High-Risk Genetic Multiple Myeloma: From Molecular Classification to Innovative Treatment with Monoclonal Antibodies and T-Cell Redirecting Therapies
Abstract
1. Introduction
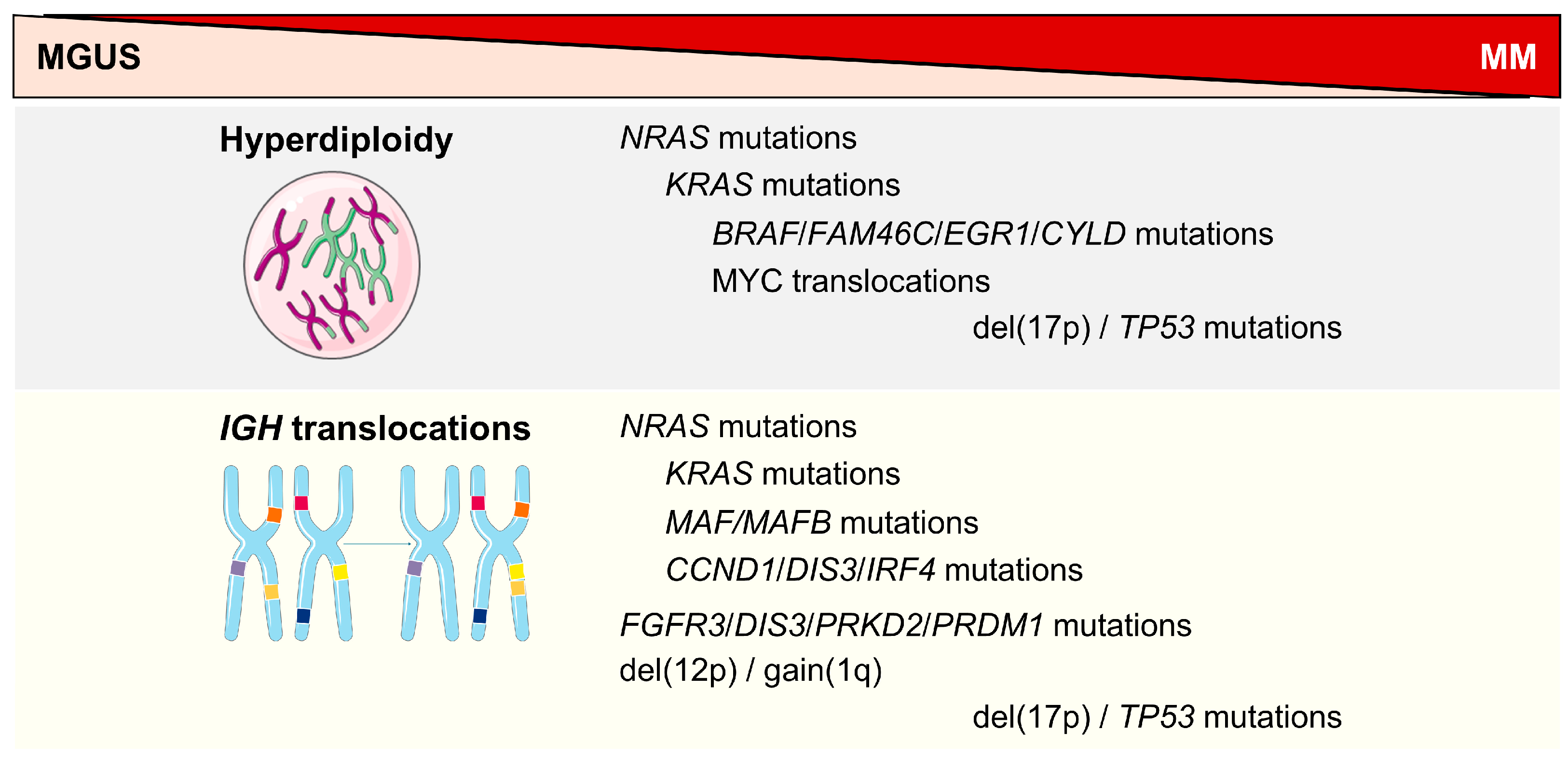
2. High-Risk Genetic Abnormalities
3. Evolution of High-Risk Genetic Classification
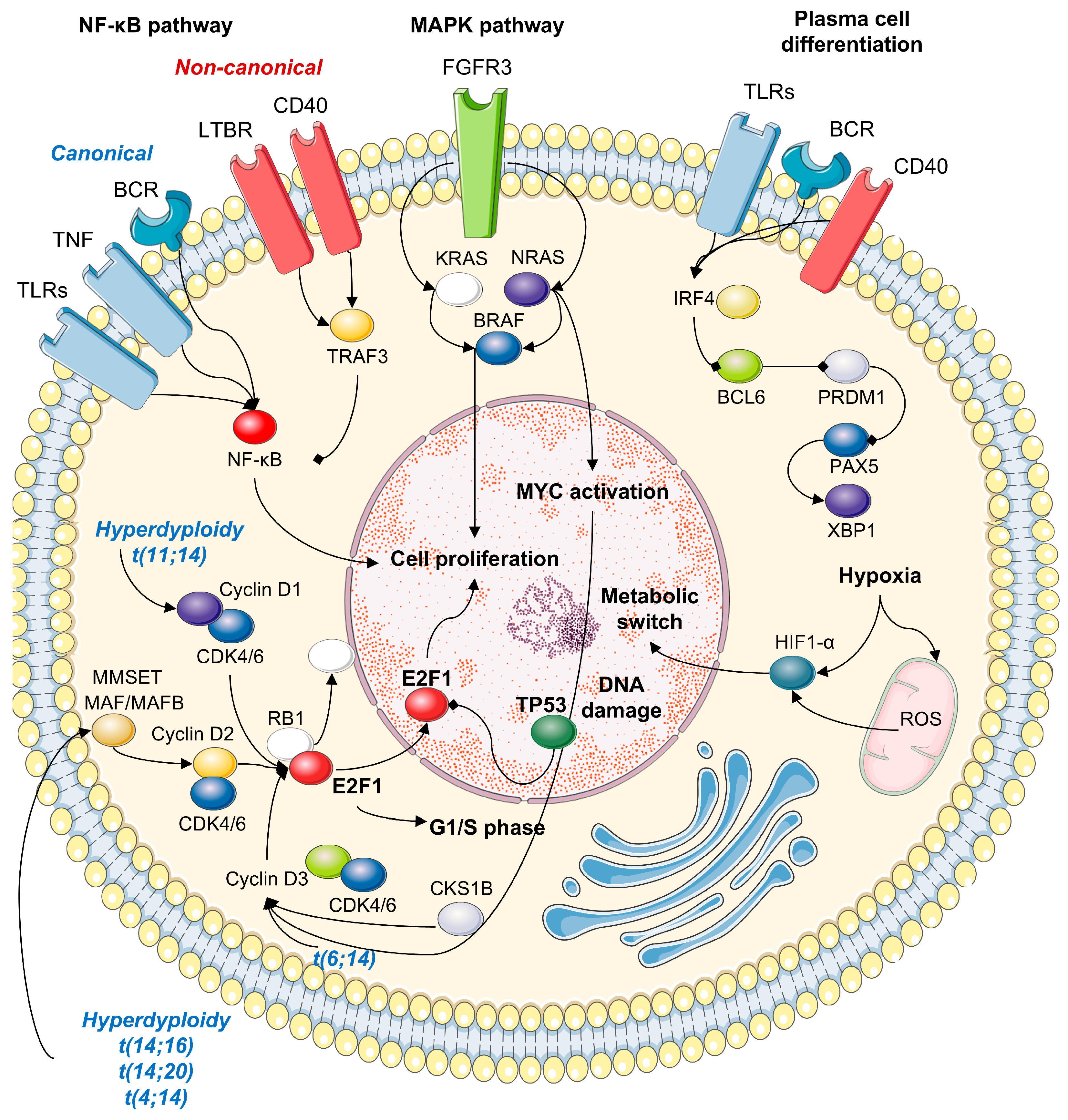
4. Molecular Mechanisms in HRMM
4.1. IGH Translocations
4.2. Methylation
4.3. MYC Translocations
4.4. Chromosome 1 Abnormalities
5. First-Line Treatments for Transplant-Eligible Patients
6. First-Line Treatment for Transplant-Ineligible Patients
7. High-Risk Genetic Relapsed/Refractory MM
8. T-Cell Redirecting Therapy for Relapsed/Refractory MM
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| MGUS | Monoclonal gammopathy of undetermined significance |
| HRMM | High-risk genetic multiple myeloma |
| FHRMM | Functional high-risk multiple myeloma |
| OS | Overall survival |
| FISH | Fluorescence in situ hybridization |
| CA | Chromosomal abnormalities |
| abn | Abnormalities |
| Sβ2M | Serum β2-microglobulin |
| IGH | Immunoglobulin heavy chain |
| FGFR3 | Fibroblast growth factor receptor 3 |
| MMSET | Multiple myeloma SET domain containing protein |
| CKS1B | Cyclin-dependent kinases regulatory subunit 1 |
| MCL1 | Myeloid cell leukemia-1 |
| ADAR1 | Adenosine deaminase acting on RNA |
| FAF1 | Fas associated factor 1 |
| CDKN | Cyclin dependent kinase inhibitor |
| ISS | International Staging System |
| R-ISS | Revised- International Staging System |
| IMWG | International Myeloma Working Group |
| EMMA | European Multiple Myeloma Academy |
| FAM46C | Family with sequence similarity 46 member C |
| EGR1 | Early growth response 1 |
| CYLD | Lysine 63 deubiquitinase |
| CCN | Cyclin |
| DIS3 | Exosome complex exonuclease RRP44 |
| IRF4 | Interferon regulatory factor 4 |
| PRKD2 | Serine/threonine-protein kinase D2 |
| PRDM1 | PR domain zinc finger protein 1 |
| CDK | Cyclin-dependent kinase |
| RB | Retinoblastoma protein |
| H3K36me2 | Histone H3 lysine 36 dimethylation |
| HDAC6 | Histone deacetylase 6 |
| HSF1 | Heat shock factor 1 |
| HSP90 | Heat shock protein 90 |
| EZH2 | Enhancer of zeste homologue 2 |
| IDH1 | Isocitrate dehydrogenase 1 |
| ANP32E | Acidic nuclear phosphoprotein 32 family member E |
| ILF2 | Interleukin enhancer binding factor 2 |
| KDM4A | Histone lysine demethylase 4A |
| HIF1α | Hypoxia-inducible factor 1α |
| RLP5 | Ribosomal protein L5 |
| NF-κB | Nuclear factor κB |
| MAPK | Mitogen-activated protein kinase |
| ASCT | Autologous stem cell transplantation |
| EHA | European Hematology Association |
| ESMO | European Society for Medical Oncology |
| MRD | Minimal residual disease |
| VRD | Bortezomib, lenalidomide and dexamethasone |
| Dara-VTD | Daratumumab-bortezomib-thalidomide-dexamethasone |
| FDA | Food and Drug Administration |
| EMA | European Medicine Agency |
| CR | Complete remission |
| IFM | Intergroupe Francophone du Myélome |
| Dara-KRd | Daratumumab, carfilzomib, lenalidomide, and dexamethasone |
| sCR | Stringent CR |
| ORR | Overall response rate |
| PFS | Progression-free survival |
| IMiDs | Immunomodulatory drugs |
| VMP | Bortezomib-melphalan-dexamethasone |
| RD | Bortezomib-dexamethasone |
| PD | Pomalidomide-dexamethasone |
| BCMA | B-cell maturation antigen |
| BiTEs | Bispecific antibodies |
| CAR | Chimeric antigen receptor |
| GPRC5D | G protein-coupled receptor class C group 5 member D |
References
- De Novellis, D.; Fontana, R.; Carobene, A.; Serio, B.; Ferrara, I.; Martorelli, M.C.; Mettivier, L.; Guariglia, R.; Luponio, S.; Ruggiero, I.; et al. Serum Free Light-Chain Ratio at Diagnosis Is Associated with Early Renal Damage in Multiple Myeloma: A Case Series Real-World Study. Biomedicines 2022, 10, 1657. [Google Scholar] [CrossRef]
- Costa, L.J.; Brill, I.K.; Omel, J.; Godby, K.; Kumar, S.K.; Brown, E.E. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017, 1, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.-J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- du Pont, S.R.; Cleynen, A.; Fontan, C.; Attal, M.; Munshi, N.; Corre, J.; Avet-Loiseau, H. Genomics of Multiple Myeloma. J. Clin. Oncol. 2017, 35, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Chretien, M.-L.; Corre, J.; Lauwers-Cances, V.; Magrangeas, F.; Cleynen, A.; Yon, E.; Hulin, C.; Leleu, X.; Orsini-Piocelle, F.; Blade, J.-S.; et al. Understanding the role of hyperdiploidy in myeloma prognosis: Which trisomies really matter? Blood 2015, 126, 2713–2719. [Google Scholar] [CrossRef]
- Fonseca, R.; Blood, E.; Rue, M.; Harrington, D.; Oken, M.M.; Kyle, R.A.; Dewald, G.W.; Van Ness, B.; Van Wier, S.A.; Henderson, K.J.; et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 2003, 101, 4569–4575. [Google Scholar] [CrossRef]
- Zamagni, E.; Barbato, S.; Cavo, M. How I treat high-risk multiple myeloma. Blood 2022, 139, 2889–2903. [Google Scholar] [CrossRef]
- Hanamura, I.; Stewart, J.P.; Huang, Y.; Zhan, F.; Santra, M.; Sawyer, J.R.; Hollmig, K.; Zangarri, M.; Pineda-Roman, M.; van Rhee, F.; et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: Incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood 2006, 108, 1724–1732. [Google Scholar] [CrossRef]
- Locher, M.; Steurer, M.; Jukic, E.; Keller, M.A.; Fresser, F.; Ruepp, C.; Wöll, E.; Verdorfer, I.; Gastl, G.; Willenbacher, W.; et al. The prognostic value of additional copies of 1q21 in multiple myeloma depends on the primary genetic event. Am. J. Hematol. 2020, 95, 1562–1571. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Fonseca, R.; Usmani, S.Z. Chromosome 1q21 abnormalities in multiple myeloma. Blood Cancer J. 2021, 11, 83. [Google Scholar] [CrossRef]
- Hebraud, B.; Leleu, X.; Lauwers-Cances, V.; Roussel, M.; Caillot, D.; Marit, G.; Karlin, L.; Hulin, C.; Gentil, C.; Guilhot, F.; et al. Deletion of the 1p32 region is a major independent prognostic factor in young patients with myeloma: The IFM experience on 1195 patients. Leukemia 2013, 28, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Schavgoulidze, A.; Talbot, A.; Perrot, A.; Cazaubiel, T.; Leleu, X.; Manier, S.; Buisson, L.; Mahéo, S.; Ferreira, L.D.S.; Pavageau, L.; et al. Biallelic deletion of 1p32 defines ultra-high-risk myeloma, but monoallelic del(1p32) remains a strong prognostic factor. Blood 2023, 141, 1308–1315. [Google Scholar] [CrossRef]
- Corre, J.; Perrot, A.; Caillot, D.; Belhadj, K.; Hulin, C.; Leleu, X.; Mohty, M.; Facon, T.; Buisson, L.; Souto, L.D.; et al. del(17p) without TP53 mutation confers a poor prognosis in intensively treated newly diagnosed patients with multiple myeloma. Blood 2021, 137, 1192–1195. [Google Scholar] [CrossRef]
- Thakurta, A.; Ortiz, M.; Blecua, P.; Towfic, F.; Corre, J.; Serbina, N.V.; Flynt, E.; Yu, Z.; Yang, Z.; Palumbo, A.; et al. High subclonal fraction of 17p deletion is associated with poor prognosis in multiple myeloma. Blood 2019, 133, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Lauwers-Cances, V.; Tournay, E.; Hulin, C.; Chretien, M.-L.; Royer, B.; Dib, M.; Decaux, O.; Jaccard, A.; Belhadj, K.; et al. Development and Validation of a Cytogenetic Prognostic Index Predicting Survival in Multiple Myeloma. J. Clin. Oncol. 2019, 37, 1657–1665. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Chiu, I.T.; Wang, Q.; Bustamante, J.; Jiang, W.; Rycaj, K.; Yi, S.S.; Li, J.H.; Kowalski-Muegge, J.; Matsui, W. Loss of p53 enhances the tumor-initiating potential and drug resistance of clonogenic multiple myeloma cells. Blood Adv. 2023, 7, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Available online: https://emma-vienna.org/sites/default/files/EMMA-2025_Booklet-web.pdf (accessed on 26 April 2025).
- Pawlyn, C.; Morgan, G.J. Evolutionary biology of high-risk multiple myeloma. Nat. Rev. Cancer 2017, 17, 543–556. [Google Scholar] [CrossRef]
- Liu, E.; Sudha, P.; Becker, N.; Jaouadi, O.; Suvannasankha, A.; Lee, K.; Abonour, R.; Abu Zaid, M.; Walker, B.A. Identifying novel mechanisms of biallelic TP53 loss refines poor outcome for patients with multiple myeloma. Blood Cancer J. 2023, 13, 144. [Google Scholar] [CrossRef]
- D’Agostino, M.; Martello, M.; De Paoli, L.; Mangiacavalli, S.; Derudas, D.; Fazio, F.; Furlan, A.; Liberatore, C.; Mele, G.; Mina, R.; et al. Overview of 1q abnormalities in multiple myeloma: Scientific opinions from Italian experts. Ann. Hematol. 2025, 104, 1443–1458. [Google Scholar] [CrossRef]
- Kalff, A.; Spencer, A. The t(4;14) translocation and FGFR3 overexpression in multiple myeloma: Prognostic implications and current clinical strategies. Blood Cancer J. 2012, 2, e89. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yano, S. Treatment Strategy for Ultra-High-Risk Multiple Myelomas with Chromosomal Aberrations Considering Minimal Residual Disease Status and Bone Marrow Microenvironment. Cancers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Giudice, V.; Ianniello, M.; De Novellis, D.; Pezzullo, L.; Petrillo, N.; Serio, B.; D’addona, M.; Della Corte, A.M.; Rizzo, M.; Cuffa, B.; et al. Non-invasive prenatal test identifies circulating cell-free DNA chromosomal abnormalities derived from clonal hematopoiesis in aggressive hematological malignancies. Clin. Exp. Med. 2024, 24, 69. [Google Scholar] [CrossRef] [PubMed]
- Chng, W.; Glebov, O.; Bergsagel, P.; Kuehl, W. Genetic events in the pathogenesis of multiple myeloma. Best. Pract. Res. Clin. Haematol. 2007, 20, 571–596. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.J.; Kumar, S. High-risk multiple myeloma: Redefining genetic, clinical, and functional high-risk disease in the era of molecular medicine and immunotherapy. Am. J. Hematol. 2024, 99, 1560–1575. [Google Scholar] [CrossRef]
- Solimando, A.G.; Da Vià, M.C.; Cicco, S.; Leone, P.; Di Lernia, G.; Giannico, D.; DeSantis, V.; Frassanito, M.A.; Morizio, A.; Delgado Tascon, J.; et al. High-Risk Multiple Myeloma: Integrated Clinical and Omics Approach Dissects the Neoplastic Clone and the Tumor Microenvironment. J. Clin. Med. 2019, 8, 997. [Google Scholar] [CrossRef]
- Abdi, J.; Chen, G.; Chang, H. Drug resistance in multiple myeloma: Latest findings and new concepts on molecular mechanisms. Oncotarget 2013, 4, 2186–2207. [Google Scholar] [CrossRef] [PubMed]
- Awada, H.; Thapa, B.; Awada, H.; Dong, J.; Gurnari, C.; Hari, P.; Dhakal, B. A Comprehensive Review of the Genomics of Multiple Myeloma: Evolutionary Trajectories, Gene Expression Profiling, and Emerging Therapeutics. Cells 2021, 10, 1961. [Google Scholar] [CrossRef]
- Nagy, Z.; Kajtár, B.; Jáksó, P.; Dávid, M.; Kosztolányi, S.; Hermesz, J.; Kereskai, L.; Pajor, L.; Alpár, D. Evolutionary sequence of cytogenetic aberrations during the oncogenesis of plasma cell disorders. Direct evidence at single cell level. Leuk. Res. 2011, 35, 1114–1116. [Google Scholar] [CrossRef]
- Manier, S.; Salem, K.Z.; Park, J.; Landau, D.A.; Getz, G.; Ghobrial, I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 100–113. [Google Scholar] [CrossRef]
- Lauring, J.; Abukhdeir, A.M.; Konishi, H.; Garay, J.P.; Gustin, J.P.; Wang, Q.; Arceci, R.J.; Matsui, W.; Park, B.H. The multiple myeloma–associated MMSET gene contributes to cellular adhesion, clonogenic growth, and tumorigenicity. Blood 2008, 111, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, E.; Minato, Y.; Maruki, H.; Asagiri, M.; Imoto, M. Regulation of FGF receptor-2 expression by transcription factor E2F-1. Oncogene 2003, 22, 5630–5635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Ahn, J.H.; Wang, G.G. Understanding histone H3 lysine 36 methylation and its deregulation in disease. Cell. Mol. Life Sci. 2019, 76, 2899–2916. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, E.; Lui, H.; Maes, K.; De Veirman, K.; Menu, E.; Vanderkerken, K.; De Bruyne, E. The Epigenome in Multiple Myeloma: Impact on Tumor Cell Plasticity and Drug Response. Front. Oncol. 2018, 8, 566. [Google Scholar] [CrossRef]
- Brito, J.L.; Walker, B.; Jenner, M.; Dickens, N.J.; Brown, N.J.; Ross, F.M.; Avramidou, A.; Irving, J.A.; Gonzalez, D.; Davies, F.E.; et al. MMSET deregulation affects cell cycle progression and adhesion regulons in t(4;14) myeloma plasma cells. Haematologica 2009, 94, 78–86. [Google Scholar] [CrossRef]
- Hanamura, I. Multiple myeloma with high-risk cytogenetics and its treatment approach. Int. J. Hematol. 2022, 115, 762–777. [Google Scholar] [CrossRef]
- Qiang, Y.-W.; Ye, S.; Chen, Y.; Buros, A.F.; Edmonson, R.; van Rhee, F.; Barlogie, B.; Epstein, J.; Morgan, G.J.; Davies, F.E. MAF protein mediates innate resistance to proteasome inhibition therapy in multiple myeloma. Blood 2016, 128, 2919–2930. [Google Scholar] [CrossRef]
- Poos, A.M.; Prokoph, N.; Przybilla, M.J.; Mallm, J.-P.; Steiger, S.; Seufert, I.; John, L.; Tirier, S.M.; Bauer, K.; Baumann, A.; et al. Resolving therapy resistance mechanisms in multiple myeloma by multiomics subclone analysis. Blood 2023, 142, 1633–1646. [Google Scholar] [CrossRef]
- Carullo, G.; Rossi, S.; Giudice, V.; Pezzotta, A.; Chianese, U.; Scala, P.; Carbone, S.; Fontana, A.; Panzeca, G.; Pasquini, S.; et al. Development of Epigenetic Modifiers with Therapeutic Potential in FMS-Related Tyrosine Kinase 3/Internal Tandem Duplication (FLT3/ITD) Acute Myeloid Leukemia and Other Blood Malignancies. ACS Pharmacol. Transl. Sci. 2024, 7, 2125–2142. [Google Scholar] [CrossRef]
- Gabrea, A.; Leif Bergsagel, P.; Michael Kuehl, W. Distinguishing primary and secondary translocations in multiple myeloma. DNA Repair. 2006, 5, 1225–1233. [Google Scholar] [CrossRef]
- Stasik, C.J.; Nitta, H.; Zhang, W.; Mosher, C.H.; Cook, J.R.; Tubbs, R.R.; Unger, J.M.; Brooks, T.A.; Persky, D.O.; Wilkinson, S.T.; et al. Increased MYC gene copy number correlates with increased mRNA levels in diffuse large B-cell lymphoma. Haematologica 2010, 95, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Pouryazdanparast, P.; Brenner, A.; Haghighat, Z.; Guitart, J.; Rademaker, A.; Gerami, P. The role of 8q24 copy number gains and c-MYC expression in amelanotic cutaneous melanoma. Mod. Pathol. 2012, 25, 1221–1226. [Google Scholar] [CrossRef]
- Sawyer, J.; Tian, E.; A Walker, B.; Weinhold, N.; Swanson, C.; Lukacs, J.L.; Binz, R.; Sammartino, G.; Thanendrarajan, S.; Schinke, C.; et al. Concurrent Amplification of MYC and 1q21 in Multiple Myeloma: Focal and Segmental Jumping Translocations of MYC. Blood 2016, 128, 3266. [Google Scholar] [CrossRef]
- Hanamura, I. Gain/Amplification of Chromosome Arm 1q21 in Multiple Myeloma. Cancers 2021, 13, 256. [Google Scholar] [CrossRef]
- Yang, G.; Li, C.; Tao, F.; Liu, Y.; Zhu, M.; Du, Y.; Fei, C.; She, Q.; Chen, J. The emerging roles of lysine-specific demethylase 4A in cancer: Implications in tumorigenesis and therapeutic opportunities. Genes. Dis. 2024, 11, 645–663. [Google Scholar] [CrossRef]
- Antero, S.; Kai, K.; Anu, K. Hypoxia-Inducible Histone Lysine Demethylases: Impact on the Aging Process and Age-Related Diseases. Aging Dis. 2016, 7, 180–200. [Google Scholar] [CrossRef]
- Boyd, K.D.; Ross, F.M.; Walker, B.A.; Wardell, C.P.; Tapper, W.J.; Chiecchio, L.; Dagrada, G.; Konn, Z.J.; Gregory, W.M.; Jackson, G.H.; et al. Mapping of Chromosome 1p Deletions in Myeloma Identifies FAM46C at 1p12 and CDKN2C at 1p32.3 as Being Genes in Regions Associated with Adverse Survival. Clin. Cancer Res. 2011, 17, 7776–7784. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, P.; Tuccillo, F.M.; Borrelli, A.; Schiattarella, A.; Buonaguro, F.M. CDK/CCN and CDKI Alterations for Cancer Prognosis and Therapeutic Predictivity. BioMed Res. Int. 2014, 2014, 361020. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322, Erratum in Ann. Oncol. 2022, 33, 117. [Google Scholar] [CrossRef]
- Medina-Herrera, A.; Sarasquete, M.E.; Jiménez, C.; Puig, N.; García-Sanz, R. Minimal Residual Disease in Multiple Myeloma: Past, Present, and Future. Cancers 2023, 15, 3687. [Google Scholar] [CrossRef]
- Neben, K.; Lokhorst, H.M.; Jauch, A.; Bertsch, U.; Hielscher, T.; van der Holt, B.; Salwender, H.; Blau, I.W.; Weisel, K.; Pfreundschuh, M.; et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood 2012, 119, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Lauwers-Cances, V.; Robillard, N.; Hulin, C.; Leleu, X.; Benboubker, L.; Marit, G.; Moreau, P.; Pegourie, B.; Caillot, D.; et al. Front-Line Transplantation Program With Lenalidomide, Bortezomib, and Dexamethasone Combination As Induction and Consolidation Followed by Lenalidomide Maintenance in Patients With Multiple Myeloma: A Phase II Study by the Intergroupe Francophone du Myélome. J. Clin. Oncol. 2014, 32, 2712–2717. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Sonneveld, P.; Attal, M.; Perrot, A.; Hulin, C.; Caillot, D.; Facon, T.; Leleu, X.; Belhadj-Merzoug, K.; Karlin, L.; Benboubker, L.; et al. Daratumumab Plus Bortezomib, Thalidomide, and Dexamethasone (D-VTd) in Transplant-eligible Newly Diagnosed Multiple Myeloma (NDMM): Subgroup Analysis of High-risk Patients (Pts) in CASSIOPEIA. Clin. Lymphoma Myeloma Leuk. 2019, 19, e2–e3. [Google Scholar] [CrossRef]
- Chari, A.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Silbermann, R.; Costa, L.J.; Anderson, L.D.; Nathwani, N.; et al. Daratumumab in transplant-eligible patients with newly diagnosed multiple myeloma: Final analysis of clinically relevant subgroups in GRIFFIN. Blood Cancer J. 2024, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Sonneveld, P.; Rodríguez-Otero, P.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. (DARA)/bortezomib/lenalidomide/dexamethasone (D-VRd) with D-R maintenance (maint) in transplant-eligible (TE) newly diagnosed myeloma (NDMM): Analysis of PERSEUS based on cytogenetic risk. In Proceedings of the EBMT 51st Annual Meeting, Florence, Italy, 30 March–2 April 2025. [Google Scholar]
- Touzeau, C.; Perrot, A.; Hulin, C.; Manier, S.; Macro, M.; Chretien, M.-L.; Karlin, L.; Escoffre, M.; Jacquet, C.; Tiab, M.; et al. Daratumumab, carfilzomib, lenalidomide, and dexamethasone with tandem transplant for high-risk newly diagnosed myeloma. Blood 2024, 143, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Roeloffzen, W.; Dimopoulos, M.A.; Rosiñol, L.; van der Klift, M.; Mina, R.; Rocafiguera, A.O.; Katodritou, E.; Wu, K.L.; Otero, P.R.; et al. Results of the Phase III Randomized Iskia Trial: Isatuximab-Carfilzomib-Lenalidomide-Dexamethasone Vs Carfilzomib-Lenalidomide-Dexamethasone As Pre-Transplant Induction and Post-Transplant Consolidation in Newly Diagnosed Multiple Myeloma Patients. Blood 2023, 142 (Suppl. 1), 4. [Google Scholar] [CrossRef]
- Leypoldt, L.B.; Tichy, D.; Besemer, B.; Hänel, M.; Raab, M.S.; Mann, C.; Munder, M.; Reinhardt, H.C.; Nogai, A.; Görner, M.; et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2024, 42, 26–37. [Google Scholar] [CrossRef]
- Cavo, M.; Salwender, H.; Rosiñol, L.; Moreau, P.; Petrucci, M.T.; Blau, I.W.; Bladé, J.; Attal, M.; Patriarca, F.; Weisel, K.; et al. Double Vs Single Autologous Stem Cell Transplantation After Bortezomib-Based Induction Regimens for Multiple Myeloma: An Integrated Analysis of Patient-Level Data from Phase European III Studies. Blood 2013, 122, 767. [Google Scholar] [CrossRef]
- Gagelmann, N.; Eikema, D.-J.; Koster, L.; Caillot, D.; Pioltelli, P.; Lleonart, J.B.; Reményi, P.; Blaise, D.; Schaap, N.; Trneny, M.; et al. Tandem Autologous Stem Cell Transplantation Improves Outcomes in Newly Diagnosed Multiple Myeloma with Extramedullary Disease and High-Risk Cytogenetics: A Study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 2134–2142. [Google Scholar] [PubMed]
- Cavo, M.; Petrucci, M.T.; Di Raimondo, F.; Zamagni, E.; Gamberi, B.; Crippa, C.; Marzocchi, G.; Grasso, M.; Ballanti, S.; Vincelli, D.I.; et al. Upfront Single Versus Double Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma: An Intergroup, Multicenter, Phase III Study of the European Myeloma Network (EMN02/HO95 MM Trial). Blood 2016, 128, 991. [Google Scholar] [CrossRef]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.-L.; Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Hari, P.; Pasquini, M.C.; Stadtmauer, E.A.; Fraser, R.; Fei, M.; Devine, S.M.; Efebera, Y.A.; Geller, N.; Horowitz, M.M.; Koreth, J.; et al. Long-term follow-up of BMT CTN 0702 (STaMINA) of postautologous hematopoietic cell transplantation (autoHCT) strategies in the upfront treatment of multiple myeloma (MM). J. Clin. Oncol. 2020, 38, 8506. [Google Scholar] [CrossRef]
- Moreau, P.; Kumar, S.K.; San Miguel, J.; Davies, F.; Zamagni, E.; Bahlis, N.; Ludwig, H.; Mikhael, J.; Terpos, E.; Schjesvold, F.; et al. Treatment of relapsed and refractory multiple myeloma: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2021, 22, e105–e118. [Google Scholar] [CrossRef]
- Cavo, M.; Gay, F.; Beksac, M.; Pantani, L.; Petrucci, M.T.; Dimopoulos, M.A.; Dozza, L.; van der Holt, B.; Zweegman, S.; Oliva, S.; et al. Autologous haematopoietic stem-cell transplantation versus bortezomib–melphalan–prednisone, with or without bortezomib–lenalidomide–dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): A multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020, 7, e456–e468. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Beksac, M.; van der Holt, B.; Aquino, S.; Ludwig, H.; Zweegman, S.; Zander, T.; Zamagni, E.; Wester, R.; et al. Consolidation and Maintenance in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2021, 39, 3613–3622. [Google Scholar] [CrossRef]
- Richardson, P.G.; Laubach, J.; Gandolfi, S.; Facon, T.; Weisel, K.; O’gorman, P. Maintenance and continuous therapy for multiple myeloma. Expert. Rev. Anticancer. Ther. 2018, 18, 751–764. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef]
- Nooka, A.K.; Kaufman, J.L.; Muppidi, S.; Langston, A.; Heffner, L.T.; Gleason, C.; Casbourne, D.; Saxe, D.; Boise, L.H.; Lonial, S. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia 2013, 28, 690–693. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Lokhorst, H.M.; Mai, E.K.; Van Der Holt, B.; Blau, I.W.; Zweegman, S.; Weisel, K.C.; Vellenga, E.; Pfreundschuh, M.; Kersten, M.J.; et al. Bortezomib before and after high-dose therapy in myeloma: Long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia 2018, 32, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Hulin, C.; Perrot, A.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Zweegman, S.; Caillon, H.; Caillot, D.; et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1378–1390. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Gay, F.; Schjesvold, F.; Beksac, M.; Hajek, R.; Weisel, K.C.; Goldschmidt, H.; Maisnar, V.; Moreau, P.; Min, C.K.; et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2019, 393, 253–264. [Google Scholar] [CrossRef]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. A plain language summary of the PERSEUS study of daratumumab plus bortezomib, lenalidomide, and dexamethasone for treating newly diagnosed multiple myeloma. Futur. Oncol. 2024, 20, 3043–3063. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Facon, T. Front-line therapies for elderly patients with transplant-ineligible multiple myeloma and high-risk cytogenetics in the era of novel agents. Leukemia 2018, 32, 1267–1276. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. VISTA trial investigators. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.V.; Belch, A.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.J.; et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood 2018, 131, 301–310. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Hoering, A.; Ailawadhi, S.; Sexton, R.; Lipe, B.; Hita, S.F.; Valent, J.; Rosenzweig, M.; Zonder, J.A.; Dhodapkar, M.; et al. Bortezomib, lenalidomide, and dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): Primary analysis of a randomised, phase 2 trial. Lancet Haematol. 2021, 8, e45–e54. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Bahlis, N.; Facon, T.; Usmani, S.Z.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Touzeau, C.; Basu, S.; Nahi, H.; Hulin, C.; et al. Daratumumab Plus Lenalidomide and Dexamethasone (D-Rd) Versus Lenalidomide and Dexamethasone (Rd) in Patients with Newly Diagnosed Multiple Myeloma (NDMM) Ineligible for Transplant: Updated Analysis of Maia. Blood 2019, 134 (Suppl. 1), 1875. [Google Scholar] [CrossRef]
- Moreau, P.; Facon, T.; Usmani, S.; Bahlis, N.J.; Raje, N.; Plesner, T.; Orlowski, R.Z.; Basu, S.; Nahi, H.; Hulin, C.; et al. Daratumumab Plus Lenalidomide and Dexamethasone (D-Rd) Versus Lenalidomide and Dexamethasone (Rd) in Transplant-Ineligible Patients (Pts) with Newly Diagnosed Multiple Myeloma (NDMM): Clinical Assessment of Key Subgroups of the Phase 3 Maia Study. Blood 2022, 140 (Suppl. 1), 7297–7300. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lúcio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Giri, S.; Grimshaw, A.; Bal, S.; Godby, K.; Kharel, P.; Djulbegovic, B.; Dimopoulos, M.A.; Facon, T.; Usmani, S.Z.; Mateos, M.-V.; et al. Evaluation of Daratumumab for the Treatment of Multiple Myeloma in Patients with High-risk Cytogenetic Factors. JAMA Oncol. 2020, 6, 1759–1765. [Google Scholar] [CrossRef]
- Mina, R.; Bonello, F.; Petrucci, M.T.; Liberati, A.M.; Conticello, C.; Ballanti, S.; Musto, P.; Olivieri, A.; Benevolo, G.; Capra, A.; et al. Carfilzomib, cyclophosphamide and dexamethasone for newly diagnosed, high-risk myeloma patients not eligible for transplant: A pooled analysis of two studies. Haematologica 2020, 106, 1079–1085. [Google Scholar] [CrossRef]
- Facon, T.; Dimopoulos, M.-A.; Leleu, X.P.; Beksac, M.; Pour, L.; Hájek, R.; Liu, Z.; Minarik, J.; Moreau, P.; Romejko-Jarosinska, J.; et al. Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 391, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Facon, T.; Hungria, V.; Bahlis, N.J.; Venner, C.P.; Braunstein, M.; Pour, L.; Martí, J.M.; Basu, S.; Cohen, Y.C.; et al. Daratumumab plus bortezomib, lenalidomide and dexamethasone for transplant-ineligible or transplant-deferred newly diagnosed multiple myeloma: The randomized phase 3 CEPHEUS trial. Nat. Med. 2025, 31, 1195–1202. [Google Scholar] [CrossRef]
- Lannes, R.; Samur, M.; Perrot, A.; Mazzotti, C.; Divoux, M.; Cazaubiel, T.; Leleu, X.; Schavgoulidze, A.; Chretien, M.-L.; Manier, S.; et al. In Multiple Myeloma, High-Risk Secondary Genetic Events Observed at Relapse Are Present from Diagnosis in Tiny, Undetectable Subclonal Populations. J. Clin. Oncol. 2023, 41, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; San-Miguel, J.; Belch, A.; White, D.; Benboubker, L.; Cook, G.; Leiba, M.; Morton, J.; Ho, P.J.; Kim, K.; et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of POLLUX. Haematologica 2018, 103, 2088–2096. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Fonseca, R.; Siegel, D.; Dimopoulos, M.A.; Špička, I.; Masszi, T.; Hájek, R.; Rosiñol, L.; Goranova-Marinova, V.; Mihaylov, G.; et al. Carfilzomib significantly improves the progression-free survival of high-risk patients in multiple myeloma. Blood 2016, 128, 1174–1180. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Bahlis, N.J.; Chng, W.-J.; Masszi, T.; Viterbo, L.; Pour, L.; Ganly, P.; Palumbo, A.; Cavo, M.; Langer, C.; et al. Ixazomib significantly prolongs progression-free survival in high-risk relapsed/refractory myeloma patients. Blood 2017, 130, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Lonial, S.; Betts, K.A.; Chen, C.; Zichlin, M.L.; Brun, A.; Signorovitch, J.E.; Makenbaeva, D.; Mekan, S.; Sy, O.; et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer 2018, 124, 4032–4043. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Dimopoulos, M.-A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Baker, R.; Martinez, G.; Min, C.-K.; et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Delimpasi, S.; Beksac, M.; Katodritou, E.; Moreau, P.; Baldini, L.; Symeonidis, A.; Bila, J.; et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 801–812. [Google Scholar] [CrossRef]
- Spicka, I.; Moreau, P.; Martin, T.G.; Facon, T.; Martinez, G.; Oriol, A.; Koh, Y.; Lim, A.; Mikala, G.; Rosiñol, L.; et al. Isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma patients with high-risk cytogenetics: IKEMA subgroup analysis. Eur. J. Haematol. 2022, 109, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.S.; Schiller, G.J.; Samaras, C.; Sebag, M.; Berdeja, J.; Ganguly, S.; Matous, J.; Song, K.; Seet, C.S.; Talamo, G.; et al. Pomalidomide, dexamethasone, and daratumumab in relapsed refractory multiple myeloma after lenalidomide treatment. Leukemia 2020, 34, 3286–3297. [Google Scholar] [CrossRef]
- Victoria, M.; Robak, P.; Hus, M.; Fu, C.; Zherebtsova, V.; Ward, C.; Ho, P.J.; de Almeida, A.C.; Hajek, R.; Kim, K.; et al. MM-557 DREAMM-7 Update: Subgroup Analyses From a Phase 3 Trial of Belantamab Mafodotin (Belamaf) + Bortezomib and Dexamethasone (BVd) vs Daratumumab, Bortezomib, and Dexamethasone (DVd) in Relapsed/Refractory Multiple Myeloma (RRMM). Clin. Lymphoma Myeloma Leuk. 2024, 24, S571. [Google Scholar] [CrossRef]
- Richard, S.; Chari, A.; Delimpasi, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Dimopoulos, M.A.; Pylypenko, H.; Auner, H.W.; et al. Selinexor, bortezomib, and dexamethasone versus bortezomib and dexamethasone in previously treated multiple myeloma: Outcomes by cytogenetic risk. Am. J. Hematol. 2021, 96, 1120–1130. [Google Scholar] [CrossRef]
- De Novellis, D.; Fontana, R.; Giudice, V.; Serio, B.; Selleri, C. Innovative Anti-CD38 and Anti-BCMA Targeted Therapies in Multiple Myeloma: Mechanisms of Action and Resistance. Int. J. Mol. Sci. 2022, 24, 645. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M.; Bahlis, N.J.; Maisel, C.; Karlin, L.; Yanovsky, A.V.; Leip, E.; Sullivan, S.T.; Viqueira, A.; Touzeau, C. Efficacy and safety of elranatamab in patients with high-risk relapsed/refractory multiple myeloma (RRMM): A subgroup analysis from MagnetisMM-3. J. Clin. Oncol. 2023, 41, e20017. [Google Scholar] [CrossRef]
- Chari, A.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; van de Donk, N.W.; Rodríguez-Otero, P.; Askari, E.; Mateos, M.-V.; Costa, L.J.; Caers, J.; et al. Talquetamab, a T-Cell–Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N. Engl. J. Med. 2022, 387, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Richter, J. Teclistamab for Multiple Myeloma: Clinical Insights and Practical Considerations for a First-in-Class Bispecific Antibody. Cancer Manag. Res. 2023, 15, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Searle, E.; Quach, H.; Wong, S.W.; Costa, L.J.; Hulin, C.; Janowski, W.; Berdeja, J.; Anguille, S.; Matous, J.V.; Touzeau, C.; et al. Teclistamab in Combination with Subcutaneous Daratumumab and Lenalidomide in Patients with Multiple Myeloma: Results from One Cohort of MajesTEC-2, a Phase1b, Multicohort Study. Blood 2022, 140 (Suppl. 1), 394–396. [Google Scholar] [CrossRef]
- Grosicki, S.; Crafoord, J.; Koh, Y.; White, D.; Mellqvist, U.-H.; Leip, E.; Kudla, A.; Finn, G.; Pruchniewski, Ł. MagnetisMM-5: An open-label, multicenter, randomized phase 3 study of elranatamab as monotherapy and in combination with daratumumab in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2022, 40, TPS8074. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Morillo, D.; Gatt, M.; Sebag, M.; Kim, K.; Min, C.-K.; Oriol, A.; Ocio, E.; Yoon, S.-S.; Cohen, Y.; et al. S190: First results from the redirectt-1 study with teclistamab (tec) + talquetamab (tal) simultaneously targeting bcma and gprc5d in patients (pts) with relapsed/refractory multiple myeloma (rrmm). HemaSphere 2023, 7, e15362d7. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Munshi, N.; Martin, T.; Usmani, S.Z.; Berdeja, J.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. S202: Cartitude-1 Final Results: Phase 1b/2 Study of Ciltacabtagene Autoleucel in Heavily Pretreated Patients with Relapsed/Refractory Multiple Myeloma. HemaSphere 2023, 7, e6102468. [Google Scholar] [CrossRef]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti–B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2023, 41, 1265–1274. [Google Scholar] [CrossRef]
- Mina, R.; Dhakal, B.; San-Miguel, J.; Kwon, M.; Purtill, D.; Magen, H.; Dutka, M.; Delforge, M.; Vij, R.; Wichert, S.; et al. MM-567 Ciltacabtagene Autoleucel vs Standard of Care in Lenalidomide-Refractory Multiple Myeloma: Phase 3 CARTITUDE-4 Subgroup Analysis by Cytogenetic Risk. Clin. Lymphoma Myeloma Leuk. 2024, 24, S572–S573. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Lan, H.; Wu, J.; Xiao, Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front. Immunol. 2023, 14, 1101495. [Google Scholar] [CrossRef] [PubMed]
| Abnormality | Frequency | Gene/Pathway | Prognostic Significance | Clinical Impact |
|---|---|---|---|---|
| All 14q32 (IGH) t(4;14) t(14;16) t(14;20) | 45–50% 10% to 15% <5% <5% | FGFR3/MMSET Upregulation MAF overexpression MAFB overexpression | Poor Uncertain; mainly poor Uncertain; mainly poor | Rapid progression. Double ASCT Double ASCT, especially with HR abn Double ASCT, especially with HR abn |
| 1q21 gain 2–3 copies ≥4 copies | 40% 20–30% 5–20% | CKS1B, MCL1, ADAR1 overexpression | Intermediate Poor | Aggressive with organ failure Double ASCT, especially with HR abn |
| 1p32 deletion Monoallelic Biallelic | 10% | FAF1/CDKN2C deficit | Poor Highly poor | Double ASCT, especially with HR abn Double ASCT + intensive maintenance |
| del(17p)/TP53 mutation Single hit Double hit | 8–15% Deletion Deletion + mutation | TP53 | Poor Highly poor | Poor sensitivity to therapy Double ASCT + intensive maintenance |
| Stage | ISS | R-ISS |
|---|---|---|
| I | Sβ2M < 3.5 mg/L Serum albumin ≥ 3.5 g/dL | Sβ2M < 3.5 mg/L Serum albumin ≥ 3.5 g/dL Standard-risk CA by iFISH Normal LDH |
| II | Sβ2M > 3.5 mg/L and serum albumin < 3.5 g/dL OR 3.5 mg/L < Sβ2M > 5.5 mg/L | Not R-ISS stage I or III |
| III | Sβ2M ≥ 5.5 mg/L | Sβ2M ≥ 5.5 mg/L and either high-risk CA by FISH OR high LDH |
| Genetic risk | ||
| R-ISS Standard-risk CA | Deletion of chromosome 17, or 17p-, translocation of chromosomes 14 and 16, or t(14;16), and translocation of chromosomes 4 and 14, or t(4;14) | |
| EMMA High genetic risk CA | del(17p) > 20% TP53 mutation Biallelic del1p32 1q gain and monoallelic del1p32 t(4;14) or t(14;16) or t(14;20) and either 1q gain or monoallelic del1p32 | |
| Study | Phase | Regimens | N. HRMM Patients | Outcomes in HRMM | General Outcomes | p-Value |
|---|---|---|---|---|---|---|
| CASSIOPEIA (Completed) | III | Dara-VTD vs. dara-VTD | 168 | CR rate: 36.6% MRD− rate: 59.8% No benefits compared to VTD | CR or better: 38.9% MRD− rate: 63.7% | NS |
| GRIFFIN (Completed) | II | Dara-VRD vs. dara-VRD | 30 | sCR rate: 18.8% MRD− rate: 37.5% No benefits compared to VRD | sCR rate: 42.4% MRD− rate: 51% | NS |
| PERSEUS (Active, not recruiting) | III | Dara-VRD vs. dara-VRD | 154 | MRD− rate: 68.4% Sustained MRD− rate: 48.7% | MRD− rate: 75.2% Sustained MRD− rate: 69.3% | 0.04 |
| ISKIA (Active, not recruiting) | III | Isa-KRD | 111 Ultra-HR: 51 | MRD− rate: 76-77% | MRD− rate: 79% in standard risk | NS |
| GMMG-CONCEPT (Active, not recruiting) | II | Isa-KRD | 125 | MRD− rate in ASCT-eligible patients: 67.7% MRD− rate in ASCT-ineligible patients: 54.2% | − | − |
| IFM 2018-04 (Active, not recruiting) | II | Dara-KRD | 50 | 30-month PFS: 80% 30-month OS: 91% ORR: 100% in patients completing 2nd ASCT | − | − |
| Study | Phase | Regimens | N. HRMM Patients | Outcomes in HRMM | General Outcomes | p-Value |
|---|---|---|---|---|---|---|
| STAMINA (Completed) | III | ASCT plus lenalidomide maintenance (auto/len) vs. ASCT plus VRd consolidation plus lenalidomide maintenance (auto/VRd) vs. tandem ASCT plus lenalidomide maintenance (auto/auto) | 223 | 6-year PFS: 43.6% and 26% for auto/auto and auto/len | 6-year PFS auto/auto: 43.9% auto/VRd: 39.7% auto/len 40.9% 6-year OS auto/auto: 73.1% auto/VRd: 74.9% auto/len 76.4% | 0.03 |
| EMN02/HO95 (Completed) | III | VCD followed by VMP or single/double ASCT | 225 | 75-month OS 54% with ASCT 30% with VMP Median PFS double vs. single ASCT: 46 vs. 27.6 months | − | − |
| MIDAS (Active, not recruiting) | III | Isa-KRD × 6 plus in standard risk (A) Isa-KRD for 6 plus lenalidomide maintenance for 3 years (B) ASCT plus isa-KRD for 2 plus lenalidomide maintenance for 3 years (C) In high-risk, ASCT plus isa-KRD for 2 plus isa-Iber for 3 years (D) In high-risk, tandem ASCT plus isa-iber for 3 years | Ongoing | − | ||
| Study | Phase | Regimens | N. HRMM Patients | Outcomes in HRMM | General Outcomes | p-Value |
|---|---|---|---|---|---|---|
| SWOG S0777 (Active,not recruiting) | III | VRD vs RD | 104 | Median PFS: 38 months vs. 16 months | Median PFS: 43 months vs. 29 months | 0.19 |
| SWOG-1211 (Active,not recruiting) | II | Elo-VRD vs. VRD | 100 | Median PFS: 31.5 months vs. 33.6 months | - | - |
| MAIA (Completed) | III | Dara-RD vs. RD | 92 | Median PFS: 45 months vs. 29 months | Median PFS: 61.9 months vs. 34.9 months | NS |
| ALCYONE (Completed) | III | Dara-VMP vs. VMP | 98 | Median PFS: 18 months vs. 18 months | 3-year PFS: 50.7% vs. 18.5% 3-year OS: 78.0% vs. 67.9% | NS |
| GMMG-CONCEPT (Active,not recruiting) | II | Isa-KRD | 125 | MRD− rate in transplant eligible: 67.7% MRD− rate in transplant ineligible: 54.2% | - | |
| IMROZ (Active,not recruiting) | III | Isa-VRD vs. VRD | 74 | Hazard ratio: 0.97 | NS | |
| CEPHEUS (Active,not recruiting) | III | Dara-VRD vs. VRD | 52 | Hazard ratio: 0.88 | NS | |
| Study | Phase | Regimens | N. HRMM Patients | Outcomes in HRMM | General Outcomes | p-Value |
|---|---|---|---|---|---|---|
| ASPIRE (Completed) | III | KRD vs. RD | 100 | Median PFS: 23 months vs. 13.9 months | Median PFS: 29.6 months vs. 19.5 months | NS |
| APOLLO (Unknown status) | III | Dara-PD vs. PD | 74 | Median PFS: 5.8 months vs. 4 months | Median PFS: 12.4 months vs. 6.9 months | NS |
| POLLUX (Completed) | III | Dara-RD vs. RD | 70 | Median PFS: 26.8 months vs. 8.3 months | Median PFS: 44.5 months vs. 17.5 months | - |
| TOURMALINE-MM1 (Completed) | III | Ixa-RD vs. RD | 137 | Median PFS: 21.4 months vs. 9.7 months | Median PFS: 20.6 months vs. 14.7 months | <0.05 |
| CANDOR (Completed) | III | Dara-KD vs. KD | 74 | Median PFS: 11.2 months vs. 7.4 months | Median PFS: 28.6 months vs. 15.2 months | NS |
| IKEMA (Completed) | III | Isa-KD vs. KD | 73 | Median PFS: not reached vs. 18.2 months | Median PFS: 35.7 months vs. 19.2 months | NS |
| DREAMM-7 (Active, not recruiting) | III | Belantamab mafodotin-VD vs. VD | 136 | Median PFS: 33.2 months vs. 10.5 months | Median PFS: 36.6 months vs. 13.4 months | − |
| BOSTON (Completed) | III | SVD vs. VD | 256 | ORR: 78% vs. 57% Median PFS: 12.9 months vs. 8.6 months | Median PFS: 13.9 months vs. 9.4 months | <0.05 |
| ICARIA (Completed) | III | Isa-PD vs. PD | 60 | ORR: 50% vs. 16.7% Median PFS: 7.5 months vs. 3.7 months | NS | |
| Study | Phase | Regimens | N. HRMM Patients | Outcomes in HRMM | General Outcomes | p-Value |
|---|---|---|---|---|---|---|
| MajesTEC-1 (Active, not recruiting) | I-II | Teclistamab | 43 | No benefits | ORR: 63% Median PFS: 11.3 months | − |
| MagnetisMM-3 (Active, not recruiting) | II | Elranatamab | 31 | ORR: 54.8% 12-month DOR: 57% | ORR: 61% Median PFS: 13.4 months | − |
| MonumenTAL-1 (Active, not recruiting) | I-II | Talquetamab | 18 | ORR: 55.6-66.7% | ORR: 64-70% | − |
| RedirecTT-1 (Active, not recruiting) | Ib | Teclistamab + talquetamab | 15 | - | ORR: 84% Median PFS: 20.9 months | − |
| KarMMa-1 (Active, not recruiting) | II | Ide-cel | 45 | Median PFS: 10.4 months | Median PFS: 8.2 months | − |
| CARTITUDE-1 (Completed) | Ib-II | Cilta-cel | 13 | ORR: 100% Median PFS: 21.1 months | ORR: 97.9% Median PFS: 34.9 months | − |
| CARTITUDE-4 (Active, not recruiting) | III | Cilta-cel vs. dara-PD/PVD | 255 | ORR: 88.5% Benefit in favor of cilta-cel | ORR: 84.6% vs. 67.3% 1-year PFS: 75.9% vs. 48.6% | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Novellis, D.; Scala, P.; Giudice, V.; Selleri, C. High-Risk Genetic Multiple Myeloma: From Molecular Classification to Innovative Treatment with Monoclonal Antibodies and T-Cell Redirecting Therapies. Cells 2025, 14, 776. https://doi.org/10.3390/cells14110776
De Novellis D, Scala P, Giudice V, Selleri C. High-Risk Genetic Multiple Myeloma: From Molecular Classification to Innovative Treatment with Monoclonal Antibodies and T-Cell Redirecting Therapies. Cells. 2025; 14(11):776. https://doi.org/10.3390/cells14110776
Chicago/Turabian StyleDe Novellis, Danilo, Pasqualina Scala, Valentina Giudice, and Carmine Selleri. 2025. "High-Risk Genetic Multiple Myeloma: From Molecular Classification to Innovative Treatment with Monoclonal Antibodies and T-Cell Redirecting Therapies" Cells 14, no. 11: 776. https://doi.org/10.3390/cells14110776
APA StyleDe Novellis, D., Scala, P., Giudice, V., & Selleri, C. (2025). High-Risk Genetic Multiple Myeloma: From Molecular Classification to Innovative Treatment with Monoclonal Antibodies and T-Cell Redirecting Therapies. Cells, 14(11), 776. https://doi.org/10.3390/cells14110776







