Cancer. Front. Immunol. 2024, 15, 1449441. [Google Scholar] [CrossRef]
- Skak, K.; Frederiksen, K.S.; Lundsgaard, D. Interleukin-21 Activates Human Natural Killer Cells and Modulates Their Surface Receptor Expression. Immunology 2008, 123, 575–583. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Jounaidi, Y. Comprehensive Snapshots of Natural Killer Cells Functions, Signaling, Molecular Mechanisms and Clinical Utilization. Signal Transduct. Target. Ther. 2024, 9, 302. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Cuff, A.O.; Sillito, F.; Dertschnig, S.; Hall, A.; Luong, T.V.; Chakraverty, R.; Male, V. The Obese Liver Environment Mediates Conversion of NK Cells to a Less Cytotoxic ILC1-Like Phenotype. Front. Immunol. 2019, 10, 2180. [Google Scholar] [CrossRef]
- Moreno-Nieves, U.Y.; Tay, J.K.; Saumyaa, S.; Horowitz, N.B.; Shin, J.H.; Mohammad, I.A.; Luca, B.; Mundy, D.C.; Gulati, G.S.; Bedi, N.; et al. Landscape of Innate Lymphoid Cells in Human Head and Neck Cancer Reveals Divergent NK Cell States in the Tumor Microenvironment. Proc. Natl. Acad. Sci. USA 2021, 118, e2101169118. [Google Scholar] [CrossRef] [PubMed]
- Moretta, L. Dissecting CD56dim Human NK Cells. Blood 2010, 116, 3689–3691. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.; Xiao, F.; Pitt, M.; Gleason, M.; McCullar, V.; Bergemann, T.L.; McQueen, K.L.; Guethlein, L.A.; Parham, P.; Miller, J.S. A Subpopulation of Human Peripheral Blood NK Cells That Lacks Inhibitory Receptors for Self-MHC Is Developmentally Immature. Blood 2007, 110, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Moura, T.; Caramelo, O.; Silva, I.; Silva, S.; Gonçalo, M.; Portilha, M.A.; Moreira, J.N.; Gil, A.M.; Laranjeira, P.; Paiva, A. Early-Stage Luminal B-like Breast Cancer Exhibits a More Immunosuppressive Tumor Microenvironment than Luminal A-like Breast Cancer. Biomolecules 2025, 15, 78. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, F.; Lu, X.; Wang, S.; Wu, M.; Chen, N.; Fan, S.; Wei, W. Peripheral NK Cell Count Predicts Response and Prognosis in Breast Cancer Patients Underwent Neoadjuvant Chemotherapy. Front. Immunol. 2024, 15, 1437193. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonteva, A.; Abdurakhmanova, M.; Bogachek, M.; Belovezhets, T.; Yurina, A.; Troitskaya, O.; Kulemzin, S.; Richter, V.; Kuligina, E.; Nushtaeva, A. The Activity of Human NK Cells Towards 3D Heterotypic Cellular Tumor Model of Breast Cancer. Cells 2025, 14, 1039. https://doi.org/10.3390/cells14141039
Leonteva A, Abdurakhmanova M, Bogachek M, Belovezhets T, Yurina A, Troitskaya O, Kulemzin S, Richter V, Kuligina E, Nushtaeva A. The Activity of Human NK Cells Towards 3D Heterotypic Cellular Tumor Model of Breast Cancer. Cells. 2025; 14(14):1039. https://doi.org/10.3390/cells14141039
Chicago/Turabian StyleLeonteva, Anastasia, Maria Abdurakhmanova, Maria Bogachek, Tatyana Belovezhets, Anna Yurina, Olga Troitskaya, Sergey Kulemzin, Vladimir Richter, Elena Kuligina, and Anna Nushtaeva. 2025. "The Activity of Human NK Cells Towards 3D Heterotypic Cellular Tumor Model of Breast Cancer" Cells 14, no. 14: 1039. https://doi.org/10.3390/cells14141039
APA StyleLeonteva, A., Abdurakhmanova, M., Bogachek, M., Belovezhets, T., Yurina, A., Troitskaya, O., Kulemzin, S., Richter, V., Kuligina, E., & Nushtaeva, A. (2025). The Activity of Human NK Cells Towards 3D Heterotypic Cellular Tumor Model of Breast Cancer. Cells, 14(14), 1039. https://doi.org/10.3390/cells14141039








