A Critical Role of Culture Medium Selection in Maximizing the Purity and Expansion of Natural Killer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of PBMCs and NK Cells
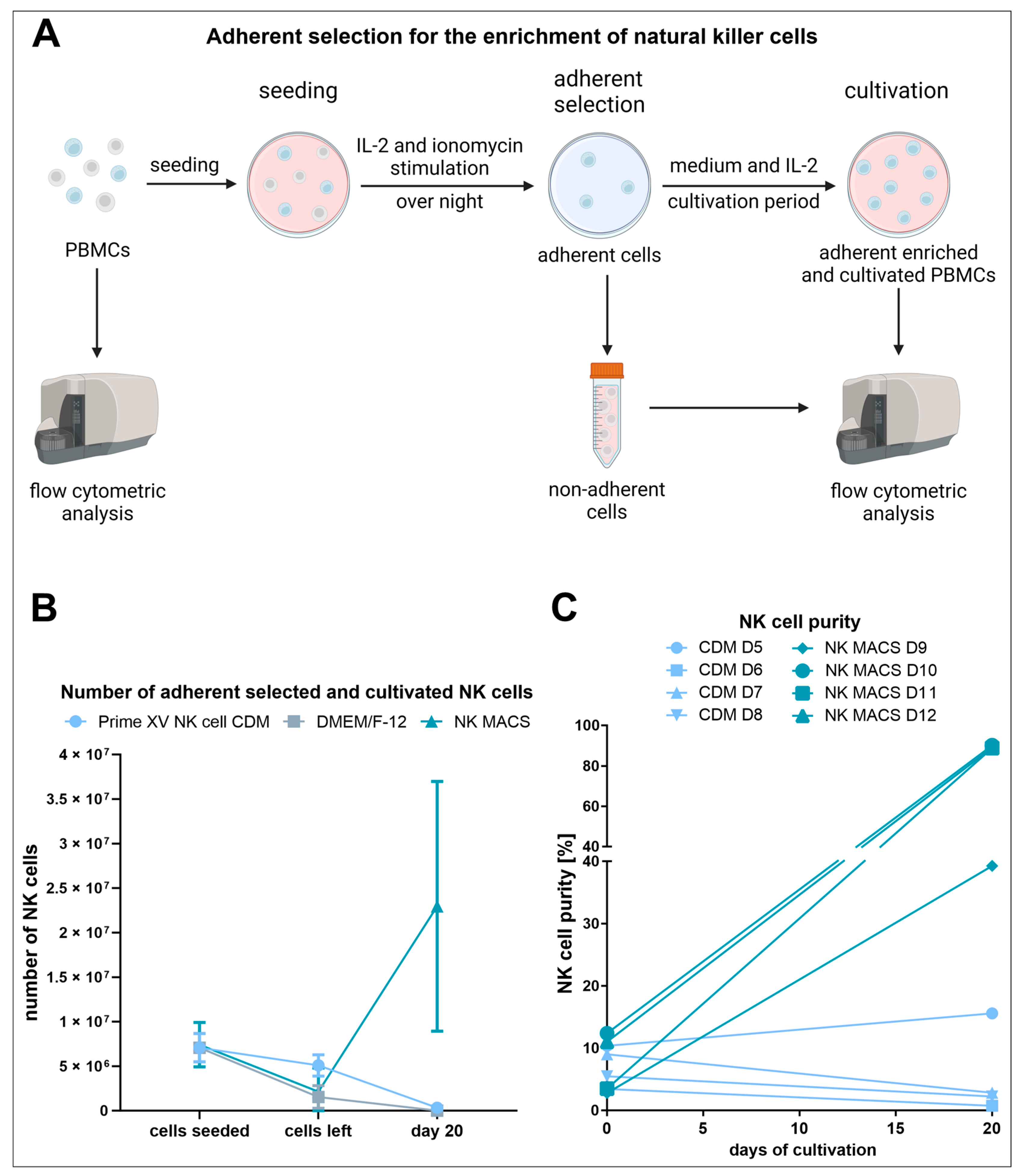
2.2. Enrichment of NK Cells through Adherent Selection
2.2.1. Seeding
2.2.2. Adherence-Based Selection
2.2.3. Cultivation of the Adherently Selected Cells
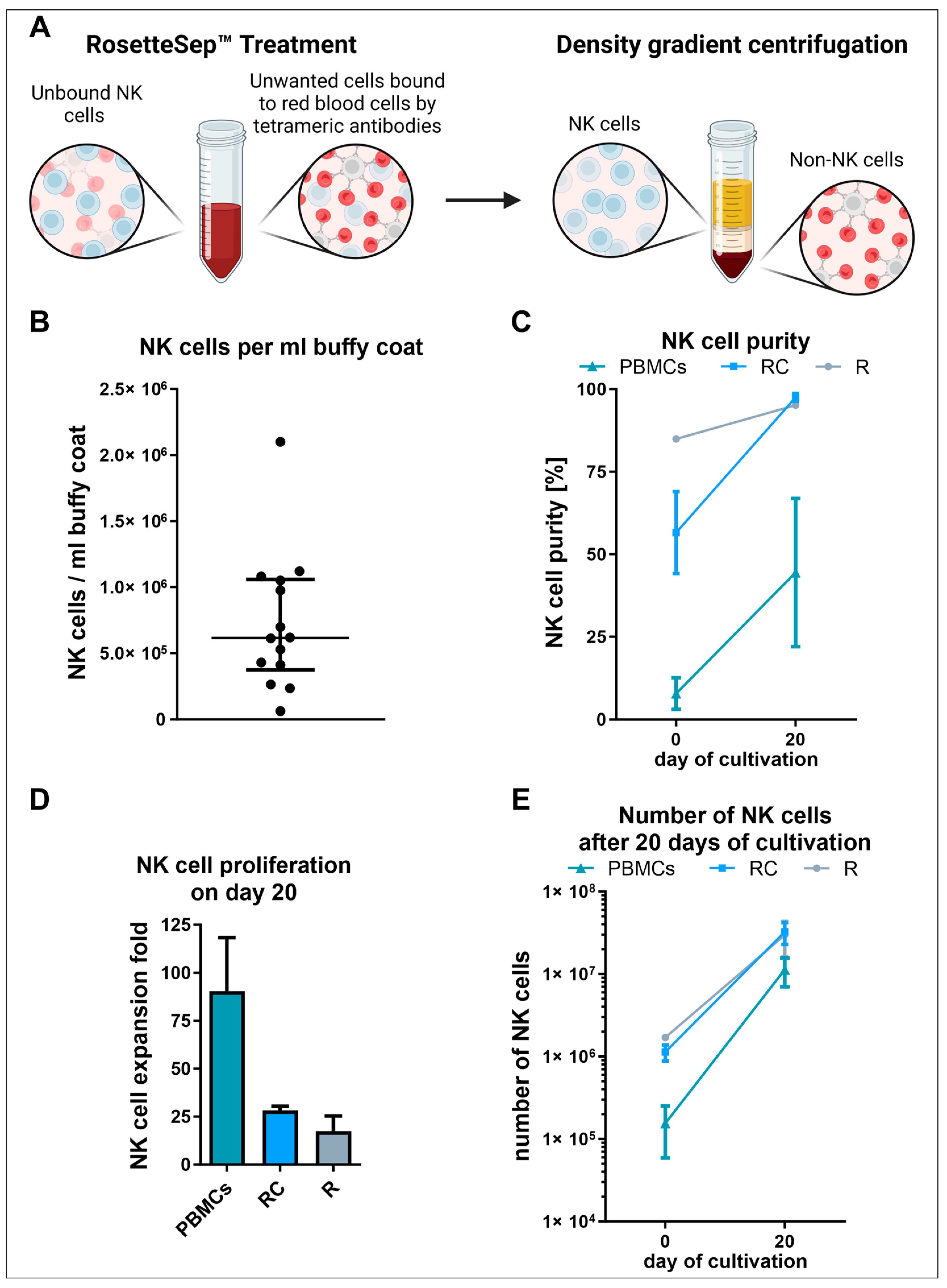
2.3. Isolation and Cultivation of RosetteSep™ Human NK Cell Enrichment Kit Purified NK Cells
2.4. Culture of NK Cells for the Proliferation Assays
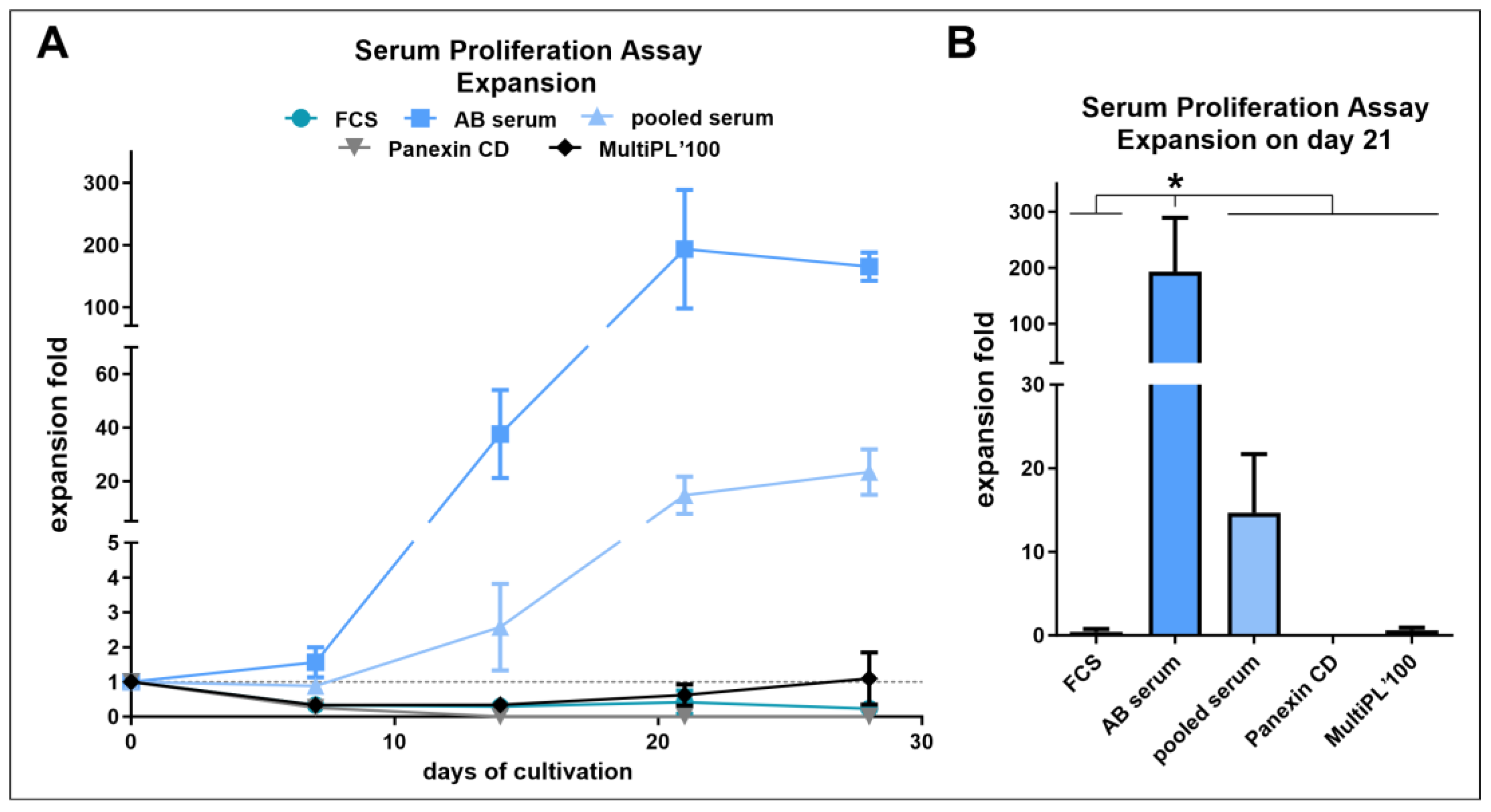
2.5. Serum and Proliferation Assay
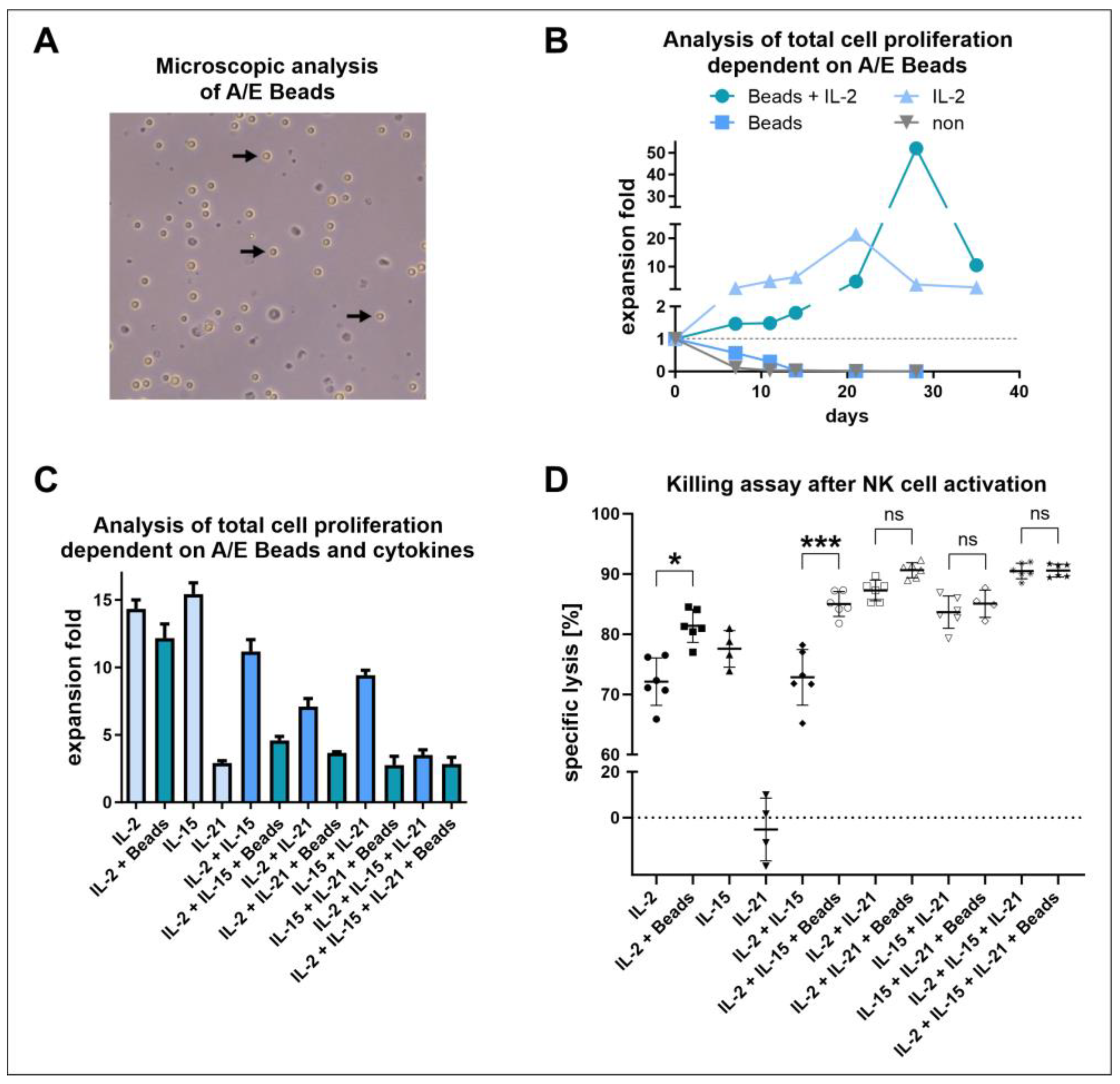
2.6. Long-Term Proliferation Assay with NK Cell Activation/Expansion Beads
2.7. Short-Term Proliferation Assay with NK Cell Activation/Expansion Beads and Cytokines
2.8. Evaluation of the Cytotoxicity of the Stimulated NK Cells
2.9. NK Cell Activating Receptor Analysis
2.10. Quantification of Cells
2.11. Flow Cytometric Analysis
2.11.1. Assessment of NK Cell Purity
(lymphocytes [%] × identified cell population [%]) × 100
2.11.2. Assessment of NK Cell Receptor Expression
2.12. NK Cell Cytotoxicity Capacity against Glioblastoma Cancer Stem Cells
2.13. Analysis and Illustration
3. Results
3.1. Adherent Selection Is Strongly Influenced by the Culture Medium, Is Highly Variable, and Leads to Low NK Cell Expansion
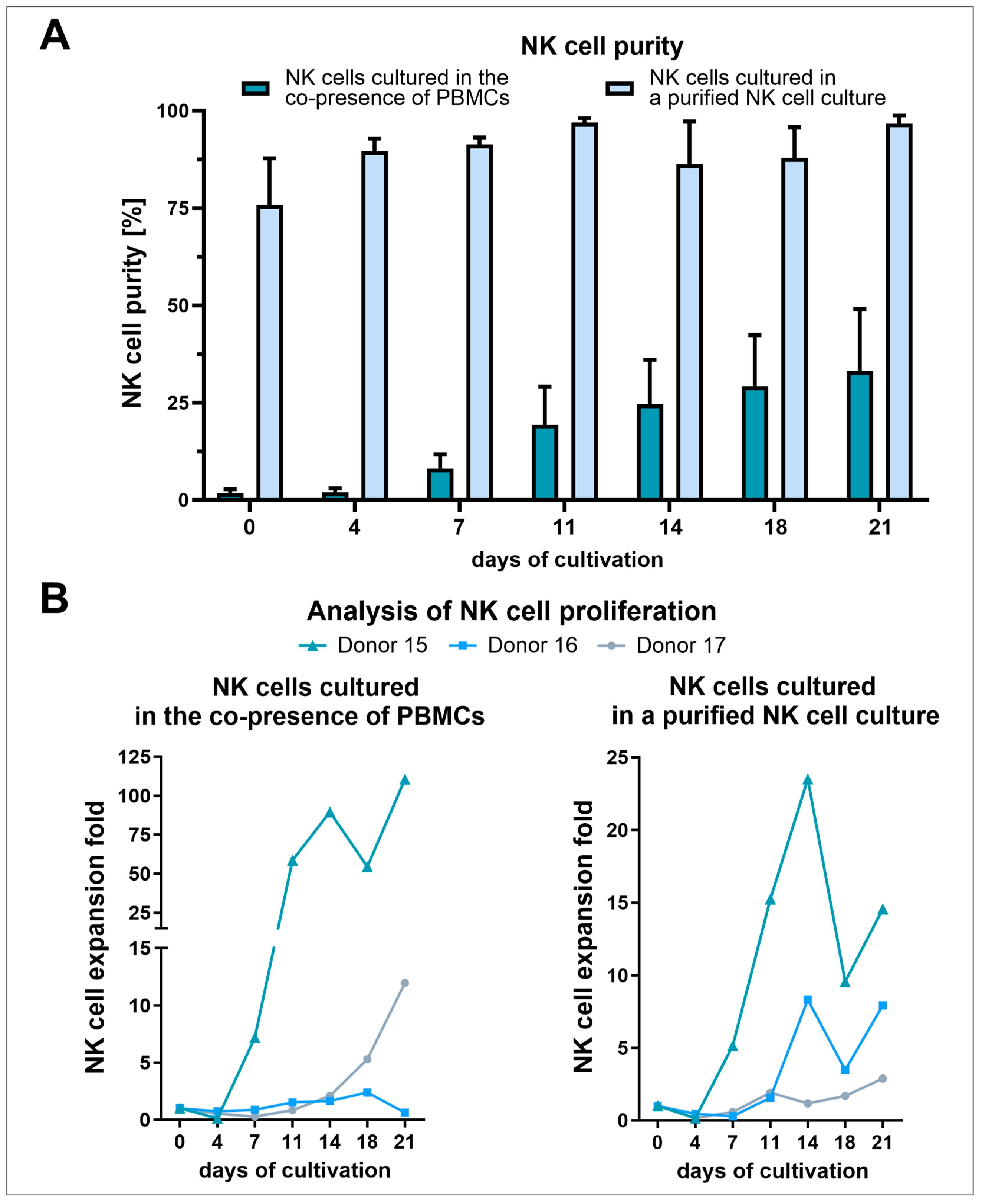
3.2. NK Cell Isolation via the RosetteSep™ Human NK Cell Enrichment Kit Yielded High Purities but Decreased NK Cell Expansion Due to the Absence of Other Stimulating PBMCs
3.3. Human AB Serum Induced the Highest Proliferation in Primary Human NK Cells
3.4. Positive Effects of NK Cell Activation/Expansion Beads Were Only Observed in Longer Cultivation Periods
3.5. IL-2 and IL-15 Alone Induced Higher Proliferation Compared to IL-21 or Combinations, including the Application of NK Cell Activation/Expansion Beads
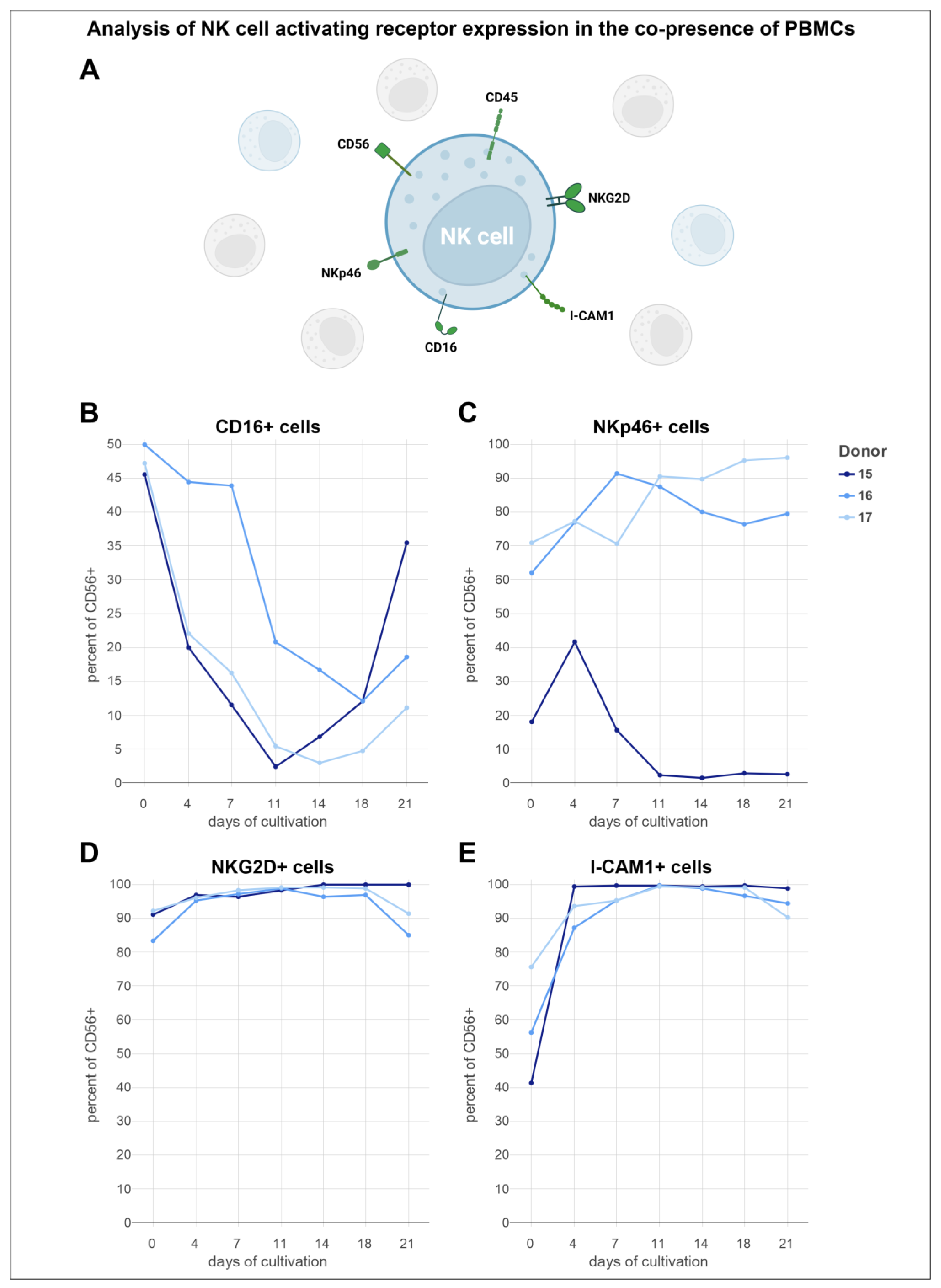
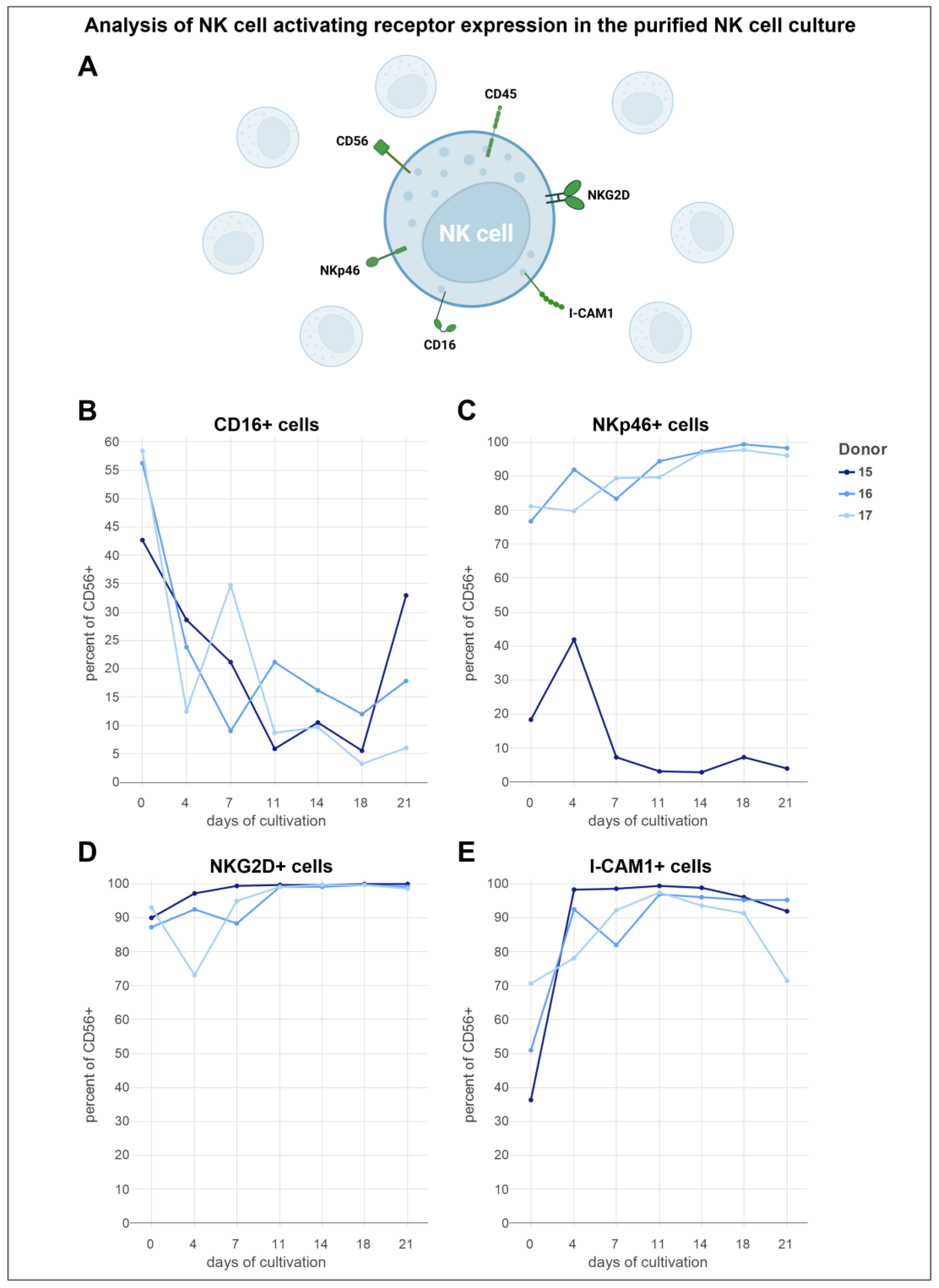
3.6. Analysis of Activating NK Cell Receptors Revealed Elevated Levels of NKp46, NKG2D, and ICAM-1, Coupled with Reduced Expression of CD16
3.7. NK Cells Cultivated in NK MACS® Medium Supplemented with Human AB Serum and Stimulated with IL-2 Potently Target and Lyse Primary Glioblastoma Cancer Stem Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herberman, R.B.; Nunn, M.E.; Holden, H.T.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 1975, 16, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Herberman, R.B.; Nunn, M.E.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer 1975, 16, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, R.; Klein, E.; Pross, H.; Wigzell, H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 1975, 5, 117–121. [Google Scholar] [CrossRef]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. NK cell receptors. Annu. Rev. Immunol. 1998, 16, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Oncol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front. Immunol. 2019, 10, 3038. [Google Scholar] [CrossRef]
- Voigt, J.; Malone, D.F.G.; Dias, J.; Leeansyah, E.; Björkström, N.K.; Ljunggren, H.-G.; Gröbe, L.; Klawonn, F.; Heyner, M.; Sandberg, J.K.; et al. Proteome analysis of human CD56neg NK cells reveals a homogeneous phenotype surprisingly similar to CD56dim NK cells. Eur. J. Immunol. 2018, 48, 1456–1469. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Jacobs, R.; Hintzen, G.; Kemper, A.; Beul, K.; Kempf, S.; Behrens, G.; Sykora, K.-W.; Schmidt, R.E. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 2001, 31, 3121–3126. [Google Scholar] [CrossRef]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Vitale, M. NK cells and cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Kärre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 319, 675–678. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Jelenčić, V.; Polić, B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell. Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Barber, D.F.; Faure, M.; Long, E.O. LFA-1 contributes an early signal for NK cell cytotoxicity. J. Immunol. 2004, 173, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Rubnitz, J.E.; Inaba, H.; Ribeiro, R.C.; Pounds, S.; Rooney, B.; Bell, T.; Pui, C.-H.; Leung, W. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 955–959. [Google Scholar] [CrossRef]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.-F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef]
- Fábián, Á.; Vereb, G.; Szöllősi, J. The hitchhikers guide to cancer stem cell theory: Markers, pathways and therapy. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2013, 83, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Unternaehrer, J.J. Epithelial-mesenchymal Transition and Cancer Stem Cells: At the Crossroads of Differentiation and Dedifferentiation. Dev. Dyn. 2019, 248, 10–20. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, A.; Huergo-Zapico, L.; Galván, J.A.; Rodrigo, L.; de Herreros, A.G.; Astudillo, A.; Gonzalez, S. Epithelial-mesenchymal transition induces an antitumor immune response mediated by NKG2D receptor. J. Immunol. 2013, 190, 4408–4419. [Google Scholar] [CrossRef]
- Tallerico, R.; Todaro, M.; Di Franco, S.; Maccalli, C.; Garofalo, C.; Sottile, R.; Palmieri, C.; Tirinato, L.; Pangigadde, P.N.; La Rocca, R.; et al. Human NK cells selective targeting of colon cancer-initiating cells: A role for natural cytotoxicity receptors and MHC class I molecules. J. Immunol. 2013, 190, 2381–2390. [Google Scholar] [CrossRef]
- Jewett, A.; Tseng, H.-C.; Arasteh, A.; Saadat, S.; Christensen, R.E.; Cacalano, N.A. Natural killer cells preferentially target cancer stem cells; role of monocytes in protection against NK cell mediated lysis of cancer stem cells. Curr. Drug Deliv. 2012, 9, 5–16. [Google Scholar] [CrossRef]
- Fujisaki, H.; Kakuda, H.; Shimasaki, N.; Imai, C.; Ma, J.; Lockey, T.; Eldridge, P.; Leung, W.H.; Campana, D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009, 69, 4010–4017. [Google Scholar] [CrossRef]
- Comans-Bitter, W.; de Groot, R.; van den Beemd, R.; Neijens, H.J.; Hop, W.C.; Groeneveld, K.; Hooijkaas, H.; van Dongen, J.J. Immunophenotyping of blood lymphocytes in childhoodReference values for lymphocyte subpopulations. J. Pediatr. 1997, 130, 388–393. [Google Scholar] [CrossRef]
- Veluchamy, J.P.; Kok, N.; van der Vliet, H.J.; Verheul, H.M.W.; de Gruijl, T.D.; Spanholtz, J. The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments. Front. Immunol. 2017, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Koehl, U.; Brehm, C.; Huenecke, S.; Zimmermann, S.-Y.; Kloess, S.; Bremm, M.; Ullrich, E.; Soerensen, J.; Quaiser, A.; Erben, S.; et al. Clinical grade purification and expansion of NK cell products for an optimized manufacturing protocol. Front. Oncol. 2013, 3, 118. [Google Scholar] [CrossRef]
- Selvan, S.R.; Dowling, J.P. “Adherent” versus Other Isolation Strategies for Expanding Purified, Potent, and Activated Human NK Cells for Cancer Immunotherapy. BioMed Res. Int. 2015, 2015, 869547. [Google Scholar] [CrossRef]
- McCrady, C.W.; Li, F.; Grant, A.J.; Merchant, R.E.; Carchman, R.A. Alteration of human lymphokine-activated killer cell activity by manipulation of protein kinase C and cytosolic Ca2+. Cancer Res. 1988, 48, 635–640. [Google Scholar]
- Robertson, M.J.; Manley, T.J.; Donahue, C.; Levine, H.; Ritz, J. Costimulatory signals are required for optimal proliferation of human natural killer cells. J. Immunol. 1993, 150, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hermann, T.E. Characterization of ionomycin as a calcium ionophore. J. Biol. Chem. 1978, 253, 5892–5894. [Google Scholar] [CrossRef]
- Kaszubowska, L.; Foerster, J.; Schetz, D.; Kmieć, Z. CD56bright cells respond to stimulation until very advanced age revealing increased expression of cellular protective proteins SIRT1, HSP70 and SOD2. Immun. Ageing 2018, 15, 31. [Google Scholar] [CrossRef]
- Ai, W.; Li, H.; Song, N.; Li, L.; Chen, H. Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int. J. Environ. Res. Public Health 2013, 10, 3834–3842. [Google Scholar] [CrossRef]
- Robertson, M.J.; Cameron, C.; Lazo, S.; Cochran, K.J.; Voss, S.D.; Ritz, J. Costimulation of human natural killer cell proliferation: Role of accessory cytokines and cell contact-dependent signals. Nat. Immun. 1996, 15, 213–226. [Google Scholar] [PubMed]
- Jouishomme, H.; Rigal, D. Proliferation and activation of human mononuclear cells induced by ionomycin in a serum-free medium. Thymus 1991, 17, 137–146. [Google Scholar] [PubMed]
- Romera-Cárdenas, G.; Thomas, L.M.; Lopez-Cobo, S.; García-Cuesta, E.M.; Long, E.O.; Reyburn, H.T. Ionomycin Treatment Renders NK Cells Hyporesponsive. PLoS ONE 2016, 11, e0150998. [Google Scholar] [CrossRef] [PubMed]
- Pierson, B.A.; McGlave, P.B.; Hu, W.S.; Miller, J.S. Natural killer cell proliferation is dependent on human serum and markedly increased utilizing an enriched supplemented basal medium. J. Hematother. 1995, 4, 149–158. [Google Scholar] [CrossRef]
- Kim, S. Ex Vivo Expansion of Highly Cytotoxic Natural Killer Cells Using Optimal Culture Medium. Ann. Lab. Med. 2022, 42, 619–620. [Google Scholar] [CrossRef]
- Fang, F.; Xie, S.; Chen, M.; Li, Y.; Yue, J.; Ma, J.; Shu, X.; He, Y.; Xiao, W.; Tian, Z. Advances in NK cell production. Cell. Mol. Immunol. 2022, 19, 460–481. [Google Scholar] [CrossRef] [PubMed]
- FUJIFILM Irvine Scientific. PRIME-XV NK Cell CDM. Available online: https://www.irvinesci.com/prime-xv-nk-cell-cdm.html (accessed on 15 April 2024).
- Moseman, J.E.; Foltz, J.A.; Sorathia, K.; Heipertz, E.L.; Lee, D.A. Evaluation of serum-free media formulations in feeder cell-stimulated expansion of natural killer cells. Cytotherapy 2020, 22, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.K.; Park, J.; Kim, S.-E.; Lim, Y.; Phan, M.-T.T.; Kim, J.; Hwang, I.; Ahn, Y.-O.; Shin, S.; Doh, J.; et al. Natural Killer Cell Expansion and Cytotoxicity Differ Depending on the Culture Medium Used. Ann. Lab. Med. 2022, 42, 638–649. [Google Scholar] [CrossRef]
- Johnson, C.D.L.; Zale, N.E.; Frary, E.D.; Lomakin, J.A. Feeder-Cell-Free and Serum-Free Expansion of Natural Killer Cells Using Cloudz Microspheres, G-Rex6M, and Human Platelet Lysate. Front. Immunol. 2022, 13, 803380. [Google Scholar] [CrossRef]
- de Rham, C.; Ferrari-Lacraz, S.; Jendly, S.; Schneiter, G.; Dayer, J.-M.; Villard, J. The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors. Arthritis Res. Ther. 2007, 9, R125. [Google Scholar] [CrossRef]
- Waters, R.S.; Perry, J.S.A.; Han, S.; Bielekova, B.; Gedeon, T. The effects of interleukin-2 on immune response regulation. Math. Med. Biol. 2018, 35, 79–119. [Google Scholar] [CrossRef]
- Tagaya, Y.; Bamford, R.N.; DeFilippis, A.P.; Waldmann, T.A. IL-15: A pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 1996, 4, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tian, Z.; Wei, H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front. Immunol. 2017, 8, 930. [Google Scholar] [CrossRef]
- Curti, B.D. Immunomodulatory and antitumor effects of interleukin-21 in patients with renal cell carcinoma. Expert Rev. Anticancer Ther. 2006, 6, 905–909. [Google Scholar] [CrossRef]
- Kasaian, M.T.; Whitters, M.J.; Carter, L.L.; Lowe, L.D.; Jussif, J.M.; Deng, B.; Johnson, K.A.; Witek, J.S.; Senices, M.; Konz, R.F.; et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: A mediator of the transition from innate to adaptive immunity. Immunity 2002, 16, 559–569. [Google Scholar] [CrossRef]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.; Gross, J.A.; Johnston, J.; Madden, K.; Xu, W.; West, J.; et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Höving, A.L.; Schmitz, J.; Schmidt, K.E.; Greiner, J.F.W.; Knabbe, C.; Kaltschmidt, B.; Grünberger, A.; Kaltschmidt, C. Human Blood Serum Induces p38-MAPK- and Hsp27-Dependent Migration Dynamics of Adult Human Cardiac Stem Cells: Single-Cell Analysis via a Microfluidic-Based Cultivation Platform. Biology 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Miltenyi Biotec. NK Cells Expansion from Human PBMCs or Isolated NK Cells: 4. Culture and Expansion of NK Cells. Available online: https://www.miltenyibiotec.com/DE-en/applications/all-protocols/nk-cell-expansion-from-human-pbmcs-or-isolated-nk-cells.html?query=:relevance:codeString:130-110-518:codeString:130-110-637:codeString:130-113-643:codeString:130-113-312:codeString:130-042-201:codeString:130-042-303:codeString:130-092-657:codeString:130-041-407:codeString:130-042-401:codeString:130-042-102:codeString:130-091-222:codeString:130-091-376:codeString:130-042-302:codeString:130-096-343:codeString:130-097-744:codeString:150-000-362:codeString:130-111-568:codeString:130-114-429 (accessed on 28 May 2024).
- Helweg, L.P.; Storm, J.; Witte, K.E.; Schulten, W.; Wrachtrup, L.; Janotte, T.; Kitke, A.; Greiner, J.F.W.; Knabbe, C.; Kaltschmidt, B.; et al. Targeting Key Signaling Pathways in Glioblastoma Stem Cells for the Development of Efficient Chemo- and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 12919. [Google Scholar] [CrossRef]
- Kolkmann, A.M.; van Essen, A.; Post, M.J.; Moutsatsou, P. Development of a Chemically Defined Medium for in vitro Expansion of Primary Bovine Satellite Cells. Front. Bioeng. Biotechnol. 2022, 10, 895289. [Google Scholar] [CrossRef] [PubMed]
- Sakwe, A.M.; Koumangoye, R.; Goodwin, S.J.; Ochieng, J. Fetuin-A ({alpha}2HS-glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells. J. Biol. Chem. 2010, 285, 41827–41835. [Google Scholar] [CrossRef] [PubMed]
- Arora, M. Cell Culture Media: A Review. Mater. Methods 2013, 3, 175. [Google Scholar] [CrossRef]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Sadoun, A.; Biarnes-Pelicot, M.; Ghesquiere-Dierickx, L.; Wu, A.; Théodoly, O.; Limozin, L.; Hamon, Y.; Puech, P.-H. Controlling T cells spreading, mechanics and activation by micropatterning. Sci. Rep. 2021, 11, 6783. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Yang, B.; Kim, Y.-S.; Park, G.M.; Kim, H.; Kim, H.; Kim, E.-J.; Hwang, Y.K.; Shin, E.-C.; Cho, S. Harnessing novel engineered feeder cells expressing activating molecules for optimal expansion of NK cells with potent antitumor activity. Cell. Mol. Immunol. 2022, 19, 296–298. [Google Scholar] [CrossRef]
- Lee, M.; Bell, C.J.M.; Rubio Garcia, A.; Godfrey, L.; Pekalski, M.; Wicker, L.S.; Todd, J.A.; Ferreira, R.C. CD56bright natural killer cells preferentially kill proliferating CD4+ T cells. Discov. Immunol. 2023, 2, kyad012. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.-A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef]
- Xiao, X.; Huang, S.; Chen, S.; Wang, Y.; Sun, Q.; Xu, X.; Li, Y. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J. Exp. Clin. Cancer Res. 2021, 40, 367. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Son, C.-H.; Koh, E.-K.; Bae, J.-H.; Kang, C.-D.; Yang, K.; Park, Y.-S. Expansion of cytotoxic natural killer cells using irradiated autologous peripheral blood mononuclear cells and anti-CD16 antibody. Sci. Rep. 2017, 7, 11075. [Google Scholar] [CrossRef]
- Denman, C.J.; Senyukov, V.V.; Somanchi, S.S.; Phatarpekar, P.V.; Kopp, L.M.; Johnson, J.L.; Singh, H.; Hurton, L.; Maiti, S.N.; Huls, M.H.; et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 2012, 7, e30264. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Badeti, S.; Tseng, H.-C.; Ma, M.T.; Liu, T.; Jiang, J.-G.; Liu, C.; Liu, D. Superior Expansion and Cytotoxicity of Human Primary NK and CAR-NK Cells from Various Sources via Enriched Metabolic Pathways. Mol. Ther. Methods Clin. Dev. 2020, 18, 428–445. [Google Scholar] [CrossRef]
- van der Valk, J.; Bieback, K.; Buta, C.; Cochrane, B.; Dirks, W.G.; Fu, J.; Hickman, J.J.; Hohensee, C.; Kolar, R.; Liebsch, M.; et al. Fetal Bovine Serum (FBS): Past—Present—Future. ALTEX-Altern. Anim. Exp. 2018, 35, 99–118. [Google Scholar] [CrossRef]
- Meggyes, M.; Nagy, D.U.; Balassa, T.; Godony, K.; Peterfalvi, A.; Szereday, L.; Polgar, B. Influence of Galectin-9 Treatment on the Phenotype and Function of NK-92MI Cells in the Presence of Different Serum Supplements. Biomolecules 2021, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Mishra, N.; Rath, G.P. Blood groups systems. Indian J. Anaesth. 2014, 58, 524–528. [Google Scholar] [CrossRef]
- Pan-Biotech. Datasheet Panexin CD: Chemically Defined Serum Replacement 2019. Available online: https://shop.pan-biotech.de/media/da/20/47/1690716244/P04-930500_DatasheetPanexinCDP04-93050P04-93100P04-930500.PDF (accessed on 28 May 2024).
- Carlens, S.; Gilljam, M.; Chambers, B.J.; Aschan, J.; Guven, H.; Ljunggren, H.-G.; Christensson, B.; Dilber, M. A new method for in vitro expansion of cytotoxic human CD3−CD56+ natural killer cells. Hum. Immunol. 2001, 62, 1092–1098. [Google Scholar] [CrossRef]
- Luhm, J.; Brand, J.-M.; Koritke, P.; Höppner, M.; Kirchner, H.; Frohn, C. Large-scale generation of natural killer lymphocytes for clinical application. J. Hematother. Stem Cell Res. 2002, 11, 651–657. [Google Scholar] [CrossRef]
- Carson, W.E.; Ross, M.E.; Baiocchi, R.A.; Marien, M.J.; Boiani, N.; Grabstein, K.; Caligiuri, M.A. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 1995, 96, 2578–2582. [Google Scholar] [CrossRef]
- Mao, Y.; van Hoef, V.; Zhang, X.; Wennerberg, E.; Lorent, J.; Witt, K.; Masvidal, L.; Liang, S.; Murray, S.; Larsson, O.; et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood 2016, 128, 1475–1489. [Google Scholar] [CrossRef]
- Skak, K.; Frederiksen, K.S.; Lundsgaard, D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008, 123, 575–583. [Google Scholar] [CrossRef]
- Zhang, C.; Kadu, S.; Xiao, Y.; Johnson, O.; Kelly, A.; O’Connor, R.S.; Lai, M.; Kong, H.; Srivatsa, S.; Tai, V.; et al. Sequential Exposure to IL21 and IL15 During Human Natural Killer Cell Expansion Optimizes Yield and Function. Cancer Immunol. Res. 2023, 11, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.; Cooper, M.A. Metabolic regulation of NK cell function: Implications for immunotherapy. Immunometabolism 2023, 5, e00020. [Google Scholar] [CrossRef]
- Brady, J.; Carotta, S.; Thong, R.P.L.; Chan, C.J.; Hayakawa, Y.; Smyth, M.J.; Nutt, S.L. The interactions of multiple cytokines control NK cell maturation. J. Immunol. 2010, 185, 6679–6688. [Google Scholar] [CrossRef]
- Brady, J.; Hayakawa, Y.; Smyth, M.J.; Nutt, S.L. IL-21 induces the functional maturation of murine NK cells. J. Immunol. 2004, 172, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-P.; Jang, Y.-Y.; Kim, S.; Koh, S.S.; Lee, J.-J.; Kim, J.-S.; Thi Phan, M.-T.; Shin, D.-J.; Shin, M.-G.; Lee, S.-H.; et al. Effect of exposure to interleukin-21 at various time points on human natural killer cell culture. Cytotherapy 2014, 16, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, C.; Liu, C.; Ma, J.; Ma, P.; Cui, H.; Tao, H.; Gao, B. Expansion of NK cells from PBMCs using immobilized 4-1BBL and interleukin-21. Int. J. Oncol. 2015, 47, 335–342. [Google Scholar] [CrossRef]
- Meza Guzman, L.G.; Keating, N.; Nicholson, S.E. Natural Killer Cells: Tumor Surveillance and Signaling. Cancers 2020, 12, 952. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, D.; Churov, A.; Fu, R. Research Progress on NK Cell Receptors and Their Signaling Pathways. Mediat. Inflamm. 2020, 2020, 6437057. [Google Scholar] [CrossRef] [PubMed]
- Demaria, O.; Gauthier, L.; Debroas, G.; Vivier, E. Natural killer cell engagers in cancer immunotherapy: Next generation of immuno-oncology treatments. Eur. J. Immunol. 2021, 51, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.-T.; Chun, S.; Kim, S.-H.; Ali, A.K.; Lee, S.-H.; Kim, S.; Kim, S.-H.; Cho, D. Natural killer cell subsets and receptor expression in peripheral blood mononuclear cells of a healthy Korean population: Reference range, influence of age and sex, and correlation between NK cell receptors and cytotoxicity. Hum. Immunol. 2017, 78, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Ollert, M.; Zimmer, J. Human CD56bright NK Cells: An Update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Guilz, N.C.; Ahn, Y.-O.; Fatima, H.; Pedroza, L.A.; Seo, S.; Soni, R.K.; Wang, N.; Egli, D.; Mace, E.M. Replication stress in activated human NK cells induces sensitivity to apoptosis. J. Immunol. 2023, 213, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Haydinger, C.D.; Ashander, L.M.; Tan, A.C.R.; Smith, J.R. Intercellular Adhesion Molecule 1: More than a Leukocyte Adhesion Molecule. Biology 2023, 12, 743. [Google Scholar] [CrossRef]
- Coënon, L.; Villalba, M. From CD16a Biology to Antibody-Dependent Cell-Mediated Cytotoxicity Improvement. Front. Immunol. 2022, 13, 913215. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.K.; Rothlein, R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 2000, 28, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Siemaszko, J.; Marzec-Przyszlak, A.; Bogunia-Kubik, K. NKG2D Natural Killer Cell Receptor-A Short Description and Potential Clinical Applications. Cells 2021, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Carlomagno, S.; Frumento, G.; Balsamo, M.; Cantoni, C.; Conte, R.; Moretta, L.; Moretta, A.; Vitale, M. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 2006, 108, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Sen Santara, S.; Lee, D.-J.; Crespo, Â.; Hu, J.J.; Walker, C.; Ma, X.; Zhang, Y.; Chowdhury, S.; Meza-Sosa, K.F.; Lewandrowski, M.; et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature 2023, 616, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Kedia-Mehta, N.; Tobin, L.; Zaiatz-Bittencourt, V.; Pisarska, M.M.; de Barra, C.; Choi, C.; Elamin, E.; O’Shea, D.; Gardiner, C.M.; Finlay, D.K.; et al. Cytokine-induced natural killer cell training is dependent on cellular metabolism and is defective in obesity. Blood Adv. 2021, 5, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
- Bähr, I.; Spielmann, J.; Quandt, D.; Kielstein, H. Obesity-Associated Alterations of Natural Killer Cells and Immunosurveillance of Cancer. Front. Immunol. 2020, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.; Hogan, A.E. Dysregulation of Natural Killer Cells in Obesity. Cancers 2019, 11, 573. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Ikei, H.; Song, C. Forest medicine research in Japan. Nihon Eiseigaku Zasshi 2014, 69, 122–135. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Li, Y.J.; Wakayama, Y.; et al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents 2008, 22, 45–55. [Google Scholar] [PubMed]
- Tsao, T.-M.; Tsai, M.-J.; Hwang, J.-S.; Cheng, W.-F.; Wu, C.-F.; Chou, C.-C.K.; Su, T.-C. Health effects of a forest environment on natural killer cells in humans: An observational pilot study. Oncotarget 2018, 9, 16501–16511. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nakadai, A.; Matsushima, H.; Miyazaki, Y.; Krensky, A.M.; Kawada, T.; Morimoto, K. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunopharmacol. Immunotoxicol. 2006, 28, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Grudzien, M.; Rapak, A. Effect of Natural Compounds on NK Cell Activation. J. Immunol. Res. 2018, 2018, 4868417. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-M.; Zhang, R.-J.; Zhu, H.; Peng, H.; Zhou, X.-P.; Hong, K.-X.; Liu, J.-L.; Chen, J.-P.; Shao, Y.-M. Comparison of the quantities and subset distributions of natural killer cells among different races. Chin. Med. J. 2010, 123, 3272–3276. [Google Scholar] [PubMed]
- Barnes, S.; Schilizzi, O.; Audsley, K.M.; Newnes, H.V.; Foley, B. Deciphering the Immunological Phenomenon of Adaptive Natural Killer (NK) Cells and Cytomegalovirus (CMV). Int. J. Mol. Sci. 2020, 21, 8864. [Google Scholar] [CrossRef]
- van der Ploeg, K.; Sottile, R.; Kontopoulos, T.; Shaffer, B.C.; Papanicolaou, G.A.; Maloy, M.A.; Cho, C.; Robinson, K.S.; Perales, M.-A.; Le Luduec, J.-B.; et al. Emergence of human CMV-induced NKG2C+ NK cells is associated with CD8+ T-cell recovery after allogeneic HCT. Blood Adv. 2023, 7, 5784–5798. [Google Scholar] [CrossRef]

| Antibody/Dye | Function/Marker |
|---|---|
| CD3-FITC | T and NKT cell marker |
| CD56-APC-A700 | NK cell marker |
| DAPI | Viability |
| Antibody/Dye | Function/Marker |
|---|---|
| CD45-VioGreen | Hematopoietic cell marker |
| CD3-PE | T and NKT cell marker |
| CD56-APC-Vio770 | NK cell marker |
| CD16-PE-Vio615 | NK cell marker |
| NKp46-Vio-Bright B515 | NK cell marker |
| NKG2D-PE-Vio770 | NK cell marker |
| ICAM-I-APC | NK cell marker |
| DAPI | Viability |
| Medium | NK Cells [%] | T + NKT Cells [%] | Number of NK Cells [×106] |
|---|---|---|---|
| Isolated PBMCs | |||
| PRIME-XV NK Cell CDM (n = 4) | 7.2% | 47.8% | 7.2 |
| (3.4–10.4%) | (46.8–71.8%) | (3.4–10.4) | |
| DMEM/F-12 (n = 4) | 7.2% | 47.8% | 7.2 |
| (3.4–10.4%) | (46.8–71.8%) | (3.4–10.4) | |
| NK MACS® (n = 4) | 7.3% | 62.2% | 7.3 |
| (2.8–12.4%) | (48.7–80.9%) | (2.8–12.4) | |
| Adherently selected and expanded cells on day 20 of cultivation | |||
| PRIME-XV NK Cell CDM (n = 4) | 7.2% | 47.8% | 7.2 |
| (3.4–10.4%) | (46.8–71.8%) | (3.4–10.4) | |
| DMEM/F-12 (n = 2) | 1.1% | 33.5% | <0.1 |
| (0–2.3%) | (1.1–65.8%) | (0–<0.1) | |
| NK MACS® (n = 4) | 89% (39.3–90.2%) | 7.2% (6.5–55.7%) | 16.2 (0.4–59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusch, N.; Storm, J.; Macioszek, A.; Kisselmann, E.; Knabbe, C.; Kaltschmidt, B.; Kaltschmidt, C. A Critical Role of Culture Medium Selection in Maximizing the Purity and Expansion of Natural Killer Cells. Cells 2024, 13, 1148. https://doi.org/10.3390/cells13131148
Kusch N, Storm J, Macioszek A, Kisselmann E, Knabbe C, Kaltschmidt B, Kaltschmidt C. A Critical Role of Culture Medium Selection in Maximizing the Purity and Expansion of Natural Killer Cells. Cells. 2024; 13(13):1148. https://doi.org/10.3390/cells13131148
Chicago/Turabian StyleKusch, Neele, Jonathan Storm, Antonia Macioszek, Ella Kisselmann, Cornelius Knabbe, Barbara Kaltschmidt, and Christian Kaltschmidt. 2024. "A Critical Role of Culture Medium Selection in Maximizing the Purity and Expansion of Natural Killer Cells" Cells 13, no. 13: 1148. https://doi.org/10.3390/cells13131148
APA StyleKusch, N., Storm, J., Macioszek, A., Kisselmann, E., Knabbe, C., Kaltschmidt, B., & Kaltschmidt, C. (2024). A Critical Role of Culture Medium Selection in Maximizing the Purity and Expansion of Natural Killer Cells. Cells, 13(13), 1148. https://doi.org/10.3390/cells13131148






