Ca2+-Dependent Processes of Innate Immunity in IBD
Abstract
1. Introduction
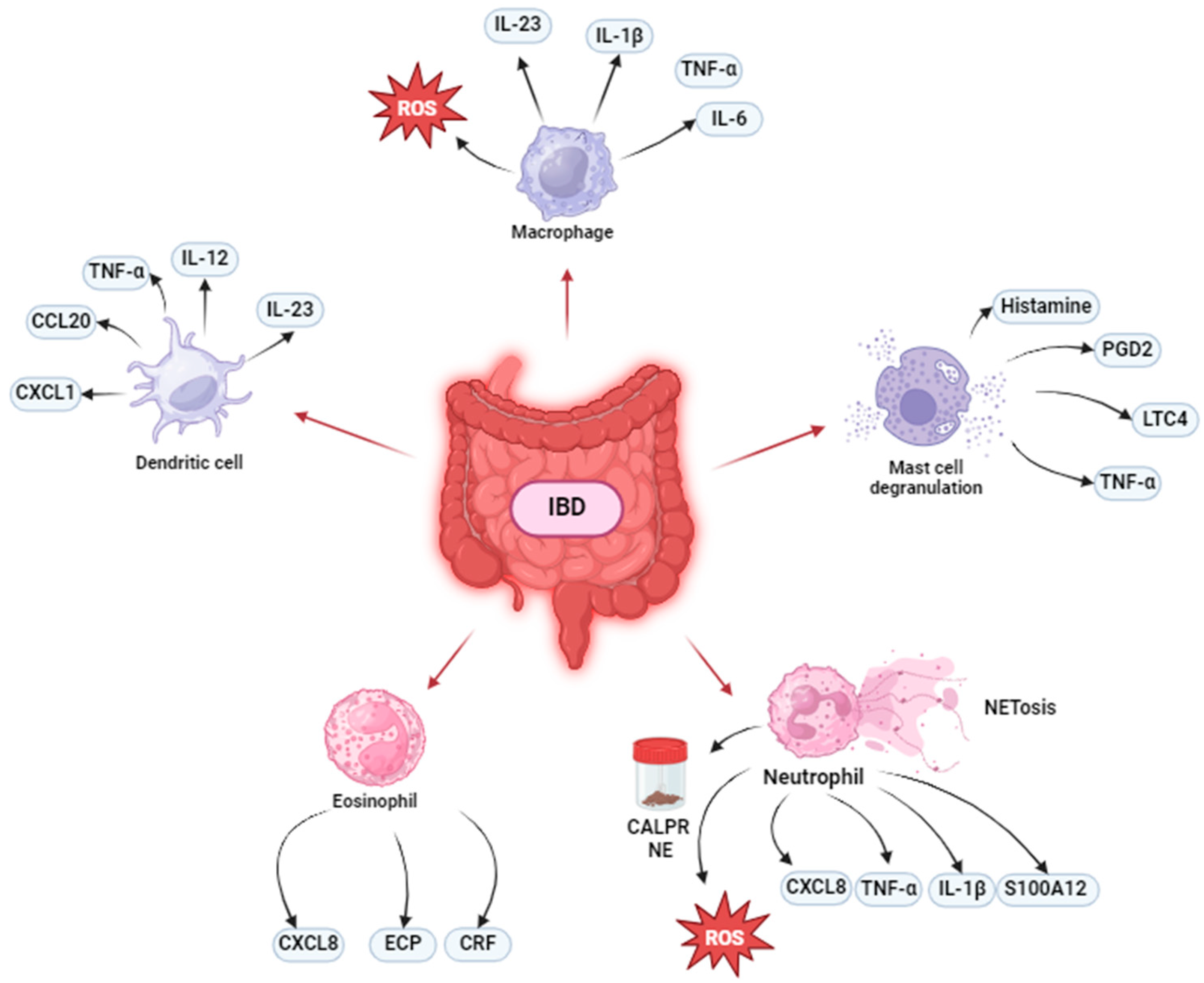
2. Innate Immunity in IBD
2.1. Monocytes
- Classical (CD14++CD16−) monocytes are large granular cells with low susceptibility to undergo apoptosis. They are the most abundant subset of monocytes in the blood and are involved in phagocytosis, antigen presentation, and cytokine production;
- Intermediate (CD14++CD16+) monocytes have intermediate levels of CD14 and CD16 expression and are involved in antigen presentation, cytokine production, and wound healing;
- Nonclassical (CD14+CD16++) monocytes have high levels of CD16 expression and low levels of CD14 expression. They are involved in patrolling the endothelium for signs of infection or damage, as well as in tissue repair and regeneration. These subsets have seemingly distinct functions within homeostasis and inflammation based on receptor expression, gene-expression profiles, and cytokine responses.
2.2. Macrophages
2.3. Dendritic Cell
2.4. Mast Cells
2.5. Granulocyte Role in IBD
2.5.1. Neutrophils
2.5.2. Eosinophils
2.5.3. Basophils
3. Ca2+ Signalling in Innate Immune Cells: Potential Therapeutic Targets for IBD
3.1. Monocytes
3.2. Macrophages
3.3. Dendritic Cells
3.4. Mast Cells
3.5. Neutrophils
3.6. Eosinophils
3.7. Basophils
4. Concluding Remarks
Funding
Conflicts of Interest
References
- GBD Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Li, M.; He, T.; Guo, S.; Zhu, L.; Tan, J.; Wang, B. Novel approaches in IBD therapy: Targeting the gut microbiota-bile acid axis. Gut Microbes 2024, 16, 2356284. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liao, S.; Lv, L.; Li, C.; Mei, Z. Intestinal Immune Imbalance is an Alarm in the Development of IBD. Mediat. Inflamm. 2023, 2023, 1073984. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, M.-F.; Liang, Y.-J.; Xu, J.; Xu, H.-M.; Nie, Y.-Q.; Wang, L.-S.; Yao, J.; Li, D.-F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- Gren, S.T.; Grip, O. Role of Monocytes and Intestinal Macrophages in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.L.; Balasuriya, G.K.; Spencer, S.J.; Hill-Yardin, E.L. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1701–1718. [Google Scholar] [CrossRef]
- Letizia, M.; Wang, Y.H.; Kaufmann, U.; Gerbeth, L.; Sand, A.; Brunkhorst, M.; Weidner, P.; Ziegler, J.F.; Bottcher, C.; Schlickeiser, S.; et al. Store-operated calcium entry controls innate and adaptive immune cell function in inflammatory bowel disease. EMBO Mol. Med. 2022, 14, e15687. [Google Scholar] [CrossRef]
- Zhou, G.; Yu, L.; Fang, L.; Yang, W.; Yu, T.; Miao, Y.; Chen, M.; Wu, K.; Chen, F.; Cong, Y.; et al. CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD. Gut 2018, 67, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ruan, G.; Cheng, Y.; Yi, A.; Chen, D.; Wei, Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front. Immunol. 2022, 13, 1055914. [Google Scholar] [CrossRef] [PubMed]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Krishnamoorthy, R.R.; Stankowska, D.L. Modulating mitochondrial calcium channels (TRPM2/MCU/NCX) as a therapeutic strategy for neurodegenerative disorders. Front. Neurosci. 2023, 17, 1202167. [Google Scholar] [CrossRef] [PubMed]
- Mareedu, S.; Million, E.D.; Duan, D.; Babu, G.J. Abnormal Calcium Handling in Duchenne Muscular Dystrophy: Mechanisms and Potential Therapies. Front. Physiol. 2021, 12, 647010. [Google Scholar] [CrossRef] [PubMed]
- Iamartino, L.; Brandi, M.L. The calcium-sensing receptor in inflammation: Recent updates. Front. Physiol. 2022, 13, 1059369. [Google Scholar] [CrossRef]
- Lin, Y.; Cui, X.; Cao, Q.; Bi, R.; Liu, Y.; Jing, D.; Yue, C.; Zhao, Q.; Wang, Y.; Liu, S.; et al. TRPC absence induces pro-inflammatory macrophages and gut microbe disorder, sensitizing mice to colitis. Int. Immunopharmacol. 2023, 115, 109655. [Google Scholar] [CrossRef]
- Morita, T.; Mitsuyama, K.; Yamasaki, H.; Mori, A.; Yoshimura, T.; Araki, T.; Morita, M.; Tsuruta, K.; Yamasaki, S.; Kuwaki, K.; et al. Gene Expression of Transient Receptor Potential Channels in Peripheral Blood Mononuclear Cells of Inflammatory Bowel Disease Patients. J. Clin. Med. 2020, 9, 2643. [Google Scholar] [CrossRef]
- Alharbi, A.F.; Parrington, J. Deciphering the Role of Endolysosomal Ca2+ Channels in Immunity. Front. Immunol. 2021, 12, 656965. [Google Scholar] [CrossRef]
- Trebak, M.; Kinet, J.-P. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Davies, J.M.; Abreu, M.T. The innate immune system and inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Faenza, I.; Blalock, W.L. Innate Immunity: A Balance between Disease and Adaption to Stress. Biomolecules 2022, 12, 737. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Cherfane, C.; Click, B.; Ramos-Rivers, C.; Koutroubakis, I.E.; Hashash, J.G.; Babichenko, D.; Tang, G.; Dunn, M.; Barrie, A.; et al. Monocytosis Is a Biomarker of Severity in Inflammatory Bowel Disease: Analysis of a 6-Year Prospective Natural History Registry. Inflamm. Bowel Dis. 2022, 28, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am. J. Transl. Res. 2016, 8, 2490–2497. [Google Scholar] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.; Diehl, L. Dendritic cells in IBD pathogenesis: An area of therapeutic opportunity? J. Pathol. 2014, 232, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.J.; Frei, S.M.; Stevens, R.L. The Multifaceted Mast Cell in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.N.; Leitinger, N. Purinergic and Calcium Signaling in Macrophage Function and Plasticity. Front. Immunol. 2014, 5, 580. [Google Scholar] [CrossRef] [PubMed]
- Babb, R.R. Cromolyn Sodium in the Treatment of Ulcerative Colitis. J. Clin. Gastroenterol. 1980, 2, 229–232. [Google Scholar] [CrossRef]
- Eliakim, R.; Karmeli, F.; Okon, E.; Rachmilewitz, D. Ketotifen effectively prevents mucosal damage in experimental colitis. Gut 1992, 33, 1498–1503. [Google Scholar] [CrossRef][Green Version]
- De Winter, B.Y.; van den Wijngaard, R.M.; de Jonge, W.J. Intestinal mast cells in gut inflammation and motility disturbances. Biochim Biophys Acta 2012, 1822, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G. Mast cells and inflammatory bowel disease. Curr. Opin. Pharmacol. 2015, 25, 45–49. [Google Scholar] [CrossRef] [PubMed]
- De Zuani, M.; Secco, C.D.; Frossi, B. Mast cells at the crossroads of microbiota and IBD. Eur. J. Immunol. 2018, 48, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Zhou, Y.-L.; Sun, H.; Zhang, Y.; Shen, C.; Wang, Z.; Xuan, B.; Zhao, Y.; Ma, Y.; Yan, Y.; et al. Microbiome and metabolome features in inflammatory bowel disease via multi-omics integration analyses across cohorts. Nat. Commun. 2023, 14, 7135. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Pathirana, W.G.W.; Chubb, S.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77–90. [Google Scholar] [PubMed]
- Barry, R.; Ruano-Gallego, D.; Radhakrishnan, S.T.; Lovell, S.; Yu, L.; Kotik, O.; Glegola-Madejska, I.; Tate, E.W.; Choudhary, J.S.; Williams, H.R.T.; et al. Faecal neutrophil elastase-antiprotease balance reflects colitis severity. Mucosal Immunol. 2020, 13, 322–333. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Liu, Y.; Li, B.; Zhang, W.; Wang, L.; Yu, M.; Zhao, X.; Du, J.; Zhang, J.; et al. Neutrophil Extracellular Traps Induce Intestinal Damage and Thrombotic Tendency in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Loktionov, A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J. Gastroenterol. 2019, 25, 3503–3526. [Google Scholar] [CrossRef]
- Yantiss, R.K. Eosinophils in the GI tract: How many is too many and what do they mean? Mod. Pathol. 2015, 28 (Suppl. S1), S7–S21. [Google Scholar] [CrossRef]
- Alhmoud, T.; Gremida, A.; Steele, D.C.; Fallahi, I.; Tuqan, W.; Nandy, N.; Ismail, M.; Altamimi, B.A.; Xiong, M.J.; Kerwin, A.; et al. Outcomes of inflammatory bowel disease in patients with eosinophil-predominant colonic inflammation. BMJ Open Gastroenterol. 2020, 7, e000373. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Bel, E.H.; Bourdin, A.; Chan, R.; Han, J.K.; Keene, O.N.; Liu, M.C.; Martin, N.; Papi, A.; Roufosse, F.; et al. From DREAM to REALITI-A and beyond: Mepolizumab for the treatment of eosinophil-driven diseases. Allergy 2022, 77, 778–797. [Google Scholar] [CrossRef] [PubMed]
- Amouzadeh-Ghadikolai, O.; Reicht, G.; Quehenberger, F.; Robier, C. Basophilia of the peripheral blood in patients with ulcerative colitis. Scand. J. Gastroenterol. 2020, 55, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Sarfati, M.; Wakahara, K.; Chapuy, L.; Delespesse, G. Mutual Interaction of Basophils and T Cells in Chronic Inflammatory Diseases. Front. Immunol. 2015, 6, 399. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Higashiyama, M.; Watanabe, C.; Tomioka, A.; Ito, S.; Tanemoto, R.; Mizoguchi, A.; Nishii, S.; Wada, A.; Sugihara, N.; et al. Proinflammatory role of basophils in oxazolone-induced chronic intestinal inflammation. J. Gastroenterol. Hepatol. 2022, 37, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Elajnaf, T.; Iamartino, L.; Mesteri, I.; Müller, C.; Bassetto, M.; Manhardt, T.; Baumgartner-Parzer, S.; Kallay, E.; Schepelmann, M. Nutritional and Pharmacological Targeting of the Calcium-Sensing Receptor Influences Chemically Induced Colitis in Mice. Nutrients 2019, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Zumerle, S.; Calì, B.; Munari, F.; Angioni, R.; Di Virgilio, F.; Molon, B.; Viola, A. Intercellular Calcium Signaling Induced by ATP Potentiates Macrophage Phagocytosis. Cell Rep. 2019, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shumilina, E.; Huber, S.M.; Lang, F. Ca2+signaling in the regulation of dendritic cell functions. Am. J. Physiol. Cell Physiol. 2011, 300, C1205–C1214. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Beaven, M.A. Regulators of Ca(2+) signaling in mast cells: Potential targets for treatment of mast cell-related diseases? Adv. Exp. Med. Biol. 2011, 716, 62–90. [Google Scholar]
- Holowka, D.; Wilkes, M.; Stefan, C.; Baird, B. Roles for Ca2+ mobilization and its regulation in mast cell functions: Recent progress. Biochem. Soc. Trans. 2016, 44, 505–509. [Google Scholar] [CrossRef]
- Chen, E.; Chuang, L.-S.; Giri, M.; Villaverde, N.; Hsu, N.-Y.; Sabic, K.; Joshowitz, S.; Gettler, K.; Nayar, S.; Chai, Z.; et al. Inflamed Ulcerative Colitis Regions Associated with MRGPRX2-Mediated Mast Cell Degranulation and Cell Activation Modules, Defining a New Therapeutic Target. Gastroenterology 2021, 160, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Banerjee, S.; Feigley, A.; Abe, H.; Blackwell, T.S.; Pozzi, A.; Hudson, B.G.; Zent, R. Salt-bridge modulates differential calcium-mediated ligand binding to integrin alpha1- and alpha2-I domains. Sci. Rep. 2018, 8, 2916. [Google Scholar] [CrossRef] [PubMed]
- Danne, C.; Skerniskyte, J.; Marteyn, B.; Sokol, H. Neutrophils: From IBD to the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Kenny, E.F.; Herzig, A.; Kruger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Gößwein, S.; Lindemann, A.; Mahajan, A.; Maueröder, C.; Martini, E.; Patankar, J.; Schett, G.; Becker, C.; Wirtz, S.; Naumann-Bartsch, N.; et al. Citrullination Licenses Calpain to Decondense Nuclei in Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 2481. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Kucharzik, T.; Kraft, M.; Vogl, T.; Sorg, C.; Domschke, W.; Roth, J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 2003, 52, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Hussain, A.; Sehmi, R.; Janssen, L. The Cycling of Intracellular Calcium Released in Response to Fluid Shear Stress Is Critical for Migration-Associated Actin Reorganization in Eosinophils. Cells 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Kolachala, V.L.; Bajaj, R.; Wang, L.; Yan, Y.; Ritzenthaler, J.D.; Gewirtz, A.T.; Roman, J.; Merlin, D.; Sitaraman, S.V. Epithelial-derived Fibronectin Expression, Signaling, and Function in Intestinal Inflammation. J. Biol. Chem. 2007, 282, 32965–32973. [Google Scholar] [CrossRef]
- Jansen, C.; Tobita, C.; Umemoto, E.U.; Starkus, J.; Rysavy, N.M.; Shimoda, L.M.N.; Sung, C.; Stokes, A.; Turner, H. Calcium-dependent, non-apoptotic, large plasma membrane bleb formation in physiologically stimulated mast cells and basophils. J. Extracell. Vesicles 2019, 8, 1578589. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Rovedatti, L.; Kaur, R.; Spencer, J.P.; Brown, J.T.; Morisset, V.D.; Biancheri, P.; Leakey, N.A.; Wilde, J.I.; Scott, L.; et al. Targeting gut T cell Ca2+ release-activated Ca2+ channels inhibits T cell cytokine production and T-box transcription factor T-bet in inflammatory bowel disease. J. Immunol. 2009, 183, 3454–3462. [Google Scholar] [CrossRef]
- Kav, T.; Akyol, A.; Aksoy, E.; Ozer, C.; Torgutalp, M.; Sivri, B. P029 Azelnidipine, a novel calcium channel blocker, ameliorates severity of colitis in DSS induced colitis in mice possibly by modulating tissue levels of TNF-alpha and IL-6. J. Crohn’s Colitis 2017, 11, S93–S94. [Google Scholar] [CrossRef]
| Cell Type | Pathogenesis | Ca2+-Involvement | Therapeutic Approach |
|---|---|---|---|
| Monocytes | ↑ CD16− monocytes ↓ CD16+ monocytes |
| |
| Macrophages | TNF—α IL-1β IL-6 IL-23/IFN-γ axis activation Increase of Th17-inducing activity |
| TNF-α blockade. a4b7 blockade. Thiopurines. IL-4. Arginase. Polyamines. SCFAs. |
| Dendritic cells | IL-12. IL-23 (Th17 cell differentiation, IL-17A and IL-17F production). TNF-α. CCL20 CXCL1 Gut microflora hyperresponsiveness. |
| |
| Mast cells | Histamine PGD2 LTs (e.g., LTC4) TNF-α |
| MC stabilizers (cromolyn sodium, ketitofen). Activation of LIMR3. Protease, tryptase and histamine inhibitors. a4β7 integrin blockade. Administration of S. cerevisiae-derived β-glucan. |
| Neutrophils | Faecal CALPR and NE TNF-α CXCL8 IL-1β ROS NETs S100A12 |
| RAGE blockade. S100A12 blockade. |
| Eosinophils | ECP CRF production induced MC degranulation Immune cells recruitment | CCR3 blockade. ECP blockade. IL-5 blockade. | |
| Basophils | TNF-α. Promotion of emergence memory IL-17+, IL-17+/IFN-γ+ Th cells. Induction of Th2, Th17, and Th17/Th1 effector responses. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palestra, F.; Memoli, G.; Ventrici, A.; Trocchia, M.; Galdiero, M.; Varricchi, G.; Loffredo, S. Ca2+-Dependent Processes of Innate Immunity in IBD. Cells 2024, 13, 1079. https://doi.org/10.3390/cells13131079
Palestra F, Memoli G, Ventrici A, Trocchia M, Galdiero M, Varricchi G, Loffredo S. Ca2+-Dependent Processes of Innate Immunity in IBD. Cells. 2024; 13(13):1079. https://doi.org/10.3390/cells13131079
Chicago/Turabian StylePalestra, Francesco, Gina Memoli, Annagioia Ventrici, Marialuisa Trocchia, Mariarosaria Galdiero, Gilda Varricchi, and Stefania Loffredo. 2024. "Ca2+-Dependent Processes of Innate Immunity in IBD" Cells 13, no. 13: 1079. https://doi.org/10.3390/cells13131079
APA StylePalestra, F., Memoli, G., Ventrici, A., Trocchia, M., Galdiero, M., Varricchi, G., & Loffredo, S. (2024). Ca2+-Dependent Processes of Innate Immunity in IBD. Cells, 13(13), 1079. https://doi.org/10.3390/cells13131079







