Mild Hyperthermia-Induced Thermogenesis in the Endoplasmic Reticulum Defines Stress Response Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Fluorescent Labeling for Measuring ER Temperature and Ca2+ Levels
2.2.1. Temperature Determination with ER Thermo Yellow (λex/λem 510/591 nm)
2.2.2. Nuclear Staining with Hoechst 33342 (λex/λem 350/461 nm)
2.2.3. Determination of ER Ca2+ Levels with Mag-Fluo-4 ER (λex/λem 493/517 nm)
2.3. Cell Fixation
2.4. Treatments
2.4.1. Heat-Shock Experiments
2.4.2. Treatment with ER Stressors
2.5. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Flow Cytometry Analysis
2.6.1. Measuring XBP1 Protein Levels
2.6.2. Measuring ER Ca2+ Levels
2.7. Fluorescence Microscopy Assays
2.7.1. ER Temperature Measurements by ER Thermo Yellow Fluorescence
2.7.2. Super-Resolution Microscopy
3. Results
3.1. Distinct Cell Types Show Differential Stress Transcriptome Profiles upon Heat Treatment
3.2. IRE1 Clustering in Response to Mild Heat Treatment of 40 °C
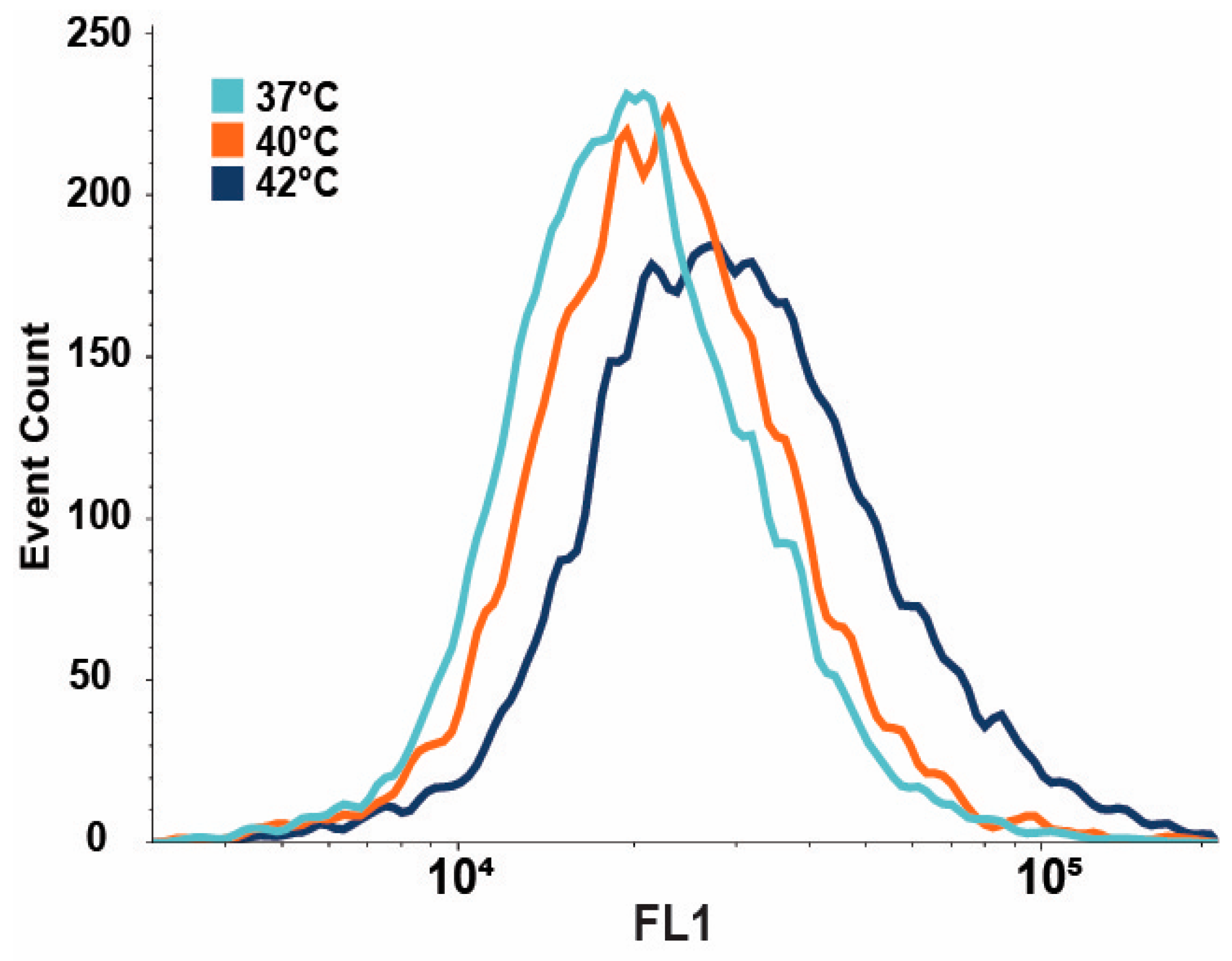
3.3. IRE1-Dependent XBP1 Expression Is Upregulated upon Mild Heat Treatment
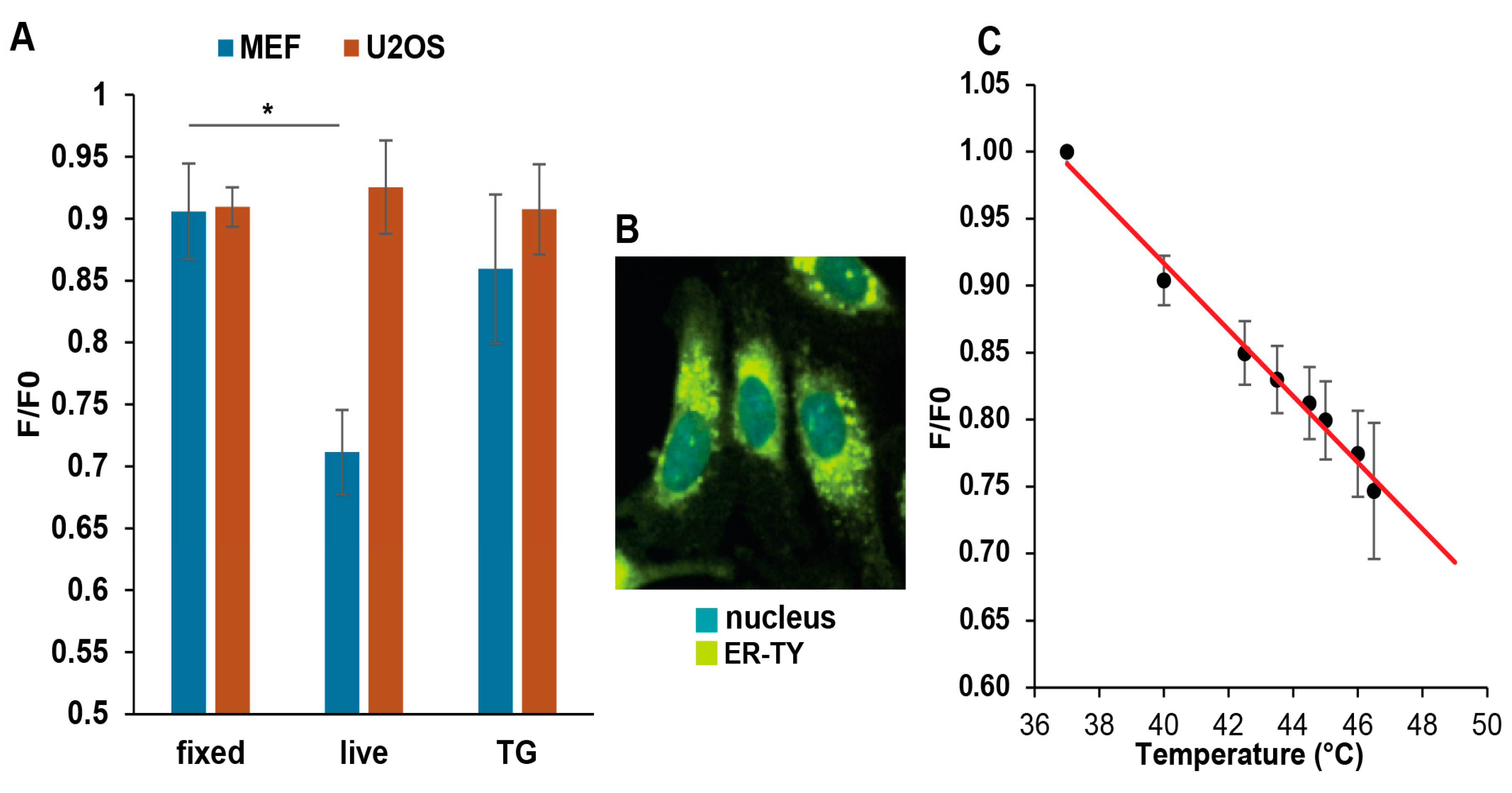
3.4. Mild Heat Could Induce Thermogenesis in the ER
3.5. ER Ca2+ Levels Decrease upon Mild Heat Treatment
4. Discussion
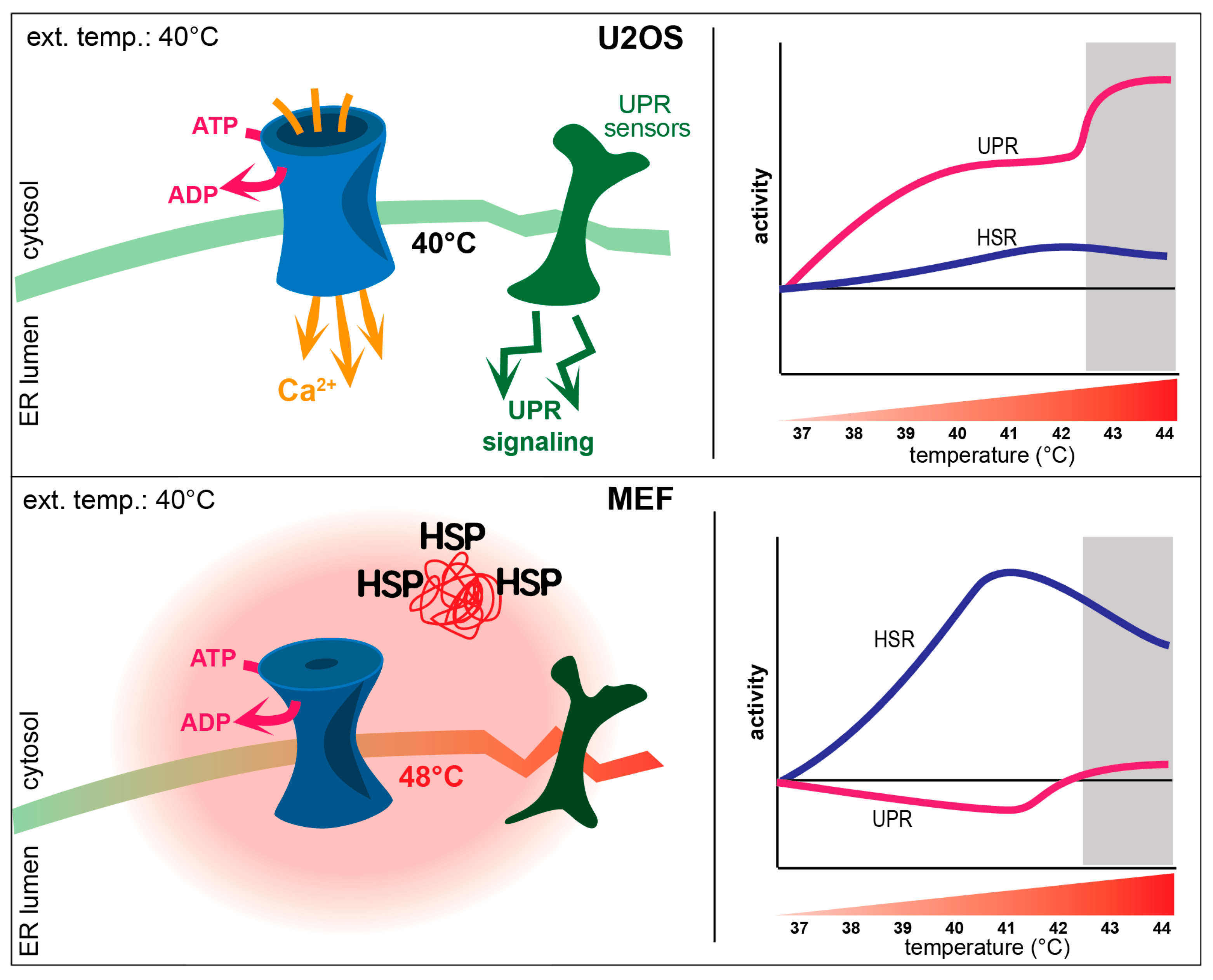
4.1. Interplay between the UPR and HSR upon Heat Stress
4.2. Mild Heat-Induced Intracellular Thermogenesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic Reticulum Stress Signalling – from Basic Mechanisms to Clinical Applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic Reticulum Stress: Molecular Mechanism and Therapeutic Targets - Signal Transduction and Targeted Therapy. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal Integration in the Endoplasmic Reticulum Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Peksel, B.; Gombos, I.; Péter, M.; Vigh, L.; Tiszlavicz, Á.; Brameshuber, M.; Balogh, G.; Schütz, G.J.; Horváth, I.; Vigh, L.; et al. Mild Heat Induces a Distinct “Eustress” Response in Chinese Hamster Ovary Cells but Does Not Induce Heat Shock Protein Synthesis. Sci. Rep. 2017, 7, 15643. [Google Scholar] [CrossRef] [PubMed]
- Tiszlavicz, Á.; Gombos, I.; Péter, M.; Hegedűs, Z.; Hunya, Á.; Dukic, B.; Nagy, I.; Peksel, B.; Balogh, G.; Horváth, I.; et al. Distinct Cellular Tools of Mild Hyperthermia-Induced Acquired Stress Tolerance in Chinese Hamster Ovary Cells. Biomedicines 2022, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance Induced at a Mild Temperature of 40°C Alleviates Heat Shock-Induced ER Stress and Apoptosis in HeLa Cells. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2015, 1853, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gupta, S.; Hu, W.; McGrath, B.C.; Cavener, D.R. Hyperthermia Induces the ER Stress Pathway. PLoS ONE 2011, 6, e23740. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6. [Google Scholar] [CrossRef]
- Lindholm, D.; Korhonen, L.; Eriksson, O.; Kõks, S. Recent Insights into the Role of Unfolded Protein Response in ER Stress in Health and Disease. Front. Cell Dev. Biol. 2017, 5, 48. [Google Scholar] [CrossRef]
- Radanović, T.; Ernst, R. The Unfolded Protein Response as a Guardian of the Secretory Pathway. Cells 2021, 10, 2965. [Google Scholar] [CrossRef]
- Ernst, R.; Ballweg, S.; Levental, I. Cellular Mechanisms of Physicochemical Membrane Homeostasis. Curr. Opin. Cell Biol. 2018, 53, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fun, X.H.; Thibault, G. Lipid Bilayer Stress and Proteotoxic Stress-Induced Unfolded Protein Response Deploy Divergent Transcriptional and Non-Transcriptional Programmes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158449. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Paquot, N.; Piette, J.; Legrand-Poels, S. Lipid Bilayer Stress in Obesity-Linked Inflammatory and Metabolic Disorders. Biochem. Pharmacol. 2018, 153, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, K.; Pesek, K.; Covino, R.; Hofbauer, H.F.; Wunnicke, D.; Hänelt, I.; Hummer, G.; Ernst, R. Activation of the Unfolded Protein Response by Lipid Bilayer Stress. Mol. Cell 2017, 67, 673–684.e8. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.B.; Roberts, L.S.; Chandra, V.; Rivera, I.G.; Nomura, D.K.; Forbes, D.J.; Niwa, M. The UPR Activator ATF6 Responds to Proteotoxic and Lipotoxic Stress by Distinct Mechanisms. Dev. Cell 2018, 46, 327–343.e7. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Taubert, S. Beyond Proteostasis: Lipid Metabolism as a New Player in Er Homeostasis. Metabolites 2021, 11, 52. [Google Scholar] [CrossRef]
- Beignon, F.; Gueguen, N.; Tricoire-Leignel, H.; Mattei, C.; Lenaers, G. The Multiple Facets of Mitochondrial Regulations Controlling Cellular Thermogenesis. Cell. Mol. Life Sci. 2022, 79, 525. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, D.; Bénit, P.; Ha, H.-H.; Keipert, S.; El-Khoury, R.; Chang, Y.-T.; Jastroch, M.; Jacobs, H.T.; Rustin, P.; Rak, M. Mitochondria Are Physiologically Maintained at Close to 50 °C. PLoS Biol. 2018, 16, e2003992. [Google Scholar] [CrossRef] [PubMed]
- Terzioglu, M.; Veeroja, K.; Montonen, T.; Ihalainen, T.O.; Salminen, T.S.; Bénit, P.; Rustin, P.; Chang, Y.-T.; Nagai, T.; Jacobs, H.T. Mitochondrial Temperature Homeostasis Resists External Metabolic Stresses; elife, 2023; 12, RP89232.
- Arai, S.; Lee, S.-C.; Zhai, D.; Suzuki, M.; Chang, Y.T. A Molecular Fluorescent Probe for Targeted Visualization of Temperature at the Endoplasmic Reticulum. Sci. Rep. 2014, 4, 6701. [Google Scholar] [CrossRef] [PubMed]
- Macherel, D.; Haraux, F.; Guillou, H.; Bourgeois, O. The Conundrum of Hot Mitochondria. Biochim. Biophys. Acta - Bioenerg. 2021, 1862, 148348. [Google Scholar] [CrossRef]
- Belyy, V.; Zuazo-Gaztelu, I.; Alamban, A.; Ashkenazi, A.; Walter, P. Endoplasmic Reticulum Stress Activates Human IRE1α through Reversible Assembly of Inactive Dimers into Small Oligomers. eLife 2022, 11, e74342. [Google Scholar] [CrossRef]
- Nougarède, A.; Tesnière, C.; Ylanko, J.; Rimokh, R.; Gillet, G.; Andrews, D.W. Improved IRE1 and PERK Pathway Sensors for Multiplex Endoplasmic Reticulum Stress Assay Reveal Stress Response to Nuclear Dyes Used for Image Segmentation. Assay Drug Dev. Technol. 2018, 16, 350–360. [Google Scholar] [CrossRef]
- Lebeau, P.F.; Platko, K.; Byun, J.H.; Austin, R.C. Calcium as a Reliable Marker for the Quantitative Assessment of Endoplasmic Reticulum Stress in Live Cells. J. Biol. Chem. 2021, 296, 100779. [Google Scholar] [CrossRef]
- Rees, E.J.; Erdelyi, M.; Schierle, G.S.K.; Knight, A.; Kaminski, C.F. Elements of Image Processing in Localization Microscopy. J. Opt. 2013, 15, 094012. [Google Scholar] [CrossRef]
- Belyy, V.; Tran, N.-H.; Walter, P. Quantitative Microscopy Reveals Dynamics and Fate of Clustered IRE1α. Proc. Natl. Acad. Sci. USA 2020, 117, 1533–1542. [Google Scholar] [CrossRef]
- Meis, L. Ca2+-ATPases (SERCA): Energy Transduction and Heat Production in Transport ATPases. J. Membr. Biol. 2002, 188, 1–9. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Hegde, R.S. Regulation of Basal Cellular Physiology by the Homeostatic Unfolded Protein Response. J. Cell Biol. 2010, 189, 783–794. [Google Scholar] [CrossRef]
- Marcu, M.G.; Doyle, M.; Bertolotti, A.; Ron, D.; Hendershot, L.; Neckers, L. Heat Shock Protein 90 Modulates the Unfolded Protein Response by Stabilizing IRE1alpha. Mol. Cell. Biol. 2002, 22, 8506–8513. [Google Scholar] [CrossRef]
- Somogyvári, M.; Khatatneh, S.; Sőti, C. Hsp90: From Cellular to Organismal Proteostasis. Cells 2022, 11, 2479. [Google Scholar] [CrossRef]
- Walczak, A.; Gradzik, K.; Kabzinski, J.; Przybylowska-Sygut, K.; Majsterek, I. The Role of the ER-Induced UPR Pathway and the Efficacy of Its Inhibitors and Inducers in the Inhibition of Tumor Progression. Oxid. Med. Cell. Longev. 2019, 2019, 5729710. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Tang, H.; Liu, Z.; Österlund, T.; Nielsen, J.; Petranovic, D. Management of the Endoplasmic Reticulum Stress by Activation of the Heat Shock Response in Yeast. FEMS Yeast Res. 2014, 14, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Arvan, P.; Zhao, X.; Ramos-Castaneda, J.; Chang, A. Secretory Pathway Quality Control Operating in Golgi, Plasmalemmal, and Endosomal Systems. Traffic Cph. Den. 2002, 3, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Alagar Boopathy, L.R.; Jacob-Tomas, S.; Alecki, C.; Vera, M. Mechanisms Tailoring the Expression of Heat Shock Proteins to Proteostasis Challenges. J. Biol. Chem. 2022, 298, 101796. [Google Scholar] [CrossRef] [PubMed]
- Shyu, P.; Ng, B.S.H.; Ho, N.; Chaw, R.; Seah, Y.L.; Marvalim, C.; Thibault, G. Membrane Phospholipid Alteration Causes Chronic ER Stress through Early Degradation of Homeostatic ER-Resident Proteins. Sci. Rep. 2019, 9, 8637. [Google Scholar] [CrossRef] [PubMed]
- Celik, C.; Lee, S.Y.T.; Yap, W.S.; Thibault, G. Endoplasmic Reticulum Stress and Lipids in Health and Diseases. Prog. Lipid Res. 2023, 89, 101198. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tapia, E.; Querol, A.; Pérez-Torrado, R. Membrane Fluidification by Ethanol Stress Activates Unfolded Protein Response in Yeasts. Microb. Biotechnol. 2018, 11, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Renne, M.F.; Ernst, R. Membrane Homeostasis beyond Fluidity: Control of Membrane Compressibility. Trends Biochem. Sci. 2023, 48, 963–977. [Google Scholar] [CrossRef]
- Kučerka, N.; Nieh, M.-P.; Katsaras, J. Fluid Phase Lipid Areas and Bilayer Thicknesses of Commonly Used Phosphatidylcholines as a Function of Temperature. Biochim. Biophys. Acta BBA - Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef]
- Pan, J.; Tristram-Nagle, S.; Kučerka, N.; Nagle, J.F. Temperature Dependence of Structure, Bending Rigidity, and Bilayer Interactions of Dioleoylphosphatidylcholine Bilayers. Biophys. J. 2008, 94, 117–124. [Google Scholar] [CrossRef]
- Kono, N.; Amin-Wetzel, N.; Ron, D. Generic Membrane-Spanning Features Endow IRE1α with Responsiveness to Membrane Aberrancy. Mol. Biol. Cell 2017, 28, 2318–2332. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of Fatty-Acid-Dependent UCP1 Uncoupling in Brown Fat Mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin Is a Newly Identified Regulator of Muscle-Based Thermogenesis in Mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.C.; Periasamy, M. Uncoupling of Sarcoendoplasmic Reticulum Calcium ATPase Pump Activity by Sarcolipin as the Basis for Muscle Non-Shivering Thermogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190135. [Google Scholar] [CrossRef] [PubMed]
- Block, B.A. Thermogenesis in Muscle. Annu. Rev. Physiol. 1994, 56, 535–577. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, R.E. Bilayer Thickness and Membrane Protein Function: An Energetic Perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M.; Feigenson, G.W. Fluorescence Quenching in Model Membranes. 3. Relationship between Calcium Adenosinetriphosphatase Enzyme Activity and the Affinity of the Protein for Phosphatidylcholines with Different Acyl Chain Characteristicst. Biochemistry 1981, 20, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Karlovská, J.; Uhríková, D.; Kučerka, N.; Teixeira, J.; Devínsky, F.; Lacko, I.; Balgavý, P. Influence of N-Dodecyl-N,N-Dimethylamine N-Oxide on the Activity of Sarcoplasmic Reticulum Ca2+-Transporting ATPase Reconstituted into Diacylphosphatidylcholine Vesicles: Effects of Bilayer Physical Parameters. Biophys. Chem. 2006, 119, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lepock, J.R. How Do Cells Respond to Their Thermal Environment? Int. J. Hyperthermia 2005, 21, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Kriszt, R.; Arai, S.; Itoh, H.; Lee, M.H.; Goralczyk, A.G.; Ang, X.M.; Cypess, A.M.; White, A.P.; Shamsi, F.; Xue, R.; et al. Optical Visualisation of Thermogenesis in Stimulated Single-Cell Brown Adipocytes. Sci. Rep. 2017, 7, 1383. [Google Scholar] [CrossRef]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-Independent Signaling Involving SERCA2b-Mediated Calcium Cycling Regulates Beige Fat Thermogenesis and Systemic Glucose Homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef]
- Török, Z.; Crul, T.; Maresca, B.; Schütz, G.J.; Viana, F.; Dindia, L.; Piotto, S.; Brameshuber, M.; Balogh, G.; Péter, M.; et al. Plasma Membranes as Heat Stress Sensors: From Lipid-Controlled Molecular Switches to Therapeutic Applications. Biochim. Biophys. Acta 2014, 1838, 1594–1618. [Google Scholar] [CrossRef] [PubMed]
- Oka, T. Stress-Induced Hyperthermia and Hypothermia. Handb. Clin. Neurol. 2018, 157, 599–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, A. Heat Shock Response Relieves ER Stress. EMBO J. 2008, 27, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Balogh, G.; Péter, M.; Glatz, A.; Gombos, I.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Key Role of Lipids in Heat Stress Management. FEBS Lett. 2013, 587, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Vigh, L.; Maresca, B.; Harwood, J.L. Does the Membrane’s Physical State Control the Expression of Heat Shock and Other Genes? Trends Biochem. Sci. 1998, 23, 369–374. [Google Scholar] [CrossRef]
- Horváth, I.; Multhoff, G.; Sonnleitner, A.; Vígh, L. Membrane-Associated Stress Proteins: More than Simply Chaperones. Biochim. Biophys. Acta BBA-Biomembr. 2008, 1778, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Saxena, S. ER Stress and the Unfolded Protein Response in Neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic Reticulum Stress, Obesity and Diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Trojanowski, N.; Castelli, L.M.; Miotto, P.M.; Amoye, F.; Ward, W.E.; Tupling, A.R.; LeBlanc, P.J. Saturation of SERCA’s Lipid Annulus May Protect against Its Thermal Inactivation. Biochem. Biophys. Res. Commun. 2017, 484, 456–460. [Google Scholar] [CrossRef]
- Kunze, C.; Luijckx, P.; Jackson, A.L.; Donohue, I. Alternate Patterns of Temperature Variation Bring about Very Different Disease Outcomes at Different Mean Temperatures. eLife 2022, 11, e72861. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dukic, B.; Ruppert, Z.; Tóth, M.E.; Hunya, Á.; Czibula, Á.; Bíró, P.; Tiszlavicz, Á.; Péter, M.; Balogh, G.; Erdélyi, M.; et al. Mild Hyperthermia-Induced Thermogenesis in the Endoplasmic Reticulum Defines Stress Response Mechanisms. Cells 2024, 13, 1141. https://doi.org/10.3390/cells13131141
Dukic B, Ruppert Z, Tóth ME, Hunya Á, Czibula Á, Bíró P, Tiszlavicz Á, Péter M, Balogh G, Erdélyi M, et al. Mild Hyperthermia-Induced Thermogenesis in the Endoplasmic Reticulum Defines Stress Response Mechanisms. Cells. 2024; 13(13):1141. https://doi.org/10.3390/cells13131141
Chicago/Turabian StyleDukic, Barbara, Zsófia Ruppert, Melinda E. Tóth, Ákos Hunya, Ágnes Czibula, Péter Bíró, Ádám Tiszlavicz, Mária Péter, Gábor Balogh, Miklós Erdélyi, and et al. 2024. "Mild Hyperthermia-Induced Thermogenesis in the Endoplasmic Reticulum Defines Stress Response Mechanisms" Cells 13, no. 13: 1141. https://doi.org/10.3390/cells13131141
APA StyleDukic, B., Ruppert, Z., Tóth, M. E., Hunya, Á., Czibula, Á., Bíró, P., Tiszlavicz, Á., Péter, M., Balogh, G., Erdélyi, M., Timinszky, G., Vígh, L., Gombos, I., & Török, Z. (2024). Mild Hyperthermia-Induced Thermogenesis in the Endoplasmic Reticulum Defines Stress Response Mechanisms. Cells, 13(13), 1141. https://doi.org/10.3390/cells13131141





