Ophthalmological Manifestations in Inflammatory Bowel Diseases: Keep an Eye on It
Abstract
1. Introduction
2. Materials and Methods
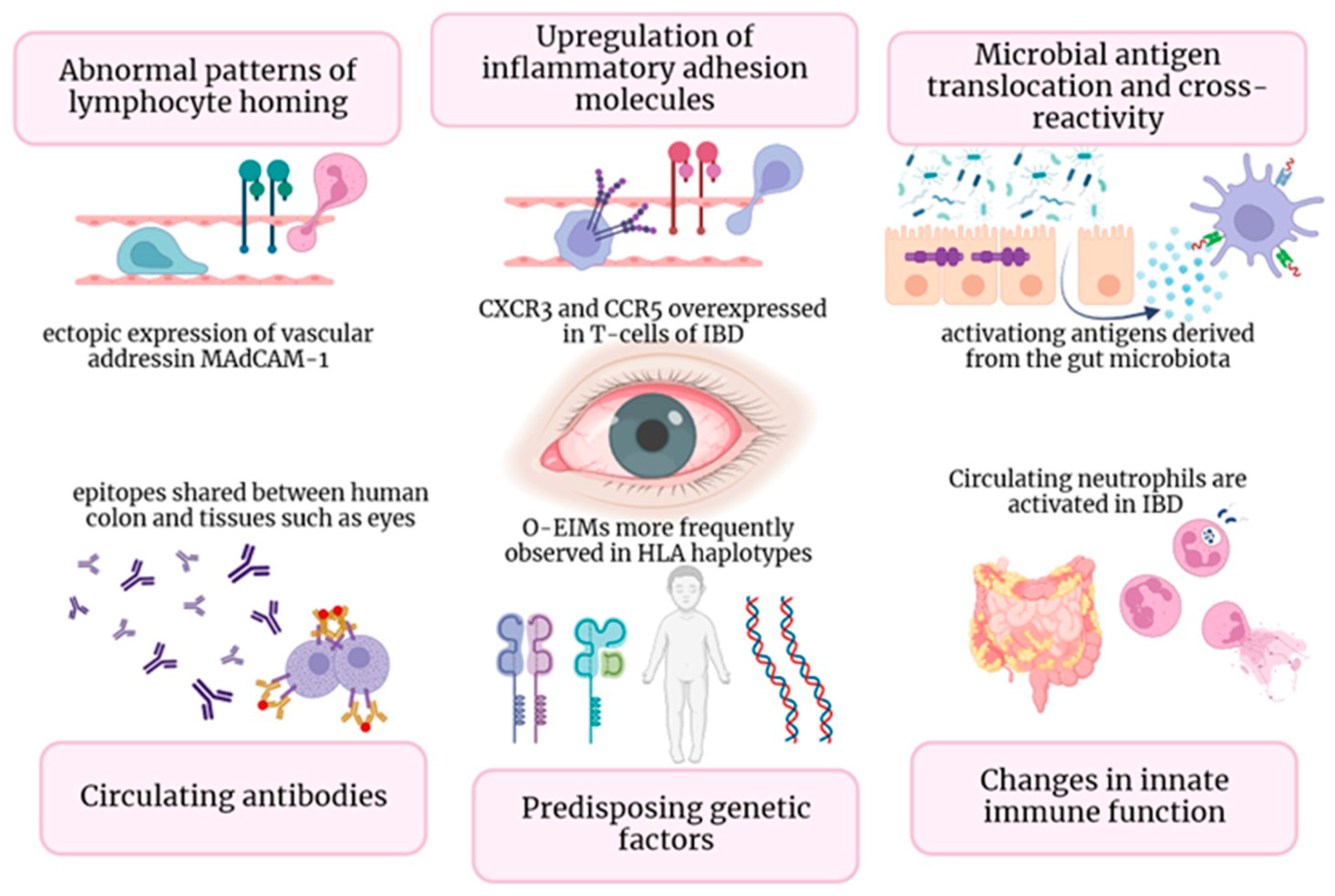
3. Pathogenesis
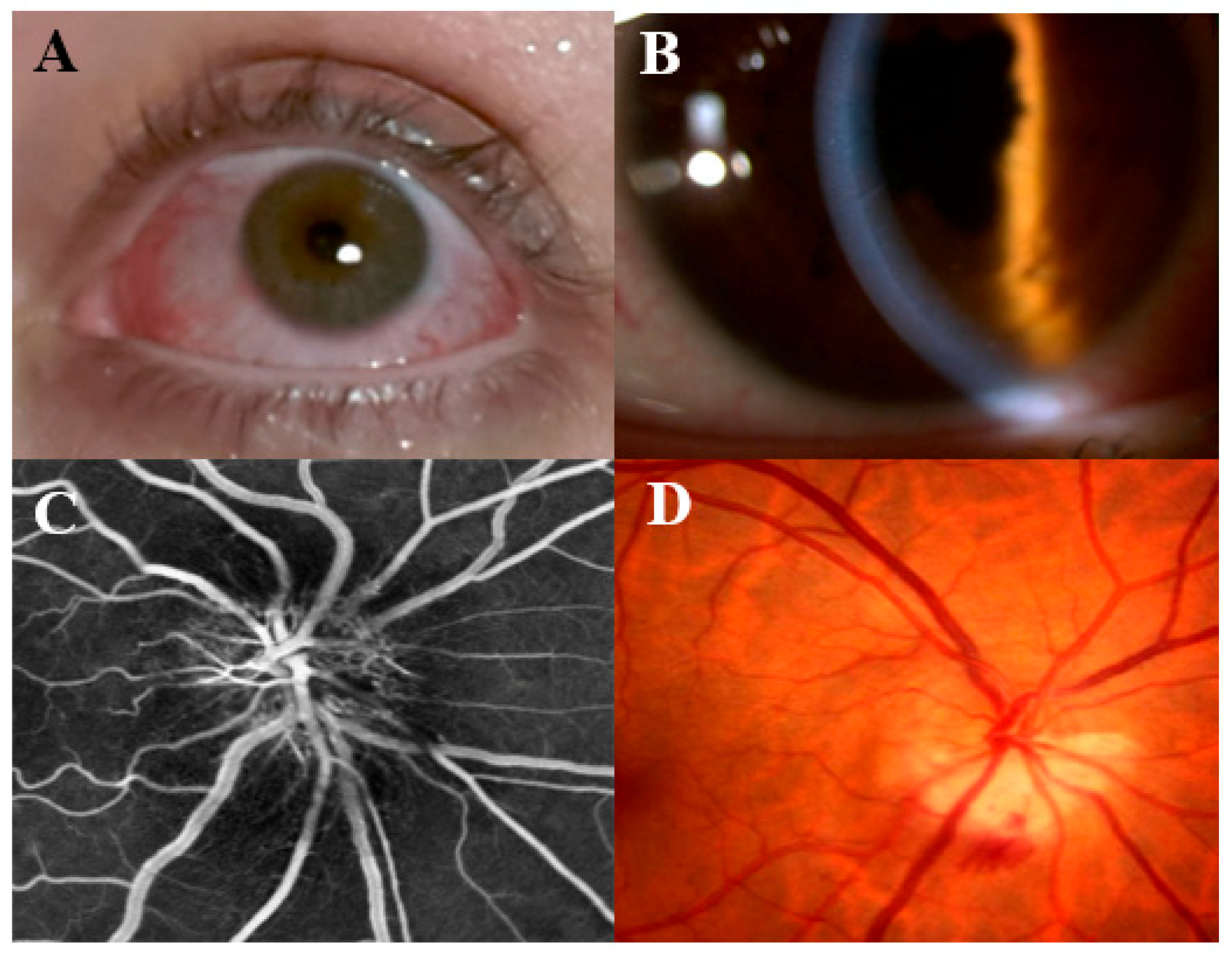
4. Episcleritis and Scleritis
4.1. Episcleritis
4.2. Scleritis
5. Uveitis
6. Corneal Disease and Other Rare Manifestations
7. Treatment
| Pathophysiology | Clinical Manifestations | Epidemiology | Therapeutic Molecules | |
|---|---|---|---|---|
| Episcleritis/scleritis | Multifactorial, mostly unknown |
|
|
|
| Uveitis | Ectopic over-expression of adhesion molecules and chemokines in extra-intestinal tissues * Bacterial antigen cross-reactivity molecular mimicry Microbial translocation |
|
|
|
| Keratitis | Altered innate Immunity |
|
|
|
| Retinal vasculitis, retinal vascular occlusions, optic neuritis | Genetic predisposition ** Environmental factors |
|
|
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.; Chan, F.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Venkatesh, P.G.K.; Navaneethan, U. Diagnosis and Therapeutic Management of Extra-Intestinal Manifestations of Inflammatory Bowel Disease. Drugs 2012, 72, 2333–2349. [Google Scholar] [CrossRef]
- Greuter, T.; Vavricka, S.R. Extraintestinal manifestations in inflammatory bowel disease–epidemiology, genetics, and pathogenesis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 307–317. [Google Scholar] [CrossRef]
- Kilic, Y.; Kamal, S.; Jaffar, F.; Sriranganathan, D.; Quraishi, M.N.; Segal, J.P. Prevalence of Extraintestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2023, 20, izad061. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Rogler, G.; Gantenbein, C.; Spoerri, M.; Vavricka, M.P.; Navarini, A.A.; French, L.; Safroneeva, E.; Fournier, N.; Straumann, A.; et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm. Bowel Dis. 2015, 21, 1794–1800. [Google Scholar] [CrossRef]
- Park, S.K.; Wong, Z.; Park, S.H.; Van Vu, K.; Bang, K.B.; Piyachaturawat, P.; Myint, T.; Hilmi, I.; Park, D.I. Extraintestinal manifestation of inflammatory bowel disease in Asian patients: A multinational study. Dig. Liver Dis. 2021, 53, 196–201. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Hedin, C.R.H.; Vavricka, S.R.; Stagg, A.J.; Schoepfer, A.; Raine, T.; Puig, L.; Pleyer, U.; Navarini, A.; Van Der Meulen-De Jong, A.E.; Maul, J.; et al. The Pathogenesis of Extraintestinal Manifestations: Implications for IBD Research, Diagnosis, and Therapy. J. Crohns Colitis 2019, 13, 541–554. [Google Scholar] [CrossRef]
- Eksteen, B.; Grant, A.J.; Miles, A.; Curbishley, S.M.; Lalor, P.F.; Hübscher, S.G.; Briskin, M.; Salmon, M.; Adams, D.H. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J. Exp. Med. 2004, 200, 1511–1517. [Google Scholar] [CrossRef]
- Horai, R.; Caspi, R.R. Cytokines in autoimmune uveitis. J. Interferon Cytokine Res. 2011, 31, 733–744. [Google Scholar] [CrossRef]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; Van Assche, G.; Van Der Merwe, S.; Vermeire, S.; et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 2016, 65, 1681–1689. [Google Scholar] [CrossRef]
- Pytrus, W.; Akutko, K.; Pytrus, T.; Turno-Kręcicka, A. A Review of Ophthalmic Complications in Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 7457. [Google Scholar] [CrossRef]
- Troncoso, L.L.; Biancardi, A.L.; Vieira de Moraes, H.J.; Zaltman, C. Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J. Gastroenterol. 2017, 23, 5836–5848. [Google Scholar] [CrossRef]
- Mintz, R.; Feller, E.R.; Bahr, R.L.; Shah, S.A. Ocular Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 135–139. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef]
- Li, J.X.; Chiang, C.C.; Chen, S.N.; Lin, J.M.; Tsai, Y.Y. The Prevalence of Ocular Extra-Intestinal Manifestations in Adults Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15683. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Das, K.M. Relationship of Extraintestinal Involvements in Inflammatory Bowel Disease New Insights into Autoimmune Pathogenesis. Dig. Dis. Sci. 1999, 44, 1–13. [Google Scholar] [CrossRef]
- Meng, Y.; Tan, Z.; Liu, C.; Dong, W.; Chen, C. Association between Inflammatory Bowel Disease and Iridocyclitis: A Mendelian Randomization Study. J. Clin. Med. 2023, 12, 1282. [Google Scholar] [CrossRef]
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Bhagat, S.; Das, K.M. A Shared and Unique Peptide in the Human Colon, Eye, and Joint Detected by a Monoclonal Antibody. Gastroenterology 1994, 107, 103–108. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Brun, L.; Ballabeni, P.; Pittet, V.; Prinz Vavricka, B.M.; Zeitz, J.; Rogler, G.; Schoepfer, A.M. Frequency and Risk Factors for Extraintestinal Manifestations in the Swiss Inflammatory Bowel Disease Cohort. Am. J. Gastroenterol. 2011, 106, 110–119. [Google Scholar] [CrossRef]
- Taylor, S.R.; McCluskey, P.; Lightman, S. The ocular manifestations of inflammatory bowel disease. Curr. Opin. Ophthalmol. 2006, 17, 538–544. [Google Scholar] [CrossRef]
- Lin, P.; Tessler, H.H.; Goldsterin, D.A. Family history of inflammatory bowel disease in patients with idiopathic ocular inflammation. Am. J. Ophthalmol. 2006, 141, 1097–1104. [Google Scholar] [CrossRef]
- Lanna, C.C.D.; Ferrari, M.D.L.A.; Rocha, S.L.; Nascimento, E.; Carvalho, M.A.P.; Cunha, A.S. A cross-sectional study of 130 Brazilian patients with Crohn’s disease and ulcerative colitis: Analysis of articular and ophthalmologic manifestations. Clin. Rheumatol. 2008, 27, 503–509. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Younis, N.; Zarif, R.; Mahfouz, R. Inflammatory bowel disease: Between genetics and microbiota. Mol. Biol. Rep. 2020, 47, 3053–3063. [Google Scholar] [CrossRef]
- Cheema, H.A.; Waheed, N.; Saeed, A.; Fayyaz, Z.; Anjum, M.N.; Alvi, M.A.; Batool, S. Very early onset inflammatory bowel disease: Spectrum of clinical presentation, diagnostic tools and outcome in children. J. Pak. Med. Assoc. 2021, 71, 2350–2354. [Google Scholar] [CrossRef]
- Naser, S.A.; Arce, M.; Khaja, A.; Fernandez, M.; Naser, N.; Elwasila, S.; Thanigachalam, S. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J. Gastroenterol. 2012, 18, 412–424. [Google Scholar] [CrossRef]
- Cho, J.H. Basic Science on the Cutting Edge the Nod2 Gene in Crohn’s Disease: Implications for Future Research into the Genetics and Immunology of Crohn’s Disease. Inflamm. Bowel Dis. 2001, 7, 271–275. [Google Scholar] [CrossRef]
- Matsuda, T.; Kambe, N.; Takimoto-Ito, R.; Ueki, Y.; Nakamizo, S.; Saito, M.K.; Takei, S.; Kanazawa, N. Potential Benefits of TNF Targeting Therapy in Blau Syndrome, a NOD2-Associated Systemic Autoinflammatory Granulomatosis. Front. Immunol. 2022, 13, 895765. [Google Scholar] [CrossRef]
- Duerr, R.H.M. The genetics of inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2002, 31, 63–76. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Deng, F. The Role of Vitamin D in Immune System and Inflammatory Bowel Disease. J. Inflamm. Res. 2022, 15, 3167–3185. [Google Scholar] [CrossRef]
- Orchard, T.R.; Chua, C.N.; Ahmad, T.; Cheng, H.; Welsh, K.I.; Jewell, D.P. Uveitis and erythema nodosum in inflammatory bowel disease: Clinical features and the role of HLA genes. Gastroenterology 2002, 123, 714–718. [Google Scholar] [CrossRef]
- Skaaby, T.; Husemoen, L.L.N.; Thuesen, B.H.; Linneberg, A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 2015, 50, 231–238. [Google Scholar] [CrossRef]
- Santeford, A.; Wiley, L.A.; Park, S.; Bamba, S.; Nakamura, R.; Gdoura, A.; Ferguson, T.A.; Rao, P.K.; Guan, J.L.; Saitoh, T.; et al. Impaired autophagy in macrophages promotes inflammatory eye disease. Autophagy 2016, 12, 1876–1885. [Google Scholar] [CrossRef]
- Horai, R.; Zárate-Bladés, C.R.; Dillenburg-Pilla, P.; Chen, J.; Kielczewski, J.L.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.C.; Yamane, H.; Honda, K.; et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity 2015, 43, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Salvador, R.; Horai, R.; Zhang, A.; Jittayasothorn, Y.; Tang, J.; Gupta, A.; Nagarajan, V.; Caspi, R.R. Too Much of a Good Thing: Extended Duration of Gut Microbiota Depletion Reverses Protection From Experimental Autoimmune Uveitis. Investig. Opthalmol. Vis. Sci. 2023, 64, 43. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Thumboo, J.; Tan, Y.K.; Wong, T.Y.; Albani, S. Eye: A Window Oppor. Rheum. Arthritis? Nat. Rev. Rheumatol. 2014, 10, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Sainz De La Maza, M.; Molina, N.; Gonzalez-Gonzalez, L.A.; Doctor, P.P.; Tauber, J.; Foster, C.S. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology 2012, 119, 43–50. [Google Scholar] [CrossRef]
- Sainz-de-la-Maza, M.; Molins, B.; Mesquida, M.; Llorenç, V.; Zarranz-Ventura, J.; Sala-Puigdollers, A.; Matas, J.; Adan, A.; Foster, C.S. Interleukin-22 serum levels are elevated in active scleritis. Acta Ophthalmol. 2016, 94, e395–e399. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Y.; Zhang, T.; Shen, Y. Identifying TNF and IL6 as potential hub genes and targeted drugs associated with scleritis: A bio-informative report. Front. Immunol. 2023, 14, 1098140. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.F.; Wright, J.P.; Murray, A.D.N. Ocular Inflammation in Crohn’s Disease. Ophthalmology 1991, 98, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Mady, R.; Grover, W.; Butrus, S. Ocular complications of inflammatory bowel disease. Sci. World J. 2015, 2015, 438402. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Shah, A.; Hassman, L.; Gutierrez, A. Ocular Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1832–1838. [Google Scholar] [CrossRef]
- Jabs, D.A.; Mudun, A.; Dunn, J.P.; Marsh, M.J. Episcleritis and Scleritis: Clinical Features and Treatment Results. Am. J. Ophthalmol. 2000, 130, 469–476. [Google Scholar] [CrossRef]
- Thomas, A.S.; Lin, P. Ocular manifestations of inflammatory bowel disease. Curr. Opin. Ophthalmol. 2016, 27, 552–560. [Google Scholar] [CrossRef]
- Braithwaite, T.; Adderley, N.J.; Subramanian, A.; Galloway, J.; Kempen, J.H.; Gokhale, K.; Cope, A.P.; Dick, A.D.; Nirantharakumar, K.; Denniston, A.K. Epidemiology of Scleritis in the United Kingdom from 1997 to 2018, Population-Based Analysis of 11 Million Patients and Association between Scleritis and Infectious and Immune-Mediated Inflammatory Disease. Arthritis Rheumatol. 2021, 73, 1267–1276. [Google Scholar] [CrossRef]
- Abdel-Aty, A.; Gupta, A.; Del Priore, L.; Kombo, N. Management of noninfectious scleritis. Ther. Adv. Ophthalmol. 2022, 14, 25158414211070879. [Google Scholar] [CrossRef]
- Sims, J. Scleritis: Presentations, disease associations and management. Postgrad. Med. J. 2012, 88, 713–718. [Google Scholar] [CrossRef]
- Murthy, S.I.; Sabhapandit, S.; Balamurugan, S.; Subramaniam, P.; Sainz-De-La-Maza, M.; Agarwal, M.; Parvesio, C. Scleritis: Differentiating infectious from non-infectious entities. Indian J. Ophthalmol. 2020, 68, 1818–1828. [Google Scholar] [CrossRef]
- Janardhana, P.; Al-Kadhi, S. A review of ophthalmic manifestations of inflammatory bowel disease and associated treatments. Curr. Opin. Ophthalmol. 2021, 32, 549–554. [Google Scholar] [CrossRef]
- Late Jameson Evans, T.P.; Eustace, P. Scleromalacia perforans associated with Crohn’s disease Treated with sodium versenate (EDTA). Br. J. Ophthalmol. 1973, 57, 330. [Google Scholar] [CrossRef]
- Satsangi, J.; Grootscholten, C.; Holt, H.; Jewell, D.P. Clinical patterns of familial inflammatory bowel disease. Gut 1996, 38, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Van Sommeren, S.; Janse, M.; Karjalainen, J.; Fehrmann, R.; Franke, L.; Fu, J.; Weersma, R.K. Extraintestinal manifestations and complications in inflammatory bowel disease: From shared genetics to shared biological pathways. Inflamm. Bowel Dis. 2014, 20, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, D.P.C.; Rothova, A.; Berge, J.C.T.; Verdijk, R.M.; van Laar, J.A.M.; Vingerling, J.R.; Schreurs, M.W.J. Current insights in the pathogenesis of scleritis. Exp. Eye Res. 2020, 197, 108078. [Google Scholar] [CrossRef]
- Wen, X.; Hu, X.; Miao, L.; Ge, X.; Deng, Y.; Bible, P.W.; Wei, L. Epigenetics, microbiota, and intraocular inflammation: New paradigms of immune regulation in the eye. Prog. Retin. Eye Res. 2018, 64, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Tsirouki, T.; Dastiridou, A.; Symeonidis, C.; Tounakaki, O.; Brazitikou, I.; Kalogeropoulos, C.; Androudi, S. A Focus on the Epidemiology of Uveitis. Ocul. Immunol. Inflamm. 2018, 26, 2–16. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, J.; Liang, C.; Wu, D.; Tan, Y.; Luo, L.; Liu, Z. Differences in the prevalence of uveitis between Crohn’s disease and ulcerative colitis: A systematic review and meta-analysis. Acta Ophthalmol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Burisch, J.; Ellul, P.; Karmiris, K.; Katsanos, K.; Allocca, M.; Bamias, G.; Barreiro-De Acosta, M.; Braithwaite, T.; Greuter, T.; et al. ECCO Guidelines on Extraintestinal Manifestations in Inflammatory Bowel Disease. J. Crohns Colitis 2023, 2023, jjad108. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.L.; Rosenbaum, J.T. Uveitis Associated with Inflammatory Bowel Disease Compared with Uveitis Associated with Spondyloarthropathy. Arch. Ophthalmol. 1997, 115, 61–64. [Google Scholar] [CrossRef]
- Biedermann, L.; Renz, L.; Fournier, N.; Rossel, J.B.; Butter, M.; Bluemel, S.; Vavricka, S.; Rogler, G.; Scharl, M.; Anderegg, C.; et al. Uveitis manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study. Ther. Adv. Gastroenterol. 2019, 12, 1756284819865142. [Google Scholar] [CrossRef]
- Miller, J.R.C.; Hanumunthadu, D. Inflammatory eye disease: An overview of clinical presentation and management. Clin. Med. J. R. Coll. Physicians Lond. 2022, 22, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.; Mishra, D.; Singh, T.R.R. Epidemiology of Ocular Manifestations in Autoimmune Disease. Front. Immunol. 2021, 12, 744396. [Google Scholar] [CrossRef]
- Vianna, R.N.G.; Ozdal, P.C.; Deschnes, J. Multifocal choroiditis—An unusual finding in Crohns disease. Eur. J. Ophthalmol. 2004, 14, 345–349. [Google Scholar] [CrossRef]
- Marrani, E.; Cimaz, R.; Lucherini, O.M.; Caputo, R.; Vitale, A.; Cantarini, L.; Simonini, G. The common NOD2/CARD15 variant P268S in patients with non-infectious uveitis: A cohort study. Pediatr. Rheumatol. 2015, 13, 38. [Google Scholar] [CrossRef]
- Campagnoli, L.I.M.; Varesi, A.; Barbieri, A.; Marchesi, N.; Pascale, A. Targeting the Gut–Eye Axis: An Emerging Strategy to Face Ocular Diseases. Int. J. Mol. Sci. 2023, 24, 13338. [Google Scholar] [CrossRef]
- Lin, P.; Bach, M.; Asquith, M.; Lee, A.Y.; Akileswaran, L.; Stauffer, P.; Davin, S.; Pan, Y.; Cambronne, E.D.; Dorris, M.; et al. HLA-B27 and human β2-microglobulin affect the gut microbiota of transgenic rats. PLoS ONE 2014, 9, e105684. [Google Scholar] [CrossRef]
- Janowitz, C.; Nakamura, Y.K.; Metea, C.; Gligor, A.; Yu, W.; Karstens, L.; Rosenbaum, J.T.; Asquith, M.; Lin, P. Disruption of intestinal homeostasis and intestinal microbiota during experimental autoimmune uveitis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 420–429. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Huang, X.; Yi, S.; Pan, S.; Zhang, Y.; Yuan, G.; Cao, Q.; Ye, X.; Li, H. Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling. Cell Rep. 2021, 36, 109726. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Nakamura, Y.K.; Janowitz, C.; Metea, C.; Asquith, M.; Karstens, L.; Rosenbaum, J.T.; Li, P. Short chain fatty acids ameliorate immune-mediated uveitis partially by altering migration of lymphocytes from the intestine. Sci. Rep. 2017, 7, 11745. [Google Scholar] [CrossRef]
- Tie, Y.; Huang, Y.; Chen, R.; Li, L.; Chen, M.; Zhang, S. Current insights on the roles of gut microbiota in inflammatory bowel disease-associated extra-intestinal manifestations: Pathophysiology and therapeutic targets. Gut Microbes 2023, 15, 2265028. [Google Scholar] [CrossRef]
- Czompa, L.; Barta, Z.; Ziad, H.; Nemeth, G.; Rentka, A.; Aszalos, Z.; Zold, E.; Gesztelyi, R.; Zsuga, J.; Szodoray, P.; et al. Corneal Manifestations of Inflammatory Bowel Disease. Semin. Ophthalmol. 2019, 34, 543–550. [Google Scholar] [CrossRef]
- Barta, Z.; Czompa, L.; Rentka, A.; Zold, E.; Remenyik, J.; Biro, A.; Gesztelyi, R.; Zsuga, J.; Szodoray, P.; Kemeny-Beke, A. Evaluation of Objective Signs and Subjective Symptoms of Dry Eye Disease in Patients with Inflammatory Bowel Disease. Biomed. Res. Int. 2019, 2019, 8310583. [Google Scholar] [CrossRef]
- Aman-Ullah, M.; Gimbel, H.V.; Purba, M.K.; Van Westenbrugge, J.A. Necrotizing keratitis after laser refractive surgery in patients with inactive inflammatory bowel disease. Case Rep. Ophthalmol. 2012, 3, 54–60. [Google Scholar] [CrossRef]
- Sainz De La Maza, M.; Foster, C.S.; Jabbur, N.S.; Baltatzis, S. Ocular Characteristics and Disease Associations in Scleritis-Associated Peripheral Keratopathy. Arch. Ophthalmol. 2002, 120, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Angioi, K.; Kaminsky, P.; Peyrin-Biroulet, L. Infliximab for severe peripheral ulcerative keratopathy revealing Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Ceresara, G.; Fogagnolo, P.; De Cillà, S.; Panizzo, V.; Danelli, P.G.; Orzalesi, N.; Rossetti, L. Corneal involvement in Crohn’s Disease: An in vivo confocal microscopy studt. Cornea 2011, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.P.; Bahar, I.; Sansanayudh, W.; Kaisermann, I.; Slomovic, A.R. Salzmann nodules—A possibile new ocular manifestation of Crohn’s disease: A case report. Cornea 2009, 28, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.M.; Spinella, R.; Aragona, P. Recurrence of Salzmann nodular degeneration of the cornea in a Crohn’s disease patient. Int. Ophthalmol. 2013, 33, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.; Asproudis, I.; Katsanos, K.H.; Dastiridou, A.I.; Aspiotis, M.; Tsianos, E.V. Orbital and optic nerve complications of inflammatory bowel disease. J. Crohns Colitis 2013, 7, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Balcer, L.J. Clinical practice. Opt. Neuritis. N. Engl. J. Med. 2006, 354, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Chung, C.H.; Sun, C.A.; Chen, P.H.; Chen, Y.H.; Liang, C.M.; Chen, J.T.; Chien, W.C.; Chen, C.L. Association between optic neuritis and inflammatory bowel disease: A population-based study. J. Clin. Med. 2021, 10, 688. [Google Scholar] [CrossRef]
- Gupta, G.; Gelfand, J.M.; Lewis, J.D. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology 2005, 129, 819–826. [Google Scholar] [CrossRef] [PubMed]
- The Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up. Arch. Neurol. 2008, 65, 727–732. [Google Scholar]
- Kowalski, T.; Mack, H.G. Ocular complications of tumour necrosis factor alpha inhibitors. Clin. Exp. Optom. 2020, 103, 148–154. [Google Scholar] [CrossRef]
- Landais, A.; Fanhan, R. Optic neuritis associated to treatment with infliximab. Presse Med. 2017, 46, 337–341. [Google Scholar] [CrossRef]
- Song, M.; Li, F.; Xie, X.; Chen, J.; Tang, M.; Tian, J.; Du, J.; Ge, Y.; Li, S.; Xu, S. Association of short-term efficacy for infliximab in rheumatoid arthritis with plasma concentration and anti-drug antibody. J. Cent. South Univ. (Med. Sci.) 2018, 43, 982–986. [Google Scholar] [CrossRef]
- Dermawan, A.; So, K.; Venugopal, K.; Picardo, S. Infliximab-induced optic neuritis. BMJ Case Rep. 2020, 13, e236041. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Chen, L.; Fraunfelder, F.W.; Ku, J.H.; Varley, C.D.; Suhler, E.; Hills, W.; Gattey, D.; Baddley, J.; Liu, L.; et al. Initiation of anti-TNF therapy and the risk of optic neuritis: From the safety assessment of biologic ThERapy (SABER) study. Am. J. Ophthalmol. 2013, 155, 183–189.e1. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, B.; Vandermeeren, Y.; Dewit, O.; Moreels, T.; de Boer, N.; Dhar, A.; Ziady, C.; Shitrit, A.; Steinwurz, F.; Jojic, N.; et al. Optic neuritis associated or not with TNF antagonists in patients with inflammatory bowel disease. J. Crohns Colitis 2016, 10, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.P.R.; Browning, A.C.; Sleep, T.J.; Webber, S.K.; McGill, J.I. A randomised, double-blind trial of topical ketorolac vs artificial tears for the treatment of episcleritis. Eye 2005, 19, 739–742. [Google Scholar] [CrossRef]
- Promelle, V.; Goeb, V.; Gueudry, J. Rheumatoid arthritis associated episcleritis and scleritis: An update on treatment perspectives. J. Clin. Med. 2021, 10, 2118. [Google Scholar] [CrossRef]
- Klein, A.; Eliakim, R. Non steroidal anti-inflammatory drugs and inflammatory bowel disease. Pharmaceuticals 2010, 3, 1084–1092. [Google Scholar] [CrossRef]
- Nevares, A.; Raut, R.; Libman, B.; Hajj-Ali, R. Noninfectious Autoimmune Scleritis: Recognition, Systemic Associations, and Therapy. Curr. Rheumatol. Rep. 2020, 22, 11. [Google Scholar] [CrossRef]
- Agrawal, R.; Lee, C.S.; Gonzalez-Lopez, J.J.; Khan, S.; Rodrigues, V.; Pavesio, C. Flurbiprofen: A Nonselective Cyclooxygenase (COX) Inhibitor for Treatment of Noninfectious, Non-necrotizing Anterior Scleritis. Ocul. Immunol. Inflamm. 2016, 24, 35–42. [Google Scholar] [CrossRef][Green Version]
- Greuter, T.; Rieder, F.; Kucharzik, T.; Peyrin-Biroulet, L.; Schoepfer, A.M.; Rubin, D.T.; Vavricka, S.R. Emerging treatment options for extraintestinal manifestations in IBD. Gut 2021, 70, 796–802. [Google Scholar] [CrossRef]
- Balasubramaniam, B.; Chong, Y.J.; Azzopardi, M.; Logeswaran, A.; Denniston, A.K. Topical Anti-Inflammatory Agents for Non-Infectious Uveitis: Current Treatment and Perspectives. J. Inflamm. Res. 2022, 15, 6439–6451. [Google Scholar] [CrossRef]
- Reddy, A.; Liu, S.H.; Brady, C.J.; Sieving, P.C.; Palestine, A.G. Corticosteroid implants for chronic non-infectious uveitis. Cochrane Database Syst. Rev. 2023, 2023. [Google Scholar] [CrossRef]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef]
- Singh, R.B.; Sinha, S.; Saini, C.; Elbasiony, E.; Thakur, S.; Agarwal, A. Recent advances in the management of non-infectious posterior uveitis. Int. Ophthalmol. 2020, 40, 3187–3207. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Scharl, M.; Gubler, M.; Rogler, G. Send Orders for Reprints to reprints@ benthamscience.net Biologics for Extraintestinal Manifestations of IBD. Curr. Drug Targets 2014, 15, 1064–1073. [Google Scholar] [CrossRef]
- Rispo, A.; Scarpa, R.; Di Girolamo, E.; Cozzolino, A.; Lembo, G.; Atteno, M.; De Falco, T.; Lo Presti, M.; Castiglione, F. Infliximab in the treatment of extra-intestinal manifestations of Crohn’s disease. Scand. J. Rheumatol. 2005, 34, 387–391. [Google Scholar] [CrossRef]
- Sharma, S.M.; Damato, E.; Hinchcliffe, A.E.; Andrews, C.D.; Myint, K.; Lee, R.; Dick, A.D. Long-term efficacy and tolerability of TNFα inhibitors in the treatment of non-infectious ocular inflammation: An 8-year prospective surveillance study. Br. J. Ophthalmol. 2021, 105, 1256–1262. [Google Scholar] [CrossRef]
- Stem, M.S.; Todorich, B.; Faia, L.J. Ocular Pharmacology for Scleritis: Review of Treatment and a Practical Perspective. J. Ocul. Pharmacol. Ther. 2017, 33, 240–246. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Dick, A.D.; Brézin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Noninfectious Uveitis. N. Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef]
- Mackensen, F.; Heinz, C.; Jakob, E.; Grewing, V.; Lorenz, H.M.; Heiligenhaus, A.; Max, R.; Becker, M.D. Randomized Controlled Study to Evaluate the Efficacy of Adalimumab in Patients with Different Forms of Refractory Uveitis. Ocul. Immunol. Inflamm. 2018, 26, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Merrill, P.T.; Jaffe, G.J.; Dick, A.D.; Kurup, S.K.; Sheppard, J.; Schlaen, A.; Pavesio, C.; Cimino, L.; Van Calster, J.; et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): A multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016, 388, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Suhler, E.B.; Adán, A.; Brézin, A.P.; Fortin, E.; Goto, H.; Jaffe, G.J.; Kaburaki, T.; Kramer, M.; Lim, L.L.; Muccioli, C.; et al. Safety and Efficacy of Adalimumab in Patients with Noninfectious Uveitis in an Ongoing Open-Label Study: VISUAL III. Ophthalmology 2018, 125, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Gubler, M.; Gantenbein, C.; Spoerri, M.; Froehlich, F.; Seibold, F.; Protic, M.; Michetti, P.; Straumann, A.; Fournier, N.; et al. Anti-TNF Treatment for Extraintestinal Manifestations of Inflammatory Bowel Disease in the Swiss IBD Cohort Study. Inflamm. Bowel Dis. 2017, 23, 1174–1181. [Google Scholar] [CrossRef]
- Löfberg, R.; Louis, E.V.; Reinisch, W.; Robinson, A.M.; Kron, M.; Camez, A.; Pollack, P.F. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn’s disease: Results from CARE. Inflamm. Bowel Dis. 2012, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Van Assche, G.; Gómez-Ulloa, D.; García-Álvarez, L.; Lara, N.; Black, C.M.; Kachroo, S. Systematic Review of Tumor Necrosis Factor Antagonists in Extraintestinal Manifestations in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 25–36.e27. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-de-Acosta, M.; Lorenzo, A.; Domìnguez-Munoz, J.E. Efficacy of adalimumab for the treatment of extraintestinal manifestations of Crohn’s disease. Rev. Esp. De Enferm. Dig. 2012, 104, 468–472. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Z.; Tao, T.; Duan, R.; Wang, X.; Su, W. TNF-α in Uveitis: From Bench to Clinic. Front. Pharmacol. 2021, 12, 740057. [Google Scholar] [CrossRef]
- Siakavellas, S.I.; Bamias, G. Tumor Necrosis Factor-like Cytokine TL1A and Its Receptors DR3 and DcR3, Important New Factors in Mucosal Homeostasis and Inflammation. Inflamm. Bowel Dis. 2015, 21, 2441–2452. [Google Scholar] [CrossRef]
- Pepple, K.L.; Lin, P. Targeting Interleukin-23 in the Treatment of Noninfectious Uveitis. Ophthalmology 2018, 125, 1977–1983. [Google Scholar] [CrossRef]
- Mugheddu, C.; Atzori, L.; Del Piano, M.; Lappi, A.; Pau, M.; Murgia, S.; Zucca, I.; Rongioletti, F. Successful ustekinumab treatment of noninfectious uveitis and concomitant severe psoriatic arthritis and plaque psoriasis. Dermatol. Ther. 2017, 30, e12527. [Google Scholar] [CrossRef]
- Letko, E.; Yeh, S.; Stephen Foster, C.; Pleyer, U.; Brigell, M.; Grosskreutz, C.L. Efficacy and Safety of Intravenous Secukinumab in Noninfectious Uveitis Requiring Steroid-Sparing Immunosuppressive Therapy. Ophthalmology 2015, 122, 939–948. [Google Scholar] [CrossRef]
- Lozano, M.J.F.; Giménez, R.S.; Fernández, M.C. Emergence of inflammatory bowel disease during treatment with secukinumab. J. Crohns Colitis 2018, 12, 1131–1133. [Google Scholar] [CrossRef]
- Țiburcă, L.; Bembea, M.; Zaha, D.C.; Jurca, A.D.; Vesa, C.M.; Rațiu, I.A.; Jurca, C.M. The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases. Curr. Issues Mol. Biol. 2022, 44, 1851–1866. [Google Scholar] [CrossRef]
- Lin, P.; Suhler, E.B.; Rosenbaum, J.T. The future of uveitis treatment. Ophthalmology 2014, 121, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Paley, M.A.; Karacal, H.; Rao, P.K.; Margolis, T.P.; Miner, J.J. Tofacitinib for refractory uveitis and scleritis. Am. J. Ophthalmol. Case Rep. 2019, 13, 53–55. [Google Scholar] [CrossRef]
- Ko, Y.T.; Wu, Y.M.; Wu, H.L.; Lai, S.C.; Dai, Y.X.; Chen, T.J.; Cherng, Y.G.; Tai, Y.H.; Kao, C.Y. Inflammatory bowel disease and the associated risk of dry eye and ocular surface injury: A nationwide matched cohort study. BMC Ophthalmol. 2023, 23, 415. [Google Scholar] [CrossRef]
- Jadon, D.R.; Corp, N.; van der Windt, D.A.; Coates, L.C.; Soriano, E.R.; Kavanaugh, A.; Raine, T.; Rieder, F.; Siebert, S.; Zummer, M.; et al. Management of Concomitant Inflammatory Bowel Disease or Uveitis in Patients with Psoriatic Arthritis: An Updated Review Informing the 2021 GRAPPA Treatment Recommendations. J. Rheumatol. 2023, 50, 438–450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorisi, G.; Vella, G.; Dal Buono, A.; Gabbiadini, R.; Busacca, A.; Loy, L.; Bezzio, C.; Vinciguerra, P.; Armuzzi, A. Ophthalmological Manifestations in Inflammatory Bowel Diseases: Keep an Eye on It. Cells 2024, 13, 142. https://doi.org/10.3390/cells13020142
Migliorisi G, Vella G, Dal Buono A, Gabbiadini R, Busacca A, Loy L, Bezzio C, Vinciguerra P, Armuzzi A. Ophthalmological Manifestations in Inflammatory Bowel Diseases: Keep an Eye on It. Cells. 2024; 13(2):142. https://doi.org/10.3390/cells13020142
Chicago/Turabian StyleMigliorisi, Giulia, Giovanna Vella, Arianna Dal Buono, Roberto Gabbiadini, Anita Busacca, Laura Loy, Cristina Bezzio, Paolo Vinciguerra, and Alessandro Armuzzi. 2024. "Ophthalmological Manifestations in Inflammatory Bowel Diseases: Keep an Eye on It" Cells 13, no. 2: 142. https://doi.org/10.3390/cells13020142
APA StyleMigliorisi, G., Vella, G., Dal Buono, A., Gabbiadini, R., Busacca, A., Loy, L., Bezzio, C., Vinciguerra, P., & Armuzzi, A. (2024). Ophthalmological Manifestations in Inflammatory Bowel Diseases: Keep an Eye on It. Cells, 13(2), 142. https://doi.org/10.3390/cells13020142







