Hydrogel-Integrated Millifluidic Systems: Advancing the Fabrication of Mucus-Producing Human Intestinal Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Decellularized and Methacrylated Small Intestinal Submucosa (dSIS-MA): Preparation, Printing, and Characterization
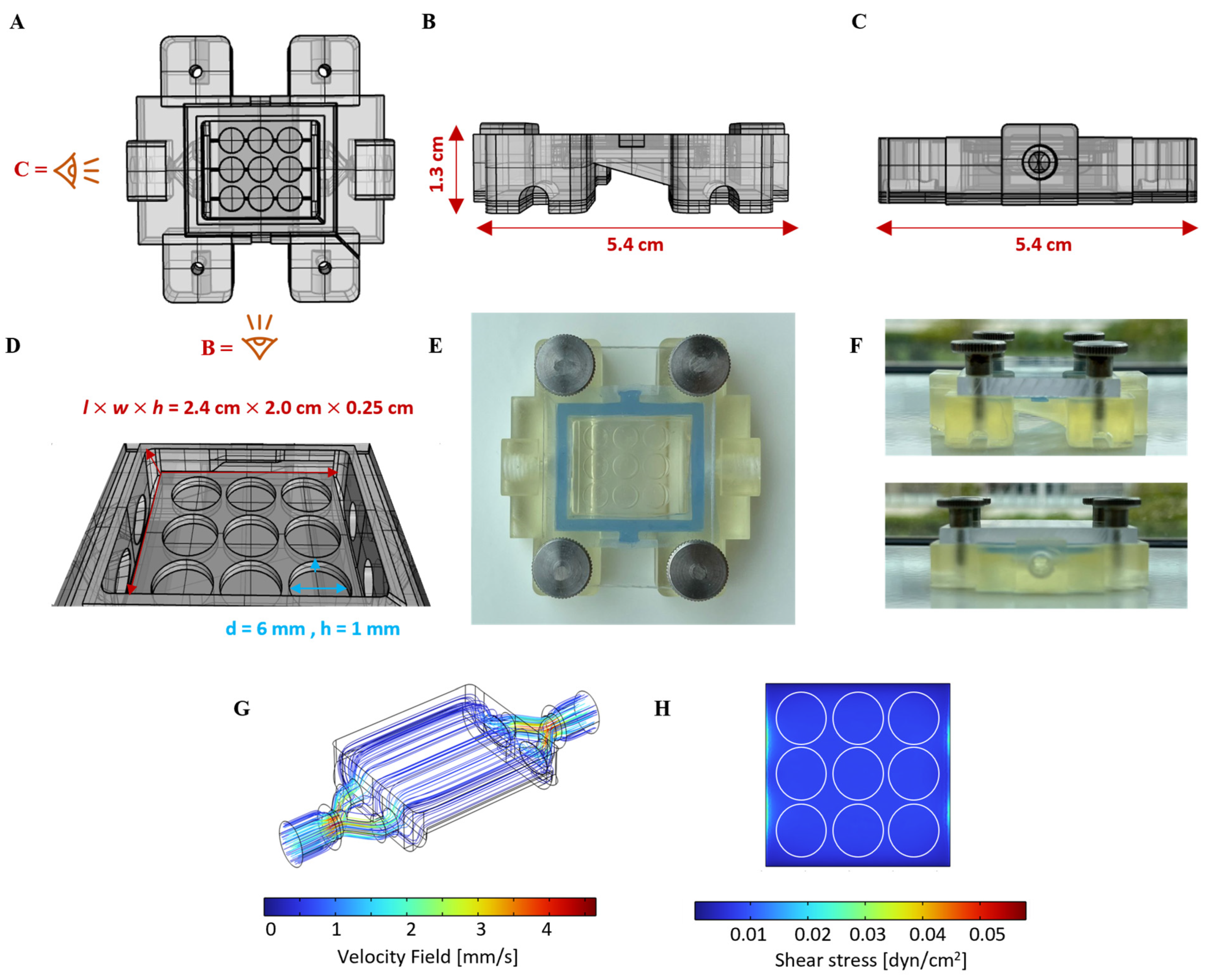
2.3. Flow Chamber Design, Fabrication, and Computational Fluid Dynamics (CFD)
2.4. Static Cell Culture
2.4.1. Culture and Seeding of HT29-MTX Cells
2.4.2. Crypt Isolation and Organoid Formation and Expansion
2.4.3. Culture of Organoid-Derived ISCs on dSIS-MA Hydrogels
2.5. Dynamic Cell Culture
2.6. Immunofluorescence Staining
2.7. Alkaline Phosphatase (ALP) Activity Assay
2.8. Mucus Quantification
2.8.1. Sample Preparation for Quantification of Mucins in Supernatants
2.8.2. Sample Preparation for Quantification of Cell-Bound Mucins
2.8.3. Mucin Quantification via Periodic Acid–Schiff Base (PAS) Reaction and Alcian Blue (AB) Staining
2.9. Statistical Analysis
3. Results and Discussion
3.1. Design of Hydrogel-Integrated Millifluidic Tissue Chamber and Flow Circuit
3.2. dSIS-MA-Based Hydrogel Scaffolds for Advanced Small Intestinal Models
3.3. Validation of the Millifluidic System with Mucus-Producing HT29-MTX Cells
3.4. Culture of Organoid-Derived ISCs on Scaffolds under Static and Dynamic Conditions
3.4.1. Formation and Culture of ISC-Based Monolayers on Scaffolds
3.4.2. Differentiation of ISC-Based Monolayers and Tissue Formation
3.4.3. Mucus Production and Secretion of ISC-Based Monolayers on Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, L.; Joyce, P.; Barnes, T.J.; Prestidge, C.A. Mimicking the Gastrointestinal Mucus Barrier: Laboratory-Based Approaches to Facilitate an Enhanced Understanding of Mucus Permeation. ACS Biomater. Sci. Eng. 2023, 9, 2819–2837. [Google Scholar] [CrossRef]
- Izadifar, Z.; Sontheimer-Phelps, A.; Lubamba, B.A.; Bai, H.; Fadel, C.; Stejskalova, A.; Ozkan, A.; Dasgupta, Q.; Bein, A.; Junaid, A.; et al. Modeling Mucus Physiology and Pathophysiology in Human Organs-on-Chips. Adv. Drug Deliv. Rev. 2022, 191, 114542. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal Mucus Components and Secretion Mechanisms: What We Do and Do Not Know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef]
- Atreya, R.; Siegmund, B. Location Is Important: Differentiation between Ileal and Colonic Crohn’s Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 544–558. [Google Scholar] [CrossRef]
- Martinez-Medina, J.N.; Ghazisaeedi, F.; Kramer, C.; Ziegler, J.F.; McParland, V.; Siegmund, B.; Jarquín-Díaz, V.H.; Fulde, M.; Forslund, S.K. Mucosal Washes Are Useful for Sampling Intestinal Mucus-Associated Microbiota Despite Low Biomass. bioRxiv 2023, 12.12.571228. [Google Scholar] [CrossRef]
- Nakamura, M.; Maeda, K.; Yamamoto, K.; Yamamura, T.; Sawada, T.; Ishikawa, E.; Kakushima, N.; Furukawa, K.; Iida, T.; Mizutani, Y.; et al. Preliminary Comparison of Endoscopic Brush and Net Catheters as the Sampling Tool to Analyze the Intestinal Mucus in the Rectum with Ulcerative Colitis Patients. Digestion 2022, 103, 232–243. [Google Scholar] [CrossRef]
- Kramer, C.; Rulff, H.; Ziegler, J.F.; Alzain, N.; Addante, A.; Kuppe, A.; Timm, S.; Schrade, P.; Bischoff, P.; Glauben, R.; et al. Ileal Mucus Viscoelastic Properties Differ in Crohn’s Disease. Mucosal Immunol. 2024. accepted. [Google Scholar] [CrossRef]
- Macierzanka, A.; Mackie, A.R.; Krupa, L. Permeability of the Small Intestinal Mucus for Physiologically Relevant Studies: Impact of Mucus Location and Ex Vivo Treatment. Sci. Rep. 2019, 9, 17516. [Google Scholar] [CrossRef]
- Martinez-Maqueda, D.; Miralles, B.; Recio, I. Ht29 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 113–124. [Google Scholar]
- Elzinga, J.; van der Lugt, B.; Belzer, C.; Steegenga, W.T. Characterization of Increased Mucus Production of Ht29-Mtx-E12 Cells Grown under Semi-Wet Interface with Mechanical Stimulation. PLoS ONE 2021, 16, e0261191. [Google Scholar] [CrossRef] [PubMed]
- Pauzuolis, M.; Samperio Ventayol, P.; Neyazi, M.; Bartfeld, S. Organoids as a Tool to Study the Impact of Heterogeneity in Gastrointestinal Epithelium on Host-Pathogen Interactions. Clin. Exp. Immunol. 2024, uxae002. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Taelman, J.; Diaz, M.; Guiu, J. Human Intestinal Organoids: Promise and Challenge. Front. Cell Dev. Biol. 2022, 10, 854740. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Chou, D.B.; Tovaglieri, A.; Ferrante, T.C.; Duckworth, T.; Fadel, C.; Frismantas, V.; Sutherland, A.D.; Jalili-Firoozinezhad, S.; Kasendra, M.; et al. Human Colon-on-a-Chip Enables Continuous in Vitro Analysis of Colon Mucus Layer Accumulation and Physiology. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 507–526. [Google Scholar] [CrossRef]
- Beaurivage, C.; Kanapeckaite, A.; Loomans, C.; Erdmann, K.S.; Stallen, J.; Janssen, R.A.J. Development of a Human Primary Gut-on-a-Chip to Model Inflammatory Processes. Sci. Rep. 2020, 10, 21475. [Google Scholar] [CrossRef]
- Co, J.Y.; Margalef-Catala, M.; Monack, D.M.; Amieva, M.R. Controlling the Polarity of Human Gastrointestinal Organoids to Investigate Epithelial Biology and Infectious Diseases. Nat. Protoc. 2021, 16, 5171–5192. [Google Scholar] [CrossRef]
- Dutton, J.S.; Hinman, S.S.; Kim, R.; Wang, Y.; Allbritton, N.L. Primary Cell-Derived Intestinal Models: Recapitulating Physiology. Trends Biotechnol. 2019, 37, 744–760. [Google Scholar] [CrossRef]
- Thorne, C.A.; Chen, I.W.; Sanman, L.E.; Cobb, M.H.; Wu, L.F.; Altschuler, S.J. Enteroid Monolayers Reveal an Autonomous Wnt and Bmp Circuit Controlling Intestinal Epithelial Growth and Organization. Dev. Cell 2018, 44, 624–633.e4. [Google Scholar] [CrossRef]
- VanDussen, K.L.; Marinshaw, J.M.; Shaikh, N.; Miyoshi, H.; Moon, C.; Tarr, P.I.; Ciorba, M.A.; Stappenbeck, T.S. Development of an Enhanced Human Gastrointestinal Epithelial Culture System to Facilitate Patient-Based Assays. Gut 2015, 64, 911–920. [Google Scholar] [CrossRef]
- Costa, J.; Ahluwalia, A. Advances and Current Challenges in Intestinal in Vitro Model Engineering: A Digest. Front. Bioeng. Biotechnol. 2019, 7, 144. [Google Scholar] [CrossRef]
- He, S.; Lei, P.; Kang, W.; Cheung, P.; Xu, T.; Mana, M.; Park, C.Y.; Wang, H.; Imada, S.; Russell, J.O.; et al. Stiffness Restricts the Stemness of the Intestinal Stem Cells and Skews Their Differentiation toward Goblet Cells. Gastroenterology 2023, 164, 1137–1151.e15. [Google Scholar] [CrossRef]
- Soofi, S.S.; Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. The Elastic Modulus of Matrigel as Determined by Atomic Force Microscopy. J. Struct. Biol. 2009, 167, 216–219. [Google Scholar] [CrossRef]
- Onfroy-Roy, L.; Hamel, D.; Foncy, J.; Malaquin, L.; Ferrand, A. Extracellular Matrix Mechanical Properties and Regulation of the Intestinal Stem Cells: When Mechanics Control Fate. Cells 2020, 9, 2629. [Google Scholar] [CrossRef]
- Wang, Y.; Gunasekara, D.B.; Reed, M.I.; DiSalvo, M.; Bultman, S.J.; Sims, C.E.; Magness, S.T.; Allbritton, N.L. A Microengineered Collagen Scaffold for Generating a Polarized Crypt-Villus Architecture of Human Small Intestinal Epithelium. Biomaterials 2017, 128, 44–55. [Google Scholar] [CrossRef]
- Schweinlin, M.; Wilhelm, S.; Schwedhelm, I.; Hansmann, J.; Rietscher, R.; Jurowich, C.; Walles, H.; Metzger, M. Development of an Advanced Primary Human in Vitro Model of the Small Intestine. Tissue Eng. Part. C Methods 2016, 22, 873–883. [Google Scholar] [CrossRef]
- Lindner, M.; Laporte, A.; Block, S.; Elomaa, L.; Weinhart, M. Physiological Shear Stress Enhances Differentiation, Mucus-Formation and Structural 3d Organization of Intestinal Epithelial Cells in Vitro. Cells 2021, 10, 2062. [Google Scholar] [CrossRef]
- Kim, H.J.; Ingber, D.E. Gut-on-a-Chip Microenvironment Induces Human Intestinal Cells to Undergo Villus Differentiation. Integr. Biol. (CAMB) 2013, 5, 1130–1140. [Google Scholar] [CrossRef]
- He, J.; Xie, X.; Xiao, Z.; Qian, W.; Zhang, L.; Hou, X. Piezo1 in Digestive System Function and Dysfunction. Int. J. Mol. Sci. 2023, 24, 12953. [Google Scholar] [CrossRef]
- He, L.; Si, G.; Huang, J.; Samuel, A.D.T.; Perrimon, N. Mechanical Regulation of Stem-Cell Differentiation by the Stretch-Activated Piezo Channel. Nature 2018, 555, 103–106. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, J.; Xu, Y.; Liu, C.; Qian, W.; Bai, T.; Hou, X. Piezo1 Regulates Intestinal Epithelial Function by Affecting the Tight Junction Protein Claudin-1 Via the Rock Pathway. Life Sci. 2021, 275, 119254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, T.; Xiong, Y.; Liu, C.; Liu, Y.; Hou, X.; Song, J. Mechanical Stimulation Activates Piezo1 to Promote Mucin2 Expression in Goblet Cells. J. Gastroenterol. Hepatol. 2021, 36, 3127–3139. [Google Scholar] [CrossRef] [PubMed]
- Almalla, A.; Elomaa, L.; Bechtella, L.; Daneshgar, A.; Yavvari, P.; Mahfouz, Z.; Tang, P.; Koksch, B.; Sauer, I.; Pagel, K.; et al. Papain-Based Solubilization of Decellularized Extracellular Matrix for the Preparation of Bioactive, Thermosensitive Pre-Gels. Biomacromolecules 2023, 24, 5620–5637. [Google Scholar] [CrossRef]
- Elomaa, L.; Gerbeth, L.; Almalla, A.; Fribiczer, N.; Daneshgar, A.; Tang, P.; Hillebrandt, K.H.; Seiffert, S.; Sauer, M.I.; Siegmund, B.; et al. Bioactive Photocrosslinkable Resin Solely Based on Refined Decellularized Small Intestine Submucosa for Vat Photopolymerization of in Vitro Tissue Mimics. Addit. Manuf. 2023, 64, 103439. [Google Scholar] [CrossRef]
- Poon, C. Measuring the Density and Viscosity of Culture Media for Optimized Computational Fluid Dynamics Analysis of in Vitro Devices. J. Mech. Behav. Biomed. Mater. 2022, 126, 105024. [Google Scholar] [CrossRef]
- Miyoshi, H.; Stappenbeck, T.S. In Vitro Expansion and Genetic Modification of Gastrointestinal Stem Cells in Spheroid Culture. Nat. Protoc. 2013, 8, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Zamora, C.Y.; Ward, E.M.; Kester, J.C.; Chen, W.L.K.; Velazquez, J.G.; Griffith, L.G.; Imperiali, B. Application of a Gut-Immune Co-Culture System for the Study of N-Glycan-Dependent Host-Pathogen Interactions of Campylobacter Jejuni. Glycobiology 2020, 30, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Donkers, J.M.; Eslami Amirabadi, H.; van de Steeg, E. Intestine-on-a-Chip: Next Level In vitro Research Model of the Human Intestine. Curr. Opin. Toxicol. 2021, 25, 6–14. [Google Scholar] [CrossRef]
- Altay, G.; Larranaga, E.; Tosi, S.; Barriga, F.M.; Batlle, E.; Fernandez-Majada, V.; Martinez, E. Self-Organized Intestinal Epithelial Monolayers in Crypt and Villus-Like Domains Show Effective Barrier Function. Sci. Rep. 2019, 9, 10140. [Google Scholar] [CrossRef] [PubMed]
- Kasendra, M.; Tovaglieri, A.; Sontheimer-Phelps, A.; Jalili-Firoozinezhad, S.; Bein, A.; Chalkiadaki, A.; Scholl, W.; Zhang, C.; Rickner, H.; Richmond, C.A.; et al. Development of a Primary Human Small Intestine-on-a-Chip Using Biopsy-Derived Organoids. Sci. Rep. 2018, 8, 2871. [Google Scholar] [CrossRef]
- Nikolaev, M.; Mitrofanova, O.; Broguiere, N.; Geraldo, S.; Dutta, D.; Tabata, Y.; Elci, B.; Brandenberg, N.; Kolotuev, I.; Gjorevski, N.; et al. Homeostatic Mini-Intestines through Scaffold-Guided Organoid Morphogenesis. Nature 2020, 585, 574–578. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human Gut-on-a-Chip Inhabited by Microbial Flora That Experiences Intestinal Peristalsis-Like Motions and Flow. Lab. Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Available online: https://formlabs.com/de/shop/materials/biomed-amber-resin/ (accessed on 16 April 2024).
- Almalla, A.; Elomaa, L.; Fribiczer, N.; Landes, T.; Tang, P.; Mahfouz, Z.; Koksch, B.; Hillebrandt, K.H.; Sauer, I.M.; Heinemann, D.; et al. Chemistry Matters: A Side-by-Side Comparison of Two Chemically Distinct Methacryloylated Decm Bioresins for Vat Photopolymerization. Biomater. Adv. 2024, 160, 213850. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Almalla, A.; Keshi, E.; Hillebrandt, K.H.; Sauer, I.M.; Weinhart, M. Rise of Tissue- and Species-Specific 3d Bioprinting Based on Decellularized Extracellular Matrix-Derived Bioinks and Bioresins. Biomater. Biosyst. 2023, 12, 100084. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of Gelma/Pcl and Decm/Pcl Resins for 3d Printing of Acellular in Vitro Tissue Scaffolds by Stereolithography. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110958. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Rodansky, E.S.; Sauder, K.L.; Horowitz, J.C.; Mih, J.D.; Tschumperlin, D.J.; Higgins, P.D. Matrix Stiffness Corresponding to Strictured Bowel Induces a Fibrogenic Response in Human Colonic Fibroblasts. Inflamm. Bowel Dis. 2013, 19, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.; Prasetyanti, P.R.; De Lau, W.; Rodermond, H.; Clevers, H.; Medema, J.P. Monoclonal Antibodies against Lgr5 Identify Human Colorectal Cancer Stem Cells. Stem Cells 2012, 30, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rodgers, G.P. Olfactomedin 4 Is Not a Precise Marker for Human Intestinal Stem Cells, but Is Involved in Intestinal Carcinogenesis. Gastroenterology 2022, 162, 1001–1004. [Google Scholar] [CrossRef]
- Yin, J.; Sunuwar, L.; Kasendra, M.; Yu, H.; Tse, C.M.; Talbot, C.C., Jr.; Boronina, T.; Cole, R.; Karalis, K.; Donowitz, M. Fluid Shear Stress Enhances Differentiation of Jejunal Human Enteroids in Intestine-Chip. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G258–G271. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Han, W.; Kim, H.; Ha, D.H.; Jang, J.; Kim, B.S.; Cho, D.W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Kasendra, M.; Luc, R.; Yin, J.; Manatakis, D.V.; Kulkarni, G.; Lucchesi, C.; Sliz, J.; Apostolou, A.; Sunuwar, L.; Obrigewitch, J.; et al. Duodenum Intestine-Chip for Preclinical Drug Assessment in a Human Relevant Model. eLife 2020, 9, 50135. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning from Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Lopes, C.; Pereira-Lima, J.; Hartmann, A. Correlation between Alcian Blue–Periodic Acid–Schiff Stain and Immunohistochemical Expression of Mucin 2 in Barrett’s Oesophagus; Blackwell Publishing Ltd.: Oxford, UK, 2004; Volume 45. [Google Scholar]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A Complex Human Gut Microbiome Cultured in an Anaerobic Intestine-on-a-Chip. Nat. Biomed. Eng. 2019, 3, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.C.; Berrie, D.; Li, J.; Liu, X.; Rickerson, C.; Mkoji, D.; Iqbal, A.; Tan, S.; Doty, A.L.; Glover, S.C.; et al. Quantitative Assessment of Intestinal Stiffness and Associations with Fibrosis in Human Inflammatory Bowel Disease. PLoS ONE 2018, 13, e0200377. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated Polymerization of Peg-Diacrylate with Lithium Phenyl-2,4,6-Trimethylbenzoylphosphinate: Polymerization Rate and Cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almalla, A.; Alzain, N.; Elomaa, L.; Richter, F.; Scholz, J.; Lindner, M.; Siegmund, B.; Weinhart, M. Hydrogel-Integrated Millifluidic Systems: Advancing the Fabrication of Mucus-Producing Human Intestinal Models. Cells 2024, 13, 1080. https://doi.org/10.3390/cells13131080
Almalla A, Alzain N, Elomaa L, Richter F, Scholz J, Lindner M, Siegmund B, Weinhart M. Hydrogel-Integrated Millifluidic Systems: Advancing the Fabrication of Mucus-Producing Human Intestinal Models. Cells. 2024; 13(13):1080. https://doi.org/10.3390/cells13131080
Chicago/Turabian StyleAlmalla, Ahed, Nadra Alzain, Laura Elomaa, Fiona Richter, Johanna Scholz, Marcus Lindner, Britta Siegmund, and Marie Weinhart. 2024. "Hydrogel-Integrated Millifluidic Systems: Advancing the Fabrication of Mucus-Producing Human Intestinal Models" Cells 13, no. 13: 1080. https://doi.org/10.3390/cells13131080
APA StyleAlmalla, A., Alzain, N., Elomaa, L., Richter, F., Scholz, J., Lindner, M., Siegmund, B., & Weinhart, M. (2024). Hydrogel-Integrated Millifluidic Systems: Advancing the Fabrication of Mucus-Producing Human Intestinal Models. Cells, 13(13), 1080. https://doi.org/10.3390/cells13131080






