Molecular Mechanisms of the Therapeutic Effect of Selenium Nanoparticles in Hepatocellular Carcinoma
Abstract
1. Introduction
2. The “Therapeutic Window” of SeNPs Determines Their Hepatoprotective Functions
3. SeNPs as Inducers of Various Forms of Cell Death Using the Example of Liver Cancer Cells
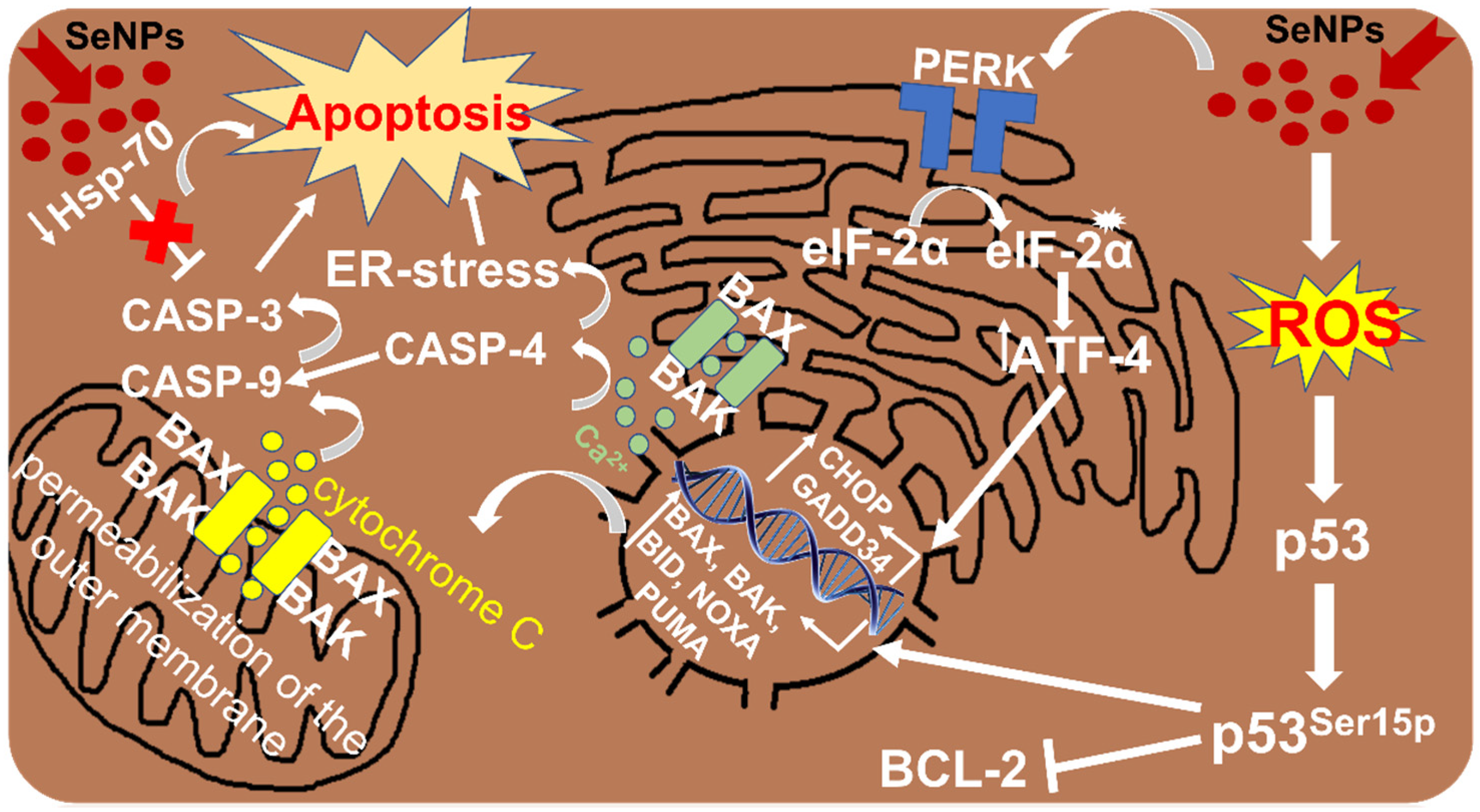
3.1. SeNPs as an Inducer of Apoptosis in Liver Cancer Cells
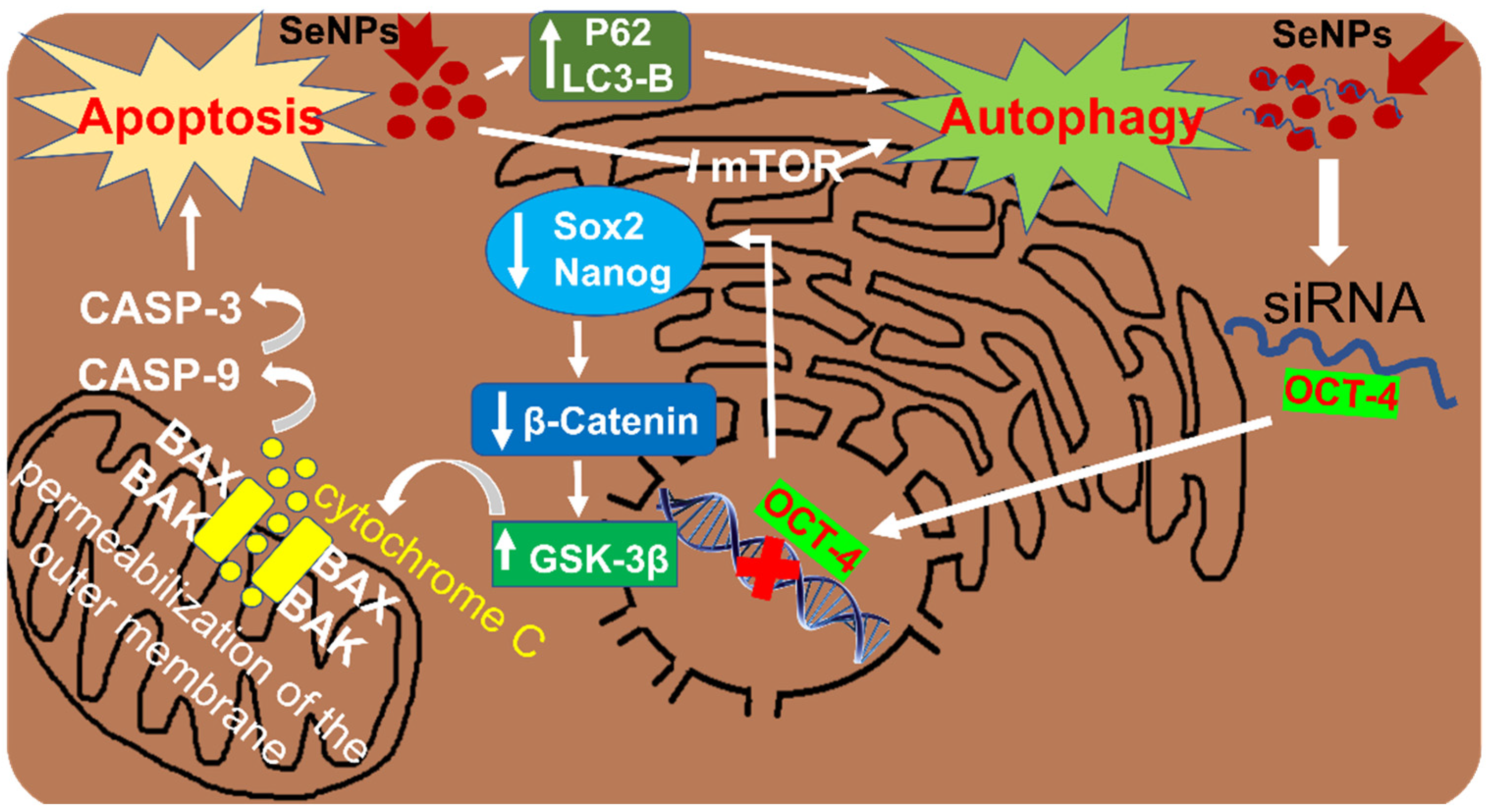
3.2. SeNPs as Inducers of Autophagy in Liver Cancer Cells
4. Signaling Pathways Activated by SeNPs in HCC
4.1. Participation of SeNPs in the Regulation of ER Stress in HCC
4.2. Participation of SeNPs in Wnt/β-Catenin Signaling in HCC
4.3. Involvement of SeNPs in the Regulation of PI3K/Akt/mTOR in HCC
4.4. SeNPs Regulate the Expression of ER-Resident Selenoproteins in HCC
5. Use of SeNPs to Improve the Effectiveness of Drugs in the Treatment of HCC
6. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nabil, A.-A.; Charles, S.; Alberto, B. Tumor Evolution as a Therapeutic Target. Cancer Discov. 2017, 7, 805–817. [Google Scholar]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef]
- Losic, B.; Craig, A.J.; Villacorta-Martin, C.; Martins-Filho, S.N.; Akers, N.; Chen, X.; Ahsen, M.E.; von Felden, J.; Labgaa, I.; DʹAvola, D.; et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat. Commun. 2020, 11, 291. [Google Scholar] [CrossRef]
- Guo, L.; Yi, X.; Chen, L.; Zhang, T.; Guo, H.; Chen, Z.; Cheng, J.; Cao, Q.; Liu, H.; Hou, C.; et al. Single-Cell DNA Sequencing Reveals Punctuated and Gradual Clonal Evolution in Hepatocellular Carcinoma. Gastroenterology 2022, 162, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in patients with advanced progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Mintz, K.; Waidely, E.; Zhou, Y.; Peng, Z.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon dots and gold nanoparticles based immunoassay for detection of alpha-L-fucosidase. Anal. Chim. Acta. 2018, 1041, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-H.; Wang, S.; Liu, S.-Y.; Chen, K.; Wu, Z.-Y.; Li, D.-F.; Mi, Y.-T.; Hu, L.-B.; Chen, Z.-W.; Zhao, X.-M. Development and in vitro study of a bi-specific magnetic resonance imaging molecular probe for hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 3030–3043. [Google Scholar] [CrossRef]
- Mohammed, E.; El-Beih, N.; El-Hussieny, E.; El-Ahwany, E.; Hassan, M.; Zoheiry, M. Effects of free and nanoparticulate curcumin on chemically induced liver carcinoma in an animal model. Arch. Med. Sci. 2021, 17, 218–227. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Huang, W. Antioxidant Capacity and Hepatoprotective Role of Chitosan-Stabilized SeNPs in Concanavalin A-Induced Liver Injury in Mice. Nutrients 2020, 12, 857. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, X.; Chen, Z.; Zeng, X.; Yue, T.; Yuan, Y. Effects of SeNPs on Preventing Patulin-Induced Liver, Kidney and Gastrointestinal Damage. Foods 2022, 11, 749. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Motaan, S.E.; Malik, N. Protective and Antioxidant Role of SeNPs and Vitamin C against Acrylamide Induced Hepatotoxicity in Male Mice. Int. J. Pharmacol. 2019, 15, 664–674. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Ghosh, P.; Biswas, J.; Bhattacharya, S. Protective effect of Selenium nanoparticle against cyclophosphamide induced hepatotoxicity and genotoxicity in Swiss albino mice. J. Biomater. Appl. 2014, 29, 303–317. [Google Scholar] [CrossRef]
- Khan, M.A.; Singh, D.; Arif, A.; Sodhi, K.K.; Singh, D.K.; Islam, S.N.; Ahmad, A.; Akhtar, K.; Siddique, H.R. Protective effect of green synthesized SeNPs against Doxorubicin induced multiple adverse effects in Swiss albino mice. Life Sci. 2022, 305, 120792. [Google Scholar] [CrossRef]
- Nhieu, J.T.; Renard, C.A.; Wei, Y.; Cherqui, D.; Zafrani, E.S.; Buendia, M.A. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am. J. Pathol. 1999, 155, 703–710. [Google Scholar] [CrossRef]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or Negative Actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Xiao, X.; Deng, H.; Lin, X.; Ali, A.S.M.; Viscardi, A.; Guo, Z.; Qiao, L.; He, Y.; Han, J. Selenium nanoparticles: Properties, preparation methods, and therapeutic applications. Chem. Biol. Interact. 2023, 378, 110483. [Google Scholar] [CrossRef]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.; Xin, N.; Wei, H.; Zhang, T.; Zhao, C. Selenium nanoparticles: Enhanced nutrition and beyond. Crit. Rev. Food Sci. Nutr. 2023, 63, 12360–12371. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Size-Dependent Cytoprotective Effects of Selenium Nanoparticles during Oxygen-Glucose Deprivation in Brain Cortical Cells. Int. J. Mol. Sci. 2022, 23, 7464. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnology 2017, 15, 4. [Google Scholar] [CrossRef]
- Menon, S.; Ks, S.D.; R, S.; S, R.; S, V.K. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the Cytotoxic Effect of Selenium Nanoparticles in Different Human Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef]
- Huang, Y.; He, L.; Liu, W.; Fan, C.; Zheng, W.; Wong, Y.S.; Chen, T. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials 2013, 34, 7106–7116. [Google Scholar] [CrossRef]
- Guo, M.; Li, Y.; Lin, Z.; Zhao, M.; Xiao, M.; Wang, C.; Xu, T.; Xiaa, Y.; Zhu, B. Surface decoration of selenium nanoparticles with curcumin induced HepG2 cell apoptosis through ROS mediated p53 and AKT signaling pathways. RSC Adv. 2017, 7, 52456–52464. [Google Scholar] [CrossRef]
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. J. Nanobiotechnol. 2017, 15, 43. [Google Scholar] [CrossRef]
- Pi, J.; Jiang, J.; Cai, H.; Yang, F.; Jin, H.; Yang, P.; Cai, J.; Chen, Z.W. GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Deliv. 2017, 24, 1549–1564. [Google Scholar] [CrossRef]
- Deng, X.; Liu, H.; Xu, Y.; Chan, L.; Xie, J.; Xiong, Z.; Tang, Z.; Yang, F.; Chen, T. Designing highly stable ferrous selenide-black phosphorus nanosheets heteronanostructure via p-Se bond for MRI-guided photothermal therapy. J. Nanobiotechnol. 2021, 19, 201. [Google Scholar] [CrossRef]
- Liu, W.; Su, J.; Shi, Q.; Wang, J.; Chen, X.; Zhang, S.; Li, M.; Cui, J.; Fan, C.; Sun, B.; et al. RGD peptide-conjugated selenium nanocomposite inhibits human glioma growth by triggering mitochondrial dysfunction and ROS-dependent MAPKs activation. Front. Bioeng. Biotechnol. 2021, 9, 781608. [Google Scholar] [CrossRef]
- Mi, X.J.; Choi, H.S.; Perumalsamy, H.; Shanmugam, R.; Thangavelu, L.; Balusamy, S.R.; Kim, Y.J. Biosynthesis and cytotoxic effect of silymarin-functionalized selenium nanoparticles induced autophagy mediated cellular apoptosis via downregulation of PI3K/Akt/mTOR pathway in gastric cancer. Phytomedicine 2022, 99, 154014. [Google Scholar] [CrossRef]
- Bjorkoy, G.; Lamark, T.; Pankiv, S.; Overvatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Z.; He, L.; Luk, K.H.; Cheung, S.T.; Wong, K.H.; Chen, T. Autophagy is an important action mode for functionalized selenium nanoparticles to exhibit anti-colorectal cancer activity. Biomater. Sci. 2018, 6, 2508–2517. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Liu, T.; Chang, Y.; Chen, T.; Li, X. Dual-targeting nanotherapeutics antagonize hyperinsulinemia-promoted tumor growth via activating cell autophagy. J. Mater. Chem. B 2019, 7, 6751–6758. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- 37. Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Zaghloul, R.A.; Abdelghany, A.M.; El Gayar, A.M. Selenium nanoparticles and quercetin suppress thioacetamide-induced hepatocellular carcinoma in rats: Attenuation of inflammation involvement. J. Biochem. Mol. Toxicol. 2022, 36, e22989. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Toxicity of repeated oral intake of organic selenium, inorganic selenium, and selenium nanoparticles: A review. J. Trace Elem. Med. Biol. 2023, 79, 127235. [Google Scholar] [CrossRef]
- Jia, X.; Li, N.; Chen, J. A subchronic toxicity study of elemental Nano-Se in Sprague-Dawley rats. Life Sci. 2005, 76, 1989–2003. [Google Scholar] [CrossRef]
- Lesnichaya, M.; Karpova, E.; Sukhov, B. Effect of high dose of selenium nanoparticles on antioxidant system and biochemical profile of rats in correction of carbon tetrachloride-induced toxic damage of liver. Colloids Surf. B Biointerfaces 2021, 197, 111381. [Google Scholar] [CrossRef]
- Ji, H.; Lou, X.; Jiao, J.; Li, Y.; Dai, K.; Jia, X. Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells. Molecules 2023, 28, 1561. [Google Scholar] [CrossRef]
- Zheng, S.; Li, X.; Zhang, Y.; Xie, Q.; Wong, Y.S.; Zheng, W.; Chen, T. PEG-nanolized ultrasmall SeNPs overcome drug resistance in hepatocellular carcinoma HepG2 cells through induction of mitochondria dysfunction. Int. J. Nanomed. 2012, 7, 3939–3949. [Google Scholar]
- Jiao, J.; Yu, J.; Ji, H.; Liu, A. Synthesis of macromolecular Astragalus polysaccharide-nano selenium complex and the inhibitory effects on HepG2 cells. Int. J. Biol. Macromol. 2022, 211, 481–489. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Simakin, A.V.; Gudkov, S.V.; Turovsky, E.A. Comparative Analysis of the Cytotoxic Effect of a Complex of SeNPs Doped with Sorafenib, “Naked” Selenium Nanoparticles, and Sorafenib on Human Hepatocyte Carcinoma HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 6641. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.; Lin, Z.; Zhao, M.; Xia, Y.; Wang, C.; Xu, T.; Zhu, B. Multifunctional SeNPs with Galangin-induced HepG2 cell apoptosis through p38 and AKT signalling pathway. R. Soc. Open Sci. 2018, 5, 180509. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, M.; Xu, T.; Li, Y.; Wang, C.; Lin, Z.; Zhao, M.; Zhu, B. siRNA-loaded selenium nanoparticle modified with hyaluronic acid for enhanced hepatocellular carcinoma therapy. Int. J. Nanomed. 2018, 13, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Xia, H.; Wang, H.; Zhu, B. Multifunctional SeNPs as carriers of HSP70 siRNA to induce apoptosis of HepG2 cells. Int. J. Nanomed. 2016, 11, 3065–3076. [Google Scholar]
- Cui, D.; Liang, T.; Sun, L.; Meng, L.; Yang, C.; Wang, L.; Liang, T.; Li, Q. Green synthesis of SeNPs with extract of hawthorn fruit induced HepG2 cells apoptosis. Pharm. Biol. 2018, 56, 528–534. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Song, H.; Cheng, J.; Qiu, W.; Hu, J.; Qiu, Z.; Wang, Q.; Chang, C.; Zheng, G.; Meng, Y. SeNPs Stabilized by β-Glucan Nanotubes from Black Fungus and Their Effects on the Proliferation, Apoptosis, and Cell Cycle of HepG2 Cells. ACS Omega 2023, 8, 45358–45368. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Overvatn, A.; Bjorkoy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Bjorkoy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Overvatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Weissman, A. The Unfolded Protein Response, Degradation from the Endoplasmic Reticulum, and Cancer. Genes. Cancer 2010, 1, 764–778. [Google Scholar] [CrossRef]
- Marciniak, S.; Ron, D. Endoplasmic Reticulum Stress Signaling in Disease. Physiol. Rev. 2006, 86, 1133–1149. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Lu, P.D.; Harding, H.P.; Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004, 167, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Kaufman, R.J. All roads lead to ATF4. Dev. Cell 2003, 4, 442–444. [Google Scholar] [CrossRef]
- Vattem, K.M.; Wek, R.C. Reinitiation involving upstream ORFs regulates ATF-4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11269–11274. [Google Scholar] [CrossRef]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF-6 and spliced by IRE1 in response to ER-stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Chu, G.C.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005, 24, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell. Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Bedard, K.; Breining, T.; Cribb, A.E. Disruption of the endoplasmic reticulum by cytotoxins in LLC-PK1 cells. Toxicol. Lett. 2005, 159, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Dourdin, N.; Wu, C.; De Veyra, T.; Elce, J.S.; Greer, P.A. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 2006, 281, 16016–16024. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L.; Oakes, S.A.; Opferman, J.T.; Cheng, E.H.; Sorcinelli, M.D.; Pozzan, T.; Korsmeyer, S.J. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science 2003, 300, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Li, C.; Hatzivassiliou, G.; Lindsten, T.; Yu, Q.C.; Yuan, J.; Thompson, C.B. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003, 162, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, J.; Katayama, T.; Eguchi, Y.; Kudo, T.; Taniguchi, M.; Koyama, Y.; Manabe, T.; Yamagishi, S.; Bando, Y.; Imaizumi, K.; et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 2004, 165, 347–356. [Google Scholar] [CrossRef]
- Schroder, M.; Kaufman, R.J. The mammalian unfolded protein response. Ann. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef]
- Anding, A.L.; Chapman, J.S.; Barnett, D.W.; Curley RWJr Clagett-Dame, M. The unhydrolyzable fenretinide analogue 4-hydroxybenzylretinone induces the proapoptotic genes GADD153 (CHOP) and Bcl-2-binding component 3 (PUMA) and apoptosis that is caspase- dependent and independent of the retinoic acid receptor. Cancer Res. 2007, 67, 6270–6277. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd 153 sensitizes cells by downregulating Bcl2 and perturbing the cellular redox state. Mol. Cell Biol. 2001, 21, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.-C.; Chen, Y.; Zhao, J.-L.; Gao, C.-C.; Han, H.; Liu, W.-C.; Qin, H.-Y. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Smits, R.; Hao, H.; He, C. Wnt/β-Catenin Signaling in Liver Cancers. Cancers 2019, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, W.; Cheng, M.; Wang, J.; Liu, Z.; He, S.; Luo, X.; Huang, W.; Chen, T.; Yan, W.; et al. Hypoxia activates Wnt/β-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma. Sci. Rep. 2017, 7, 40446. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.L.; Weis, W.I. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Takada, K.; Zhu, D. Targeting Wnt/β-catenin pathway for drug therapy. Med. Drug Discov. 2020, 8, 100066. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Wang, Q.; Liu, P.; Xiao, Y.; Wu, P.; Wang, Y.; Chen, B.; Liu, Z.; Liu, Q. Lgr5-mediated p53 Repression through PDCD5 leads to doxorubicin resistance in Hepatocellular Carcinoma. Theranostics 2019, 9, 2967. [Google Scholar] [CrossRef] [PubMed]
- Koni, M.; Pinnarò, V.; Brizzi, M.F. The Wnt signalling pathway: A tailored target in cancer. Int. J. Mol. Sci. 2020, 21, 7697. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Alsinet, C.; Savic, R.; Cabellos, L.; Toffanin, S.; Hoshida, Y.; Villanueva, A.; Minguez, B.; Newell, P.; Tsai, H.W.; et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin. Cancer Res. 2012, 18, 4997–5007. [Google Scholar] [CrossRef]
- Wong, C.M.; Fan, S.T.; Ng, I.O. beta-Catenin mutation and overexpression in hepatocellular carcinoma: Clinicopathologic and prognostic significance. Cancer 2001, 92, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chao, C.C. Identification of the beta-catenin/JNK/prothymosin-alpha axis as a novel target of sorafenib in hepatocellular carcinoma cells. Oncotarget 2015, 6, 38999–39017. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lisby, A.; Ma, C.; Lo, N.; Ehmer, U.; Hayer, K.E.; Furth, E.E.; Viatour, P. Promotion of growth factor signaling as a critical function of β-catenin during HCC progression. Nat. Commun. 2019, 10, 1909. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lin, Z.; Li, Y.; Zhao, M.; Wang, C.; Guo, M.; Zhang, B.; Zhu, B. Targeted delivery of siRNA using RGDfC-conjugated functionalized SeNPs for anticancer therapy. J. Mater. Chem. B 2017, 5, 6941–6952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Yang, X.; Chen, Y.; Jiang, Y.; Wang, S.J.; Li, Y.; Wang, X.Q.; Meng, Y.; Zhu, M.M.; Ma, X.; et al. Phytother. Res. 2017, 31, 680–688. [Google Scholar]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Pompura, S.L.; Dominguez-Villar, M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukocyte Biol. 2018, 103, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prever, L.; Hirsch, E.; Gulluni, F. Targeting PI3K/AKT/mTOR signaling pathway in breast cancer. Cancers 2021, 13, 3517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Xu, J.; Zhu, J.; Ding, K. Inhibition of cathepsin S induces autophagy and apoptosis in human glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K and JNK signaling pathways. Toxicol. Lett. 2014, 228, 248–259. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, T.; Sun, W.; Wang, H.; Yin, F.; Wang, Z.; Zuo, D.; Sun, M.; Zhou, Z.; Lin, B.; et al. Arsenic sulfide induces apoptosis and autophagy through the activation of ROS/JNK and suppression of Akt/mTOR signaling pathways in osteosarcoma. Free Radic. Biol. Med. 2017, 106, 24–37. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, C.; Huang, M.Y.; Li, W.Y.; Hu, G.Q. Cinobufagin induces autophagy-mediated cell death in human osteosarcoma U2OS cells through the ROS/JNK/p38 signaling pathway. Oncol. Rep. 2016, 36, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Son, K.M.; Kim, K.Y.; Yu, S.N.; Park, S.G.; Kim, Y.W.; Nam, H.W.; Suh, J.T.; Ji, J.H.; Ahn, S.C. Deoxypodophyllotoxin induces cytoprotective autophagy against apoptosis via inhibition of PI3K/AKT/mTOR pathway in osteosarcoma U2OS cells. Pharmacol. Rep. 2017, 69, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.T.; Qin, Y.; Zhou, Z.W.; He, Z.X.; Zhang, X.; Yang, T.; Yang, Y.X.; Wang, D.; Qiu, J.X.; Zhou, S.F. Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK-and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells. Drug Des. Dev. Ther. 2015, 9, 1601. [Google Scholar]
- Wang, R.; Ha, K.Y.; Dhandapani, S.; Kim, Y.J. Biologically synthesized black ginger-selenium nanoparticle induces apoptosis and autophagy of AGS gastric cancer cells by suppressing the PI3K/Akt/mTOR signaling pathway. J. Nanobiotechnology 2022, 20, 441. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.J.; Le, H.M.; Lee, S.; Park, H.R.; Kim, Y.J. Silymarin-Functionalized SeNPs Prevent LPS-Induced Inflammatory Response in RAW264.7 Cells through Downregulation of the PI3K/Akt/NF-κB Pathway. ACS Omega 2022, 7, 42723–42732. [Google Scholar] [CrossRef] [PubMed]
- Rabah, H.M.; Mohamed, D.A.; Mariah, R.A.; Abd El-Khalik, S.R.; Khattab, H.A.; AbuoHashish, N.A.; Abdelsattar, A.M.; Raslan, M.A.; Farghal, E.E.; Eltokhy, A.K. Novel insights into the synergistic effects of SeNPs and metformin treatment of letrozole—Induced polycystic ovarian syndrome: Targeting PI3K/Akt signalling pathway, redox status and mitochondrial dysfunction in ovarian tissue. Redox Rep. 2023, 28, 2160569. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, Y.P.; Goltyaev, M.V.; Gorbacheva, O.S.; Novoselov, S.V.; Varlamova, E.G.; Fesenko, E.E. Influence of Sodium Selenite on the mRNA Expression of the Mammalian Selenocysteine-Containing Protein Genes in Testicle and Prostate Cancer Cells. Dokl. Biochem. Biophys. 2018, 480, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G. Participation of selenoproteins localized in the ER in the processes occurring in this organelle and in the regulation of carcinogenesis-associated processes. J. Trace Elem. Med. Biol. 2018, 48, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Goltyaev, M.V.; Kuznetsova, J.P. Effect of Sodium Selenite on Gene Expression of SELF, SELW, and TGR Selenoproteins in Adenocarcinoma Cells of the Human Prostate. Mol. Biol. 2018, 52, 519–526. (In Russian) [Google Scholar] [CrossRef]
- Goltyaev, M.V.; Mal’tseva, V.N.; Varlamova, E.G. Expression of ER-resident selenoproteins and activation of cancer cells apoptosis mechanisms under ER-stress conditions caused by methylseleninic acid. Gene 2020, 755, 144884. [Google Scholar] [CrossRef]
- Goltyaev, M.V.; Varlamova, E.G.; Novoselov, S.V.; Fesenko, E.E. Activation of Signal Pathways of Apoptosis under Conditions of Prolonged ER-Stress Caused by Exposure of Mouse Testicular Teratoma Cells to Selenium-Containing Compounds. Dokl. Biochem. Biophys. 2020, 490, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Turovsky, E.A. The main cytotoxic effects of methylseleninic acid on various cancer cells. Int. J. Mol. Sci. 2021, 22, 6614. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M.W.; Hoffmann, P.R. Endoplasmic reticulum–resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium. 2018, 70, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Varlamova, E.G. Mechanism of Ca2+-Dependent Pro-Apoptotic Action of Selenium Nanoparticles, Mediated by Activation of Cx43 Hemichannels. Biology 2021, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Mal’tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and Anti-Inflammatory Properties of Selenium-Containing Agents: Their Role in the Regulation of Defense Mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Goltyaev, M.V.; Novoselov, S.V.; Varlamova, E.G. Effects of Sodium Selenite and Dithiothreitol on Expression of Endoplasmic Reticulum Selenoproteins and Apoptosis Markers in MSF7 Breast Adenocarcinoma Cells. Mol. Biol. 2022, 56, 135–146. (In Russian) [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Novoselov, V.I.; Fesenko, E.E. Cloning, intracellular localization, and expression of the mammalian selenocysteine-containing protein SELENOI (SelI) in tumor cell lines. Dokl. Biochem. Biophys. 2017, 476, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Xu, I.M.-J.; Chiu, D.K.-C.; Leibold, J.; Tse, A.P.-W.; Bao, M.H.-R.; Yuen, V.W.-H.; Chan, C.Y.-K.; Lai, R.K.-H.; Chin, D.W.-C.; et al. Induction of oxidative stress through inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology 2019, 69, 1768–1786. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; de Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.F.H. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef]
- Liu, Q.; Qian, Y.; Li, P.; Zhang, S.; Liu, J.; Sun, X.; Fulham, M.; Feng, D.; Huang, G.; Lu, W.; et al. (131)I-Labeled Copper Sulfide-Loaded Microspheres to Treat Hepatic Tumors via Hepatic Artery Embolization. Theranostics 2018, 8, 785–799. [Google Scholar] [CrossRef]
- Lewis, A.L.; Gonzalez, M.V.; Lloyd, A.W.; Hall, B.; Tang, Y.; Willis, S.L.; Leppard, S.W.; Wolfenden, L.C.; Palmer, R.R.; Stratford, P.W. DC bead: In vitro characterization of a drug-delivery device for transarterial chemoembolization. J. Vasc. Interv. Radiol. JVIR 2006, 17 Pt 1, 335–342. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Chung, Y.H.; Han, G.; Yoon, J.H.; Yang, J.; Wang, J.; Shao, G.L.; Kim, B.I.; Lee, T.Y.; Chao, Y. Interim analysis of START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int. J. Cancer 2013, 132, 2448–2458. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. The lancet. Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef]
- Kroschinsky, F.; Stolzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 89. [Google Scholar] [CrossRef]
- Hutson, T.E.; Al-Shukri, S.; Stus, V.P.; Lipatov, O.N.; Shparyk, Y.; Bair, A.H.; Rosbrook, B.; Andrews, G.I.; Vogelzang, N.J. Axitinib versus sorafenib in first-line metastatic renal cell carcinoma: Overall survival from a randomized phase III trial. Clin. Genitourin. Cancer 2017, 15, 72–76. [Google Scholar] [CrossRef]
- Zheng, L.; Li, C.; Huang, X.; Lin, X.; Lin, W.; Yang, F.; Chen, T. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials 2019, 216, 119220. [Google Scholar] [CrossRef] [PubMed]
- Al-Noshokaty, T.M.; Mesbah, N.M.; Abo-Elmatty, D.M.; Abulsoud, A.I.; Abdel-Hamed, A.R. Selenium nanoparticles overcomes sorafenib resistance in thioacetamide induced hepatocellular carcinoma in rats by modulation of mTOR, NF-κB pathways and LncRNA-AF085935/GPC3 axis. Life Sci. 2022, 303, 120675. [Google Scholar] [CrossRef]
- Wu, D.; Wang, H.; Hou, X.; Chen, H.; Ma, Y.; Hou, Y.; Hong, J. Effects of gold core size on regulating the performance of doxorubicin-conjugated gold nanoparticles. Nano Res. 2018, 11, 3396–3410. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Li, B.; Hou, Y.; Yang, J.; Yi, L. Development of a novel morphological paclitaxel-loaded PLGA microspheres for effective cancer therapy: In vitro and in vivo evaluations. Drug Deliv. 2018, 25, 166–177. [Google Scholar] [CrossRef]
- Acharya, S.; Nithyananthan, S.; Thirunavukkarasu, C. Selenium Nanoparticles Show Anticancer Activity Through Regulation of HIF-1α and HIF-2α Under Hypoxic Condition in Liver Cancer Cells. DNA Cell Biol. 2023, 42, 433–444. [Google Scholar] [CrossRef]
| Nanoparticle Composition | Object of Study | Molecular Mechanisms Activated by Nanoparticles | Form of Cell Death | Ref. |
|---|---|---|---|---|
| SeNPs or SeSo Selenium nanoparticles or sorafenib + selenium nanoparticles | HepG2 cells | Promote an increase in the expression of a number of pro-apoptotic genes, including GADD34, BAK, BAX, PUMA, CASP-3 and CASP-4; activate ER stress through the PERK signaling pathway; cause dose-dependent generation of various calcium signals | Apoptosis | [45] |
| Cur-SeNPs Curcumin + selenium nanoparticles | HepG2 cells | Activate the PI3K/Akt/mTOR pathway | Apoptosis | [27] |
| siRNA-PEI-SeNPs Small interfering RNA + polyethylenimine + selenium nanoparticles | HepG2 cells | Reduce the expression of HSP70; increase the activity of CASP-3 and the cleavage PARP | Apoptosis | [48] |
| LP-SeNPs Laminarin + selenium nanoparticles | HepG2 cells | Increase BAX mRNA expression and CASP-9 cleavage; decrease BCL-2 levels | Apoptosis | [49] |
| HE-SeNPs Hawthorn fruit extract + selenium nanoparticles | HepG2 cells | Increase the level of CASP-9; decrease the level of BCL-2; induce intracellular oxidative stress and mitochondrial dysfunction | Apoptosis | [50] |
| BFP-SeNPs Triple-helix β-glucan + selenium nanoparticles | HepG2 cells | Inhibit cell proliferation through cell cycle arrest in the S phase; cause condensation of nuclear chromatin and severe nuclear shrinkage; increase ROS; decrease mitochondrial membrane potential | Apoptosis | [51] |
| siRNA-RGDfC-SeNPs Small interfering RNA + peptide + selenium nanoparticles | HepG2 cells | KD of Oct4, which is accompanied by a decrease in the expression of Sox-2, Nanog, β-catenin and GSK-3β; activate Wnt/β-catenin signaling; reduce the expression of mTOR, AKT and PI3K; activate LC3-II and p62 | Autophagy | [86] |
| QCT-SeNPs Quercetin + selenium nanoparticles | Rat model of TAA-induced HCC | Increase oxidative stress; dysregulate the oncogenic p53/β-catenin/cyclin D signaling pathway | Apoptosis or autophagy | [39] |
| So + SeNPs Sorafenib + selenium nanoparticles or sorafenib + selenium nanoparticles + radiation | HepG2 cells or mice with HCC | Reduce the expression of CD34 and Ki67; increase the growth of CASP-3. With radiation, hydrogel led to improved protein, se-rum creatinine, cholesterol and blood glucose levels. | Apoptosis | [120] |
| So + SeNPs Sorafenib + selenium nanoparticles | Mouse model of TAA-induced HCC | Decrease angiogenesis and metastasis, affecting the mTOR and NF-kB pathways | Apoptosis | [121] |
| DOX + HA-SeNPs Doxorubicin + hyaluronic acid + selenium nanoparticles | HepG2 cells | Suppress proliferation; promote the production of ROS; induce apoptosis through activation of CASP-3 pathways | Apoptosis | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varlamova, E.G. Molecular Mechanisms of the Therapeutic Effect of Selenium Nanoparticles in Hepatocellular Carcinoma. Cells 2024, 13, 1102. https://doi.org/10.3390/cells13131102
Varlamova EG. Molecular Mechanisms of the Therapeutic Effect of Selenium Nanoparticles in Hepatocellular Carcinoma. Cells. 2024; 13(13):1102. https://doi.org/10.3390/cells13131102
Chicago/Turabian StyleVarlamova, Elena G. 2024. "Molecular Mechanisms of the Therapeutic Effect of Selenium Nanoparticles in Hepatocellular Carcinoma" Cells 13, no. 13: 1102. https://doi.org/10.3390/cells13131102
APA StyleVarlamova, E. G. (2024). Molecular Mechanisms of the Therapeutic Effect of Selenium Nanoparticles in Hepatocellular Carcinoma. Cells, 13(13), 1102. https://doi.org/10.3390/cells13131102







