Hypothalamic Median Eminence Thyrotropin-Releasing Hormone-Degrading Ectoenzyme Activity Is Dispensable for Basal Thyroid Axis Activity in Lean Rodents
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histochemistry for β-Gal in Mouse Brain Slices
2.3. Immunofluorescence Detection of Vimentin and TRH-DE in Rats, and β-Gal and Vimentin in Mouse Brain Slices
2.4. Triple Immunofluorescence for Vimentin, TRH, and TRH-DE in Rat Brain Slices
2.5. Microinjection into the Rat Brain Third Ventricle (3V)
2.6. Analysis of AAV Ability to Transduce Rat Tanycytes In Vivo
2.7. Efficiency of AAV1-TRH-DE and -TRH-DEt Vectors to Induce Changes in TRH-DE Activity in Cell Culture
2.8. Overexpression of TRH-DE and TRH-DEt in Rats
2.9. Hormone Assays
2.10. TRH-DE, Thyroliberinase, and Aminopeptidase N (APN) Activities
2.11. Genomic DNA PCR
2.12. End-Point RT-PCR
2.13. RT-qPCR
2.14. Data Presentation and Statistical Analyses
3. Results
3.1. Localization of TRH-DE Immunoreactivity in the ME of the Adult Rat
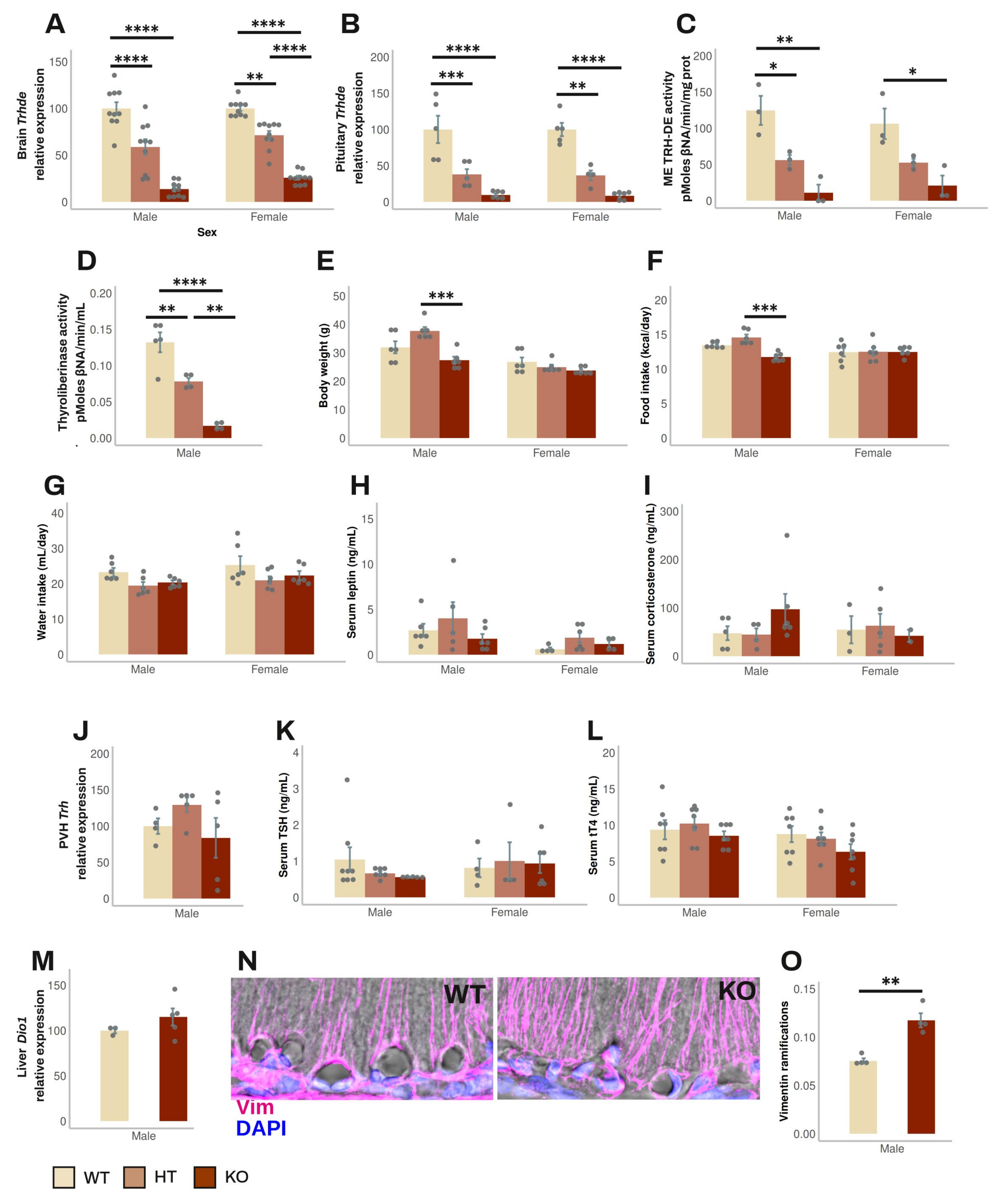
3.2. Effect of Global Elimination of Trhde Expression on Basal HPT Axis Activity in Mice
3.3. Identification of AAV Serotypes Whose Injection into Rat 3V Transduces ME Tanycytes
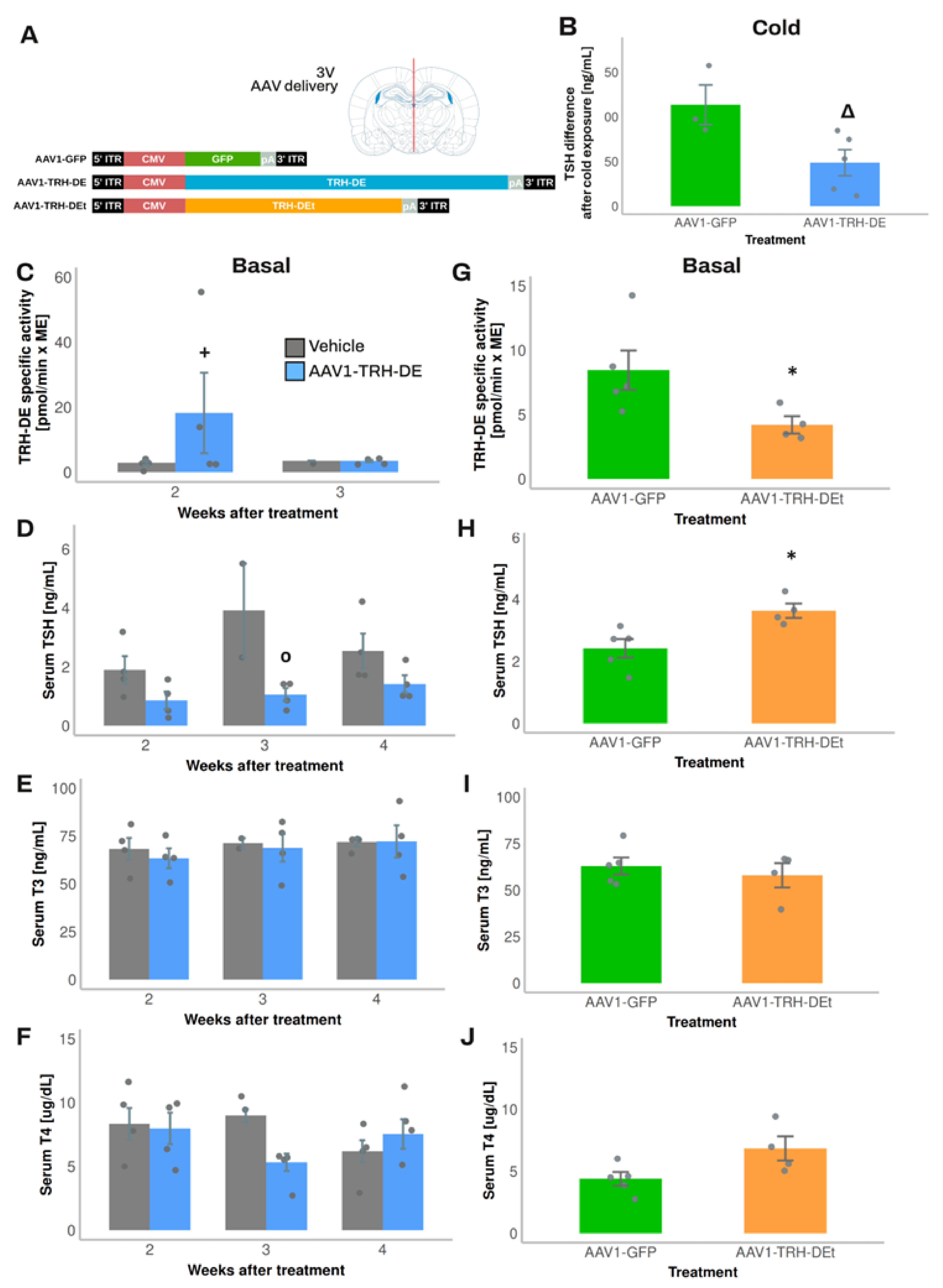
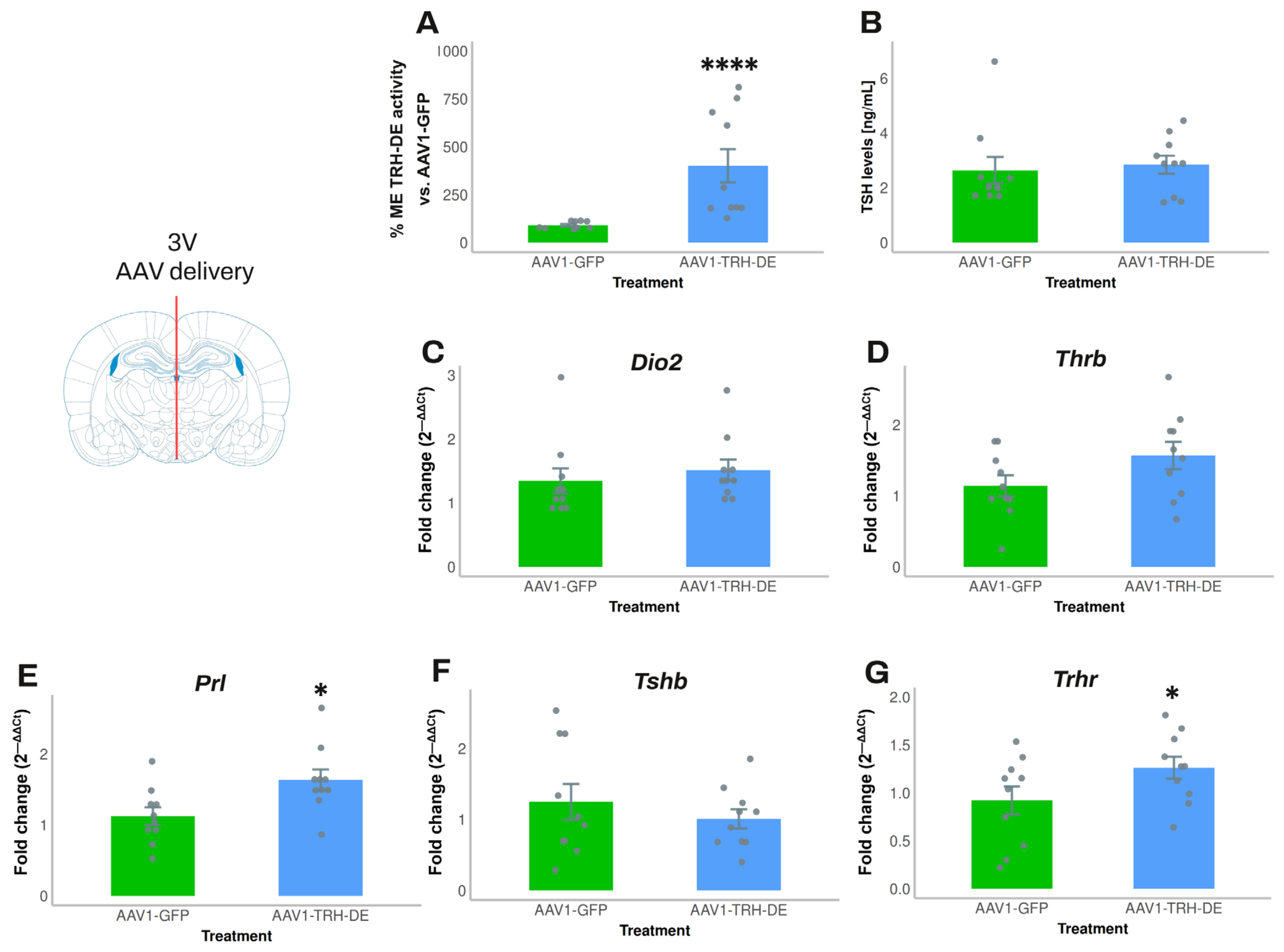
3.4. Temporal Dynamics of over- or Under-Expression of TRH-DE Induced by AAV1 Constructions and Influence on TSH Secretion and HPT Axis Parameters
3.5. TRH-DE Activity in the ME and Basal Serum TSH Concentration Are Negatively Correlated in Adult Male Rats
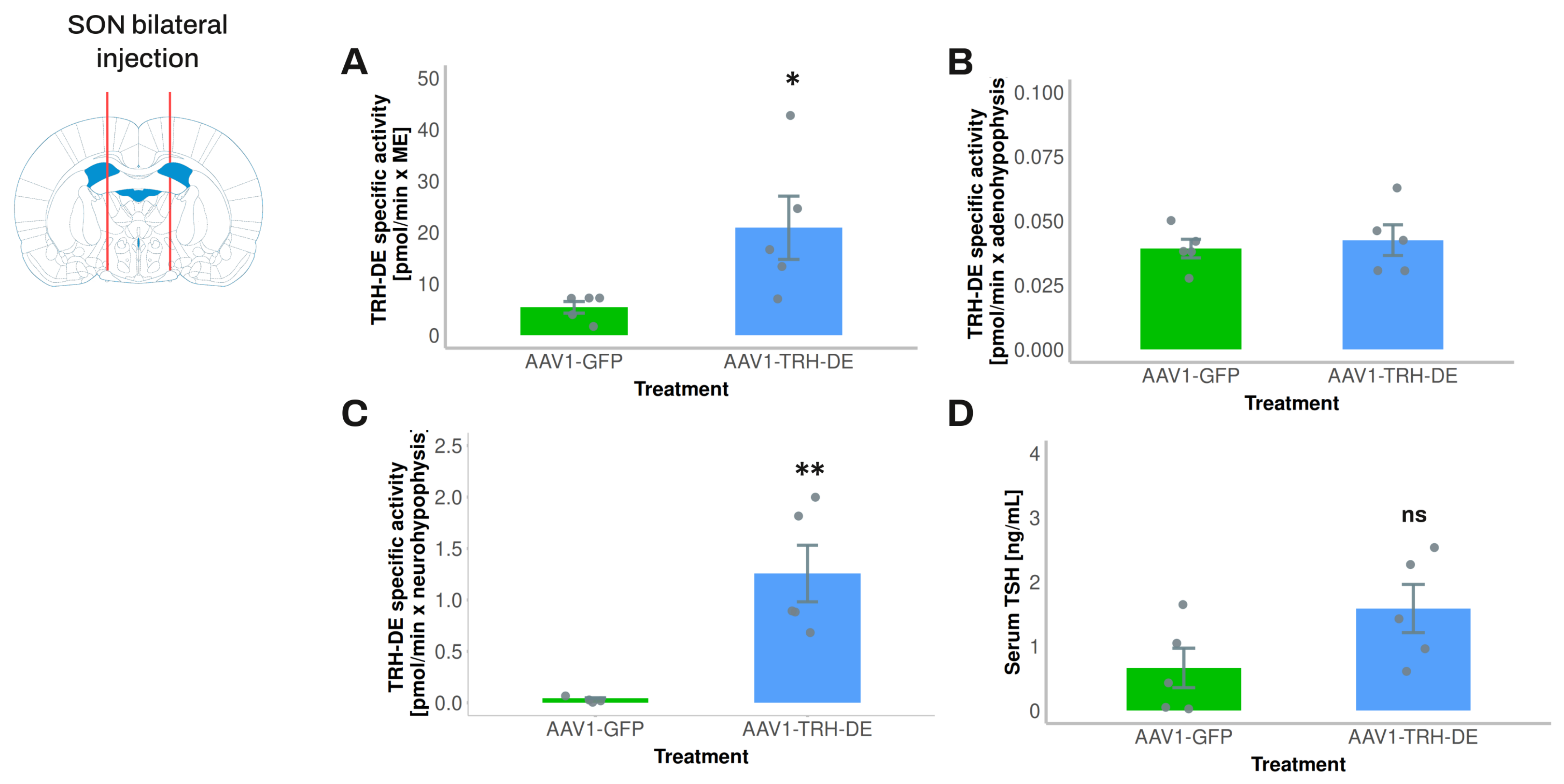
3.6. Exploring the Influence of Posterior Pituitary TRH-DE Activity on Basal Serum TSH Concentration in Rats
4. Discussion
4.1. TRH-DE Is Close to the Putative Sites of TRH Release in the Rat ME
4.2. Two Models for Long-Term Modification of TRH-DE Activity
4.3. ME TRH-DE Controls the Phasic Output of TSH
4.4. Long-Term Alteration of ME TRH-DE Activity Has Either No or a Small Impact on Basal Serum Concentration of TSH and TH
4.5. Long-Term Alteration of Trhde Expression Impacts Parameters That Might Control the Basal Activity of the HPT Axis in ME and Anterior Pituitary
4.6. Multiple Evidence Indicates That Trhde Expression Is Not Critical for the Control of the Basal Activity of the HPT Axis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiecker, C. The Origins of the Circumventricular Organs. J. Anat. 2018, 232, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Guerra, M.; Peruzzo, B.; Blázquez, J.L. Tanycytes: A Rich Morphological History to Underpin Future Molecular and Physiological Investigations. J. Neuroendocrinol. 2019, 31, e12690. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.M.; Blázquez, J.L.; Guerra, M. The Design of Barriers in the Hypothalamus Allows the Median Eminence and the Arcuate Nucleus to Enjoy Private Milieus: The Former Opens to the Portal Blood and the Latter to the Cerebrospinal Fluid. Peptides 2010, 31, 757–776. [Google Scholar] [CrossRef]
- Page, R.B.; Leure-Dupree, A.E.; Bergland, R.M. The Neurohypophyseal Capillary Bed II. Specializations within Median Eminence. Am. J. Anat. 1978, 153, 33–65. [Google Scholar] [CrossRef]
- Silverman, A.J.; Hoffman, D.L.; Zimmerman, E.A. The Descending Afferent Connections of the Paraventricular Nucleus of the Hypothalamus (PVN). Brain Res. Bull. 1981, 6, 47–61. [Google Scholar] [CrossRef]
- Baker, R.A.; Herkenham, M. Arcuate Nucleus Neurons That Project to the Hypothalamic Paraventricular Nucleus: Neuropeptidergic Identity and Consequences of Adrenalectomy on mRNA Levels in the Rat. J. Comp. Neurol. 1995, 358, 518–530. [Google Scholar] [CrossRef]
- Tóth, Z.E.; Palkovits, M. Distributions of Periventricular Projections of the Paraventricular Nucleus to the Median Eminence and Arcuate Nucleus. Brain Res. 1998, 802, 294–297. [Google Scholar] [CrossRef]
- Ben-Shlomo, A.; Melmed, S. Hypothalamic Regulation of Anterior Pituitary Function. In The Pituitary; Elsevier: Amsterdam, The Netherlands, 2011; pp. 21–45. ISBN 978-0-12-380926-1. [Google Scholar]
- Shioda, S.; Nakai, Y. Immunocytochemical Localization of TRH and Autoradiographic Determination of 3H-TRH-Binding Sites in the Arcuate Nucleus-Median Eminence of the Rat. Cell Tissue Res. 1983, 228, 475–487. [Google Scholar] [CrossRef]
- Ishikawa, K.; Taniguchi, Y.; Inoue, K.; Kurosumi, K.; Suzuki, M. Immunocytochemical Delineation of Thyrotrophic Area: Origin of Thyrotropin-Releasing Hormone in the Median Eminence. Neuroendocrinology 1988, 47, 384–388. [Google Scholar] [CrossRef]
- Fekete, C.; Lechan, R.M. Central Regulation of Hypothalamic-Pituitary-Thyroid Axis Under Physiological and Pathophysiological Conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef]
- Joseph-Bravo, P.; Jaimes-Hoy, L.; Uribe, R.-M.; Charli, J.-L. 60 YEARS OF NEUROENDOCRINOLOGY: TRH, the First Hypophysiotropic Releasing Hormone Isolated: Control of the Pituitary–Thyroid Axis. J. Endocrinol. 2015, 226, T85–T100. [Google Scholar] [CrossRef] [PubMed]
- Chiamolera, M.I.; Wondisford, F.E. Thyrotropin-Releasing Hormone and the Thyroid Hormone Feedback Mechanism. Endocrinology 2009, 150, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Salas-Lucia, F.; Fekete, C.; Sinkó, R.; Egri, P.; Rada, K.; Ruska, Y.; Gereben, B.; Bianco, A.C. Axonal T3 Uptake and Transport Can Trigger Thyroid Hormone Signaling in the Brain. eLife 2023, 12, e82683. [Google Scholar] [CrossRef]
- Sánchez, E.; Vargas, M.A.; Singru, P.S.; Pascual, I.; Romero, F.; Fekete, C.; Charli, J.-L.; Lechan, R.M. Tanycyte Pyroglutamyl Peptidase II Contributes to Regulation of the Hypothalamic-Pituitary-Thyroid Axis through Glial-Axonal Associations in the Median Eminence. Endocrinology 2009, 150, 2283–2291. [Google Scholar] [CrossRef]
- Farkas, E.; Varga, E.; Kovács, B.; Szilvásy-Szabó, A.; Cote-Vélez, A.; Péterfi, Z.; Matziari, M.; Tóth, M.; Zelena, D.; Mezriczky, Z.; et al. A Glial-Neuronal Circuit in the Median Eminence Regulates Thyrotropin-Releasing Hormone-Release via the Endocannabinoid System. iScience 2020, 23, 100921. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.; Lazcano, I.; Sánchez-Jaramillo, E.; Uribe, R.M.; Jaimes-Hoy, L.; Joseph-Bravo, P.; Charli, J.-L. Tanycytes and the Control of Thyrotropin-Releasing Hormone Flux Into Portal Capillaries. Front. Endocrinol. 2019, 10, 401. [Google Scholar] [CrossRef]
- Müller-Fielitz, H.; Stahr, M.; Bernau, M.; Richter, M.; Abele, S.; Krajka, V.; Benzin, A.; Wenzel, J.; Kalies, K.; Mittag, J.; et al. Tanycytes Control the Hormonal Output of the Hypothalamic-Pituitary-Thyroid Axis. Nat. Commun. 2017, 8, 484. [Google Scholar] [CrossRef]
- Hsieh, K.P.; Martin, T.F. Thyrotropin-Releasing Hormone and Gonadotropin-Releasing Hormone Receptors Activate Phospholipase C by Coupling to the Guanosine Triphosphate-Binding Proteins Gq and G11. Mol. Endocrinol. 1992, 6, 1673–1681. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, X.; Gershengorn, M. Thyrotropin-Releasing Hormone Receptors—Similarities and Differences. J. Mol. Endocrinol. 2003, 30, 87–97. [Google Scholar] [CrossRef]
- Charli, J.-L.; Rodríguez-Rodríguez, A.; Hernández-Ortega, K.; Cote-Vélez, A.; Uribe, R.M.; Jaimes-Hoy, L.; Joseph-Bravo, P. The Thyrotropin-Releasing Hormone-Degrading Ectoenzyme, a Therapeutic Target? Front. Pharmacol. 2020, 11, 640. [Google Scholar] [CrossRef]
- Tang, T.; Li, L.; Tang, J.; Li, Y.; Lin, W.Y.; Martin, F.; Grant, D.; Solloway, M.; Parker, L.; Ye, W.; et al. A Mouse Knockout Library for Secreted and Transmembrane Proteins. Nat. Biotechnol. 2010, 28, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Zhou, R. β-Galactosidase Staining of LacZ Fusion Proteins in Whole Tissue Preparations. In Neural Development; Zhou, R., Mei, L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 1018, pp. 189–197. ISBN 978-1-62703-443-2. [Google Scholar]
- Paxinos, G.; Watson, S.J. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: Cambridge, MA, USA, 1986; Volume 10, ISBN 012 547 6213. [Google Scholar]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, Compact, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Berlin/Heidelberg, Germany, 2008; ISBN 978-0-12-374244-5. [Google Scholar]
- Jamur, M.C.; Oliver, C. Permeabilization of Cell Membranes. In Immunocytochemical Methods and Protocols; Oliver, C., Jamur, M.C., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 588, pp. 63–66. ISBN 978-1-58829-463-0. [Google Scholar]
- Heuer, H.; Schäfer, M.K.-H.; Bauer, K. The Thyrotropin-Releasing Hormone-Degrading Ectoenzyme: The Third Element of the Thyrotropin-Releasing Hormone-Signaling System. Thyroid 1998, 8, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Lechan, R.M.; Jackson, I.M.D. Immunohistochemical Localization of Thyrotropin-Releasing Hormone in the Rat Hypothalamus and Pituitary*. Endocrinology 1982, 111, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Anderson, G.; Forrest, D.; Galton, V.A.; Gereben, B.; Kim, B.W.; Kopp, P.A.; Liao, X.H.; Obregon, M.J.; Peeters, R.P.; et al. American Thyroid Association Guide to Investigating Thyroid Hormone Economy and Action in Rodent and Cell Models: Report of the American Thyroid Association Task Force on Approaches and Strategies to Investigate Thyroid Hormone Economy and Action. Thyroid 2014, 24, 88–168. [Google Scholar] [CrossRef]
- Joseph-Bravo, P.; Charli, J.L.; Palacios, J.M.; Kordon, C. Effect of Neurotransmitters on the in Vitro Release of Immunoreactive Thyrotropin-Releasing Hormone from Rat Mediobasal Hypothalamus*. Endocrinology 1979, 104, 801–806. [Google Scholar] [CrossRef]
- Joseph-Bravo, P.; Lazcano, I.; Jaimes-Hoy, L.; Gutierrez-Mariscal, M.; Sanchez-Jaramillo, E.; Uribe, R.M.; Charli, J.-L. Sexually Dimorphic Dynamics of Thyroid Axis Activity during Fasting in Rats. Front. Biosci 2020, 25, 1305–1323. [Google Scholar] [CrossRef]
- Kelly, J.A.; Slator, G.R.; Tipton, K.F.; Williams, C.H.; Bauer, K. Development of a Continuous, Fluorometric Coupled Enzyme Assay for Thyrotropin-Releasing Hormone-Degrading Ectoenzyme. Anal. Biochem. 1999, 274, 195–202. [Google Scholar] [CrossRef]
- Limaye, A.; Cho, K.; Hall, B.; Khillan, J.S.; Kulkarni, A.B. Genotyping Protocols for Genetically Engineered Mice. Curr. Protoc. 2023, 3, e929. [Google Scholar] [CrossRef]
- Chomzynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. In CRAN: Contributed Packages; The R Foundation: Indianapolis, IN, USA, 2020. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Bauer, K. Regulation of Degradation of Thyrotropin Releasing Hormone by Thyroid Hormones. Nature 1976, 259, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Schmitmeier, S.; Thole, H.; Bader, A.; Bauer, K. Purification and Characterization of the Thyrotropin-releasing Hormone (TRH)-degrading Serum Enzyme and Its Identification as a Product of Liver Origin. Eur. J. Biochem. 2002, 269, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-Wide Atlas of Gene Expression in the Adult Mouse Brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef]
- Haan, N.; Goodman, T.; Najdi-Samiei, A.; Stratford, C.M.; Rice, R.; El Agha, E.; Bellusci, S.; Hajihosseini, M.K. Fgf10-Expressing Tanycytes Add New Neurons to the Appetite/Energy-Balance Regulating Centers of the Postnatal and Adult Hypothalamus. J. Neurosci. 2013, 33, 6170–6180. [Google Scholar] [CrossRef]
- Cui, C.; Wani, M.A.; Wight, D.; Kopchick, J.; Stambrook, P.J. Reporter Genes in Transgenic Mice. Transgenic Res. 1994, 3, 182–194. [Google Scholar] [CrossRef]
- Zhuo, L.; Theis, M.; Alvarez-Maya, I.; Brenner, M.; Willecke, K.; Messing, A. hGFAP-cre Transgenic Mice for Manipulation of Glial and Neuronal Function in Vivo. Genesis 2001, 31, 85–94. [Google Scholar] [CrossRef]
- Friedman, T.C.; Wilk, S. The Effect of Inhibitors of Prolyl Endopeptidase and Pyroglutamyl Peptide Hydrolase on TRH Degradation in Rat Serum. Biochem. Biophys. Res. Commun. 1985, 132, 787–794. [Google Scholar] [CrossRef]
- Cetin, A.; Komai, S.; Eliava, M.; Seeburg, P.H.; Osten, P. Stereotaxic Gene Delivery in the Rodent Brain. Nat. Protoc. 2006, 1, 3166–3173. [Google Scholar] [CrossRef]
- Müller-Fielitz, H.; Schwaninger, M. The Role of Tanycytes in the Hypothalamus-Pituitary-Thyroid Axis and the Possibilities for Their Genetic Manipulation. Exp. Clin. Endocrinol. Diabetes 2020, 128, 388–394. [Google Scholar] [CrossRef]
- Campbell, J.N.; Macosko, E.Z.; Fenselau, H.; Pers, T.H.; Lyubetskaya, A.; Tenen, D.; Goldman, M.; Verstegen, A.M.J.; Resch, J.M.; McCarroll, S.A.; et al. A Molecular Census of Arcuate Hypothalamus and Median Eminence Cell Types. Nat. Neurosci. 2017, 20, 484–496. [Google Scholar] [CrossRef]
- Chávez-Gutiérrez, L.; Bourdais, J.; Aranda, G.; Vargas, M.A.; Matta-Camacho, E.; Ducancel, F.; Segovia, L.; Joseph-Bravo, P.; Charli, J. A Truncated Isoform of Pyroglutamyl Aminopeptidase II Produced by Exon Extension Has Dominant-negative Activity. J. Neurochem. 2005, 92, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Hefco, E.; Krulich, L.; Illner, P.; Larsen, P.R. Effect of Acute Exposure to Cold on the Activity of the Hypothalamic-Pituitary-Thyroid System. Endocrinology 1975, 97, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.; Redondo, L.; Charli, J.-L.; Joseph-Bravo, P. Suckling and Cold Stress Rapidly and Transiently Increase TRH mRNA in the Paraventricular Nucleus. Neuroendocrinology 1993, 58, 140–145. [Google Scholar] [CrossRef]
- Fujimoto, J.; Straub, R.E.; Gershengorn, M.C. Thyrotropin-Releasing Hormone (TRH) and Phorbol Myristate Acetate Decrease TRH Receptor Messenger RNA in Rat Pituitary GH 3 Cells: Evidence That Protein Kinase-C Mediates the TRH Effect. Mol. Endocrinol. 1991, 5, 1527–1532. [Google Scholar] [CrossRef]
- Yang, J.; Tashjian, A.H. Regulation of Endogenous Thyrotropin-Releasing Hormone (TRH) Receptor Messenger RNA by TRH in GH4C1 Cells. Mol. Endocrinol. 1993, 7, 753–758. [Google Scholar] [CrossRef]
- White, B.A.; Bancroft, F.C. Epidermal Growth Factor and Thyrotropin-Releasing Hormone Interact Synergistically with Calcium to Regulate Prolactin mRNA Levels. J. Biol. Chem. 1983, 258, 4618–4622. [Google Scholar] [CrossRef]
- Kim, S. Ppcor: An R Package for a Fast Calculation to Semi-Partial Correlation Coefficients. Commun. Stat. Appl. Methods 2015, 22, 665–674. [Google Scholar] [CrossRef]
- Vargas, M.; Mendez, M.; Cisneros, M.; Joseph-Bravo, P.; Charli, J.L. Regional Distribution of the Membrane-Bound Pyroglutamate Amino Peptidase-Degrading Thyrotropin-Releasing Hormone in Rat Brain. Neurosci. Lett. 1987, 79, 311–314. [Google Scholar] [CrossRef]
- Fiedler, J.; Jara, P.; Luza, S.; Dorfman, M.; Grouselle, D.; Rage, F.; Lara, H.E.; Arancibia, S. Cold Stress Induces Metabolic Activation of Thyrotrophin-Releasing Hormone-Synthesising Neurones in the Magnocellular Division of the Hypothalamic Paraventricular Nucleus and Concomitantly Changes Ovarian Sympathetic Activity Parameters. J. Neuroendocrinol. 2006, 18, 367–376. [Google Scholar] [CrossRef]
- Ciosek, J. Vasopressin and Oxytocin Release as Influenced by Thyrotropin-Releasing Hormone in Euhydrated and Dehydrated Rats. J. Physiol. Pharmacol. 2002, 53, 423–437. [Google Scholar]
- Thompson, D.; Odufuwa, A.E.; Brissette, C.A.; Watt, J.A. Transcriptome and Methylome of the Supraoptic Nucleus Provides Insights into the Age-Dependent Loss of Neuronal Plasticity. Front. Aging Neurosci. 2023, 15, 1223273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Leshkowitz, D.; Blechman, J.; Levkowitz, G. Single-Cell Molecular and Cellular Architecture of the Mouse Neurohypophysis. eNeuro 2020, 7, ENEURO.0345-19.2019. [Google Scholar] [CrossRef] [PubMed]
- Ryder, P.V.; Lerit, D.A. RNA Localization Regulates Diverse and Dynamic Cellular Processes. Traffic 2018, 19, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Gala, D.S.; Titlow, J.S.; Teodoro, R.O.; Davis, I. Far from Home: The Role of Glial mRNA Localization in Synaptic Plasticity. RNA 2023, 29, 153–169. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Cook, W.H.; Young, D. AAV Targeting of Glial Cell Types in the Central and Peripheral Nervous System and Relevance to Human Gene Therapy. Front. Mol. Neurosci. 2021, 13, 618020. [Google Scholar] [CrossRef]
- Redding, T.W.; Schally, A.V. Studies on the Inactivation of Thyrotropin-Releasing Hormone (TRH). Exp. Biol. Med. 1969, 131, 415–420. [Google Scholar] [CrossRef]
- Leppäluoto, J.; Ranta, T.; Lybeck, H.; Varis, R. Effect of TRH, and Short-term Exposure to Experimental Stress or Cold on the Serum Immunoassayable TSH Concentration in the Rat. Acta Physiol. Scand. 1974, 90, 640–644. [Google Scholar] [CrossRef]
- Jobin, M.; Ferland, L.; Côté, J.; Labrie, F. Effect of Exposure to Cold on Hypothalamic TRH Activity and Plasma Levels of TSH and Prolactin in the Rat. Neuroendocrinology 1975, 18, 204–212. [Google Scholar] [CrossRef]
- Arancibia, S.; Tapia-Arancibia, L.; Assenmacher, I.; Astier, H. Direct Evidence of Short-Term Cold-Induced TRH Release in the Median Eminence of Unanesthetized Rats. Neuroendocrinology 1983, 37, 225–228. [Google Scholar] [CrossRef]
- Abdalla, S.M.; Bianco, A.C. Defending Plasma T3 Is a Biological Priority. Clin. Endocrinol. 2014, 81, 633–641. [Google Scholar] [CrossRef]
- Yamada, M.; Saga, Y.; Shibusawa, N.; Hirato, J.; Murakami, M.; Iwasaki, T.; Hashimoto, K.; Satoh, T.; Wakabayashi, K.; Taketo, M.M.; et al. Tertiary Hypothyroidism and Hyperglycemia in Mice with Targeted Disruption of the Thyrotropin-Releasing Hormone Gene. Proc. Natl. Acad. Sci. USA 1997, 94, 10862–10867. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ozawa, A.; Kohno, D.; Saito, K.; Buyandalai, B.; Yamada, S.; Horiguchi, K.; Nakajima, Y.; Shibusawa, N.; Harada, A.; et al. The Hypothalamic Paraventricular Nucleus Is the Center of the Hypothalamic–Pituitary–Thyroid Axis for Regulating Thyroid Hormone Levels. Thyroid 2021, 32, thy.2021.0444. [Google Scholar] [CrossRef] [PubMed]
- Kővári, D.; Penksza, V.; Szilvásy-Szabó, A.; Sinkó, R.; Gereben, B.; Mackie, K.; Fekete, C. Tanycyte Specific Ablation of Diacylglycerol Lipase Alpha Stimulates the Hypothalamic-pituitary-thyroid Axis by Decreasing the Endocannabinoid Mediated Inhibition of TRH Release. J. Neuroendocrinol. 2022, 34, e13079. [Google Scholar] [CrossRef]
- Rabeler, R.; Mittag, J.; Geffers, L.; Rüther, U.; Leitges, M.; Parlow, A.F.; Visser, T.J.; Bauer, K. Generation of Thyrotropin-Releasing Hormone Receptor 1-Deficient Mice as an Animal Model of Central Hypothyroidism. Mol. Endocrinol. 2004, 18, 1450–1460. [Google Scholar] [CrossRef]
- Zeng, H.; Schimpf, B.A.; Rohde, A.D.; Pavlova, M.N.; Gragerov, A.; Bergmann, J.E. Thyrotropin-Releasing Hormone Receptor 1-Deficient Mice Display Increased Depression and Anxiety-Like Behavior. Mol. Endocrinol. 2007, 21, 2795–2804. [Google Scholar] [CrossRef]
- Fonseca, T.L.; Correa-Medina, M.; Campos, M.P.O.; Wittmann, G.; Werneck-de-Castro, J.P.; Arrojo E Drigo, R.; Mora-Garzon, M.; Ueta, C.B.; Caicedo, A.; Fekete, C.; et al. Coordination of Hypothalamic and Pituitary T3 Production Regulates TSH Expression. J. Clin. Investig. 2013, 123, 1492–1500. [Google Scholar] [CrossRef]
- Luongo, C.; Martin, C.; Vella, K.; Marsili, A.; Ambrosio, R.; Dentice, M.; Harney, J.W.; Salvatore, D.; Zavacki, A.M.; Larsen, P.R. The Selective Loss of the Type 2 Iodothyronine Deiodinase in Mouse Thyrotrophs Increases Basal TSH but Blunts the Thyrotropin Response to Hypothyroidism. Endocrinology 2015, 156, 745–754. [Google Scholar] [CrossRef]
- Forrest, D.; Hanebuth, E.; Smeyne, R.J.; Everds, N.; Stewart, C.L.; Wehner, J.M.; Curran, T. Recessive Resistance to Thyroid Hormone in Mice Lacking Thyroid Hormone Receptor Beta: Evidence for Tissue-Specific Modulation of Receptor Function. EMBO J. 1996, 15, 3006–3015. [Google Scholar] [CrossRef]
- Wu, Z.; Hernandez, A. Thyroid Hormone Clearance in the Paraventricular Nucleus of Male Mice Regulates Lean Mass and Physical Activity. Neuroendocrinology 2024, 114, 1066–1076. [Google Scholar] [CrossRef]
- Weintraub, B.D.; Gesundheit, N.; Taylor, T.; Gyves, P.W. Effect of TRH on TSH Glycosylation and Biological Action. Ann. NY Acad. Sci. 1989, 553, 205–213. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, J.; Jones, K.E.; Gerald, C.; Suzuki, Y.; Hogan, P.G.; Chin, W.W.; Tashjian, A.H. Molecular Cloning of a Complementary Deoxyribonucleic Acid Encoding the Thyrotropin-Releasing Hormone Receptor and Regulation of Its Messenger Ribonucleic Acid in Rat GH Cells. Endocrinology 1992, 130, 3529–3536. [Google Scholar] [CrossRef] [PubMed]
- Scalabrino, G.A.; Hogan, N.; O’Boyle, K.M.; Slator, G.R.; Gregg, D.J.; Fitchett, C.M.; Draper, S.M.; Bennett, G.W.; Hinkle, P.M.; Bauer, K.; et al. Discovery of a Dual Action First-in-Class Peptide That Mimics and Enhances CNS-Mediated Actions of Thyrotropin-Releasing Hormone. Neuropharmacology 2007, 52, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Vargas, M.A.; Uribe, R.M.; Pascual, I.; Lazcano, I.; Yiotakis, A.; Matziari, M.; Joseph-Bravo, P.; Charli, J.-L. Anterior Pituitary Pyroglutamyl Peptidase II Activity Controls TRH-Induced Prolactin Release. Peptides 2008, 29, 1953–1964. [Google Scholar] [CrossRef]
- Yoo, S.; Cha, D.; Kim, S.; Jiang, L.; Cooke, P.; Adebesin, M.; Wolfe, A.; Riddle, R.; Aja, S.; Blackshaw, S. Tanycyte Ablation in the Arcuate Nucleus and Median Eminence Increases Obesity Susceptibility by Increasing Body Fat Content in Male Mice. Glia 2020, 68, 1987–2000. [Google Scholar] [CrossRef]
- Narumi, S. Genome-Wide Association Studies for Thyroid Physiology and Diseases. Endocr. J. 2023, 70, 9–17. [Google Scholar] [CrossRef]
- Wittmann, G.; Füzesi, T.; Liposits, Z.; Lechan, R.M.; Fekete, C. Distribution and Axonal Projections of Neurons Coexpressing Thyrotropin-releasing Hormone and Urocortin 3 in the Rat Brain. J. Comp. Neurol. 2009, 517, 825–840. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rodríguez, A.; Uribe, R.M.; Cote-Vélez, A.; Joseph-Bravo, P.; Charli, J.-L. Hypothalamic Median Eminence Thyrotropin-Releasing Hormone-Degrading Ectoenzyme Activity Is Dispensable for Basal Thyroid Axis Activity in Lean Rodents. Cells 2025, 14, 725. https://doi.org/10.3390/cells14100725
Rodríguez-Rodríguez A, Uribe RM, Cote-Vélez A, Joseph-Bravo P, Charli J-L. Hypothalamic Median Eminence Thyrotropin-Releasing Hormone-Degrading Ectoenzyme Activity Is Dispensable for Basal Thyroid Axis Activity in Lean Rodents. Cells. 2025; 14(10):725. https://doi.org/10.3390/cells14100725
Chicago/Turabian StyleRodríguez-Rodríguez, Adair, Rosa María Uribe, Antonieta Cote-Vélez, Patricia Joseph-Bravo, and Jean-Louis Charli. 2025. "Hypothalamic Median Eminence Thyrotropin-Releasing Hormone-Degrading Ectoenzyme Activity Is Dispensable for Basal Thyroid Axis Activity in Lean Rodents" Cells 14, no. 10: 725. https://doi.org/10.3390/cells14100725
APA StyleRodríguez-Rodríguez, A., Uribe, R. M., Cote-Vélez, A., Joseph-Bravo, P., & Charli, J.-L. (2025). Hypothalamic Median Eminence Thyrotropin-Releasing Hormone-Degrading Ectoenzyme Activity Is Dispensable for Basal Thyroid Axis Activity in Lean Rodents. Cells, 14(10), 725. https://doi.org/10.3390/cells14100725





