FGF-Mediated Axon Guidance: Role of Downstream Signaling Pathways in Cytoskeletal Control
Abstract
1. Introduction
2. The Background of FGFs Transferring Signals Mechanism
2.1. FGFs and the Role of FGFs in Nervous System
2.2. Fibroblast Growth Factor Receptors (FGFRs)
2.3. Interaction Between FGFs and FGFRs
3. Effects of Cytoskeleton on Axon Guidance
3.1. Microfilaments
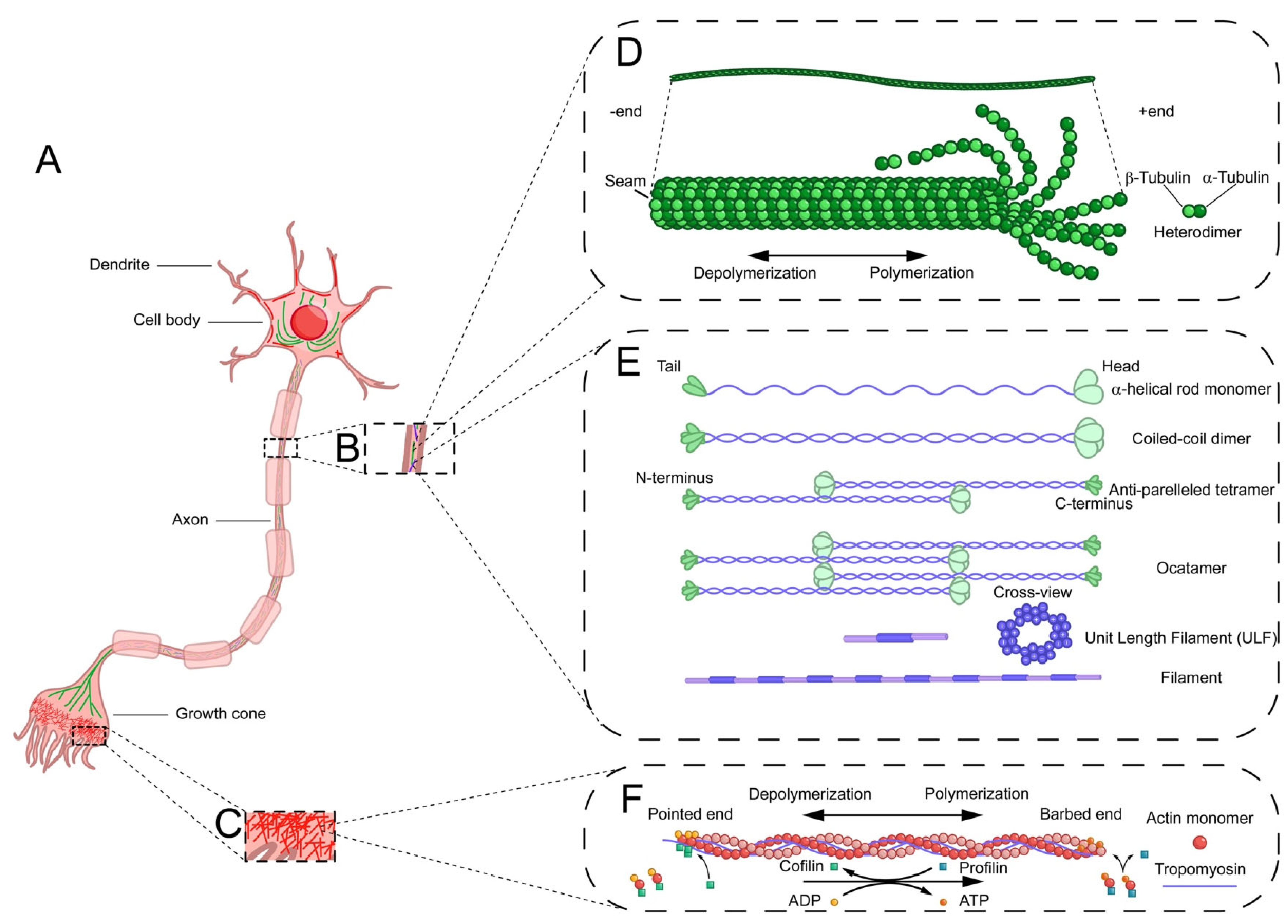
3.1.1. Microfilaments Structure
3.1.2. Microfilaments Regulatory Proteins
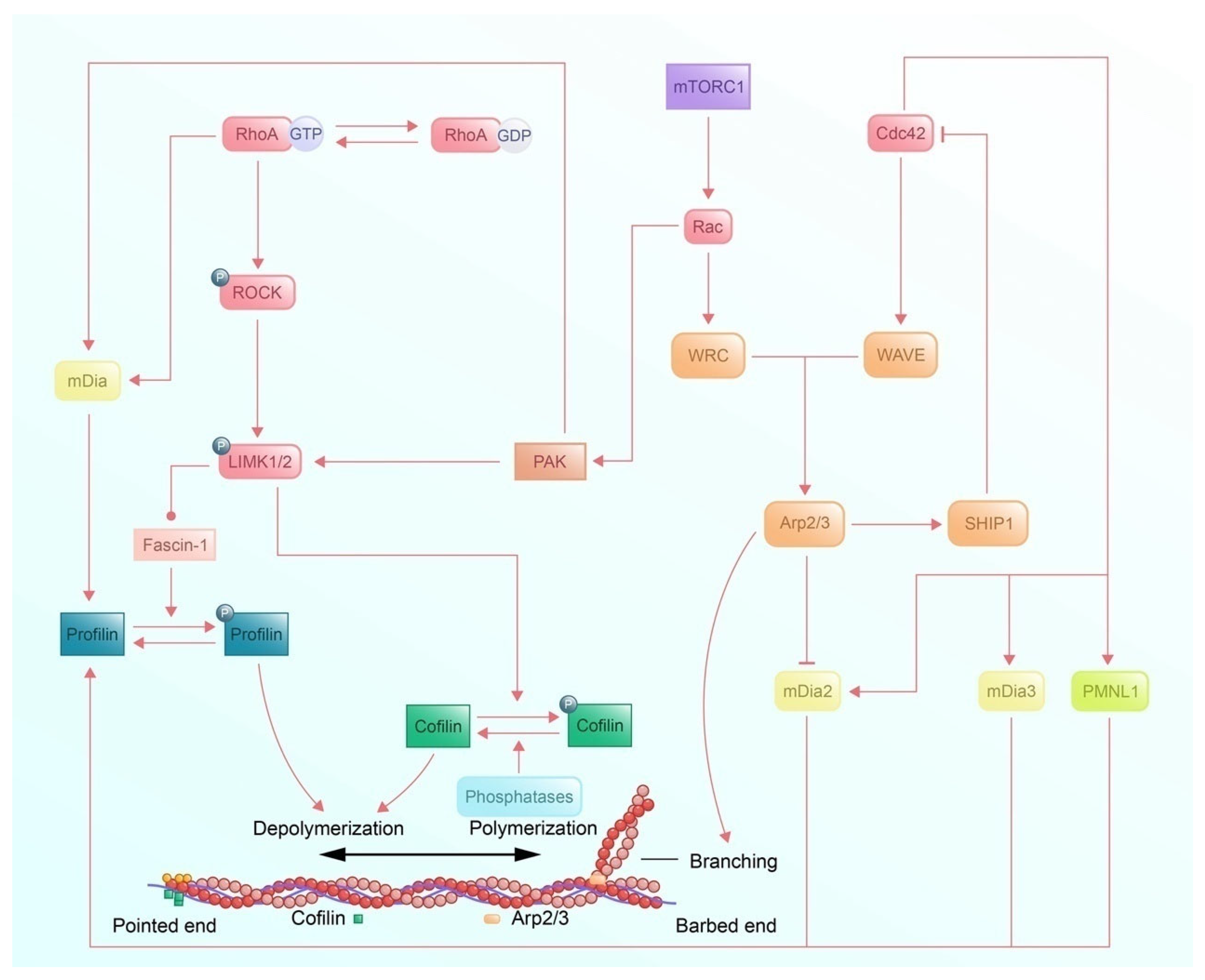
3.1.3. Signaling Pathways of Microfilaments Regulatory Protein and Regulation of Axon Guidance
3.2. Microtubule
3.2.1. Microtubule Structure
3.2.2. Dynamic Instability
3.2.3. Regulatory Proteins
3.2.4. Signaling Pathways
3.3. Intermediate Filaments
3.3.1. Intermediate Filament Structure
3.3.2. Intermediate Filaments Regulatory Proteins
4. Downstream Signaling Pathways
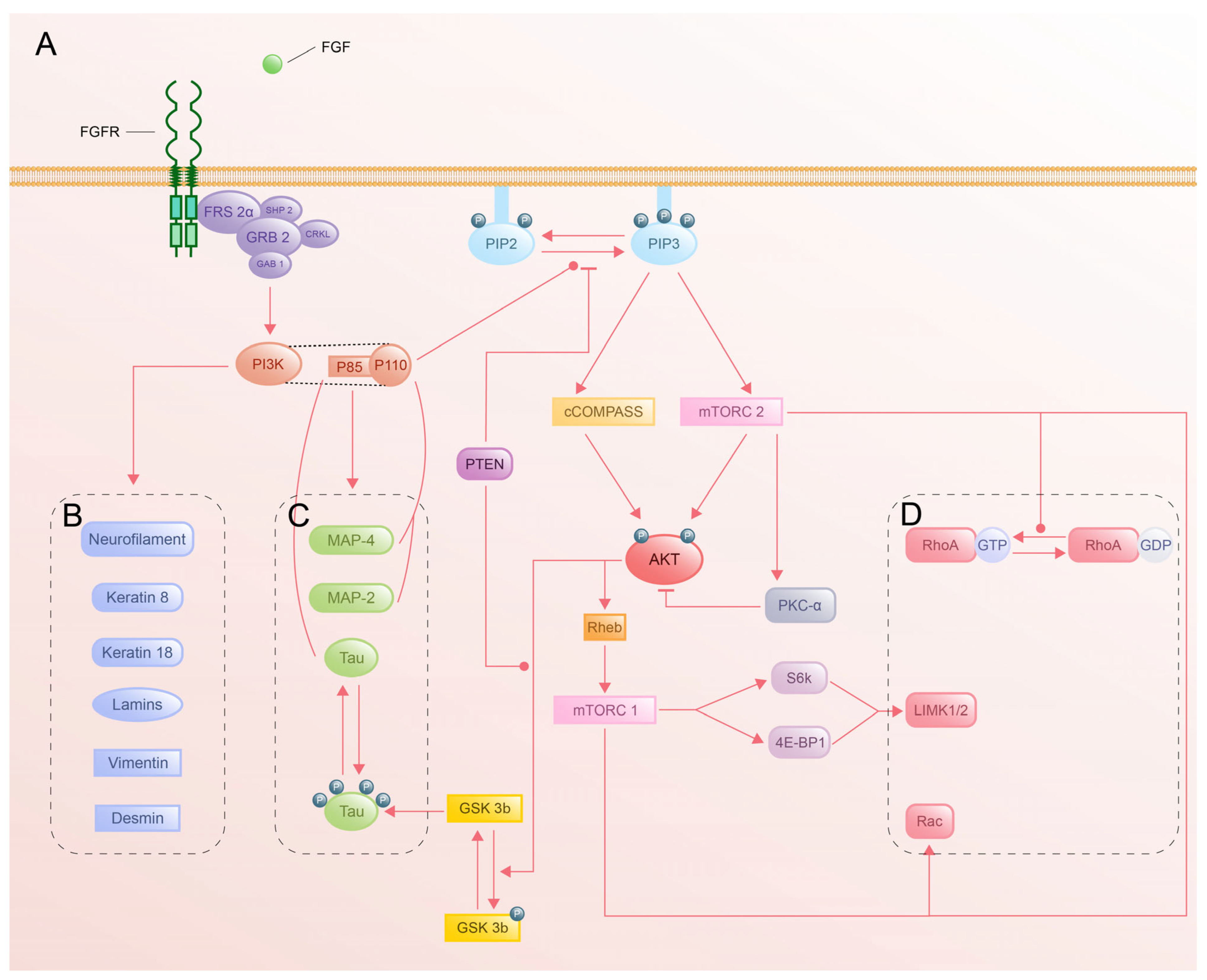
4.1. PI3K-Akt Pathway
4.1.1. Activation of PI3K-Akt by FGFR
4.1.2. Structural and Functional Complexity of PI3K
4.1.3. Akt Activation and Downstream Targets
4.1.4. Cytoskeletal Regulation by PI3K-Akt
4.2. JAK-STAT Pathway
4.2.1. FGF Signaling and JAK-STAT Activation
4.2.2. JAK-STAT Signaling Pathway by FGFR Phosphorylation
4.2.3. Cytoskeletal Regulation via Activation of STAT
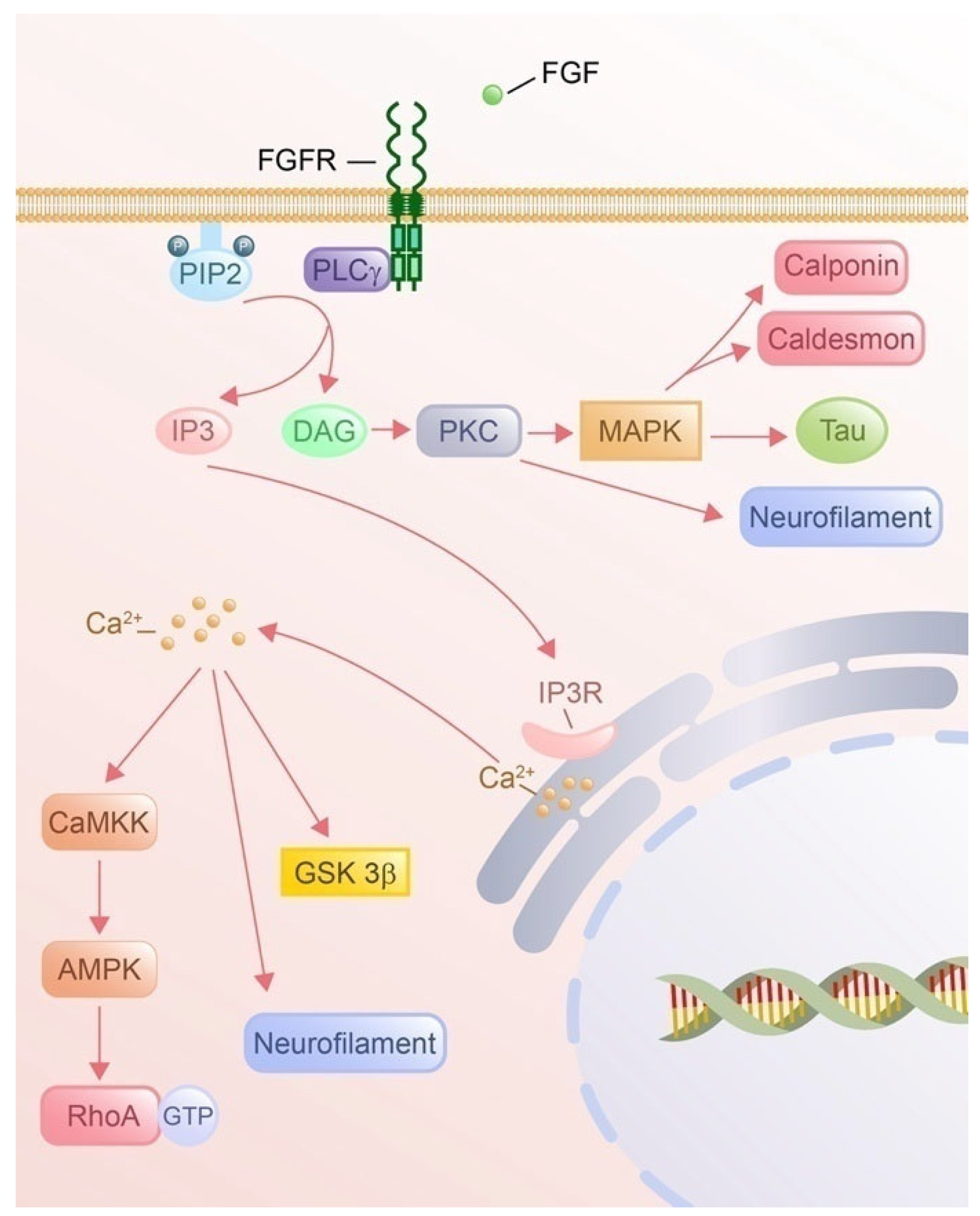
4.3. PLCγ Pathway
4.3.1. PLCγ Signaling Pathway by FGF Phosphorylation
4.3.2. IP3 Signaling and Cytoskeletal Remodeling
4.3.3. DAG Signaling and Cytoskeletal Remodeling
4.4. RAS-MAPK Pathway
4.4.1. Activation of RAS-MAPK by FGF Signaling
4.4.2. Cytoskeletal Remodeling via RAS-MAPK Pathway
5. Discussion of FGF-Mediated Axon Guidance Mechanism
6. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tessier-Lavigne, M.; Goodman, C.S. The molecular biology of axon guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, T.W.; Bargmann, C.I. Dynamic regulation of axon guidance. Nat. Neurosci. 2001, 4, 1169–1176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Belov, A.A.; Mohammadi, M. Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb. Perspect. Biol. 2013, 5, a015958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goetz, R.; Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 2013, 14, 166–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flippot, R.; Kone, M.; Magné, N.; Vignot, S. FGF/FGFR signalling: Implication in oncogenesis and perspectives. Bull. Cancer 2015, 102, 516–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quinn, C.C.; Pfeil, D.S.; Wadsworth, W.G. CED-10/Rac1 mediates axon guidance by regulating the asymmetric distribution of MIG-10/lamellipodin. Curr. Biol. 2008, 18, 808–813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tojima, T.; Hines, J.H.; Henley, J.R.; Kamiguchi, H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat. Rev. Neurosci. 2011, 12, 191–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quinn, C.C.; Wadsworth, W.G. Axon guidance: Asymmetric signaling orients polarized outgrowth. Trends Cell Biol. 2008, 18, 597–603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eswarakumar, V.P.; Lax, I.; Schlessinger, J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor. Rev. 2005, 16, 139–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlessinger, J.; Plotnikov, A.N.; Ibrahimi, O.A.; Eliseenkova, A.V.; Yeh, B.K.; Yayon, A.; Linhardt, R.J.; Mohammadi, M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol. Cell 2000, 6, 743–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katoh, M.; Nakagama, H. FGF receptors: Cancer biology and therapeutics. Med. Res. Rev. 2014, 34, 280–300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katoh, M.; Katoh, M. Evolutionary conservation of CCND1-ORAOV1-FGF19-FGF4 locus from zebrafish to human. Int. J. Mol. Med. 2003, 12, 45–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uhlrich, S.; Lagente, O.; Lenfant, M.; Courtois, Y. Effect of heparin on the stimulation of non-vascular cells by human acidic and basic FGF. Biochem. Biophys. Res. Commun. 1986, 137, 1205–1213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldfarb, M. Fibroblast growth factor homologous factors: Evolution, structure, and function. Cytokine Growth Factor. Rev. 2005, 16, 215–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stifani, N. Motor neurons and the generation of spinal motor neuron diversity. Front. Cell. Neurosci. 2014, 8, 293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shirasaki, R.; Lewcock, J.W.; Lettieri, K.; Pfaff, S.L. FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron 2006, 50, 841–853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webber, C.A.; Hyakutake, M.T.; McFarlane, S. Fibroblast growth factors redirect retinal axons in vitro and in vivo. Dev. Biol. 2003, 263, 24–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Webber, C.A.; Chen, Y.Y.; Hehr, C.L.; Johnston, J.; McFarlane, S. Multiple signaling pathways regulate FGF-2-induced retinal ganglion cell neurite extension and growth cone guidance. Mol. Cell. Neurosci. 2005, 30, 37–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, E.C.; Dalton, P.D.; Shoichet, M.S.; Tator, C.H. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials 2006, 27, 519–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grumbles, R.M.; Casella, G.T.B.; Rudinsky, M.J.; Wood, P.M.; Sesodia, S.; Bent, M.; Thomas, C.K. Long-term delivery of FGF-6 changes the fiber type and fatigability of muscle reinnervated from embryonic neurons transplanted into adult rat peripheral nerve. J. Neurosci. Res. 2007, 85, 1933–1942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yabut, O.R.; Arela, J.; Gomez, H.G.; Castillo, J.G.; Ngo, T.; Pleasure, S.J. Aberrant FGF signaling promotes granule neuron precursor expansion in SHH subgroup infantile medulloblastoma. eLife 2025, 13, 100767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Lv, Z.; Huang, H.; Yu, S.; Xiao, L.; Li, X.; Li, G.; Liu, F. FGF3 from the Hypothalamus Regulates the Guidance of Thalamocortical Axons. Dev. Neurosci. 2020, 42, 208–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Lv, Z.; Huang, H.; Li, M.; Xiao, L.; Li, X.; Li, G.; Liu, F. FGF10 regulates thalamocortical axon guidance in the developing thalamus. Neurosci. Lett. 2020, 716, 134685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, F.; Pogoda, H.-M.; Pearson, C.A.; Ohyama, K.; Löhr, H.; Hammerschmidt, M.; Placzek, M. Direct and indirect roles of Fgf3 and Fgf10 in innervation and vascularisation of the vertebrate hypothalamic neurohypophysis. Development 2013, 140, 1111–1122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Umemori, H.; Linhoff, M.W.; Ornitz, D.M.; Sanes, J.R. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 2004, 118, 257–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irving, C.; Malhas, A.; Guthrie, S.; Mason, I. Establishing the trochlear motor axon trajectory: Role of the isthmic organiser and Fgf8. Development 2002, 129, 5389–5398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamauchi, K.; Mizushima, S.; Tamada, A.; Yamamoto, N.; Takashima, S.; Murakami, F. FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. J. Neurosci. 2009, 29, 4044–4055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clegg, J.M.; Parkin, H.M.; Mason, J.O.; Pratt, T. Heparan Sulfate Sulfation by Hs2st Restricts Astroglial Precursor Somal Translocation in Developing Mouse Forebrain by a Non-Cell-Autonomous Mechanism. J. Neurosci. 2019, 39, 1386–1404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, T.W.; Piao, J.; Bocchi, V.D.; Koo, S.Y.; Choi, S.J.; Chaudhry, F.; Yang, D.; Cho, H.S.; Hergenreder, E.; Perera, L.R.; et al. Enhanced yield and subtype identity of hPSC-derived midbrain dopamine neuron by modulation of WNT and FGF18 signaling. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, E.; Teku, G.N.; Boza-Serrano, A.; Russ, K.; Berns, M.; Deierborg, T.; Lamas, N.J.; Wichterle, H.; Rothstein, J.; Henderson, C.E.; et al. FGF family members differentially regulate maturation and proliferation of stem cell-derived astrocytes. Sci. Rep. 2019, 9, 9610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, X.; Xie, Z.; Zhao, J.; Lu, W.; Zhu, Z.; Chen, M.; Huang, Z.; Ying, Y.; Fu, Y.; Xu, J.; et al. FGF20 promotes spinal cord injury repair by inhibiting the formation of necrotic corpuscle P-MLKL/P-RIP1/P-RIP3 in neurons. J. Cell Mol. Med. 2024, 28, e70109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakayama, Y.; Miyake, A.; Nakagawa, Y.; Mido, T.; Yoshikawa, M.; Konishi, M.; Itoh, N. Fgf19 is required for zebrafish lens and retina development. Dev. Biol. 2008, 313, 752–766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, X.; Hu, J.; Li, Y.; Zhuyun Yang, Z.; Zhu, H.; Zhou, L.; et, al. The cell adhesion molecule L1 regulates the expression of FGF21 and enhances neurite outgrowth. Brain Res. 2013, 1530, 13–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marebwa, B.K.; Adams, R.J.; Magwood, G.S.; Kindy, M.; Wilmskoetter, J.; Wolf, M.; Bonilha, L. Fibroblast growth factor23 is associated with axonal integrity and neural network architecture in the human frontal lobes. PLoS ONE 2018, 13, e0203460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 2006, 281, 15694–15700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, W.C.; Lin, X.; Torres, J. The strong dimerization of the transmembrane domain of the fibroblast growth factor receptor (FGFR) is modulated by C-terminal juxtamembrane residues. Protein Sci. 2009, 18, 450–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avivi, A.; Yayon, A.; Givol, D. A novel form of FGF receptor-3 using an alternative exon in the immunoglobulin domain III. FEBS Lett. 1993, 330, 249–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beenken, A.; Eliseenkova, A.V.; Ibrahimi, O.A.; Olsen, S.K.; Mohammadi, M. Plasticity in interactions of fibroblast growth factor 1 (FGF1) N terminus with FGF receptors underlies promiscuity of FGF1. J. Biol. Chem. 2012, 287, 3067–3078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trueb, B. Biology of FGFRL1, the fifth fibroblast growth factor receptor. Cell. Mol. Life Sci. 2011, 68, 951–964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ornitz, D.M.; Xu, J.; Colvin, J.S.; McEwen, D.G.; MacArthur, C.A.; Coulier, F.; Gao, G.; Goldfarb, M. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 1996, 271, 15292–15297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, H.-W.; Jin, H.-S.; Eom, Y.-B. FGFRL1 and FGF genes are associated with height, hypertension, and osteoporosis. PLoS ONE 2022, 17, e0273237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steinberg, F.; Zhuang, L.; Beyeler, M.; Kälin, R.E.; Mullis, P.E.; Brändli, A.W.; Trueb, B. The FGFRL1 receptor is shed from cell membranes, binds fibroblast growth factors (FGFs), and antagonizes FGF signaling in Xenopus embryos. J. Biol. Chem. 2010, 285, 2193–2202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rieckmann, T.; Zhuang, L.; Flück, C.E.; Trueb, B. Characterization of the first FGFRL1 mutation identified in a craniosynostosis patient. Biochim. Biophys. Acta 2009, 1792, 112–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allen, B.L.; Filla, M.S.; Rapraeger, A.C. Role of heparan sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition. J. Cell Biol. 2001, 155, 845–858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pye, D.A.; Vives, R.R.; Turnbull, J.E.; Hyde, P.; Gallagher, J.T. Heparan sulfate oligosaccharides require 6-O-sulfation for promotion of basic fibroblast growth factor mitogenic activity. J. Biol. Chem. 1998, 273, 22936–22942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, M.; Li, J.-P. Heparan sulfate proteoglycan—A common receptor for diverse cytokines. Cell Signal 2019, 54, 115–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nugent, M.A.; Iozzo, R.V. Fibroblast growth factor-2. Int. J. Biochem. Cell Biol. 2000, 32, 115–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plotnikov, A.N.; Schlessinger, J.; Hubbard, S.R.; Mohammadi, M. Structural basis for FGF receptor dimerization and activation. Cell 1999, 98, 641–650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stauber, D.J.; DiGabriele, A.D.; Hendrickson, W.A. Structural interactions of fibroblast growth factor receptor with its ligands. Proc. Natl. Acad. Sci. USA 2000, 97, 49–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pellegrini, L.; Burke, D.F.; von Delft, F.; Mulloy, B.; Blundell, T.L. Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin. Nature 2000, 407, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chua, C.C.; Rahimi, N.; Forsten-Williams, K.; Nugent, M.A. Heparan sulfate proteoglycans function as receptors for fibroblast growth factor-2 activation of extracellular signal-regulated kinases 1 and 2. Circ. Res. 2004, 94, 316–323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, G.; Goldfarb, M. Heparin can activate a receptor tyrosine kinase. EMBO J. 1995, 14, 2183–2190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venero Galanternik, M.; Kramer, K.L.; Piotrowski, T. Heparan Sulfate Proteoglycans Regulate Fgf Signaling and Cell Polarity during Collective Cell Migration. Cell Rep. 2015, 10, 414–428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pretorius, D.; Richter, R.P.; Anand, T.; Cardenas, J.C.; Richter, J.R. Alterations in heparan sulfate proteoglycan synthesis and sulfation and the impact on vascular endothelial function. Matrix Biol. Plus 2022, 16, 100121. [Google Scholar] [CrossRef] [PubMed]
- Schultz, V.; Suflita, M.; Liu, X.; Zhang, X.; Yu, Y.; Li, L.; Green, D.E.; Xu, Y.; Zhang, F.; DeAngelis, P.L.; et al. Heparan Sulfate Domains Required for Fibroblast Growth Factor 1 and 2 Signaling through Fibroblast Growth Factor Receptor 1c. J. Biol. Chem. 2017, 292, 2495–2509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumura, Y.; Aizawa, H.; Shiraki-Iida, T.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 1998, 242, 626–630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiraki-Iida, T.; Aizawa, H.; Matsumura, Y.; Sekine, S.; Iida, A.; Anazawa, H.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998, 424, 6–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onishi, K.; Miyake, M.; Hori, S.; Onishi, S.; Iida, K.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Tanaka, N.; Fujimoto, K. γ-Klotho is correlated with resistance to docetaxel in castration-resistant prostate cancer. Oncol. Lett. 2020, 19, 2306–2316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumoto, M.; Ogawa, N.; Fukuda, T.; Bando, Y.; Nishimura, T.; Usuda, J. Protein interaction networks characterizing the A549 cells Klotho transfected are associated with activated pro-apoptotic Bim and suppressed Wnt/β-catenin signaling pathway. Sci. Rep. 2024, 14, 2130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuro-o, M. Klotho and βKlotho. Adv. Exp. Med. Biol. 2012, 728, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.-C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hori, S.; Miyake, M.; Tatsumi, Y.; Morizawa, Y.; Nakai, Y.; Onishi, S.; Onishi, K.; Iida, K.; Gotoh, D.; Tanaka, N.; et al. Gamma-Klotho exhibits multiple roles in tumor growth of human bladder cancer. Oncotarget 2018, 9, 19508–19524. [Google Scholar] [CrossRef] [PubMed]
- Beenken, A.; Mohammadi, M. The structural biology of the FGF19 subfamily. Adv. Exp. Med. Biol. 2012, 728, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, L.; Sun, J.; Huang, Z.; Fang, M.; Zinkle, A.; Liu, X.; Lu, J.; Pan, Z.; Wang, Y.; et al. Structural basis for FGF hormone signalling. Nature 2023, 618, 862–870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, N.; Tohyama, K.; Yamashiro, S. Mechanostress resistance involving formin homology proteins: G- and F-actin homeostasis-driven filament nucleation and helical polymerization-mediated actin polymer stabilization. Biochem. Biophys. Res. Commun. 2018, 506, 323–329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selby, C.C.; Bear, R.S. The structure of actin-rich filaments of muscles according to x-ray diffraction. J. Biophys. Biochem. Cytol. 1956, 2, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Astbury, W.T.; Perry, S.V.; Reed, R.; Spark, L.C. An electron microscope and X-ray study of actin: I. Electron microscope 1947. Biochim. Biophys. Acta 1989, 1000, 163–176. [Google Scholar] [PubMed]
- Depue, R.H.; Rice, R.V. F-ACTIN IS A RIGHT-HANDED HELIX. J. Mol. Biol. 1965, 12, 302–303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Egelman, E.H. The structure of F-actin. J. Muscle Res. Cell Motil. 1985, 6, 129–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujii, T.; Iwane, A.H.; Yanagida, T.; Namba, K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature 2010, 467, 724–728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jegou, A.; Romet-Lemonne, G. The many implications of actin filament helicity. Semin. Cell Dev. Biol. 2020, 102, 65–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Mezgueldi, M. Tropomyosin dynamics. J. Muscle Res. Cell Motil. 2014, 35, 203–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oosterheert, W.; Boiero Sanders, M.; Funk, J.; Prumbaum, D.; Raunser, S.; Bieling, P. Molecular mechanism of actin filament elongation by formins. Science 2024, 384, eadn9560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carman, P.J.; Barrie, K.R.; Rebowski, G.; Dominguez, R. Structures of the free and capped ends of the actin filament. Science 2023, 380, 1287–1292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghasemi, F.; Cao, L.; Mladenov, M.; Guichard, B.; Way, M.; Jégou, A.; Romet-Lemonne, G. Regeneration of actin filament branches from the same Arp2/3 complex. Sci. Adv. 2024, 10, eadj7681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chou, S.Z.; Chatterjee, M.; Pollard, T.D. Mechanism of actin filament branch formation by Arp2/3 complex revealed by a high-resolution cryo-EM structureof the branch junction. Proc. Natl. Acad. Sci. USA 2022, 119, e2206722119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vemula, V.; Huber, T.; Ušaj, M.; Bugyi, B.; Månsson, A. Myosin and gelsolin cooperate in actin filament severing and actomyosin motor activity. J. Biol. Chem. 2021, 296, 100181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zweifel, M.E.; Courtemanche, N. Profilin’s Affinity for Formin Regulates the Availability of Filament Ends for Actin Monomer Binding. J. Mol. Biol. 2020, 432, 166688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kardos, R.; Pozsonyi, K.; Nevalainen, E.; Lappalainen, P.; Nyitrai, M.; Hild, G. The effects of ADF/cofilin and profilin on the conformation of the ATP-binding cleft of monomeric actin. Biophys. J. 2009, 96, 2335–2343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schutt, C.E.; Karlén, M.; Karlsson, R. A structural model of the profilin-formin pacemaker system for actin filament elongation. Sci. Rep. 2022, 12, 20515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, F.; Metz, I.; Rust, M.B. Regulation of actin filament assembly and disassembly in growth cone motility and axon guidance. Brain Res. Bull. 2023, 192, 21–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wioland, H.; Frémont, S.; Guichard, B.; Echard, A.; Jégou, A.; Romet-Lemonne, G. Actin filament oxidation by MICAL1 suppresses protections from cofilin-induced disassembly. EMBO Rep. 2021, 22, e50965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, V.W.; Nadkarni, A.V.; Brieher, W.M. Catastrophic actin filament bursting by cofilin, Aip1, and coronin. J. Biol. Chem. 2020, 295, 13299–13313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hall, A. Rho GTPases and the actin cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, M.; Nagano, T.; Chikama, T.; Nishida, T. Role of the small GTP-binding protein rho in epithelial cell migration in the rabbit cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 941–947. [Google Scholar] [PubMed] [PubMed Central]
- Vigil, D.; Kim, T.Y.; Plachco, A.; Garton, A.J.; Castaldo, L.; Pachter, J.A.; Dong, H.; Chen, X.; Tokar, B.; Campbell, S.L.; et al. ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res. 2012, 72, 5338–5347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, J.; Pan, Y.; Zheng, X.; Zhu, C.; Zhang, Y.; Shi, G.; Yao, L.; Chen, Y.; Xu, N. Comparative Study of ROCK1 and ROCK2 in Hippocampal Spine Formation and Synaptic Function. Neurosci. Bull. 2019, 35, 649–660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mertsch, S.; Thanos, S. Opposing signaling of ROCK1 and ROCK2 determines the switching of substrate specificity and the mode of migration of glioblastoma cells. Mol. Neurobiol. 2014, 49, 900–915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bourne, H.R.; Sanders, D.A.; McCormick, F. The GTPase superfamily: A conserved switch for diverse cell functions. Nature 1990, 348, 125–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuhlmann, N.; Wroblowski, S.; Knyphausen, P.; de Boor, S.; Brenig, J.; Zienert, A.Y.; Meyer-Teschendorf, K.; Praefcke, G.J.K.; Nolte, H.; Krüger, M.; et al. Structural and Mechanistic Insights into the Regulation of the Fundamental Rho Regulator RhoGDIα by Lysine Acetylation. J. Biol. Chem. 2016, 291, 5484–5499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanifa, M.; Singh, M.; Randhawa, P.K.; Jaggi, A.S.; Bali, A. A focus on Rho/ROCK signaling pathway: An emerging therapeutic target in depression. Eur. J. Pharmacol. 2023, 946, 175648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishizaki, T.; Maekawa, M.; Fujisawa, K.; Okawa, K.; Iwamatsu, A.; Fujita, A.; Watanabe, N.; Saito, Y.; Kakizuka, A.; Morii, N.; et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996, 15, 1885–1893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsui, T.; Amano, M.; Yamamoto, T.; Chihara, K.; Nakafuku, M.; Ito, M.; Nakano, T.; Okawa, K.; Iwamatsu, A.; Kaibuchi, K.; et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996, 15, 2208–2216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakagawa, O.; Fujisawa, K.; Ishizaki, T.; Saito, Y.; Nakao, K.; Narumiya, S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996, 392, 189–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, W.; Wen, J.; Chen, Z. Distinct Roles of ROCK1 and ROCK2 on the Cerebral Ischemia Injury and Subsequently Neurodegenerative Changes. Pharmacology 2020, 105, 3–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greathouse, K.M.; Boros, B.D.; Deslauriers, J.F.; Henderson, B.W.; Curtis, K.A.; Gentry, E.G.; Herskowitz, J.H. Distinct and complementary functions of rho kinase isoforms ROCK1 and ROCK2 in prefrontal cortex structural plasticity. Brain Struct. Funct. 2018, 223, 4227–4241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, L.; Dai, F.; Tang, L.; Bao, X.; Liu, Z.; Huang, C.; Zhang, T.; Yao, W. Distinct Roles For ROCK1 and ROCK2 in the Regulation of Oxldl-Mediated Endothelial Dysfunction. Cell Physiol. Biochem. 2018, 49, 565–577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujisawa, K.; Fujita, A.; Ishizaki, T.; Saito, Y.; Narumiya, S. Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J. Biol. Chem. 1996, 271, 23022–23028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arber, S.; Barbayannis, F.A.; Hanser, H.; Schneider, C.; Stanyon, C.A.; Bernard, O.; Caroni, P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998, 393, 805–809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freyd, G.; Kim, S.K.; Horvitz, H.R. Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature 1990, 344, 876–879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, N.; Higuchi, O.; Ohashi, K.; Nagata, K.; Wada, A.; Kangawa, K.; Nishida, E.; Mizuno, K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 1998, 393, 809–812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.-J.; Zhang, T.; Chen, S.; Cheng, D.; Wu, C.; Wang, X.; Duan, D.; Zhu, L.; Lou, H.; Gong, Z.; et al. The noncanonical role of the protease cathepsin D as a cofilin phosphatase. Cell Res. 2021, 31, 801–813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niwa, R.; Nagata-Ohashi, K.; Takeichi, M.; Mizuno, K.; Uemura, T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell 2002, 108, 233–246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jayo, A.; Parsons, M.; Adams, J.C. A novel Rho-dependent pathway that drives interaction of fascin-1 with p-Lin-11/Isl-1/Mec-3 kinase (LIMK) 1/2 to promote fascin-1/actin binding and filopodia stability. BMC Biol. 2012, 10, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klein, O.; Krier-Burris, R.A.; Lazki-Hagenbach, P.; Gorzalczany, Y.; Mei, Y.; Ji, P.; Bochner, B.S.; Sagi-Eisenberg, R. Mammalian diaphanous-related formin 1 (mDia1) coordinates mast cell migration and secretion through its actin-nucleating activity. J. Allergy Clin. Immunol. 2019, 144, 1074–1090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kühn, S.; Geyer, M. Formins as effector proteins of Rho GTPases. Small GTPases 2014, 5, e29513. [Google Scholar] [CrossRef] [PubMed]
- Lammers, M.; Meyer, S.; Kühlmann, D.; Wittinghofer, A. Specificity of interactions between mDia isoforms and Rho proteins. J. Biol. Chem. 2008, 283, 35236–35246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero, S.; Le Clainche, C.; Didry, D.; Egile, C.; Pantaloni, D.; Carlier, M.-F. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 2004, 119, 419–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goode, B.L.; Eck, M.J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007, 76, 593–627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmer, N.J.; Barrie, K.R.; Dominguez, R. Mechanisms of actin filament severing and elongation by formins. Nature 2024, 632, 437–442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, J.; Welch, W.J.; Diprospero, N.A.; Diamond, M.I. Phosphorylation of profilin by ROCK1 regulates polyglutamine aggregation. Mol. Cell. Biol. 2008, 28, 5196–5208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, J.; Diamond, M.I. Protein phosphatase 1 dephosphorylates profilin-1 at Ser-137. PLoS ONE 2012, 7, e32802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bieling, P.; Rottner, K. From WRC to Arp2/3: Collective molecular mechanisms of branched actin network assembly. Curr. Opin. Cell Biol. 2023, 80, 102156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Molinie, N.; Gautreau, A. The Arp2/3 Regulatory System and Its Deregulation in Cancer. Physiol. Rev. 2018, 98, 215–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, H.; Qi, D.; Guan, X.; Jiang, G.; Liao, Z.; Zhang, X.; Chen, P.; Li, N.; Wu, M. c-Abl tyrosine kinase regulates neutrophil crawling behavior under fluid shear stress via Rac/PAK/LIMK/cofilin signaling axis. J. Cell Biochem. 2018, 119, 2806–2817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beli, P.; Mascheroni, D.; Xu, D.; Innocenti, M. WAVE and Arp2/3 jointly inhibit filopodium formation by entering into a complex with mDia2. Nat. Cell Biol. 2008, 10, 849–857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chua, X.L.; Tong, C.S.; Su, M.; Xu, X.J.; Xiao, S.; Wu, X.; Wu, M. Competition and synergy of Arp2/3 and formins in nucleating actin waves. Cell Rep. 2024, 43, 114423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a022608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nogales, E.; Whittaker, M.; Milligan, R.A.; Downing, K.H. High-resolution model of the microtubule. Cell 1999, 96, 79–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brito, C.; Serna, M.; Guerra, P.; Llorca, O.; Surrey, T. Transition of human γ-tubulin ring complex into a closed conformation during microtubule nucleation. Science 2024, 383, 870–876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mandelkow, E.M.; Schultheiss, R.; Rapp, R.; Müller, M.; Mandelkow, E. On the surface lattice of microtubules: Helix starts, protofilament number, seam, and handedness. J. Cell Biol. 1986, 102, 1067–1073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nogales, E.; Zhang, R. Visualizing microtubule structural transitions and interactions with associated proteins. Curr. Opin. Struct. Biol. 2016, 37, 90–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, J.L.; Pražák, V.; Vasishtan, D.; Siggel, M.; Hentzschel, F.; Binder, A.M.; Pietsch, E.; Kosinski, J.; Frischknecht, F.; Gilberger, T.W.; et al. Variable microtubule architecture in the malaria parasite. Nat. Commun. 2023, 14, 1216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yau, K.W.; van Beuningen, S.F.B.; Cunha-Ferreira, I.; Cloin, B.M.C.; van Battum, E.Y.; Will, L.; Schätzle, P.; Tas, R.P.; van Krugten, J.; Katrukha, E.A.; et al. Microtubule minus-end binding protein CAMSAP2 controls axon specification and dendrite development. Neuron 2014, 82, 1058–1073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bettencourt-Dias, M.; Glover, D.M. Centrosome biogenesis and function: Centrosomics brings new understanding. Nat. Rev. Mol. Cell. Biol. 2007, 8, 451–463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alushin, G.M.; Lander, G.C.; Kellogg, E.H.; Zhang, R.; Baker, D.; Nogales, E. High-resolution microtubule structures reveal the structural transitions in αβ-tubulin upon GTP hydrolysis. Cell 2014, 157, 1117–1129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rice, L.M.; Moritz, M.; Agard, D.A. Microtubules form by progressively faster tubulin accretion, not by nucleation-elongation. J. Cell Biol. 2021, 220, e202012079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seetharaman, S.; Vianay, B.; Roca, V.; Farrugia, A.J.; De Pascalis, C.; Boëda, B.; Dingli, F.; Loew, D.; Vassilopoulos, S.; Bershadsky, A.; et al. Microtubules tune mechanosensitive cell responses. Nat. Mater. 2022, 21, 366–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yildiz, A.; Zhao, Y. Dyneins. Curr. Biol. 2023, 33, R1274–R1279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atkins, M.; Nicol, X.; Fassier, C. Microtubule remodelling as a driving force of axon guidance and pruning. Semin. Cell Dev. Biol. 2023, 140, 35–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flyvbjerg, H.; Jobs, E.; Leibler, S. Kinetics of self-assembling microtubules: An “inverse problem” in biochemistry. Proc. Natl. Acad. Sci. USA 1996, 93, 5975–5979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buck, K.B.; Zheng, J.Q. Growth cone turning induced by direct local modification of microtubule dynamics. J. Neurosci. 2002, 22, 9358–9367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mack, T.G.; Koester, M.P.; Pollerberg, G.E. The microtubule-associated protein MAP1B is involved in local stabilization of turning growth cones. Mol. Cell. Neurosci. 2000, 15, 51–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koester, M.P.; Müller, O.; Pollerberg, G.E. Adenomatous polyposis coli is differentially distributed in growth cones and modulates their steering. J. Neurosci. 2007, 27, 12590–12600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alberico, E.O.; Zhu, Z.C.; Wu, Y.-F.O.; Gardner, M.K.; Kovar, D.R.; Goodson, H.V. Interactions between the Microtubule Binding Protein EB1 and F-Actin. J. Mol. Biol. 2016, 428, 1304–1314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mimori-Kiyosue, Y.; Grigoriev, I.; Lansbergen, G.; Sasaki, H.; Matsui, C.; Severin, F.; Galjart, N.; Grosveld, F.; Vorobjev, I.; Tsukita, S.; et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 2005, 168, 141–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hahn, I.; Voelzmann, A.; Parkin, J.; Fülle, J.B.; Slater, P.G.; Lowery, L.A.; Sanchez-Soriano, N.; Prokop, A. Tau, XMAP215/Msps and Eb1 co-operate interdependently to regulate microtubule polymerisation and bundle formation in axons. PLoS Genet. 2021, 17, e1009647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farmer, V.J.; Zanic, M. TOG-domain proteins. Curr. Biol. 2021, 31, R499–R501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puri, D.; Ponniah, K.; Biswas, K.; Basu, A.; Dey, S.; Lundquist, E.A.; Ghosh-Roy, A. Wnt signaling establishes the microtubule polarity in neurons through regulation of Kinesin-13. J. Cell Biol. 2021, 220, e202005080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friel, C.T.; Welburn, J.P. Parts list for a microtubule depolymerising kinesin. Biochem. Soc. Trans. 2018, 46, 1665–1672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, J.T.; Belsham, H.R.; Rathbone, A.J.; Wickstead, B.; Gell, C.; Friel, C.T. The family-specific α4-helix of the kinesin-13, MCAK, is critical to microtubule end recognition. Open Biol. 2016, 6, 160223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beaudet, D.; Berger, C.L.; Hendricks, A.G. The types and numbers of kinesins and dyneins transporting endocytic cargoes modulate their motility and response to tau. J. Biol. Chem. 2024, 300, 107323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sloboda, R.D.; Dentler, W.L.; Rosenbaum, J.L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry 1976, 15, 4497–4505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murphy, D.B.; Borisy, G.G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc. Natl. Acad. Sci. USA 1975, 72, 2696–2700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishida, K.; Matsumura, K.; Tamura, M.; Nakamichi, T.; Shimamori, K.; Kuragano, M.; Kabir, A.M.R.; Kakugo, A.; Kotani, S.; Nishishita, N.; et al. Effects of three microtubule-associated proteins (MAP2, MAP4, and Tau) on microtubules’ physical properties and neurite morphology. Sci. Rep. 2023, 13, 8870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanai, Y.; Takemura, R.; Oshima, T.; Mori, H.; Ihara, Y.; Yanagisawa, M.; Masaki, T.; Hirokawa, N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J. Cell Biol. 1989, 109, 1173–1184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanai, Y.; Chen, J.; Hirokawa, N. Microtubule bundling by tau proteins in vivo: Analysis of functional domains. EMBO J. 1992, 11, 3953–3961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takemura, R.; Okabe, S.; Umeyama, T.; Kanai, Y.; Cowan, N.J.; Hirokawa, N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J. Cell Sci. 1992, 103 Pt 4, 953–964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feizabadi, M.S.; Castillon, V.J. The Effect of Tau and Taxol on Polymerization of MCF7 Microtubules In Vitro. Int. J. Mol. Sci. 2022, 23, 677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeGiosio, R.A.; Needham, P.G.; Andrews, O.A.; Tristan, H.; Grubisha, M.J.; Brodsky, J.L.; Camacho, C.; Sweet, R.A. Differential regulation of MAP2 by phosphorylation events in proline-rich versus C-terminal domains. FASEB J. 2023, 37, e23194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.-H.; Rhoades, E. Heterogeneous Tau-Tubulin Complexes Accelerate Microtubule Polymerization. Biophys. J. 2017, 112, 2567–2574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadavath, H.; Hofele, R.V.; Biernat, J.; Kumar, S.; Tepper, K.; Urlaub, H.; Mandelkow, E.; Zweckstetter, M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc. Natl. Acad. Sci. USA 2015, 112, 7501–7506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cassimeris, L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 2002, 14, 18–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belmont, L.D.; Mitchison, T.J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell 1996, 84, 623–631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curmi, P.A.; Andersen, S.S.; Lachkar, S.; Gavet, O.; Karsenti, E.; Knossow, M.; Sobel, A. The stathmin/tubulin interaction in vitro. J. Biol. Chem. 1997, 272, 25029–25036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howell, B.; Larsson, N.; Gullberg, M.; Cassimeris, L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin. Mol. Biol. Cell 1999, 10, 105–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Paolo, G.; Antonsson, B.; Kassel, D.; Riederer, B.M.; Grenningloh, G. Phosphorylation regulates the microtubule-destabilizing activity of stathmin and its interaction with tubulin. FEBS Lett. 1997, 416, 149–152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Y.; Eng, C.H.; Schmoranzer, J.; Cabrera-Poch, N.; Morris, E.J.S.; Chen, M.; Wallar, B.J.; Alberts, A.S.; Gundersen, G.G. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 2004, 6, 820–830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eng, C.H.; Huckaba, T.M.; Gundersen, G.G. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol. Biol. Cell 2006, 17, 5004–5016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Wojnacki, J.; Quassollo, G.; Bordenave, M.D.; Unsain, N.; Martínez, G.F.; Szalai, A.M.; Pertz, O.; Gundersen, G.G.; Bartolini, F.; Stefani, F.D.; et al. Dual spatio-temporal regulation of axon growth and microtubule dynamics by RhoA signaling pathways. J. Cell Sci. 2024, 137, 261970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeeshan, M.; Rea, E.; Abel, S.; Vukušić, K.; Markus, R.; Brady, D.; Eze, A.; Rashpa, R.; Balestra, A.C.; Bottrill, A.R.; et al. Plasmodium ARK2 and EB1 drive unconventional spindle dynamics, during chromosome segregation in sexual transmission stages. Nat. Commun. 2023, 14, 5652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moriwaki, T.; Goshima, G. Five factors can reconstitute all three phases of microtubule polymerization dynamics. J. Cell Biol. 2016, 215, 357–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsu, J.-L.; Leu, W.-J.; Hsu, L.-C.; Ho, C.-H.; Liu, S.-P.; Guh, J.-H. Phosphodiesterase Type 5 Inhibitors Synergize Vincristine in Killing Castration-Resistant Prostate Cancer Through Amplifying Mitotic Arrest Signaling. Front. Oncol. 2020, 10, 1274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Chen, S.; Shen, F.; Long, D.; Yu, T.; Wu, M.; Lin, X. In vitro neutralization of autocrine IL-10 affects Op18/stathmin signaling in non-small cell lung cancer cells. Oncol. Rep. 2019, 41, 501–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alesi, G.N.; Jin, L.; Li, D.; Magliocca, K.R.; Kang, Y.; Chen, Z.G.; Shin, D.M.; Khuri, F.R.; Kang, S. RSK2 signals through stathmin to promote microtubule dynamics and tumor metastasis. Oncogene 2016, 35, 5412–5421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Xu, L.; Shen, Y.; Wang, L.; Lai, X.; Hu, H. Qingxin Kaiqiao Fang decreases Tau hyperphosphorylation in Alzheimer’s disease via the PI3K/Akt/GSK3β pathway in vitro and in vivo. J. Ethnopharmacol. 2024, 318 Pt B, 117031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Torregrossa, R.; Whiteman, M.; et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ackmann, J.; Brüge, A.; Gotina, L.; Lim, S.; Jahreis, K.; Vollbrecht, A.-L.; Kim, Y.K.; Pae, A.N.; Labus, J.; Ponimaskin, E. Structural determinants for activation of the Tau kinase CDK5 by the serotonin receptor 5-HT7R. Cell Commun. Signal. 2024, 22, 233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matenia, D.; Mandelkow, E.-M. The tau of MARK: A polarized view of the cytoskeleton. Trends Biochem. Sci. 2009, 34, 332–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pollard, T.D.; Goldman, R.D. Overview of the Cytoskeleton from an Evolutionary Perspective. Cold Spring Harb. Perspect. Biol. 2018, 10, a030288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eibauer, M.; Weber, M.S.; Kronenberg-Tenga, R.; Beales, C.T.; Boujemaa-Paterski, R.; Turgay, Y.; Sivagurunathan, S.; Kraxner, J.; Köster, S.; Goldman, R.D.; et al. Vimentin filaments integrate low-complexity domains in a complex helical structure. Nat. Struct. Mol. Biol. 2024, 31, 939–949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pekny, M.; Lane, E.B. Intermediate filaments and stress. Exp. Cell Res. 2007, 313, 2244–2254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doganyigit, Z.; Eroglu, E.; Okan, A. Intermediate filament proteins are reliable immunohistological biomarkers to help diagnose multiple tissue-specific diseases. Anat. Histol. Embryol. 2023, 52, 655–672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, X.; Qiu, Y.; Gao, Y.; Wan, D.; Zhu, H. A Subtle Network Mediating Axon Guidance: Intrinsic Dynamic Structure of Growth Cone, Attractive and Repulsive Molecular Cues, and the Intermediate Role of Signaling Pathways. Neural Plast. 2019, 2019, 1719829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutour-Provenzano, G.; Etienne-Manneville, S. Intermediate filaments. Curr. Biol. 2021, 31, R522–R529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guzenko, D.; Chernyatina, A.A.; Strelkov, S.V. Crystallographic Studies of Intermediate Filament Proteins. Subcell. Biochem. 2017, 82, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, P.-J.; Stalmans, G.; Lilina, A.V.; Fiala, J.; Novak, P.; Herrmann, H.; Strelkov, S.V. Molecular Interactions Driving Intermediate Filament Assembly. Cells 2021, 10, 2457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eldirany, S.A.; Lomakin, I.B.; Ho, M.; Bunick, C.G. Recent insight into intermediate filament structure. Curr. Opin. Cell Biol. 2021, 68, 132–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Snider, N.T.; Omary, M.B. Post-translational modifications of intermediate filament proteins: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 163–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraxner, J.; Köster, S. Influence of phosphorylation on intermediate filaments. Biol. Chem. 2023, 404, 821–827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Badowski, C.; Benny, P.; Verma, C.S.; Lane, E.B. Desmoplakin CSM models unravel mechanisms regulating the binding to intermediate filaments and putative therapeutics for cardiocutaneous diseases. Sci. Rep. 2024, 14, 23206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X.; Lin, Y.; Kato, M.; Mori, E.; Liszczak, G.; Sutherland, L.; Sysoev, V.O.; Murray, D.T.; Tycko, R.; McKnight, S.L. Transiently structured head domains control intermediate filament assembly. Proc. Natl. Acad. Sci. USA 2021, 118, e2022121118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Birkenberger, L.; Ip, W. Properties of the desmin tail domain: Studies using synthetic peptides and antipeptide antibodies. J. Cell Biol. 1990, 111 Pt 1, 2063–2075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inaba, H.; Yamakawa, D.; Tomono, Y.; Enomoto, A.; Mii, S.; Kasahara, K.; Goto, H.; Inagaki, M. Regulation of keratin 5/14 intermediate filaments by CDK1, Aurora-B, and Rho-kinase. Biochem. Biophys. Res. Commun. 2018, 498, 544–550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makihara, H.; Inaba, H.; Enomoto, A.; Tanaka, H.; Tomono, Y.; Ushida, K.; Goto, M.; Kurita, K.; Nishida, Y.; Kasahara, K.; et al. Desmin phosphorylation by Cdk1 is required for efficient separation of desmin intermediate filaments in mitosis and detected in murine embryonic/newborn muscle and human rhabdomyosarcoma tissues. Biochem. Biophys. Res. Commun. 2016, 478, 1323–1329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dabbaghizadeh, A.; Paré, A.; Cheng-Boivin, Z.; Dagher, R.; Minotti, S.; Dicaire, M.-J.; Brais, B.; Young, J.C.; Durham, H.D.; Gentil, B.J. The J Domain of Sacsin Disrupts Intermediate Filament Assembly. Int. J. Mol. Sci. 2022, 23, 15742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, J.; Lowthert, L.A.; Ghori, N.; Omary, M.B. The 70-kDa heat shock proteins associate with glandular intermediate filaments in an ATP-dependent manner. J. Biol. Chem. 1995, 270, 915–922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, R.; Song, J.; Ruze, R.; Chen, Y.; Yin, X.; Wang, C.; Zhao, Y. SQLE promotes pancreatic cancer growth by attenuating ER stress and activating lipid rafts-regulated Src/PI3K/Akt signaling pathway. Cell Death Dis. 2023, 14, 497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rezaei, S.; Nikpanjeh, N.; Rezaee, A.; Gholami, S.; Hashemipour, R.; Biavarz, N.; Yousefi, F.; Tashakori, A.; Salmani, F.; Rajabi, R.; et al. PI3K/Akt signaling in urological cancers: Tumorigenesis function, therapeutic potential, and therapy response regulation. Eur. J. Pharmacol. 2023, 955, 175909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Browaeys-Poly, E.; Perdereau, D.; Lescuyer, A.; Burnol, A.-F.; Cailliau, K. Akt interaction with PLC(gamma) regulates the G(2)/M transition triggered by FGF receptors from MDA-MB-231 breast cancer cells. Anticancer. Res. 2009, 29, 4965–4969. [Google Scholar] [PubMed] [PubMed Central]
- Kouhara, H.; Hadari, Y.R.; Spivak-Kroizman, T.; Schilling, J.; Bar-Sagi, D.; Lax, I.; Schlessinger, J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 1997, 89, 693–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.-L.J.; Bertolesi, G.E.; Dueck, S.; Hehr, C.L.; McFarlane, S. The Expression of Key Guidance Genes at a Forebrain Axon Turning Point Is Maintained by Distinct Fgfr Isoforms but a Common Downstream Signal Transduction Mechanism. eNeuro 2019, 6, ENEURO.0086-19.2019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakinuma, N.; Roy, B.C.; Zhu, Y.; Wang, Y.; Kiyama, R. Kank regulates RhoA-dependent formation of actin stress fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J. Cell Biol. 2008, 181, 537–549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, X.; Xu, G.; Lin, Z.; Zou, F.; Liu, S.; Zhang, Y.; Fu, W.; Jiang, J.; Ma, X.; Song, J. Engineered exosomes enriched in netrin-1 modRNA promote axonal growth in spinal cord injury by attenuating inflammation and pyroptosis. Biomater. Res. 2023, 27, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, Y.; Kanyo, J.; Wilson, R.; Bathla, S.; Cardozo, P.L.; Tong, L.; Qin, S.; Fuentes, L.A.; Pinheiro-de-Sousa, I.; Huynh, T.; et al. Subcellular proteomics and iPSC modeling uncover reversible mechanisms of axonal pathology in Alzheimer’s disease. Nat. Aging 2025, 5, 504–527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schaeper, U.; Gehring, N.H.; Fuchs, K.P.; Sachs, M.; Kempkes, B.; Birchmeier, W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 2000, 149, 1419–1432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clark, J.F.; Soriano, P. FRS2-independent GRB2 interaction with FGFR2 is not required for embryonic development. Biol. Open 2023, 12, bio059942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Y.; Kakudo, N.; Morimoto, N.; Lai, F.; Taketani, S.; Kusumoto, K. Fibroblast growth factor-2 stimulates proliferation of human adipose-derived stem cells via Src activation. Stem Cell Res. Ther. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishida, T.; Ito, J.-I.; Nagayasu, Y.; Yokoyama, S. FGF-1-induced reactions for biogenesis of apoE-HDL are mediated by src in rat astrocytes. J. Biochem. 2009, 146, 881–886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fox, M.; Mott, H.R.; Owen, D. Class IA PI3K regulatory subunits: p110-independent roles and structures. Biochem. Soc. Trans. 2020, 48, 1397–1417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fontana, F.; Giannitti, G.; Marchesi, S.; Limonta, P. The PI3K/Akt Pathway and Glucose Metabolism: A Dangerous Liaison in Cancer. Int. J. Biol. Sci. 2024, 20, 3113–3125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahl, K.D.; Almeida, A.R.; Hathaway, H.A.; Bourne, J.; Brown, T.L.; Finseth, L.T.; Wood, T.L.; Macklin, W.B. mTORC2 Loss in Oligodendrocyte Progenitor Cells Results in Regional Hypomyelination in the Central Nervous System. J. Neurosci. 2023, 43, 540–558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, Z.; Fang, W.; Wu, B.; He, M.; Li, S.; Wei, J.; Hao, Y.; Jin, L.; Liu, M.; Zhang, X.; et al. Targeting Smurf1 to block PDK1-Akt signaling in KRAS-mutated colorectal cancer. Nat. Chem. Biol. 2025, 21, 59–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Xu, W.; Wang, D.; Wang, L.; Fang, Q.; Wan, X.; Zhang, J.; Hu, Y.; Li, H.; Zhang, J.; et al. 4R Tau Modulates Cocaine-Associated Memory through Adult Dorsal Hippocampal Neurogenesis. J. Neurosci. 2021, 41, 6753–6774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Illenberger, S.; Drewes, G.; Trinczek, B.; Biernat, J.; Meyer, H.E.; Olmsted, J.B.; Mandelkow, E.M.; Mandelkow, E. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. J. Biol. Chem. 1996, 271, 10834–10843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thapa, N.; Chen, M.; Cryns, V.L.; Anderson, R. A p85 isoform switch enhances PI3K activation on endosomes by a MAP4- and PI3P-dependent mechanism. Cell Rep. 2024, 43, 114119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.; Hai, Z.; Hou, L.; Liu, Y.; Zhang, D.; Zhou, X. Baicalin Attenuates Panton-Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 14520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pagano, A.; Breuzard, G.; Parat, F.; Tchoghandjian, A.; Figarella-Branger, D.; De Bessa, T.C.; Garrouste, F.; Douence, A.; Barbier, P.; Kovacic, H. Tau Regulates Glioblastoma Progression, 3D Cell Organization, Growth and Migration via the PI3K-AKT Axis. Cancers 2021, 13, 5818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fortier, A.-M.; Van Themsche, C.; Asselin, E.; Cadrin, M. Akt isoforms regulate intermediate filament protein levels in epithelial carcinoma cells. FEBS Lett. 2010, 584, 984–988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Fang, F.; Chen, S.; Jing, X.; Zhuang, Y.; Xie, Y. Dual efficacy of Fasudil at improvement of survival and reinnervation of flap through RhoA/ROCK/PI3K/Akt pathway. Int. Wound J. 2022, 19, 2000–2011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Schoeps, B.; Yao, D.; Zhang, Z.; Schuck, K.; Tissen, V.; Jäger, C.; Schlitter, A.M.; van der Kammen, R.; Ludwig, C.; et al. mTORC1 and mTORC2 Converge on the Arp2/3 Complex to Promote KrasG12D-Induced Acinar-to-Ductal Metaplasia and Early Pancreatic Carcinogenesis. Gastroenterology 2021, 160, 1755–1770.e17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, T.; Umetsu, A.; Tamanoi, F. Characterization of the Rheb-mTOR signaling pathway in mammalian cells: Constitutive active mutants of Rheb and mTOR. Methods Enzymol. 2008, 438, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Labrèche, C.; Cook, D.P.; Abou-Hamad, J.; Pascoal, J.; Pryce, B.R.; Al-Zahrani, K.N.; Sabourin, L.A. Periostin gene expression in neu-positive breast cancer cells is regulated by a FGFR signaling cross talk with TGFβ/PI3K/AKT pathways. Breast Cancer Res. 2021, 23, 107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masri, J.; Bernath, A.; Martin, J.; Jo, O.D.; Vartanian, R.; Funk, A.; Gera, J. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 2007, 67, 11712–11720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoi, C.S.L.; Xiong, W.; Rebay, I. Retinal Axon Guidance Requires Integration of Eya and the Jak/Stat Pathway into Phosphotyrosine-Based Signaling Circuitries in Drosophila. Genetics 2016, 203, 1283–1295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, P.; Huang, T.; Zou, Q.; Liu, D.; Wang, Y.; Tan, X.; Wei, Y.; Qiu, H. FGFR2 Promotes Expression of PD-L1 in Colorectal Cancer via the JAK/STAT3 Signaling Pathway. J. Immunol. 2019, 202, 3065–3075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, K.-O.; Chen, Y.; Po, H.-M.; Lok, K.-C.; Gong, K.; Ip, N.Y. Identification of the Jak/Stat proteins as novel downstream targets of EphA4 signaling in muscle: Implications in the regulation of acetylcholinesterase expression. J. Biol. Chem. 2004, 279, 13383–13392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Godoi, M.A.; Camilli, A.C.; Gonzales, K.G.A.; Costa, V.B.; Papathanasiou, E.; Leite, F.R.M.; Guimarães-Stabili, M.R. JAK/STAT as a Potential Therapeutic Target for Osteolytic Diseases. Int. J. Mol. Sci. 2023, 24, 10290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, B.; Cai, J.-P.; Luo, Y.-L.; Chen, C.; Zhang, S. The Specific Roles of JAK/STAT Signaling Pathway in Sepsis. Inflammation 2015, 38, 1599–1608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goto, K.; Chiba, Y.; Matsusue, K.; Hattori, Y.; Maitani, Y.; Sakai, H.; Kimura, S.; Misawa, M. The proximal STAT6 and NF-kappaB sites are responsible for IL-13- and TNF-alpha-induced RhoA transcriptions in human bronchial smooth muscle cells. Pharmacol. Res. 2010, 61, 466–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Hu, X.; Boumsell, L.; Ivashkiv, L.B. IFN-gamma and STAT1 arrest monocyte migration and modulate RAC/CDC42 pathways. J. Immunol. 2008, 180, 8057–8065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jablonka, S.; Dombert, B.; Asan, E.; Sendtner, M. Mechanisms for axon maintenance and plasticity in motoneurons: Alterations in motoneuron disease. J. Anat. 2014, 224, 3–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selvaraj, B.T.; Frank, N.; Bender, F.L.P.; Asan, E.; Sendtner, M. Local axonal function of STAT3 rescues axon degeneration in the pmn model of motoneuron disease. J. Cell Biol. 2012, 199, 437–451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mar, F.M.; Simões, A.R.; Rodrigo, I.S.; Sousa, M.M. Inhibitory Injury Signaling Represses Axon Regeneration After Dorsal Root Injury. Mol. Neurobiol. 2016, 53, 4596–4605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moreno-Manzano, V.; Rodríguez-Jiménez, F.J.; García-Roselló, M.; Laínez, S.; Erceg, S.; Calvo, M.T.; Ronaghi, M.; Lloret, M.; Planells-Cases, R.; Sánchez-Puelles, J.M.; et al. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells 2009, 27, 733–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sutherland, D.J.; Pujic, Z.; Goodhill, G.J. Calcium signaling in axon guidance. Trends Neurosci. 2014, 37, 424–432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Jia, Y.-C.; Cui, K.; Li, N.; Zheng, Z.-Y.; Wang, Y.-Z.; Yuan, X.-B. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 2005, 434, 894–898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gasperini, R.; Choi-Lundberg, D.; Thompson, M.J.W.; Mitchell, C.B.; Foa, L. Homer regulates calcium signalling in growth cone turning. Neural Dev. 2009, 4, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Browaeys-Poly, E.; Blanquart, C.; Perdereau, D.; Antoine, A.-F.; Goenaga, D.; Luzy, J.-P.; Chen, H.; Garbay, C.; Issad, T.; Cailliau, K.; et al. Grb14 inhibits FGF receptor signaling through the regulation of PLCγ recruitment and activation. FEBS Lett. 2010, 584, 4383–4388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishiyama, M.; Hoshino, A.; Tsai, L.; Henley, J.R.; Goshima, Y.; Tessier-Lavigne, M.; Poo, M.-M.; Hong, K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 2003, 423, 990–995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hutchins, B.I.; Klenke, U.; Wray, S. Calcium release-dependent actin flow in the leading process mediates axophilic migration. J. Neurosci. 2013, 33, 11361–11371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markovinovic, A.; Martín-Guerrero, S.M.; Mórotz, G.M.; Salam, S.; Gomez-Suaga, P.; Paillusson, S.; Greig, J.; Lee, Y.; Mitchell, J.C.; Noble, W.; et al. Stimulating VAPB-PTPIP51 ER-mitochondria tethering corrects FTD/ALS mutant TDP43 linked Ca2+ and synaptic defects. Acta Neuropathol. Commun. 2024, 12, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pierozan, P.; Zamoner, A.; Soska, Â.K.; de Lima, B.O.; Reis, K.P.; Zamboni, F.; Wajner, M.; Pessoa-Pureur, R. Signaling mechanisms downstream of quinolinic acid targeting the cytoskeleton of rat striatal neurons and astrocytes. Exp. Neurol. 2012, 233, 391–399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Khalil, R.A. Evolving mechanisms of vascular smooth muscle contraction highlight key targets in vascular disease. Biochem Pharmacol. 2018, 153, 91–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ekinci, F.J.; Shea, T.B. Free PKC catalytic subunits (PKM) phosphorylate tau via a pathway distinct from that utilized by intact PKC. Brain Res. 1999, 850, 207–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antoine-Bertrand, J.; Duquette, P.M.; Alchini, R.; Kennedy, T.E.; Fournier, A.E.; Lamarche-Vane, N. p120RasGAP Protein Mediates Netrin-1 Protein-induced Cortical Axon Outgrowth and Guidance. J. Biol. Chem. 2016, 291, 4589–4602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meier, C.; Anastasiadou, S.; Knöll, B. Ephrin-A5 suppresses neurotrophin evoked neuronal motility, ERK activation and gene expression. PLoS ONE 2011, 6, e26089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elowe, S.; Holland, S.J.; Kulkarni, S.; Pawson, T. Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol. Cell. Biol. 2001, 21, 7429–7441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, K.; Xiang, Y.; Wang, X.; Wang, Q.; Zhong, M.; Wang, S.; Wang, X.; Fan, J.; Kitazato, K.; Wang, Y. Epidermal growth factor receptor-PI3K signaling controls cofilin activity to facilitate herpes simplex virus 1 entry into neuronal cells. mBio 2014, 5, e00958-e13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guise, S.; Braguer, D.; Carles, G.; Delacourte, A.; Briand, C. Hyperphosphorylation of tau is mediated by ERK activation during anticancer drug-induced apoptosis in neuroblastoma cells. J. Neurosci. Res. 2001, 63, 257–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thananthirige, K.P.M.; Chitranshi, N.; Basavarajappa, D.; Rajput, R.; Abbasi, M.; Palanivel, V.; Gupta, V.B.; Paulo, J.A.; Koronyo-Hamaoui, M.; Mirzaei, M.; et al. Tau modulation through AAV9 therapy augments Akt/Erk survival signalling in glaucoma mitigating the retinal degenerative phenotype. Acta Neuropathol. Commun. 2024, 12, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sindi, R.A.; Harris, W.; Arnott, G.; Flaskos, J.; Lloyd Mills, C.; Hargreaves, A.J. Chlorpyrifos- and chlorpyrifos oxon-induced neurite retraction in pre-differentiated N2a cells is associated with transient hyperphosphorylation of neurofilament heavy chain and ERK 1/2. Toxicol. Appl. Pharmacol. 2016, 308, 20–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dent, E.W.; Barnes, A.M.; Tang, F.; Kalil, K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J. Neurosci. 2004, 24, 3002–3012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conway, C.D.; Howe, K.M.; Nettleton, N.K.; Price, D.J.; Mason, J.O.; Pratt, T. Heparan sulfate sugar modifications mediate the functions of slits and other factors needed for mouse forebrain commissure development. J. Neurosci. 2011, 31, 1955–1970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
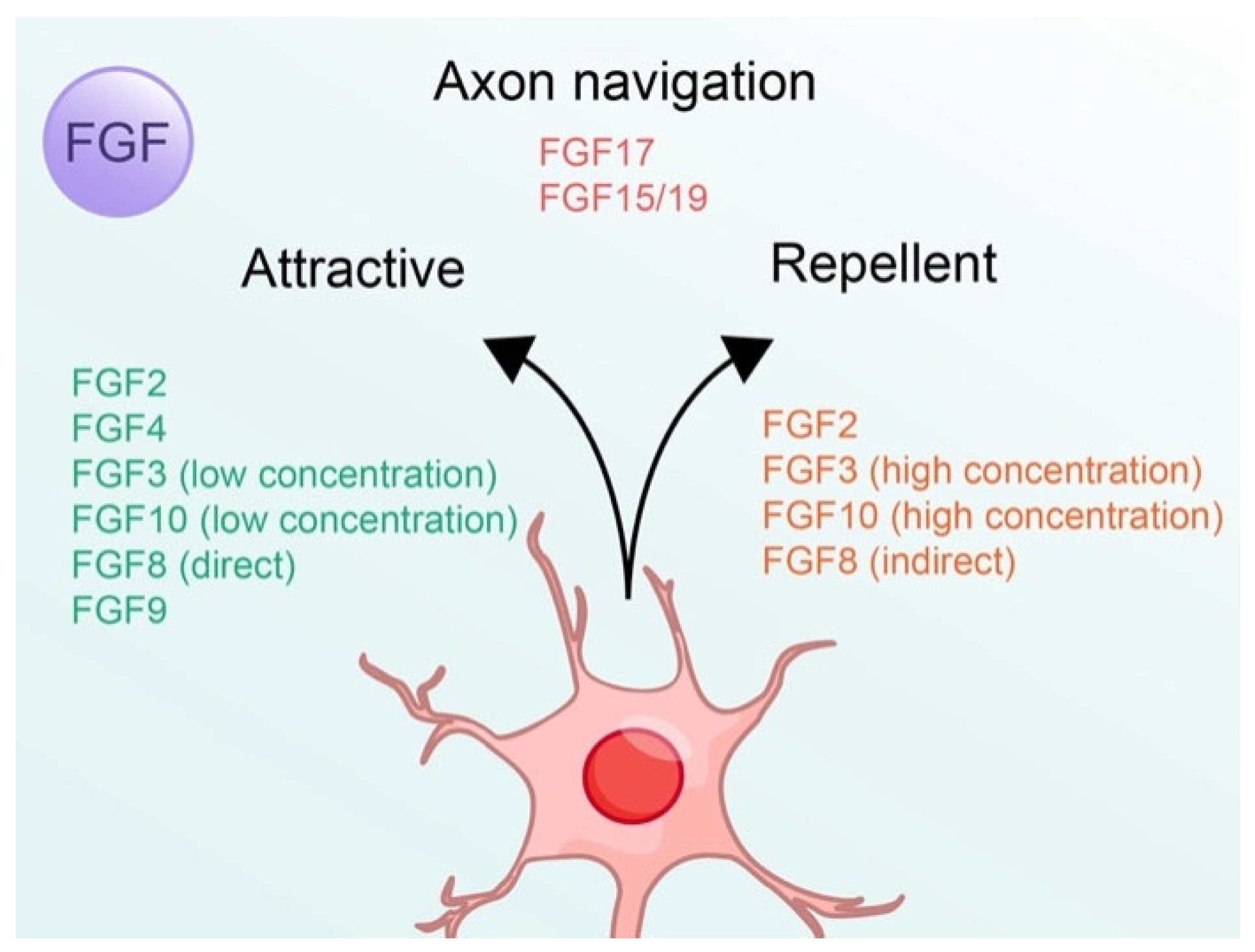
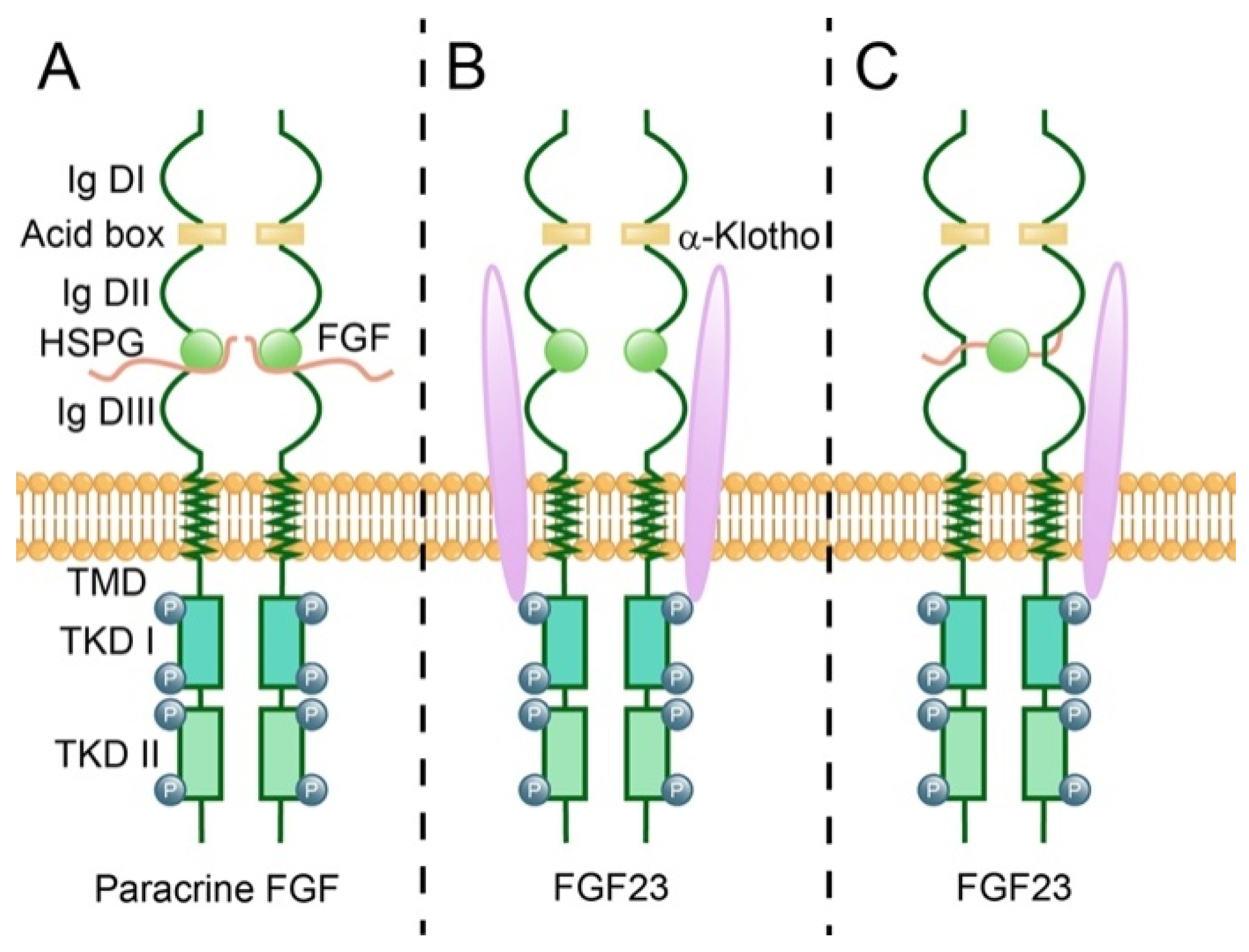




| Classification | Subfamily | FGFs | High Affinity FGFRs [38] | Effects on Neurons |
|---|---|---|---|---|
| Paracrine (Autocrine) | FGF1 subfamily | FGF1 | FGFR1–4 | Axon regeneration [22] |
| FGF2 | FGFR1, 2c, 3c, 4 | Axon guidance: attractive/repellent [18,19,20,21] | ||
| FGF4 subfamily | FGF4 | FGFR1c, 2c, 3c, 4 | Axon guidance: Attractive [18,19] | |
| FGF5 | FGFR1c, 2c, 3c, 4 | Regulation [24] | ||
| FGF6 | FGFR1c, 2c, 4 | Inhibition of axon regeneration [23] | ||
| FGF7 subfamily | FGF3 | FGFR1b, 2b | Axon guidance: attractive (low concentration)/repellent (high concentration) [25,27] | |
| FGF7 | FGFR2b | Synapse differentiation [28] | ||
| FGF10 | FGFR1b, 2b | Axon guidance: attractive (low concentration)/repellent (high concentration) [26,27] Synapse differentiation [28] | ||
| FGF22 | FGFR1b, 2b | Synapse differentiation [28] | ||
| FGF8 subfamily | FGF8 | FGFR1c, 2c, 3c, 4 | Axon guidance: attractive (directly)/repellent (indirectly) [29,30] | |
| FGF17 | FGFR2c, 3c, 4 | Axon navigation (indirectly) [31] | ||
| FGF18 | FGFR3c, 4 | Increase of neuron number [32,33] | ||
| FGF9 subfamily | FGF9 | FGFR2c, 3b, 3c | Axon guidance: Attractive [19] | |
| FGF16 | FGFR2c, 3b | Maturation [33] | ||
| FGF20 | FGFR1c, 2b, 2c, 3b, 3c, 4 | Axon regeneration [18,34] | ||
| Intracrine | FGF11 subfamily | FGF11 | - | - |
| FGF12 | - | - | ||
| FGF13 | - | - | ||
| FGF14 | - | - | ||
| Endocrine | FGF15/19 subfamily | FGF15/19 | FGFR1c, 2c, 3c, 4 | Axon navigation [35] |
| FGF21 | FGFR1c, 2, 4 | Axon outgrowth [36] | ||
| FGF23 | FGFR2c, 4 | Axon loss [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Gao, H.; Liu, F. FGF-Mediated Axon Guidance: Role of Downstream Signaling Pathways in Cytoskeletal Control. Cells 2025, 14, 777. https://doi.org/10.3390/cells14110777
Li J, Gao H, Liu F. FGF-Mediated Axon Guidance: Role of Downstream Signaling Pathways in Cytoskeletal Control. Cells. 2025; 14(11):777. https://doi.org/10.3390/cells14110777
Chicago/Turabian StyleLi, Jiyuan, Hanqi Gao, and Fang Liu. 2025. "FGF-Mediated Axon Guidance: Role of Downstream Signaling Pathways in Cytoskeletal Control" Cells 14, no. 11: 777. https://doi.org/10.3390/cells14110777
APA StyleLi, J., Gao, H., & Liu, F. (2025). FGF-Mediated Axon Guidance: Role of Downstream Signaling Pathways in Cytoskeletal Control. Cells, 14(11), 777. https://doi.org/10.3390/cells14110777






