Global Transcriptomic and Characteristics Comparisons between Mouse Fetal Liver and Bone Marrow Definitive Erythropoiesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Mice
2.2. Fluorescence-Activated Cell Sorting
2.3. Cytospin Preparation and MGG (May–Grunwald–Giemsa) Staining
2.4. Single Cell Culture of Erythroid Progenitors In Vitro
2.5. Cell Division Tracing Assay
2.6. RNA-Seq and Real-Time PCR Analyses
2.7. In Vivo 5-Ethynyl-2′-Deoxyuridine Incorporation Assay
2.8. Statistics
3. Results
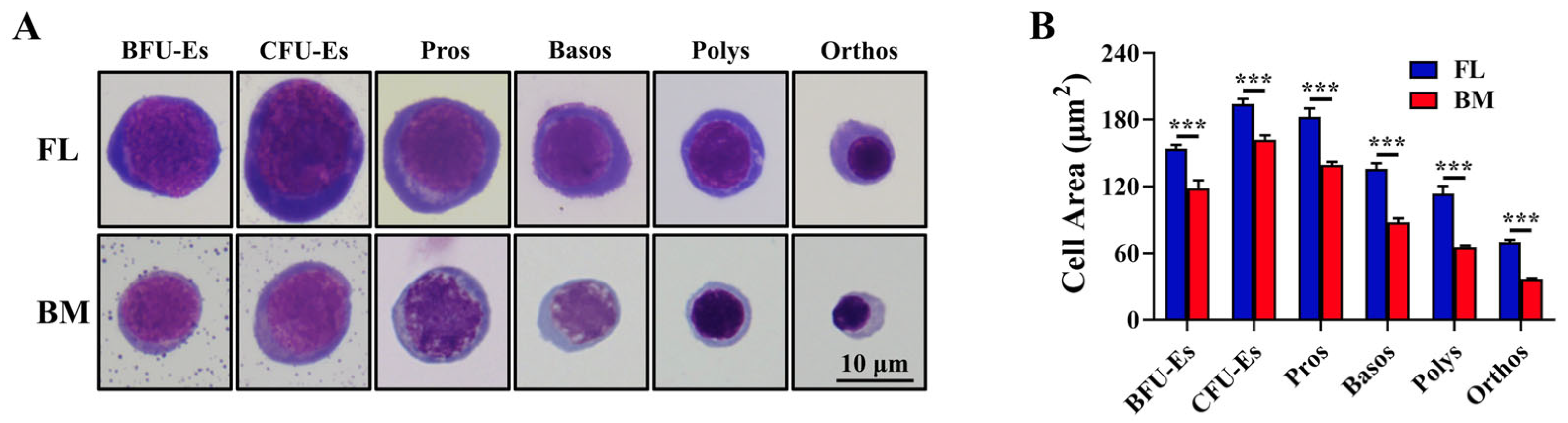
3.1. FL Definitive Erythroid Progenitors and Precursors at All Maturational Stages Were Larger Than Their BM Counterparts
3.2. Higher Proliferation Capability of FL BFU-E Cells
3.3. GO Terms Related to Cell Cycle Were Upregulated in FL BFU-Es Than in BM BFU-Es
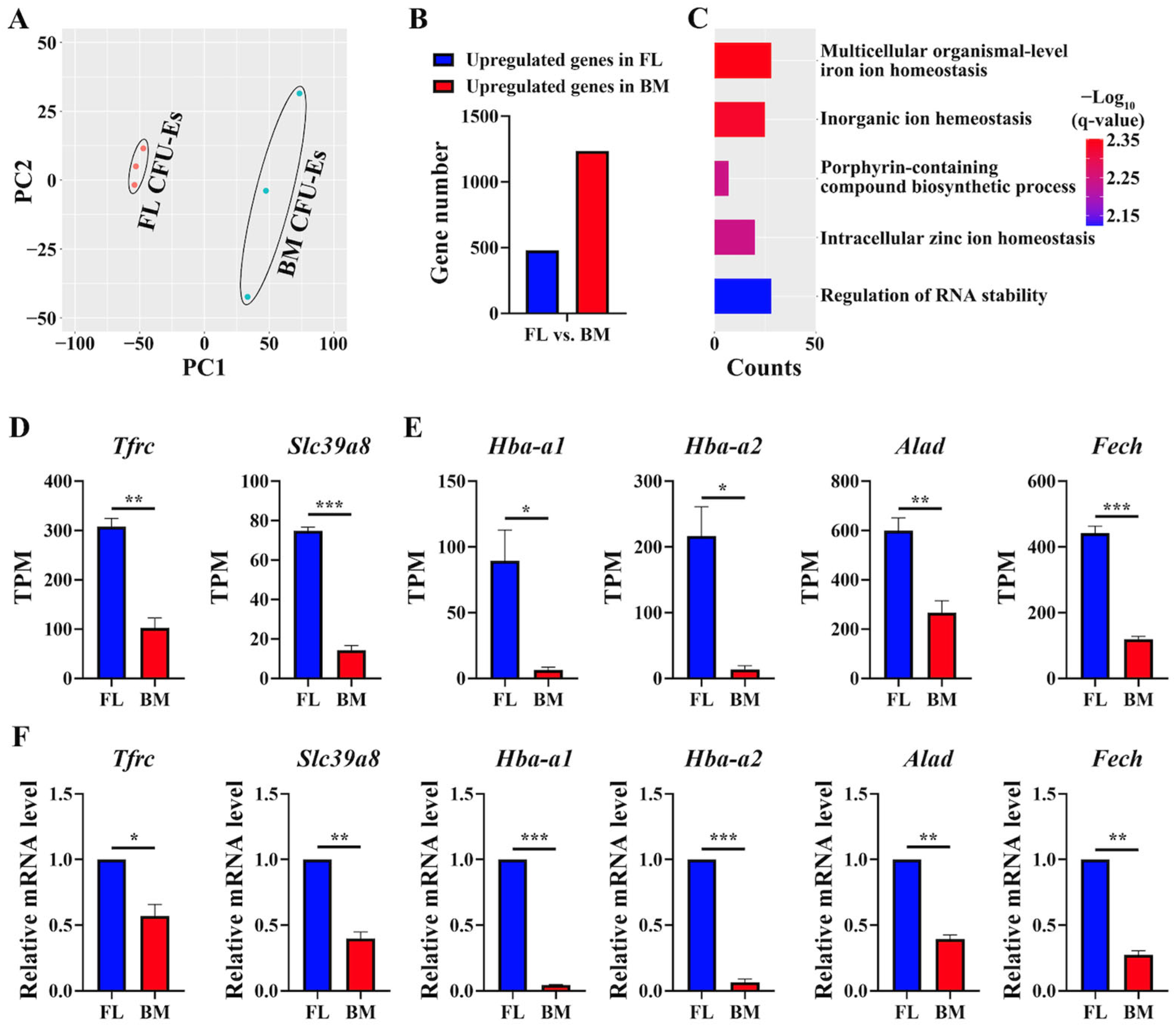
3.4. GO Terms Involved in Heme/Hemoglobin Biosynthesis Were Upregulated in FL CFU-Es Compared to BM CFU-Es
3.5. GO Terms Involved in Immune Response/Immunoregulation Were Upregulated in BM Progenitors Than in FL Progenitors
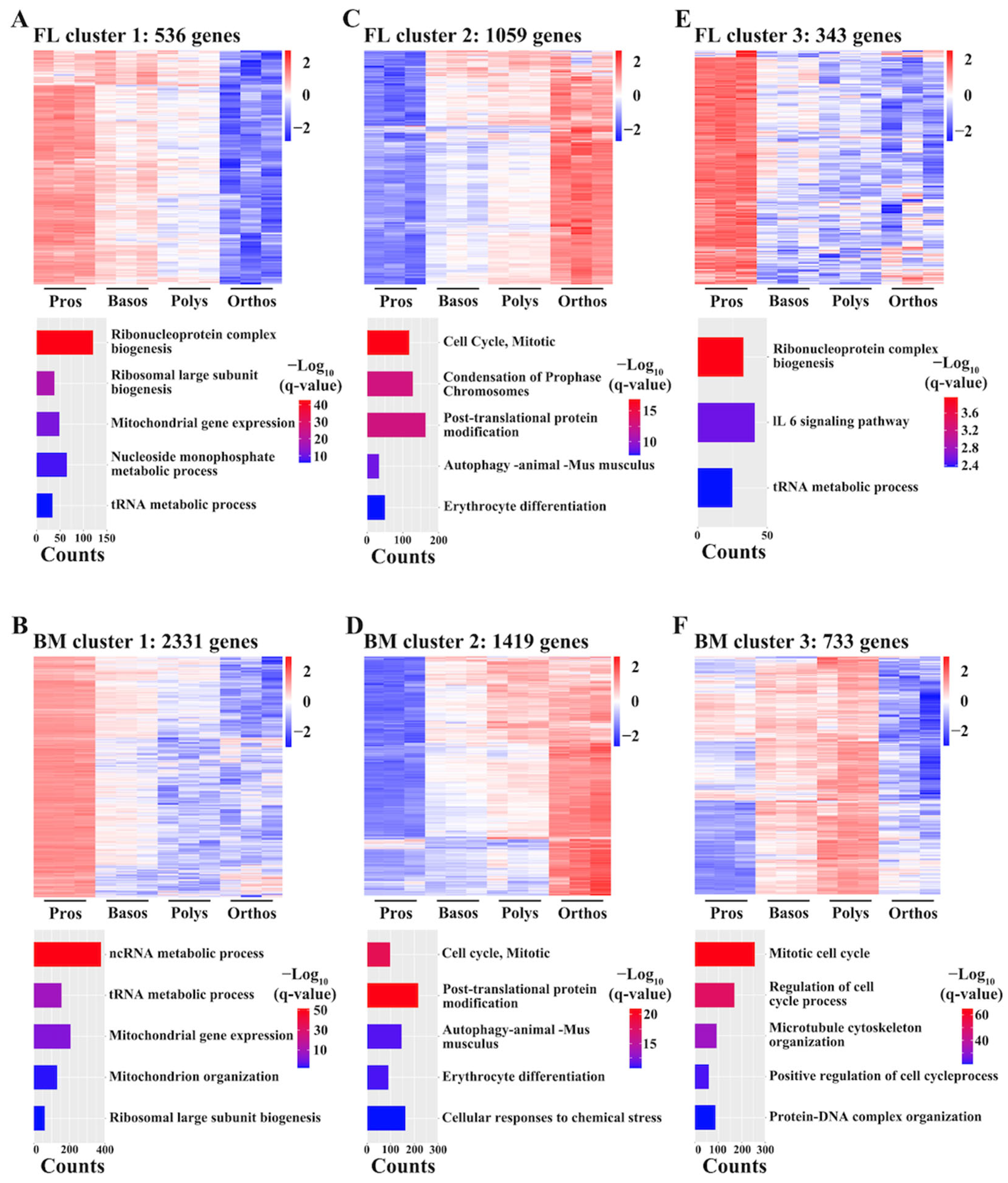
3.6. Overall Conserved Temporal Patterns in Gene Expression during FL and BM Terminal Erythropoiesis
3.7. Cluster Analyses Suggest Decrease in Protein Synthesis and Increase in Autophagy during Both FL and BM Terminal Erythropoiesis
3.8. Upregulation of GO Terms Involved in Translation and TCA Cycle in FL Erythroblasts and Upregulation of GO Terms Involved in Response to Virus in BM Erythroblasts
3.9. Specifically Expressed Genes in FL or BM Erythroblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Palis, J.; Robertson, S.; Kennedy, M.; Wall, C.; Keller, G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 1999, 126, 5073–5084. [Google Scholar] [CrossRef] [PubMed]
- Palis, J.; Yoder, M.C. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp. Hematol. 2001, 29, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.H.; Vacaru, A.; Nieves, J. Erythroid development in the mammalian embryo. Blood Cells Mol. Dis. 2013, 51, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Dzierzak, E.; Philipsen, S. Erythropoiesis: Development and differentiation. Cold Spring Harb. Perspect. Med. 2013, 3, a011601. [Google Scholar] [CrossRef] [PubMed]
- Palis, J. Ontogeny of erythropoiesis. Curr. Opin. Hematol. 2008, 15, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, P.D.; Greenfest-Allen, E.; Frame, J.M.; Bushnell, T.P.; Malik, J.; McGrath, K.E.; Stoeckert, C.J.; Palis, J. Ontogeny of erythroid gene expression. Blood 2013, 121, E5–E13. [Google Scholar] [CrossRef] [PubMed]
- Palis, J. Primitive and definitive erythropoiesis in mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Liu, D.; Gao, C.; Han, Y.; Guo, X.; Qu, X.; Li, W.; Zhang, S.; Geng, J.; et al. EpoR-tdTomato-Cre mice enable identification of EpoR expression in subsets of tissue macrophages and hematopoietic cells. Blood 2021, 138, 1986–1997. [Google Scholar] [CrossRef]
- Flygare, J.; Rayon Estrada, V.; Shin, C.; Gupta, S.; Lodish, H.F. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood 2011, 117, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Ginzburg, Y.; Li, H.; Xue, F.; De Franceschi, L.; Chasis, J.A.; Mohandas, N.; An, X. Quantitative analysis of murine terminal erythroid differentiation in vivo: Novel method to study normal and disordered erythropoiesis. Blood 2013, 121, e43–e49. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, E.G.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, C.; Liu, Y.; Zhang, H.; Wang, S.; Zhao, H.; Bao, W.; Guo, X.; Vinchi, F.; Lobo, C.; et al. Hemolysis-driven IFNα production impairs erythropoiesis by negatively regulating EPO signaling in sickle cell disease. Blood 2024, 143, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, J.; Heck, S.; Chasis, J.A.; An, X.; Mohandas, N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA 2009, 106, 17413–17418. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Gao, X.; Barrasa, M.I.; Li, H.; Elmes, R.R.; Peters, L.L.; Lodish, H.F. PPAR-alpha and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature 2015, 522, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Stellacci, E.; Di Noia, A.; Di Baldassarre, A.; Migliaccio, G.; Battistini, A.; Migliaccio, A.R. Interaction between the glucocorticoid and erythropoietin receptors in human erythroid cells. Exp. Hematol. 2009, 37, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Pastori, V.; Pozzi, S.; Labedz, A.; Ahmed, S.; Ronchi, A.E. Role of Nuclear Receptors in Controlling Erythropoiesis. Int. J. Mol. Sci. 2022, 23, 2800. [Google Scholar] [CrossRef]
- Ma, T.L.; Copland, J.A.; Brasier, A.R.; Thompson, E.A. A novel glucocorticoid receptor binding element within the murine c-myc promoter. Mol. Endocrinol. 2000, 14, 1377–1386. [Google Scholar] [CrossRef]
- Bretones, G.; Delgado, M.D.; León, J. Myc and cell cycle control. Bba-Gene Regul. Mech. 2015, 1849, 506–516. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yang, B.; Zhang, M.; Guo, W.W.; Wu, Z.Y.; Wang, Y.; Jia, L.; Li, S.; Xie, W.; Yang, D.; et al. lncRNA Epigenetic Landscape Analysis Identifies as an Oncogenic lncRNA that Interacts with MYC and Promotes Cell-Cycle Progression in Cancer. Cancer Cell 2018, 33, 706–720.e9. [Google Scholar] [CrossRef] [PubMed]
- La, T.; Chen, S.; Guo, T.; Zhao, X.H.; Teng, L.; Li, D.D.; Carnell, M.; Zhang, Y.Y.; Feng, Y.C.; Cole, N.; et al. Visualization of endogenous p27 and Ki67 reveals the importance of a c-Myc-driven metabolic switch in promoting survival of quiescent cancer cells. Theranostics 2021, 11, 9605–9622. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Ryu, M.S. Cellular Zinc Deficiency Impairs Heme Biosynthesis in Developing Erythroid Progenitors. Nutrients 2023, 15, 281. [Google Scholar] [CrossRef]
- Gálvez-Peralta, M.; He, L.; Jorge-Nebert, L.F.; Wang, B.; Miller, M.L.; Eppert, B.L.; Afton, S.; Nebert, D.W. ZIP8 Zinc Transporter: Indispensable Role for Both Multiple-Organ Organogenesis and Hematopoiesis In Utero. PLoS ONE 2012, 7, e36055. [Google Scholar] [CrossRef]
- Xu, C.; He, J.; Wang, H.; Zhang, Y.; Wu, J.; Zhao, L.; Li, Y.; Gao, J.; Geng, G.; Wang, B.; et al. Single-cell transcriptomic analysis identifies an immune-prone population in erythroid precursors during human ontogenesis. Nat. Immunol. 2022, 23, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, H.; Gad, H.H.; Eskildsen-Larsen, S.; Despres, P.; Hartmann, R. The Oligoadenylate Synthetase Family: An Ancient Protein Family with Multiple Antiviral Activities. J. Interf. Cytok. Res. 2011, 31, 41–47. [Google Scholar] [CrossRef]
- Nagasawa, T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J. Mol. Med. 2014, 92, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; Liu, J.; Wang, H.; Hillyer, C.D.; Blanc, L.; An, X.; Mohandas, N. Dynamic changes in murine erythropoiesis from birth to adulthood: Implications for the study of murine models of anemia. Blood Adv. 2021, 5, 16–25. [Google Scholar] [CrossRef]
- Burns, E.R.; Reed, L.J.; Wenz, B. Volumetric Erythrocyte Macrocytosis Induced by Hydroxyurea. Am. J. Clin. Pathol. 1986, 85, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, V.G.; Ludwig, L.S.; Sicinska, E.; Xu, J.; Bauer, D.E.; Eng, J.C.; Patterson, H.C.; Metcalf, R.A.; Natkunam, Y.; Orkin, S.H.; et al. Cyclin D3 coordinates the cell cycle during differentiation to regulate erythrocyte size and number. Gene Dev. 2012, 26, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Jayapal, S.R.; Wang, C.Q.; Bisteau, X.; Caldez, M.J.; Lim, S.; Tergaonkar, V.; Osato, M.; Kaldis, P. Hematopoiesis specific loss of Cdk2 and Cdk4 results in increased erythrocyte size and delayed platelet recovery following stress. Haematologica 2015, 100, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.O.; Rogers, C.; Ganiatsas, S.; Landsberg, R.L.; Trimarchi, J.M.; Dandapani, S.; Brugnara, C.; Erdman, S.; Schrenzel, M.; Bronson, R.T.; et al. E2F4 is essential for normal erythrocyte maturation and neonatal viability. Mol. Cell 2000, 6, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.; Bejder, J.; Pop, R.; Gellatly, K.; Hwang, Y.; Scalf, S.M.; Eastman, A.; Chen, J.J.; Zhu, L.J.; Heuberger, J.; et al. Epor Stimulates Rapid Cycling and Larger Red Cells during Mouse and Human Erythropoiesis. Nat. Commun. 2021, 12, 7334. [Google Scholar] [CrossRef] [PubMed]
- Teramo, K.A.; Klemetti, M.M.; Widness, J.A. Robust increases in erythropoietin production by the hypoxic fetus is a response to protect the brain and other vital organs. Pediatr. Res. 2018, 84, 807–812. [Google Scholar] [CrossRef]
- Teramo, K.A.; Widness, J.A.; Clemons, G.K.; Voutilainen, P.; Mckinlay, S.; Schwartz, R. Amniotic-Fluid Erythropoietin Correlates with Umbilical Plasma Erythropoietin in Normal and Abnormal Pregnancy. Obstet. Gynecol. 1987, 69, 710–716. [Google Scholar] [PubMed]
- Lloyd, A.C. The Regulation of Cell Size. Cell 2013, 154, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Mohanasundaram, P.; Coelho-Rato, L.S.; Modi, M.K.; Urbanska, M.; Lautenschläger, F.; Cheng, F.; Eriksson, J.E. Cytoskeletal vimentin regulates cell size and autophagy through mTORC1 signaling. PLoS Biol. 2022, 20, e3001737. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.Q.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, X.; Jaenisch, R.; Lodish, H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Socolovsky, M.; Gross, A.W.; Lodish, H.F. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: Functional analysis by a flow cytometry-based novel culture system. Blood 2003, 102, 3938–3946. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Chang, E.; Kashefiaazam, M.; Rogaev, E.I.; Piatyszek, M.A.; Shay, J.W.; Harley, C.B. Telomere Shortening Is Associated with Cell-Division in-Vitro and in-Vivo. Exp. Cell Res. 1995, 220, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Kumar, R.; Albanell, J.; Pettengell, R.; Han, W.; Moore, M.A.S. Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells. Blood 1997, 90, 182–193. [Google Scholar] [CrossRef]
- Boyle, C.; Lansdorp, P.M.; Edelstein-Keshet, L. Predicting the number of lifetime divisions for hematopoietic stem cells from telomere length measurements. iScience 2023, 26, 107053. [Google Scholar] [CrossRef]
- Labbé, R.F.; Vreman, H.J.; Stevenson, D.K. Zinc protoporphyrin: A metabolite with a mission. Clin. Chem. 1999, 45, 2060–2072. [Google Scholar] [CrossRef]
- Jeng, S.S.; Chen, Y.H. Association of Zinc with Anemia. Nutrients 2022, 14, 4918. [Google Scholar] [CrossRef] [PubMed]
- Kelkitli, E.; Ozturk, N.; Aslan, N.A.; Kilic-Baygutalp, N.; Bayraktutan, Z.; Kurt, N.; Bakan, N.; Bakan, E. Serum zinc levels in patients with iron deficiency anemia and its association with symptoms of iron deficiency anemia. Ann. Hematol. 2016, 95, 751–756. [Google Scholar] [CrossRef]
- Dobkin, J.; Mangalmurti, N.S. Immunomodulatory roles of red blood cells. Curr. Opin. Hematol. 2022, 29, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S.; Ertelt, J.M.; Kinder, J.M.; Jiang, T.T.; Zhang, X.Z.; Xin, L.J.; Chaturvedi, V.; Strong, B.S.; Qualls, J.E.; Steinbrecher, K.A.; et al. Immunosuppressive CD71(+) erythroid cells compromise neonatal host defence against infection. Nature 2013, 504, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S. New insight into an old concept: Role of immature erythroid cells in immune pathogenesis of neonatal infection. Front. Immunol. 2014, 5, 376. [Google Scholar] [CrossRef]
- An, X.; Schulz, V.P.; Li, J.; Wu, K.; Liu, J.; Xue, F.; Hu, J.; Mohandas, N.; Gallagher, P.G. Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood 2014, 123, 3466–3477. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, S.; Wang, Y.; Huang, Y.; Gao, C.; Guo, X.; Chen, L.; Zhao, H.; An, X. Comprehensive Characterization and Global Transcriptome Analyses of Human Fetal Liver Terminal Erythropoiesis. Genom. Proteom. Bioinform. 2023, 21, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hale, J.; Jaffray, J.; Li, J.; Wang, Y.; Huang, Y.; An, X.; Hillyer, C.; Wang, N.; Kinet, S.; et al. Developmental differences between neonatal and adult human erythropoiesis. Am. J. Hematol. 2018, 93, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Montel-Hagen, A.; Blanc, L.; Boyer-Clavel, M.; Jacquet, C.; Vidal, M.; Sitbon, M.; Taylor, N. The Glut1 and Glut4 glucose transporters are differentially expressed during perinatal and postnatal erythropoiesis. Blood 2008, 112, 4729–4738. [Google Scholar] [CrossRef] [PubMed]
- Montel-Hagen, A.; Sitbon, M.; Taylor, N. Erythroid glucose transporters. Curr. Opin. Hematol. 2009, 16, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.L.; Conn, G.L. RNA regulation of the antiviral protein 2′-5′-oligoadenylate synthetase. Wires RNA 2019, 10, e1534. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, S.; Hartmann, R.; Kjeldgaard, N.O.; Justesen, J. Gene structure of the murine 2′-5′-oligoadenylate synthetase family. Cell. Mol. Life Sci. 2002, 59, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.J.; He, Z.M.; Li, Y.Y.; Yang, R.R.; Yan, M.; Shang, X.; Cao, J.M. Role of OAS gene family in COVID-19 induced heart failure. J. Transl. Med. 2023, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Wickramage, I.; VanWye, J.; Max, K.; Lockhart, J.H.; Hortu, I.; Mong, E.F.; Canfield, J.; Lamabadu Warnakulasuriya Patabendige, H.M.; Guzeloglu-Kayisli, O.; Inoue, K.; et al. SINE RNA of the imprinted miRNA clusters mediates constitutive type III interferon expression and antiviral protection in hemochorial placentas. Cell Host Microbe 2023, 31, 1185–1199.e1110. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Goff, M.A.; Froggatt, H.M.; Lim, J.K.; Heaton, N.S. GPER1 is required to protect fetal health from maternal inflammation. Science 2021, 371, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Foudi, A.; Jarrier, P.; Zhang, Y.Y.; Wittner, M.; Geay, J.F.; Lecluse, Y.; Nagasawa, T.; Vainchenker, W.; Louache, F. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironmemt in CXCR4−/− chimeric mice. Blood 2006, 107, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Zhang, H.; Wang, Y.; Wang, S.; Guo, X.; Han, Y.; Zhao, H.; An, X. Global Transcriptomic and Characteristics Comparisons between Mouse Fetal Liver and Bone Marrow Definitive Erythropoiesis. Cells 2024, 13, 1149. https://doi.org/10.3390/cells13131149
Gao C, Zhang H, Wang Y, Wang S, Guo X, Han Y, Zhao H, An X. Global Transcriptomic and Characteristics Comparisons between Mouse Fetal Liver and Bone Marrow Definitive Erythropoiesis. Cells. 2024; 13(13):1149. https://doi.org/10.3390/cells13131149
Chicago/Turabian StyleGao, Chengjie, Huan Zhang, Yaomei Wang, Shihui Wang, Xinhua Guo, Yongshuai Han, Huizhi Zhao, and Xiuli An. 2024. "Global Transcriptomic and Characteristics Comparisons between Mouse Fetal Liver and Bone Marrow Definitive Erythropoiesis" Cells 13, no. 13: 1149. https://doi.org/10.3390/cells13131149
APA StyleGao, C., Zhang, H., Wang, Y., Wang, S., Guo, X., Han, Y., Zhao, H., & An, X. (2024). Global Transcriptomic and Characteristics Comparisons between Mouse Fetal Liver and Bone Marrow Definitive Erythropoiesis. Cells, 13(13), 1149. https://doi.org/10.3390/cells13131149




