Exercise Training Differentially Affects Skeletal Muscle Mitochondria in Rats with Inherited High or Low Exercise Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Echocardiography
2.3. Assessment of Aerobic Exercise Capacity
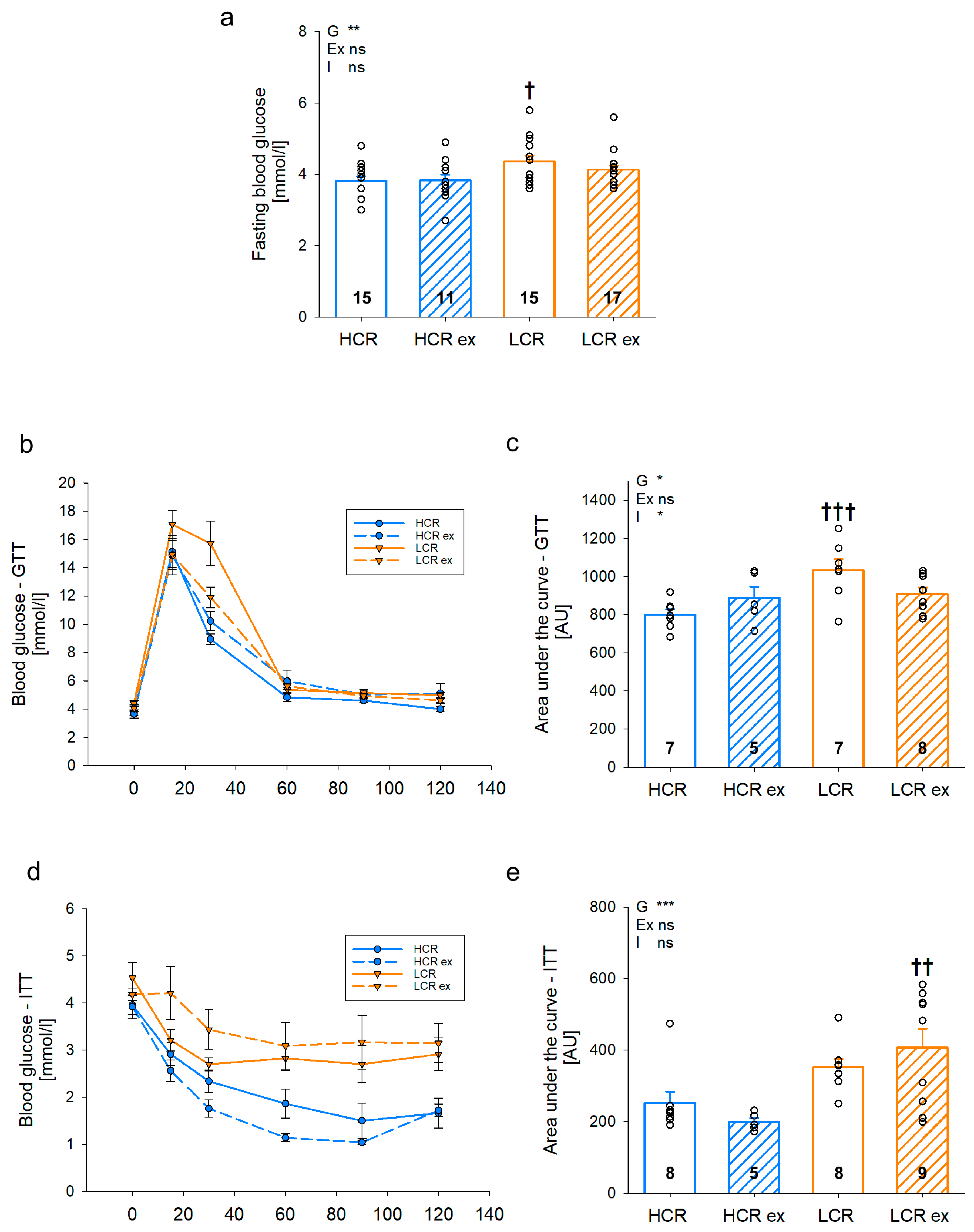
2.4. Glucose and Insulin Tolerance Test
2.5. Aerobic Interval Training
2.6. Isolation of Mitochondria
2.7. Mitochondrial Morphometry
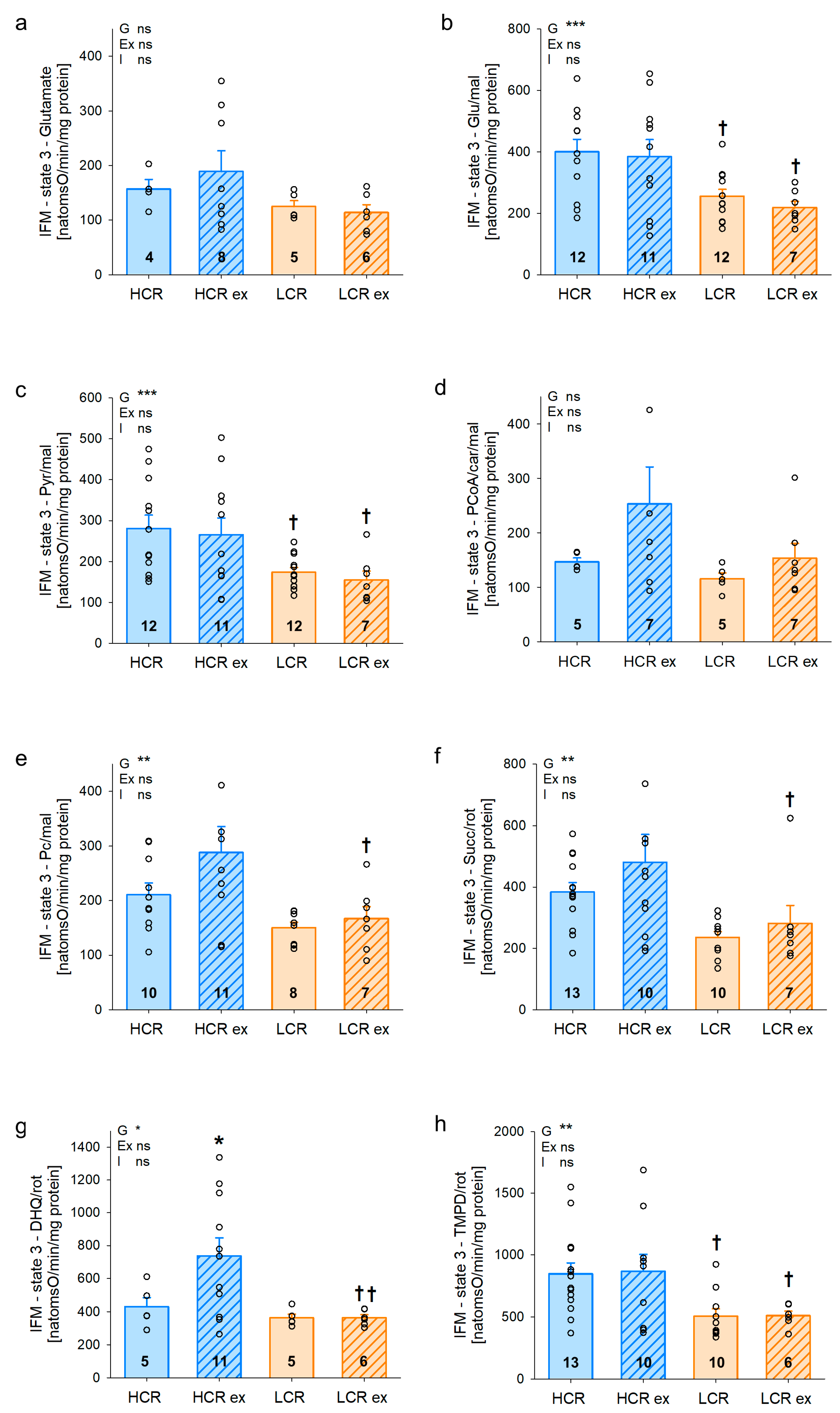
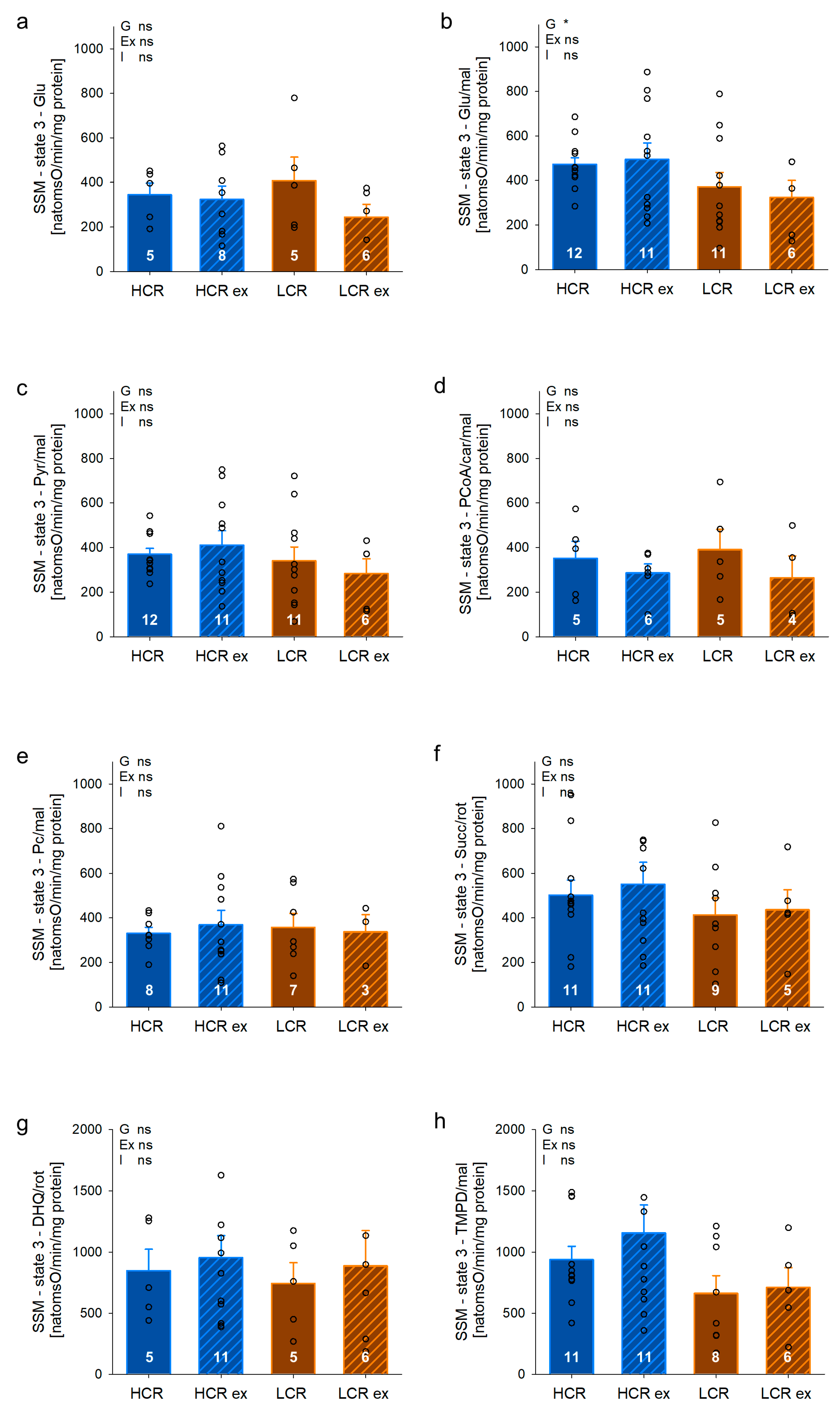
2.8. Assessment of Mitochondrial Respiratory Rates
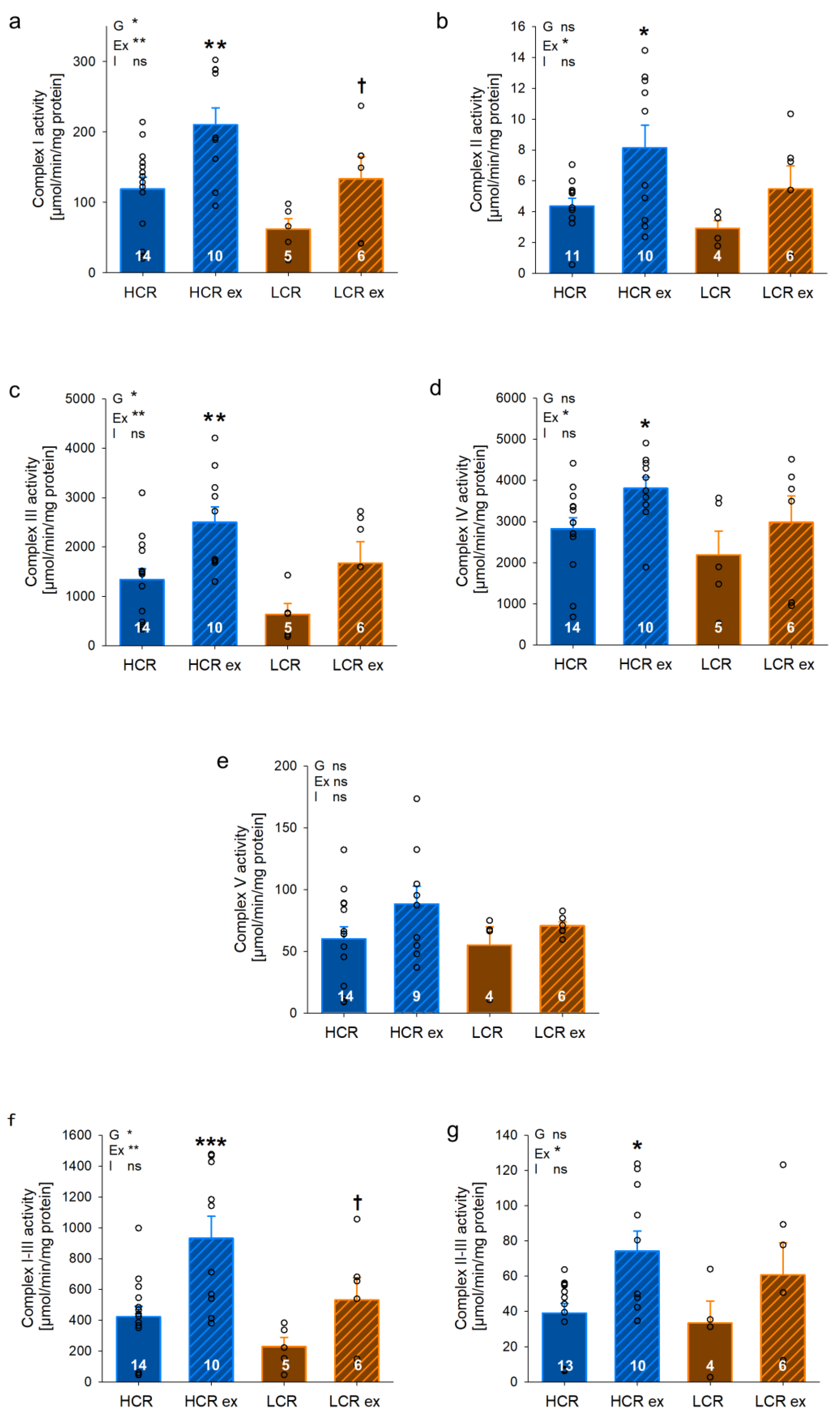
2.9. Determination of Isolated Complex Activities
2.10. Statistical Analysis
3. Results
4. Discussion
4.1. Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kokkinos, P.; Myers, J.; Kokkinos, J.P.; Pittaras, A.; Narayan, P.; Manolis, A.; Karasik, P.; Greenberg, M.; Papademetriou, V.; Singh, S. Exercise Capacity and Mortality in Black and White Men. Circulation 2008, 117, 614–622. [Google Scholar] [CrossRef]
- Mandsager, K.; Harb, S.; Cremer, P.; Phelan, D.; Nissen, S.E.; Jaber, W. Association of Cardiorespiratory Fitness with Long-Term Mortality among Adults Undergoing Exercise Treadmill Testing. JAMA Netw. Open 2018, 1, e183605. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, L.G.; Haskell, W.L.; Johnson, J.L.; Whaley, F.S.; Criqui, M.H.; Sheps, D.S. Physical Fitness as a Predictor of Cardiovascular Mortality in Asymptomatic North American Men. The Lipid Research Clinics Mortality Follow-Up Study. N. Engl. J. Med. 1988, 319, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, R.; Dausin, C.; Claus, P.; Bogaert, J.; Dymarkowski, S.; Goetschalckx, K.; Ghekiere, O.; Van De Heyning, C.M.; Van Herck, P.; Paelinck, B.; et al. Lifelong Endurance Exercise and Its Relation with Coronary Atherosclerosis. Eur. Heart J. 2023, 44, 2388–2399. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.A.; Monk, A.M.; Upham, P.A.; Bergenstal, R.M. Low Cardiorespiratory Fitness in People at Risk for Type 2 Diabetes: Early Marker for Insulin Resistance. Diabetol. Metab. Syndr. 2009, 1, 8. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women: A Meta-Analysis. JAMA J. Am. Med. Assoc. 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Huertas, J.R.; Casuso, R.A.; Agustín, P.H.; Cogliati, S. Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm. Oxid. Med. Cell. Longev. 2019, 2019, 7058350. [Google Scholar] [CrossRef]
- Bouchard, C.; Lesage, R.; Lortie, G.; Simoneau, J.A.; Hamel, P.; Boulay, M.R.; Pérusse, L.; Thériault, G.; Leblanc, C. Aerobic Performance in Brothers, Dizygotic and Monozygotic Twins. Med. Sci. Sports Exerc. 1986, 18, 639–646. [Google Scholar] [CrossRef]
- Koch, L.G.; Britton, S.L. Artificial Selection for Intrinsic Aerobic Endurance Running Capacity in Rats. Physiol. Genom. 2001, 5, 45–52. [Google Scholar] [CrossRef]
- Koch, L.G.; Kemi, O.J.; Qi, N.; Leng, S.X.; Bijma, P.; Gilligan, L.J.; Wilkinson, J.E.; Wisløff, H.; Høydal, M.A.; Rolim, N.; et al. Intrinsic Aerobic Capacity Sets a Divide for Aging and Longevity. Circ. Res. 2011, 109, 1162–1172. [Google Scholar] [CrossRef]
- Wisløff, U.; Najjar, S.M.; Ellingsen, Ø.; Haram, P.M.; Swoap, S.; Al-Share, Q.; Fernström, M.; Rezaei, K.; Lee, S.J.; Koch, L.G.; et al. Cardiovascular Risk Factors Emerge After Artificial Selection for Low Aerobic Capacity. Science 2005, 307, 418–420. [Google Scholar] [CrossRef]
- Walsh, B.; Hooks, R.B.; Hornyak, J.E.; Koch, L.G.; Britton, S.L.; Hogan, M.C. Enhanced Mitochondrial Sensitivity to Creatine in Rats Bred for High Aerobic Capacity. J. Appl. Physiol. 2006, 100, 1765–1769. [Google Scholar] [CrossRef]
- Bleck, C.K.; Kim, Y.; Willingham, T.B.; Glancy, B. Subcellular Connectomic Analyses of Energy Networks in Striated Muscle. Nat. Commun. 2018, 9, 5111. [Google Scholar] [CrossRef]
- Willingham, T.B.; Ajayi, P.T.; Glancy, B. Subcellular Specialization of Mitochondrial Form and Function in Skeletal Muscle Cells. Front. Cell Dev. Biol. 2021, 9, 757305. [Google Scholar] [CrossRef]
- Holmuhamedov, E.L.; Oberlin, A.; Short, K.; Terzic, A.; Jahangir, A. Cardiac Subsarcolemmal and Interfibrillar Mitochondria Display Distinct Responsiveness to Protection by Diazoxide. PLoS ONE 2012, 7, e44667. [Google Scholar] [CrossRef]
- Heyne, E.; Schrepper, A.; Doenst, T.; Schenkl, C.; Kreuzer, K.; Schwarzer, M. High-Fat Diet Affects Skeletal Muscle Mitochondria Comparable to Pressure Overload-Induced Heart Failure. J. Cell. Mol. Med. 2020, 24, 6741–6749. [Google Scholar] [CrossRef]
- Karavirta, L.; Häkkinen, A.; Sillanpää, E.; García-López, D.; Kauhanen, A.; Haapasaari, A.; Alen, M.; Pakarinen, A.; Kraemer, W.J.; Izquierdo, M.; et al. Effects of Combined Endurance and Strength Training on Muscle Strength, Power and Hypertrophy in 40–67-Year-Old Men. Scand. J. Med. Sci. Sports 2011, 21, 402–411. [Google Scholar] [CrossRef]
- Bell, G.J.; Syrotuik, D.; Martin, T.P.; Burnham, R.; Quinney, H.A. Effect of Concurrent Strength and Endurance Training on Skeletal Muscle Properties and Hormone Concentrations in Humans. Eur. J. Appl. Physiol. 2000, 81, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Huiberts, R.O.; Wüst, R.C.I.; van der Zwaard, S. Concurrent Strength and Endurance Training: A Systematic Review and Meta-Analysis on the Impact of Sex and Training Status. Sports Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Zacharewicz, E.; Martin, B.J.; Haikalis, M.E.; Skelly, L.E.; Tarnopolsky, M.A.; Murphy, R.M.; Gibala, M.J. Superior Mitochondrial Adaptations in Human Skeletal Muscle after Interval Compared to Continuous Single-Leg Cycling Matched for Total Work. J. Physiol. 2017, 595, 2955–2968. [Google Scholar] [CrossRef] [PubMed]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Training-Induced Changes in Mitochondrial Content and Respiratory Function in Human Skeletal Muscle. Sports Med. 2018, 48, 1809–1828. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Syrotuik, D.G.; Bell, G.J. The Effect of Concurrent Endurance and Strength Training on Quantitative Estimates of Subsarcolemmal and Intermyofibrillar Mitochondria. Int. J. Sports Med. 2002, 23, 33–39. [Google Scholar] [CrossRef][Green Version]
- Høydal, M.A.; Wisløff, U.; Kemi, O.J.; Ellingsen, O. Running Speed and Maximal Oxygen Uptake in Rats and Mice: Practical Implications for Exercise Training. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 753–760. [Google Scholar] [CrossRef]
- Purves, R.D. Optimum Numerical Integration Methods for Estimation of Area-Under-the-Curve (AUC) and Area-Under-the-Moment-Curve (AUMC). J. Pharmacokinet. Biopharm. 1992, 20, 211–226. [Google Scholar] [CrossRef]
- Schwarzer, M.; Molis, A.; Schenkl, C.; Schrepper, A.; Britton, S.L.; Koch, L.G.; Doenst, T. Genetically Determined Exercise Capacity Affects Systemic Glucose Response to Insulin in Rats. Physiol. Genom. 2021, 53, 395–405. [Google Scholar] [CrossRef]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Biochemical Properties of Subsarcolemmal and Interfibrillar Mitochondria Isolated from Rat Cardiac Muscle. J. Biol. Chem. 1977, 252, 8731–8739. [Google Scholar] [CrossRef]
- Srere, P. [1] Citrate synthase:[EC 4.1. 3.7. Citrate Oxaloacetate-Lyase (CoA-acetylating)]. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1969; Volume 13, pp. 3–11. [Google Scholar]
- Dabkowski, E.R.; Williamson, C.L.; Bukowski, V.C.; Chapman, R.S.; Leonard, S.S.; Peer, C.J.; Callery, P.S.; Hollander, J.M. Diabetic Cardiomyopathy-Associated Dysfunction in Spatially Distinct Mitochondrial Subpopulations. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H359–H369. [Google Scholar] [CrossRef]
- Schwarzer, M.; Osterholt, M.; Lunkenbein, A.; Schrepper, A.; Amorim, P.; Doenst, T. Mitochondrial Reactive Oxygen Species Production and Respiratory Complex Activity in Rats with Pressure Overload-Induced Heart Failure. J. Physiol. 2014, 592, 3767–3782. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.-C.; Shao, J.; Wang, H.; Lokhnygina, Y. Sample Size Calculations in Clinical Research; CRC Press: New York, NY, USA, 2017; Volume 58. [Google Scholar]
- Seifert, E.L.; Bastianelli, M.; Aguer, C.; Moffat, C.; Estey, C.; Koch, L.G.; Britton, S.L.; Harper, M.E. Intrinsic Aerobic Capacity Correlates with Greater Inherent Mitochondrial Oxidative and H2O2 Emission Capacities without Major Shifts in Myosin Heavy Chain Isoform. J. Appl. Physiol. 2012, 113, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, J.Y.; Van den Bergh, F.; Collins, N.L.; Bowers, M.; Beard, D.A.; Burant, C.F. Higher Mitochondrial Oxidative Capacity is the Primary Molecular Differentiator in Muscle of Rats with High and Low Intrinsic Cardiorespiratory Fitness. Mol. Metab. 2023, 76, 101793. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.J.; Stepto, N.K.; Koch, L.G.; Britton, S.L.; Hawley, J.A. Divergent Skeletal Muscle Respiratory Capacities in Rats Artificially Selected for High and Low Running Ability: A role for Nor1? J. Appl. Physiol. 2012, 113, 1403–1412. [Google Scholar] [CrossRef]
- Lai, N.; Kummitha, C.M.; Rosca, M.G.; Fujioka, H.; Tandler, B.; Hoppel, C.L. Isolation of Mitochondrial Subpopulations from Skeletal Muscle: Optimizing Recovery and Preserving Integrity. Acta Physiol. 2019, 225, e13182. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.M.; Thapa, D.; Shepherd, D.L. Physiological and Structural Differences in Spatially Distinct Subpopulations of Cardiac Mitochondria: Influence of Cardiac Pathologies. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1–H14. [Google Scholar] [CrossRef]
- Schwarzer, M.; Schrepper, A.; Amorim, P.A.; Osterholt, M.; Doenst, T. Pressure Overload Differentially Affects Respiratory Capacity in Interfibrillar and Subsarcolemmal Mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H529–H537. [Google Scholar] [CrossRef]
- Srisakuldee, W.; Makazan, J.; Nickel, B.; Zhang, F.; Thliveris, J.; Pasumarthi, K.; Kardami, E. The FGF-2-Triggered Protection of Cardiac Subsarcolemmal Mitochondria from Calcium Overload is Mitochondrial Connexin 43-Dependent. Cardiovasc. Res. 2014, 103, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical Activity Advice Only or Structured Exercise Training and Association with HbA1c Levels in Type 2 Diabetes: A Systematic Review and Meta-Analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.G.; Green, C.L.; Lee, A.D.; Hornyak, J.E.; Cicila, G.T.; Britton, S.L. Test of the Principle of Initial Value in Rat Genetic Models of Exercise Capacity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R466–R472. [Google Scholar] [CrossRef] [PubMed]
- Wisløff, U.; Helgerud, J.; Kemi, O.J.; Ellingsen, O. Intensity-Controlled Treadmill Running in Rats: VO(2 Max) and Cardiac Hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1301–H1310. [Google Scholar] [CrossRef]
- Schoepe, M.; Schrepper, A.; Schwarzer, M.; Osterholt, M.; Doenst, T. Exercise Can Induce Temporary Mitochondrial and Contractile Dysfunction Linked to Impaired Respiratory Chain Complex Activity. Metabolism 2012, 61, 117–126. [Google Scholar] [CrossRef]
- Wang, Y.; Wisloff, U.; Kemi, O.J. Animal Models in the Study of Exercise-Induced Cardiac Hypertrophy. Physiol. Res. 2010, 59, 633–644. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Koziel, A.; Woyda-Ploszczyca, A.; Celichowski, J.; Jarmuszkiewicz, W. Endurance Training Increases the Efficiency of Rat Skeletal Muscle Mitochondria. Pflügers Arch. Eur. J. Physiol. 2016, 468, 1709–1724. [Google Scholar] [CrossRef]
- Shi, M.; Ellingsen, Ø.; Bathen, T.F.; Høydal, M.A.; Koch, L.G.; Britton, S.L.; Wisløff, U.; Stølen, T.O.; Esmaeili, M. Skeletal Muscle Metabolism in Rats with Low and High Intrinsic Aerobic Capacity: Effect of Aging and Exercise Training. PLoS ONE 2018, 13, e0208703. [Google Scholar] [CrossRef]
- Lessard, S.J.; Rivas, D.A.; Stephenson, E.J.; Yaspelkis, B.B., 3rd; Koch, L.G.; Britton, S.L.; Hawley, J.A. Exercise Training Reverses Impaired Skeletal Muscle Metabolism Induced by Artificial Selection for Low Aerobic Capacity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R175–R182. [Google Scholar] [CrossRef]
- Naples, S.P.; Borengasser, S.J.; Rector, R.S.; Uptergrove, G.M.; Morris, E.M.; Mikus, C.R.; Koch, L.G.; Britton, S.L.; Ibdah, J.A.; Thyfault, J.P. Skeletal Muscle Mitochondrial and Metabolic Responses to a High-Fat Diet in Female Rats Bred for High and Low Aerobic Capacity. Appl. Physiol. Nutr. Metab. 2010, 35, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Judge, S.; Jang, Y.M.; Smith, A.; Selman, C.; Phillips, T.; Speakman, J.R.; Hagen, T.; Leeuwenburgh, C. Exercise by Lifelong Voluntary Wheel Running Reduces Subsarcolemmal and Interfibrillar Mitochondrial Hydrogen Peroxide Production in the Heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1564–R1572. [Google Scholar] [CrossRef]
- Kavazis, A.N.; Alvarez, S.; Talbert, E.; Lee, Y.; Powers, S.K. Exercise training induces a cardioprotective phenotype and Alterations in Cardiac Subsarcolemmal and Intermyofibrillar Mitochondrial Proteins. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H144–H152. [Google Scholar] [CrossRef] [PubMed]
- Kavazis, A.N.; McClung, J.M.; Hood, D.A.; Powers, S.K. Exercise Induces a Cardiac Mitochondrial Phenotype That Resists Apoptotic Stimuli. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H928–H935. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Lüthi, P.; Claassen, H.; Weibel, E.R.; Howald, H. Ultrastructure of Normal Human Skeletal Muscle; a Morphometric Analysis in Controls and Men Trained in Long-Distance Running. Hoppe Seylers Z. Physiol. Chem. 1973, 354, 229–230. [Google Scholar] [PubMed]
- Koves, T.R.; Noland, R.C.; Bates, A.L.; Henes, S.T.; Muoio, D.M.; Cortright, R.N. Subsarcolemmal and Intermyofibrillar Mitochondria Play Distinct Roles in Regulating Skeletal Muscle Fatty Acid Metabolism. Am. J. Physiol. Cell Physiol. 2005, 288, C1074–C1082. [Google Scholar] [CrossRef] [PubMed]
- Bizeau, M.E.; Willis, W.T.; Hazel, J.R. Differential Responses to Endurance Training in Subsarcolemmal and Intermyofibrillar Mitochondria. J. Appl. Physiol. 1998, 85, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Krieger, D.A.; Tate, C.A.; McMillin-Wood, J.; Booth, F.W. Populations of Rat Skeletal Muscle Mitochondria after Exercise and Immobilization. J. Appl. Physiol. Respir. Env. Exerc. Physiol. 1980, 48, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Roussel, D.; Lhenry, F.; Ecochard, L.; Sempore, B.; Rouanet, J.L.; Favier, R. Differential Effects of Endurance Training and Creatine Depletion on Regional Mitochondrial Adaptations in Rat Skeletal Muscle. Biochem. J. 2000, 350 Pt 2, 547–553. [Google Scholar] [CrossRef]
- Gemmink, A.; Schrauwen, P.; Hesselink, M.K.C. Exercising Your Fat (Metabolism) into Shape: A Muscle-Centred View. Diabetologia 2020, 63, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Daemen, S.; van Polanen, N.; Hesselink, M.K.C. The Effect of Diet and Exercise on Lipid Droplet Dynamics in Human Muscle Tissue. J. Exp. Biol. 2018, 221, jeb167015. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.B.; Murach, K.A.; Dyar, K.A.; Zierath, J.R. Exercise Metabolism and Adaptation in Skeletal Muscle. Nat. Rev. Mol. Cell Biol. 2023, 24, 607–632. [Google Scholar] [CrossRef]
- Bye, A.; Høydal, M.A.; Catalucci, D.; Langaas, M.; Kemi, O.J.; Beisvag, V.; Koch, L.G.; Britton, S.L.; Ellingsen, Ø.; Wisløff, U. Gene expression Profiling of Skeletal Muscle in Exercise-Trained and Sedentary Rats with Inborn High and Low VO2max. Physiol. Genom. 2008, 35, 213–221. [Google Scholar] [CrossRef]
- Overmyer, K.A.; Evans, C.R.; Qi, N.R.; Minogue, C.E.; Carson, J.J.; Chermside-Scabbo, C.J.; Koch, L.G.; Britton, S.L.; Pagliarini, J.; Coon, J.J.; et al. Maximal Oxidative Capacity during Exercise Is Associated with Skeletal Muscle Fuel Selection and Dynamic Changes in Mitochondrial Protein Acetylation. Cell Metab. 2015, 21, 468–478. [Google Scholar] [CrossRef]
- Zhang, Y.; Kumarasamy, S.; Mell, B.; Cheng, X.; Morgan, E.E.; Britton, S.L.; Vijay-Kumar, M.; Koch, L.G.; Joe, B. Vertical Selection for Nuclear and Mitochondrial Genomes Shapes Gut Microbiota and Modifies Risks for Complex Diseases. Physiol. Genom. 2020, 52, 1–14. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Coyle, E.F. Adaptations of Skeletal Muscle to Endurance Exercise and Their Metabolic Consequences. J. Appl. Physiol. Respir. Env. Exerc. Physiol. 1984, 56, 831–838. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Jain, S.S.; McFarlan, J.T.; Snook, L.A.; Chabowski, A.; Bonen, A. Exercise- and Training-Induced Upregulation of Skeletal Muscle Fatty Acid Oxidation Are Not Solely Dependent on Mitochondrial Machinery and Biogenesis. J. Physiol. 2013, 591, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.-M.; Fritzen, A.M.; Kiens, B. Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Melanson, E.L.; MacLean, P.S.; Hill, J.O. Exercise Improves Fat Metabolism in Muscle but Does Not Increase 24-h Fat Oxidation. Exerc. Sport. Sci. Rev. 2009, 37, 93–101. [Google Scholar] [CrossRef]
- Silva, L.A.; Tromm, C.B.; Doyenart, R.; Thirupathi, A.; Silveira, P.C.L.; Pinho, R.A. Effects of Different Frequencies of Physical Training on Electron Transport Chain and Oxidative Damage in Healthy Mice. Mot. Rev. De. Educ. Física 2018, 24, e101804. [Google Scholar] [CrossRef]
- Greggio, C.; Jha, P.; Kulkarni, S.S.; Lagarrigue, S.; Broskey, N.T.; Boutant, M.; Wang, X.; Conde Alonso, S.; Ofori, E.; Auwerx, J.; et al. Enhanced Respiratory Chain Supercomplex Formation in Response to Exercise in Human Skeletal Muscle. Cell Metab. 2017, 25, 301–311. [Google Scholar] [CrossRef]
- Sukala, W.R.; Page, R.; Cheema, B.S. Exercise Training in High-Risk Ethnic Populations with Type 2 Diabetes: A Systematic Review of Clinical Trials. Diabetes Res. Clin. Pract. 2012, 97, 206–216. [Google Scholar] [CrossRef]
- Lewis, D.A.; Kamon, E.; Hodgson, J.L. Physiological Differences between Genders. Implications for Sports Conditioning. Sports Med. 1986, 3, 357–369. [Google Scholar] [CrossRef]



| HCR (n) | HCR ex (n) | LCR (n) | LCR ex (n) | G | E | I | |
|---|---|---|---|---|---|---|---|
| Age [weeks] | 15.1 ± 0.3 (15) | 15.6 ± 0.1 (11) | 15.3 ± 0.2 (12) | 15.4 ± 0.3 (7) | ns | ns | ns |
| Body weight [g] | 177 ± 3 (14) | 204 ± 8 ** (11) | 213 ± 6 ††† (12) | 204 ± 8 (6) | ** | ns | ** |
| Tibia length (TL) [mm] | 34.1 ± 0.4 (15) | 34.5 ± 0.2 (11) | 34.8 ± 0.4 (12) | 35.1 ± 0.4 (6) | ns | ns | ns |
| Heart weight/TL [mg/mm] | 17.2 ± 0.3 (15) | 20.3 ± 0.6 *** (11) | 17.4 ± 0.6 (12) | 18.2 ± 0.6 † (6) | ns | *** | * |
| Lung weight/TL [mg/mm] | 24.9 ± 0.4 (15) | 27.9 ± 0.7 ** (10) | 25.9 ± 0.7 (12) | 26.2 ± 1.2 (6) | ns | * | ns |
| Liver weight/TL [g/mm] | 181 ± 3 (15) | 233 ± 10 *** (11) | 211 ± 10 †† (12) | 208 ± 11 (6) | ns | ** | ** |
| Heart rate [beats/min] | 380 ± 14 (8) | 339 ± 7 * (6) | 384 ± 11 (8) | 370 ± 12 (10) | ns | * | ns |
| IVSd + LVPWd [mm] | 3.14 ± 0.11 (8) | 3.25 ± 0.18 (6) | 3.34 ± 0.11 (8) | 3.37 ± 0.09 (10) | ns | ns | ns |
| LVIDd [mm] | 6.41 ± 0.16 (8) | 6.92 ± 0.13 (6) | 6.10 ± 0.19 (8) | 6.42 ± 0.16 † (10) | * | * | ns |
| E/E’ | 14.2 ± 0.6 (8) | 15.1 ± 0.6 (6) | 14.4 ± 1.0 (8) | 17.5 ± 1.1 (10) | ns | ns | ns |
| FS [%] | 41.8 ± 2.1 (8) | 41.6 ± 2.9 (6) | 53.6 ± 2.6 ††† (8) | 47.6 ± 2.1 (10) | *** | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyne, E.; Zeeb, S.; Junker, C.; Petzinna, A.; Schrepper, A.; Doenst, T.; Koch, L.G.; Britton, S.L.; Schwarzer, M. Exercise Training Differentially Affects Skeletal Muscle Mitochondria in Rats with Inherited High or Low Exercise Capacity. Cells 2024, 13, 393. https://doi.org/10.3390/cells13050393
Heyne E, Zeeb S, Junker C, Petzinna A, Schrepper A, Doenst T, Koch LG, Britton SL, Schwarzer M. Exercise Training Differentially Affects Skeletal Muscle Mitochondria in Rats with Inherited High or Low Exercise Capacity. Cells. 2024; 13(5):393. https://doi.org/10.3390/cells13050393
Chicago/Turabian StyleHeyne, Estelle, Susanne Zeeb, Celina Junker, Andreas Petzinna, Andrea Schrepper, Torsten Doenst, Lauren G. Koch, Steven L. Britton, and Michael Schwarzer. 2024. "Exercise Training Differentially Affects Skeletal Muscle Mitochondria in Rats with Inherited High or Low Exercise Capacity" Cells 13, no. 5: 393. https://doi.org/10.3390/cells13050393
APA StyleHeyne, E., Zeeb, S., Junker, C., Petzinna, A., Schrepper, A., Doenst, T., Koch, L. G., Britton, S. L., & Schwarzer, M. (2024). Exercise Training Differentially Affects Skeletal Muscle Mitochondria in Rats with Inherited High or Low Exercise Capacity. Cells, 13(5), 393. https://doi.org/10.3390/cells13050393






